PURPOSE
The need for an individualized management of indolent clinical forms in mantle cell lymphoma (MCL) is increasingly recognized. We hypothesized that a tailored treatment with ibrutinib in combination with rituximab (IR) could obtain significant responses in these patients.
METHODS
This is a multicenter single-arm, open-label, phase II study with a two-stage design conducted in 12 Spanish GELTAMO sites (ClinicalTrials.gov identifier: NCT02682641). Previously untreated MCL patients with indolent clinical forms defined by the following criteria were eligible: no disease-related symptoms, nonblastoid variants, Ki-67 < 30%, and largest tumor diameter ≤ 3 cm. Both leukemic non-nodal and nodal subtypes were recruited. Patients received ibrutinib 560 mg once daily and a total of eight doses of rituximab 375 mg/m2. Ibrutinib could be discontinued after 2 years in the case of sustained undetectable minimal residual disease (MRD). The primary end point was the complete response (CR) rate achieved after 12 cycles according to Lugano criteria.
RESULTS
Fifty patients with MCL (male 66%; median age 65 years) were enrolled. After 12 cycles of treatment, 42 (84%; 95% CI, 74 to 94) patients had an overall response, including 40 (80%; 95% CI, 69 to 91) with CR. Moreover, undetectable MRD in peripheral blood was achieved in 87% (95% CI, 77 to 97) of cases. At 2 years, 24 of 35 evaluable patients (69%) could discontinue ibrutinib because of undetectable MRD. Four patients had disease progression; three were non-nodal MCL and carried high genomic complexity and TP53 mutations at enrollment. No unexpected toxicity was seen except one patient with severe aplastic anemia.
CONCLUSION
Frontline IR combination achieves a high rate of CRs and undetectable MRD in indolent clinical forms of MCL. Discontinuation seems appropriate in cases with undetectable MRD, except for TP53-mutated cases.
INTRODUCTION
Mantle cell lymphoma (MCL) is usually considered an aggressive lymphoid neoplasm; however, some patients show an indolent clinical course with long survival times even in the absence of intensive therapy.1 In the past two decades, different studies have recognized a molecular subtype known as leukemic non-nodal (nnMCL), because of a clinical presentation mainly characterized by splenomegaly and leukemic involvement without a significant enlargement of lymph nodes,2,3 often combined with an indolent behavior. These cases have particular biologic features such as mutations in IGHV genes and possibly different cell of origin.2-5
CONTEXT
Key Objective
Indolent clinical forms of mantle cell lymphoma (MCL) could deserve an individualized management. For the first time, the IMCL-2015 evaluates a tailored frontline treatment using ibrutinib in combination with rituximab (IR). In addition, a minimal residual disease (MRD)–driven time-limited treatment is incorporated, which is a clear novelty in this disorder.
Knowledge Generated
The IR is highly active in indolent clinical forms of MCL patients, achieving high rates of complete responses and undetectable MRD, which allows treatment interruption in the majority of responders. Of note, no differences have been observed among non-nodal MCL or conventional MCL molecular subtypes. However, patients with TP53 mutations have a higher risk of early progression and poor survival.
Relevance (J.W. Friedberg)
These provocative data on an MRD-driven time-limited treatment for patients with indolent MCL warrant further investigation in prospective randomized clinical trials.**Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
From the clinical stand point, asymptomatic patients with MCL, usually with low tumor burden and proliferation, could be managed with observation and deferring therapy until disease progression.6-12 In retrospective studies, this approach has been shown to be safe with no compromise for the long-term outcome of the patients. However, there is no consensus on how to define these patients with indolent MCL nor on how to manage them. Regarding treatment, the MCL therapeutic landscape has rapidly evolved in the past few years with the introduction of targeted drugs.13 Its combinations either with immunochemotherapy or with other targeted approaches in a chemotherapy-free way are currently being extensively investigated in both MCL relapse14-18 and frontline.17,19,20
The use of minimal residual disease (MRD), which has demonstrated an outstanding prognostic value in immunochemotherapy-based clinical trials,21 needs to be evaluated in depth with new targeted therapies in MCL. Nevertheless, it seems reasonable to incorporate this parameter to try to limit the duration of the otherwise indefinite administration of new drugs.
The IMCL-2015 study aimed to evaluate the combination of ibrutinib with rituximab (IR) in the upfront treatment of indolent clinical forms of MCL by means of a MRD-driven approach to limit treatment duration. By using a clinical definition to select indolent MCL cases, we intended to provide an easy ground to allow future comparisons although a deep biologic characterization of these cases should be performed.
METHODS
Patients and Trial Design
The Spanish Lymphoma Group (GELTAMO [Grupo Español de Linfomas y Trasplante Autólogo de Médula Ósea]) carried out an open-label, multicenter, single-arm, phase II study in patients with untreated indolent clinical forms of MCL. Patients were enrolled in 12 centers according to the following key eligibility criteria: age 18 years or older; confirmed MCL diagnosis according to WHO diagnosis, excluding blastoid variants and/or Ki-67 > 30%; previously untreated; asymptomatic and with an Eastern Cooperative Oncology Group performance status score of 0 or 1; and leukemic non-nodal forms but also other clinical presentations as long as the largest diameter of the lymph nodes did not exceed 3 cm. In addition, stable disease for at least 3 months with no signs of progression or immediate need of treatment was also required (Protocol, online only) The study was approved by the ethics committees of the centers and conducted according to the Declaration of Helsinki and International Conference on Harmonisation guidelines for Good Clinical Practice. All patients provided written informed consent. This trial is registered with ClinicalTrials.gov identifier: NCT02682641.
Ethics Committee Approval
The GELTAMO-sponsored IMCL-2015 study, NCT02682641 and EudraCT: 2015-004158-17, was first approved by the ethics committee of the Hospital Clínic of Barcelona in January 26, 2016.
Treatment and Assessments
Patients received four weekly doses of rituximab at 375 mg/m2 intravenously during the first cycle of 28 days (days 1, 8, 15, and 22 of cycle 1) followed by four more doses at day one of every other cycle (cycles 3, 5, 7, and 9) up to a total of eight doses. Ibrutinib was given orally at a fixed continuous dose of 560 mg once daily and discontinued after 2 years of treatment in the case of sustained undetectable MRD (at least for 6 months at two consecutive determinations), otherwise until progression or unacceptable toxicity. Adverse events (AEs) were evaluated according to the Common Toxicity Criteria for adverse Events (CTCAE, version 4.03). The screening workup and the assessment of response after cycle 12 (C12) included a positron emission tomography-computed tomography (PET-CT), bone marrow (BM) aspirate, and biopsy. Histologic review and peripheral blood (PB) sample collection for genomic studies were centralized at Hospital Clínic of Barcelona. Lugano response criteria were applied and included a central review of PET-CT by the GELTAMO imaging working group. For the detection of MRD, samples of PB and BM were centralized at Hospital Clínico Universitario de Salamanca. EuroMRD protocols22 were applied for allele-specific oligonucleotide real-time quantitative polymerase chain reaction and when needed, next-generation sequencing–based protocols,23 to allow at least a sensitivity of 10−5. Patients were monitored with MRD studies and CT scans every 6 months for a maximum planned follow-up of 7 years. All genetic analyses were performed as previously described4,5 and are detailed in the Data Supplement (online only).
