PURPOSE
Graft-versus-host disease (GVHD) causes morbidity and mortality following allogeneic hematopoietic cell transplantation. Naive T cells (TN) cause severe GVHD in murine models. We evaluated chronic GVHD (cGVHD) and other outcomes in three phase II clinical trials of TN-depletion of peripheral blood stem-cell (PBSC) grafts.
METHODS
One hundred thirty-eight patients with acute leukemia received TN-depleted PBSC from HLA-matched related or unrelated donors following conditioning with high- or intermediate-dose total-body irradiation and chemotherapy. GVHD prophylaxis was with tacrolimus, with or without methotrexate or mycophenolate mofetil. Subjects received CD34-selected PBSC and a defined dose of memory T cells depleted of TN. Median follow-up was 4 years. The primary outcome of the analysis of cumulative data from the three trials was cGVHD.
RESULTS
cGVHD was very infrequent and mild (3-year cumulative incidence total, 7% [95% CI, 2 to 11]; moderate, 1% [95% CI, 0 to 2]; severe, 0%). Grade III and IV acute GVHD (aGVHD) occurred in 4% (95% CI, 1 to 8) and 0%, respectively. The cumulative incidence of grade II aGVHD, which was mostly stage 1 upper gastrointestinal GVHD, was 71% (95% CI, 64 to 79). Recipients of matched related donor and matched unrelated donor grafts had similar rates of grade III aGVHD (5% [95% CI, 0 to 9] and 4% [95% CI, 0 to 9]) and cGVHD (7% [95% CI, 2 to 13] and 6% [95% CI, 0 to 12]). Overall survival, cGVHD-free, relapse-free survival, relapse, and nonrelapse mortality were, respectively, 77% (95% CI, 71 to 85), 68% (95% CI, 61 to 76), 23% (95% CI, 16 to 30), and 8% (95% CI, 3 to 13) at 3 years.
CONCLUSION
Depletion of TN from PBSC allografts results in very low incidences of severe acute and any cGVHD, without apparent excess risks of relapse or nonrelapse mortality, distinguishing this novel graft engineering strategy from other hematopoietic cell transplantation approaches.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) can cure patients with advanced hematologic malignancies.1,2 αβT cells in the graft that recognize recipient alloantigens promote engraftment by attacking host hematopoietic and immune cells and diminish relapse by killing neoplastic blood cells, thereby mediating the graft-versus-leukemia (GVL) effect. Unfortunately, alloreactive T cells can damage normal host tissues, causing graft-versus-host disease (GVHD) and necessitating pharmacologic immunosuppression or T-cell–depleted grafts. GVHD and immunosuppression both contribute to morbidity and mortality and are barriers to a broader application of HCT.3 Chronic GVHD (cGVHD), which occurs in 30%-60% of patients receiving unmanipulated grafts, often requires prolonged immunosuppression and causes nonrelapse mortality (NRM) and a reduced quality of life.4 cGVHD is especially problematic in recipients of peripheral blood stem-cell (PBSC) grafts; yet, PBSCs remain the most prevalent graft source.5 Complete graft T-cell–depletion (pan-TCD) reduces GVHD but delays immune reconstitution, increases opportunistic infections, and may reduce survival.6 In vivo partial T-cell–depletion with antithymocyte globulin (ATG) or anti–T-lymphocyte globulin (ATLG), impairment of alloreactive T cells using post-HCT cyclophosphamide (PTCy), and other strategies have also reduced GVHD, but each has limitations and cGVHD remains problematic.6-12
CONTEXT
Key Objective
Chronic graft-versus-host disease (cGVHD) occurs in 30%-60% of patients with leukemia who receive unmanipulated donor HLA-matched peripheral blood stem-cell (PBSC) allografts and is a major source of morbidity and mortality. In mouse models, the depletion of antigen-inexperienced naive T cells (TN) from grafts reduced GVHD. Importantly, the remaining memory T cells retained antileukemia activity. We, therefore, conducted clinical trials to determine whether the depletion of TN from PBSC grafts would reduce cGVHD.
Knowledge Generated
In 138 recipients of HLA-matched TN-depleted PBSC, the incidence of cGVHD was very low (7%), resulting in a 3-year cGVHD-free, relapse-free survival of 68%. These rates were similar across subgroups, including age, related or unrelated grafts, leukemia type, and conditioning regimen intensity.
Relevance
Depletion of TN from donor PBSC is a promising and widely applicable strategy for reducing cGVHD, which warrants comparison to other graft-versus-host disease-reduction strategies in randomized clinical trials.
αβT cells include naive (TN), effector (TE), and memory (TM) subsets, distinguishable by surface phenotype.13 We and others demonstrated that TN caused severe GVHD in murine models, whereas TM caused milder or no GVHD and retained graft-versus-tumor activity.14-20 Informed by these results and human in vitro studies demonstrating enrichment of alloreactive T cells in TN,21 we developed a graft engineering strategy to selectively deplete TN from granulocyte colony-stimulating factor–mobilized PBSC using immunomagnetic beads targeting CD45RA, which is expressed on all TN but absent on most TM.22 An initial clinical trial supported the hypothesis that TN-depletion of PBSC would mitigate GVHD and protect patients from severe opportunistic infections by retaining graft pathogen-specific TM.23
We now report our experience with TN-depleted HCT in 138 patients with acute leukemia and myelodysplastic syndrome treated on three prospective phase II clinical trials. TN-depletion resulted in low rates of severe acute GVHD (aGVHD) and exceptionally low rates of cGVHD.
METHODS
Study Design and Participants
Subjects were enrolled in three phase II trials of TN-depleted PBSC (Data Supplement 1 [online only] and Clinical Trial Protocols NCT00914940, NCT01858740, and NCT02220985 [online only]). The preliminary results for the first 35 patients in NCT00914940 were reported previously.23 All patients enrolled in NCT00914940 (n = 41) and NCT01858740 (n = 20), and 77 of 84 patients enrolled in NCT02220985, received TN-depleted grafts. Donor apheresis products for seven patients on NCT02220985 did not meet the Protocol specified criteria for cell selection; by Protocol, those patients received unmanipulated PBSC (Fig 1).
FIG 1.
