Abstract
Cell-based therapies to the brain are promising for the treatment of multiple brain disorders including neurodegeneration and cancers. In order to access the brain parenchyma, there are multiple physiological barriers that must be overcome depending on the route of delivery. Specifically, the blood-brain barrier has been a major difficulty in drug delivery for decades, and it still presents a challenge for the delivery of therapeutic cells. Other barriers, including the blood-cerebrospinal fluid barrier and lymphatic-brain barrier are less explored, but may offer specific challenges or opportunities for therapeutic delivery. Here we discuss the barriers to the brain and the strategies currently in place to deliver cell-based therapies, including engineered T cells, dendritic cells, and stem cells, to treat diseases. With a particular focus on cancers, we also highlight the current ongoing clinical trials that use cell-based therapies to treat disease, many of which show promise at treating some of the deadliest illnesses.
Keywords: Brain, cancer, T-cells, drug delivery, blood brain barrier, neural stem cells
Graphical Abstract

1. Introduction to Cell-based therapies
Cell-based therapy involves the delivery of cells to circulation or tissues for the treatment of a multitude of diseases. In the brain, cell-based therapies have been developed to treat disorders including cancers, Parkinson’s, Alzheimer’s, traumatic brain injury and other neurodegenerative diseases. Stem cells can be applied to replace damaged neural cells, immune cells can be engineered to fight disease, or other types of cells with unique homing properties may act as therapeutic carriers. Regardless of the disease and the purpose of the cellular delivery, cells face similar challenges at the barriers that exists between and within the brain and the rest of the body. Here we discuss cells as therapeutics and discuss barriers in the brain and strategies for bypassing them.
2. Types of cell-based therapies
2.1. Immune cells
Immune cell delivery is a growing option in the battle against brain cancers. These cells can be reprogrammed or engineered to target antigens present on tumor cells. Since the central nervous system (CNS) tightly controls the entry of immune cells into the brain, delivery of these cells often requires novel strategies. Despite this, T cells, dendritic cells, Natural killer T cells, and others have been implemented clinically1. Engineered T cells with a chimeric antigen receptor (CAR T cells) have been created against a number of tumors found in the brain. Though immune cells have primarily been applied in cancer, there are potential applications across a range of neurological disorders2. Brain tumors pose unique obstacles for CAR T cell homing due to the selective properties of the blood-brain barrier (BBB) and the blood-cerebrospinal fluid barrier. These barriers regulate extravasation limiting immune entry in the brain or CSF due to the presence of endothelial cell layers with tight junctions and the glia limitans. From circulation, CAR T cells must first adhere to vascular endothelium via a multitude of integrins, adhesion molecules, and chemokines3. CAR macrophages leverage the enhanced extracellular matrix degradation ability of the macrophage to better migrate into and infiltrate dense tumors. Macrophages are an important source of matrix metalloproteases and can significantly alter the tumor environment. CAR macrophages are also able to promote the ability of antigen presentation and enhance the cytotoxic effects of T cells. Compared to CAR-T, CAR macrophages have a limited time in circulation and less non-tumor toxicity. Although CAR macrophages have great potential to become an efficient cancer immunotherapy, many obstacles need to be overcome including reduced proliferation compared to CAR T, their accumulation in the liver, potential patient toxicity at high numbers delivered, and, like CAR T cells, insufficient antigen expression on tumor cells4.
Another option in addition to CAR T cell therapy is CAR natural killer cell (NK-cell) therapy. Compared to the CAR-T cells, the risk of on-target/off-tumor toxicity to normal tissues is relatively low due to a limited lifespan of CAR-NK cells in the circulation. Besides killing tumor target cells in the CAR-dependent manner, CAR-NK cells can potentially eliminate cancer cells in a CAR-independent manner. CAR-NK cells still possess their natural cytotoxic activity against tumor cells and can be activated through CAR-independent mechanisms, such as NCRs, NKG2D, co-stimulatory receptor DNAM-1 (CD226), and certain activating KIRs. Moreover, NK cells can eliminate tumor cells through CD16-mediated antibody-dependent cellular cytotoxicity (ADCC). Because of this, CAR-modified NK cells may be able to efficiently eradicate a heterogeneous tumor, in which some tumor cells do not express CAR-targeted antigen, via both CAR-dependent and NK cell receptor-dependent mechanisms.
There are challenges regarding the potential for use of dendritic cells to treat tumors as well. Immature dendritic cells are not functionally ideal for the loading of antigens because they are unable to activate lymphocytes until an inflammatory signal or pathogen induces their maturation5. Some argue that ex vivo maturation of DCs through CD40L or interferon (IFN)-γ is necessary prior to vaccine administration to ensure proper antigen presentation and T cell activation6–9. Others argue that maturation occurs naturally, and that no prior stimulus is required10. In the process of maturation, DCs lose their ability to uptake and process antigens. They also exchange their immature molecular signature for a mature CD83+ phenotype, increasing expression of MHC-antigen complexes, lymphocyte costimulatory molecules, TNF and TNF-receptor molecules, and many chemokines and chemokine receptors to aid in T-cell recruitment and DC navigation to lymphoid tissues5.
2.2. Stem cells
Stem cells are promising for neurodegeneration to potentially replace damaged neurons within critical brain regions. These therapies involve the use of induced pluripotent stem cells (iPSCs) and neural stem cells (NSCs) and can be injected directly into damaged regions of the brain. For therapeutic application utilizing NSCs, it is important to be cognizant of the molecular processing required once cell-mediated delivery of a therapeutic has reached the target site. For example, an antibody-drug conjugate, would be endocytosed into cells and sequestered within endosomes in the cytoplasm. A curative amount of active drug must then traffic from the endosome to the intracellular organelle that is sensitive to the cytotoxic effect of the drug. INSCs as delivery vehicles, however, may not be subject to endosomal compartmentalization, but other characteristics must be considered, such as how efficiently the prodrugs or activated forms of these drugs cross cell membranes and how the permeability characteristics of each form of the drug might affect overall antitumor efficacy11.
3. Barriers in the brain
3.1. The Blood-Brain Barrier.
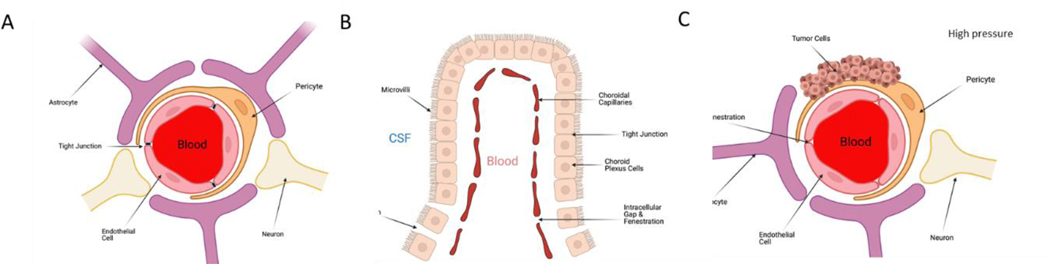
The blood-brain barrier (BBB, Figure 1A) impedes the entrance of substances from the blood to the brain to maintain brain homeostasis12–14 and is comprised of multiple cell types with unique features. Brain capillary endothelial cells (ECs) lack fenestrations, thus limiting the diffusion of small molecules and proteins across the capillary wall. Efflux transporters located in brain capillary ECs, further limit substances entering the brain. Interendothelial junctions, such as adherens junctions, tight junctions, and gap junctions, link these ECs to a continuous barrier that severely restrict the penetration of water-soluble substances12,15. Adherens junctions primarily regulate the permeability of the BBB. Tight junctions play a vital role in sustaining the BBB by controlling tissue homeostasis12,16. Gap junctions, composed of six connexin molecules, direct electrical and chemical communication between ECs. The ECs are further surrounded by pericytes, astrocytes and their associated basal membrane, creating physically tight brain capillaries and which form the impermeable BBB12–14.
Figure 1: Barriers in the brain.
A) The blood brain barrier (BBB) is a diffusion barrier that impedes the entrance of substances from the blood to the brain to maintain brain homeostasis B) The blood-cerebrospinal fluid barrier is formed by modified epithelial cells. It is located between the choroid plexus and the arachnoid membrane. C) Abnormal angiogenesis in tumors occurs due to an overproduction of proangiogenic factors. This leads to the formation of blood vessels that lack a normal physiological structure. Combined with heightened intratumoral pressure, this barrier is a unique impediment to drug delivery
While preclinical work on treatments and their ability to bypass the BBB is conducted on rodents, there are key differences between the BBB of humans and other species that can affect translatability. The activity of P-gp, a protein that is responsible for the efflux of many compounds from BECs to the blood. Proteomic studies in different species have shown that, with regard to the expression of P-gp protein, the human BBB is closer to cynomolagus monkey and marmoset BBBs than to rodent BBBs. PET studies have shown that the penetration efficiencies of P-gp substrates, [18F]-altanserin and [11C] -R205171, were 4.5- and 8.6-fold greater in humans than in rodents, respectively. These results can be explained by lower P-gp function in the human BBB than in rodent BBBs17. Similarly, other proteins affiliated with BBB access, including multidrug resistance-associated protein 4 (MRP4), monocarboxylate transporter 1 (MCT1/SLC16A1), L-type amino acid transporter (LAT1/SLC7A5), and organic anion transporter 3 (OAT3/SLC22A8) are expressed at higher levels in the mouse/rat BBB than in the human BBB, whereas glucose transporter 1 (SLC2A1/GLUT-1) and insulin receptor (INSR) were less expressed. The lower expression of the organic anion transporters MRP4 and OAT3 in the human BBB indicates that humans might efflux/transport some drugs less efficiently. The lower expression of LAT1 in humans than in rodents suggests that the transport of amino acids and drugs, such as levodopa and melphalan, from the blood to the brain is lower in humans than in rodents17. There are similar effects with many other transporters implicated in BBB crossing, and thus, it is important that when developing targeting moieties and cells to understand these differences when scaling up therapeutics. For a fuller accounting of these inter-species differences, please see Aday, et al.17
3.2. The Blood-Cerebrospinal Fluid Barrier
The blood-cerebrospinal fluid barrier (Figure 1B) is formed by modified epithelial cells which line specific locations around the brain. The choroid plexus is located within the ventricular system and is composed, in part, of a cuboidal epithelial barrier with fenestrated vasculature. It is responsible for the secretion and movement of CSF that is driven by specialized ependymal cells that have cilia that beat in unison to move CSF through the vast ventricular system. The CSF occupies the ventricular systems and the subarachnoid space, having extensive two-way communication with the CNS interstitial fluid. The choroid plexus is involved in CNS homeostasis by aiding in controlling the production and composition of CSF and brain interstitial fluid. This is important because most waste products from the interstitial fluid are transported to the CSF and exchange between blood and CSF at the choroid plexus is affected by local blood flow, fenestrated capillaries, and the substantial surface area created by membrane folding and microvilli18,19.
Following immune activation, adhesion molecules and chemokines are activated in the choroid plexus to support homing of CD4 T cells20. In the CSF, resting T cells are activated in response to brain-draining antigens and migration of naïve T cells to the brain parenchyma, which is mediated by blood-born inflammatory cytokines (CXCL12, IL6, CXCL10, INFγ) and activation of adhesion molecules (ICAM-1, VCAM-1) expressed on apical site of choroid plexus20. Furthermore, T cells undergo stimulation and proliferation in the choroid plexus, facilitating leukocyte recruitment and antigen presentation modulating cell-mediated immunity in the CNS. It was demonstrated that migration of effector T cells (Th1) through the choroid plexus was more efficient than naïve CD4 T cells21. Thus, the choroid plexus is a CNS compartment which is rapidly responds to inflammation/injury and triggers immune signaling offering an important interface for cellular interactions20.
3.3. The Blood-Tumor Barrier
Abnormal angiogenesis in tumors occurs due to an overproduction of proangiogenic factors22,23. This leads to the formation of blood vessels that lack a normal physiological structure. The abnormal structure of these blood vessels result in a compromised BBB, otherwise known as the Blood-Tumor Barrier (BTB, Figure 1C). Because of the leaky structure of the BTB, larger molecules can pass through it22,24–26. However, because of increased intratumoral pressure, there are limits to what can move freely into and around the space. BTB permeability and transport, however, varies between brain sites in multifocal disease and within single lesions22,27.
3.4. Lymphatic-Brain Barrier.
The development of vascular catastrophes is accompanied by activation of brain clearance from toxic blood products via the cervical lymphatics28,29. Re-discovered in 2015, the lymphatics that line the meninges surrounding the brain tissue drain biomolecules and allow cellular trafficking from the brain to the deep cervical lymph nodes. The blockade of this lymphatic pathway aggravates the severity of brain edema and contributes to an elevation of intracranial pressure after stroke28–30. Further, modulation of the meningeal lymphatics can affect immunotherapeutic outcomes, with increased lymphangiogenesis generally enhancing the efficacy of immunotherapies such as checkpoint inhibitors hinting at the role of this barrier in immune trafficking and delivery to the brain31.
