Abstract
Polymyalgia rheumatica (PMR) is an inflammatory rheumatic disease characterized by stiffness and aching mainly in the shoulders, neck and hip girdles. The underlying pathogenesis of PMR involves myeloid lineage activation with a high expression of pattern recognition receptors. In addition, vaccination against severe acute respiratory syndrome coronavirus 2 with mRNA-1273 functions as both an immunogen and intrinsic adjuvant. It leads to the activation of innate immunity, resulting in antibody production. We herein report the first case of PMR-like syndrome seven days after mRNA-1273 vaccination. Reassuringly, the symptoms, such as pain of the neck, shoulder girdle and pelvic girdle, as well as elevated inflammatory markers were resolved within a month without glucocorticoid or immunosuppressant administration.
Keywords: polymyalgia rheumatica, giant cell arteritis, SARS-CoV-2, COVID-19, vaccine
Introduction
In the COVID-19 pandemic era, vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has a great impact on its management. Indeed, the high efficacy in preventing COVID-19 and lack of safety concerns concerning the mRNA-1273 SARS-CoV-2 vaccine aside from transient reactions was confirmed in a large-scale clinical trial (1). The seasonal influenza vaccine has been reported to induce polymyalgia rheumatica (PMR) and giant cell arteritis (GCA) (2), and thus far, there have been three reports of PMR following BNT162b2 administration (3,4).
We herein report a patient who developed PMR-like syndrome after mRNA-1273 vaccination that resolved within a month without immunosuppressive agent administration.
Case Report
A 70-year-old man presented with a sudden onset of severe pain and stiffness of the neck, shoulder girdle, pelvic girdle, and thigh with restricted motion 7 days after receiving the second dose of the mRNA-1273 vaccine. Before that, the patient had developed a low-grade fever and slight malaise after the second injection. Those symptoms had been progressing, with the additional development of a headache, jaw claudication, and scalp tenderness simultaneously. The patient had previously been healthy without any medication and no family history of connective tissue disorders.
A physical examination revealed tenderness of shoulder and hip joints, and upper arm with no temporal artery tenderness. The laboratory findings indicated a C-reactive protein (CRP) level of 15.14 mg/dL, erythrocyte sedimentation rate (ESR) of 75 mm/h, and matrix metalloproteinase-3 of 73.4 ng/mL. Rheumatoid factor, anti-cyclic citrullinated peptide antibody, anti-nucleolar antibody, myeloperoxidase-anti-neutrophil cytoplasmic antibody (ANCA), and proteinase 3-ANCA were negative. His serological tests demonstrated negative findings for immunoglobulin (Ig) M antibody for parvovirus B19 and positive findings for IgG antibody for cytomegalovirus, indicating no association of these viruses with the symptoms.
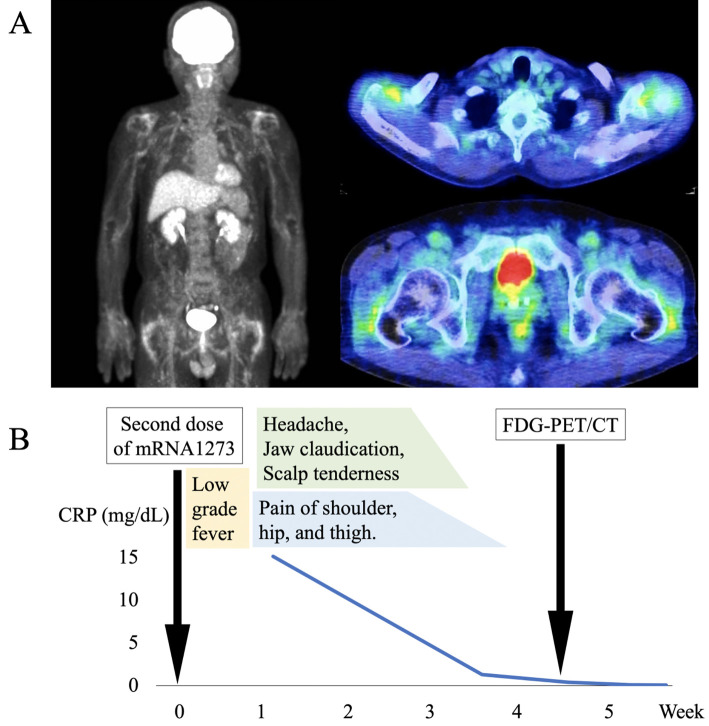
Ultrasound revealed mild bilateral biceps tenosynovitis. Gadolinium-enhanced head magnetic resonance imaging failed to detect any wall thickening or stenoses of the temporal arteries. The fluorine-18 fluorodeoxyglucose uptake on positron emission tomography/computed tomography (PET/CT) was increased around the bilateral shoulder joints, hip joints, greater trochanter interspinous bursa, and ischial tuberosity with no signs of temporal arteritis or large-vessel vasculitis (Figure A). Contrast-enhanced CT and upper gastrointestinal endoscopy showed no sign of malignancy. The patient met the classification criteria for PMR (5,6), and the imaging findings were typical for PMR.