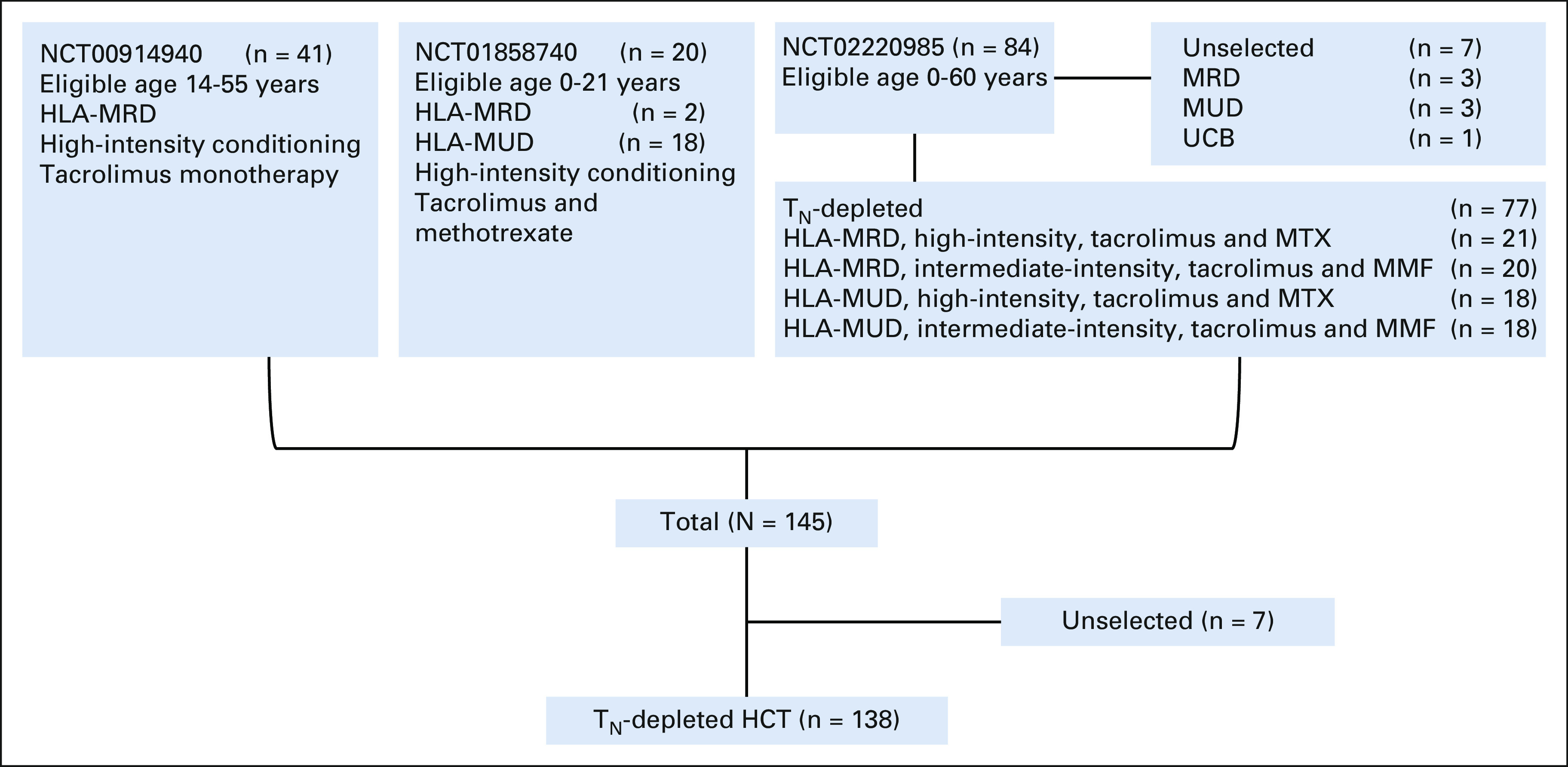
Study flow diagram. HCT, hematopoietic cell transplantation; MMF, mycophenolate mofetil; MRD, matched related donors; MTX, methotrexate; MUD, matched unrelated donors; TN, naive T cells; UCB, umbilical cord blood.
Patients provided informed consent in accordance with the Declaration of Helsinki. The study was performed after approval by the institutional review boards and the US Food and Drug Administration (Investigational Device Exemption 14160 and Investigational New Drug 15673), and in accord with an assurance approved by the Department of Health and Human Services.
Eligible patients were those referred for allogeneic HCT for a high predicted risk of relapse following chemotherapy alone. Inclusion and exclusion criteria are in Data Supplement 1. NCT00914940, NCT01858740, and NCT02220985 enrolled patients age 14-55, 0-21, and 0-60 years, respectively.
Procedures
Conditioning for NCT00914940 and NCT01858740 was high-intensity with fludarabine (25 mg/m2 once per day for 5 days), thiotepa (5 mg/kg once per day for 2 days), and total-body irradiation (1,320 cGy).24 In NCT02220985, younger patients mostly received the same high-intensity conditioning, whereas patients age ≥ 50 years or with comorbidities received intermediate-intensity myeloablative conditioning with fludarabine (30 mg/m2 once per day for 5 days), cyclophosphamide (50 mg/kg once per day for 1 day), thiotepa (5 mg/kg once per day for 2 days), and total-body irradiation (400 cGy25; Data Supplement 1). Patients then received HLA-matched, CD34-selected PBSC, followed immediately by CD45RA-depleted cells from the CD34-negative fraction. NCT00914940 was restricted to patients with HLA-matched related donors (MRD) and used tacrolimus for GVHD prophylaxis, whereas NCT01858740 and NCT02220985 included patients with MRD or HLA-matched unrelated donors (MUD) and used tacrolimus plus methotrexate (high-intensity conditioning) or tacrolimus plus mycophenolate mofetil (intermediate-intensity conditioning).
GVHD was treated according to institutional standard practices with systemic and/or topical corticosteroids, continuation of tacrolimus, and additional therapies as deemed necessary. Duration of systemic corticosteroids (0.5-2 mg/kg prednisone per day) and subsequent taper were determined by treating physicians. Antimicrobial prophylaxis is in Data Supplement 1.
Each trial used the same graft engineering method (Data Supplement 1).22 Donors received granulocyte colony-stimulating factor daily for 5 days, followed by PBSC collection by apheresis. Cell selection was initiated if ≥ 5 × 106 CD34+ cells/kg were collected and the presence of CD3+CD45RA−CD45RO+ TM was confirmed. Positive selection of CD34+ progenitor cells was followed by depletion of CD45RA+ cells from the CD34-negative fraction using anti-CD45RA beads (Miltenyi Biotec; Data Supplement 1). All trials targeted CD34+ cell doses of ≥ 5.0 × 106 cells/kg. In NCT00914940, ≤ 7.5 × 104 TN/kg was targeted on the basis of estimates that more would cause GVHD. As this was consistently achieved, a goal of ≤ 5 × 104 TN/kg was set in NCT01858740 and NCT02220985. In each trial, 107 CD3+ T cells/kg was targeted to provide sufficient TM to facilitate immune reconstitution.
Outcomes
The primary outcome of the analysis of cumulative data was cGVHD diagnosed by 2014 National Institutes of Health Consensus Criteria (Data Supplement 1).26 Secondary outcomes were graft failure, grade III-IV aGVHD within 1 year of HCT (Data Supplement 1),27 overall survival (OS), relapse, NRM, and survival free of moderate or severe cGVHD or relapse (cGVHD-free, relapse-free survival [CRFS]). The end point definition details and competing risks are in Data Supplement 1. Expert physicians graded aGVHD and reviewed cGVHD diagnosis and grading. Biopsies were performed to confirm skin and GI GVHD in most cases (Data Supplement 1). Donor chimerism was monitored by molecular techniques (Data Supplement 1).
Statistical Analysis
Data were analyzed as of December 2020. Probabilities of OS, relapse-free survival (RFS), CRFS, and GVHD-free, relapse-free survival (GRFS) were estimated with the Kaplan-Meier method. Probabilities of engraftment, death not preceded by relapse, recurrent malignancy, aGVHD and cGVHD, permanent discontinuation of systemic corticosteroids, and discontinuation of all systemic immune suppression were summarized by cumulative incidence estimates with competing risks (Data Supplement 1). The relationship between aGVHD and relapse was explored using a Cox proportional hazards model with aGVHD as a time-dependent covariate, adjusting for relapse-risk group and conditioning intensity. Clinical outcome analyses were conducted using R 3.6.0.
RESULTS
Subjects and Graft Engineering
From December 2009 to March 2020, 145 patients age 1-60 years with acute leukemia or advanced myelodysplastic syndrome were enrolled at one of three centers on one of three phase II trials of TN-depleted PBSC grafts (Data Supplement 1). All patients received Protocol-specified conditioning and post-HCT pharmacologic immunosuppression. Donor PBSC aphereses meeting requirements for cell selection were available for 138 of 145 patients; these patients received TN-depleted grafts meeting release criteria (Data Supplement 1). The characteristics and outcomes of the recipients of unmanipulated PBSC (n = 6) or umbilical cord blood (n = 1) and an intent-to-treat analysis are in Data Supplement 1.