4. Cell delivery strategies
There are a number of injection strategies for cell-based therapies in the context of the brain. Brain tumors offer the bulk of the studies for therapeutic injection and thus will be the primary focus.. Direct injection encompasses the injection of cells into the body using a needle and a syringe or through a delivery device such as a port or catheter/reservoir system. The various ways to administer cells locoregionally in brain cancers via direct injection to the brain include intracerebroatumoral injection (ICT) wherein cells are infused directly into the tumor or resection cavity and/or intracerebroventricular injection (ICV), whereby cells are delivered to the CSF via the lateral ventricle. The CSF circulates throughout the brain from the ventricles and around the outer cortex. As such, this fluid bathes the brain and interacts with all cells and tissues within the space, eventually exiting through numerous routes. Strategies to deliver drugs or therapies into the CSF can benefit from this enhanced circulation. Treatments delivered in this manner are injected into the spinal canal or the subarachnoid space so it reaches the cerebrospinal fluid. We demonstrated that NSCs administered via ICV routes can migrate efficiently toward single or multiple tumor foci. ICV delivery has been used to enable repeat administrations for patients through an Ommaya reservoir, potentially resulting in improved therapeutic outcomes32.
Intravenous injection is the most easily accessible route of injection for patients, with infusion into the bloodstream. With the BBB and blood CSF barrier offering significant impediments to CNS entry, this route is seemingly the least efficient method for cell-based deliveries. However, it is the least invasive administration method and can be enhanced with the addition of targeting methods or potential other approaches discussed later in this article.
4.1. Implications of CAR T Cell Delivery Strategies
Locoregional delivery strategies can bypass some of the anatomical barriers of the BBB to improve the trafficking of therapeutic cells to brain tumors. One method of locoregional delivery for CAR T cell therapies is intracerebrotumoral (ICT) administration where CAR T cells are delivered into a tumor bed or resection cavity3. ICT delivery requires implantation of a reservoir or catheter delivery device that is typically placed during surgical resection or biopsy, with the reservoir accessible under the scalp and the catheter placed to drain into the tumor bed or cavity33. These devices need to be monitored closely due to the risk of infection, however they are generally well tolerated by patients3.
Studies comparing routes of delivery for preclinical models of glioblastoma (GBM) and primary brain tumors have shown that local ICT outperforms intravenous delivery of CAR T cells34,35. Brown et al. engineered IL13BBζ CAR T cells in an in vivo xenograft brain tumor model with IL13Rα2+ PBT030–2 cells. The multifocal model involved injection of PBT030–2 cells to both the right and left forebrains of NSG mice. Mice were then treated intratumorally via ICT delivery with CAR T cells with five days of engraftment. ICT-administered IL13Rα2-CAR T cells resulted in long-term survival in orthotopic GBM models36. In preclinical studies where CAR T cells were labeled with the radionuclide 89Zr and followed by PET imaging in mice, it was shown that CAR T cells delivered into the brain parenchyma remain localized in the brain37. Locoregional CNS delivery of CAR T cells may also reduce the risk of systemic toxicities associated with CAR T cells. In clinical human vaccine trials of EGFRvIII- and HER2-CAR T cells, IV infusion increased the risk of serious pulmonary toxicities, and in the most severe cases resulted in death38,39. With these results, it can be assumed that locoregional delivery may limit intravenous first-pass pulmonary toxicity and off-tumor targeting of other systemic tissues3. Together these studies suggest that locoregional delivery may improve trafficking, infiltration, and safety of CAR T cells for the treatment of brain tumors3,33,38.
Intracerebroventricular (ICV) administration of CAR T cells into the CSF via the ventricular system. bypasses all but the glia limitans in delivery to the brain parenchyma3. For ICV delivery, the reservoir or catheter delivery device drains into the CSF and circulates with the natural fluid circulation driven by blood pressure and ependymal beating33. In Priceman et al., BBM1 tumor cells were prepared in HBSS−/− and injected orthotopically in the brain parenchyma of female NSG mice via stereotactic injection. Once flux signals reached desired levels, HER2-CAR T cells were prepared in PBS and mice were treated by ICV injection. A single dose of HER2-CAR T cells delivered ICV was more effective than a 10-fold higher dose delivered IV in orthotopic models of brain metastatic breast cancer40. When comparing locoregional delivery routes in a multifocal GBM model where tumors were implanted in contralateral hemispheres, ICV exhibited improved targeting of this multifocal disease36. In preclinical studies in which CAR T cells were labeled with the radionuclide 89Zr and followed by PET imaging in mice, ICV-delivered cells distributed throughout the CNS over 6 days of monitoring by PET imaging. Direct infusion into the CSF via intraventricular delivery achieved a complete clinical response in one patient with multifocal disease, including a distal lesion in the spine, illustrating the surveillance of CAR T cells throughout the CNS3.
For CAR T Cell administration, IV delivery is the most common approach to date for hematological and solid cancers. Melanoma-targeted and CD19-CAR T cells delivered intravenously into humans have been shown to traffic to the brain and eliminate malignant disease41–43. Despite this, as mentioned earlier, locoregional delivery, including ICT And ICV delivery, outperforms intravenous delivery of CAR T cells. There is also a higher chance of systemic toxicities associated with CAR T cells for IV delivery compared to locoregional delivery3. In clinical human vaccine trials of EGFRvIII- and HER2-CAR T cells, IV infusion increased the risk of serious pulmonary toxicities, and in the most severe cases resulted in death38,39. Therefore, from the current data we have, the use of locoregional delivery offers a number of benefits compared to IV injection for brain disorders.
4.2. Dendritic Cell Injection Strategies
Dendritic cells have been delivered via multiple routes for malignancies within and outside of the CNS. Intradermal44, intralymphatic44, intracranial45, intranodal46, and subcutaneous injections47 of DCs all drain to lymph nodes and induce greater T cell mediated immunity against tumor antigens compared to intravenous injections in pre-clinical models5. Fong et al. demonstrated in human clinical trials that intradermal and intralymphatic administrations of DCs induce Th1 immunity with greater frequency than intravenous administration in prostate cancer. The advantages of intradermal administration are its simplicity, lack of transfusion reactions, and the frequency of IFN-ɣ responses, especially where induction of Th1 immunity is desired. However, intradermally injected DCs migrate out of the skin very inefficiently44.
Microinjection of cognate antigens into the brain leads to accumulation of DCs in the CNS45. In Karman et al., DCs differentiated from mouse bone marrow were injected via ICT. These cognate antigen-loaded DCs were able to migrate to cervical lymph nodes, transport cognate antigens out of the CNS, induce a primary immune response in the periphery, and instruct cognate antigen-specific T cells to home to the brain. This peripheral migration resulted in CD8+ T cells homing to the brain45. Thus, the administration of intracerebral DCs can lead to more systemic immune activation and affect disease outcomes in the brain.
Outside of the CNS, much attention has been given to the intranodal or perinodal administration of DCs, as lymph nodes are major processing centers responsible for antigen presentation and T cell activation48. Intranodal application of immature or mature DCs leads to substantial migration of DCs to distant lymph nodes, as soon as one hour after vaccination into humans46. Only mature DCs reach the T cell areas of the lymph nodes, however, little or no difference is observed between the migration to lymph nodes. Intranodal injection resulted in a rather variable migration in both cell populations. This may be due to the partial destruction of the lymph node architecture caused by the direct injection of the DCs into the lymph node, causing the migration of DCs to distant lymph nodes through the lymphatic vessels46. Nonetheless, intranodal vaccination has a major advantage over intradermal vaccination due to an increased number of DCs getting to the lymph nodes49. In a phase I/II clinical trial, twenty-four patients with recurrent malignant glioma were resistant to the standard maximum therapy. Dendritic cells were injected intradermally, or both intratumorally and intradermally. With the combined intratumoral-intradermal treatment, there was an increased percentage of NK cells and increased T cell-mediated antitumor activity7. This type of strategy may show promise in targeting the deep cervical lymph nodes, known to drain the brain, and thus warrants further exploration.
Intradermal, intralymphatic, intracranial, intranodal, and subcutaneous injections of DCs induce greater T cell mediated immunity against tumor antigens than intravenous injections in pre-clinical models44–47. Radioisotope tracing studies have shown that intravenously delivered DCs accumulate within the spleen and liver. This method results in the greatest humoral anti-tumor response as indicated by increased tumor antigen specific antibodies5. Despite this, as mentioned earlier, intradermal and intralymphatic administrations of DCs induce Th1 immunity with greater frequency than intravenous administration in humans. Also, the capacity of DCs to immunize have demonstrated superiority of subcutaneous injection over intravenous injection in humans for the induction of cytotoxic T cells44. The efficiency of DCs administered subcutaneously to induce an immune response is higher when compared with intravenously.-injected DCs and correlates with the preferential accumulation of DCs in lymph nodes after subcutaneous injection47. Though these cells have been examined via multiple delivery routes, more work is needed to understand the translatability of these injection strategies to brain malignancies.
4.3. Neural Stem Cell Injection Strategies
Direct injection via ICT into established GBM has been the mainstay of cytotoxic NSC delivery for treatment as it leads to efficient NSC transplant and robust tumor killing11,50,51. NSCs injected into the resection cavity in a xenograft model of GBM52 cleared in 7 days and the remaining cells only extended survival of tumor-bearing mice by one week53 In contrast, directly injecting NSCs into the immunosuppressive tumor niche improves the survival of human NSC transplants and neglects their defining ability to scavenge distant tumor foci54. This behavior can be mimicked by implanting therapeutic NSCs outside the established GBM55,56. This slows tumor progression but this method is not as pronounced as when therapeutic NSCs are entirely co-localized with the GBM cells. Transplanting NSCs encapsulated in hydrogel scaffolds increased the intracavity persistence of therapeutic NSCs, improved tumor killing, and extended the survival of tumor-bearing mice to over 63 days post-transplant. The mechanisms driving the loss of directly injected NSCs from the resection cavity and the resultant effects on efficacy are unclear, therefore, it is important to continue to study and find new strategies to effectively transplant cytotoxic NSCs into GBM patients57.
We and others have previously demonstrated in a mouse glioma model that therapeutic NSCs will home to sites of brain tumors and injury following intracranial and intravenous injection, and can exert a therapeutic effect, as detected by a decrease in tumor burden58. Notwithstanding our previous success with these approaches, each presents some challenges. Intravenous administration has obvious clinical advantages because of the ease of administration of one or more rounds of NSCs, and because NSCs can reach multiple tumor sites from the bloodstream. However, the number of injected cells that are able to traffic to the lesion is relatively small (0.5–1.0%) when compared to intracranially injected NSCs. Intracerebral injection is associated with higher rates of engraftment (1–10% or more), but this procedure is more complex and more invasive. As an alternative to IV and IC administration, we have begun to explore the feasibility of intranasal (IN) administration of NSCs and MSCs for targeting of brain tumors and injury59. The IN route of administration has been shown to be efficient for delivery of polypeptides such as insulin for treatment of Alzheimer’s disease60. More recently, IN-administered mesenchymal stem cells have been shown to successfully migrate to the brain in a rat model of Parkinson’s disease61. Use of IN delivery to target and treat brain tumors could overcome the complications of IC and IV injections by eliminating the need for invasive neurosurgical implantation of cells and avoiding the dispersal of cells throughout the body that can result in unwanted systemic exposure. Thus, IN delivery of therapeutic NSCs represents a promising, non- invasive method for treatment of malignant brain tumors and brain injury that has substantial advantages over current methods of administration61.
5. Novel strategies for overcoming barriers to increase delivery
In addition to injection strategies, there are novel approaches to overcome the difficulties in delivering cell-based therapies to the brain. Clinical and preclinical application of these techniques offer promise though can often be difficult to implement. Here we analyze a few strategies for better cell-based therapy delivery.