End Points
The primary end point was the complete response (CR) rate achieved at 12 months of treatment (after completion of C12) according to Lugano criteria in the intention-to-treat population. Secondary end points included the safety and the tolerability of the IR combination, overall response (OR) and the rate of undetectable MRD, progression-free survival (PFS), duration of response, and overall survival (OS).
Statistical Analysis
The hypothesis was that ibrutinib and rituximab combination (IR) would achieve at least a 50% CR rate compared with 30% usually obtained with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. We used Simon's two-stage optimal design with error probabilities α and β of .05 and .2, respectively, to fix the final figure at 50 patients that included a 10% increase because of potential dropouts. The first stage would consist of 15 patients. If five or less CRs were observed, the trial had to be terminated; otherwise, recruitment could continue. Nineteen or more CRs in 46 evaluable patients had to be observed to meet the primary end point of the study.
An amendment allowed accepting a response evaluation at 6 months instead of 12 months, by using CT scan and BM aspirate, with the goal of ensuring an adequate treatment activity to justify the movement to stage II without penalizing excessively the recruitment time. All the CRs assessed at 6 months in stage I were later confirmed at 12 months according to the initial description of the protocol. Both primary and safety analyses were performed by intention to treat in all 50 patients included in the study.
Descriptive statistics were used to assess the baseline characteristics of the patients. The survival analysis was performed by the Kaplan-Meier method, and differences were assessed by the log-rank test. P values < .05 were considered statistically significant. All statistical analyses were performed using IBM SPSS version 25 and R version 3.6.0.
RESULTS
Patients
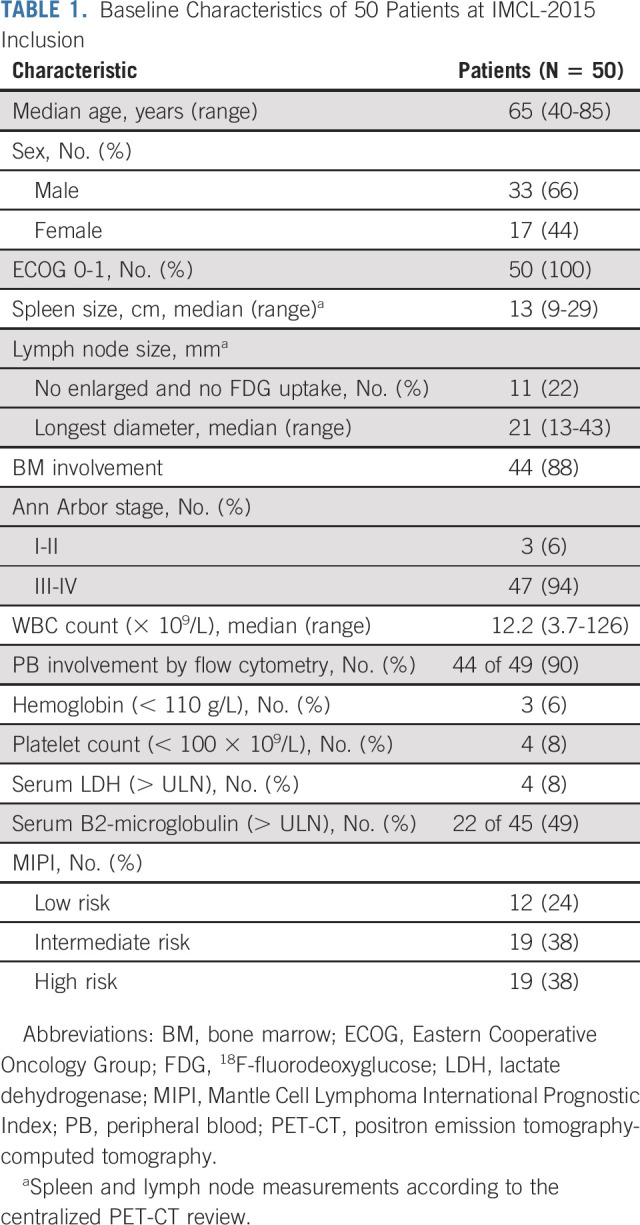
Between June 7, 2016, and December 10, 2019, 50 patients with untreated indolent clinical forms of MCL were enrolled in 12 GELTAMO centers to receive the IR combination. Baseline characteristics are summarized in Table 1. The median observation time from diagnosis to therapy was 7.9 (3-107) months.
TABLE 1.
Baseline Characteristics of 50 Patients at IMCL-2015 Inclusion
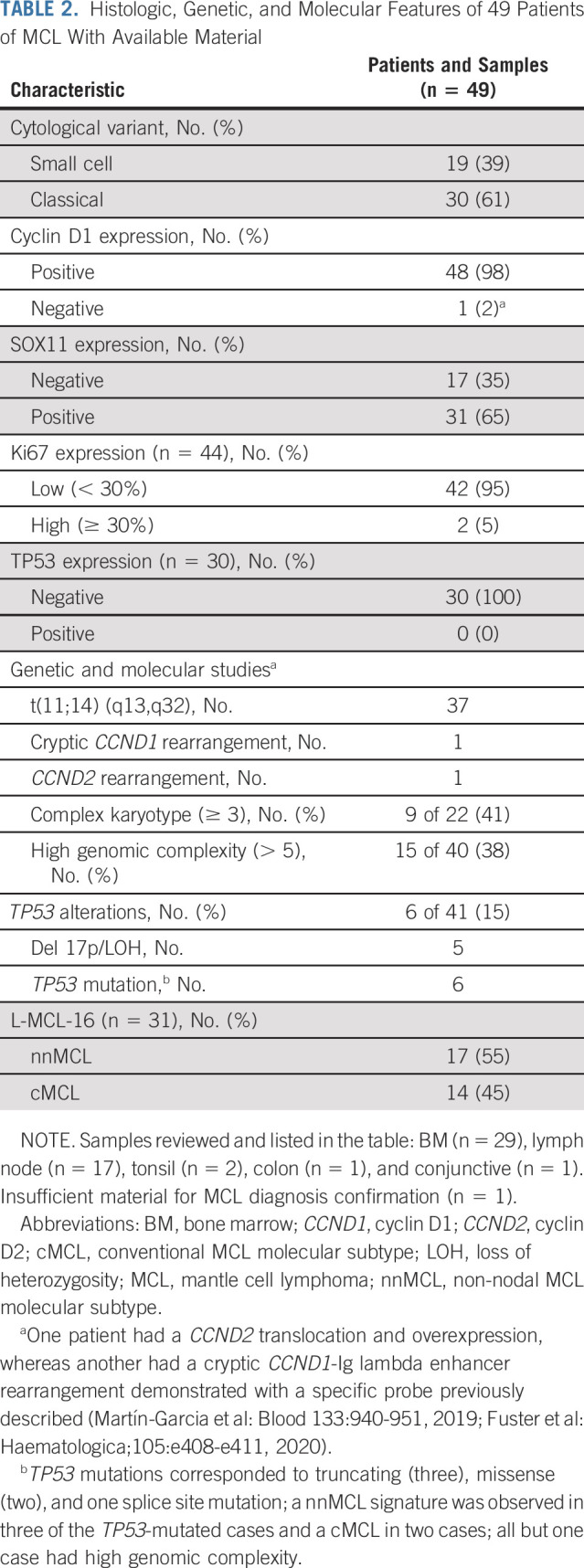
Histologic, genetic, and molecular data of 49 patients with available material are detailed in Table 2 and the Data Supplement. As required, no blastoid forms were identified and Ki-67 when evaluable was below 30%, except in two cases (34% and 50%) after centralized review. The L-MCL16 assay4 could be performed in 31 cases, in which 17 (55%) had the nnMCL gene expression profile and 14 (45%) had a conventional MCL (cMCL) signature. Among patients with a leukemic clinical presentation, with or without splenomegaly or small lymph nodes, 68% had a nnMCL profile. The main initial features according to TP53 mutational status and molecular subtypes are shown in the Data Supplement.
