Abstract
Spectinomycin resistance in clinical isolates of Neisseria meningitidis and Neisseria gonorrhoeae was found to be due to mutations G1064C and C1192U (Escherichia coli numbering) in 16S rRNA genes, respectively.
Among Neisseria species, only N. meningitidis and N. gonorrhoeae are considered primary pathogens (8). Strains of N. gonorrhoeae are always pathogenic, whereas strains of N. meningitidis, in addition to causing acute meningitis and septicemia, can also colonize the oro- and nasopharynx of a healthy carrier. Development of drug resistance in N. gonorrhoeae has led to concern about the almost inevitable increase of resistance in N. meningitidis (8). Cephalosporins and fluoroquinolones are the two classes of antibiotics recommended for primary therapy (8). Resistance to alternative therapy such as chloramphenicol for meningococcal infections (5) or spectinomycin for gonococcal infections (1) has been reported.
In gram-positive bacteria, resistance to spectinomycin, although much less common than resistance to other aminoglycoside-aminocyclitol antibiotics, is usually due to production of an aminoglycoside 9-O-nucleotidyltransferase of type I (9, 12). Recently, a spectinomycin phosphotransferase has been reported for the gram-negative Legionella pneumophila (16). In Escherichia coli, spectinomycin resistance has been shown to be due to mutations in helix 34 of 16S rRNA (15). This helix consists of an upper and a lower stem separated by an internal loop containing two uracil residues.
We report mutations responsible for spectinomycin resistance in 16S rRNA genes of clinical isolates of N. meningitidis and N. gonorrhoeae.
Since 1988, surveillance of antibiotic resistance in clinical isolates of Neisseria spp. has been conducted at the National Center for Meningococci, Institut Pasteur, Paris, France, by disk-agar diffusion. Out of more than 16,800 clinical isolates, a single N. meningitidis isolate, LNP16311, and four N. gonorrhoeae isolates were found to be resistant to spectinomycin. The five strains of Neisseria spp. remained susceptible to penicillins, cephalosporins, tetracyclines, macrolides, rifamycins, and quinolones. Among the three N. gonorrhoeae strains isolated from urethritis patients in Gabon (LNP8205, Libreville, May 1989; LNP8919 and LNP8920, Franceville, March 1990), only strain LNP8205, as well as strain LNP9455, isolated from a urethritis patient in November 1990 at Saint Louis Hospital in Paris, was studied further.
N. meningitidis LNP16311, serogroup Y, was isolated in 1998 from the rhinopharynx of a 71-year-old male in Macon, France. A spectinomycin-resistant transformant, N. meningitidis BM4417, obtained after transformation of strain BM4377 (5, 13) with total DNA from LNP16311, was included in the study. Spectinomycin-susceptible N. meningitidis LNP15908 and BM4377 and N. gonorrhoeae LNP6910 were used as controls.
Total DNA from N. meningitidis LNP16311 and BM4417 and from N. gonorrhoeae LNP8205 and LNP9455 was transferred to nitrocellulose sheets (Nytran; Schleicher & Schuell, Dassel, Germany) and hybridized to 32P-labeled ant(9)-I- and aph-specific probes (Radiochemical Centre, Amersham, England). Lack of hybridization suggested that spectinomycin resistance in these strains was not due to acquisition of known genes.
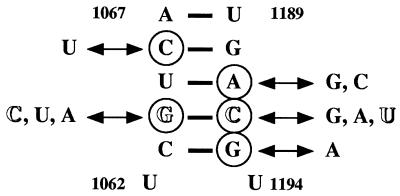
In structural models of E. coli 16S rRNA, spectinomycin resistance mutations are located in the upper stem of helix 34 (Fig. 1) (7), which is formed by base pairing of regions 1046 to 1067 and 1189 to 1211 (E. coli numbering [2, 3]). Comparison of the sequence of 16S rRNA rrs genes from E. coli (GenBank accession no. V00348) to those of N. meningitidis (Sanger Centre; http://www.sanger.ac.uk/) indicated 79% identity with contig 407.
FIG. 1.
