Abstract
Purpose
To describe two cases of Acanthamoeba keratitis diagnosed and treated at the epithelial stage of disease and to underscore the importance of early diagnosis on prognosis.
Observations
Case 1 is a 28-year-old male who developed Acanthamoeba keratitis after prolonged contact lens wear. Case 2 is a 43-year-old male with poor contact lens hygiene who was initially misdiagnosed and treated for herpetic keratitis. Both cases presented with epitheliopathy and were successfully treated with corneal epithelial debridement and topical anti-amoebic therapy, with complete avoidance of deeper extension of infection and associated complications.
Conclusion and importance
Epithelial stage Acanthamoeba keratitis represents a critical window of opportunity to achieve rapid cure. Acanthamoeba epitheliopathy may be mistaken for other conditions such as herpetic keratitis, contact lens overwear, or dry eye. Given worsening prognosis following delayed diagnosis, it is important for clinicians to be suspicious of Acanthamoeba keratitis in all contact lens wearers who develop elevated epitheliopathy.
Keywords: Acanthamoeba keratitis, Contact lenses
1. Introduction
Acanthamoeba species are ubiquitous single-celled protozoans found in soil, dust, air, and water. Acanthamoeba keratitis (AK) is a severe infection of the cornea that is strongly associated with contact lens wear and poor contact lens hygiene practices.1 An estimated 85% of AK cases in the United States occur in contact lens users, with an incidence of 1–33 cases per million contact lens wearers.2 The incidence of AK is increasing in the United States and the United Kingdom and is also expected to increase globally in the coming years due to widespread use of contact lenses. Multiple retrospective case series have shown that early diagnosis of AK is associated with improved visual outcomes.3 Delayed diagnosis and deeper parasite invasion within the cornea are associated with worse outcomes including prolonged clinical course, severe vision loss, need for keratoplasty, and even enucleation.3 When the parasite is located within the corneal epithelium but has yet to invade deeper stroma, complete epithelial debridement can remove nearly all viable microbes, increase the likelihood of rapid microbiologic cure, and prevent severe sight-threatening complications.4 Here we present two cases of bilateral epithelial AK that were cured with epithelial debridement and topical anti-amoebic therapy.
1.1. Case 1
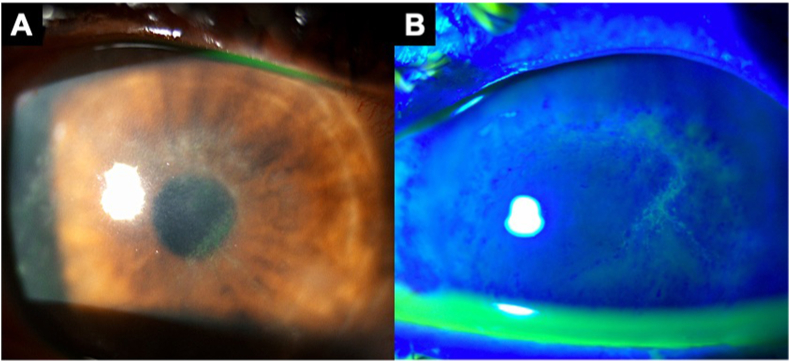
A 28-year-old man with no notable ocular history presented with 2 weeks of gradually worsening redness, blurred vision, and photophobia in the left eye. The patient had a history of bi-weekly soft contact lens (SCL) wear, but was often unsure how long he continued wearing old SCLs. Two weeks prior to presentation, the patient began experiencing eye irritation and redness after staying awake wearing SCLs for over 24 hours. He stopped wearing SCLs after symptom onset, but symptoms persisted. Seven days prior to referral to our institution, the patient had been seen by an optometrist who prescribed him moxifloxacin 0.5% every 2 hours and tobramycin 0.3% every 12 hours in the left eye which did not improve symptoms. On presentation, the patient had spectacle-corrected distance Snellen visual acuity of 20/20 in the right eye and 20/50 in the left eye, with intraocular pressure of 10 mmHg in the right eye and 23 mmHg in the left eye. Slit lamp examination of the right cornea was notable for flat, <0.1mm diameter