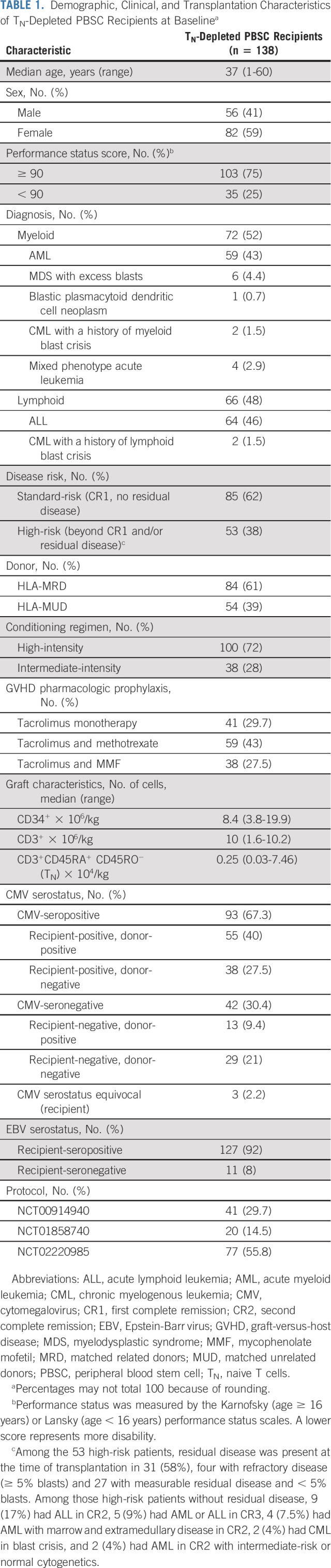
Cell selection targets were achieved for all 138 TN-depleted PBSC recipients. Grafts contained a median of 8.4 × 106 CD34+ cells/kg (3.8-20 × 106) and 1 × 107 CD3+ T cells/kg (1.6-10.2 × 106), including a median of 2,500 TN/kg (300-74,600; interquartile range [IQR], 1,500-4,100; 95% < 10,000 TN/kg; Table 1). Products administered across trials, institutions, and from MRD and MUD sources did not significantly differ (Data Supplement 1).
TABLE 1.
Demographic, Clinical, and Transplantation Characteristics of TN-Depleted PBSC Recipients at Baselinea
Donor Cell Engraftment, Chimerism, and Immune Reconstitution
Neutrophil engraftment occurred at a median of 15 days (9-29) and platelet counts exceeded 20,000/mm3 without transfusion at a median of 14 days (8-111; Data Supplement 1). Six patients died during the first 100 days, before neutrophil (n = 1) or platelet engraftment (n = 6). Myeloid (CD33+) cells were ≥ 95% donor-derived in all recipients at most or all time points. CD3+ T cells were ≥ 95% donor-derived in most recipients at all time points (Data Supplement 1). There were no graft rejections. Two patients developed secondary graft failure at nine and 16 months, one with 100% donor myeloid and T-cell chimerism and one with 100% donor myeloid and stable mixed T-cell chimerism (50%-70%).
Lymphocyte recovery was similar to that observed in the unmanipulated PBSC graft recipients (Data Supplement 1), and the numbers of CD4+ and CD8+ T cells exceeded the values reported for pan-TCD in the early post-HCT period.23,28 CD8+ and CD4+ TN were generally not observed during the first 6 months post-HCT, as expected.
Graft-Versus-Host Disease
The cumulative incidences of grades III and IV aGVHD were 4% (95% CI, 1 to 8) and 0%, respectively (Fig 2A). The cumulative incidence of grade II aGVHD, which was mostly stage 1 (upper) gastrointestinal GVHD, was 71% (95% CI, 64 to 79; Fig 2B, Data Supplement 1). The incidences of aGVHD are summarized by subgroups in Data Supplement 1. The incidence of grade III aGVHD was very low, regardless of graft source (MRD, 5% [95% CI, 0 to 9]; MUD, 4% [95% CI, 0 to 9]), or conditioning intensity (high-intensity, 5% [95% CI, 1 to 9]; intermediate-intensity, 3% [95% CI, 0 to 8]). The clinical pattern of aGVHD, stage, and response to corticosteroids are in Data Supplement 1. Systemic treatment with agents other than corticosteroids was required in only two subjects. The cumulative incidence of stage 1, 2, 3, and 4 gastrointestinal aGVHD was 69% (95% CI, 61 to 77), 2.2 (95% CI, 0 to 4.7), 1.5% (95% CI, 0 to 3.5), and 0.7% (95% CI, 0 to 2.2), respectively. Although stage 1 gastrointestinal aGVHD, manifested by anorexia and nausea, occurred frequently, it responded to prednisone within seven days in most patients (Data Supplement 1). Thirteen subjects with gastrointestinal aGVHD required only topical beclomethasone with or without budesonide. Of note, grade II-III aGVHD was associated with decreased risks of relapse and death (Fig 3, Data Supplement 1).
FIG 2.
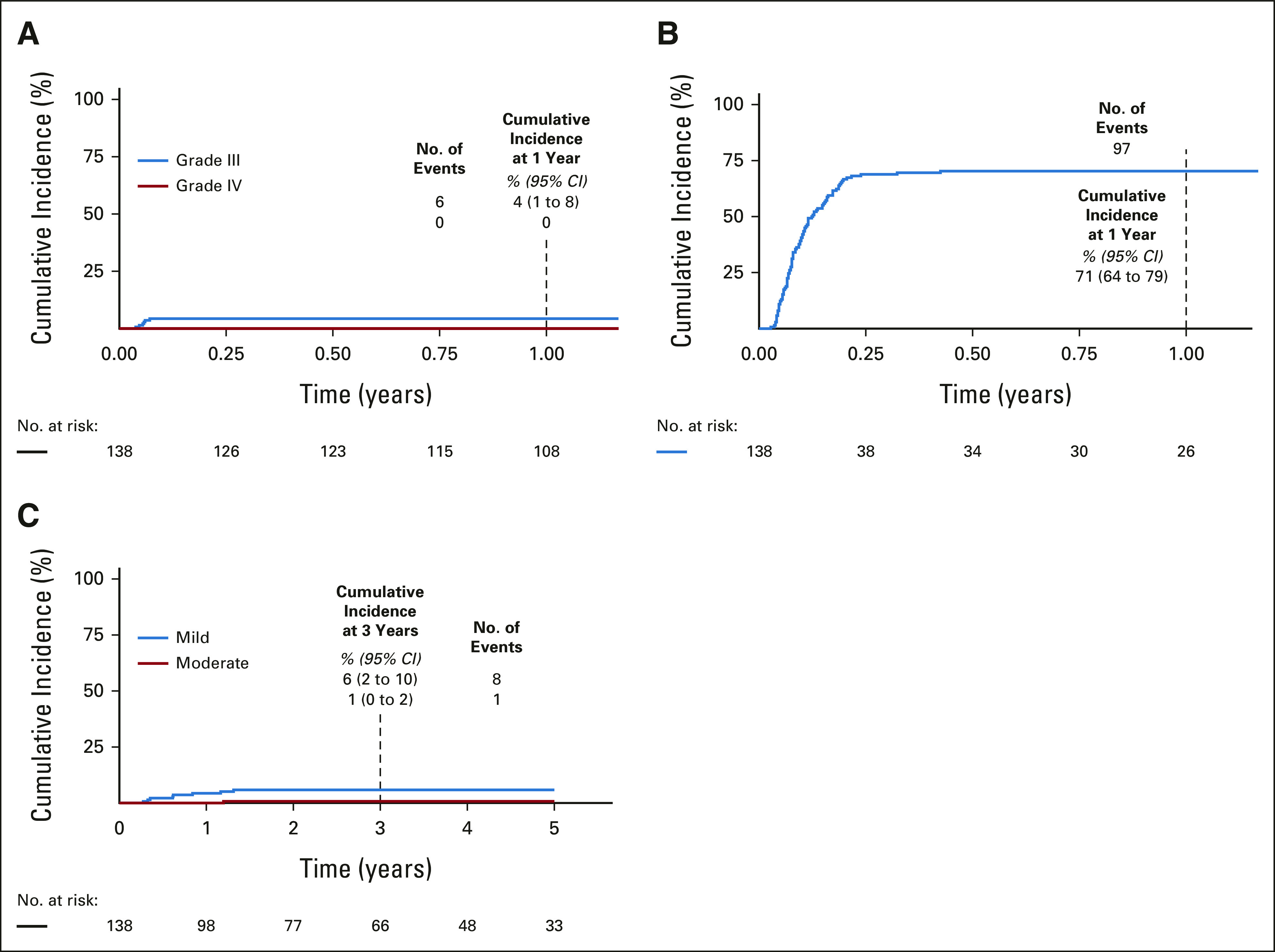
Cumulative incidence of (A) grade III-IV aGVHD, (B) grade II aGVHD, and (C) cGVHD. aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease.
FIG 3.