5.1. Convection Enhanced Delivery
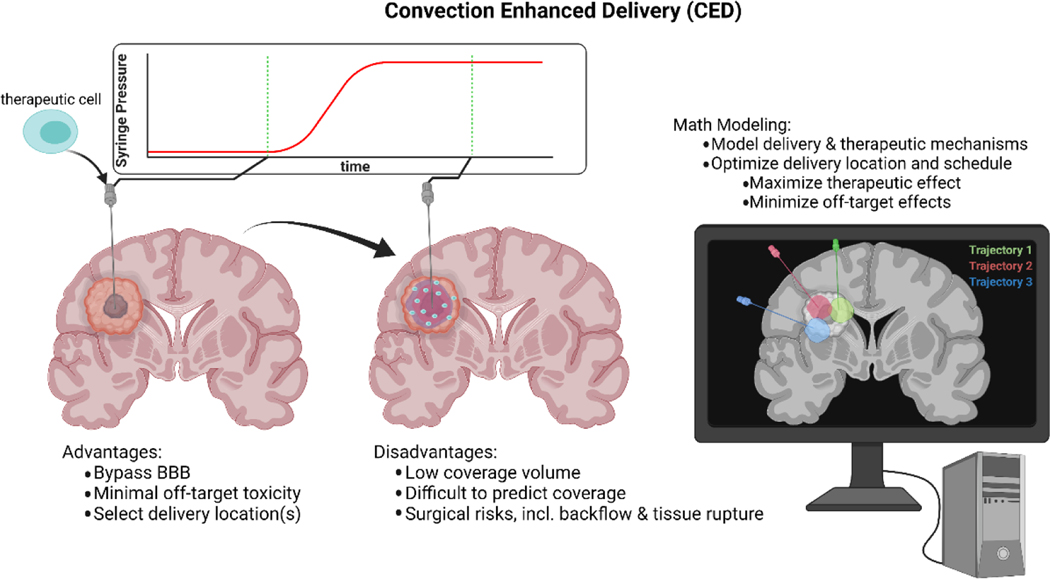
A form of direct injection that avoids the many barriers of delivery to brain interstitial space while increasing distribution of therapies, is convection-enhanced delivery (CED, Figure 2). In this method, the therapeutic of interest is delivered via intracranial catheter, surgically inserted through the skull, with placement directly within the site of interest (i.e. disease location, or resection cavity). The catheter is then connected to a syringe pump, and a pressure gradient is applied (thereby supplying convection) to deliver the therapeutic at a relatively constant rate and high local doses and can overcome the high pressures of the BTB. As the agent is not delivered through the bloodstream, the recipient is therefore at lower risk for off-target toxicities62.
Figure 2: Convection enhanced delivery of therapeutic cells.
Briefly, a catheter is surgically placed in the cranium to delivery therapeutic cells via convection supplied by a pressure source. Cells travel along the convection front, seeding the tumor. CED allows for directly bypassing the BBB, allowing for minimal systemic exposure to potentially toxic therapy, and increasing therapeutic exposure. However, CED is limited in that the small distribution volume is often not sufficient to cover the entire tumor, and comes with surgical risks including backflow along the catheter and tissue rupture. In order to maximize tumor coverage and therapeutic effect of this delivery modality, mathematical modeling may be employed to predict both coverage volume and the therapeutic mechanism of the cells.
While CED directly bypasses the vast majority of barriers outlined in this paper, it is still known to have several limitations, preventing it from being widely applied in the clinic. Catheter placement for CED is notoriously difficult, requiring both precise mathematical fluid dynamical modeling and veteran neurosurgical experience63. The bolus of therapeutic, while carried by a convection front supplied by a syringe pump, is still only capable of covering a region of roughly 2.3 times the volume infused64. This coverage volume is often much smaller than aggressive primary and secondary brain tumor volumes, and higher tumor coverage is then left to passive diffusion, resting-state CSF flow, and in the case of cell-based therapies, cell motility. In order to achieve higher tumor coverage, a larger pressure gradient (and therefore larger convection front) may be applied, but with the additional risks of tissue rupture and backflow along the catheter, and increased hydrostatic stresses on therapeutic cells to be delivered. For molecular therapeutics, flow rates of up to 50 uL/min may be achieved without backflow. However, the risk of rupture and leakage into subarachnoid and ventricular space is significant above rates of 3 uL/min63. Some trials utilize multiple catheters to achieve higher tumor coverage, while others pick a single linear catheter trajectory and deliver cell boli as the catheter is slowly inserted or removed62,65. Cell-based therapy is unique, in that the therapeutic particle (i.e. the cell) is many orders of magnitude larger than the single molecule drugs typically delivered with CED. This means that a cell carried on an advection-front would more easily become trapped in the brain parenchyma than a molecular- or nano-scale therapeutic66. Thus, the success of cell-based therapies hinges on the assumption of immune cell motility. In this case, a wider distribution of cells could provide significant benefit by allowing a region of interest to be more evenly seeded by therapeutic cells62. While the risk of tissue rupture and backflow are significant at high infusion rates, Atik et al. have successfully delivered CAR T cells to the mouse brain at a rate greater than 8uL/min utilizing a hyaluronic acid gel as a cell media67. Cell motility may be able to overcome the limited infusion rates, and compensate for limited coverage due to convection alone67.
5.2. Catheter design for convection enhanced delivery
A serious risk in CED is reflux, or solute backflow, along the catheter. This risk increases with increasing particle size (i.e. cells), as more pressure is required to achieve the same coverage volume due to increased flow resistance68. Additionally, the risk of reflux increases with larger catheter diameter69. To mitigate these risks, many catheter manufacturers utilize a stepped design, providing a physical barrier to reflux.
Tumor coverage is a difficult challenge to overcome in convection enhanced delivery, especially so for large particles such as cells, which require great advective transport to not only carry, but also push through the small pores of the brain parenchyma. To more distribute cells throughout the tumor, surgical strategies such as usage of multiple catheters, or multiple smaller cell infusions performed along the catheter trajectory may be implemented62. Some catheter designs take these methods for inspiration. One example is a multi-directional or arborizing catheter, which once implanted, extends one or multiple smaller catheters from the tip at varying angles in order to achieve a larger and more uniform coverage70,71. Porous catheter designs have a semipermeable segment near the catheter tip, allowing for multidirectional seeding of cells at positions along the catheter without the need for multiple infusions along the same catheter trajectory72. Instead of large cell clusters at a single focal point, arborizing, branched, or porous catheters allow for a large number of foci spread throughout the target tissue, and may prove useful for delivery of cell-based therapies.
5.3. Mathematical Modeling
In a review of clinical trials involving the use of drug delivery via convection enhancement, Jagangiri et al. state that a lack of mathematical modeling in surgical planning could be one of several reasons why the use of convection enhancement has been limited to clinical trials63. Since the publication of this review, a number of models of fluid transport due to convection enhancement have been published73–76. Raghavan have published a number of research articles on the development of a model of convection-based delivery of drugs73,74. There are numerous modeling niches to explore, including modeling of bulk-fluid transport derived from CED, backflow along the catheter trajectory, molecular targeting, medical image-derived fluid dynamical material property determination, etc. We direct the mathematically inclined reader to Vendel et. al (2019) for those interested in further details77. In the present work, we primarily discuss methods of mathematical modeling which apply primarily to the sub-field of cell therapy.
The mathematical challenges of modeling cell therapy are unique, as the particle size is large in comparison to those typically modeled in the field. Models developed by Therataxis, are explicitly designed for the delivery of small molecules73,74. Even models written for nano-scale particles, including a drag factor to account for slow transport through the parenchyma66,75, could be insufficient to model the transport of cells upon delivery. Convection-driven transport of large particles in the CNS (relative to pore size) remains largely understudied in the biological sciences, although future endeavors into this field may take their inspiration from particle transport theory used in petroleum and geological engineering68,78.
With cell-based therapy, two unique modeling aspects present themselves; first is modeling the interaction of the therapeutic cell with that of the target cell. Sahoo et. al have demonstrated that a predator-prey model of immune cells and tumor cells is able to describe the tumor volume dynamics in patients receiving CAR-T cells therapy in the brain79. This model is not only capable of describing the dynamics, but is also able to provide mathematical insight into the differences between responding and non-responding populations. In particular, they develop a modified predator-prey system to describe in vitro and in vivo cell dynamics, wherein the CAR-T cells act as predators and cancer cells act as prey79. The model consists of logistic tumor growth, CAR-T-mediated tumor death, stimulation of CAR-T growth (or death) by tumor cells, and natural CAR-T cell death. This model was applied to multiple primary glioma lines in an in vitro setting, as well as three lesions from a patient enrolled in a CAR-T clinical trial. Using phase field analysis, the authors describe three primary regimes of CAR-T response to therapy: treatment success, treatment failure, and pseudo-response. Under this model, treatment success occurs when the CAR-T death rate is significantly lower than the tumor cell proliferation rate, and CAR-T cells are stimulated to reproduce upon killing tumor cells. Treatment failure occurs in the model when CAR-T death rate surpasses the CAR-T proliferation rate, which is hypothesized to be mediated by CAR-T action on tumor cells. Finally, the pseudo-response regime occurs when the CAR-T cell proliferation is stimulated by cancer cell death, but cancer cell death exceeds the rate of CAR-T cell proliferation. In this final regime, the population of CAR-T and tumor cells oscillate and approach a stable equilibrium. The authors demonstrate that CAR-T-mediated tumor killing rate is positively related to the density of CAR-T antigen expression by the tumor. In a single patient, the predator-prey model accurately predicts treatment success in two lesions, and failure in a third79.
The second aspect of mathematical modeling unique to cell therapy is cell motility. Munson et. al hypothesize that tumor cell invasion could be made worse by providing strong chemical gradients stemming from the convection front used to deliver therapies80,81. By this same logic, therapeutic cell motility could also migrate along a chemical gradient created through CED, enabling therapeutic immune cells to infiltrate the tumor along the same convection streamlines the tumor would use to invade healthy tissue. As such, models of interstitial fluid flow (IFF) are needed for predicting the direction of chemical gradients. Raghavan and others have developed advection-diffusion models of fluid flow during CED administration73,74. These models utilized patient-specific material properties, derived from diffusion-weighted MR imaging82. These models first estimate an IFF field during active infusion, and then predict the distribution of therapeutic agent within the parenchyma. In one study, researchers were able to predict the distribution volume of drug in pig brains with distribution volume concordance percentage ranging from 75%−95% at the final time point74. To apply these results to cell delivery, the underlying model would have to be adjusted to reflect the size of the therapeutic cells, and the potential for therapeutic cells to block interstitial pores.
The distribution of therapeutic cells in the tumor is also affected by the IFF field at resting pressure (after the infusion is completed). Kingsmore et. al have developed a model to determine the resting-state IFF field from contrast-enhanced dynamic MR imaging, which is validated by phantoms in vitro and in silico80. The authors were capable of determining the IFF field of maximum likelihood by inverting a finite-difference implementation of the advection diffusion equation to fit the concentration of contrast agent measured via DCE-MRI. The authors determine both the IFF and apparent diffusivity fields in a murine brain glioma stem cell xenograft model. When compared to the distribution of Evans Blue in vivo, regions of dye pooling corresponded to the regions predicted from IFF mapping. The resulting IFF fields were heterogeneous, indicating that movement of tumor cells along concentration gradients would be non-trivial to predict from tumor geometry alone both in preclinical models and in patients80,83. Applying these models to delivery of CAR-T cells would allow for modeling of bulk-fluid transport of cells upon delivery, and inform chemotaxis models of both tumor and immune cell motility.
The combination of immune and tumor cell dynamics, along with cell motility provide the foundation for future development of mathematically optimized delivery. If a treatment objective function can be derived, the forward models of active and resting-state IFF, as well as immune/tumor cell dynamics will constrain the optimization of this objective function for individual patients. Jarrett et al. describe the development of clinical objective functions, wherein the authors investigate the control parameters clinically available, and realistic outcomes that can be achieved84. In the case of cell therapy, the control variables include the method and location(s) of cell delivery, cell dosage, and scheduling. Through the use of Optimal Control Theory85,86, these variables may be used to determine the course of treatment under the constraints of the procedure. In the case of immunotherapy, these constraints would be the number of cells available, the risk and cost to the patient, and enforcement of the patient’s extended survival. By adjusting the mathematical importance of each of these constraints, various mathematically optimal treatment schedules may be developed and investigated for an individual, allowing physicians to select a treatment strategy which would be most effective for each individual84.
