Abstract
Standfirst: Some “species differences” between mouse and human can be diminished simply by housing mice at warmer temperatures. Failure to strategically turn up the thermostat may undermine translation of findings in mice into insights on human metabolic diseases.
For the convenience of their human experimentalists and caretakers, and because it is less expensive, mice are generally housed at environmental temperatures below their thermoneutrality. Our comfort and fiscal frugalness come at a cost that may include appropriate interpretation of findings, and even the translatability of experimental findings to human physiology and disease. For practical and scientific reasons, mice have become the model organism of choice for a huge swath of biomedical research. Mice thrive and breed well in a laboratory environment, can be housed economically, and powerful experimental approaches are now widely available to alter gene expression and to specifically evaluate molecular, cellular, tissue or systems biology. Importantly, basic metabolic and physiological mechanisms are generally conserved between these species. For example, mice are omnivores, eat in discrete bouts and the mouse gastrointestinal tract largely develops and functions like that of humans.
Despite these clear advantages, there are a number of barriers to use of mice to understand and treat human disease. We often think of these barriers as “species differences”. That is to say a difference that relates to the genomes of mice due to their evolution on a different path than humans(1). For example, mice produce a form of heparin-binding EGF-like growth factor that, unlike humans, does not bind to diphtheria toxin. This genomic difference renders mice insensitive to toxic effects of diphtheria toxin and makes them a poor model for understanding effects of diphtheria toxin in humans. However, these differences do allow for selective ablation of cells in mice through expression and activation of human heparin-binding EGF-like growth factor in specific cell types (2). Thus, when used cleverly, differences between species can be exploited to gain insight into the similarities.
Unlike humans, most laboratory mice are mildly cold-stressed.
While it is clear we should take genetic divergences between species into consideration, we should also be aware that disparities in results can arise from how experiments are performed. One factor that deserves further scrutiny is environmental temperature – in the modern world, humans spend much of their time at temperatures close to thermoneutrality, whereas mice are generally housed well below their thermoneutral zone. Thermoneutrality is the ambient temperature range for which energy is only expended to maintain basal metabolic rate. When environmental temperature is cooler than thermoneutrality, warm blooded organisms burn extra energy to maintain their core body temperature. At temperatures above thermoneutrality, organisms spend additional energy to cool themselves.
Mice and humans both defend core body temperatures of ~37°C; however, peripheral tissues and appendages of both species are cooler and highly subject to environmental temperatures. Although mice and humans share many behavioral and physiological mechanisms for regulating heat loss, such as vasodilation/constriction of blood vessels in skin, a true “species difference” comes from the tail of mice, which accounts for ~5 to 8% of total body heat loss (3). Homeothermic animals can adapt to a wide range of temperatures, indeed mice survive quite nicely at −20°C as long as they have food, nest materials and time to adapt (4); however, mice and humans prefer to live in temperatures at or slightly below thermoneutrality (5). Factors that influence thermoneutrality include body size, shape and composition, age, sex, clothing or fur, temperature acclimation and energy expenditure (6), and together these factors determine that thermoneutrality for mice is generally higher (~29 – 32°C) than that of humans (~22 °C). Since mice are typically housed in rooms that are kept at temperatures that are ideal for human staff (20–22°C), mice are almost always at temperatures below their thermoneutrality. Consequently, mice use considerably more energy to maintain their core body temperature than humans.
The degree to which mice experience cool temperatures also depends on their housing conditions. Mice housed in groups huddle together to preserve warmth. Mice are avid nest builders, in part to provide protection against cold temperatures (7). Finally, ventilated cage racks, which limit contamination between cages, increase convective heat loss, thus effectively lowering the effective temperature experienced by caged mice. Unfortunately, there is undoubtedly considerable variation in perceived environmental temperature for mice between research settings, which generally is not quantified nor included in research methods.
Environmental temperature profoundly influences physiology and pathophysiology of mice.
There are important differences in the physiology of mice housed at thermoneutrality from those housed at room temperature. For example, mice have much higher heart rates than humans but the size of this difference is highly dependent on housing temperature. Mice at thermoneutrality have a heart rate of ~375 bpm, which increases to ~575 bpm for mice at 22°C (8). Hence, prominent differences in heart rate between humans and mice are partly due to housing conditions rather than a “species difference.” Importantly, heart rate is highly correlated with blood pressure, which is also elevated at cooler temperatures (8). A key mechanism for cool adaptation in mice is to elevate sympathetic drive, which increases heart rate, and is easily observed as higher norepinephrine content and turnover in adipose depots (9). Although the vagus nerve is the predominant regulator of heart rate in humans, the importance of vagal tone in mice is revealed only when mice are maintained at thermoneutrality (10). Thus, what looks like a “species difference” at room temperature disappears when species are compared at thermoneutral temperatures (see Figure 1).