Secondary structure of the upper stem of 16S rRNA helix 34 of E. coli (2). Transversion G1064C and transition C1192U (E. coli numbering) in spectinomycin-resistant N. meningitidis and N. gonorrhoeae, respectively, are indicated by open letters. The locations of other spectinomycin resistance single mutations, A1191G,C and G1193A in Chlamydomonas reinhardtii (6), A1191C in N. tabacum chloroplast (17), G1193A in Nicotiana undulata chloroplast (4), and G1064U,C,A in E. coli (3), and of double mutations G1064U-C1192A, G1064C-C1192G, and G1064A-C1192U in E. coli (3) are indicated by circles.
A region internal to rrs genes, including helix 34, of spectinomycin-susceptible and -resistant N. meningitidis and N. gonorrhoeae was explored by PCR using a DNA Thermal Cycler (model 2400; Perkin-Elmer Cetus, Norwalk, Conn.) and total DNA as a template. Two heptadecadeoxynucleotides (F.980, 5′CTTACCTGGTCTTGACA3′, and R.1353, 5′CGATTACTAGCGATTCC3′; E. coli numbering) designed from contig 407 and synthesized by the methoxy-phosphoramidite method (Unité de Chimie Organique, Institut Pasteur) allowed amplification of fragments with the predicted size (data not shown).
Double-stranded sequencing of the 373-bp PCR products was performed by the dideoxynucleotide chain termination method (14) with a T7 Sequenase PCR product sequencing kit (Amersham, Little Chalfont, Buckinghamshire, England), the 17-mer primers, and α-35S-dATP (Amersham). The sequence of helix 34 (positions 1046 to 1067 and 1189 to 1211, E. coli numbering) from Neisseria strains was identical to that of E. coli except at position 1201, located in the lower stem of helix 34, where a transversion converted the adenine present in E. coli to a cytosine in Neisseria spp.
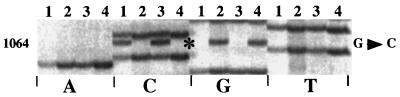
The sequence of helix 34 in the spectinomycin-resistant N. meningitidis clinical isolate LNP16311 differed from that of susceptible strain LNP15908 by a guanine-to-cytosine transversion at position 1064 (E. coli numbering [Fig. 1]). An identical substitution was found in the spectinomycin-resistant transformant BM4417 relative to BM4377. In both cases, the sequence of the amplification product obtained directly without cloning did not display any ambiguity (Fig. 2). These data indicate that, like in LNP16311, each of the three rrs genes (10) in N. meningitidis BM4377 has been altered, an observation compatible with the small number of rrn operons in this species. Mutations at this position conferring spectinomycin resistance have been described for E. coli (G1064U,C,A [3]) and Nicotiana tabacum chloroplast (G1064A [4]).
FIG. 2.
Sequence of the region corresponding to E. coli positions 1062 to 1067 of the PCR-amplified rRNA genes of N. meningitidis strains. Lanes 1, spectinomycin-resistant LNP16311; lanes 2, spectinomycin-susceptible LNP15908; lanes 3, spectinomycin-resistant transformant BM4417; lanes 4, spectinomycin-susceptible BM4377. The mutated position is indicated by an asterisk.
Spectinomycin-resistant N. gonorrhoeae LNP8205 differed from susceptible LNP6910 by a cytosine-to-thymine transition at position 1192 (E. coli numbering [Fig. 1 and data not shown]). Similar mutations conferring spectinomycin resistance have been described for E. coli (C1192U [15] and C1192U,G,A [11]) and N. tabacum chloroplast (C1192U [17]).
In conclusion, spectinomycin resistance in N. meningitidis and N. gonorrhoeae was due to mutations already found in the 16S rRNA genes. Spectinomycin alone is used in infections due to N. gonorrhoeae, in particular in the case of a high prevalence of β-lactamase-producing strains (1), and is likely to be responsible for emergence of resistance. The reason for the mutation in N. meningitidis, against which spectinomycin is not used, remains unknown.
Acknowledgments
We thank J. M. Alonso and M. Guibourdenche for the gift of strains.
This work was supported in part by a Bristol-Myers Squibb Unrestricted Biomedical Research Grant in Infectious Diseases.