Figure.
(A) The pathological uptake of fluorine-18 fluorodeoxyglucose on positron emission tomography/computed tomography at the bilateral shoulder joints, hip joints, greater trochanter interspinous bursa, and ischial tuberosity, with no signs of temporal arteritis or large-vessel vasculitis. (B) The clinical course of the present patient. FDG-PET/CT: fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography
Fortunately, on monitoring, the symptoms and levels of CRP and ESR abated with only acetaminophen up to 1,500 mg/day (Figure B). One month after the onset, the patient's condition was resolved without any immunomodulatory interventions.
Discussion
To our knowledge, this is the first case of PMR-like syndrome possibly induced by mRNA-1273 vaccination that resolved in a month with acetaminophen alone. Although a causal relationship was not established, a case/non-case study using the WHO global individual case safety report database recently revealed 147 cases of GCA, 290 cases of PMR, and 9 cases of both GCA with PMR among 1,295,482 reports concerning adverse reactions to COVID-19 vaccination (7). In the study, the median [interquartile range] duration from vaccination to the disease onset was 4 [1-14] days, and the ratios of mRNA vaccine and viral vector vaccine were 61.9% and 37.4%, respectively (7). Manzo et al. reported a case of PMR-like symptoms occurring one day after the first dose of the BNT162b2 vaccine treated by prednisone to control inflammation (3). In addition, Cadiou et al. reported three cases of PMR or GCA possibly induced or exacerbated by COVID-19 vaccination (4). Of those, 1 patient with new-onset PMR started to suffer left shoulder pain 2 weeks after the first dose of the BNT162b2 vaccine and was treated with prednisone 20 mg daily (4). In our case, the duration between the second dose of the mRNA-1273 vaccine and the onset of PMR-like symptoms was seven days. The immune responses, including neutralizing antibody responses and T cell responses have been revealed to differ for each COVID-19 vaccines (8). Although the antibody titer was higher in the mRNA-1273 group than in the BNT162b2 group, both vaccines work in almost the same fashion (8). Another study also confirmed higher immune responses after mRNA-273 than after BNT162b2 vaccination (9). The differing immunogenicity, kinetics, and mRNA content between mRNA-1273 and BNT162b2 (8,9) might result in differences in the clinical courses and management of PMR-like symptoms caused by the vaccines.
The current standard management strategy for both PMR and GCA consists of glucocorticoid administration (10). Fortunately, the condition in our patient with PMR that was probably associated with mRNA-1273 vaccination resolved spontaneously, but all other reported cases of PMR and GCA following COVID-19 vaccination have been treated with glucocorticoids (3,4). A retrospective study of 58 PMR patients revealed a possible association between the disease onset and environmental factors, such as vaccination or infection, in 26% of patients (11). That study also demonstrated differences in the clinical course based on the presence of an environmental trigger, with self-limiting clinical courses expected in cases of PMR after influenza vaccination (2). Importantly, the report also suggested that the presence of the HLA-DRB1*13:01 allele might increase the risk of post-influenza vaccination PMR and GCA, and PMR and GCA patients may be able to be stratified thus, allowing for the optimization of the management based on the presence of a specific genetic background or environmental trigger. Although elucidating the causal association between PMR and vaccination is still a matter of debate, our case suggests that some do not require immunosuppressant treatment. We should initiate treatment for patients at the appropriate timing, but careful monitoring without medication may be another option.
Although we were unable to confirm a definite association between COVID-19 vaccination and PMR, common mechanisms of vaccination and PMR may explain the associations between COVID-19 vaccination and PMR. Even though influenza vaccine-associated PMR is said to be a spectrum of autoimmune/autoinflammatory syndrome induced by adjuvants (2), mRNA-1273 contains synthetic mRNA, lipids, tromethamine, tromethamine hydrochloride, acetic acid, sodium acetate, and sucrose without any conventional adjuvants (12). However, mRNA vaccines play a role as both immunogen and intrinsic adjuvant, activating innate immunity via the complement pathway and Toll-like receptors (13). The pathogenesis of PMR is also associated with the activation of innate immunity and proinflammatory cytokine production by dendritic cells, B cells, and T cells (14). Intriguingly, mRNA-1273 elicits antibodies against SARS-CoV-2 spike protein through the activation of CD4+ T helper 1 cells, follicular helper T cells, and CD8+ T cells and proinflammatory cytokines (13). This similarity might explain the relationship between mRNA-1273 vaccination and PMR.