TABLE 2.
Histologic, Genetic, and Molecular Features of 49 Patients of MCL With Available Material
The CONSORT diagram of the patients is shown in Figure 1. The data cutoff date was May 28, 2021. Stage I of the study completed recruitment of 15 patients in April 2017. When six patients achieved a CR with no early discontinuations, recruitment continued to complete the 50 patients originally planned. Forty-three patients received the eight intended doses of rituximab, and seven patients between four and seven doses. The dose of ibrutinib was reduced in six patients (12%), and four cases (8%) required early discontinuation; thus, at 1 year, 46 patients were receiving ibrutinib treatment.
FIG 1.
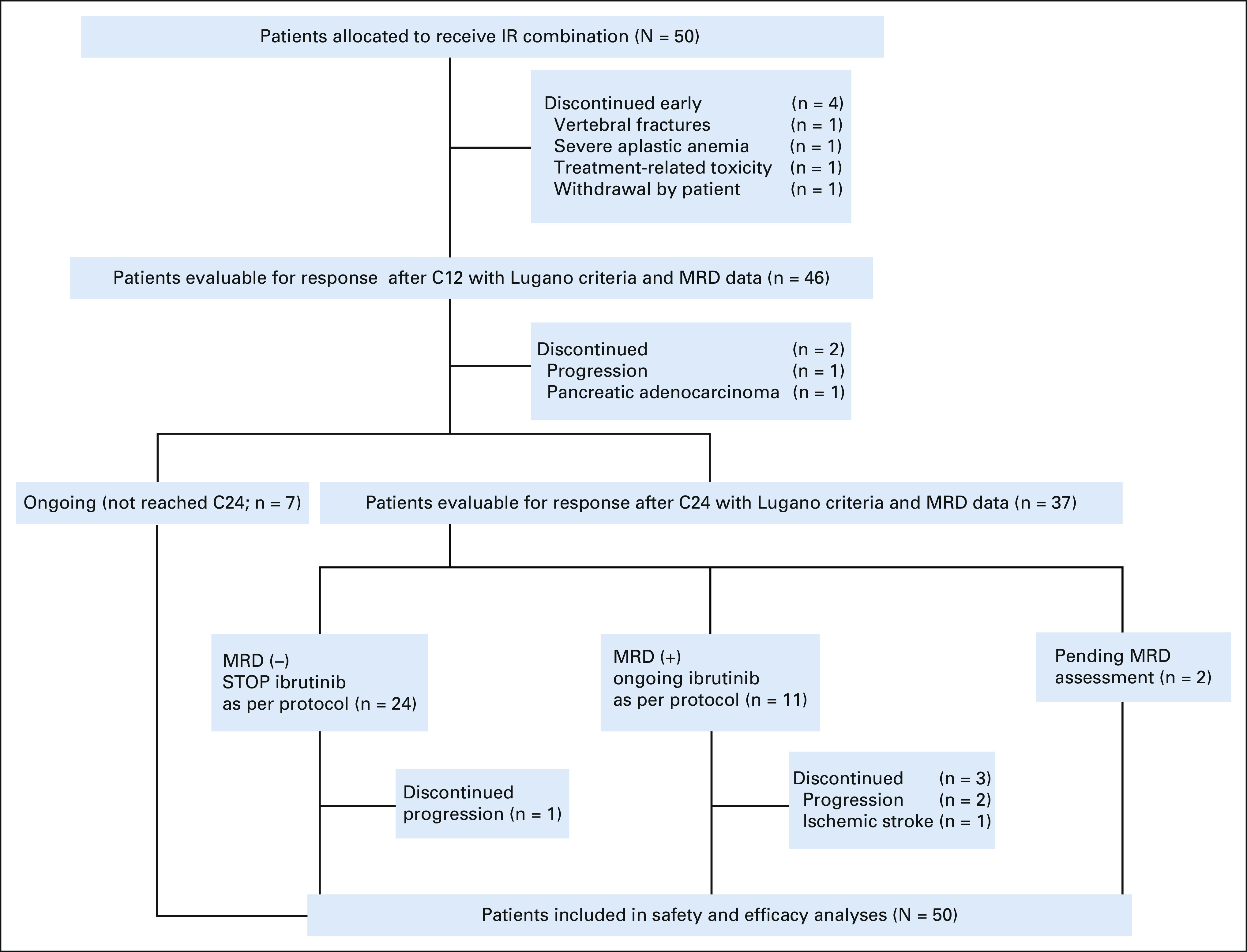
CONSORT diagram of IMCL-2015. C12, cycle 12; C24, cycle 24; IR, ibrutinib, rituximab combination; MRD, minimal residual disease.
Efficacy
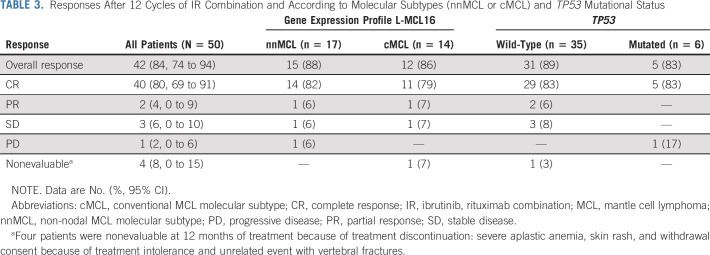
After 12 cycles of treatment, 46 patients were evaluated according to Lugano criteria. The remaining four patients had early discontinuation between 1.6 and 5.5 months of ongoing treatment because of severe aplastic anemia, accidental vertebral fractures, skin rash, and withdrawal of consent because of treatment intolerance. The centralized evaluation showed 42 of 50 patients (84%; 95% CI, 74 to 94) responding to treatment, with 40 (80%; 95% CI, 69 to 91) patients achieving a CR (Table 3). No clinical or biologic variables, including Mantle Cell Lymphoma International Prognostic Index (MIPI), β2-microglobulin, molecular subtypes, TP53 mutational status, or high genomic complexity, predicted CR achievement at 12 months. Response rates according to molecular subtypes and TP53 mutational status are listed in Table 3, and for genomic complexity, in the Data Supplement.