REFERENCES
- 1.Boslego J W, Tramont E C, Takafuji E T, Diniega B M, Mitchell B S, Small J W, Khan W N, Stein D C. Effect of spectinomycin use on the prevalence of spectinomycin-resistant and penicillinase-producing Neisseria gonorrhoeae. N Engl J Med. 1987;317:272–278. doi: 10.1056/NEJM198707303170504. [DOI] [PubMed] [Google Scholar]
- 2.Brimacombe R, Atmadja J, Stiege W, Schuler D. A detailed model of the three-dimensional structure of Escherichia coli 16S ribosomal RNA in situ in the 30S subunit. J Mol Biol. 1988;199:115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- 3.Brink M F, Brink G, Verbeet M P, de Boer H A. Spectinomycin interacts specifically with the residues G1064 and C1192 in 16S rRNA, thereby potentially freezing this molecule into an inactive conformation. Nucleic Acids Res. 1994;22:325–331. doi: 10.1093/nar/22.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fromm H, Edelman M, Aviv D, Galun E. The molecular basis for rDNA-dependent spectinomycin resistance in Nicotiana chloroplasts. EMBO J. 1987;6:3233–3237. doi: 10.1002/j.1460-2075.1987.tb02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galimand M, Gerbaud G, Guibourdenche M, Riou J-Y, Courvalin P. High-level chloramphenicol resistance in Neisseria meningitidis. N Engl J Med. 1998;339:868–874. doi: 10.1056/NEJM199809243391302. [DOI] [PubMed] [Google Scholar]
- 6.Harris E H, Burkhart B D, Gillham N W, Boynton J E. Antibiotic resistance mutations in the chloroplast 16S and 23S rRNA genes of Chlamydomonas reinhardtii: correlation of genetic and physical maps of the chloroplast genome. Genetics. 1989;123:281–292. doi: 10.1093/genetics/123.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johanson U, Hughes D. A new mutation in 16S rRNA of Escherichia coli conferring spectinomycin resistance. Nucleic Acids Res. 1995;23:464–466. doi: 10.1093/nar/23.3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knapp J S, Rice R J. Neisseria and Branhamella. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 324–340. [Google Scholar]
- 9.LeBlanc D J, Lee L N, Inamine J M. Cloning and nucleotide base sequence analysis of a spectinomycin adenyltransferase AAD(9) determinant from Enterococcus faecalis. Antimicrob Agents Chemother. 1991;35:1804–1810. doi: 10.1128/aac.35.9.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S-L, Hessel A, Sanderson K E. Genomic mapping with I-Ceu I, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc Natl Acad Sci USA. 1993;90:6874–6878. doi: 10.1073/pnas.90.14.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makosky P C, Dahlberg A E. Spectinomycin resistance at site 1192 in 16S ribosomal RNA of E. coli: an analysis of three mutants. Biochimie. 1987;69:885–889. doi: 10.1016/0300-9084(87)90216-1. [DOI] [PubMed] [Google Scholar]
- 12.Murphy E. Nucleotide sequence of a spectinomycin adenyltransferase AAD(9) determinant from Staphylococcus aureus and its relationship to AAD(3")(9) Mol Gen Genet. 1985;200:33–39. doi: 10.1007/BF00383309. [DOI] [PubMed] [Google Scholar]
- 13.Nassif X, Puaoi D, So M. Transposition of Tn1545-Δ3 in the pathogenic neisseriae: a genetic tool for mutagenesis. J Bacteriol. 1991;173:2147–2154. doi: 10.1128/jb.173.7.2147-2154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigmund C D, Ettayebi M, Morgan E A. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1984;12:4653–4663. doi: 10.1093/nar/12.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suter T M, Viswanathan V K, Cianciotto N P. Isolation of a gene encoding a novel spectinomycin phosphotransferase from Legionella pneumophila. Antimicrob Agents Chemother. 1997;41:1385–1388. doi: 10.1128/aac.41.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svab Z, Maliga P. Mutation proximal to the tRNA binding region of the Nicotiana plastid 16S rRNA confers resistance to spectinomycin. Mol Gen Genet. 1991;228:316–319. doi: 10.1007/BF00282483. [DOI] [PubMed] [Google Scholar]