5.4. Focused ultrasound (FUS) and BBB opening
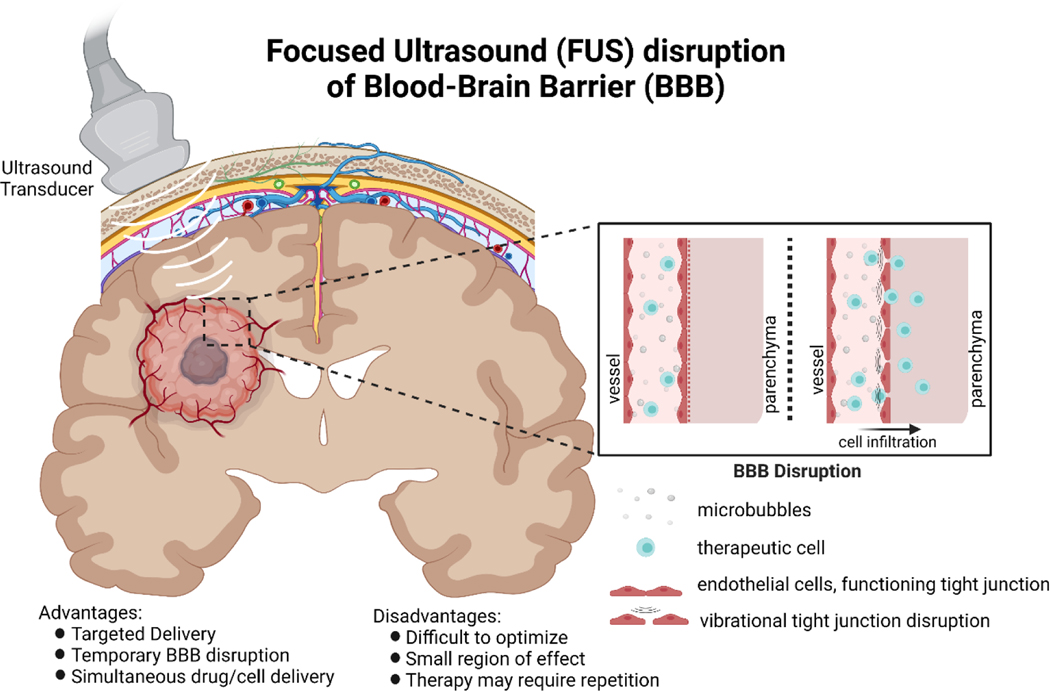
Focused ultrasound coupled with microbubbles is a method that can be used to temporarily open the blood brain barrier in an image-guided manner while limiting unwanted inflammation (Figure 3). This can result in transient delivery of otherwise impassable biomolecules, particles, and other therapies to increase total delivery load, and has shown success at increasing drug delivery to the brain87. A few successful examples of FUS coupled with cellular therapy delivery show promise for diseases in the brain. Natural killer-92 (NK-92) cells were delivered to mouse brain after FUS-mediated BBB opening88. These cells accumulated near sites of BBB opening with success, and in subsequent studies increased survival in a murine model of metastatic breast cancer89. Neural stem cells have also been successfully delivered via FUS-mediated BBB opening to the hippocampus of rats using MR-guided imaging90. Subsequent work has loaded neural progenitor cells with magnetic nanoparticles prior to delivery. This enables magnetic localization of the stem cells and increases retention within the brains of rats at the site of delivery compared to non-magnetic cells overcoming one of the primary limitations of delivery of NSCs to brain91. Optimization of FUS-mediated BBB opening is a multifaceted engineering problem. Tight junction integrity may be disrupted both by thermal and vibrational effects, though thermal BBB disruption (and its associated frequency range) has potential for permanent BBB disruption and tissue damage92. As such, vibrational disruption of tight junctions via vibration and cavitation of microbubbles is preferred (frequencies 0.2–1.5 MHz), as normal BBB function typically returns within hours92,93. FUS-mediated BBB opening offers the option to specifically deliver cells to locations within the brain, offering potential for targeted delivery across multiple brain disorders.
Figure 3: Focused ultrasound disruption of the blood-brain barrier.
Briefly, ultrasound microbubbles are injected into the blood stream, and are locally excited in the desired region of delivery by an external ultrasound transducer. Vibrational disturbances temporarily disrupt tight junctions forming the blood-brain barrier, allowing for therapeutic cells to enter the brain parenchyma. As the BBB disruption is only temporary, the number of cells delivered may be smaller compared to direct delivery methods (e.g. CED), and may require repetition to achieve the desired therapeutic effect.
5.5. Targeting cells
There different methods for the targeting of cell-based therapies to glioma cells. One of those methods was researched by Punganuru et al where they inhibited the MDM2 oncogene for targeted brain tumor therapy. A common feature of glioblastomas and lower-grade gliomas is a disruption of the p53 pathway, leading to the emergence of oncogenic genomes. Under normal conditions, the MDM2 feedback loop regulates the level of p53 activity and the duration of p53 activation in response to DNA damage and various metabolic and pathological stresses by targeting p53 for degradation through its intrinsic E3 ubiquitin ligase activity. Since MDM2 is an important hub for cell survival, growth, invasion, and DNA repair it is an optimal therapeutic target for GBM. MDM2 inhibition has emerged as a prime therapeutic strategy to reactivate the p53 pathway, leading to cell cycle arrest, increased apoptosis, and decreased tumor growth. Punganuru et al discovered a new class of MDM2 inhibitor, SP-141, a first-in-class MDM2 inhibitor that directly binds to the MDM2 protein, inhibits MDM2 expression, and induces its autoubiquitination and proteasomal degradation. SP-141 can also cross the blood–brain barrier fairly well and inhibit MDM2 in the presence or absence of wild-type p53, The postive results of Punganuru et al’s study support the further development of SP-141 as MDM2-targeted therapeutic strategies for combating brain tumors94.
Chlorotoxin (CLTX), a 36-amino acid peptide isolated from the venom of the death stalker scorpion, was used by Wang et al to target CAR T cells to GBMs. Results from this study showed that CLTX binds to a broad variety of GBM cells. First, CLTX binding to freshly-dissociated tumor cells from surgical resection specimens was evaluated. Primary brain tumor (PBT) cells were examined by flow cytometry for binding of Cy5.5-conjugated CLTX peptide (CLTX-Cy5.5) and compared with expression of IL13Rα2, HER2 and EGFR, three targets being clinically evaluated in CAR T cell therapies for GBMs. Of 22 tumor samples, greater than 80% of cells bound CLTX. CLTX-Cy5.5 binding appeared independent of other antigens, and was observed on tumors with both high and low expression of IL13Rα2, HER2 and EGFR. CLTX-Cy5.5 binding to low-passage patient-derived GBM tumor sphere (PBT-TS) lines expanded under conditions favoring a cancer stem cell-like phenotype was also examined. 18 out of 19 PBT-TS lines showed greater than 80% CLTX-Cy5.5 binding. Within GBM tumors, stem-like cells (GSCs) display self-renewal and tumor-initiation capacity, so Wang et al examined the effectiveness of CLTX binding with respect to this stem cell-like population. In freshly-dissociated primary PBTs, GSCs were distinguished from other GBM cells by expression of surface markers CD133 or CD44. CLTX-Cy5.5 binding was somewhat higher in CD133+ GSCs, but also evident in more differentiated CD133- cells. No significant difference was observed between CD44+ and CD44- GBM cells. In another approach, Wang et al used PBT-TS lines and varied culture conditions to favor GSCs or alternatively to promote differentiation. Differentiation led to reduced expression of CD133, however, CLTX-Cy5.5 binding was not affected in comparison to GSCs. Combined, these studies demonstrated that CLTX binding, while showing some preference for CD133+ GSCs in freshly dispersed tumor samples, remains robust on both stem-like and more differentiated GBM cells. These results demonstrated the broad GBM binding potential of CLTX, showing promise in investigating its use to redirect CAR T cell immunotherapy95.
Nonaka et al identified a peptide they designated as IF7 that binds to annexin A1 (Anxa1), a specific endothelial cell-surface marker of malignant tumors. Upon intravenous injection into tumor-bearing mice, IF7- conjugated anticancer drugs suppressed growth of tumors in mouse models, suggesting that, after binding to Anxa1 on tumor vasculature surface, IF7 is transported across endothelial cells from the luminal surface to the basal membrane via transcytosis and released to the tumor stroma. The Anxa1 N-terminal domain was shown to be on the surface of tumor vasculature surface in the mouse, however, this domain is not expressed as a full-length Anxa1 but exist as peptide fragment.. Data reported in this study suggest that IF7 and IF7-conjugated chemicals are transported across endothelial cells by transcytosis from the luminal surface to basal stroma. We conclude that IF7 binding to some, if not all, Anxa1 fragments containing MC16 domain mediates transcytosis. Appearance of IF7 fluorescence in brain tumor stroma is evidence that IF7 crossed the BBB. The presence of fluorescent signals in tumor cells also suggests that IF7 is actively taken up by cancer cells, further suggesting that the IF7 receptor is expressed on the surface of brain tumor cells. The binding properties of IF7 to Anxa1 shows promise as a targeting method for immunotherapies to treat GBMs96. Thus, targeting of cell-based therapies can both enhance their efficacy towards final target cells as well as enhance their localization and ability to cross the brain barriers.
6. State of the Field/Clinical Trials
Across the country, cell based therapies have been implemented in clinical trials to treat a number of diseases with varying success. The state of the field is promising, and here we outline a few of the clinical applications of these therapies. A summary can be found in Table 1.
Table 1.
A summary of the clinical applications of cell-based therapies implemented in clinical trials in treating a number of diseases
| Cell | Target Antigen | Trial | Aim | Product | Route of Delivery | Antigen Loss | TME | Toxicity | Patient Outcome |
|---|---|---|---|---|---|---|---|---|---|
| CAR-T | IL13Rα2 |
NCT00730613 City of Hope 66, 68 |
First to evaluate intracranial delivery of IL13Rα2 CAR in rGBM | IL13(E13Y)-CD3ζ CD8+ CTL clones |
ICT | IL13Rα2 negative/low (1 patient tested) | Transient inflammatory response/necrosis at tumor site by MRI | No DLTs | 3 pts evaluated Median OS 10.9 mo Increased necrotic volume at treatment site (1 pt) Decreased IL13Rα2 expression (1 pt) |
| CAR-T | IL13Rα2 |
NCT01082926 City of Hope 67 |
First off-the-shelf allogeneic CAR T cells for rGBM. Evaluated feasibility of [18F]FHBG gene reporter imaging to monitor T-cell distribution in rGBM | IL13(E13Y)-CD3ζ [18F]FHBG HSV1-tk Glucocorticoid-receptor-depleted allogeneic CD8+ CTL clone |
ICT | Not reported | Not reported | No DLTs | 6 pts evaluated [18F]FHBG gene reporter allowed longitudinal imaging of ICT CAR-T Clinical outcomes not reported |
| CAR-T | IL13Rα2 |
NCT02208362 City of Hope69 |
Evaluate safety of ICV and dual ICT-ICV CAR delivery in rGBM | IL13(E13Y)-41BBζ Memory-derived T cells |
ICT, ICV and dual ICT-ICV | IL13Rα2 negative/low tumors (1 patient reported) | Increased CD3+ CD14+ and CD15+ immune cells and inflammatory cytokines | No DLTs | Case study demonstrated CAR-Ts mediate complete response that was durable for 7.5 mo |
| CAR-T | EGFRvIII |
NCT02664363 Duke University |
Utilizing TMZ-DI as a preconditioning lymphodepleting regimen and treatment for GBM | KLuc-EGFRvIII-expressing T cells | IV | Not reported | EGFRvIII-positive GBM. Pre-conditioned with TMZ-DI | Not reported | Terminated, July 2020, no results available |
| CAR-T | HER2 |
NCT04660929 Carisma Therapeutics, Inc. https://pubmed.ncbi.nlm.nih.gov/32361713/ https://www.nature.com/articles/d43747-020-01096-y https://clinicaltrials.gov/ct2/show/NCT04660929 |
Phase 1 study of CAR macrophages in HER2 overexpressing solid tumors. | THP-1 cell line transduced with a first-generation anti-CD19 CAR encoding the CD3ζ intracellular domain. | IV | Not reported | CAR macrophages are activated by tumor-associated antigen engagement with the CAR, signaling via CD3-ζ to phagocytose the tumor cell and release cytokines and chemokines that ‘warm up’ the TME. | Not reported | Ongoing |
| CAR-Macrophage | Mesothelin |
NCT03608618 MaxCyte, Inc. https://clinicaltrials.gov/ct2/show/NCT03608618 |
Phase 1 study on the use of of MCY-M11 as treatment for platinum resistant high grade serous adenocarcinoma and peritoneal mesothelioma | Mesothelin-CAR-expressing mRNA-targeted CAR macrophages | Intraperitoneal | Not reported | Not reported | Not reported | Ongoing |
| CAR-NK | CD33 |
NCT02944162 PersonGen BioTherapeutics (Suzhou) Co., Ltd. https://clinicaltrials.gov/ct2/show/NCT02944162 |
Study genetically engineered NK92 cell therapy to treat CD33 positive acute myeloid leukemias | Anti-CD33 CAR-NK cells | IV | Not reported | Not reported | Not reported | Recruitment status unknown |
| CAR-NK | NKG2D ligands |
NCT03415100 The Third Affiliated Hospital of Guangzhou Medical University https://www.clinicaltrials.gov/ct2/show/NCT03415100 |
Study to evaluate the safety and feasibility of CAR-NK cell treatment for metastatic solid tumors. | NKG2D-Ligand Targeted CAR-NK Cells | IV | Not reported | Not reported | Not reported | Recruitment status unknown |
| CAR-NK | CD19+ |
NCT03056339 M.D. Anderson Cancer Center https://clinicaltrials.gov/ct2/show/NCT03056339 |
Study the use of CAR-CD19-CD28-zeta-2A-iCasp9-IL15-transduced CB-NK cells to treat relapsed/refractory CD19+ B lymphoid malignancies. | CAR-CD19-CD28-zeta-2A-iCasp9-IL15-transduced CB-NK cells | IV | Not reported | Not reported | Not reported | Actively recruiting |
| NSC | N/A |
NCT01172964 City of Hope |
Determine the safety and feasibility of intracerebral administration of NSCs with oral 5-FC to treat recurrent high-grade gliomas. | E. coli CD-expressing genetically modified neural stem cells | ICT | Not reported | Not reported | Not reported | Completed, no results posted |
| NSC | N/A |
NCT03072134 Northwestern University |
Phase I study of neural stem cell based virotherapy in combination with standard radiation and chemotherapy to treat with newly diagnosed malignant glioma | NSC-CRAd-Survivin-pk7 | ICT | Not reported | Not reported | Not reported | Ongoing |
| NSC | N/A |
NCT03629275 ReNeuron, Ltd. |
Study of the use of CTX0E03 neural stem cells to treat moderate to moderately severe disability as a result of an ischemic stroke | CTX0E03 neural stem cells | ICT | Not reported | Not reported | Not reported | Ongoing |
| Dendritic | Autologous tumor cells |
NCT04277221 Safe Save Medical Cell Sciences & Technology Co.,Ltd. |
Phase III study on the use of ADCTA immunotherapy with standard therapy to treat recurrent GBM. | ADCTA-SSI-G1 | Subcutaneous | Not reported | Not reported | Not reported | Actively recruiting |
| Dendritic | MGMT-promotor methylation |
NCT03548571 Oslo University Hospital |
Study of dendritic cell therapy to treat IDH wild-type, MGMT-promotor methylated glioblastoma | Dendritic cells transfected with mRNA of autologous tumor stem cells, survivin, and hTERT | Intradermal | Not reported | Not reported | Not reported | Actively recruiting |
6.1. CAR Therapy
Of five CAR T clinical trials performed at the City of Hope National Medical Center, two were focused on recurrent GBM targeting IL13Rα2 and three trials were focused in either IL13Rα2+, HER2+ GBM, or HER2+ breast metastases in the brain. Patients with malignant brain tumors were enrolled following confirmation of the expression of IL13Rα2 or HER2 followed by leukapheresis to collect peripheral blood mononuclear cells in order to create an autologous CAR T cell product. Patients then underwent surgery for placement of a reservoir or catheter delivery device for intraventricular, intratumoral, or intracavitary use. During the treatment that often consisted of 3–4 weekly cycles of infusions, immunologic correlative studies were performed to assess the persistence of the CAR T cells in the CSF and peripheral blood, monitor for evidence of activation of the endogenous immune system, and describe changes in cytokine levels in the CSF and peripheral blood. Repetitive dosing affords a larger total dose of cells, introduction of functional CAR T cells in a way that extends the therapeutic window in the immunosuppressive tumor microenvironment, while limiting unintended toxicities of larger bolus dosing3.