TABLE 3.
Responses After 12 Cycles of IR Combination and According to Molecular Subtypes (nnMCL or cMCL) and TP53 Mutational Status
Sequential MRD determinations in PB and BM are detailed in the Data Supplement. After 12 cycles of IR, 40 of 46 evaluable patients (87%; 95% CI, 77 to 97) achieved undetectable MRD in PB and 28 of 43 evaluable patients (65%; 95% CI, 51 to 78) also in BM. Focusing on the 40 patients with CR, MRD was undetectable in PB in 37 of 40 (93%; 95% CI, 86 to 100) assessed patients and also in BM, in 26 of 37 (70%; 95% CI, 55 to 85) patients. No significant differences in terms of molecular response were seen according to the clinicobiologic variables mentioned above.
Outcome
Thirty-seven patients had reached 24 cycles of ibrutinib. As shown in the CONSORT diagram, two additional patients had discontinued the trial because of disease progression and pancreatic adenocarcinoma, respectively, and seven had not yet reached 24 cycles of therapy. In addition, MRD was under evaluation in two cases. As per protocol, ibrutinib was discontinued in 24 of 35 evaluable cases (69%) because of response with sustained undetectable MRD after C24. Among the 24 patients who have discontinued treatment, three cases stopped ibrutinib earlier because of intolerance and two cases were considered in PR and SD after cycle 12 evaluation, respectively. At the last cutoff, 19 patients continued having undetectable MRD in PB (the maximum MRD follow-up after planned discontinuation was 34 months), whereas five converted to detectable MRD in PB between 3 and 20 months from discontinuation. Only one clinical relapse has been detected after MRD conversion within those 24 patients (Data Supplement).
All four patients with disease progression at the last follow-up were preceded by persistence or reappearance of detectable MRD in PB. After a median follow-up of 36 months for surviving patients, the estimated PFS at 36 months was 93% (95% CI, 86 to 100; Fig 2A). Only MIPI and TP53 mutational status showed a significant impact on PFS, with no differences by molecular subtype (nnMCL v cMCL), genomic complexity (Figures 2B-2E), or MRD status at C12 (Data Supplement). Multivariate analysis was not performed as the proportional hazards assumption was not met. The event-free survival at 36 months was 76% (95% CI, 64 to 89; Fig 2A).
FIG 2.
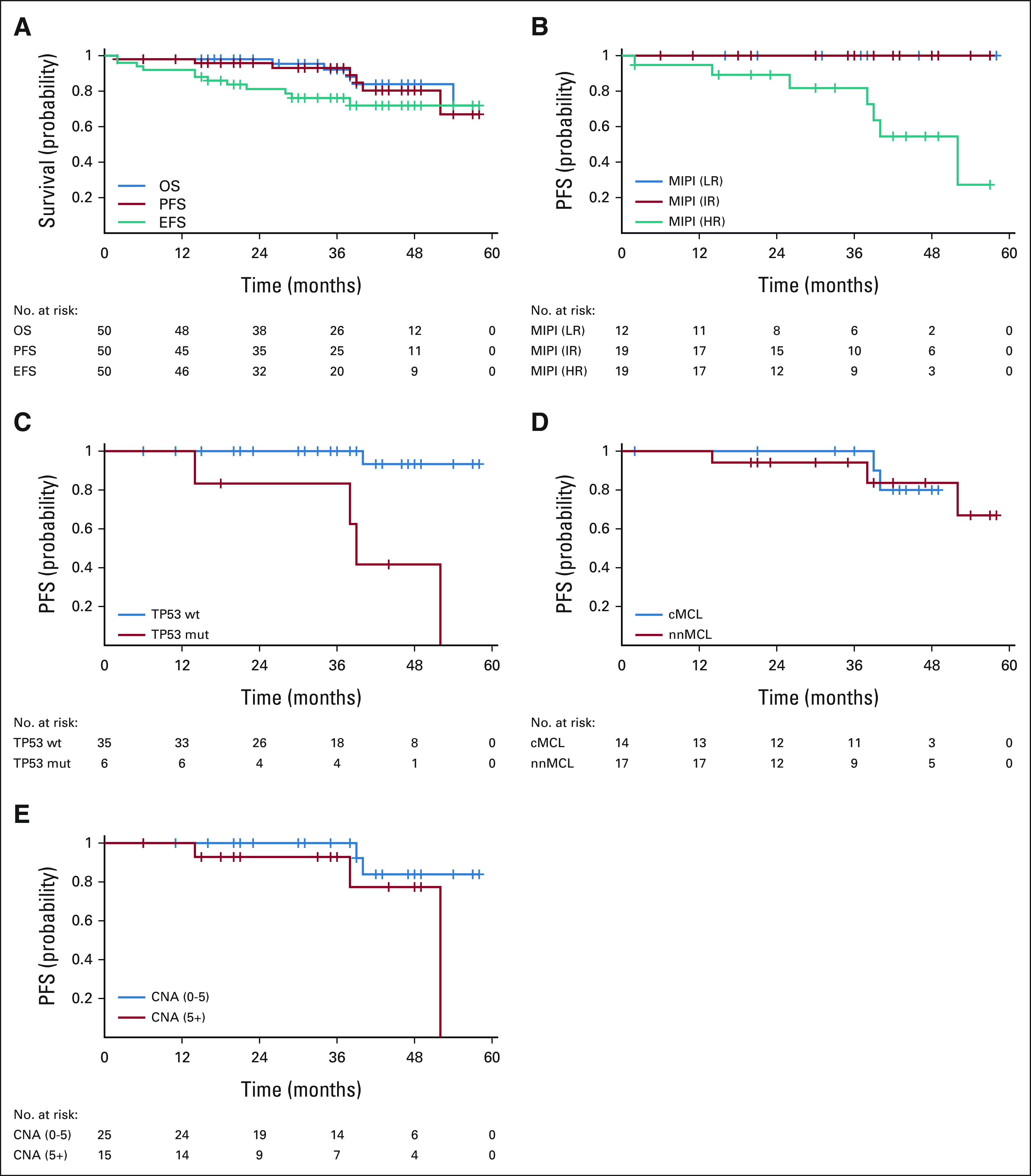
Survival in the IMCL-2015 study. (A) OS, PFS, and EFS (medians not reached). (B) PFS according to MIPI was significantly different in the high-risk group (P = .002). (C) PFS in 41 patients with TP53 mutational status was significantly poorer for TP53-mutated cases (P = .0001). No significant differences in PFS were observed: (D) according to the molecular variant (cMCL and nnMCL assessed in 31 patients) or (E) according to the genomic complexity available in 40 patients. PFS was measured from the treatment start date until disease progression or death whichever occurs first. OS was measured from the treatment start date to the date of death or last follow-up. EFS was measured from the treatment start date to the failure of treatment or death as a result of any cause or the last follow-up. A total of 12 patients had an event as follows: unacceptable toxicity, six cases (treatment-related, two of the patients presented an ulterior progression); disease progression, two cases; pancreatic adenocarcinoma, one case (SUSAR); severe aplastic anemia, one case (SUSAR); vertebral fractures, one case (unrelated); and ischemic stroke, one case (unrelated). cMCL, conventional MCL; CNA, copy number alterations; EFS, event-free survival; HR, high risk; IR, intermediate risk; LR, low risk; MCL, mantle cell lymphoma; MIPI, Mantle cell lymphoma International Prognostic Index; mut, mutated; nnMCL, non-nodal MCL; OS, overall survival; PFS, progression-free survival; wt, wild-type.
