PURPOSE
The US Food and Drug Administration approved abemaciclib in combination with endocrine therapy (ET) for the adjuvant treatment of adult patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative, node-positive, early breast cancer (EBC) at high risk of recurrence and a Ki-67 score ≥ 20%.
PATIENTS AND METHODS
The approval was based on monarchE, a phase III, open-label, 2-cohort, multicenter trial of patients with EBC randomly assigned to receive abemaciclib plus ET (n = 2,808) or ET alone (n = 2,829). Abemaciclib was given at 150 mg orally twice daily for 2 years.
RESULTS
Invasive disease-free survival (IDFS) in the intent-to-treat population was statistically significant at the second IDFS interim analysis (IA; March 2020; hazard ratio [HR; 95% CI], 0.747 [0.598 to 0.932]; P = .0096); however, only 12.5% of patients had completed adjuvant therapy, and the HR for overall survival (OS) was > 1. A prespecified, controlled analysis of IDFS in patients with Ki-67 ≥ 20% in cohort 1 was statistically significant at the final IDFS analysis (July 2020; HR [95% CI], 0.643 [0.475 to 0.872]; P = .0042). At the first OS IA (April 2021), the majority of patients had completed adjuvant therapy, IDFS remained consistent, and potential detriment in OS was not observed for this subgroup (HR [95% CI], 0.767 [0.511 to 1.152]). The HR for OS in the intent-to-treat population at OS IA remained > 1 (HR [95% CI], 1.091 [0.818 to 1.455]). More patients in the abemaciclib plus ET arm experienced treatment emergent adverse events (all grades 98.4% v 88.8%, grade 3 ≥ 49.7% v 16.3%).
CONCLUSION
The approval of abemaciclib in adjuvant EBC was limited to patients with high risk of recurrence and Ki-67 ≥ 20%, given their favorable benefit:risk with a statistically significant IDFS advantage and no observed detriment on survival.
INTRODUCTION
Over the past decade, the treatment of patients with early breast cancer (EBC) has been substantially affected by six US Food and Drug Administration (FDA) approvals in the neoadjuvant and adjuvant settings.1 Four of these approvals, pertuzumab (in the neoadjuvant and adjuvant settings), neratinib, and trastuzumab emtansine, were for patients with hormone receptor–negative, human epidermal growth factor receptor 2 (HER2)–positive breast cancer, pembrolizumab was for patients with triple-negative breast cancer in the neoadjuvant and adjuvant settings, and most recently abemaciclib was approved for the treatment of patients with hormone receptor–positive (HR+), HER2-negative (HER2–) breast cancer in the adjuvant setting.2-7 The majority of these approvals were based on statistically significant and clinically meaningful improvements in either invasive disease-free survival (IDFS) or event-free survival. Since trials in EBC may take a decade or more to mature, a meaningful improvement in overall survival (OS) could take as long to be apparent; none of these approvals required that OS be statistically significant at the time of submission.4,6,7 However, interim OS was always assessed for consistency of effect to be certain there were no signs of potential detriment.
CONTEXT
Key Objective
Until abemaciclib's approval in early breast cancer (EBC), none of the previous US Food and Drug Administration (FDA) approvals in 16 years have included approval for patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative EBC. This summary presents FDA's analysis of data from the monarchE trial, which was the basis for the approval of abemaciclib + endocrine therapy (ET) in the adjuvant setting and highlights review considerations.
Knowledge Generated
Invasive disease-free survival was significantly improved in the intent-to-treat population, but overall survival, although immature, showed a potential detriment in survival. The FDA indication for abemaciclib + ET was limited to the prespecified population with Ki-67 ≥ 20% in cohort 1 where invasive disease-free survival remained consistent and no potential detriment in overall survival was observed. FDA contemporaneously approved a Ki-67 companion diagnostic.
Relevance
In EBC, use abemaciclib + ET in patients at high risk of recurrence on the basis of monarchE cohort 1 criteria and whose tumors are Ki-67 ≥ 20% on the basis of an FDA-approved test.
