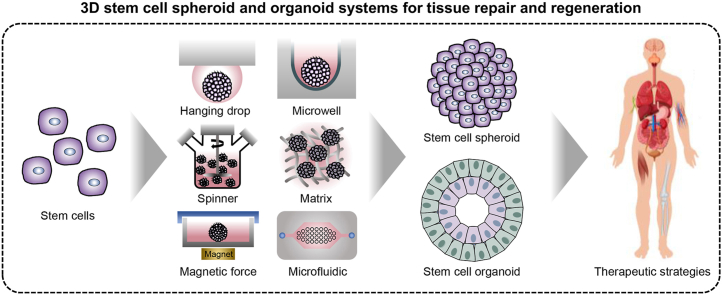
Abstract
Three-dimensional (3D) stem cell culture systems have attracted considerable attention as a way to better mimic the complex interactions between individual cells and the extracellular matrix (ECM) that occur in vivo. Moreover, 3D cell culture systems have unique properties that help guide specific functions, growth, and processes of stem cells (e.g., embryogenesis, morphogenesis, and organogenesis). Thus, 3D stem cell culture systems that mimic in vivo environments enable basic research about various tissues and organs. In this review, we focus on the advanced therapeutic applications of stem cell-based 3D culture systems generated using different engineering techniques. Specifically, we summarize the historical advancements of 3D cell culture systems and discuss the therapeutic applications of stem cell-based spheroids and organoids, including engineering techniques for tissue repair and regeneration.
Keywords: 3D stem cell culture, Spheroid, Organoid, Tissue repair, Tissue regeneration
Graphical abstract
Highlights
-
•
Timeline and historical evolution of stem cell culture systems were reviewed.
-
•
Engineering techniques for formation of stem cell spheroids and organoids were reviewed.
-
•
Advanced therapeutic strategies of stem cells-based spheroids and organoids for tissue repair and regeneration were reviewed.
-
•
Perspective on stem cells-based 3D therapeutic strategies were proposed.
1. Introduction
Progressing from two-dimensional (2D) monolayer cell culture to three-dimensional (3D) cell culture requires the use of cell based-models that mimic the function of living tissues and organs [1,2]. Moreover, 3D cell culture systems have great potential for narrowing the gap between cell-based methods and animal models for studying the development of novel drugs and therapeutics, as well as the repair and replacement of tissues and organs [3,4]. Previously, various cells have been cultivated in 2D monolayers on cell culture plates or other platforms and utilized not only for biological research but also for the development of therapeutic applications for damaged tissues and organs [5,6]. However, monolayer cells do not reflect the in vivo microenvironment because they lack exposure to the extracellular matrix (ECM) and physiological relevance, which may cause abnormal cell metabolism and protein expression [7,8]. To address this issue, 3D cell culture systems have attracted considerable attention as a strategy to better mimic in vivo conditions, such as the complex cell-cell and cell-ECM interactions [9]. Additionally, 3D cell cultures, such as aggregates, spheroids, and organoids, provide unique properties to guide the specific functions and growth of cells (e.g., embryogenesis, morphogenesis, and organogenesis). Thus, 3D cell culture is a tool for creating in vivo-like environments that enable basic research of various tissues and organs.
In 3D cell culture models, spheroids are simple clusters of a broad range of cell types including tumor spheroids, embryoid bodies, hepatospheres, neurospheres, and mammospheres [10]. Cell spheroids are widely used as multicellular 3D models that form because of the tendency of adherent cells to aggregate into higher cell densities. The multicellular spheroid, which allows for abundant chemical and mechanical interactions, was investigated in various ex vivo tissue models and presented gradients for nutrients, gases, growth factors, and signal factors [8]. Stem cells in the form of spheroids have great application potential in tissue engineering and regenerative medicine because of their extensive abilities, such as self-renewal, generation of differentiated progeny, and protein secretion [[11], [12], [13]]. For this reason, recently, stem cell-based spheroids have been utilized in tissue engineering and regenerative medicine as transplantable treatments targeting bone, cartilage, tendon, muscle, dental, nerve, blood vessel, and skin tissue. Furthermore, the application of various engineering approaches to forming or transplanting stem cell spheroids can improve the therapeutic effects [[14], [15], [16], [17], [18], [19], [20]]. The engineered stem cell spheroid is crucial not only for activating functions of stem cells residing in 3D niche microenvironments, in which the complex interactions between ECM molecules are induced, but also for overcoming the issue of biocompatibility in scaffolds and biomaterials [21,22].
Accurately controlling stem cell differentiation and formation of tissue in vitro is essential for development, organogenesis, and tissue homeostasis to achieve in vivo-like tissues or organs. Organoids, miniature organ models generated in vitro, are complex 3D stem cells [e.g., adult stem cell (ASC), embryonic stem cell (ESC), and induced pluripotent stem cell (iPSC)] culture systems that mimic the specific structural and functional characteristics of a native organ [23,24]. Recently, transplantable organoids have emerged as materials with the potential to replace and regenerate tissue or organs [23,25,26]. In modern medicine, it is possible to replace damaged or dysfunctional tissue with healthy tissue through homologous transplantation. Therefore, other types of tissue sources are required because of the limitations of providing healthy tissues and immune rejection response challenges. Organoids generated using stem cells derived from the patient tissue enable the formation of an isogenic tissue having identical genes. Specifically, in case of the pluripotent stem cell, utilizing the iPSCs derived from easily accessible human biological samples (e.g., skin tissue, blood, and urine) facilitates mass organoid production without the ethical issues surrounding human ESCs [27]. Thus, the development and production of organoids may result in improved replacements and regeneration tools for advanced therapeutic applications.
In this review, we focus on the recently advanced therapeutic applications of stem cells, spheroids, and organoids in 3D culture systems using engineering tools to improve tissue repair and regeneration. First, we introduce the timeline and historical origin of stem cell, spheroid, and organoid technology. We subsequently describe the therapeutic applications of stem cells in various tissues. We introduce and describe commonly used formation methods, engineering techniques, and therapeutic applications of stem cell spheroids for tissue repair and regeneration. We describe the recent advances in technology to guide matured organoids and therapeutic applications for tissue repair and regeneration. Finally, we discuss the future opportunities, challenges, and perspectives regarding 3D stem cell culture systems, including spheroids and organoids, for improving therapeutic applications.