Six patients have died so far, four cases because of MCL progression and the other two as a result of pancreatic adenocarcinoma and SARS-CoV-2 infection, respectively. The OS at 36 months was 92% (95% CI, 84 to 100; Fig 2A). MIPI and TP53 mutational status also had a significant impact on OS (Data Supplement).
Interestingly, three of the four patients with disease progression had a nnMCL subtype and carried high genomic complexity with TP53 mutations. The remaining case was a cMCL with wild-type TP53 and without genomic complexity. Only one of these patients was eligible for active salvage treatment, but was refractory to different immunochemotherapy and new targeted drugs.
TP53-Mutated MCL Patients
Focusing on the six TP53-mutated cases included in this study, five achieved a CR, including four with undetectable MRD that allowed discontinuation of ibrutinib as per protocol in two cases. All but one of the patients eventually converted to detectable MRD. Clinical progression has been observed in three cases with a median PFS of 38.5 months (95% CI, 37 to 39; v not reached for wild-type TP53 cases; P = .0001; Fig 2C). Three TP53-mutated patients have died because of disease progression in two cases and from SARS-CoV-2 infection in one case that also had detectable MRD. The median OS of TP53-mutated cases was significantly lower than that of wild-type cases (38.5 months; 95% CI, 37 to 39 v not reached, respectively, P = .0002).
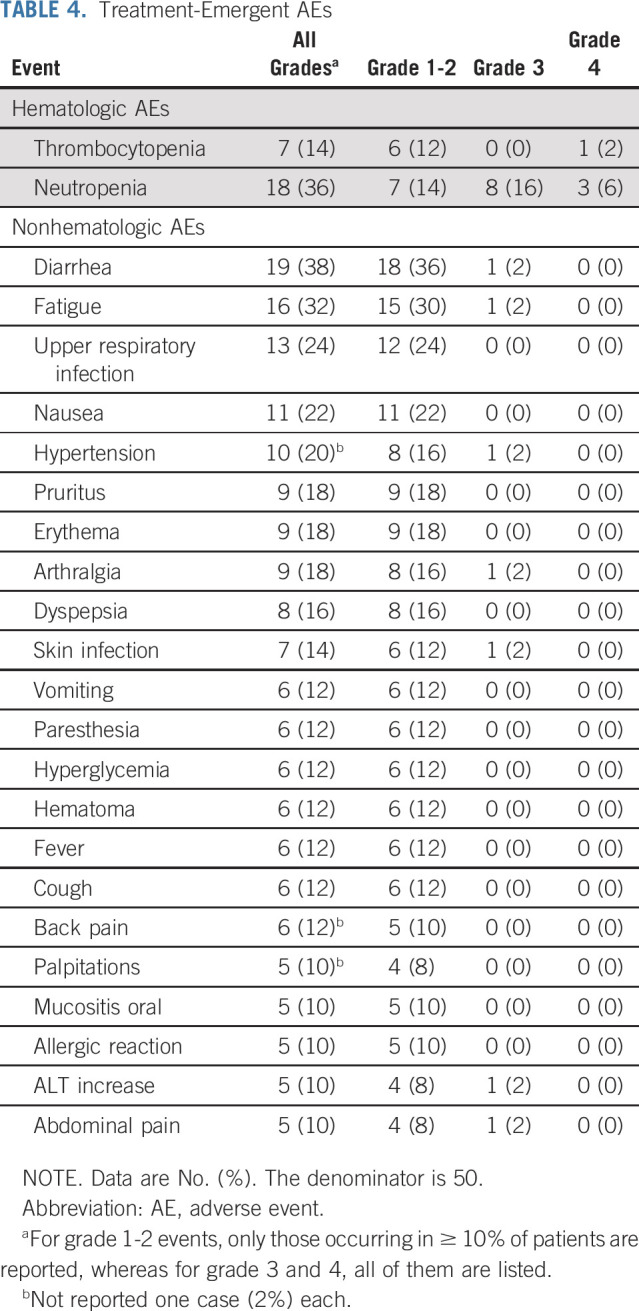
Safety
The most common treatment-related AEs were diarrhea (38%), neutropenia (36%), fatigue (32%), upper respiratory infection (24%), nausea (22%), and arterial hypertension (20%; Table 4). Grade ≥ 3-4 AEs were predominantly hematologic toxicity (22%). At the last cutoff, six patients had discontinued ibrutinib because of intolerance. Atrial fibrillation and stroke were seen in 2 and 1 cases, respectively. Three neoplasms, basocellular carcinoma, pancreatic adenocarcinoma, and bladder carcinoma at 1, 12, and 40 months, from the onset of treatment were observed in two patients. Severe aplastic anemia was observed in one case at 5.5 months of IR treatment. At that time, the patient was in CR by CT imaging, with undetectable MRD in both PB and BM. Despite IR treatment withdrawal, the severe aplasia did not recover and required treatment with antithymocyte globulin followed by allogeneic stem-cell transplantation. The patient is alive and in CR.
TABLE 4.
Treatment-Emergent AEs
DISCUSSION
The present study shows that the IR combination achieves a high rate of complete and molecular responses as upfront treatment for indolent clinical forms of MCL. This is a MRD-driven study that results in a time-limited treatment with the IR combination for the majority of patients. The discontinuation of ibrutinib therapy after 2 years seems reasonable in this selected population, particularly in patients without TP53 mutation.
The primary end point of the study was met: IR combination proved to be very active in this setting, with 80% of CR at 1 year, including a high proportion of undetectable MRD. Further conclusions regarding the potential effectiveness of this combination in the frontline treatment are limited because of the study design, a nonrandomized phase II study lacking a comparator arm. However, these results compare favorably with what we could expect from established high-dose treatment approaches, indeed with a most favorable toxicity profile. Moreover, these excellent data should be taken with caution as the MCL population herein evaluated was favorably selected and, more importantly, a longer term follow-up is needed to see how sustained such responses are. It is of note that the outstanding efficacy was achieved with only eight doses of rituximab and a MRD-driven duration of ibrutinib. Treatment was limited to 2 years in about 70% of responders. This aspect of the IMCL-2015 study differs from the majority of currently ongoing studies in which new targeted drugs are usually used until disease progression.