The abemaciclib approval represents the first adjuvant approval of a kinase inhibitor of cyclin-dependent kinase 4 and 6, and is the first adjuvant approval for patients with HR+, HER2– EBC in 16 years. Although many patients with resectable HR+, HER2– EBC will be cured, patients with high-risk EBC are at risk for recurrence and death from metastatic disease. Many factors are considered when determining which patients with HR+, HER2– breast cancer are at high risk of recurrence including size and histologic grade of the tumor, as well as the number of involved lymph nodes.8 Ki-67 is a nuclear protein and a marker of cellular proliferation, which has also been shown to be a prognostic factor of clinical outcomes in EBC.9-11 Here, we present the FDA analysis of patient-level information submitted as raw and derived data sets, important review considerations, and how these provided the basis for approval of abemaciclib.
PATIENTS AND METHODS
Clinical Trial Design
The ongoing monarchE (ClinicalTrials.gov identifier: NCT03155997)12 trial is a phase III, multicenter, randomized, open-label trial in adult patients, with node-positive, HR+, HER2– EBC who completed definitive locoregional therapy (with or without neoadjuvant or adjuvant chemotherapy) and are at high risk of disease recurrence on the basis of clinical or pathologic features or Ki-67 score comparing abemaciclib plus endocrine therapy (ET) to ET alone (Fig 1). Random assignment was stratified by region, prior chemotherapy, and menopausal status. The trial enrolled patients in the following two cohorts:
Cohort 1: patients with ≥ 4 pathologic positive axillary lymph nodes (pALN) or 1-3 pALN and at least one of the following: tumor histologic grade 3 or tumor size ≥ 50 mm.
Cohort 2: patients with 1-3 pALN and Ki-67 score ≥ 20%.
FIG 1.
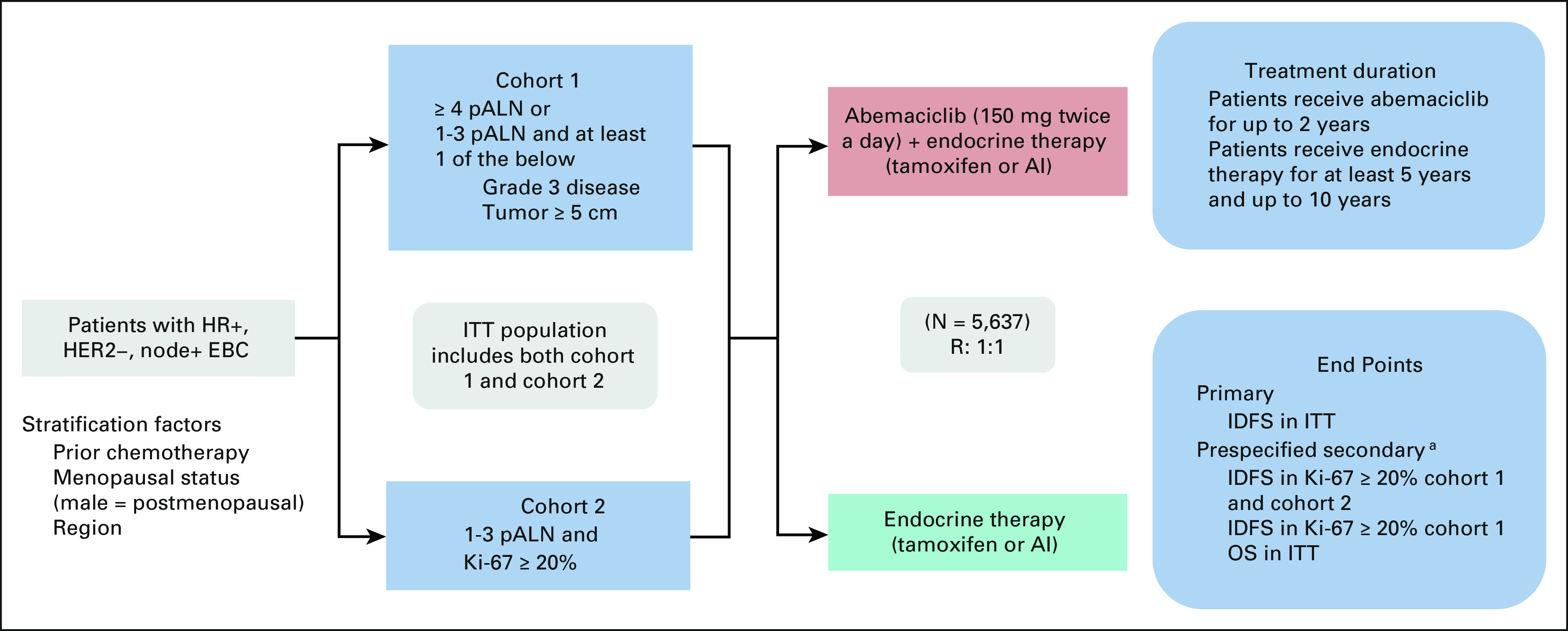