2. Historical evolution of 3D cell culture systems
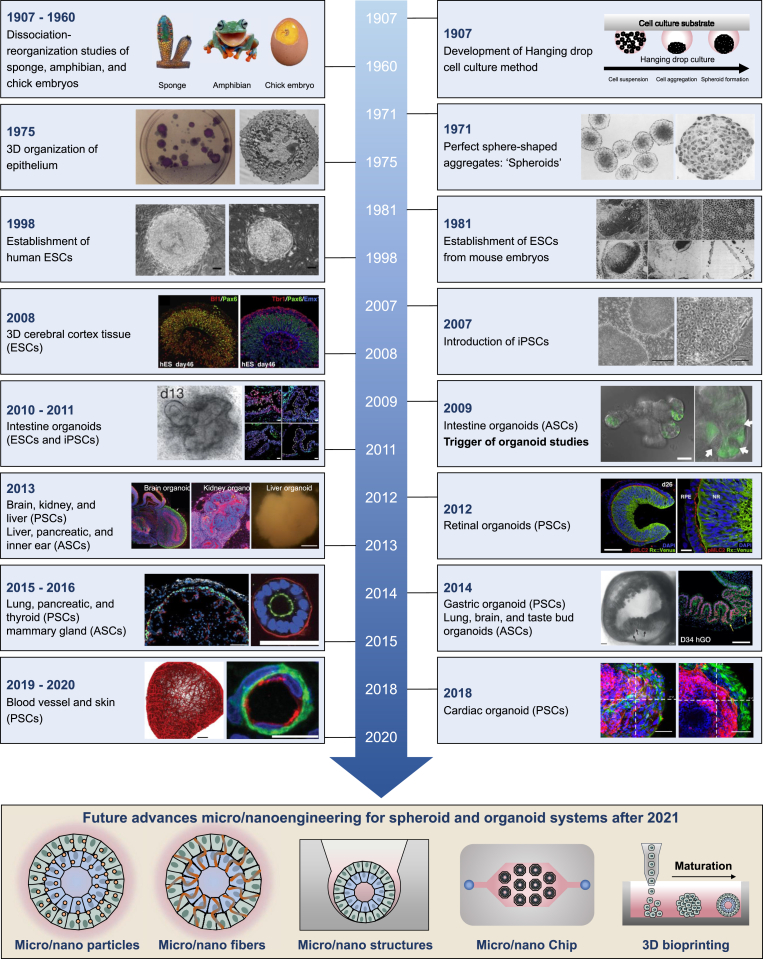
Although it has only been a few years since the full-scale development of 3D cell culture systems, 3D cell culture has a history of about 100 years (Fig. 1). In 1907, Wilson et al. described the first attempt of reorganization of an organism by demonstrating the ability of dissociated sponge cells to self-organize a whole organism [28]. That same year, the hanging drop method was established by Ross Harrison et al., while exploring ways to culture and maintain frog embryo nerve fibers in vitro [29]. Dissociation-reaggregation experiments were conducted by Holtfreter et al. with dissociated amphibian pronephros in 1944 [30] and by Weiss and Taylor with multiple organs from an embryonic chick in 1960 [31]. In 1971, multicellular spheroids with nearly perfect sphere-shaped aggregates were first established using Chinese hamster V79 lung cells grown in suspension in tissue culture [32]. In 1975, Rheinwald et al. showed that single cells of the keratinizing line grow into colonies, each consisting of a stratified squamous epithelium [33,34]. Martin et al. first reported the successful isolation and establishment of mouse ESCs, a pluripotent stem cell (PSC) line, from early mouse embryos in 1981, following which research related to stem cells accelerated [35]. Thomson et al. successfully isolated and cultured human ESCs derived from human blastocysts in 1998 [36]. The Yamanaka group first established iPSCs by reprogramming mouse and human fibroblasts, significantly influencing stem cell and organoid research from 2006 to 2007 [37,38]. In 2008, Eiraku et al. generated self-organized and polarized 3D cerebral cortical tissue from mouse and human embryonic stem cells [39]. In 2009, Sato et al. reported that 3D intestinal organoids formed by adult mouse intestinal stem cells isolated from primary intestinal tissue [40]. This impactful study is the first report on establishing 3D organoid culture derived from stem cells. After reporting this study [40], many researchers actively attempted to develop various organoids, such as those of the intestine (2011), stomach (2010, 2014), liver (2013), inner ear (2013), pancreas (2013, 2015), lung (2014, 2015), kidney (2013), thyroid (2015), brain (2013), retina (2012), breast (2016), taste bud (2014), heart (2018), blood vessel (2019), and skin derived from ASCs and PSCs [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60]]. The methods of developing organoids are well-established, and currently, efforts are being devoted to developing mature organoid systems. Recently, microengineering and nanoengineering techniques have attracted great attention. In the near future, applying microengineering- and nanoengineering-based platforms, such as particles, fibers, structural cues, fluidic chips, and 3D bioprinting, at the micro- and nanoscale may facilitate increased production, improved reproducibility, and development of highly matured organoid systems. Fig. 1 summarizes the various organoids formed by various stem cells and advances in microengineering and nanoengineering of 3D cell culture systems.
Fig. 1.
Timeline of historical advancements and future advances in 3D cell culture systems. A summary of representative studies and breakthroughs leading to the establishment of various 3D culture systems has been presented. Reproduced with permission from Ref. [[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60]]. Reorganization of dissociated sponge cells, amphibian pronephros, and multiple organs from an embryonic chick was first conducted. Reproduced with permission from Ref. [[28], [29], [30]]. The hanging drop method was established. Reproduced with permission from Ref. [29]. Perfect sphere-shaped aggregates were first established. Reproduced with permission from Ref. [32]. 3D organization of epithelium was conducted. Reproduced with permission from Ref. [33,34]. ESCs from mouse embryos were established. Reproduced with permission from Ref. [35]. Human ESCs were isolated and cultured. Reproduced with permission from Ref. [36]. Human and mouse iPSC were first established. Reproduced with permission from Ref. [37,38]. 3D intestinal organoids were formed by mouse intestinal stem cells. Reproduced with permission from Ref. [40]. Many researchers actively attempted to develop various organoids, such as intestine (2011), stomach (2010, 2014), liver (2013), inner ear (2013), pancreas (2013, 2015), lung (2014, 2015), kidney (2013), thyroid (2015), brain (2013), retina (2012), breast (2016), taste bud (2014), heart (2018), blood vessel (2019), and skin derived from ASCs and PSCs. Reproduced with permission from Ref. [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60]]. In the near future, applying microengineering- and nanoengineering-based platforms may facilitate increased production, improved reproducibility, and development of highly matured organoid systems.