oval sub-epithelial opacities in the mid-peripheral cornea. Slit lamp examination of the left eye was notable for 2+ conjunctival injection, ciliary flush with tortuous vessels temporally, and multiple curvilinear arrangements of elevated, irregular corneal epithelium. These epithelial lesions had adjacent areas of positive and negative staining (Fig. 1). The left cornea also had multiple <0.1mm diameter oval-shaped flat foci of subepithelial opacification. Neither eye had keratoneuritis, deeper stromal infiltrates, or a ring infiltrate. Corneal epithelial scrapings from each eye were separately sent for bacterial culture, fungal culture, and Acanthamoeba culture consisting of non-nutrient agar with E. coli overlay. Bacterial and fungal cultures did not show growth of viable organisms by 1 week. Acanthamoeba culture was positive at 2 days in the left eye and negative in the right eye. The patient was diagnosed with early stage AK in the left eye. Though there was no definitive microbiological confirmation of AK in the right eye, the patient underwent empiric therapy for AK bilaterally based on strong clinical suspicion. Total corneal epithelial debridement was performed in both eyes, and treatment with chlorhexidine 0.02% every 1 hour in both eyes was initiated. Chlorhexidine 0.02% was stopped after 5 days and the patient was started on polyhexamethylene biguanide (PHMB) 12.5mg/0.1mL every 1 hour which was tapered over several weeks. The patient's corneal epithelium had fully healed by 3 days post-debridement. His subepithelial infiltrates had fully resolved by 5 days post-debridement. At the last date of follow-up 3 months after presentation, his spectacle-corrected visual acuity was 20/20 in each eye with no sign of recurrence.
Fig. 1.
A 28-year-old male with a history of soft contact lens overwear presented with 2 weeks of progressive redness, irritation, and photophobia in the left eye. Symptoms had not improved with topical antibiotics. Slit lamp examination showed diffuse multifocal epitheliopathy with elevated granular epithelial changes with negative fluorescein staining as well as adjacent areas of positive fluorescein staining (A,B). The patient underwent immediate total epithelial debridement. Epithelial scrapings in the left eye confirmed diagnosis of Acanthamoeba keratitis. The patient made complete visual recovery after several weeks of anti-amoebic therapy.
1.2. Case 2
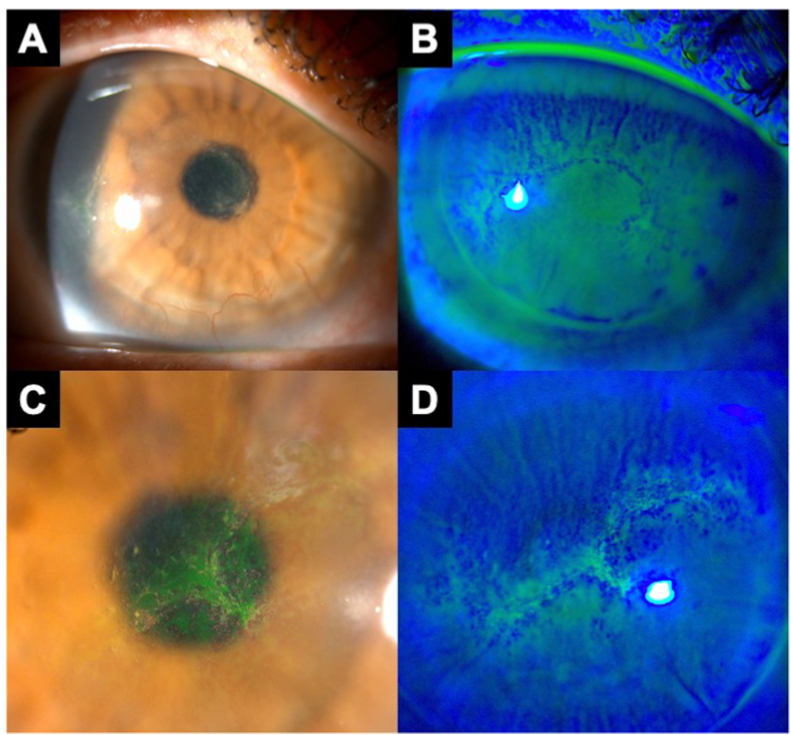
A 43-year-old man with a history of keratectasia following laser-assisted in situ keratomileusis (LASIK) presented with 1 week of itching, surface irritation, and mild photophobia in both eyes that had not improved with over-the-counter tetryzoline. He had a history of rigid gas permeable lens wear with poor hygiene, including showering in his RGPs and storing lenses in the same contact lens solution for several weeks. On presentation, the patient had pinhole visual acuity of 20/100 in the right eye and 20/150 in the left eye. Slit lamp examination was notable for well-positioned LASIK flaps, 2+ conjunctival papillae, and 3+ punctate epithelial erosions in both eyes. We also noted inferior corneal neovascularization in the right eye. The patient was diagnosed with contact lens overwear but was also advised about the possibility of early contact lens-related infection. He was advised to stop RGP wear, use frequent lubrication, start ketotifen 0.025%, and return for evaluation in 3 days. The patient was then lost to follow-up for 2 weeks. During this time, he resumed RGP wear and