Relative risks of (A) relapse and (B) death in naive T cells-depleted peripheral blood stem cell recipients who developed acute graft-versus-host disease (grade II-III) compared with those who did not. HCT, hematopoietic cell transplantation; MRD, matched related donors; MUD, matched unrelated donors.
Three-year cumulative incidences of mild, moderate, and severe cGVHD were 6% (95% CI, 2 to 10), 1% (95% CI, 0 to 2) and 0%, respectively (Fig 2C). Six of nine patients with cGVHD completed prednisone at a median of 667 days (259-1,366) post-HCT; the others required only tacrolimus (n = 2) or topical corticosteroids (n = 1). Two of the six who received prednisone required additional therapies (Data Supplement 1). The incidences of cGVHD are summarized by subgroup in Data Supplement 1. The incidence of cGVHD was similar in patients who received TN-depleted MRD (7% [95% CI, 2 to 13]) or MUD (6% [95% CI, 0 to 12]) PBSC. Of the 94 patients who were alive at 2 years post-HCT, three were receiving systemic immunosuppression for cGVHD management.
Relapse and Survival
Median follow-up of surviving patients was 1,485 days (262-1,826 days). The 3-year cumulative incidence of relapse was 23% (95% CI, 16 to 30; Fig 4A). Relapse was 27% (95% CI, 17 to 37) and 17% (95% CI, 7 to 27) in recipients of MRD or MUD grafts, 19% (95% CI, 11 to 27) and 35% (95% CI, 18 to 52) after high-intensity or intermediate-intensity conditioning, 27% (95% CI, 17 to 38) and 19% (95% CI, 9 to 28) in those with myeloid malignancies or lymphoid leukemia, and 18% (95% CI, 7 to 29) and 26% (95% CI, 17 to 36) in those age < 30 and ≥ 30 years, respectively (Data Supplement 1). The median time to relapse was 206 days (56-1,091; IQR, 111-343 days). Among the 31 patients who relapsed, 90% were not on prednisone at the time and most had discontinued (10 of 31) or were rapidly tapered off immunosuppression without developing GVHD (16 of 18 tapered successfully, two flared GVHD, and three had rapid leukemia progression precluding a taper). Of the 31 patients who relapsed, the median survival from relapse was 273 days (4-1,615 days to death or last follow-up; IQR, 85-590 days). Twenty-one relapsed patients were treated with donor lymphocyte infusions or other immunotherapies; 11 were subsequently in complete remission for at least 6 months (Data Supplement 1).
FIG 4.
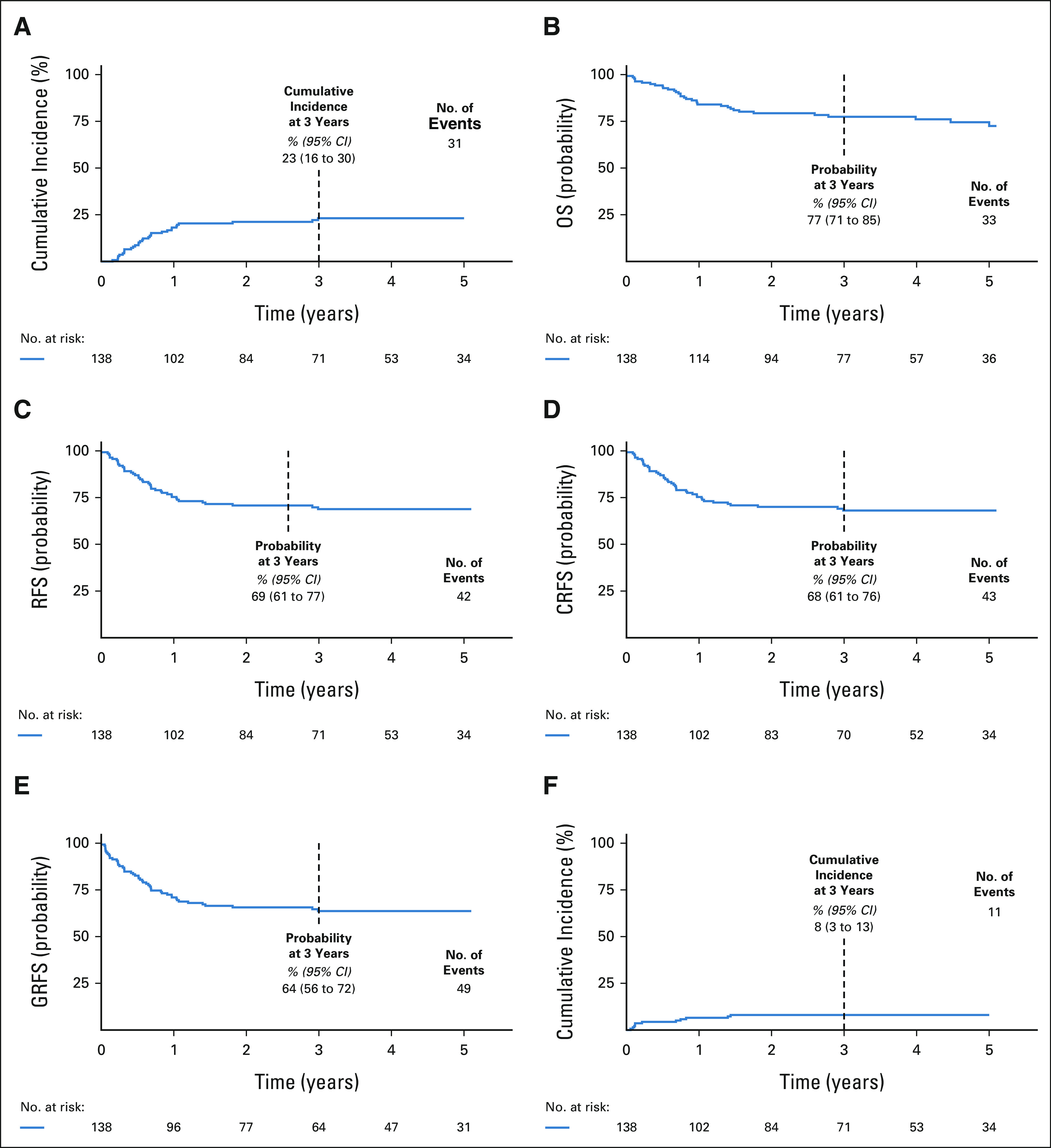
Relapse and survival. (A) Cumulative incidence of relapse, (B) probability of OS, (C) probability of RFS, (D) probability of CRFS, (E) probability of GRFS, and (F) cumulative incidence of NRM. CRFS, cGVHD-free, relapse-free survival; GRFS, GVHD-free, relapse-free survival; NRM, nonrelapse mortality; OS, overall survival; RFS, relapse-free survival.