3. 2D-cultured stem cell for tissue repair and regeneration
Transplantation of stem cells can be utilized for the treatment of diseases, as well as the regeneration of tissues and organs, instead of using complex surgical procedures or tissue/organ transplantation [61]. Stem cells have several unique properties and capabilities, including continuous high self-renewal activity, multi-lineage differentiation capacity, re-population of a host, and secretion of reparative paracrine factors, making them an ideal cell source for cell-based therapy, regenerative medicine, and tissue engineering [12,62]. In recent decades, tissues engineered using a variety of stem cells have been applied to epithelial surfaces (e.g., skin, cornea, and mucosal membranes) and hard tissues (e.g., bone, tooth enamel, dentin, and cementum) [12]. Because of their limitless therapeutic potential, stem cells continue to receive tremendous public, scientific, and clinical interest. In this section, we provide an overview of a range of stem cell-based therapeutic applications for tissue regeneration and repair that are applied as the transplantation of only stem cells with controlled functions or engineered scaffolds combined with stem cells.
Various engineering approaches are used to provide the environment and conditions to improve adhesion, proliferation, differentiation, migration, and paracrine factor secretion of stem cells used in the regeneration of various tissues (e.g., bone, cartilage, tendon, muscle, nerve, tooth, and skin). Microengineered and nanoengineered platforms and biomaterials have been utilized to modulate the behaviors of stem cells and enhance their function by providing them with specific microenvironments (Fig. 1 and Table 1) [[63], [64], [65]]. For example, micro- and nano-scale structured materials and hydrogel matrix have been proposed as effective controllers for the modulation of cellular behaviors and functions [[66], [67], [68]].
Table 1.
Stem cell therapy for tissue repair and regeneration.
| Target tissue | Cell type (cell source) | Transplantation method | Material | Outcomes | Ref. |
|---|---|---|---|---|---|
| Bone | BMSC (rat) | Collagen sponge vehicle loaded with BMSCs | Treatment with N-acetyl-l-cysteine | Enhanced new bone formation via treatment with N-acetyl-l-cysteine after 5 weeks | [73] |
| BMSC (human) | Integrin-specific hMSC-encapsulated hydrogel | 4-arm PEG macromers with terminal maleimide groups (PEG-4MAL) and GFOGER peptides | Enhanced inflammation inhibition, vascularization, and bone formation | [70] | |
| DPSC (human) | Transplantation of nanospike array loaded with DPSCs | PEGDMA nanospike | Significantly promoted the regeneration of cranial bone defect | [63] | |
| Periosteal stem cell (mouse) | Implantation of periosteal stem cells in Matrigel | Matrigel | Enhanced periosteal bone formation and normal cortical architecture | [74] | |
| BMSC (murine) | Injection of microporous hydrogel loaded with BMSCs | GelMA hydrogel | Bone tissue volume/total tissue volume >30%, bone mineral density >500 mg/cc, trabecular thickness >200 μm, and trabecular separation/spacing <0.5 mm | [72] | |
| Cartilage | BMSC (rabbit) | Injection of thermosensitive hydrogel loaded with BMSCs | Copolymers of PA-PEG-PA and PAF-PEG-PAF | Transparent tissue filling with smooth and consecutive surface | [80] |
| ICRS macroscopic score: 9.19 | |||||
| Histological score: 9.92 | |||||
| ADMSC (human) | Injection of ADMSC-loaded microbot with electromagnetic field control | PLGAMicroscaffold with ferumoxytol and chitosan | Low expression of proinflammatory genes and significant increase in COLII expression | [246] | |
| MSC (human) | Injection of MSCs encapsulated in hydrogel | Hyper-branched polyPEGDA and thiolated hyaluronic acid (HA) | ICRS macroscopic score: 12.68 ± 2.11 | [81] | |
| Reduced defect area: 0.148 ± 0.074 mm2 | |||||
| Total histological score: 20.05 ± 1.73 | |||||
| ASC (rabbit) | Implantation of cartilage extracellular matrix (ECM)-derived particles loaded with ASCs | Decellularized porcine knee articular cartilage | High ICRS macroscopic score, contact stiffness, reduced modulus, histological score, and bone volume | [82] | |
| Tendon | BMSC (dog) | Engineered tendon-fibrocartilage-bone composite and BMSC cell sheet | Decellularized canine patellar tendons | Improved collagen fiber organization and increased new fibrocartilage formation | [86] |
| BMSC (rat) | Aligned collagen fiber scaffold loaded with BMSCs | Collagen | Improved scoring, thickness, and weight of tendon; normal Achilles functional index; high quality of repair, as per histological score | [88] | |
| Tendon stem cell (TSC) (rat) | Implantation of scaffold loaded with TSCs | Biomimetic parallel-aligned collagen scaffold | Compact regeneration; smooth structure; more distributed structure; massive, spindle-shaped, tenocyte-like cells; and aligned collagen fibrous structure | [65] | |
| BMSC (rabbit) | Extracellular matrix scaffold and BMSC sheet | Decellularized bone-fibrocartilage-tendon tissue of rabbit | Bone tissue volume/total tissue volume >50%, trabecular thickness >35 μm, trabecular number <15/mm, high histological score, high failure load, and high stiffness | [87] | |