Trial schema of monarchE. Study design of monarchE, including key eligibility criteria and stratification factors, two enrollment cohorts, treatments arms (A = experimental—ET with abemaciclib v B = control—ET alone), treatment duration, and study end points. aA gated hierarchical testing strategy to control for type 1 error included three additional end points, IDFS in patients with Ki-67 score ≥ 20% from cohorts 1 and 2, followed by IDFS in patients with a Ki-67 score ≥ 20% from cohort 1 alone, and finally OS in the ITT population. AI, aromatase inhibitor; EBC, early breast cancer; ET, endocrine therapy; HER2–, human epidermal growth factor receptor 2–negative; HR+, hormone receptor–positive; IDFS, invasive disease-free survival; ITT, intent-to-treat; OS, overall survival; pALN, pathologic positive axillary lymph nodes; R, random assignment.
All patients in cohort 1 with available untreated breast tumor samples were tested retrospectively at central sites using the Ki-67 IHC MIB-1 pharmDx (Dako Omnis, Carpinteria, CA; hereafter referred to as Ki-67 CDx) assay to establish whether the Ki-67 score was ≥ 20% or < 20%. Patients in cohort 2 had breast tumor samples tested centrally with the same assay to be eligible for enrollment.13
Random assignment occurred within a maximum of 16 months following definitive breast cancer surgery. Patients received abemaciclib (150 mg orally twice daily) for up to 2 years plus ET or adjuvant ET per physician's choice of either an aromatase inhibitor or tamoxifen (with or without a gonadotrophin-releasing hormone agonist per standard of care) for a total ET duration of at least 5 and for up to 10 years, if deemed medically appropriate.
Statistical Analysis
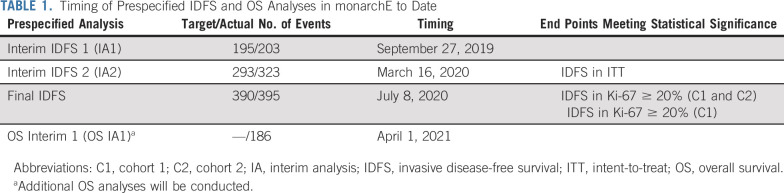
The study was powered to test the intent-to-treat (ITT) population for IDFS. The ITT population represented all of cohort 1 and cohort 2. A gated hierarchical testing strategy included three additional end points: IDFS in patients with Ki-67 score ≥ 20% from cohorts 1 and 2, IDFS in patients with a Ki-67 score ≥ 20% from cohort 1 alone, and OS in the ITT population. Two interim analyses and one final efficacy analysis for IDFS were planned, as well as two interim analyses and one final OS analysis (Table 1).
TABLE 1.