It is unknown whether concomitant medicines, such as temozolomide (TMZ), dexamethasone, and bevacizumab, which are commonly used for brain tumor management, may impact CAR T cell activity. In a syngeneic mouse model of GBM, lymphodepleting preconditioning with a dose-intensified regimen of TMZ (TMZ-DI), but not standard TMZ dose regimens prompted enhanced EGFRvIII-CAR expansion and persistence in circulation, and tumor eradication97. Based on these findings, an ongoing EGFRvIII-CAR T cell clinical trial at Duke University (NCT02664363) is utilizing TMZ-DI. While it is reasonable to assume that lymphodepletion will also augment CAR T cell clinical responses for brain tumors, its therapeutic benefit requires further clinical validation3. Dexamethasone (Dex) is known to inhibit priming of T cell immune response by dendritic cells and was shown to be deleterious to vaccine trials for GBM at very low levels. In preclinical mouse models, intratumorally delivered CAR T cells were strongly suppressed by levels above 5 mg/kg. Bevacizumab is FDA approved for the treatment of recurrent GBM and acts as a ligand-sink against the vascular endothelial growth factor receptor (VEGF). Bevacizumab may augment CAR T cell therapy by inhibiting the immune suppressive effects of VEGF, as well as promote tumor lymphocyte trafficking3. Preclinical models in neuroblastoma have demonstrated enhanced efficacy of combination bevacizumab and GD2 CAR T cells. Combination treatment resulted in high infiltration of GD2-CAR T cells which resulted in increased IFNγ production. Bevacizumab’s role in enhancing lymphocyte trafficking and blocking VEGF immunosuppressive effects may be a promising combination therapy with CARs for treatment of solid tumors. Whether there is enhanced clinical efficacy in GBM and other brain tumors still requires validation3.
An advantage of CAR-NK cells is their use as an “off-the-shelf” product, eliminating the need for a personalized and patient-specific product which is necessary for current CAR-T cell therapies. As such, as of June 2020, there are nineteen studies registered on clinicaltrials.gov evaluating the safety and efficacy of CAR-NK cells in cancer patients including twelve Phase I/II trials under active patient recruitment and two completed Phase I/II trials. The clinical results from most of these trials are currently pending, with only three studies published, in non-CNS disease98 99,100 101,102 103,104, These initial studie demonstrates feasibility, initial efficacy and very attractive safety profile of the cord blood CAR-NK cell immunotherapy, but no data exists clinically yet for addressing brain neoplasms98.
6.2. Neural Stem Cell Therapy
The tumor-homing capacity of tumoricidal NSCs creates a powerful drug delivery platform that provides access to invasive cancer foci which traditional surgery, chemotherapy, and radio-therapy cannot typically access. NSCs can be engineered with a wide range of therapeutic agents, and typically achieve tumor reductions of 70–90% in preclinical models. As of January 2021, according to clinicaltrials.gov, there has been one completed and one ongoing clinical trial involving NSC therapies to treat brain tumors. The completed trial in high grade glioma focused on the use of genetically-modified NSCs that convert 5-fluorocytosine into the chemotherapy agent, fluorouracil, at sites of tumor in the brain may be an effective treatment for glioma. Currently, the results of this trial have not been published105. The ongoing trial focuses on studying the delivery of a novel oncolytic adenovirus via a neural stem cell line in combination with radiation and chemotherapy is well-suited for evaluation in newly diagnosed malignant gliomas106. A clinical trial studying the use of NSCs to treat ischemic stroke is also currently ongoing and involves intracerebral injection of CTX0E03 neural stem cells into patients with persistent disability 6–24 months following an ischemic stroke107. NSCs are injected in an area adjacent to the lesion and patients are followed for recovery in addition to standard physical therapy. While NSCs are a growing area of clinical exploration, we have yet to see whether delivery of these cells to the brain is efficient at ameliorating disease.
6.3. Dendritic Cell Therapy
With the various methods of administration for DCs in the treatment of brain tumors, there are numerous completed and ongoing clinical trials. There are two phase III clinical trials involving the usage of DCs for therapeutic usage against brain tumors. Autologous dendritic cell tumor antigen (ADCTA) is an individualized cell immunotherapy generated by co-culturing autologous dendritic cells derived from peripheral blood mononuclear cells with autologous tumor cells to evoke tumor-specific immune response. The focus of phase III of this trial is to test the efficacy and safety of ADCTA immunotherapy plus standard of care therapy versus standard of care alone in patients with recurrent GBM108. In the second trial, PBMCs are harvested via autologous leukapheresis for enrichment of PBMCs followed by ex vivo generation and preservation of DCs. To generate tumor-specific DCs, tumor biopsies mRNA is harvested from cultured glioma stem cells from patient biopsies. Patients receive three intradermal injections of DCs transfected with mRNA from autologous tumor stem cells, survivin and hTERT at a time. This vaccination will continue for as long as there are vaccines available. This experimental therapy is coupled with standard of care therapies and the results of this trial could result in a treatment for GBM that is more efficient than the current standard treatment109.
Sipuleucel-T (Provenge), is a cell-based, immunostimulant, cancer immunotherapy for metastatic, asymptomatic, hormone-refractory prostate cancer (HRPC) developed by Dendreon Pharmaceuticals, LLC110,111. Provenge was approved by the U.S. Food and Drug Administration (FDA) on April 29, 2010, to treat asymptomatic or minimally symptomatic metastatic HRPC including those cancers which have metastasized to the brain112–114. Shortly afterward, sipuleucel-T was added to the compendium of cancer treatments published by the National Comprehensive Cancer Network (NCCN) as a “category 1” treatment for HRPC, the NCCN’s highest recommendation level. The NCCN Compendium is used by Medicare and major health care insurance providers to decide whether a treatment should be reimbursed115. During the course of treatment using Provenge, the patient’s white blood cells, primarily dendritic cells, are extracted in a leukapheresis procedure. The blood product is sent to a production facility and incubated with the fusion protein, PA2024, which consists of the antigen, prostatic acid phosphatase (PAP), and the immune signaling factor, granulocyte-macrophage colony stimulating factor (GM-CSF), which helps APCs to mature. The activated blood product, APC8015, is returned from the production facility to the infusion center and reinfused into the patient116,117. Sipuleucel-T showed overall survival benefit to patients in three double-blind randomized phase III clinical trials, D9901117, D9902a118,119, and IMPACT116 There are ongoing clinical trials combining this therapy with other therapies for better efficacy, however, the benefit of such a therapy against brain cancers is still limited120,121. Thus, dendritic cell therapies are by far the most advanced against cancers, though there is still limited data for their use in the brain. As clinical trials conclude, we may be able to better understand how to implement these and other cell-based therapies more regularly.
7. Summary
The brain has been a formidable target for drug delivery for decades, and cell-based therapies are subject to many of the same limitations. However, there are a number strategies that are showing promise in the clinic against multiple diseases, yet the research on optimization of delivery strategies is still relatively young. Interestingly, by leveraging methods to enhance drug delivery that have previously been developed, cells can be better delivered into the brain and bypass the multiple complex barriers. These strategies include bypassing the barriers through locoregional infusions or barrier opening. However, the more exciting prospects for these therapeutics is to exploit their natural biological properties to better their delivery, efficacy, and targeting capacity as well as combining mathematical approaches to provide patient-specific information and outcomes. Lastly, though the BBB is the primary barrier that has been targeted to overcome, the (re)discovery of the meningeal lymphatics offers a potential novel entry point into the brain, that is particularly suited to immune cell recruitment and trafficking. Thus, though the use of cell-based therapies is relatively new and the brain still presents a difficult delivery challenge, the existing and potential strategies for bypassing these barriers is promising as is evident from the early clinical successes.
Acknowledgements:
Figures were created using Biorender with a license to JMM.
Funding: Funding provided to JMM from the National Cancer Institute R37CA222563. Funding provided to JMM, RCR, and CEB from the National Institute of Neurological Disorders and Stroke R01NS115971.
Footnotes
Declarations
Ethical Statement: No animal or human studies were carried out by the authors for this article.
Ethics approval and consent to participate: Not Applicable
Consent for publication: Not applicable
Availability of data and material: Not applicable.
Competing interest: This review was written without competing interests.
