Abstract
The patient had suffered from both proximal and distal limb weakness since her early childhood, without the involvement of ocular or respiratory muscles. Repetitive nerve stimulation (RNS) at 3 Hz showed significant decrement in the area and amplitude of the compound muscle action potential (CMAP) on the right abductor digiti minimi (26%) and trapezius (17%). Whole-exon sequencing revealed two novel heterozygous mutations (p.Q1406Rfs*29 and p.R1521H) in the LG1 domain of agrin, which were deemed likely pathogenic for congenital myasthenic syndromes (CMS) according to a bioinformatics analysis. The patient showed remarkable improvement after treatment with salbutamol. This case expanded the mutation spectrum of AGRN.
Keywords: AGRN, congenital myasthenic syndromes, compound heterozygous mutation
Introduction
Congenital myasthenic syndromes (CMS) are a group of inherited disorders caused by mutations in genes encoding proteins maintaining the functional integrity of the neuromuscular junction (NMJ) transmission (1,2). Typical manifestations of CMS include fatigable ocular, facial and limb weakness presenting in the neonatal period or early childhood. Respiratory and bulbar muscles are involved in a small minority of patients (2,3).
The treatment strategy is case-based and mainly depends on the subtype of CMS but usually consists of acetylcholinesterase (AChEI), 3,4-diaminopyridine (3,4-DAP), ephedrine, albuterol or a combine of these (4,5). Mutations in at least 30 genes have been linked to CMS (6), including DOK7, RAPSN, lipoprotein receptor-related protein 4 (LRP4), muscle-specific tyrosine kinase (MuSK) and AGRN (2). Agrin, encoded by AGRN, is a heparan sulfate proteoglycan secreted by nerve terminals that stabilizes the structure and function of the NMJ by activating the postsynaptic agrin-LRP4-MuSK-Dok-7 complex (7). Among genes, an AGRN mutation is one of the rarest causes of CMS (2,6).
We herein report a case of CMS with new compound heterozygous mutations in ARGN. Written informed consent was obtained from the patient. This study was approved by the Research Ethics Committees of Sir Run Run Shaw Hospital, Zhejiang University.
Case Report
A 15-year-old girl was admitted to our department. During early childhood she had shown difficulty keeping up with her peers in physical activities and experienced frequent falls. She also had difficulty lifting her arms. She reported no disturbance of ptosis, sensation, breathing or swallowing. The weakness of limbs showed no fluctuation and progressed gradually. The patient was born in a non-consanguineous family, and she denied any family history of movement disorders.
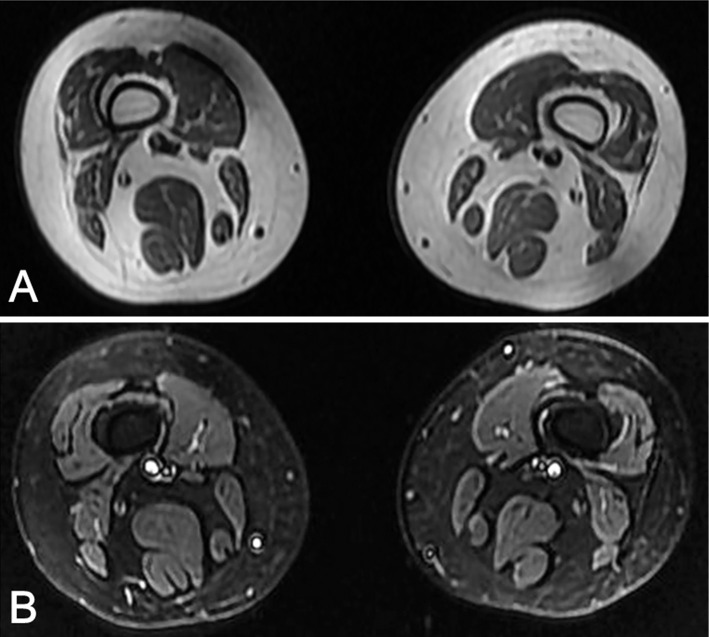