Timing of Prespecified IDFS and OS Analyses in monarchE to Date
RESULTS
Efficacy
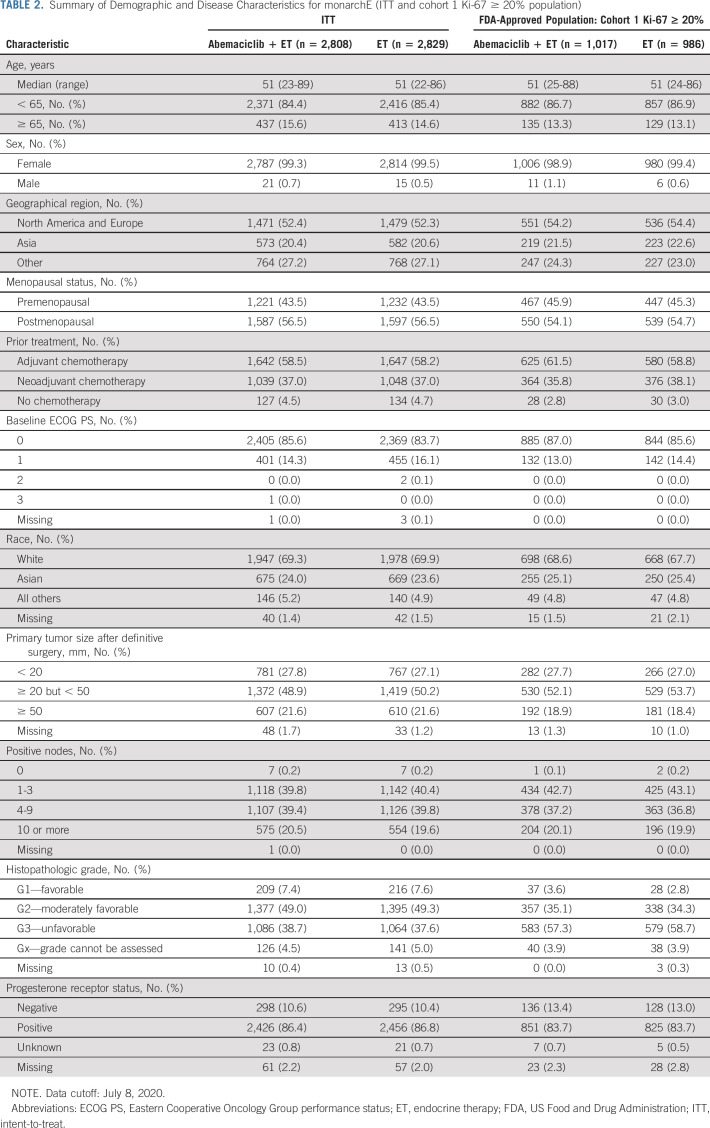
A CONSORT diagram detailing the breakdown of randomly assigned patients by arm and Ki-67 status in each cohort is shown in Figure 2. A summary of the baseline demographic and disease characteristics of the monarchE in the ITT population and in the cohort 1 Ki-67 ≥ 20% population is presented in Table 2.
FIG 2.
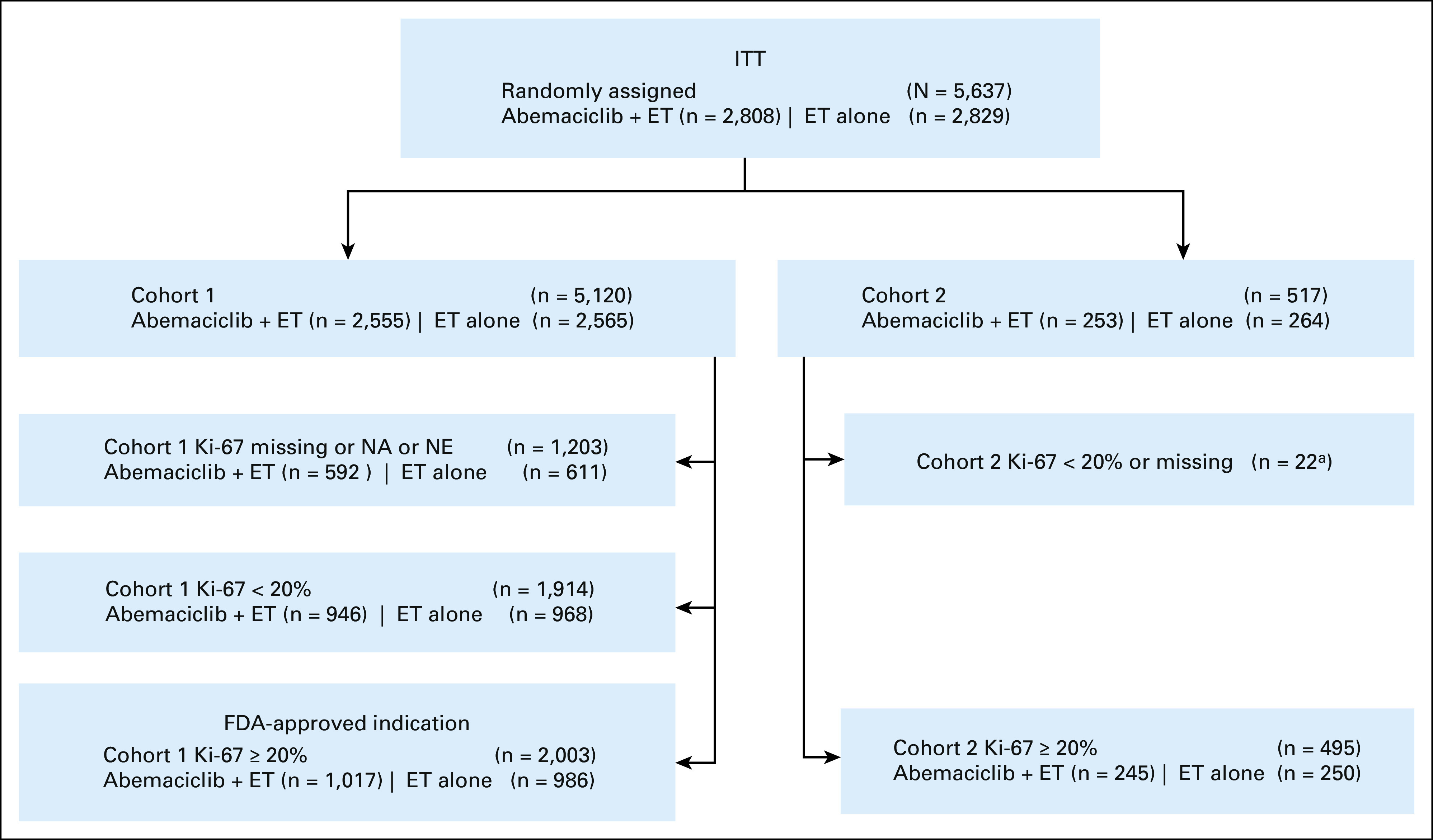
CONSORT diagram showing the enrollment of subjects, their allocation to each cohort, Ki-67 status and treatment arm, and how they are analyzed in the monarchE trial. aIncorrect cohort assignment. ET, endocrine therapy; FDA, US Food and Drug Administration; ITT, intent-to-treat; NA, not applicable; NE, not evaluable.
TABLE 2.
Summary of Demographic and Disease Characteristics for monarchE (ITT and cohort 1 Ki-67 ≥ 20% population)
The primary efficacy end point of monarchE was IDFS in the ITT population. The primary end point was deemed statistically significant at the prespecified second interim