| Muscle | Muscle stem cells (MuSCs) | Transplantation of + decellularized muscle tissue 3D scaffold loaded with MuSCs | 3D scaffold (decellularized muscle tissue) | Bioconstruct made with human MuSCs and MRCs can generate functional muscle tissue in VML model. | [90] |
| iPSC-CM (human), MSC (human) | hiPSCs-CMs injected intramyocardially, and implantation of 3D-printed scaffold loaded with MSCs | PCL and porcine heart-derived decellularized extracellular matrix bioink | Improved cardiac function and capillary density, and reduced scar formation | [91] | |
| Nerve | Neural crest stem cell (NCSC) (human) | Injection of NCSCs | Electrical stimulation | Promoted axon regeneration and myelination | [95] |
| ASC (rat) | Implantation of ASC sheets | – | Improved the functional recovery, improved reinnervation, and prevented atrophy | [94] | |
| Periodontal ligament stem cell (PDLSC), gingival mesenchymal stem cell (GMSC) | Implantation of stem cell-encapsulated hydrogel | Alginate and hyaluronic acid hydrogel | Higher expression levels of neurogenic‐related genes, higher cell densities, and greater number of cell colonies | [96] | |
| iPSC derived NSC (murine) | Implantation of stem cell encapsulated- hydrogel | GelMA hydrogels | Reduced cavity areas, lesser collagen deposition, decreased inflammation, and promoted axonal regeneration | [97] | |
| Tooth | DPSC | Implantation of tooth slice and scaffolds loaded with DPSCs | Human DPSCs culture conditions containing human serum (DPSCs-HS) | DPSCs-HS produced a robust angiogenic response and regeneration of dentin equivalent to DPSCs-FBS. | [101] |
| DPSC | Implantation of human tooth root canal with DPSC constructs | Scaffold-free 3D cell constructs composed of DPSCs | Pulp-like tissues with rich blood vessels were formed within the human root canal 6 weeks after implantation | [102] | |
| Deciduous pulp stem cell (human) | Implantation of hDPSCs | – | Increased the length of the root, reduced the width of the apical foramen, and regeneration of dental pulp tissue containing sensory nerves (human patients) | [103] | |
| DPSC (human) | Implantation of injectable hydrogel encapsulating hDPSCs | Alginate and laponite hydrogel microspheres | Regeneration of rich microvessels and neotissue | [100] | |
| Skin | Gingival MSC (human) | Transplantation of 3D-printed scaffolds | Medical grade polycaprolactone | Least contraction, least scar area, accelerated wound closure, and most differentiated epithelium | [106] |
| ASC (human and mouse) | Injection of hydrogel encapsulating ASCs | Hyperbranched PEGDA and thiolated gelatin-based hydrogel | Accelerated chronic wound closure, enhanced neovascularization, and reduced inflammation in diabetic wound model | [105] | |
| ASC (rat) | Injection of hydrogel encapsulating ADSCs | Hyperbranched multi-acrylated PEG macromers and thiolated hyaluronic acid | Inhibition of inflammation, promotion of angiogenesis, and re-epithelialization in diabetic wound model | [107] | |
| MSC (human) | Transplantation of pre-vascularized hMSC cell sheets | – | Smallest contraction, best preservation of skin appendages, highest number and area of microvessels, lowest inflammatory reactions, and a morphology that more closely resembles normal skin | [108] | |
| ASC (human) | Transplantation of ASC sheets | – | Enhanced immunomodulatory and antifibrotic capabilities, and reduced scar formation | [109] |
3.1. Bone tissue regeneration
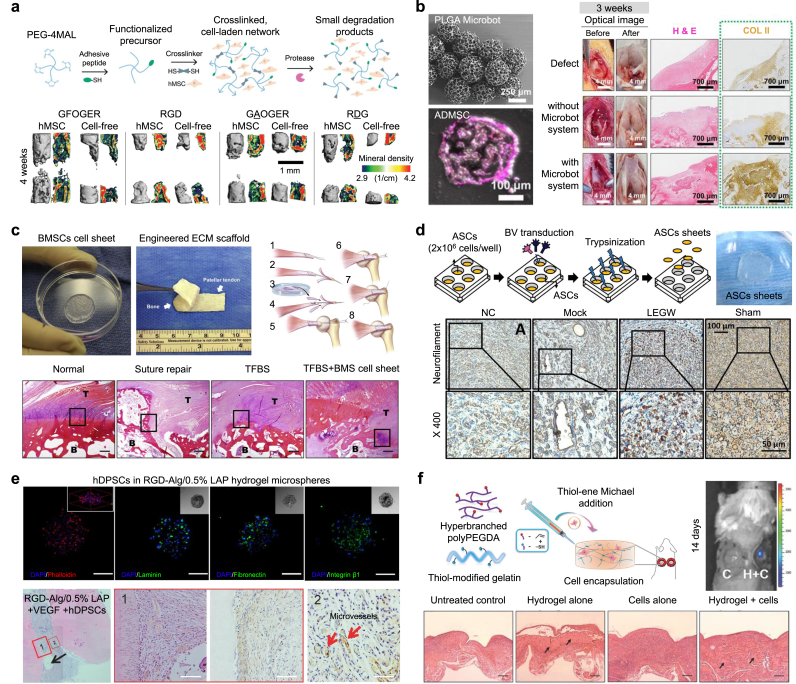
Bone regeneration is one of the major targets of stem cell-based therapy in orthopedic fields. The restoration of extensive bone loss and defects remains an unresolved challenge in modern medicine [69]. Clark et al. synthesized poly(ethylene glycol) (PEG) hydrogels based on 4-arm PEG macromers with terminal maleimide groups (PEG-4MAL) presenting GFOGER, a peptide targeting the α2β1 integrin (Fig. 2a) [70]. Human bone marrow-derived stem cells (BMSCs) encapsulated in PEG-4MAL hydrogels presenting GFOGER showed enhanced cell adhesion, paracrine secretions, and osteoblastic differentiation. Compared with the effects of other combined peptides in murine bone defects, the hydrogels promoted increased survival, retention, and osteo-reparative function of encapsulated BMSCs. Regarding bone tissue regeneration, the hydrogels improved the inflammation inhibition, vascularization, gene expression, and bone formation in vivo. Synthetic hydrogel systems are potentially suitable as in vivo stem cell carriers because of their biocompatibility, injectability, and versatility in presenting bioactive functionalities [70,71]. To improve the bone regeneration capacity, a system using gelatin methacrylamide (GelMA) hydrogel with porous microspheres, BMSCs preconditioned by N-acetyl-L cysteine, a poly(ethylene glycol) dimethacrylate (PEGDMA) polymer-based hydrogel nanopillar array, and periosteal stem cells was developed and investigated as an engineered stem cell carrier and stem cell therapy tool [63,[72], [73], [74]]. In addition, it has been reported that not only simple nanoscale structures, but also nanostructural scaffolds combined with various nanocomposites significantly promote the stem cell differentiation and the bone regeneration [[75], [76], [77]]. Although stem cell and engineered biomaterials have great potential for the treatment of bone loss and defects, maintaining therapeutic efficacy by delivering the microenvironment with stem cells currently remains a major challenge [69,78].
Fig. 2.