A neurological examination showed that the cranial and axial muscles were spared. Atrophy of her arms and legs was not obvious. The power of the bilateral upper and lower limbs was Medical Research Council (MRC) grade 4-/5 proximally and 4+/5 distally. Tendon reflexes, muscle tone and sensation examination were entirely normal. The Babinski sign was negative. Laboratory tests showed that the levels of creatine kinase and lactic dehydrogenase were normal. Complete blood count (CBC), erythrocyte sedimentation rate (ESR), electrolyte, thyroid function, antinuclear antibodies, anti-acetylcholine receptor (AChR) and anti-MuSK antibodies were normal. Electromyography (EMG) presented no spontaneous potentials such as positive sharp waves and fibrillations. Motor unit potential (MUP) revealed decreased time courses and amplitudes, which pointed to a myogenic disorder. The conduction velocities of both sensory and motor nerves were normal. The amplitudes of compound muscle action potential (CMAP) and sensory nerve action potential (SNAP) were also within normal ranges. Repetitive nerve stimulation (RNS) at 3 Hz revealed decrement in the area and amplitude of the fourth CMAP compared to the first CMAP on the right abductor digiti minimi (26%) and trapezius (17%), without increment at 30-Hz stimulation. Post-exercise facilitation was absent. A stimulated jitter analysis was performed on the right extensor digitorum communis. Twenty single-muscle fiber action potentials (ASFAPs) were recorded with a mean consecutive difference (MCD) of 87 μs (normal <50 μs). Eleven (55%) ASFAPs showed increased jitter, and 3 (15%) were blocked. Electrocardiography and an echocardiogram showed normal results. Magnetic resonance imaging (MRI) of her legs indicated extensive muscle atrophy with fatty replacement (Fig. 1).
Figure 1.
Thigh MR of the patient showed extensive muscle atrophy with fatty replacement. (A) T1-weighted image. (B) STIR image.
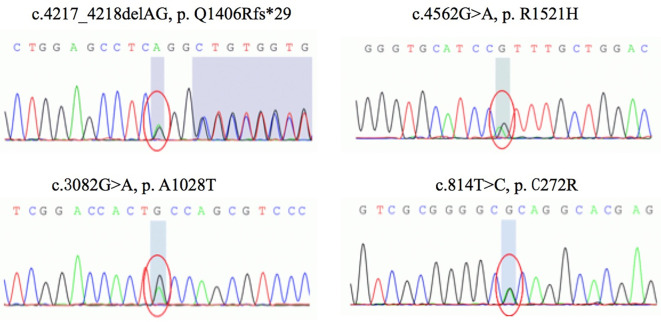
A blood sample was collected from the patient, and genomic DNA was extracted from peripheral leukocytes using the standard method. Whole-exome sequencing demonstrated 4 novel heterozygous variants in the AGRN gene (NM 198576.3) located in exon 24 (c.4217_4218delAG, p.Q1406Rfs*29), exon 26 (c.4562G>A, p.R1521H), exon 18 (c.3082G>A, p.A1028T) and exon 5 (c.814T>C, p.C272R), respectively (Fig. 2). All the mutations were verified by Sanger sequencing. A co-segregate analysis in the family revealed that mutations c.4217_4218delAG and c.3082G>A were inherited from the father, and mutations c.4562G>A and c.814T>C were inherited from the mother. Of the mutations inherited from the father, both were absent from the ExAC, dbSNP, 1000G, and gnomAD databases. The mutation c.4217_4218delAG (p.Q1406Rfs*29) caused a shift in the normal frame with the generation of a premature stop codon. In silico analyses of the mutation c.3082G>A (p.A1028T) revealed that the mutation was not located in a conserved site and was predicted to be tolerable by most in silico pathogenicity prediction tools in VarCards, which provides functional prediction scores on more than 20 different algorithms (http://varcards.biols.ac.cn). Of the mutations inherited from the mother, allele frequency of mutation c.4562G>A in gnomAD databases was 0.0001370 and c.814T>C was not found in the public normal variant database list above. Both mutations p.R1521H and p.C272R were highly conserved (GERP++ scored 4.51 and 4.69, respectively) and predicted to be deleterious by most in silico tools listed in VarCards (18/23 and 22/23, respectively).