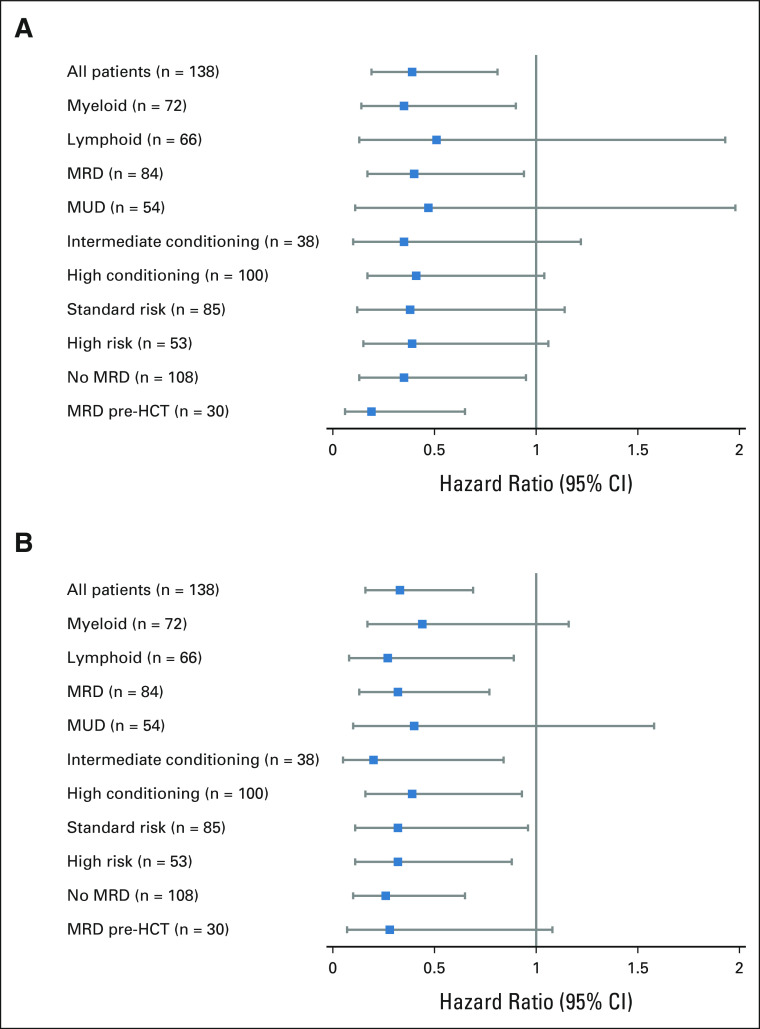
OS, RFS, CRFS, and GRFS at 3 years were 77% (95% CI, 71 to 85), 69% (95% CI, 61 to 77), 68% (95% CI, 61 to 76), and 64% (95% CI, 56 to 72), respectively (Figs 4B-4E). These probabilities are summarized by subgroup in the Data Supplement (OS, RFS, CRFS, and GRFS) and Figure 5 (CRFS). In recipients of intermediate-intensity conditioning (n = 38; median age, 54 years; 15-60, IQR, 50-57), the OS, RFS, CRFS, and GRFS at 3 years were 78% (95% CI, 66 to 93), 60% (95% CI, 45 to 80), 57% (95% CI, 41 to 78), and 54% (95% CI, 38 to 75), respectively (Data Supplement 1, Fig 5). An intent-to-treat analysis is in Data Supplement 1.
FIG 5.
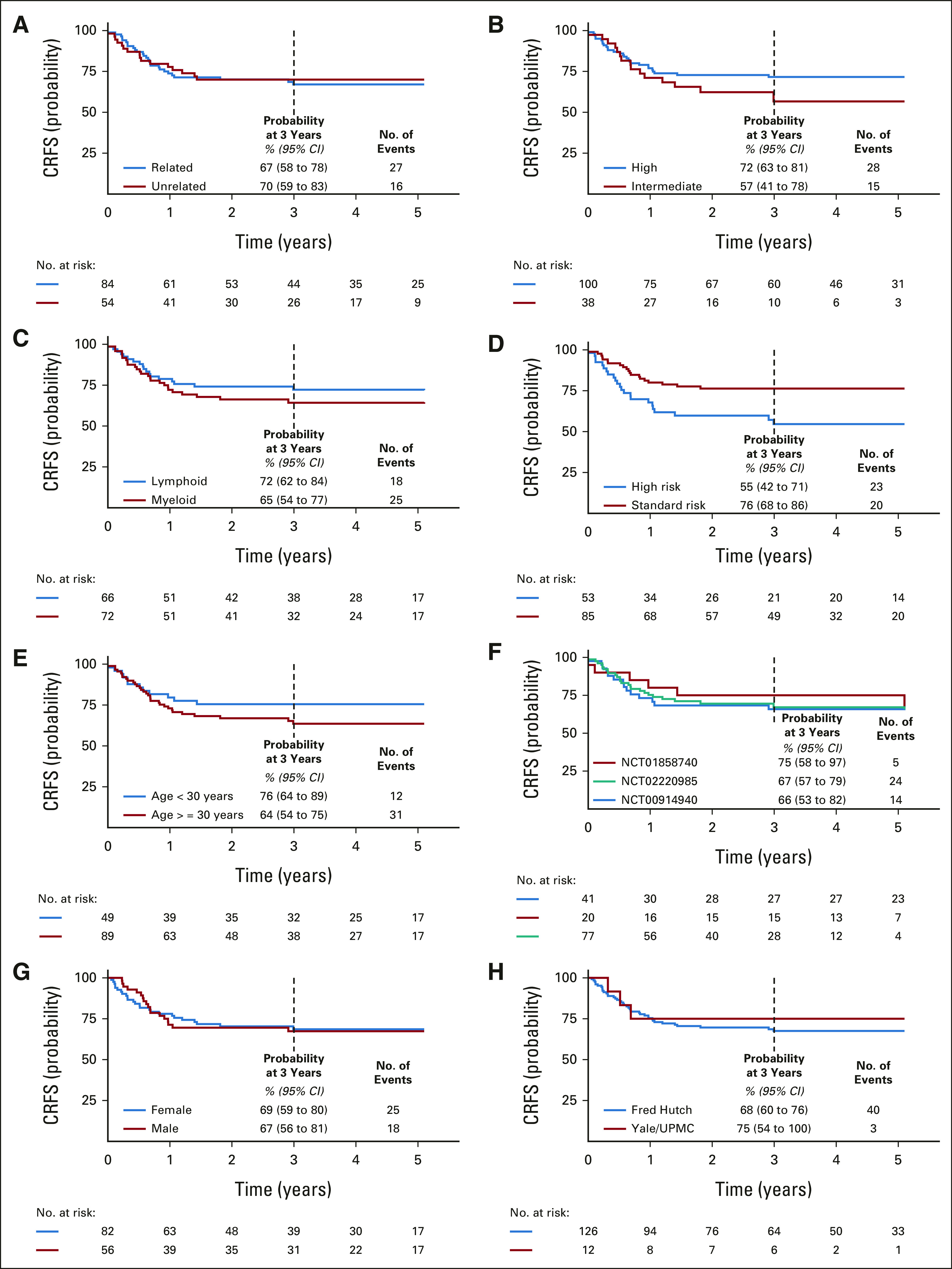
CRFS in subject subgroups. Probability of CRFS by (A) donor source, (B) conditioning intensity, (C) lymphoid or myeloid malignancy, (D) disease risk, (E) age, (F) clinical trial, (G) sex, and (H) institution. CRFS, cGVHD-free, relapse-free survival; UPMC, University of Pittsburgh Medical Center.
NRM, Causes of Death, Adverse Events, and Infectious Complications
Among recipients of TN-depleted grafts, the cumulative incidences of NRM at 100 days and 3 years were 4% (95% CI, 1 to 8) and 8% (95% CI, 3 to 13), respectively (Fig 4F). The 3-year NRM estimates were 6% (95% CI, 1 to 11) and 11% (95% CI, 3 to 20) for recipients of MRD and MUD grafts, 9% (95% CI, 3 to 15) and 5% (95% CI, 0 to 13) for recipients of high-intensity and intermediate-intensity conditioning, and 6% (95% CI, 0 to 13) and 9% (95% CI, 3 to 15) in those age < 30 and ≥ 30 years, respectively (Data Supplement 1). No serious reactions occurred with cell infusions (Data Supplement 1). Types and frequencies of grades 3-5 nonhematologic adverse effects were typical of myeloablative HCT recipients (Data Supplement 2, online only). NRM causes are in Data Supplement 1.
Infections are detailed in Data Supplement 1. Epstein-Barr virus (EBV) reactivation was uncommon (EBV ≥ 1,000 copies/mL plasma in three [2.1%] subjects); only one of 138 subjects required rituximab to manage EBV reactivation. One and two subjects had human herpesvirus 6 (> 1,000 copies/mL) or adenovirus (> 300 copies/mL) reactivations. The cumulative incidence of cytomegalovirus reactivation (≥ 500 IU/mL) at day 180 (12%) was similar to that reported after pan-TCD (11.6%) and T-cell–replete (9.6%) HCT.6 Possible, probable, or proven cytomegalovirus disease occurred in 4.3% of subjects.
DISCUSSION
Here, we report on 138 recipients of TN-depleted HLA-matched allografts. Cell processing was reliable using widely available commercial technology. aGVHD was mild and corticosteroid-responsive; use of second-line agents to treat aGVHD was rarely required. Strikingly, only 7% of patients developed cGVHD, which was also mostly mild and steroid-responsive. The decrement in cGVHD was not associated with an apparent increase in relapse or fatal infections, yielding favorable OS, RFS, and CRFS rates regardless of graft source, conditioning intensity, or patient age.