References
- (1).Weber EW; Maus MV; Mackall CL The Emerging Landscape of Immune Cell Therapies. Cell 2020, 181 (1), 46–62. 10.1016/j.cell.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Hu X; Leak RK; Thomson AW; Yu F; Xia Y; Wechsler LR; Chen J Promises and Limitations of Immune Cell-Based Therapies in Neurological Disorders. Nat. Rev. Neurol. 2018, 14 (9), 559–568. 10.1038/s41582-018-0028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Akhavan D; Alizadeh D; Wang D; Weist MR; Shepphird JK; Brown CE CAR T Cells for Brain Tumors: Lessons Learned and Road Ahead. Immunol. Rev. 2019, 290 (1), 60–84. 10.1111/imr.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Chen Y; Yu Z; Tan X; Jiang H; Xu Z; Fang Y; Han D; Hong W; Wei W; Tu J CAR-Macrophage: A New Immunotherapy Candidate against Solid Tumors. Biomed. Pharmacother. 2021, 139, 111605. 10.1016/j.biopha.2021.111605. [DOI] [PubMed] [Google Scholar]
- (5).Kim W; Liau LM Dendritic Cell Vaccines for Brain Tumors. Neurosurg Clin N Am 2010, 21 (1), 139–157. 10.1016/j.nec.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).De Vleeschouwer S; Fieuws S; Rutkowski S; Van Calenbergh F; Van Loon J; Goffin J; Sciot R; Wilms G; Demaerel P; Warmuth-Metz M; Soerensen N; Wolff JEA; Wagner S; Kaempgen E; Van Gool SW Postoperative Adjuvant Dendritic Cell-Based Immunotherapy in Patients with Relapsed Glioblastoma Multiforme. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14 (10), 3098–3104. 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- (7).Yamanaka R; Homma J; Yajima N; Tsuchiya N; Sano M; Kobayashi T; Yoshida S; Abe T; Narita M; Takahashi M; Tanaka R Clinical Evaluation of Dendritic Cell Vaccination for Patients with Recurrent Glioma: Results of a Clinical Phase I/II Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11 (11), 4160–4167. 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- (8).Rutkowski S; De Vleeschouwer S; Kaempgen E; Wolff JEA; Kühl J; Demaerel P; Warmuth-Metz M; Flamen P; Van Calenbergh F; Plets C; Sörensen N; Opitz A; Van Gool SW Surgery and Adjuvant Dendritic Cell-Based Tumour Vaccination for Patients with Relapsed Malignant Glioma, a Feasibility Study. Br. J. Cancer 2004, 91 (9), 1656–1662. 10.1038/sj.bjc.6602195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).De Vleeschouwer S; Van Calenbergh F; Demaerel P; Flamen P; Rutkowski S; Kaempgen E; Wolff JE; Plets C; Sciot R; Van Gool SW Transient Local Response and Persistent Tumor Control in a Child with Recurrent Malignant Glioma: Treatment with Combination Therapy Including Dendritic Cell Therapy. Case Report. J. Neurosurg. 2004, 100 (5 Suppl Pediatrics), 492–497. 10.3171/ped.2004.100.5.0492. [DOI] [PubMed] [Google Scholar]
- (10).Barratt-Boyes SM; Zimmer MI; Harshyne LA; Meyer EM; Watkins SC; Capuano S; Murphey-Corb M; Falo LD; Donnenberg AD Maturation and Trafficking of Monocyte-Derived Dendritic Cells in Monkeys: Implications for Dendritic Cell-Based Vaccines. J. Immunol. Baltim. Md 1950 2000, 164 (5), 2487–2495. 10.4049/jimmunol.164.5.2487. [DOI] [PubMed] [Google Scholar]
- (11).Aboody KS; Najbauer J; Danks MK Stem and Progenitor Cell-Mediated Tumor Selective Gene Therapy. Gene Ther. 2008, 15 (10), 739–752. 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- (12).Dong X Current Strategies for Brain Drug Delivery. Theranostics 2018, 8 (6), 1481–1493. 10.7150/thno.21254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Pehlivan SB Nanotechnology-Based Drug Delivery Systems for Targeting, Imaging and Diagnosis of Neurodegenerative Diseases. Pharm. Res. 2013, 30 (10), 2499–2511. 10.1007/s11095-013-1156-7. [DOI] [PubMed] [Google Scholar]
- (14).Guerra M; Blázquez JL; Rodríguez EM Blood-Brain Barrier and Foetal-Onset Hydrocephalus, with a View on Potential Novel Treatments beyond Managing CSF Flow. Fluids Barriers CNS 2017, 14 (1), 19. 10.1186/s12987-017-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Komarova YA; Kruse K; Mehta D; Malik AB Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ. Res. 2017, 120 (1), 179–206. 10.1161/CIRCRESAHA.116.306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lécuyer M-A; Saint-Laurent O; Bourbonnière L; Larouche S; Larochelle C; Michel L; Charabati M; Abadier M; Zandee S; Haghayegh Jahromi N; Gowing E; Pittet C; Lyck R; Engelhardt B; Prat A Dual Role of ALCAM in Neuroinflammation and Blood-Brain Barrier Homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (4), E524–E533. 10.1073/pnas.1614336114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Aday S; Cecchelli R; Hallier-Vanuxeem D; Dehouck MP; Ferreira L Stem Cell-Based Human Blood–Brain Barrier Models for Drug Discovery and Delivery. Trends Biotechnol. 2016, 34 (5), 382–393. 10.1016/j.tibtech.2016.01.001. [DOI] [PubMed] [Google Scholar]
- (18).Warren KE Beyond the Blood:Brain Barrier: The Importance of Central Nervous System (CNS) Pharmacokinetics for the Treatment of CNS Tumors, Including Diffuse Intrinsic Pontine Glioma. Front. Oncol. 2018, 8. 10.3389/fonc.2018.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ghersi-Egea J-F; Strazielle N; Catala M; Silva-Vargas V; Doetsch F; Engelhardt B Molecular Anatomy and Functions of the Choroidal Blood-Cerebrospinal Fluid Barrier in Health and Disease. Acta Neuropathol. (Berl.) 2018, 135 (3), 337–361. 10.1007/s00401-018-1807-1. [DOI] [PubMed] [Google Scholar]
- (20).Strominger I; Elyahu Y; Berner O; Reckhow J; Mittal K; Nemirovsky A; Monsonego A The Choroid Plexus Functions as a Niche for T-Cell Stimulation Within the Central Nervous System. Front. Immunol. 2018, 9. 10.3389/fimmu.2018.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Wiatr M; Stump-Guthier C; Latorre D; Uhlig S; Weiss C; Ilonen J; Engelhardt B; Ishikawa H; Schwerk C; Schroten H; Tenenbaum T; Rudolph H Distinct Migratory Pattern of Naive and Effector T Cells through the Blood–CSF Barrier Following Echovirus 30 Infection. J. Neuroinflammation 2019, 16 (1), 232. 10.1186/s12974-019-1626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Arvanitis CD; Askoxylakis V; Guo Y; Datta M; Kloepper J; Ferraro GB; Bernabeu MO; Fukumura D; McDannold N; Jain RK Mechanisms of Enhanced Drug Delivery in Brain Metastases with Focused Ultrasound-Induced Blood–Tumor Barrier Disruption. Proc. Natl. Acad. Sci. 2018, 115 (37), E8717–E8726. 10.1073/pnas.1807105115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Jain RK Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell 2014, 26 (5), 605–622. 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kabraji S; Ni J; Lin NU; Xie S; Winer EP; Zhao JJ Drug Resistance in HER2-Positive Breast Cancer Brain Metastases: Blame the Barrier or the Brain? Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24 (8), 1795–1804. 10.1158/1078-0432.CCR-17-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kodack DP; Askoxylakis V; Ferraro GB; Sheng Q; Badeaux M; Goel S; Qi X; Shankaraiah R; Cao ZA; Ramjiawan RR; Bezwada D; Patel B; Song Y; Costa C; Naxerova K; Wong CSF; Kloepper J; Das R; Tam A; Tanboon J; Duda DG; Miller CR; Siegel MB; Anders CK; Sanders M; Estrada MV; Schlegel R; Arteaga CL; Brachtel E; Huang A; Fukumura D; Engelman JA; Jain RK The Brain Microenvironment Mediates Resistance in Luminal Breast Cancer to PI3K Inhibition through HER3 Activation. Sci. Transl. Med. 2017, 9 (391). 10.1126/scitranslmed.aal4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Askoxylakis V; Ferraro GB; Kodack DP; Badeaux M; Shankaraiah RC; Seano G; Kloepper J; Vardam T; Martin JD; Naxerova K; Bezwada D; Qi X; Selig MK; Brachtel E; Duda DG; Huang P; Fukumura D; Engelman JA; Jain RK Preclinical Efficacy of Ado-Trastuzumab Emtansine in the Brain Microenvironment. J. Natl. Cancer Inst. 2016, 108 (2). 10.1093/jnci/djv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Lockman PR; Mittapalli RK; Taskar KS; Rudraraju V; Gril B; Bohn KA; Adkins CE; Roberts A; Thorsheim HR; Gaasch JA; Huang S; Palmieri D; Steeg PS; Smith QR Heterogeneous Blood-Tumor Barrier Permeability Determines Drug Efficacy in Experimental Brain Metastases of Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16 (23), 5664–5678. 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Semyachkina-Glushkovskaya O; Postnov D; Kurths J Blood–Brain Barrier, Lymphatic Clearance, and Recovery: Ariadne’s Thread in Labyrinths of Hypotheses. Int. J. Mol. Sci. 2018, 19 (12). 10.3390/ijms19123818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Caversaccio M; Peschel O; Arnold W Connections between the Cerebrospinal Fluid Space and the Lymphatic System of the Head and Neck in Humans. In Intracranial and Intralabyrinthine Fluids; Ernst A, Marchbanks R, Samii M, Eds.; Springer: Berlin, Heidelberg, 1996; pp 123–128. 10.1007/978-3-642-80163-1_15. [DOI] [Google Scholar]
- (30).Si J; Chen L; Xia Z Effects of Cervical-Lymphatic Blockade on Brain Edema and Infarction Volume in Cerebral Ischemic Rats. Chin. J. Physiol. 2006, 49 (5), 258–265. [PubMed] [Google Scholar]
- (31).Song E; Mao T; Dong H; Boisserand LSB; Antila S; Bosenberg M; Alitalo K; Thomas J-L; Iwasaki A VEGF-C-Driven Lymphatic Drainage Enables Immunosurveillance of Brain Tumours. Nature 2020, 577 (7792), 689–694. 10.1038/s41586-019-1912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Gutova M; Flores L; Adhikarla V; Tsaturyan L; Tirughana R; Aramburo S; Metz M; Gonzaga J; Annala A; Synold TW; Portnow J; Rockne RC; Aboody KS Quantitative Evaluation of Intraventricular Delivery of Therapeutic Neural Stem Cells to Orthotopic Glioma. Front. Oncol. 2019, 9. 10.3389/fonc.2019.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Cohen-Pfeffer JL; Gururangan S; Lester T; Lim DA; Shaywitz AJ; Westphal M; Slavc I Intracerebroventricular Delivery as a Safe, Long-Term Route of Drug Administration. Pediatr. Neurol. 2017, 67, 23–35. 10.1016/j.pediatrneurol.2016.10.022. [DOI] [PubMed] [Google Scholar]
- (34).Donovan LK; Delaidelli A; Joseph SK; Bielamowicz K; Fousek K; Holgado BL; Manno A; Srikanthan D; Gad AZ; Van Ommeren R; Przelicki D; Richman C; Ramaswamy V; Daniels C; Pallota JG; Douglas T; Joynt ACM; Haapasalo J; Nor C; Vladoiu MC; Kuzan-Fischer CM; Garzia L; Mack SC; Varadharajan S; Baker ML; Hendrikse L; Ly M; Kharas K; Balin P; Wu X; Qin L; Huang N; Stucklin AG; Morrissy AS; Cavalli FMG; Luu B; Suarez R; De Antonellis P; Michealraj A; Rastan A; Hegde M; Komosa M; Sirbu O; Kumar SA; Abdullaev Z; Faria CC; Yip S; Hukin J; Tabori U; Hawkins C; Aldape K; Daugaard M; Maris JM; Sorensen PH; Ahmed N; Taylor MD Locoregional Delivery of CAR T Cells to the Cerebrospinal Fluid for Treatment of Metastatic Medulloblastoma and Ependymoma. Nat. Med. 2020, 26 (5), 720–731. 10.1038/s41591-020-0827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Theruvath J; Sotillo E; Mount CW; Graef CM; Delaidelli A; Heitzeneder S; Labanieh L; Dhingra S; Leruste A; Majzner RG; Xu P; Mueller S; Yecies DW; Finetti MA; Williamson D; Johann PD; Kool M; Pfister S; Hasselblatt M; Frühwald MC; Delattre O; Surdez D; Bourdeaut F; Puget S; Zaidi S; Mitra SS; Cheshier S; Sorensen PH; Monje M; Mackall CL Locoregionally Administered B7-H3-Targeted CAR T Cells for Treatment of Atypical Teratoid/Rhabdoid Tumors. Nat. Med. 2020, 26 (5), 712–719. 10.1038/s41591-020-0821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Brown CE; Aguilar B; Starr R; Yang X; Chang W-C; Weng L; Chang B; Sarkissian A; Brito A; Sanchez JF; Ostberg JR; D’Apuzzo M; Badie B; Barish ME; Forman SJ Optimization of IL13Rα2-Targeted Chimeric Antigen Receptor T Cells for Improved Anti-Tumor Efficacy against Glioblastoma. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26 (1), 31–44. 10.1016/j.ymthe.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Weist MR; Starr R; Aguilar B; Chea J; Miles JK; Poku E; Gerdts E; Yang X; Priceman SJ; Forman SJ; Colcher D; Brown CE; Shively JE PET of Adoptively Transferred Chimeric Antigen Receptor T Cells with 89Zr-Oxine. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59 (10), 1531–1537. 10.2967/jnumed.117.206714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Goff SL; Morgan RA; Yang JC; Sherry RM; Robbins PF; Restifo NP; Feldman SA; Lu Y-C; Lu L; Zheng Z; Xi L; Epstein M; McIntyre LS; Malekzadeh P; Raffeld M; Fine HA; Rosenberg SA Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor-Transduced T Cells Targeting EGFRvIII in Patients With Glioblastoma. J. Immunother. Hagerstown Md 1997 2019, 42 (4), 126–135. 10.1097/CJI.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Morgan RA; Yang JC; Kitano M; Dudley ME; Laurencot CM; Rosenberg SA Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced with a Chimeric Antigen Receptor Recognizing ERBB2. Mol. Ther. J. Am. Soc. Gene Ther. 2010, 18 (4), 843–851. 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Priceman SJ; Tilakawardane D; Jeang B; Aguilar B; Murad JP; Park AK; Chang W-C; Ostberg JR; Neman J; Jandial R; Portnow J; Forman SJ; Brown CE Regional Delivery of Chimeric Antigen Receptor-Engineered T Cells Effectively Targets HER2+ Breast Cancer Metastasis to the Brain. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24 (1), 95–105. 10.1158/1078-0432.CCR-17-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Hong JJ; Rosenberg SA; Dudley ME; Yang JC; White DE; Butman JA; Sherry RM Successful Treatment of Melanoma Brain Metastases with Adoptive Cell Therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16 (19), 4892–4898. 10.1158/1078-0432.CCR-10-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Lee DW; Kochenderfer JN; Stetler-Stevenson M; Cui YK; Delbrook C; Feldman SA; Fry TJ; Orentas R; Sabatino M; Shah NN; Steinberg SM; Stroncek D; Tschernia N; Yuan C; Zhang H; Zhang L; Rosenberg SA; Wayne AS; Mackall CL T Cells Expressing CD19 Chimeric Antigen Receptors for Acute Lymphoblastic Leukaemia in Children and Young Adults: A Phase 1 Dose-Escalation Trial. Lancet Lond. Engl. 2015, 385 (9967), 517–528. 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Abramson JS; McGree B; Noyes S; Plummer S; Wong C; Chen Y-B; Palmer E; Albertson T; Ferry JA; Arrillaga-Romany IC Anti-CD19 CAR T Cells in CNS Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2017, 377 (8), 783–784. 10.1056/NEJMc1704610. [DOI] [PubMed] [Google Scholar]