Figure 2.
Genetic results of the patient: Sequence chromatograms of compound heterozygous mutations: c.4217_4218delAG (p.Q1406Rfs*29), c.4562G>A (p.R1521H), c.3082G>A (p.A1028T), and c.814T>C (p.C272R).
According to the clinical features and laboratory tests, the patient was diagnosed with CMS. Initially, pyridostigmine was administered, but the symptoms slightly worsened after one week. Thus, we changed the treatment to salbutamol (2 mg/time, 3 times daily) and the symptoms markedly improved after 2 weeks. We checked the decrement in RNS and single-fiber EMG approximately six months after salbutamol treatment. The RNS decrement at 3 Hz showed a significant improvement (7.7% on the right abductor digiti minimi and 10.8% on the trapezius). A stimulated jitter analysis on the right extensor digitorum communis revealed that the ASFAPs were all within the normal range and none were blocked, with a mean MCD of 24.7 μs.
Discussion
The clinical features suggestive for CMS due to AGRN mutations include fatigable weakness of the limb, ocular, facial, and/or bulbar muscles (6,8). However, typical clinical symptoms did not present in all patients, which points to a specific CMS syndrome. The typical EMG of CMS caused by defects in AGRN includes a decremental response to slow (2-3 Hz) RNS and positive single-fiber EMG (3,9). A reduced CMAP at rest and an incremental CMAP following exercise were found in some cases (8). On clinical manifestation, our patient shared features with previously reported cases.
Agrin is encoded by AGRN, which induces the aggregation of postsynaptic proteins at the NMJ and maintenance of the NMJ in skeletal muscle (10). Agrin binds to laminins via the N-terminal agrin (NtA) domain and interacts with α-dystroglycan and LRP4 through its C-terminal (10). Agrin contains three laminin G-like (LG) domains in its C-terminal: LG1, LG2, and LG3. The LG domain has a critical role in the activation of the LRP4-MuSK-Doc 7 complex, which is essential for postsynaptic differentiation and maintaining the structure of the adult NMJ junction (11). Eighteen different AGRN mutations have been reported, the majority of which were located in the NtA, LG2, and LG3 domains (3). Previous studies revealed that the binding of agrin to α-dystroglycan was regulated by alternative mRNA splicing at A and B sites within the LG2 and LG3 domains (10,12,13). The implication of the LG1 domain in the formation of neuromuscular is not completely understood.
To date, only two mutations (p.R1509W and p.Ala1506Thr) have been described in the LG1 domain as associated with a phenotype of CMS in two isolated patients (14,15). Both patients presented with proximal weakness greater than distal weakness and scoliosis of the spine. One patient also showed ptosis of both eyelids, weakness in chewing and swallowing, and physical retardation. However, only one missense mutation (p.R1509W) in the LG1 domain was functionally characterized in CMS. It was shown that p.R1509W had an effect on AChR clustering rather than on MuSK activation. In addition, the study also revealed that the LG1 domain was essential for anchoring agrin to the NMJ by α-dystroglycan or other muscle-specific cell surface molecules (15). The p.Q1406Rfs*29 and p.R1521H mutant AGRN reported here are both located in the LG1 domain. A mutation p.Q1406Rfs*29 causes a premature stop codon, leading to the loss of partial LG1 and the remaining agrin protein domains. The mutation p.R1521H is close to p.R1509W and may share a similar pathogenicity, which affects the anchoring of agrin to the NMJ by α-dystroglycan or other muscle-specific cell surface molecules. We therefore speculate that the two mutations discovered in the present study - p.Q1406Rfs*29 and p.R1521H - may damage the formation and maintenance of the NMJ through the agrin pathway, leading to CMS. However, further functional studies are needed to determine the exact functional role of these two mutations.