Vaccination remains essential for overcoming the COVID-19 pandemic. While this report warns of the risk of developing PMR-like syndrome induced by the vaccine, it also notes that the symptoms are transient and encourages physicians to continue to promote the vaccine.
Author's disclosure of potential Conflicts of Interest (COI).
Toshihiko Komai: Honoraria, Tanabe Mitsubishi, Kissei, Pfizer, Amgen, Chugai, GlaxoSmithKlin; Research Funding, GlaxoSmithKline. Bunki Natsumoto: Research funding, GlaxoSmithKline. Hirofumi Shoda: Honoraria, Pfizer, Bristol-Myers Squibb, Eli Lilly, Sanofi, AbbVie, GlaxoSmithKline, Gilead, Boehringer Ingelheim, Jansen, Novartis, Chugai, Takeda, Astellas, Eisai, Asahi Kasei and Daiichi-Sankyo; Research funding, Novartis. Keishi Fujio: Honoraria, Tanabe Mitsubishi, Bristol-Myers Squibb, Eli Lilly, Chugai, Jansen, Pfizer, Ono, AbbVie, Ayumi, Astellas, Sanofi, Novartis, Daiichi Sankyo, Eisai, Asahi Kasei and AstraZeneca; Research funding, Tanabe Mitsubishi, Bristol-Myers Squibb, Eli Lilly, Chugai, Eisai, Tsumura and Asahi Kasei.
References
- 1.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: 403-416, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liozon E, Parreau S, Filloux M, et al. Giant cell arteritis or polymyalgia rheumatica after influenza vaccination: a study of 12 patients and a literature review. Autoimmun Rev 20: 102732, 2021. [DOI] [PubMed] [Google Scholar]
- 3.Manzo C, Natale M, Castagna A. Polymyalgia rheumatica as uncommon adverse event following immunization with COVID-19 vaccine: a case report and review of literature. Aging Med (Milton) 4: 234-238, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadiou S, Perdriger A, Ardois S, et al. SARS-CoV-2, polymyalgia rheumatica and giant cell arteritis: COVID-19 vaccine shot as a trigger? Comment on: “Can SARS-CoV-2 trigger relapse of polymyalgia rheumatica?” by Manzo et al. Joint Bone Spine 2021;88:105150. Joint Bone Spine 89: 105282, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasgupta B, Cimmino MA, Kremers HM, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheum 64: 943-954, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Bird HA, Esselinckx W, Dixon AS, Mowat AG, Wood PH. An evaluation of criteria for polymyalgia rheumatica. Ann Rheum Dis 38: 434-439, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mettler C, Jonville-Bera A-P, Grandvuillemin A, Treluyer J-M, Terrier B, Chouchana L. Risk of giant cell arteritis and polymyalgia rheumatica following COVID-19 vaccination: a global pharmacovigilance study. Rheumatology (Oxford). Forthcoming. [DOI] [PubMed] [Google Scholar]
- 8.Collier A-RY, Yu J, McMahan K, et al. Differential kinetics of immune responses elicited by COVID-19 vaccines. N Engl J Med 385: 2010-2012, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA 326: 1533-1535, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camellino D, Matteson EL, Buttgereit F, Dejaco C. Monitoring and long-term management of giant cell arteritis and polymyalgia rheumatica. Nat Rev Rheumatol 16: 481-495, 2020. [DOI] [PubMed] [Google Scholar]
- 11.Falsetti P, Conticini E, Acciai C, et al. Polymyalgia rheumatica following infective triggersor vaccinations: a different subset of disease? Reumatologia/Rheumatology 58: 76-80, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klimek L, Novak N, Cabanillas B, Jutel M, Bousquet J, Akdis CA. Allergenic components of the mRNA-1273 vaccine for COVID-19: possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy 76: 3307-3313, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiPiazza AT, Leist SR, Abiona OM, et al. COVID-19 vaccine mRNA-1273 elicits a protective immune profile in mice that is not associated with vaccine-enhanced disease upon SARS-CoV-2 challenge. Immunity 54: 1869-1882.e6, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dejaco C, Brouwer E, Mason JC, Buttgereit F, Matteson EL, Dasgupta B. Giant cell arteritis and polymyalgia rheumatica: current challenges and opportunities. Nat Rev Rheumatol 13: 578-592, 2017. [DOI] [PubMed] [Google Scholar]