did not experience improvements in symptoms. He saw an optometrist who diagnosed him with herpetic keratitis and started him on oral valaciclovir and ganciclovir gel. A few days later, the patient was started on besifloxacin 0.6% and loteprednol etabonate 0.5% in both eyes. He returned to our institution for evaluation after experiencing worsening pain. Repeat slit lamp examination revealed diffuse non-confluent granular pseudodendritiform lesions with intervening normal epithelium in both eyes (Fig. 2). He did not have stromal infiltrates, keratoneuritis, corneal edema, or keratic precipitates. The patient was diagnosed with epithelial AK, underwent corneal culture and epithelial debridement in both eyes, and began treatment with chlorhexidine 0.02% every 1 hour in addition to continuing besifloxacin 0.6% every 1 hour in both eyes. Loteprednol etabonate 0.5% and ganciclovir gel were stopped. Corneal epithelium scrapings from each eye were sent for Acanthamoeba culture and were both positive. Both eyes' RGP storage cases were separately submitted for Acanthamoeba culture and were each positive. The patient was continued on chlorhexidine 0.02% which was slowly tapered and stopped after 12 weeks. His corneal epithelium had fully healed in both eyes by 5 days post-debridement with no additional lesions compared to his baseline examination. At the last date of follow-up 7 months after presentation, his spectacle-corrected visual acuity was 20/30 in the right eye and 20/70 in the left eye.
Fig. 2.
A 43-year-old male with a history of rigid gas permeable lens use and poor contact lens hygiene presented with 1 week of itching, foreign body sensation, and mild photophobia in both eyes. After being lost to follow-up for 2 weeks and taking topical ganciclovir and loteprednol, he returned with worsening of symptoms. Slit lamp examination of the right eye showed elevated epithelial changes with negative fluorescein staining in a curvilinear configuration over a large area of the cornea (A,B). Slit lamp examination of the left eye showed similar findings, with adjacent areas of positive and negative fluorescein staining (C,D). The cornea had no keratoneuritis, deeper stromal infiltrates, or ring infiltrate. The patient underwent bilateral epithelial debridement, and both scrapings sent for Acanthamoeba culture were positive. The patient recovered baseline visual acuity after 12 weeks of anti-amoebic therapy.
2. Discussion
Acanthamoeba sp. exists in two forms: an active trophozoite form that contributes to active disease, and a dormant cystic form that can cause persistent infection.1,5 Acanthamoeba adhesion to the ocular surface is the first step in infection and is controlled by a number of adhesion proteins and cell surface molecules.5 Contact lens wear is the most common risk factor for the development of AK.5, 6, 7, 8 Swimming in contact lenses and inadequate or irregular disinfection of contact lenses can increase the risk of developing AK, but patients who practice proper contact lens hygiene may still contract AK.5 Contact lens wear can cause microtrauma to the epithelium and upregulate glycoproteins which serve as binding sites for trophozoites. Bound trophozoites then release cytopathic factors that cause epithelial destruction and allow for stromal invasion.1,5 AK behaves differently than bacteria or fungi in that it can sometimes linger in the epithelium or ocular surface before invading deeper. Therefore, catching AK at this early stage represents a critical opportunity to prevent severe worsening.