By contrast, perhaps a small part of the patients included in the IMCL-2015 study might not have required treatment. The definition and management of the so-called indolent clinical forms of MCL patients is currently an open question with scarce and mostly retrospective available evidence.1,6-12,24 Moreover, the different policies in the selection of patients to apply observation and the lack of reliable biomarkers hamper having a benchmark where to make any consistent comparison. In most of these series,6-12 a median time to treatment between 1 and 2 years has been described, and only 7% of initially observed cases had long-term observation (> 5 years) in one study.6
Regarding previously reported experiences with targeted combinations, the IR regimen has already been tested in relapsed/refractory patients with MCL.14,18 Although no direct comparison with single-agent ibrutinib is available, the results of the combination seem superior with CR in 58% of cases and a median PFS of 43 months in the absence of unexpected toxicity.18 The very recently published results for the frontline IR combination in older patients20 with MCL showing the best OR rate of 92% and CR rate of 68%, together with the data from the present study, give some evidence supporting its combination rather than the use of single-agent ibrutinib alone although these data are not available in frontline treatment. Both studies showed 3-year PFS and OS around 90%. The results of the ongoing randomized phase III studies, ENRICH (ISRCTN 11038174) and MANGROVE (NCT04002297), are eagerly awaited to clarify the role of the BTK inhibitors and rituximab combination in the upfront treatment of MCL. Combinations with immunomodulators have been tested as well. The IR combination herein reported with a more favorably selected population of asymptomatic patients with MCL compares very well with the data reported for R-lenalidomide, OR 91% and CR 63%, in a small series of 38 patients with favorable characteristics.19
Our study also provides detailed MRD information for the IR combination, to our knowledge, showing for the first time, the possibility of applying a MRD-guided treatment cessation. We observed that molecular responses with IR combination were achieved rapidly, especially in PB, most likely because of rituximab treatment. In some cases, MRD response was achieved over time. It is an open question whether cases with detectable MRD could have benefited from a longer exposure to rituximab, as disease progression was preceded by detectable MRD in all cases. Despite the fact that the follow-up is still short, many of the patients in whom ibrutinib was stopped after C24 because of sustained undetectable MRD continue in a molecular response for more than 2 years after discontinuation. Overall, these data are very promising and suggest that time-limited MRD-guided treatment with new targeted therapies in MCL is feasible and worth exploring in future studies.
After a median follow-up of 3 years, four clinical progressions (8%) have been observed. Interestingly, three of the four cases had a nnMCL variant and carried high genomic complexity with TP53 mutation, which could explain the poor outcome. However, no significant differences in the outcome have been observed so far between nnMCL and cMCL molecular subtypes or according to genomic complexity. Although the present data indicate a noteworthy activity of the IR combination in TP53-mutated MCL cases in terms of CR, similar to what have been described in other studies using ibrutinib-based combinations in the relapsed and refractory setting,15-17 the duration of the responses seems to be lower than that in TP53 wild-type cases, with this translating into a significantly poorer median PFS and OS (Fig 2C and Data Supplement). Thus, in practical terms, TP53-mutated cases seem to be not particularly suitable for IR discontinuation, even with undetectable MRD, since most likely these patients may relapse in a short period of time. Nonetheless, the median PFS and OS observed in our study for TP53-mutated cases are much better than those described in the series of patients treated with an intensive immunochemotherapy approach, including high-dose cytarabine and autologous stem-cell transplantation.25 This could be explained either by an improved activity of targeted IR treatment over intensive immunochemotherapy in TP53-mutated cases or by a highly selected MCL population with favorable clinical characteristics.
The toxicity seen with the IR combination in first-line was expected, with a predominance of hematologic toxicity, where up to 22% of patients experienced grade 3-4 neutropenia. This figure is superior than that observed with IR in older patients20 with MCL (8%), and this could be explained by the enriched presence of cases with leukemic involvement in our trial. Gastrointestinal symptoms and fatigue were also frequent, although in general with low or mild intensity, and seemed to be less frequently observed than when the IR combination was given in the relapsed and refractory setting14 or in frontline in older patients,20 which could be attributed to the better general condition of our patients. We observed few AEs of special interest such as hypertension or atrial fibrillation. The limited duration of the treatment could also contribute to the few AEs observed and therefore a lower discontinuation from the study than the one observed in older patients with MCL for IR combination.20 Despite that, up to 12% of patients had to discontinue treatment during follow-up because of ibrutinib intolerance and an additional 8% because of other reasons, including severe aplastic anemia and pancreatic adenocarcinoma, among others.
The IMCL-2015 study has shown the high activity, in terms of CR and undetectable MRD, of the upfront IR combination in indolent clinical forms of MCL following a MRD-driven approach to limit the extent of treatment. The duration of molecular and clinical responses after ibrutinib discontinuation will be a key aspect to define the final role of such individualized approaches. Some patients, particularly those with a TP53 mutation, may not be good candidates for treatment discontinuation, even in the case of molecular response. Future randomized studies are guaranteed to explore the final role of targeted therapies in the frontline treatment of patients with MCL.
ACKNOWLEDGMENT
The authors would like to thank the patients and their families, and study investigators and coordinators—among them, Carlos Grande, Lucia Palacios, and Silvia Ruíz—for their contribution to the study. Partial support of biological and MRD studies was obtained from grants Instituto de Salud Carlos III (ISCIII) PI19/00887 (to A.L.-G. and E.G.) and PI17/01061 (to S.B.), Fundació La Marató de TV3 grant 201904-30 (to S.B.), Ministerio de Ciencia e Innovación (MCI) RTI2018-094274-B-I00 (to E.C.), National Institutes of Health (NIH) 1P01CA229100 (to E.C.) and FEDER: European Regional Development Fund “Una manera de hacer Europa” (to E.C. and S.B.). A personal grant FEHH-Janssen 2021 to Alejandro Medina-Herrera from Fundación Española de Hematología y Hemoterapia is acknowledged. E.C. is an ICREA Academia Researcher.