IDFS analysis (hazard ratio [HR] = 0.747; 95% CI, 0.598 to 0.932; P = .0096) based upon 83% of expected events when 26.4% of patients had either completed (12.5%) or discontinued (13.9%) 2 years of adjuvant therapy.
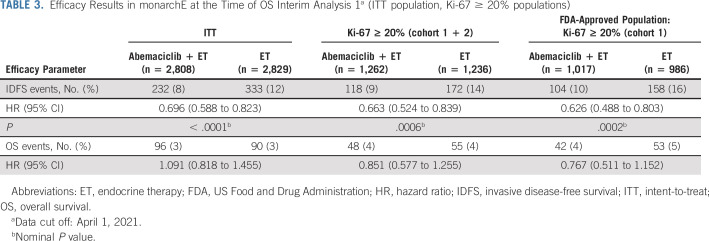
The prespecified statistical hierarchy included IDFS in the Ki-67 ≥ 20% population from cohorts 1 and 2 and IDFS in the Ki-67 ≥ 20% population for cohort 1. Both were met at the final IDFS analysis ([IDFS HR = 0.691; 95% CI, 0.519 to 0.920; P = .0111] and [IDFS HR = 0.643; 95% CI, 0.475 to 0.872; P = .0042, respectively]) when 41% of patients had either completed (25.5%) or discontinued (15.5%) 2 years of adjuvant therapy. The final hierarchically tested secondary end point was OS in the ITT population, which remains immature at the first interim OS analysis (OS IA1; HR = 1.091; 95% CI, 0.818 to 1.455) with 89.6% of patients having completed (72.2%) or discontinued (17.4%) the 2-year treatment period (Table 3 provides IDFS and OS results from this time point in ITT and the two controlled subgroups).
TABLE 3.