Thus far, no missense mutations in the flollistatin domain, where p.C272R is located, have been reported. As the p.Q1406Rfs*29 and p.R1521H mutants were likely pathogenic variants causing CMS, we did not functionally characterize the potential effect of p.C272R. Further studies are also required to explore the functional role of p.C272R.
The present findings broadened the spectrum of ARGN mutations in CMS and highlighted the need to screen ARGN in patients suspected of having CMS, which may aid in the early diagnosis and treatment.
The authors state that they have no Conflict of Interest (COI).
Financial Support
The study was funded by Zhejiang Provincial Science and Technology Department (2016C34006), the Foundation of Zhejiang Administration of Traditional Chinese Medicine (2015ZA061).
References
- 1.Finsterer J. Congenital myasthenic syndromes. Orphanet J Rare Dis 14: 57, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engel AG, Shen XM, Selcen D, Sine SM. Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol 14: 420-434, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gan SY, Yang HY, Xiao T, Pan Z, Wu L. AGRN gene mutation leads to congenital myasthenia syndromes: a pediatric case report and literature review. Neuropediatrics 51: 364-367, 2020. [DOI] [PubMed] [Google Scholar]
- 4.Vanhaesebrouck AE, Beeson D. The congenital myasthenic syndromes: expanding genetic and phenotypic spectrums and refining treatment strategies. Curr Opin Neurol 32: 696-703, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vrinten C, van der Zwaag AM, Weinreich SS, Scholten RJ, Verschuuren JJ. Ephedrine for myasthenia gravis, neonatal myasthenia and the congenital myasthenic syndromes. Cochrane Database Syst Rev 2014: CD010028, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohno K, Ohkawara B, Ito M. Agrin-LRP4-MuSK signaling as a therapeutic target for myasthenia gravis and other neuromuscular disorders. Expert Opin Ther Targets 21: 949-958, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Farmakidis C, Pasnoor M, Barohn RJ, Dimachkie MM. Congenital myasthenic syndromes: a clinical and treatment approach. Curr Treat Options Neurol 20: 36, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Nicole S, Chaouch A, Torbergsen T, et al. Agrin mutations lead to a congenital myasthenic syndrome with distal muscle weakness and atrophy. Brain 137: 2429-2443, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Xi J, Yan C, Liu WW, et al. Novel SEA and LG2 Agrin mutations causing congenital myasthenic syndrome. Orphanet J Rare Dis 12: 182, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scotton P, Bleckmann D, Stebler M, et al. Activation of muscle-specific receptor tyrosine kinase and binding to dystroglycan are regulated by alternative mRNA splicing of agrin. J Biol Chem 281: 36835-36845, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Tezuka T, Inoue A, Weatherbee SD, et al. The MuSK activator agrin has a separate role essential for postnatal maintenance of neuromuscular synapses. Proc Natl Acad Sci 111: 16556-16561, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsim KW, Ruegg MA, Escher G, Kröger S, McMahan UJ. cDNA that encodes active agrin. Neuron 8: 677-689, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Rupp F, Ozçelik T, Linial M, Peterson K, Francke U, Scheller R. Structure and chromosomal localization of the mammalian agrin gene. J Neurosci 12: 3535-3544, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang A, Xiao Y, Huang P, et al. Novel NtA and LG1 mutations in agrin in a single patient causes congenital myasthenic syndrome. Front Neurol 11: 239, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohkawara B, Shen XM, Selcen D, et al. Congenital myasthenic syndrome-associated agrin variants affect clustering of acetylcholine receptors in a domain-specific manner. JCI Insight 5: e132023, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]