Eva Giné
Honoraria: Gilead, Kite Pharma, Janssen, Genmab
Consulting or Advisory Role: Gilead, Kite Pharma
Research Funding: Janssen
Travel, Accommodations, Expenses: Gilead, Kite Pharma
Fátima de la Cruz
Honoraria: BeiGene, Takeda, Kyowa Kirin International, AbbVie, Janssen, AstraZeneca Spain
Consulting or Advisory Role: BeiGene, EUSA Pharma, Kyowa Kirin International, Roche, AbbVie, Janssen Oncology
Speakers' Bureau: Janssen, AbbVie
Travel, Accommodations, Expenses: AbbVie, Takeda, Janssen, AbbVie
Javier López Jimenez,
Consulting or Advisory Role: Roche, Genentech, AbbVie, Janssen Oncology, Gilead Sciences
Speakers' Bureau: Roche, Genentech, AbbVie, Pfizer
Research Funding: Roche, Genentech, AbbVie
Travel, Accommodations, Expenses: Gilead Sciences, Janssen Oncology, AbbVie
Alejandro Martín García-Sancho
Honoraria: Roche, Janssen-Cilag, Celgene, Servier, Gilead Sciences, Takeda, Eusa Pharma, Novartis
Consulting or Advisory Role: Roche, Celgene, MorphoSys, Kyowa Kirin, Iqone, EUSA Pharma, Gilead Sciences, Novartis, Servier, Incyte
Expert Testimony: Gilead Sciences
Travel, Accommodations, Expenses: Roche, Celgene, Servier
M. José Terol
Consulting or Advisory Role: Janssen, AbbVie
Research Funding: Gilead Sciences
Travel, Accommodations, Expenses: Janssen, Roche, Gilead Sciences, AbbVie
Eva González Barca
Honoraria: Janssen, AbbVie, Takeda, Roche, Incyte, EUSA Pharma
Consulting or Advisory Role: Janssen, AbbVie, Gilead Sciences, Kiowa, EUSA Pharma, Incyte, Lilly, BeiGene, Novartis
Travel, Accommodations, Expenses: Janssen, EUSA Pharma
Adolfo de la Fuente
Honoraria: BSM, AbbVie, Astellas Pharma, Incyte, Pfizer
Consulting or Advisory Role: BMS
Research Funding: Novartis (Inst)
Xavier Setoain
Honoraria: General Electric
Consulting or Advisory Role: Qbiotech, Lemer Pax
Speakers' Bureau: Lemer Pax
Patents, Royalties, Other Intellectual Property: Patent and Royalties with the medical device Epijet with the company Lemer Pax
Travel, Accommodations, Expenses: Takeda, General Electric
Amanda Rotger
Employment: ITM Oncologics
Honoraria: ITM Oncologics, AAA HealthCare
Travel, Accommodations, Expenses: ITM Oncologics
Alejandro Medina Herrera
Research Funding: Janssen
Travel, Accommodations, Expenses: Diagnostica Longwood
Ramón García Sanz
Honoraria: Janssen, Takeda, Amgen, BeiGene, Novartis, Astellas Pharma
Consulting or Advisory Role: Janssen
Research Funding: Gilead Sciences (Inst), Incyte (Inst), Astellas Pharma
Patents, Royalties, Other Intellectual Property: BIOMED 2 primers (Inst)
Travel, Accommodations, Expenses: Janssen, Takeda (I)
Other Relationship: Spanish Society of Hematology (SEHH)
Ferran Nadeu
Honoraria: Janssen
Elías Campo
Honoraria: EUSA Pharma, AstraZeneca
Consulting or Advisory Role: Illumina, AbbVie
Patents, Royalties, Other Intellectual Property: Author on a patent licensed to NanoString Technologies
Armando López-Guillermo
Honoraria: Roche
Consulting or Advisory Role: Roche, Gilead, Kite Pharma, Celgene, Bristol Myers Squibb, Incyte, Takeda, Kern Pharma, Pfizer, Janssen
Research Funding: Janssen, Roche, Celgene, Bristol Myers Squibb
Travel, Accommodations, Expenses: Roche, Kite/Gilead
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 61st Annual Meeting of the American Society of Hematology, Orlando, FL, December 7-10, 2019 (oral); LXII Congreso Nacional de la SEHH Virtual, Pamplona, Spain, October 26-30, 2020 (oral); and 16-ICML, International Conference on Malignant Lymphoma Virtual Edition, Lugano, Switzerland, June 18-22, 2021 (oral).
SUPPORT
The funding for the IMCL-2015 was obtained through unrestricted Janssen Clinical Investigator-Initiated Study (IIS) Research Support. Janssen was not involved in the protocol writing, data collection, and analysis or interpretation of results. Janssen was informed of the development of the IMCL-2015 study and reviewed the manuscript before submission.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The Spanish Lymphoma Group (GELTAMO, Grupo Español de Linfomas y Trasplante Autólogo de Médula Ósea) supports the ethical obligation of responsible sharing of data from clinical trials. The study Protocol, informed consent form, and amendments of the study Protocol will be provided. Deidentified individual participant data reported in the manuscript will be shared under the terms of a Data Use Agreement and may only be used for approved proposals. Request may be made to egine@clinic.cat.
AUTHOR CONTRIBUTIONS
Conception and design: Eva Giné, Armando López-Guillermo
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: Eva Giné, Xavier Setoain, Sonia Rodríguez, Alejandro Medina-Herrera, Ramón García-Sanz, Ferran Nadeu, Silvia Beá, Elias Campo, Armando López-Guillermo
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ibrutinib in Combination With Rituximab for Indolent Clinical Forms of Mantle Cell Lymphoma (IMCL-2015): A Multicenter, Open-Label, Single-Arm, Phase II Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eva Giné
Honoraria: Gilead, Kite Pharma, Janssen, Genmab
Consulting or Advisory Role: Gilead, Kite Pharma
Research Funding: Janssen
Travel, Accommodations, Expenses: Gilead, Kite Pharma
Fátima de la Cruz
Honoraria: BeiGene, Takeda, Kyowa Kirin International, AbbVie, Janssen, AstraZeneca Spain
Consulting or Advisory Role: BeiGene, EUSA Pharma, Kyowa Kirin International, Roche, AbbVie, Janssen Oncology
Speakers' Bureau: Janssen, AbbVie
Travel, Accommodations, Expenses: AbbVie, Takeda, Janssen, AbbVie
Javier López Jimenez,
Consulting or Advisory Role: Roche, Genentech, AbbVie, Janssen Oncology, Gilead Sciences
Speakers' Bureau: Roche, Genentech, AbbVie, Pfizer
Research Funding: Roche, Genentech, AbbVie
Travel, Accommodations, Expenses: Gilead Sciences, Janssen Oncology, AbbVie
Alejandro Martín García-Sancho
Honoraria: Roche, Janssen-Cilag, Celgene, Servier, Gilead Sciences, Takeda, Eusa Pharma, Novartis
Consulting or Advisory Role: Roche, Celgene, MorphoSys, Kyowa Kirin, Iqone, EUSA Pharma, Gilead Sciences, Novartis, Servier, Incyte
Expert Testimony: Gilead Sciences
Travel, Accommodations, Expenses: Roche, Celgene, Servier
M. José Terol
Consulting or Advisory Role: Janssen, AbbVie
Research Funding: Gilead Sciences
Travel, Accommodations, Expenses: Janssen, Roche, Gilead Sciences, AbbVie
Eva González Barca
Honoraria: Janssen, AbbVie, Takeda, Roche, Incyte, EUSA Pharma
Consulting or Advisory Role: Janssen, AbbVie, Gilead Sciences, Kiowa, EUSA Pharma, Incyte, Lilly, BeiGene, Novartis
Travel, Accommodations, Expenses: Janssen, EUSA Pharma
Adolfo de la Fuente
Honoraria: BSM, AbbVie, Astellas Pharma, Incyte, Pfizer
Consulting or Advisory Role: BMS
Research Funding: Novartis (Inst)
Xavier Setoain
Honoraria: General Electric
Consulting or Advisory Role: Qbiotech, Lemer Pax
Speakers' Bureau: Lemer Pax
Patents, Royalties, Other Intellectual Property: Patent and Royalties with the medical device Epijet with the company Lemer Pax
Travel, Accommodations, Expenses: Takeda, General Electric
Amanda Rotger
Employment: ITM Oncologics
Honoraria: ITM Oncologics, AAA HealthCare
Travel, Accommodations, Expenses: ITM Oncologics
Alejandro Medina Herrera
Research Funding: Janssen
Travel, Accommodations, Expenses: Diagnostica Longwood
Ramón García Sanz
Honoraria: Janssen, Takeda, Amgen, BeiGene, Novartis, Astellas Pharma
Consulting or Advisory Role: Janssen
Research Funding: Gilead Sciences (Inst), Incyte (Inst), Astellas Pharma
Patents, Royalties, Other Intellectual Property: BIOMED 2 primers (Inst)
Travel, Accommodations, Expenses: Janssen, Takeda (I)
Other Relationship: Spanish Society of Hematology (SEHH)
Ferran Nadeu
Honoraria: Janssen
Elías Campo
Honoraria: EUSA Pharma, AstraZeneca
Consulting or Advisory Role: Illumina, AbbVie
Patents, Royalties, Other Intellectual Property: Author on a patent licensed to NanoString Technologies
Armando López-Guillermo
Honoraria: Roche
Consulting or Advisory Role: Roche, Gilead, Kite Pharma, Celgene, Bristol Myers Squibb, Incyte, Takeda, Kern Pharma, Pfizer, Janssen
Research Funding: Janssen, Roche, Celgene, Bristol Myers Squibb
Travel, Accommodations, Expenses: Roche, Kite/Gilead
No other potential conflicts of interest were reported.