Efficacy Results in monarchE at the Time of OS Interim Analysis 1a (ITT population, Ki-67 ≥ 20% populations)
Safety
Evaluation of the safety of abemaciclib plus ET for the adjuvant treatment of node-positive, HR+, HER– EBC was from 5,591 patients who received abemaciclib plus ET or ET alone in monarchE. Abemaciclib was administered at the dose of 150 mg twice daily, consistent with the recommended dosage in the approved US prescribing information14 for metastatic breast cancer. The duration of abemaciclib treatment was 2 years. More patients in the abemaciclib plus ET arm experienced treatment-emergent adverse events compared with patients in the ET arm (98.4% v 88.8%); the most common (≥ 20%) of these were diarrhea, infections, neutropenia, fatigue, leukopenia, nausea, anemia, and headache. Patients receiving abemaciclib plus ET experienced increased grade ≥ 3 adverse events compared with those treated with ET alone (49.7% v 16.3%). More patients on the abemaciclib plus ET arm had serious adverse events (15.2% v 8.8%) and adverse events leading to discontinuation of abemaciclib study treatment versus endocrine monotherapy in the control arm (18.5% v 1.1%, respectively).
Companion Diagnostic
The Ki-67 CDx with a cutoff of ≥ 20% was used in monarchE to centrally test untreated breast tumor samples, retrospectively in cohort 1 and prospectively in cohort 2.13 The Ki-67 pharmDx Score is the percentage of viable tumor cells in the invasive cancer component showing nuclear Ki-67 staining of any intensity. Analytical performance reviewed by FDA included sensitivity, specificity, precision, external reproducibility, robustness, and stability of the Ki-67 CDx (with Ki-67 pharmDx Score ≥ 20% cutoff) in specimen from patients with breast cancer. Ki-67 CDx reproducibility results (between-site, within-site and between-day, between-observer, and within-observer) from three sites met the acceptance criteria of negative percent agreement and positive percent agreement and overall percent agreement ≥ 85% at the lower bound of a 95% CI for the ≥ 20% cutoff. Robustness of the Ki-67 CDx was evaluated using breast carcinoma specimens to assess the potential impact of process variations on assay performance at critical stages of the assay workflow (eg, tissue thickness, target retrieval solution pH values, etc). Agreement estimates were calculated for the ≥ 20% cutoff. Overall, the robustness studies demonstrated that the use of Ki-67 CDx on breast carcinoma specimens produces consistent results under the conditions tested when scored at the ≥ 20% cutoff. The clinical performance of the Ki-67 CDx was demonstrated in monarchE where patients with Ki-67 pharmDX Score ≥ 20% as identified by the Ki-67 CDx demonstrated a statistically significant and clinically meaningful improvement in IDFS.
DISCUSSION
Although monarchE demonstrated a statistically significant improvement of IDFS in the ITT population at interim analysis 2 (IA2), only 12.5% of patients had completed the planned 2-year adjuvant treatment. At the final IDFS analysis, the results were still immature with only 25.5% of patients completing the 2 years of planned therapy. With an additional 9 months of follow-up at OS IA1, approximately 72.2% of patients had completed 2 years of study treatment and these results were able to be more readily interpreted in a benefit:risk framework. However, despite IDFS in the ITT population being statistically significant and favoring abemaciclib plus ET at all analyses, the OS analysis at all time points (IA2 data cutoff March 2020, final IDFS data cutoff July 2020, and OS IA1 data cutoff April 2021) favored the ET only arm, with the HR point estimates > 1, indicating a potential survival detriment in a curable population. Across multiple solid tumor malignancies, FDA considers IDFS to be an end point that demonstrates direct clinical benefit, as this end point is a direct measure of disease and is clinically relevant with recurrence (local or metastatic), signaling a change in disease status with resulting morbidity and mortality. Although still considered a long-term outcome measure, IDFS also has the benefit of earlier assessment than survival and thus can decrease the time of trials and required sample size. However, when assessing end points earlier than survival, particularly in a curative setting such as adjuvant breast cancer, FDA always examines the survival data to confirm there is not a detrimental effect. In this case, although immature, the OS HR in the ITT population favored the control arm, despite the statistical improvement in IDFS and thus did not support a broad approval. Consistent IDFS results were observed across exploratory subgroups such as > 3 pALN, grade 3 histology, and tumor size > 5 cm, regardless of Ki-67 score.