Stem cell therapies for regeneration and repair of various tissues. (a) Bone tissue regeneration and repair using hBMSC-encapsulated 4-arm poly (ethylene glycol) (PEG) maleimide hydrogels. hMSC in GFOGER-conjugated hydrogels showed higher levels of new bone formation (live animal μCT). Reproduced with permission from Ref. [70]. (b) Cartilage tissue regeneration and repair using human adipose-derived mesenchymal stem cell (hADMSC)-loaded magnetic PLGA microrobot systems (SEM image of microbot system and Confocal images of hADMSC-microrobot). Microrobot system improved the cartilage regeneration by enhancing the delivery of the hADMSC-microrobot (optical images, H&E staining, and Collagen type II staining). Reproduced with permission from Ref. [64]. (c) Tendon tissue regeneration and repair using decellularized tendon extracellular matrix (ECM) combined with BMSCs cell sheet (images of the cell sheet, dECM scaffold, and Surgical procedure). These scaffolds demonstrated well-orientated collagen, fewer inflammatory cells and, spindle-shaped nuclei (H&E staining). Reproduced with permission from Ref. [86]. (d) Nerve tissue regeneration and repair using baculovirus vector -transduced adipose-derived stem cell (ADSC) sheets (ADSCs sheet image). Neurofilament protein was significantly deposited. Reproduced with permission from Ref. [94]. (e) Dental tissue regeneration and repair using the injectable hybrid RGD-alginate/laponite hydrogel microspheres by encapsulating human dental pulp stem cells (hDPSCs) (confocal images). The hydrogel improved neotissue regeneration (H&E, DMP-1, and CD31 staining). Reproduced with permission from Ref. [100]. (f) Skin tissue regeneration and repair using the ADSC-encapsulated injectable PEGDA/thiolated gelatin hydrogel. ADSC-encapsulated hydrogel promoted the host cell filtration and granulation tissue remodeling (H&E staining). Reproduced with permission from Ref. [105].
3.2. Cartilage tissue regeneration
Cartilage tissue is difficult to self-repair after injury due to its avascular structure and low metabolic activity [79]. Stem cells with exogenous biochemical or mechanical stimulations, and the engineered scaffolds in stem cell-based therapies, have demonstrated significant advances in cartilage regeneration. Go et al. developed a magnetic microrobot system (size: 357.55 ± 18.57 μm) with 3D porous structure (size: 43.85 ± 13.39 μm) by combining a poly(l-glutamic acid) (PLGA) microscaffold, ferumoxytol, and chitosan to achieve targeted stem cell delivery for knee cartilage regeneration (Fig. 2b) [64]. Human adipose-derived mesenchymal stem cells (ADMSCs) were loaded into the microbot systems after incubating for 24 h. Most ADMSC-loaded microbots were delivered and positioned to the targeted defect area of the rabbit knee cartilage by controlling the electromagnetic actuation system. The expression of COLII was significantly increased after injection of the hADMSC-loaded microrobot compared with the non-injected group, at 2 and 3 weeks after injection. Recently, engineering approaches for cartilage regeneration have been widely studied, including local delivery platforms for stem cells such as thermosensitive polypeptide poly(l-alanine-co-l-phenylalanine)-block-poly(ethylene glycol)-block-poly(l-alanine-co-l-phenylalanine) hydrogels, hyper-branched poly(ethylene glycol) diacrylate (PEGDA)/hyaluronic acid (HA) hydrogel, and decellularized porcine knee articular cartilage ECM-derived particles [[80], [81], [82]]. Although the cartilage therapeutic effect of stem cell-based scaffolds has shown good efficacy in repairing tissues, approaches that improve angiogenesis, anti-inflammatory, tissue formation potential, and increase cell viability when compared to individual cells are still needed.
3.3. Tendon and muscle tissue regeneration
Tendon injuries are treated by the conservative treatment which aims to relieve pain and surgical repair [83,84]. Stem cell-based therapies provides great promise for tendon regeneration due to their high proliferative, synthetic and immunomodulatory activities, as well as their potential to differentiate into target cell types [85]. Liu et al. reported combining novel biomaterials with decellularized canine patellar tendon ECM including tendon-to-bone interfaces and BMSC sheets for tendon-to-bone healing of the rotator cuff (Fig. 2c) [86]. The composite of BMSC sheets and decellularized tendon ECM implanted in canine non-weight-bearing models promoted rotator cuff healing, as demonstrated by anatomic structure, collagen organization, and biomechanical strength [86]. Recently, decellularization engineering has offered a more natural approach (i.e., using tissue-derived ECM) to mimic the morphology and composition of bone-tendon inserts. Tang et al. developed decellularized rabbit bone-fibrocartilage-tendon scaffolds combined with autologous BMSC sheets for tendon-to-bone healing by regenerating a robust fibrocartilage layer and collagen fibers, arranged in the direction of traction [87]. For functional regeneration and repair of tendons, biomimetic scaffolds, such as parallel-aligned collagen fiber scaffolds loaded with recombinant periostin and collagen fiber membranes of various orientations (established using counter-rotating extrusion), have been used [65,88,89]. Quarta et al. suggested a 3D bioconstruct scaffold composed of decellularized tibialis anterior muscle tissue cultured with muscle stem cells and other muscle resident cells using a perfusing bioreactor to improve the treatment of volumetric muscle loss injury [90]. Park et al. developed porcine heart-derived decellularized ECM bioink for fabricating 3D-printed scaffolds that allowed seeding of human mesenchymal stem cells (MSCs). The authors proposed a concomitant method that exploits the advantages of cardiomyocytes derived from human iPSCs and human MSC-loaded scaffolds to improve cardiac repair in a rat myocardial infarction model [91]. Biomimetic scaffolds combined with stem cells provide enhanced muscle tissue of the largely defected esophagus [89]. Despite tremendous efforts and advances in tendon regeneration, it presents clinical difficulties because it does not respond well to degenerative tendon treatment and requires long-term rehabilitation [92].
3.4. Nerve tissue regeneration
Nerve damage can cause great morbidity in people who suffer from loss of sensation, loss of movement, chronic pain, or a combination of deficits [93]. Hsu et al. proposed the use of stem cell sheets comprising adipose-derived stem cells (ADSCs) generated by constructing a Cre/loxP-based hybrid baculovirus vector, which enabled intracellular formation of an episomal DNA minicircle for effective transduction of cells and prolonged expression of functional glial cell line-derived neurotrophic factor capable of recruiting Schwann cells (Fig. 2d) [94]. Implantation of the hybrid baculovirus-engineered stem cell sheets significantly improved nerve repair, as indicated by the enhanced functional recovery, nerve reinnervation, electrophysiological functionality, Schwann cell proliferation and infiltration, axon regeneration, myelination, and angiogenesis [94]. In addition, strategies for functional nerve regeneration using human neural crest stem cell differentiation controlled by electrical stimulation platforms, human periodontal ligament- and gingiva-derived mesenchymal stem cells encapsulated in alginate/hyaluronic acid hydrogels, and iPSC-derived neural stem cells encapsulated in GelMA hydrogels have been reported [[95], [96], [97]]. However, three-dimensional nerve regeneration still requires therapies that more accurately mimic the structure of autologous nerve grafts and efficiently form blood vessels to maintain the viability of transplanted cells [98].