Prior studies of pharmacologic approaches for GVHD reduction with unmanipulated grafts have reported low rates of severe aGVHD but have not reduced cGVHD to the levels observed with TN-depletion (Data Supplement 1). In the recent multiarm Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1301 randomized controlled trial (RCT) of GVHD prophylaxis methods, the arm using bone marrow transplantation with PTCy for GVHD prophylaxis reported a 2-year incidence of moderate-to-severe cGVHD of 27%, similar to the tacrolimus and methotrexate arm (33.7%).6 Other studies of PTCy in HLA-matched HCT reported cGVHD and moderate-to-severe cGVHD rates of 13%-42% and up to 30%, respectively (Data Supplement 1). In RCTs, ATG or ATLG reduced aGVHD, cGVHD, or both; however, cGVHD and moderate-severe cGVHD rates were 16%-34% and 8%-16%, respectively (Data Supplement 1). In our trials, severe aGVHD rates were comparable to those observed with the addition of sitagliptin29 abatacept,30 ATG or ATLG to standard immunosuppression with unmanipulated HCT, and to bone marrow transplantation with PTCy.6 However, overall (7%) and moderate-to-severe (1%) cGVHD were less frequent with TN-depletion.
Ex vivo pan-TCD reduces both aGVHD and cGVHD (Data Supplement 1).28,31 In BMT CTN 1301, the pan-TCD PBSC arm had a low 2-year incidence of cGVHD (22.5% total and 8.9% moderate-to-severe)6 but high NRM (21.5%), partially because of opportunistic infections. By contrast, with TN-depletion, cGVHD was very infrequent and NRM was only 8% at 3 years, reflecting the paucity of fatal opportunistic infections. In particular, high-level EBV reactivation, which can lead to fatal lymphoproliferative disorders and is more common with pan-TCD,6,28 was rare in TN-depleted graft recipients, consistent with the transfer of donor EBV-specific TM. The higher NRM with pan-TCD could be partially because of the administration of ATG combined with ex-vivo graft lymphocyte depletion. By contrast, TN-depleted grafts were used without ATG, thereby preserving graft TM. The 8% incidence of NRM in TN-depleted HCT is similar or lower than NRM reported in other forms of HCT (Data Supplement 1).
Although cGVHD and severe aGVHD rates were low with TN-depletion, a syndrome of corticosteroid-responsive stage 1 upper GI aGVHD, characterized by nausea and anorexia and associated with a mild increase in apoptotic epithelial cells, was frequently observed. This syndrome was associated with protection from relapse and death, suggesting a connection to GVL and an alloimmune mechanism. Given that aGVHD is the greatest risk factor for cGVHD,32 the disconnect between aGVHD and cGVHD in TN-depleted HCT implies fundamental functional differences between the progeny of alloreactive TM and TN, such that TM cause only limited aGVHD and rare cGVHD, yet provide some protection from relapse.
T cell receptor (TCR)–repertoire–dependent and –independent differences between TN and TM have been studied in mouse GVHD models.18-20,33-36 TN have a more diverse TCR repertoire and a greater frequency of minor histocompatibility (H) antigen-reactive T cells.21 The remaining TM in TN-depleted grafts may target fewer minor H antigens with fewer unique TCRs targeting each. This might generate a sufficient alloresponse to induce limited aGVHD and some GVL but be generally insufficient to initiate or sustain cGVHD.37 Other cell-intrinsic, repertoire-independent differences between TN and TM, such as where they traffic and their potential for clonal expansion and differentiation, may also contribute to their disparate capacities to induce severe or cGVHD.18-20,34-36 Testing these hypotheses in humans will require the ability to broadly characterize minor H antigen-specific T cells in tissues of HCT recipients, which is not practical with current technologies.
Relative to published cohorts that received myeloablative conditioning and unmanipulated MUD or MRD grafts, an increase in leukemia relapse was not observed in TN-depleted graft recipients (Data Supplement 1), suggesting GVL activity was at least partially retained. Although (62%) of our subjects were in CR1 without measurable residual disease, such patients remain at risk of relapse, with 3-year relapse rates of 22% reported in 235 patients transplanted with AML in remission with no measurable disease by multiparametric flow cytometry,38 and relapse of 20% reported even in patients with AML transplanted in a measurable residual disease-negative remission by highly sensitive next-generation sequencing.39 Thus, the 23% cumulative incidence of relapse in our cohort, and 17% among our CR1 measurable residual disease-negative subjects, is consistent with previous experience. Randomized trials comparing TN-depletion to other strategies would better clarify any impact of TN-depletion on relapse.
Drugs used to prevent and treat GVHD may limit the use and efficacy of promising engineered T-cell immunotherapies for post-HCT relapse.40-42 Because of the shorter duration of pharmacologic immunosuppression, TN-depleted HCT may facilitate such post-HCT immunotherapies. Notably, immunosuppression was discontinued in most of our patients with early relapse without subsequent GVHD, suggesting that routine immunosuppression tapering after TN-depleted HCT could be accelerated.
The strengths of our research include the low rate of cGVHD and high CRFS in a sizable cohort of 138 patients across three clinical trials, observed across major subgroups including recipients of MRD and MUD grafts, high- and intermediate-intensity conditioning regimens, and immunosuppression with tacrolimus monotherapy or tacrolimus with methotrexate or MMF. Our results support the use of graft TN-depletion as a broadly applicable approach for cGVHD reduction (Data Supplement 1, Fig 5).
Study limitations include the possibility that the lower total T-cell dose administered with TN-depleted grafts might explain the cGVHD reduction. Although the T-cell dose in our trials (107 CD3+ cells/kg) is less than in unselected PBSC grafts (around 20-30 × 107/kg), it is in the same range as the CD3+ content of bone marrow grafts (2-3 × 107/kg). In the randomized trial of PBSC versus BMT5 for which the T-cell doses were subsequently published,43 the incidence of cGVHD at 2 years in the PBSC group was 53% with a median T-cell dose of 24.6 × 107/kg, compared with 41% with a median T-cell dose of 2.3 × 107/kg in the BMT group. If a 90% difference in the T-cell content of grafts (24.6 × 107 to 2.3 × 107/kg) is associated with a decrease in cGVHD of 53% to 41%, it seems highly unlikely that a 95% reduction in total T cells (24.6 × 107 to 1.0 × 107/kg) would result in a cGVHD rate of < 10%, and much more likely that the multilog reduction of the TN content of the graft was responsible.
We acknowledge the limitations of single-arm phase II studies, including the risk of type 1 errors. Given the size and composition of our cohorts and the magnitude of the reduction in cGVHD observed across all trials and subgroups, it is unlikely that a randomized trial would disprove that TN-depletion results in a much lower incidence of cGVHD (7% [95% CI, 2 to 11]) than unselected PBSC transplantation with tacrolimus and methotrexate GVHD prophylaxis. Historical cGVHD rates at Fred Hutch, the center that enrolled > 90% of the patients, were 45% for MUD and 42% for MRD recipients.44 Rates of cGVHD reported in the literature have remained stable with incidences of all cGVHD and moderate-severe cGVHD of 37%-45% and 34%-36%, respectively, reported in the control arms of phase III RCTs between 2019 and 2021 (Data Supplement 1). However, randomized trials will be important to confirm beyond doubt the cGVHD reduction and to definitively determine how relapse, survival, and composite end points such as GRFS in recipients of TN-depleted HCT compare to patients treated with standard HCT and other GVHD-reduction strategies. To address this, we have initiated two phase II RCTs. NCT03970096 compares TN-depleted PBSCT to T-cell–replete PBSCT with tacrolimus and methotrexate or with PTCy, with the goal of determining whether TN-depleted HSC is sufficiently promising to justify comparing it to other approaches in a pivotal phase III RCT. NCT03779854 is a Pediatric Transplantation and Cellular Therapy Consortium trial that compares TN-depleted PBSCT to T-cell–replete BMT with tacrolimus and methotrexate.