In both the final IDFS analysis and the OS IA1, the remaining subgroups in the statistical hierarchy, Ki-67 score ≥ 20% from cohorts 1 and 2 and Ki-67 score ≥ 20% from cohort 1, each demonstrated a statistically significant IDFS, which are deepening with time and OS HRs that numerically favored the abemaciclib plus ET arm with HR < 1 (see Table 3 for results from the OS IA1 time point). However, cohort 2's enrollment began approximately 11 months after cohort 1, and patients had to have known Ki-67 score to be randomly assigned. When evaluating cohort 2 alone, which represented a limited sample size (n = 517 or 9% of total patients), an exploratory ad hoc analysis of IDFS revealed a small number of events, making it difficult to interpret the IDFS HR, which also had a wide CI. Thus, the statistically significant IDFS improvement in the Ki-67 ≥ 20% population seen in cohorts 1 and 2 combined was driven by the patients in cohort 1. Considering that patients treated with abemaciclib plus ET experienced more toxicities than patients who were treated with ET alone, this added toxicity was not deemed justified for patients in cohort 2 on the basis of the current data available for evaluation. Testing of IDFS in the entire cohort 1 patient population was not prespecified in the statistical analysis plan, although FDA routinely performs assessments of treatment effects across clinically important subgroups whether prespecified or not as part of our comprehensive review. Here, an exploratory ad hoc analysis of all patients in cohort 1 could not rule out a potential detriment in survival; thus, the approval did not include patients from cohort 1 with a Ki-67 score < 20%.
The only population appropriate for approval of abemaciclib plus ET was patients in cohort 1 (patients with high-risk clinicopathologic tumor features) and Ki-67 ≥ 20%. Follow-up for the OS end point in the monarchE trial continues, and as additional information accumulates, the benefit:risk considerations may evolve with potential for labeling updates to broaden or narrow the indication. For instance, if the OS data on maturity turn out to be unfavorable for the ITT population or the indicated population, then that would be a signal for a potential detriment for this curable population and may warrant a withdrawal of the current approval.
Given the population appropriate for approval was selected by Ki-67, a CDx approved concurrently with the adjuvant approval was required for the safe and effective use of abemaciclib. The Ki-67 CDx was clinically validated in the monarchE trial and was used to identify the patient population with a positive risk:benefit assessment and ultimately the indicated population. The availability of an FDA-approved CDx should address issues regarding assessment of Ki-67 as a barrier for use of abemaciclib plus ET in the appropriate population of patients with EBC at high risk of recurrence.
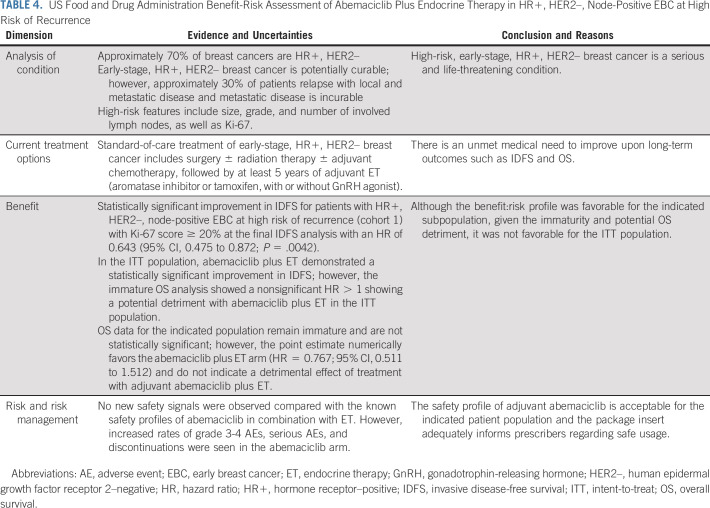
In conclusion, overall, the benefit:risk assessment (Table 4) of abemaciclib plus ET as adjuvant treatment of patients with HR+, HER2–, node-positive EBC at high risk of recurrence and a Ki-67 score ≥ 20% is favorable. For this indicated population, abemaciclib plus ET demonstrated a statistically significant and clinically meaningful improvement in IDFS. These patients have an unmet medical need for treatments that improve long-term outcomes. Although median duration of follow-up is short and OS results are immature, there does not appear to be a detrimental effect from adjuvant treatment with abemaciclib plus ET in this select high-risk population.
TABLE 4.