3.5. Dental tissue regeneration
The restoration of tooth defect and loss are treated by replacing the tissues with biocompatible materials such as metal, ceramic, resin, and titanium implant, but these materials have limitations for perfectly reproducing the functions of teeth [99]. Zhang et al. developed injectable hybrid RGD-alginate/laponite hydrogel microspheres to co-encapsulate human dental pulp stem cells (DPSCs) and vascular endothelial growth factor (VEGF) (Fig. 2e) [100]. After subcutaneous implantation with tooth slices in a mouse model for 1 month, the human DPSC-laden RGD-alginate/laponite + VEGF microspheres significantly promoted the regeneration of pulp-like tissues, in addition to the formation of new microvessels [100]. To regenerate dentin and dental pulp tissue, some studies demonstrated the potential of DPSCs isolated and expanded in human serum, DPSC constructs, and autologous human deciduous pulp stem cells [[101], [102], [103]]. Since dental tissue is exposed to significant blood flow, fluid movement, and physical stimuli, it is necessary to effectively implant stem cells into the treatment site while immobilizing them for vascular regeneration and tissue repair [104].
3.6. Skin tissue regeneration
Skin tissue-related diseases are the easiest and most frequent diseases in the human body. Dong et al. developed an injectable PEG–gelatin hydrogel with highly tunable properties from a multifunctional PEG-based hyperbranched polymer and a commercially available thiolated gelatin for encapsulating murine ADSCs (Fig. 2f) [105]. The proposed in situ-formed hydrogel significantly improved cell retention, enhanced angiogenesis, and accelerated wound closure in a murine wound healing model. The engineered hydrogels, 3D-printed scaffolds, and stem cell sheets were utilized for efficient wound healing [[106], [107], [108], [109]]. The future challenges for stem cell-based skin tissue regeneration are rapid healing, scar-free and comprehensive regeneration of skin appendages including hair follicles and sweat glands [110].
Currently, the most commonly proposed stem cell therapy in clinical trials is performing in vitro expansion of a sufficient number of autologous stem cells isolated from the patient and then injecting them into the target site [61]. However, the stem cells transplanted into the target site have critical limitations, such as low efficiency (i.e., cells can be easily washed out of the target tissue) and failure to maintain viability and function (e.g., self-renewal, multi-lineage differentiation, recruitment, and paracrine secretion) in the host tissue. This has resulted in limited success in restoring damaged tissues and organs, especially in large-scale tissue repair or regeneration. Therefore, effective delivery systems that regulate the survival, behavior, and function of stem cells are required to efficiently transplant stem cells to the target tissue sites. Recently, many studies demonstrated that the 3D-cultured stem cells enhanced viability, differentiation, paracrine secretion, and tissue regeneration compared to 2D-cultured stem cells [[111], [112], [113], [114], [115], [116], [117]]. Cardiac stem cell spheroids promoted the secretion of growth factors and the expression of cardiomyocyte-specific markers [112]. The spheroids improved cell engraftment and survival within the myocardium and enhanced neovascularization and myocardial regeneration, resulting in myocardial infarction recovery compared to single cell. Therefore, 3D-cultured stem cells can be good alternatives to stem cell transplantation due to more realistic biochemical and biomechanical microenvironments compared to 2D-cultured stem cells.
4. 3D cell culture for stem cell spheroids and organoids
Spheroids formed by aggregating stem cells are the result of the self-assembly behaviors of single cells in suspension due to embryogenesis, morphogenesis, and organogenesis. The formation of spheroids involves complex homogeneous and heterogeneous binding of cell adhesion molecules, ECM proteins, and integrins [118]. Stem cell spheroid assembly occurs in multiple steps [8]. First, single cells are drawn closer to form loosely adhesive cell spheroids because ECM fibers and complementary binding of the peripheral cell surface to integrins encourages preliminary aggregation. Next, cadherin on the cell membrane surface induces tight connections between the aggregated cells because of the homophilic cadherin binding of peripheral cells. Finally, early cell assemblies formed by both pathways generate contractile forces via rearrangement of actin stress fibers, leading to compression and the formation of mature spheroids. After these processes, strong, compact multicellular spheroids are formed. 3D cell culture systems can be classified into three major organization and structural types of spheroids, multicellular spheroids, and organoids depending on their complexity [119]. Spheroids are generally considered as 3D cell aggregates generated from a single cell type or various cell types but cannot completely mimic complex contacts with other cell types [120]. Stem cell-derived organoids have a similar phenotype to in vivo, which has higher tissue complexity than spheroids. Although the differences between spheroids and organoids are vaguely defined and inconsistent among researchers, stem cell spheroids are usually close to the meaning of simple cell aggregates and can be utilized in a series of processes for developing organoids from stem cells [121,122]. Especially, whereas internal developmental processes drive organoid formation, spheroids develop primarily through cell-to-cell adhesion. Stem cell-based organoids can be formed by providing the appropriate physical and biochemical signal for differentiation and development of organ-like phenotype or embryoid body after the formation of stem cell-based aggregates and spheroids.
In the following contents, we reviewed the engineering techniques and therapeutic applications for tissue regeneration and organ repair of stem cell spheroids and organoids. Engineered platforms for stem cell spheroid culture require providing the environments that promote aggregation of stem cells and for cellular self-organization of the aggregated cells. Furthermore, it is important to high-throughput fabrication of spheroids with defined size and composition. Stem cell spheroids formed by engineering tools could maintain the stemness properties of stem cells and enhance their multilineage differentiation capacities. Organoids differ from spheroids in that they must have the characteristics of a fully differentiated organ. Therefore, the organoid culture platforms should provide the environments that promote and regulate the differentiation of stem cells to produce mature organoids like native organs. Spheroids produced under optimal culture conditions should promote regeneration by improving the proper differentiation of stem cells and paracrine effects when transplanted into tissues or organs. Otherwise, mature organoids can be inserted into a part of an organ to replace organ functions and be applied to tissue regeneration. Spheroids are mainly applied to tissue regeneration, and organoids are mainly applied to organ function recovery.