- (44).Fong L; Brockstedt D; Benike C; Wu L; Engleman EG Dendritic Cells Injected via Different Routes Induce Immunity in Cancer Patients. J. Immunol. Baltim. Md 1950 2001, 166 (6), 4254–4259. 10.4049/jimmunol.166.6.4254. [DOI] [PubMed] [Google Scholar]
- (45).Karman J; Ling C; Sandor M; Fabry Z Initiation of Immune Responses in Brain Is Promoted by Local Dendritic Cells. J. Immunol. Baltim. Md 1950 2004, 173 (4), 2353–2361. 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- (46).De Vries IJM; Krooshoop DJEB; Scharenborg NM; Lesterhuis WJ; Diepstra JHS; Van Muijen GNP; Strijk SP; Ruers TJ; Boerman OC; Oyen WJG; Adema GJ; Punt CJA; Figdor CG Effective Migration of Antigen-Pulsed Dendritic Cells to Lymph Nodes in Melanoma Patients Is Determined by Their Maturation State. Cancer Res. 2003, 63 (1), 12–17. [PubMed] [Google Scholar]
- (47).Eggert AA; Schreurs MW; Boerman OC; Oyen WJ; de Boer AJ; Punt CJ; Figdor CG; Adema GJ Biodistribution and Vaccine Efficiency of Murine Dendritic Cells Are Dependent on the Route of Administration. Cancer Res. 1999, 59 (14), 3340–3345. [PubMed] [Google Scholar]
- (48).Barratt-Boyes SM; Figdor CG Current Issues in Delivering DCs for Immunotherapy. Cytotherapy 2004, 6 (2), 105–110. 10.1080/14653240410005258. [DOI] [PubMed] [Google Scholar]
- (49).Giuliano AE; Kirgan DM; Guenther JM; Morton DL Lymphatic Mapping and Sentinel Lymphadenectomy for Breast Cancer. Ann. Surg. 1994, 220 (3), 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Hingtgen S; Kasmieh R; Elbayly E; Nesterenko I; Figueiredo J-L; Dash R; Sarkar D; Hall D; Kozakov D; Vajda S; Fisher PB; Shah K A First-Generation Multi-Functional Cytokine for Simultaneous Optical Tracking and Tumor Therapy. PLOS ONE 2012, 7 (7), e40234. 10.1371/journal.pone.0040234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Hingtgen SD; Kasmieh R; van de Water J; Weissleder R; Shah K A Novel Molecule Integrating Therapeutic and Diagnostic Activities Reveals Multiple Aspects of Stem Cell-Based Therapy. Stem Cells Dayt. Ohio 2010, 28 (4), 832–841. 10.1002/stem.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Hingtgen S; Figueiredo J-L; Farrar C; Duebgen M; Martinez-Quintanilla J; Bhere D; Shah K Real-Time Multi-Modality Imaging of Glioblastoma Tumor Resection and Recurrence. J. Neurooncol. 2013, 111 (2), 153–161. 10.1007/s11060-012-1008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Kauer TM; Figueiredo J-L; Hingtgen S; Shah K Encapsulated Therapeutic Stem Cells Implanted in the Tumor Resection Cavity Induce Cell Death in Gliomas. Nat. Neurosci. 2012, 15 (2), 197–204. 10.1038/nn.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Shah K; Hingtgen S; Kasmieh R; Figueiredo JL; Garcia-Garcia E; Martinez-Serrano A; Breakefield X; Weissleder R Bimodal Viral Vectors and in Vivo Imaging Reveal the Fate of Human Neural Stem Cells in Experimental Glioma Model. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28 (17), 4406–4413. 10.1523/JNEUROSCI.0296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Aboody KS; Najbauer J; Metz MZ; D’Apuzzo M; Gutova M; Annala AJ; Synold TW; Couture LA; Blanchard S; Moats RA; Garcia E; Aramburo S; Valenzuela VV; Frank RT; Barish ME; Brown CE; Kim SU; Badie B; Portnow J Neural Stem Cell-Mediated Enzyme-Prodrug Therapy for Glioma: Preclinical Studies. Sci. Transl. Med. 2013, 5 (184). 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Shah K; Bureau E; Kim D-E; Yang K; Tang Y; Weissleder R; Breakefield XO Glioma Therapy and Real-Time Imaging of Neural Precursor Cell Migration and Tumor Regression. Ann. Neurol. 2005, 57 (1), 34–41. 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- (57).Bagó JR; Sheets KT; Hingtgen SD Neural Stem Cell Therapy for Cancer. Methods San Diego Calif 2016, 99, 37–43. 10.1016/j.ymeth.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Barish ME; Herrmann K; Tang Y; Argalian Herculian S; Metz M; Aramburo S; Tirughana R; Gutova M; Annala A; Moats RA; Goldstein L; Rockne RC; Gutierrez J; Brown CE; Ghoda L; Aboody KS Human Neural Stem Cell Biodistribution and Predicted Tumor Coverage by a Diffusible Therapeutic in a Mouse Glioma Model. Stem Cells Transl. Med. 2017, 6 (6), 1522–1532. 10.1002/sctm.16-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Li G; Bonamici N; Dey M; Lesniak MS; Balyasnikova IV Intranasal Delivery of Stem Cell-Based Therapies for the Treatment of Brain Malignancies. Expert Opin. Drug Deliv. 2018, 15 (2), 163–172. 10.1080/17425247.2018.1378642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Lu M-H; Ji W-L; Chen H; Sun Y-Y; Zhao X-Y; Wang F; Shi Y; Hu Y-N; Liu B-X; Wu J-W; Xu D-E; Zheng J-W; Liu C-F; Ma Q-H Intranasal Transplantation of Human Neural Stem Cells Ameliorates Alzheimer’s Disease-Like Pathology in a Mouse Model. Front. Aging Neurosci. 2021, 13, 650103. 10.3389/fnagi.2021.650103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Ii WHF Noninvasive Intranasal Stem Cells Bypass the Blood-Brain Barrier to Target the Brain to Treat Parkinson’s Disease, Stroke, MS, Brain Tumors, Cerebral Ischemia, Alzheimer’s and Other CNS Disorders. J. Nat. Sci. 2015, 1 (1), 23. [Google Scholar]
- (62).Potts MB; Silvestrini MT; Lim DA Devices for Cell Transplantation into the Central Nervous System: Design Considerations and Emerging Technologies. Surg. Neurol. Int. 2013, 4 (Suppl 1), S22–S30. 10.4103/2152-7806.109190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Jahangiri A; Chin AT; Flanigan PM; Chen R; Bankiewicz K; Aghi MK Convection-Enhanced Delivery in Glioblastoma: A Review of Preclinical and Clinical Studies. J. Neurosurg. 2017, 126 (1), 191–200. 10.3171/2016.1.JNS151591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Rosenbluth KH; Martin AJ; Bringas J; Bankiewicz KS Evaluation of Pressure-Driven Brain Infusions in Nonhuman Primates by Intra-Operative 7 Tesla MRI. J. Magn. Reson. Imaging 2012, 36 (6), 1339–1346. 10.1002/jmri.23771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Mehta AM; Sonabend AM; Bruce JN Convection-Enhanced Delivery. Neurotherapeutics 2017, 14 (2), 358–371. 10.1007/s13311-017-0520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Jain RK Transport of Molecules in the Tumor Interstitium: A Review. Cancer Res. 1987, 47 (12), 3039–3051. [PubMed] [Google Scholar]
- (67).Atik AF; Suryadevara CM; Schweller RM; West JL; Healy P; Ii JEH; Congdon KL; Sanchez-Perez L; McLendon RE; Archer GE; Fecci P; Sampson JH Hyaluronic Acid Based Low Viscosity Hydrogel as a Novel Carrier for Convection Enhanced Delivery of CAR T Cells. J. Clin. Neurosci. 2018, 56, 163–168. 10.1016/j.jocn.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Zhang T; Murphy MJ; Yu H; Bagaria HG; Yoon KY; Neilson BM; Bielawski CW; Johnston KP; Huh C; Bryant SL Investigation of Nanoparticle Adsorption During Transport in Porous Media. SPE J. 2015, 20 (04), 667–677. 10.2118/166346-PA. [DOI] [Google Scholar]
- (69).Morrison PF; Chen MY; Chadwick RS; Lonser RR; Oldfield EH Focal Delivery during Direct Infusion to Brain: Role of Flow Rate, Catheter Diameter, and Tissue Mechanics. Am. J. Physiol. 1999, 277 (4), R1218–1229. 10.1152/ajpregu.1999.277.4.R1218. [DOI] [PubMed] [Google Scholar]
- (70).Elenes EY; Mehta JN; Hsu F-C; Whitlow CT; Debinski W; Rossmeisl J; Tatter S; Rylander CG Convection-Enhanced Arborizing Catheter System Improves Local/Regional Delivery of Infusates Versus a Single-Port Catheter in Ex Vivo Porcine Brain Tissue. J. Eng. Sci. Med. Diagn. Ther. 2020, 4 (011003). 10.1115/1.4048935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Silvestrini MT; Yin D; Coppes VG; Mann P; Martin AJ; Larson PS; Starr PA; Gupta N; Panter SS; Desai TA; Lim DA Radially Branched Deployment for More Efficient Cell Transplantation at the Scale of the Human Brain. Stereotact. Funct. Neurosurg. 2013, 91 (2), 92–103. 10.1159/000343213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Raghavan R; Odland RM Theory of Porous Catheters and Their Applications in Intraparenchymal Infusions. Biomed. Phys. Eng. Express 2017, 3 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Brady M; Raghavan R; Sampson J Determinants of Intraparenchymal Infusion Distributions: Modeling and Analyses of Human Glioblastoma Trials. Pharmaceutics 2020, 12 (9), 895. 10.3390/pharmaceutics12090895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Raghavan R; Brady M Predictive Models for Pressure-Driven Fluid Infusions into Brain Parenchyma. Phys. Med. Biol. 2011, 56 (19), 6179–6204. 10.1088/0031-9155/56/19/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Woodall RT; Ii DAH; Abdelmalik MRA; Wu C; Feng X; Phillips WT; Bao A; Hughes TJR; Brenner AJ; Yankeelov TE Integrating Quantitative Imaging and Computational Modeling to Predict the Spatiotemporal Distribution of 186Re Nanoliposomes for Recurrent Glioblastoma Treatment. In Medical Imaging 2019: Physics of Medical Imaging; International Society for Optics and Photonics, 2019; Vol. 10948, p 109483M. 10.1117/12.2512867. [DOI] [Google Scholar]
- (76).Zhan W; Wang C-H Convection Enhanced Delivery of Chemotherapeutic Drugs into Brain Tumour. J. Controlled Release 2018, 271, 74–87. 10.1016/j.jconrel.2017.12.020. [DOI] [PubMed] [Google Scholar]
- (77).Vendel E; Rottschäfer V; de Lange ECM The Need for Mathematical Modelling of Spatial Drug Distribution within the Brain. Fluids Barriers CNS 2019, 16 (1), 12. 10.1186/s12987-019-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Yu J; Berlin JM; Lu W; Zhang L; Kan AT; Zhang P; Walsh EE; Work SN; Chen W; Tour JM; Wong MS; Tomson MB Transport Study of Nanoparticles for Oilfield Application; OnePetro, 2010. 10.2118/131158-MS. [DOI] [Google Scholar]
- (79).Sahoo P; Yang X; Abler D; Maestrini D; Adhikarla V; Frankhouser D; Cho H; Machuca V; Wang D; Barish M; Gutova M; Branciamore S; Brown CE; Rockne RC Mathematical Deconvolution of CAR T-Cell Proliferation and Exhaustion from Real-Time Killing Assay Data. J. R. Soc. Interface 2020, 17 (162), 20190734. 10.1098/rsif.2019.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Kingsmore KM; Vaccari A; Abler D; Cui SX; Epstein FH; Rockne RC; Acton ST; Munson JM MRI Analysis to Map Interstitial Flow in the Brain Tumor Microenvironment. APL Bioeng. 2018, 2 (3), 031905. 10.1063/1.5023503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Stine CA; Munson JM Convection-Enhanced Delivery: Connection to and Impact of Interstitial Fluid Flow. Front. Oncol. 2019, 9. 10.3389/fonc.2019.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Zhan W; Baena F. R. y; Dini D Effect of Tissue Permeability and Drug Diffusion Anisotropy on Convection-Enhanced Delivery. Drug Deliv. 2019, 26 (1), 773–781. 10.1080/10717544.2019.1639844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Chatterjee K; Atay N; Abler D; Bhargava S; Sahoo P; Rockne RC; Munson JM Utilizing Dynamic Contrast-Enhanced Magnetic Resonance Imaging (DCE-MRI) to Analyze Interstitial Fluid Flow and Transport in Glioblastoma and the Surrounding Parenchyma in Human Patients. Pharmaceutics 2021, 13 (2). 10.3390/pharmaceutics13020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Jarrett AM; Faghihi D; Ii DAH; Lima EABF; Virostko J; Biros G; Patt D; Yankeelov TE Optimal Control Theory for Personalized Therapeutic Regimens in Oncology: Background, History, Challenges, and Opportunities. J. Clin. Med. 2020, 9 (5). 10.3390/jcm9051314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Lenhart S; Workman JT Optimal Control Applied to Biological Models; CRC Press, 2007. [Google Scholar]
- (86).Modelling Optimization and Control of Biomedical Systems; Pistikopoulos, E. N., Nascu, I., Velliou, E. G., Eds.; 2017.