US Food and Drug Administration Benefit-Risk Assessment of Abemaciclib Plus Endocrine Therapy in HR+, HER2–, Node-Positive EBC at High Risk of Recurrence
Flora Mulkey
Employment: BeiGene (I)
No other potential conflicts of interest were reported.
See accompanying article on page 1142
DISCLAIMER
This is a US Government work. There are no restrictions on its use.
CLINICAL TRIAL INFORMATION
M.R. and C.O. contributed equally to this work.
DATA SHARING STATEMENT
The sharing of individual patient data from each participating trial will be subject to the policies and procedures of the institutions and groups that did the original studies.
AUTHOR CONTRIBUTIONS
Conception and design: Melanie Royce, Christy Osgood, Richard Pazdur, Julia A. Beaver, Laleh Amiri-Kordestani
Administrative support: Soma Ghosh, Richard Pazdur
Collection and assembly of data: Christy Osgood, Reena Philip, Bronwyn D. Mixter
Data analysis and interpretation: Melanie Royce, Christy Osgood, Flora Mulkey, Erik Bloomquist, William F. Pierce, Arpita Roy, Shyam Kalavar, Soma Ghosh, Shenghui Tang, Julia A. Beaver, Laleh Amiri-Kordestani
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
FDA Approval Summary: Abemaciclib With Endocrine Therapy for High-Risk Early Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Flora Mulkey
Employment: BeiGene (I)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Arora S, Narayan P, Osgood CL, et al. U.S. FDA drug approvals for breast cancer: A decade in review. Clin Cancer Res. 2022;28:1072–1086. doi: 10.1158/1078-0432.CCR-21-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 3. Howie LJ, Scher NS, Amiri-Kordestani L, et al. FDA approval summary: Pertuzumab for adjuvant treatment of HER2-positive early breast cancer. Clin Cancer Res. 2019;25:2949–2955. doi: 10.1158/1078-0432.CCR-18-3003. [DOI] [PubMed] [Google Scholar]
- 4. Singh H, Walker AJ, Amiri-Kordestani L, et al. U.S. Food and Drug Administration approval: Neratinib for the extended adjuvant treatment of early-stage HER2-positive breast cancer. Clin Cancer Res. 2018;24:3486–3491. doi: 10.1158/1078-0432.CCR-17-3628. [DOI] [PubMed] [Google Scholar]
- 5. Wedam S, Fashoyin-Aje L, Gao X, et al. FDA approval summary: Ado-trastuzumab emtansine for the adjuvant treatment of HER2-positive early breast cancer. Clin Cancer Res. 2020;26:4180–4185. doi: 10.1158/1078-0432.CCR-19-3980. [DOI] [PubMed] [Google Scholar]
- 6. Schmid P, Cortes J, Dent R, et al. VP7-2021: KEYNOTE-522: Phase III study of neoadjuvant pembrolizumab + chemotherapy vs. placebo + chemotherapy, followed by adjuvant pembrolizumab vs. placebo for early-stage TNBC. Ann Oncol. 2021;32:1198–1200. [Google Scholar]
- 7. Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571–1581. doi: 10.1016/j.annonc.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 8. Todd JH, Dowle C, Williams MR, et al. Confirmation of a prognostic index in primary breast cancer. Br J Cancer. 1987;56:489–492. doi: 10.1038/bjc.1987.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Viale G, Giobbie-Hurder A, Regan MM, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: Results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569–5575. doi: 10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies—Improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fasching PA, Gass P, Häberle L, et al. Prognostic effect of Ki-67 in common clinical subgroups of patients with HER2-negative, hormone receptor-positive early breast cancer. Breast Cancer Res Treat. 2019;175:617–625. doi: 10.1007/s10549-019-05198-9. [DOI] [PubMed] [Google Scholar]
- 12. Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node- positive, high-risk, early breast cancer (monarchE) J Clin Oncol. 2020;38:3987–3998. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ki-67 IHC MIB-1 pharmDX (Dako Omnis) device. https://www.accessdata.fda.gov/cdrh_docs/pdf21/P210026C.pdf
- 14.VERZENIO™ (Abemaciclib) Tablets, for Oral Use. US prescribing information; 2018. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=208855 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sharing of individual patient data from each participating trial will be subject to the policies and procedures of the institutions and groups that did the original studies.