Epithelial AK is often mistaken for herpetic epithelial keratitis, contact lens overwear, or dry eye due to the similarity of slit lamp examination findings, but subtle clues can help in its diagnosis.8 Epithelial AK is often described using the term “pseudodendrites,” a non-specific label used to describe the variable elevated epithelial changes that can occur in AK and other conditions such as herpetic keratitis and contact lens overwear. Misdiagnosis of AK as herpetic keratitis is particularly common and consequential, as patients are sometimes treated with topical corticosteroids that can rapidly worsen AK infection in the absence of anti-amoebic therapy.7 In an animal model of AK, the use of topical dexamethasone was associated with accelerated trophozoite excystment and proliferation, significant cytopathic effect on corneal epithelial cells, and more severe keratitis at all time points compared to untreated animals.9 In a recent case series from our institution, a plurality of patients with culture-confirmed AK presented with pseudodendrites and/or were initially misdiagnosed as having herpetic keratitis, highlighting an opportunity for improved care.10 Pseudodendrites were more common than the more well-known findings of keratoneuritis or ring infiltrates. There are subtle differences that can help differentiate pseudodendritiform lesions in herpetic keratitis from those of AK. Both herpetic and AK epithelial keratitis can feature curvilinear arrangements of elevated epithelium with negative fluorescein staining. However, diffuse granular (i.e. spaced apart) epithelial changes as shown in Case 1 are less likely to be herpetic and are more consistent with AK (Fig. 1B). Both conditions can feature consolidated epitheliopathy, as shown in Case 2 (Fig. 2). However, new onset of pseudodendritiform lesions in a contact lens wearer should raise concern for AK. Both herpetic epithelial keratitis and AK epithelial keratitis can be associated with flat, oval subepithelial opacities (Fig. 1A).
Epithelial basement membrane dystrophy (EBMD; also known as anterior basement membrane dystrophy) can cause negative fluorescein staining due to irregularity of the underlying basement membrane. However, EBMD ridges are typically associated with sharp-edged, continuous lines of negative staining that are often in a shelved or scalloped configuration. In AK epitheliopathy, the linear elevations seldom occur in a narrow line but have greater width than in EBMD (Fig. 2C). On close inspection, even a single long “line” of negative staining in AK often contains numerous non-continuous foci of negatively staining elevations with intervening normal epithelium. Unlike the distinctly shelved or scalloped configurations of EBMD, AK epithelial lesions can be either diffusely scattered across a large area of the cornea or arranged in a gently looping curvilinear configuration that also takes up significant corneal surface area.
Diagnostic techniques for AK include confocal microscopy, Acanthamoeba plate culture, polymerase chain reaction (PCR), and smear slides using corneal scraping/biopsy.5,8 In vivo confocal microscopy can be used to quickly make a tentative diagnosis of AK as laboratory results may take weeks to obtain. Modern confocal microscopes have spatial resolutions of 2–4 μm, allowing for clear visualization of individual Acanthamoeba double-walled cysts or trophozoites.5 However, confocal microscopy is not available in all clinics, is subjective and user-dependent, and is unable to evaluate the entire cornea due to the limited field of view.11 Plate culture is typically performed using non-nutrient broth with E. coli overlay and is the classic technique for Acanthamoeba diagnosis, but requires sampling of a sufficient volume of infected tissue via corneal scraping or corneal biopsy in order to obtain a reliable diagnosis. Previous studies have shown that the effectiveness of isolating Acanthamoeba in culture can range from 26 to 64%.12,13 As such, PCR and smear slides are often used in conjunction with culture to increase diagnostic sensitivity. Numerous studies have reported cases of AK diagnosis by PCR with negative cultures.14, 15, 16 Compared to later stages of AK with deeper stromal infections, epithelial stage AK may be easier to diagnose via corneal scraping due to the higher volume of easily accessible infected tissue on the corneal surface. In contrast, later stage AK infections with deeper stromal invasion may require deeper scraping or more invasive approaches such as corneal biopsy in order to obtain sufficient volume of infected specimen. Epithelial stage AK may therefore represent a critical window of opportunity where diagnostic testing is both higher yield and less invasive. Future research should compare yield of diagnostic techniques across different stages of AK.
The most classic symptom of AK is “pain out of proportion to exam findings.” However, this finding may not apply to early stages of the disease when infection is limited to the epithelium, making the disease even harder to identify early on.1,6 The absence of pain should not exclude AK.10 Taking a comprehensive history is key to early diagnosis. Above all, asking patients about a history of CL wear and poor CL hygiene can be helpful in differentiating between AK and other conditions. Contact lens wearers with new onset of elevated epitheliopathy and failure to improve after 1 week of contact lens holiday and lubrication should be referred to a cornea specialist for consideration of Acanthamoeba testing including culture, PCR, smear, and/or confocal microscopy.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any
Personal information that could lead to the identification of the patient.