5. Engineering techniques for formation of stem cell spheroids
The generation of stem cell spheroids is essentially based on the common principle of self-assembly. The process of cell self-organization occurs within the test tube if the cells cannot attach to the substrate surface, and thus, must interact with each other [10]. The tight connection, cell-cell communication, and cell-ECM communication within cell spheroids contributes to the control of stem cell behaviors and functions (e.g., viability, stimuli responsiveness, and protein secretion), leading to considerable differences compared to monolayer cell cultures [123]. In addition, the high-throughput nature of generating stem cell spheroids with uniform size and composition is an important factor in fabrication [6]. To maximize the functionality of stem cells and cell-cell and cell-ECM connections when culturing 3D spheroids, various platforms based on commonly used spheroid formation methods (e.g., hanging drop, well plate, spinner flask, hydrogel matrix, magnet, and microfluidic chip) have been developed. In this section, we introduce the stem cell spheroid formation methods and platforms that have been developed and improved upon (Table 2 and Fig. 3). 3D cell culture system can be classified into two main types of scaffold-based system (e.g., hydrogel matrix, well plate, and microfluidic chip) and scaffold-free system (e.g., hanging drop, rotation methods, and magnetic force). Since scaffold-free systems do not use biomaterials or ECM components to support cell-to-cell adhesion and migration, cell-to-cell connectivity must be considered. Scaffold systems should consider the composition of various biological and synthetic materials with different porosity, permeability, surface chemistry, and stiffness to mimic the microenvironment of a specific tissue to promote cell growth and migration.
Table 2.
Engineering techniques for stem cell spheroid formation.
| Formation method | Culture platform | Platform materials | Structure | Features | Stem cell(Derived species) | Ref. |
|---|---|---|---|---|---|---|
| Hanging drop | Lid of a Petri dish | PolystyreneSolution coating | Square wettable surface | Superhydrophobic patternwith wettable regions | Adipose-derived stem cells(human) | [126] |
| Automated microfluidic device | Water white glass substrates | Through-hole and well | 2.4-mm wells | BMSCs (mouse) | [127] | |
| Pressure-assisted network for droplet accumulation system | Polycarbonatesheet, polyethylene terephthalate | Through-hole well plate | Balanced control of the internal and surrounding pressure | MSCs | [128] | |
| Bio-inspired superhydrophobic substrate | Silicon (Si) | Vertically aligned nanowires | Length: 25 μm | ADSCs (Human) | [17] | |
| Cell printer | – | – | 2 nL or fewer than five cells | ESCs | [129] | |
| Hydrogel scaffold | Sponge-like hydrogel | Gellan gum, silk fibroin | Micropore structures | Porosity: 90 ± 0.7% Pore wall thickness: 6.6 μm | ADSCs (human) | [150] |
| Sandpaper-embossed microstructure | Tetronic-tyramine | Microstructure | Surface roughness from 100 to 200 μm | ADSCs (human) | [148] | |
| Porous microstructure | Poly(l-glutamic acid), polyethylene glycol | Porous microstructure | Pore size: 275–375 μm | ADSCs (human) | [149] | |
| 3D bioprinted matrix | Chitosan methacrylate and polyvinyl alcohol hybrid microparticle ink | Biomimetic microstructures | Scaffold pore size: 250 μm Particle size: 197 μm | BMSCs (rat) | [147] | |
| Microwells | Microstructure | Tet-TA polymer | lotus seedpod-inspired microwell | 200 or 400 μm in width with various depths | ADSCs (human) | [133] |
| Microstructure | Alginate | Hexagonal well array | 100-, 200-, and 400-μm wells | MSCs (mouse) | [134] | |
| Microstructure | PEG hydrogel | Inverted-pyramidal opening well | Diameter: 200 μm | MSCs (human) | [135] | |
| Microstructure | Agarose | Round-bottom microwells | Diameter: 2 mm | iPSCs (human) | [132] | |
| ESCs (human) | ||||||
| Microstructure | Collagen, Matrigel | Micro-honeycomb structures | 100- and 300-μm holes | ESCs (mouse) | [20] | |
| Spiner and rotational methods | Multi-trap acoustic levitation | PDMS, ultrasonic transducer | Cylindrical cavity | Diameter: 5 mm | ADSCs (human) | [139] |
| Height: 10 mm | ||||||
| Pellet culture with nanofiber | Poly(l-lactic acid) (PLLA) | Mineralized fragmented nanofiber | Diameter: 1–2.5 μm | ADSCs (human) | [247] | |
| Length: 60 μm | ||||||
| Pellet culture with nanofiber | Poly(l-lactic acid) (PLLA) | Fragmented nanofiber | Diameter: 400–800 nm | MSCs (human) | [248] | |
| Length: 50–100 μm | ||||||
| Pellet culture with nanofiber | Poly(l-lactic acid) (PLLA), platelet-derived growth | Fragmented nanofiber | Diameter: 5.4 μm | ADSCs (human) | [249] | |
| factor (PDGF), bio-mineral | Length: 100–150 μm | |||||
| Microfluidics | Hydrogel encapsulation | Poly(vinyl alcohol) (PVA) | Microcapsule | Smaller than 200 μm | BMSCs (human) | [162] |
| Droplet-microfluidic platform, micro anchors hole | Agarose | Encapsulation | Diameter: 680 μm | MSCs (human) | [18] | |
| Magnetic field | Magnetic nanoparticles | Iron oxide core (Fe3O4) coated in polydimethylamine | Nanoparticle | Diameter: 200 nm | BMSCs (human) | [15] |
| Magnetic nanoparticles | Iron salts | Nanoparticle | Diameter: 8 nm | ESCs (mouse) | [19] | |
| Magnetic nanoparticles | Gold and iron oxide nanoparticles crosslinked by poly-l-lysine | Nanoparticle | Size: 50 nm | Neural crest-derived mesenchymal stem cells (human) DPSCs (human) | [157] |
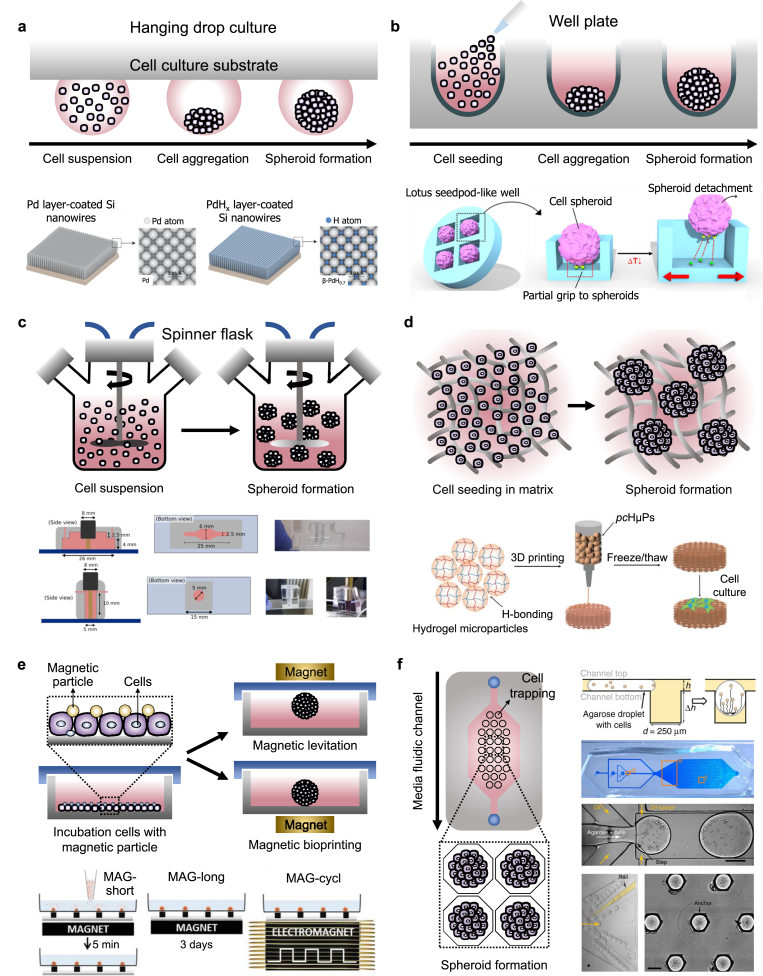
Fig. 3.