Figure 1:
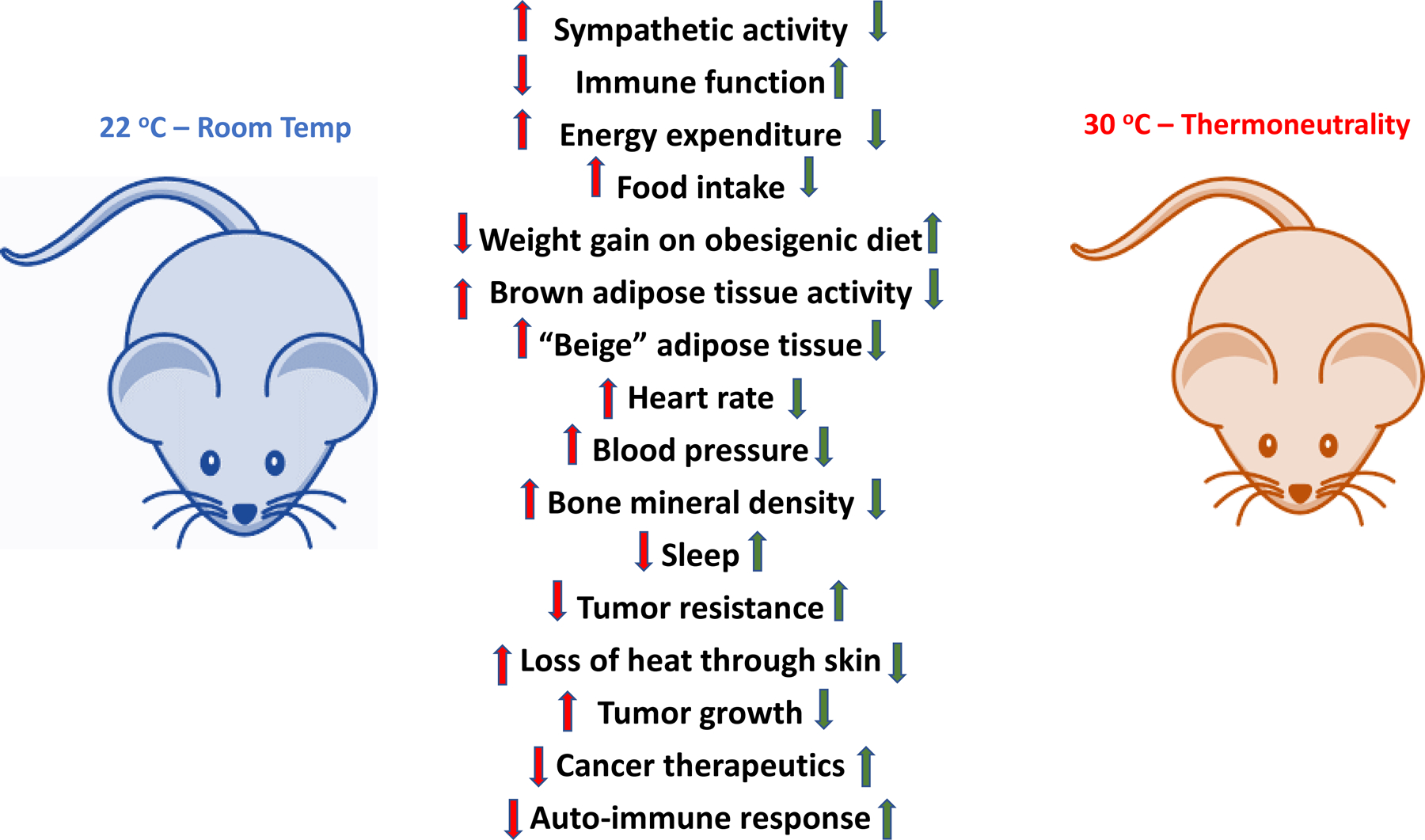
Room Temperature vs Thermoneutrality in mouse
A comparison of a variety of physiological systems in the mouse under either typical, room temperature housing conditions versus mice housed close to their thermoneutral temperature. Green arrows indicate that the parameter appears to be closer to human when the mouse is housed at thermoneutral conditions. This implies that for many experiments, housing mice at thermoneutral conditions would increase translatability of mouse experiments to humans.