- (87).Burgess A; Hynynen K Drug Delivery across the Blood-Brain Barrier Using Focused Ultrasound. Expert Opin. Drug Deliv. 2014, 11 (5), 711–721. 10.1517/17425247.2014.897693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Alkins R; Burgess A; Ganguly M; Francia G; Kerbel R; Wels WS; Hynynen K Focused Ultrasound Delivers Targeted Immune Cells to Metastatic Brain Tumors. Cancer Res. 2013, 73 (6), 1892–1899. 10.1158/0008-5472.CAN-12-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Alkins R; Burgess A; Kerbel R; Wels WS; Hynynen K Early Treatment of HER2-Amplified Brain Tumors with Targeted NK-92 Cells and Focused Ultrasound Improves Survival. Neuro-Oncol. 2016, 18 (7), 974–981. 10.1093/neuonc/nov318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Burgess A; Ayala-Grosso CA; Ganguly M; Jordão JF; Aubert I; Hynynen K Targeted Delivery of Neural Stem Cells to the Brain Using MRI-Guided Focused Ultrasound to Disrupt the Blood-Brain Barrier. PLOS ONE 2011, 6 (11), e27877. 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Shen W-B; Anastasiadis P; Nguyen B; Yarnell D; Yarowsky PJ; Frenkel V; Fishman PS Magnetic Enhancement of Stem Cell-Targeted Delivery into the Brain Following MR-Guided Focused Ultrasound for Opening the Blood-Brain Barrier. Cell Transplant. 2017, 26 (7), 1235–1246. 10.1177/0963689717715824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Burgess A; Hynynen K Drug Delivery across the Blood–Brain Barrier Using Focused Ultrasound. Expert Opin. Drug Deliv. 2014, 11 (5), 711–721. 10.1517/17425247.2014.897693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Deng Z; Sheng Z; Yan F Ultrasound-Induced Blood-Brain-Barrier Opening Enhances Anticancer Efficacy in the Treatment of Glioblastoma: Current Status and Future Prospects. J. Oncol. 2019, 2019, e2345203. 10.1155/2019/2345203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Punganuru SR; Arutla V; Zhao W; Rajaei M; Deokar H; Zhang R; Buolamwini JK; Srivenugopal KS; Wang W Targeted Brain Tumor Therapy by Inhibiting the MDM2 Oncogene: In Vitro and In Vivo Antitumor Activity and Mechanism of Action. Cells 2020, 9 (7), 1592. 10.3390/cells9071592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Wang D; Starr R; Chang W-C; Aguilar B; Alizadeh D; Wright SL; Yang X; Brito A; Sarkissian A; Ostberg JR; Li L; Shi Y; Gutova M; Aboody K; Badie B; Forman SJ; Barish ME; Brown CE Chlorotoxin-Directed CAR T Cells for Specific and Effective Targeting of Glioblastoma. Sci. Transl. Med. 2020, 12 (533). 10.1126/scitranslmed.aaw2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Nonaka M; Suzuki-Anekoji M; Nakayama J; Mabashi-Asazuma H; Jarvis DL; Yeh J-C; Yamasaki K; Akama TO; Huang C-T; Campos AR; Nagaoka M; Sasai T; Kimura-Takagi I; Suwa Y; Yaegashi T; Shibata TK; Sugihara K; Nishizawa-Harada C; Fukuda M; Fukuda MN Overcoming the Blood–Brain Barrier by Annexin A1-Binding Peptide to Target Brain Tumours. Br. J. Cancer 2020, 123 (11), 1633–1643. 10.1038/s41416-020-01066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Suryadevara CM; Desai R; Abel ML; Riccione KA; Batich KA; Shen SH; Chongsathidkiet P; Gedeon PC; Elsamadicy AA; Snyder DJ; Herndon JE; Healy P; Archer GE; Choi BD; Fecci PE; Sampson JH; Sanchez-Perez L Temozolomide Lymphodepletion Enhances CAR Abundance and Correlates with Antitumor Efficacy against Established Glioblastoma. Oncoimmunology 2018, 7 (6), e1434464. 10.1080/2162402X.2018.1434464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Xie G; Dong H; Liang Y; Ham JD; Rizwan R; Chen J CAR-NK Cells: A Promising Cellular Immunotherapy for Cancer. EBioMedicine 2020, 59. 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).PersonGen BioTherapeutics (Suzhou) Co., Ltd. Clinical Investigation of Chimeric CD(Cluster of Differentiation)33 Antigen Receptor-Modified NK92 Cells in Relapsed and/or Refractory Acute Myeloid Leukemias; Clinical trial registration; NCT02944162; clinicaltrials.gov, 2016. [Google Scholar]
- (100).Tang X; Yang L; Li Z; Nalin AP; Dai H; Xu T; Yin J; You F; Zhu M; Shen W; Chen G; Zhu X; Wu D; Yu J First-in-Man Clinical Trial of CAR NK-92 Cells: Safety Test of CD33-CAR NK-92 Cells in Patients with Relapsed and Refractory Acute Myeloid Leukemia. Am. J. Cancer Res. 2018, 8 (6), 1083–1089. [PMC free article] [PubMed] [Google Scholar]
- (101).The Third Affiliated Hospital of Guangzhou Medical University. Pilot Study of NKG2D-Ligand Targeted CAR-NK Cells in Patients With Metastatic Solid Tumours; Clinical trial registration; NCT03415100; clinicaltrials.gov, 2018. [Google Scholar]
- (102).Xiao L; Cen D; Gan H; Sun Y; Huang N; Xiong H; Jin Q; Su L; Liu X; Wang K; Yan G; Dong T; Wu S; Zhou P; Zhang J; Liang W; Ren J; Teng Y; Chen C; Xu XH Adoptive Transfer of NKG2D CAR MRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27 (6), 1114–1125. 10.1016/j.ymthe.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).M.D. Anderson Cancer Center. Dose Escalation Study Phase I/II of Umbilical Cord Blood-Derived CAR-Engineered NK Cells in Conjunction With Lymphodepleting Chemotherapy in Patients With Relapsed/Refractory B-Lymphoid Malignancies; Clinical trial registration; NCT03056339; clinicaltrials.gov, 2020. [Google Scholar]
- (104).Liu E; Marin D; Banerjee P; Macapinlac HA; Thompson P; Basar R; Nassif Kerbauy L; Overman B; Thall P; Kaplan M; Nandivada V; Kaur I; Nunez Cortes A; Cao K; Daher M; Hosing C; Cohen EN; Kebriaei P; Mehta R; Neelapu S; Nieto Y; Wang M; Wierda W; Keating M; Champlin R; Shpall EJ; Rezvani K Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382 (6), 545–553. 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).City of Hope Medical Center. A Pilot Feasibility Study of Oral 5-Fluorocytosine and Genetically-Modified Neural Stem Cells Expressing E.Coli Cytosine Deaminase for Treatment of Recurrent High Grade Gliomas; Clinical trial registration study/NCT01172964; clinicaltrials.gov, 2017. [Google Scholar]
- (106).Lesniak M Neural Stem Cell Oncolytic Adenoviral Virotherapy of Newly Diagnosed Malignant Glioma; Clinical trial registration; NCT03072134; clinicaltrials.gov, 2020. [Google Scholar]
- (107).ReNeuron Limited. A Randomized, Placebo-Controlled Study of the Efficacy and Safety of Intracerebral Stem Cells (CTX0E03) in Subjects With Disability Following an Ischemic Stroke; Clinical trial registration; NCT03629275; clinicaltrials.gov, 2020. [Google Scholar]
- (108).Safe Save Medical Cell Sciences & Technology Co.,Ltd. Autologous Dendritic Cell / Tumor Antigen (ADCTA-SSI-G1) for Adjuvant Immunotherapy in Standard Treatment of Recurrent Glioblastoma Multiforme (GBM): A Multi-Center, Open-Label, Randomized Phase III Clinical Trial; Clinical trial registration; NCT04277221; clinicaltrials.gov, 2020. [Google Scholar]
- (109).Vik-Mo E Open Label Randomized Phase II/III Trial of Dendritic Cell Immunotherapy Against Cancer Stem Cells in Glioblastoma Patients Receiving Standard Therapy (DEN-STEM); Clinical trial registration; NCT03548571; clinicaltrials.gov, 2019. [Google Scholar]
- (110).Plosker GL Sipuleucel-T: In Metastatic Castration-Resistant Prostate Cancer. Drugs 2011, 71 (1), 101–108. 10.2165/11206840-000000000-00000. [DOI] [PubMed] [Google Scholar]
- (111).Laus R; Ruegg CL; Wu H Immunostimulatory Composition. US6210662B1, April 3, 2001.
- (112).Richwine L UPDATE 3-U.S. FDA OKs Dendreon’s Prostate Cancer Vaccine. Reuters. April 29, 2010.
- (113).Research, C. for B. E. and. PROVENGE (Sipuleucel-T). FDA; 2019. [Google Scholar]
- (114).Research, C. for B. E. and. Approved Products - PROVENGE (sipuleucel-T) https://wayback.archive-it.org/7993/20170722071307/https://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm210012.htm (accessed 2021 −06 −08).
- (115).Drugs and Biologics Compendium https://www.nccn.org/compendia-templates/compendia/drugs-and-biologics-compendia (accessed 2021 −06 −08).
- (116).Kantoff PW; Higano CS; Shore ND; Berger ER; Small EJ; Penson DF; Redfern CH; Ferrari AC; Dreicer R; Sims RB; Xu Y; Frohlich MW; Schellhammer PF; IMPACT Study Investigators. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363 (5), 411–422. 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- (117).Small EJ; Schellhammer PF; Higano CS; Redfern CH; Nemunaitis JJ; Valone FH; Verjee SS; Jones LA; Hershberg RM Placebo-Controlled Phase III Trial of Immunologic Therapy with Sipuleucel-T (APC8015) in Patients with Metastatic, Asymptomatic Hormone Refractory Prostate Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24 (19), 3089–3094. 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- (118).PS1 Late Breaking Immunotherapy (APC8015) for Androgen Independent Prostate Cancer (AIPC): Final Progression and Survival Data from a Second Phase 3 Trial. Eur. J. Cancer Suppl. 2005, 3 (4), 1. 10.1016/S1359-6349(05)82004-X. [DOI] [Google Scholar]
- (119).New treatment options for patients with prostate cancer http://www.eurekalert.org/pub_releases/2005-11/foec-nto110205.php (accessed 2021 −06 −08).
- (120).Dendreon. Autologous PAP-Loaded Dendritic Cell Vaccine (Sipuleucel-T, APC8015, Provenge®) in Patients With Non-Metastatic Prostate Cancer Who Experience PSA Elevation Following Radical Prostatectomy: A Randomized, Controlled, Double-Blind Trial; Clinical trial registration; NCT00779402; clinicaltrials.gov, 2017. [Google Scholar]
- (121).Prostate Oncology Specialists, Inc. Phase 1 Study of Sipuleucel-T and Ipilimumab in Combination for Advanced Prostate Cancer; Clinical trial registration; NCT01832870; clinicaltrials.gov, 2017. [Google Scholar]