ACKNOWLEDGMENT
The authors would like to thank the following people for their contributions to this work: Cindy Hirano (regulatory support), Rekha Suresh (data management), Jennifer Adrian (cell therapy laboratory), Melissa Dela Pena (cell therapy laboratory), Andrew Mackie (cell therapy laboratory), Mary Jo Buffo (cell therapy laboratory), Joan Suver (patient care), Kyle Woodward (laboratory studies), Reema Jain (laboratory studies), Diego Archilla (laboratory studies), and Jacqueline Diaz (laboratory studies).
Marie Bleakley
Stock and Other Ownership Interests: HighPass Bio
Consulting or Advisory Role: HighPass Bio, Orca Bio
Research Funding: HighPass Bio
Patents, Royalties, Other Intellectual Property: TCRs specific for minor histocompatibility antigen HA-1 and uses thereof US20190211076A1 and international. Patent issued January 21, 2020. US10,538,574. Inventor on patent. Held by Fred Hutchinson Cancer Research Center
Other Relationship: Miltenyi Biotec
Alison Sehgal
Speakers' Bureau: OncLive
Research Funding: Kite/Gilead, Juno Therapeutics
Melinda A. Biernacki
Employment: Outpace Bio (I), Lyell Immunopharma (I)
Stock and Other Ownership Interests: Lyell Immunopharma (I), Outpace Bio (I)
Patents, Royalties, Other Intellectual Property: My partner receives royalties from a patent held by the Fred Hutchinson Cancer Research Center pertaining to nanomaterials for mRNA delivery. There is no relationship between the research in the current publication and the technology in the patent (I)
Elizabeth F. Krakow
Research Funding: HighPass Bio (Inst)
Ann Dahlberg
Research Funding: Jazz Pharmaceuticals, Atara Biotherapeutics
Paul J. Martin
Stock and Other Ownership Interests: Procter & Gamble
Honoraria: Janssen/Pharmacyclics, Therakos
Consulting or Advisory Role: Pfizer, Mesoblast, Rigel, Talaris, Malinckrodt
Research Funding: AltruBio (Inst)
Paul A. Carpenter
Honoraria: Johnson and Johnson Corporation
Consulting or Advisory Role: Janssen Scientific Affairs
Research Funding: Pharmacyclics, Janssen, Incyte
Mary E. Flowers
Honoraria: Astellas Pharma, Mallinckrodt
Research Funding: Pharmacyclics, Incyte
Theodore A. Gooley
Consulting or Advisory Role: Kiadis Pharma, Pharmacyclics, Regimmune
Brent L. Wood
Honoraria: Amgen, Seattle Genetics, AbbVie, Janssen, Amgen, Astellas Pharma, Roche Diagnostics, Beckman Coulter
Consulting or Advisory Role: Sysmex
Research Funding: Amgen (Inst), Seattle Genetics (Inst), Pfizer (Inst), Juno Therapeutics (Inst), BiolineRx (Inst), Biosight (Inst), Stemline Therapeutics (Inst), Janssen Oncology (Inst), Novartis, Kite, a Gilead Company (Inst), Macrogenics (Inst)
Travel, Accommodations, Expenses: Amgen
Stanley R. Riddell
Employment: Lyell Immunopharma
Leadership: Lyell Immunopharma
Stock and Other Ownership Interests: Lyell Immunopharma (Inst), Adaptive Biotechnologies
Consulting or Advisory Role: Lyell Immunopharma
Research Funding: Lyell Immunopharma
Patents, Royalties, Other Intellectual Property: Patents, licensing fees, and royalties from Bristol Myers Squibb, Lyell, and Deverra. Patents, licensing fees, and royalties from Bristol Myers Squibb, Lyell, and Deverra (Inst)
Warren D. Shlomchik
Employment: Bluesphere Bio
Stock and Other Ownership Interests: Bluesphere Bio
Consulting or Advisory Role: Bluesphere Bio
Research Funding: Bluesphere Bio
Patents, Royalties, Other Intellectual Property: T-cell receptors
No other potential conflicts of interest were reported.
See accompanying editorial on page 1139
DISCLAIMER
These funding bodies did not play a role in the collection, analysis, interpretation of the data, writing of the report, or decision to submit the paper for publication.
PRIOR PRESENTATION
Cumulative interim data from the first 70 patients were presented in an oral abstract at the American Society of Hematology meeting, 2016.
SUPPORT
There was no commercial sponsor for this research. Supported by NIH Grant Nos. CA18029, CA15704, HL121568-01A1, CA 136551, and by the NIH Rapid Access to Intervention Development (project 298). M. B. was the Damon Runyon-Richard Lumsden Foundation Clinical Investigator, supported in part by the Damon Runyon Cancer Research and Richard Lumsden Foundations (CI-57-11), and received support from the National Cancer Institute (K23CA154532) and from a Special Fellowship in Clinical Research from the Leukemia and Lymphoma Society. W.D.S. was the recipient of support from the Burroughs Wellcome Fund and was a Leukemia and Lymphoma Society Clinical Scholar.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.01755.
AUTHOR CONTRIBUTIONS
Conception and design: Marie Bleakley, Stanley R. Riddell, Warren D. Shlomchik
Financial support: Marie Bleakley
Administrative support: Marie Bleakley, Warren D. Shlomchik
Provision of study materials or patients: Marie Bleakley, Alison Sehgal, Stuart Seropian, Elizabeth F. Krakow, Paul J. Martin, Mary E. Flowers, Keith Loeb, Warren D. Shlomchik
Collection and assembly of data: Marie Bleakley, Alison Sehgal, Stuart Seropian, Elizabeth F. Krakow, Heather Persinger, Barbara Hilzinger, Paul J. Martin, Paul A. Carpenter, Mary E. Flowers, Brent L. Wood, Shelly Heimfeld, Warren D. Shlomchik
Data analysis and interpretation: Marie Bleakley, Alison Sehgal, Stuart Seropian, Melinda A. Biernacki, Elizabeth F. Krakow, Ann Dahlberg, Paul J. Martin, Paul A. Carpenter, Mary E. Flowers, Jenna Voutsinas, Theodore A. Gooley, Keith Loeb, Brent L. Wood, Shelly Heimfeld, Warren D. Shlomchik
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Naive T-Cell Depletion to Prevent Chronic Graft-Versus-Host Disease
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Marie Bleakley
Stock and Other Ownership Interests: HighPass Bio
Consulting or Advisory Role: HighPass Bio, Orca Bio
Research Funding: HighPass Bio
Patents, Royalties, Other Intellectual Property: TCRs specific for minor histocompatibility antigen HA-1 and uses thereof US20190211076A1 and international. Patent issued January 21, 2020. US10,538,574. Inventor on patent. Held by Fred Hutchinson Cancer Research Center
Other Relationship: Miltenyi Biotec
Alison Sehgal
Speakers' Bureau: OncLive
Research Funding: Kite/Gilead, Juno Therapeutics
Melinda A. Biernacki
Employment: Outpace Bio (I), Lyell Immunopharma (I)
Stock and Other Ownership Interests: Lyell Immunopharma (I), Outpace Bio (I)
Patents, Royalties, Other Intellectual Property: My partner receives royalties from a patent held by the Fred Hutchinson Cancer Research Center pertaining to nanomaterials for mRNA delivery. There is no relationship between the research in the current publication and the technology in the patent (I)
Elizabeth F. Krakow
Research Funding: HighPass Bio (Inst)
Ann Dahlberg
Research Funding: Jazz Pharmaceuticals, Atara Biotherapeutics
Paul J. Martin
Stock and Other Ownership Interests: Procter & Gamble
Honoraria: Janssen/Pharmacyclics, Therakos
Consulting or Advisory Role: Pfizer, Mesoblast, Rigel, Talaris, Malinckrodt
Research Funding: AltruBio (Inst)
Paul A. Carpenter
Honoraria: Johnson and Johnson Corporation
Consulting or Advisory Role: Janssen Scientific Affairs
Research Funding: Pharmacyclics, Janssen, Incyte
Mary E. Flowers
Honoraria: Astellas Pharma, Mallinckrodt
Research Funding: Pharmacyclics, Incyte
Theodore A. Gooley
Consulting or Advisory Role: Kiadis Pharma, Pharmacyclics, Regimmune
Brent L. Wood
Honoraria: Amgen, Seattle Genetics, AbbVie, Janssen, Amgen, Astellas Pharma, Roche Diagnostics, Beckman Coulter
Consulting or Advisory Role: Sysmex
Research Funding: Amgen (Inst), Seattle Genetics (Inst), Pfizer (Inst), Juno Therapeutics (Inst), BiolineRx (Inst), Biosight (Inst), Stemline Therapeutics (Inst), Janssen Oncology (Inst), Novartis, Kite, a Gilead Company (Inst), Macrogenics (Inst)
Travel, Accommodations, Expenses: Amgen
Stanley R. Riddell
Employment: Lyell Immunopharma
Leadership: Lyell Immunopharma
Stock and Other Ownership Interests: Lyell Immunopharma (Inst), Adaptive Biotechnologies
Consulting or Advisory Role: Lyell Immunopharma
Research Funding: Lyell Immunopharma
Patents, Royalties, Other Intellectual Property: Patents, licensing fees, and royalties from Bristol Myers Squibb, Lyell, and Deverra. Patents, licensing fees, and royalties from Bristol Myers Squibb, Lyell, and Deverra (Inst)
Warren D. Shlomchik
Employment: Bluesphere Bio
Stock and Other Ownership Interests: Bluesphere Bio
Consulting or Advisory Role: Bluesphere Bio
Research Funding: Bluesphere Bio
Patents, Royalties, Other Intellectual Property: T-cell receptors
No other potential conflicts of interest were reported.