Activation of sympathetic drive has profound effects on other aspects of mouse physiology. Activation of the sympathetic nervous system results in suppression of the immune system as energy is directed from the immune system to heat generation. While such a response is adaptive to short-term cold exposure, chronic activation of sympathetic drive results in an immune system that operates differently than at thermoneutrality. Immune cell metabolism, fever, and response to auto-immune disease are all increased at thermoneutrality (11, 12). This observation has impacted the cancer field where thermoneutrality is well-known to confer resistance to growth of a wide variety of cancers, and to increase efficacy of cancer therapies that rely on immune cell function (12, 13).
Housing temperature impacts not only development of metabolic diseases, but also the potential to assess treatment strategies. Increased heat loss from their larger surface area to volume ratio causes mice to burn proportionally more energy to maintain core body temperature than humans (14). This difference is exacerbated when mice are housed at temperatures below thermoneutrality, with increased metabolic rate and activation of brown adipose tissue impacting their body weight and composition (15). Growing appreciation for the role the immune system plays in metabolic disease has spawned a field termed immuno-metabolism. However, as in immuno-oncology, our ability to move from mouse to human is greatly limited by studying mice with chronically activated sympathetic nervous systems. For example, mice on a high-fat diet housed at thermoneutrality gain more adipose tissue, accumulate more liver lipids, have elevated glucose intolerance, with more adipose tissue inflammation than mice housed at room temperature (16). It is simply hard to imagine that these differences do not color our view of metabolic disease progression and limit our ability to apply these lessons to human disease.
The bottom line is that housing temperature is the most prominent example of a “species difference” that is produced by the nature of the experiments rather than genetic divergence. While production of ever more sophisticated genetic mouse models improves ability to “humanize” mice, the most important step to make mice more similar to human comparators is to house them closer to thermoneutrality. However, addressing issues of housing temperature comprehensively and consistently comes at a high cost. Housing mice in thermoregulated chambers is expensive and adds considerably to the labor of even simple experiments and renders some complicated physiological experiments exceptionally difficult. Raising the temperature of mouse housing rooms is often not possible given the HVAC systems designed to maintain mandated levels of air changes. Working in a room set for mouse thermoneutrality (~30°C) is a considerable challenge to individuals wearing appropriate PPE and can even be dangerous under some conditions. What is less clear is under what circumstances would it be necessary to go to 30°C or would smaller increases in housing temperature be sufficient.
When is housing mice at thermoneutrality warranted?
The ability of appetite suppressants to cause weight loss in humans was predicted using obese mice under standard housing conditions, implying that mechanisms for regulating food intake are not uniformly disrupted to the point of misinforming human interventions. This is despite the fact that ambient temperature can have rapid and profound impact on daily food intake of mice (17). However, for mice in which there are changes in body composition that are independent of food intake, it is important to evaluate these mice at thermoneutrality in addition to room temperature.
Consider the situation where a genetic or pharmacologic perturbation results in increased whole-body metabolism, protection from obesity and elevated beige and brown adipose tissue thermogenesis. Although it could be that the treatment directly stimulates adaptive thermogenesis, it could also be that the treatment acts to reduce insulative properties of the skin, and thus indirectly stimulates beige and brown fat activity secondary to heat loss. In this case, if the treatment also protects against obesity when mice are housed at thermoneutrality, results are more likely to be translatable to humans, than if protection against obesity is only observed at room temperature. In addition, manipulations of the immune system that are found to alter metabolic function and which are impacted by increased sympathetic tone need to be tested at thermoneutrality to assess whether they can plausibly be linked to metabolic regulation in humans.
Many “species differences” between mouse and human can be addressed experimentally - failure to do so in a systematic way has the potential to undermine translation of findings in mice into insights on human disease and treatment for no other reason than a failure to change the thermostat.
Acknowledgments
Writing of this commentary was supported by NIH grants DK089503 and DK117821 to RJS, and DK121759, DK125513, and AG069795 to OAM.
Footnotes
Competing interests
RJS has received research support from Novo Nordisk, AstraZeneca, Pfizer, Kintai and consulting fees from Novo Nordisk, Kintai, Scohia and has equity positions in Zafgen, Calibrate Health and Rewind. OAM has received research support from Novo Nordisk, Regeneron, AstraZenica, and Agilent.