REFERENCES
- 1.Swerdlow SH, Campo E, Seto M, et al. in Swerdlow SH, Campo E, Harris NL, et al (eds): WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues (rev ed 4). Lyon, France, IARC. 2017. Mantle cell lymphoma; pp. 285–290. [Google Scholar]
- 2. Orchard J, Garand R, Davis Z, et al. A subset of t(11;14) lymphoma with mantle cell features displays mutated IgVH genes and includes patients with good prognosis, nonnodal disease. Blood. 2003;101:4975–4981. doi: 10.1182/blood-2002-06-1864. [DOI] [PubMed] [Google Scholar]
- 3. Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. J Clin Invest. 2012;122:3416–3423. doi: 10.1172/JCI61272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clot G, Jares P, Giné E, et al. A gene signature that distinguishes conventional and leukemic nonnodal mantle cell lymphoma helps predict outcome. Blood. 2018;132:413–422. doi: 10.1182/blood-2018-03-838136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nadeu F, Martin-Garcia D, Clot G, et al. Genomic and epigenomic insights into the origin, pathogenesis, and clinical behavior of mantle cell lymphoma subtypes. Blood. 2020;136:1419–1432. doi: 10.1182/blood.2020005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27:1209–1213. doi: 10.1200/JCO.2008.19.6121. [DOI] [PubMed] [Google Scholar]
- 7. Abrahamsson A, Albertsson-Lindblad A, Brown PN, et al. Real world data on primary treatment for mantle cell lymphoma: A Nordic Lymphoma Group observational study. Blood. 2014;124:1288–1295. doi: 10.1182/blood-2014-03-559930. [DOI] [PubMed] [Google Scholar]
- 8. Cohen JB, Han X, Jemal A, et al. Deferred therapy is associated with improved overall survival in patients with newly diagnosed mantle cell lymphoma. Cancer. 2016;122:2356–2363. doi: 10.1002/cncr.30068. [DOI] [PubMed] [Google Scholar]
- 9. Abrisqueta P, Scott DW, Slack GW, et al. Observation as the initial management strategy in patients with mantle cell lymphoma. Ann Oncol. 2017;28:2489–2495. doi: 10.1093/annonc/mdx333. [DOI] [PubMed] [Google Scholar]
- 10. Calzada O, Switchenko JM, Maly JJ, et al. Deferred treatment is a safe and viable option for selected patients with mantle cell lymphoma. Leuk Lymphoma. 2018;59:2862–2870. doi: 10.1080/10428194.2018.1455973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar A, Ying Z, Alperovich A, et al. Clinical presentation determines selection of patients for initial observation in mantle cell lymphoma. Haematologica. 2019;104:e163–6. doi: 10.3324/haematol.2018.201350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCulloch R, Smith A, Wainman B, et al. 40% of females with mantle cell lymphoma are managed with initial observation: Results from the MCL biobank observational study. Blood. 2019;134 suppl; abstr 2821. [Google Scholar]
- 13. Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: A single-centre, open-label, phase 2 trial. Lancet Oncol. 2016;17:48–56. doi: 10.1016/S1470-2045(15)00438-6. [DOI] [PubMed] [Google Scholar]
- 15. Jerkeman M, Eskelund CW, Hutchings M, et al. Ibrutinib, lenalidomide, and rituximab in relapsed or refractory mantle cell lymphoma (PHILEMON): A multicentre, open-label, singlearm, phase 2 trial. Lancet Haematol. 2018;5:e109–116. doi: 10.1016/S2352-3026(18)30018-8. [DOI] [PubMed] [Google Scholar]
- 16. Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211–1223. doi: 10.1056/NEJMoa1715519. [DOI] [PubMed] [Google Scholar]
- 17. Le Gouill S, Morschhauser F, Chiron D, et al. Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: A phase 1/2 trial. Blood. 2021;137:877–887. doi: 10.1182/blood.2020008727. [DOI] [PubMed] [Google Scholar]
- 18. Jain P, Romaguera J, Srour SA, et al. Four-year follow-up of a single arm, phase II clinical trial of ibrutinib with rituximab (IR) in patients with relapsed/refractory mantle cell lymphoma (MCL) Br J Haematol. 2018;182:404–411. doi: 10.1111/bjh.15411. [DOI] [PubMed] [Google Scholar]
- 19. Ruan J, Martin P, Christos P, et al. Five year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood. 2018;132:2016–2025. doi: 10.1182/blood-2018-07-859769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jain P, Zhao S, Lee HJ, et al. Ibrutinib with rituximab in first-line treatment of older patients with mantle cell lymphoma. J Clin Oncol. 2022;40:202. 212. doi: 10.1200/JCO.21.01797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pott C. Minimal residual disease detection in mantle cell lymphoma: Technical aspects and clinical relevance. Semin Hematol. 2011;48:172–184. doi: 10.1053/j.seminhematol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 22. van Dongen JJ, Langerak AW, Brüggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 concerted action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 23. Kotrova M, Darzentas N, Pott C, et al. Next-generation sequencing technology to identify minimal residual disease in lymphoid malignancies. Methods Mol Biol. 2021;2185:95–111. doi: 10.1007/978-1-0716-0810-4_7. [DOI] [PubMed] [Google Scholar]
- 24. Dreyling M, Campo E, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv62–iv71. doi: 10.1093/annonc/mdx223. [DOI] [PubMed] [Google Scholar]
- 25. Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130:1903–1910. doi: 10.1182/blood-2017-04-779736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Spanish Lymphoma Group (GELTAMO, Grupo Español de Linfomas y Trasplante Autólogo de Médula Ósea) supports the ethical obligation of responsible sharing of data from clinical trials. The study Protocol, informed consent form, and amendments of the study Protocol will be provided. Deidentified individual participant data reported in the manuscript will be shared under the terms of a Data Use Agreement and may only be used for approved proposals. Request may be made to egine@clinic.cat.