REFERENCES
- 1. Pidala J, Djulbegovic B, Anasetti C, et al. Allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia (ALL) in first complete remission. Cochrane Database Syst Rev. 2011:CD008818. doi: 10.1002/14651858.CD008818.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: Systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 4. Hamilton BK, Storer BE, Wood WA, et al. Disability related to chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2020;26:772–777. doi: 10.1016/j.bbmt.2019.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luznik L, Pasquini MC, Logan B, et al. Randomized phase III BMT CTN trial of calcineurin inhibitor-free chronic graft-versus-host disease interventions in myeloablative hematopoietic cell transplantation for hematologic malignancies. J Clin Oncol. 2022;40:356–368. doi: 10.1200/JCO.21.02293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker I, Panzarella T, Couban S, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: A randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–173. doi: 10.1016/S1470-2045(15)00462-3. [DOI] [PubMed] [Google Scholar]
- 8. Kroger N, Solano C, Wolschke C, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374:43–53. doi: 10.1056/NEJMoa1506002. [DOI] [PubMed] [Google Scholar]
- 9. Soiffer RJ, Kim HT, McGuirk J, et al. Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. 2017;35:4003–4011. doi: 10.1200/JCO.2017.75.8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanakry CG, O'Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanakry CG, Tsai HL, Bolanos-Meade J, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124:3817–3827. doi: 10.1182/blood-2014-07-587477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mielcarek M, Furlong T, O'Donnell PV, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–1508. doi: 10.1182/blood-2015-10-672071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: Intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 14. Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112:101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen BJ, Cui X, Sempowski GD, et al. Transfer of allogeneic CD62L- memory T cells without graft-versus-host disease. Blood. 2004;103:1534–1541. doi: 10.1182/blood-2003-08-2987. [DOI] [PubMed] [Google Scholar]
- 16. Dutt S, Tseng D, Ermann J, et al. Naive and memory T cells induce different types of graft-versus-host disease. J Immunol. 2007;179:6547–6554. doi: 10.4049/jimmunol.179.10.6547. [DOI] [PubMed] [Google Scholar]
- 17. Chen BJ, Deoliveira D, Cui X, et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007;109:3115–3123. doi: 10.1182/blood-2006-04-016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng H, Matte-Martone C, Li H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111:2476–2484. doi: 10.1182/blood-2007-08-109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng H, Matte-Martone C, Jain D, et al. Central memory CD8+ T cells induce graft-versus-host disease and mediate graft-versus-leukemia. J Immunol. 2009;182:5938–5948. doi: 10.4049/jimmunol.0802212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juchem KW, Anderson BE, Zhang C, et al. A repertoire-independent and cell-intrinsic defect in murine GVHD induction by effector memory T cells. Blood. 2011;118:6209–6219. doi: 10.1182/blood-2011-01-330035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bleakley M, Otterud BE, Richardt JL, et al. Leukemia-associated minor histocompatibility antigen discovery using T-cell clones isolated by in vitro stimulation of naive CD8+ T cells. Blood. 2010;115:4923–4933. doi: 10.1182/blood-2009-12-260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bleakley M, Heimfeld S, Jones LA, et al. Engineering human peripheral blood stem cell grafts that are depleted of naive T cells and retain functional pathogen-specific memory T cells. Biol Blood Marrow Transplant. 2014;20:705–716. doi: 10.1016/j.bbmt.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bleakley M, Heimfeld S, Loeb KR, et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest. 2015;125:2677–2689. doi: 10.1172/JCI81229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: Sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ponce DM, Sauter C, Devlin S, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19:799–803. doi: 10.1016/j.bbmt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 28. Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: Results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17:1343–1351. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farag SS, Abu Zaid M, Schwartz JE, et al. Dipeptidyl peptidase 4 inhibition for prophylaxis of acute graft-versus-host disease. N Engl J Med. 2021;384:11–19. doi: 10.1056/NEJMoa2027372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watkins B, Qayed M, McCracken C, et al. Phase II trial of costimulation blockade with abatacept for prevention of acute GVHD. J Clin Oncol. 2021;39:1865–1877. doi: 10.1200/JCO.20.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30:3194–3201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 33. Anderson BE, Tang AL, Wang Y, et al. Enhancing alloreactivity does not restore GVHD induction but augments skin graft rejection by CD4(+) effector memory T cells. Eur J Immunol. 2011;41:2782–2792. doi: 10.1002/eji.201141678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson BE, Taylor PA, McNiff JM, et al. Effects of donor T-cell trafficking and priming site on graft-versus-host disease induction by naive and memory phenotype CD4 T cells. Blood. 2008;111:5242–5251. doi: 10.1182/blood-2007-09-107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang P, Wu J, Deoliveira D, et al. Allospecific CD4(+) effector memory T cells do not induce graft-versus-host disease in mice. Biol Blood Marrow Transplant. 2012;18:1488–1499. doi: 10.1016/j.bbmt.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang W, Mo W, Jiang J, et al. Donor allospecific CD44(high) central memory T cells have decreased ability to mediate graft-vs.-host disease. Front Immunol. 2019;10:624. doi: 10.3389/fimmu.2019.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Bergen CA, van Luxemburg-Heijs SA, de Wreede LC, et al. Selective graft-versus-leukemia depends on magnitude and diversity of the alloreactive T cell response. J Clin Invest. 2017;127:517–529. doi: 10.1172/JCI86175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Araki D, Wood BL, Othus M, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: Time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol. 2016;34:329–336. doi: 10.1200/JCO.2015.63.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hourigan CS, Dillon LW, Gui G, et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol. 2020;38:1273–1283. doi: 10.1200/JCO.19.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chapuis AG, Egan DN, Bar M, et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med. 2019;25:1064–1072. doi: 10.1038/s41591-019-0472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith M, Zakrzewski J, James S, et al. Posttransplant chimeric antigen receptor therapy. Blood. 2018;131:1045–1052. doi: 10.1182/blood-2017-08-752121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dossa RG, Cunningham T, Sommermeyer D, et al. Development of T-cell immunotherapy for hematopoietic stem cell transplantation recipients at risk of leukemia relapse. Blood. 2018;131:108–120. doi: 10.1182/blood-2017-07-791608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waller EK, Logan BR, Harris WA, et al. Improved survival after transplantation of more donor plasmacytoid dendritic or naive T cells from unrelated-donor marrow grafts: Results from BMTCTN 0201. J Clin Oncol. 2014;32:2365–2372. doi: 10.1200/JCO.2013.54.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Woolfrey A, Lee SJ, Gooley TA, et al. HLA-allele matched unrelated donors compared to HLA-matched sibling donors: Role of cell source and disease risk category. Biol Blood Marrow Transplant. 2010;16:1382–1387. doi: 10.1016/j.bbmt.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.01755.