Financial Support
No funding or grant support.
Authorship
All authors attest that they met the current ICMJE criteria for Authorship.
Declaration of competing interest
No conflicting relationship exists for any author.
Acknowledgments
None.
References
- 1.Lacerda AG de, Lira M. Acanthamoeba keratitis: a review of biology, pathophysiology and epidemiology. Ophthalmic Physiol Opt. doi: 10.1111/opo.12752. [DOI] [PubMed]
- 2.U.S. Centers for Disease Control and Prevention Acanthamoeba: Sources of Infection and Risk Factors. https://www.cdc.gov/parasites/acanthamoeba/infection-sources.html
- 3.Tu E.Y., Joslin C.E., Sugar J., Shoff M.E., Booton G.C. Prognostic factors affecting visual outcome in Acanthamoeba keratitis. Ophthalmology. 2008;115(11):1998–2003. doi: 10.1016/j.ophtha.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks J.G., Coster D.J., Badenoch P.R. Acanthamoeba keratitis. Resolution after epithelial debridement. Cornea. 1994;13(2):186–189. [PubMed] [Google Scholar]
- 5.Maycock N.J.R., Jayaswal R. Update on acanthamoeba keratitis: diagnosis, treatment, and outcomes. Cornea. 2016;35(5):713–720. doi: 10.1097/ICO.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 6.Alkharashi M., Lindsley K., Law H.A., Sikder S. Medical interventions for acanthamoeba keratitis. Cochrane Database Syst Rev. 2015;2 doi: 10.1002/14651858.CD010792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dart J.K.G., Saw V.P.J., Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol. 2009;148(4):487–499. doi: 10.1016/j.ajo.2009.06.009. e2. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzo-Morales J., Khan N.A., Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. doi: 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClellan K., Howard K., Niederkorn J.Y., Alizadeh H. Effect of steroids on acanthamoeba cysts and trophozoites. Invest Ophthalmol Vis Sci. 2001;42(12):2885–2893. [PubMed] [Google Scholar]
- 10.Shah YS, Stroh IG, Zafar S, et al. Delayed diagnoses of Acanthamoeba keratitis at a tertiary care medical centre. Acta Ophthalmol. Published online February 14, 2021. doi:10.1111/aos.14792. [DOI] [PubMed]
- 11.Shiraishi A., Uno T., Oka N., Hara Y., Yamaguchi M., Ohashi Y. In vivo and in vitro laser confocal microscopy to diagnose acanthamoeba keratitis. Cornea. 2010;29(8):861–865. doi: 10.1097/ICO.0b013e3181ca36b6. [DOI] [PubMed] [Google Scholar]
- 12.Parmar D.N., Awwad S.T., Petroll W.M., Bowman R.W., McCulley J.P., Cavanagh H.D. Tandem scanning confocal corneal microscopy in the diagnosis of suspected acanthamoeba keratitis. Ophthalmology. 2006;113(4):538–547. doi: 10.1016/j.ophtha.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Bacon A.S., Frazer D.G., Dart J.K., Matheson M., Ficker L.A., Wright P. A review of 72 consecutive cases of Acanthamoeba keratitis, 1984-1992. Eye (Lond) 1993;7(Pt 6):719–725. doi: 10.1038/eye.1993.168. [DOI] [PubMed] [Google Scholar]
- 14.Kandori M., Inoue T., Takamatsu F., et al. Two cases of acanthamoeba keratitis diagnosed only by real-time polymerase chain reaction. Cornea. 2010;29(2):228–231. doi: 10.1097/ICO.0b013e3181a39020. [DOI] [PubMed] [Google Scholar]
- 15.Itahashi M., Higaki S., Fukuda M., Mishima H., Shimomura Y. Utility of real-time polymerase chain reaction in diagnosing and treating acanthamoeba keratitis. Cornea. 2011;30(11):1233–1237. doi: 10.1097/ICO.0b013e3182032196. [DOI] [PubMed] [Google Scholar]
- 16.Thompson P.P., Kowalski R.P., Shanks R.M.Q., Gordon Y.J. Validation of real-time PCR for laboratory diagnosis of Acanthamoeba keratitis. J Clin Microbiol. 2008;46(10):3232–3236. doi: 10.1128/JCM.00908-08. [DOI] [PMC free article] [PubMed] [Google Scholar]