REFERENCES
- 1.Perlman RL. Mouse models of human disease: An evolutionary perspective. Evol Med Public Health 2016;2016(1):170–6. Epub 2016/04/29. doi: 10.1093/emph/eow014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol 2001;19(8):746–50. Epub 2001/08/02. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 3.Skop V, Liu N, Guo J, Gavrilova O, Reitman ML. The contribution of the mouse tail to thermoregulation is modest. Am J Physiol Endocrinol Metab 2020;319(2):E438–E46. Epub 2020/07/22. doi: 10.1152/ajpendo.00133.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Sui W, Zhang M, Dong M, Lim S, Seki T, Guo Z, Fischer C, Lu H, Zhang C, Yang J, Zhang M, Wang Y, Cao C, Gao Y, Zhao X, Sun M, Sun Y, Zhuang R, Samani NJ, Zhang Y, Cao Y. Switching harmful visceral fat to beneficial energy combustion improves metabolic dysfunctions. JCI Insight 2017;2(4):e89044. Epub 2017/02/28. doi: 10.1172/jci.insight.89044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One 2012;7(3):e32799. Epub 2012/04/06. doi: 10.1371/journal.pone.0032799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kingma B, Frijns A, van Marken Lichtenbelt W. The thermoneutral zone: implications for metabolic studies. Front Biosci (Elite Ed) 2012;4:1975–85. Epub 2011/12/29. doi: 10.2741/518. [DOI] [PubMed] [Google Scholar]
- 7.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. Impact of nesting material on mouse body temperature and physiology. Physiol Behav 2013;110–111:87–95. Epub 2013/01/15. doi: 10.1016/j.physbeh.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Maloney SK, Fuller A, Mitchell D, Gordon C, Overton JM. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda) 2014;29(6):413–20. Epub 2014/11/05. doi: 10.1152/physiol.00029.2014. [DOI] [PubMed] [Google Scholar]
- 9.Cui X, Nguyen NL, Zarebidaki E, Cao Q, Li F, Zha L, Bartness T, Shi H, Xue B. Thermoneutrality decreases thermogenic program and promotes adiposity in high-fat diet-fed mice. Physiol Rep 2016;4(10). Epub 2016/05/28. doi: 10.14814/phy2.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, Overton JM. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol Heart Circ Physiol 2008;294(4):H1581–8. Epub 2008/02/05. doi: 10.1152/ajpheart.01000.2007. [DOI] [PubMed] [Google Scholar]
- 11.Leigh ND, Kokolus KM, O’Neill RE, Du W, Eng JW, Qiu J, Chen GL, McCarthy PL, Farrar JD, Cao X, Repasky EA. Housing Temperature-Induced Stress Is Suppressing Murine Graft-versus-Host Disease through beta2-Adrenergic Receptor Signaling. J Immunol 2015;195(10):5045–54. Epub 2015/10/16. doi: 10.4049/jimmunol.1500700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vialard F, Olivier M. Thermoneutrality and Immunity: How Does Cold Stress Affect Disease? Front Immunol 2020;11:588387. Epub 2020/12/18. doi: 10.3389/fimmu.2020.588387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, Repasky EA. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci U S A 2013;110(50):20176–81. Epub 2013/11/20. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasza I, Adler D, Nelson DW, Eric Yen CL, Dumas S, Ntambi JM, MacDougald OA, Hernando D, Porter WP, Best FA, Alexander CM. Evaporative cooling provides a major metabolic energy sink. Mol Metab 2019;27:47–61. Epub 2019/07/16. doi: 10.1016/j.molmet.2019.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasza I, Suh Y, Wollny D, Clark RJ, Roopra A, Colman RJ, MacDougald OA, Shedd TA, Nelson DW, Yen MI, Yen CL, Alexander CM. Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet 2014;10(8):e1004514. Epub 2014/08/08. doi: 10.1371/journal.pgen.1004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stemmer K, Kotzbeck P, Zani F, Bauer M, Neff C, Muller TD, Pfluger PT, Seeley RJ, Divanovic S. Thermoneutral housing is a critical factor for immune function and diet-induced obesity in C57BL/6 nude mice. Int J Obes (Lond) 2015;39(5):791–7. Epub 2014/10/29. doi: 10.1038/ijo.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deem JD, Faber CL, Pedersen C, Phan BA, Larsen SA, Ogimoto K, Nelson JT, Damian V, Tran MA, Palmiter RD, Kaiyala KJ, Scarlett JM, Bruchas MR, Schwartz MW, Morton GJ. Cold-induced hyperphagia requires AgRP neuron activation in mice. Elife 2020;9. Epub 2020/12/16. doi: 10.7554/eLife.58764. [DOI] [PMC free article] [PubMed] [Google Scholar]