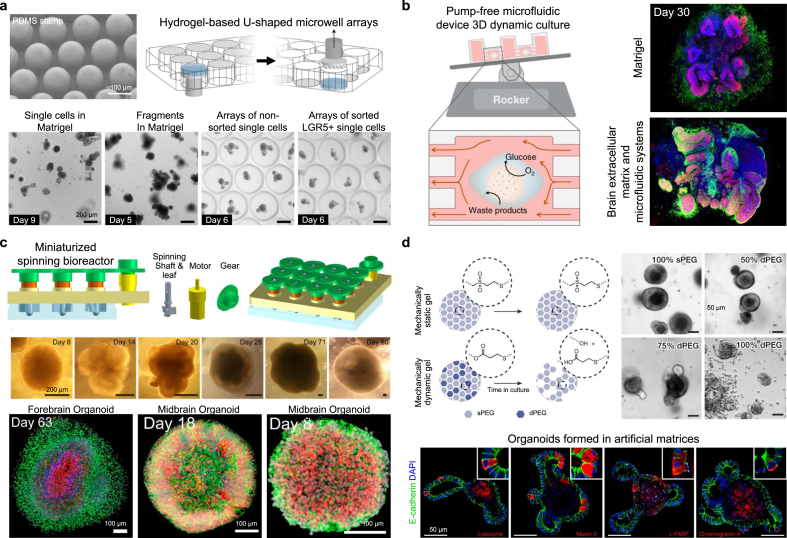
Commonly used formation methods and advanced engineering formation methods for stem cell spheroids. (a) Representative schematic of hanging drop culture method and engineered hanging drop methods using bio-inspired superhydrophobic surfaces with palladium layer onto vertically aligned silicon nanowires. Reproduced with permission from Ref. [17]. (b) Representative schematic of microwell plate formation method and engineered microwell plate using lotus seedpod-structured Tet-TA polymer hydrogel coated with fibronectin. Reproduced with permission from Ref. [133]. (c) Representative schematic of spinner flask levitation culture method and engineered levitation formation method using multi-trap acoustic levitation platforms using a PDMS chip and an ultrasound transducer. Reproduced with permission from Ref. [139]. (d) Representative schematic of hydrogel matrix formation method and engineered hydrogel matrix formation method using the 3D-printed matrix of chitosan methacrylate and polyvinyl alcohol hybrid hydrogel microparticles. Reproduced with permission from Ref. [147]. (e) Representative schematic of magnetic force-derived formation method and engineered magnetic-based formation method using microfabricated magnetic patterns and magnetic nanoparticles. Reproduced with permission from Ref. [19]. (f) Representative schematic of microfluidic chip-based formation method and engineered method using a droplet-trapping microfluidic chip. Reproduced with permission from Ref. [18].
5.1. Hanging drop methods
The hanging drop method is a well-established method commonly used for the formation of 3D cell spheroids that utilizes gravitational force to induce cellular assembly. Single cells are aggregated at the tip of small to medium droplets that are hung on the cell culture substrate using the hydrophilic and surface tension properties of the liquid. Hanging drop methods have several advantages including simplicity, consistency, lack of matrix requirements, scalability for high throughput production, and the ability to produce spheroids in a small number of cells [124]. Due to advances in liquid automated handling systems and robotics, the combination of automated systems and hanging drop platforms enables the simultaneous fabrication of large numbers of 3D cellular constructs [125]. However, the hanging drop method has some limitations in replacing cell culture medium without any negative effects on spheroids and using large droplets of the medium. In addition, the hanging drop methods combined with automated systems have disadvantages in that it requires high cost and complex processes. Based on these characteristics, bio-inspired superhydrophobic surfaces having vertically aligned silicon nanowires coated with a palladium layer using sputter deposition have been developed via aqueous electroless etching of silicon (Fig. 3a) [17]. The developed superhydrophobic surfaces have an extremely large water contact angle (>150°) in both ambient air and hydrogen, enabling the control of the size of hADSC spheroids. Compared to spheroids generated using spinner flask suspension culture and hanging drop culture on the lid of a petri dish, the spheroids fabricated on the developed superhydrophobic surfaces exhibited a more uniform size distribution. Utilizing a patterned superhydrophobic surface with polystyrene with wettable regions has been proposed for the production and culture of hADSC spheroids [126]. A hanging drop culture system for producing stem cell spheroids was engineered using a digital microfluidic device, coated with indium tin oxide, parylene-C, and Cytop, having an automated liquid-handling system that facilitated in situ cell spheroid culture [127]. The pressure-assisted network for droplet accumulation systems was proposed to form a consistent and uniform hanging drop array for quick and increased production of human umbilical cord blood-derived MSC spheroids [128]. The valve-based bioprinting process was reported to maintain hESC viability, be accurate enough to produce spheroids of uniform size, and allow printed cells to maintain their pluripotency [129].
5.2. Well plate methods
Well plates can be easily handled without complex processes for spheroid formation and can control the uniform size and cell composition compared to other spheroid formation methods [130]. The surface of commonly used low cell attachment plates has modified hydrophobicity to promote strong and fast aggregation of cells compared with traditional cell culture plates [131]. Regarding commercial low cell attachment well plates for stem cell aggregation, a lack of control over the homogeneity of the environmental factors to which individual cells are exposed makes them unsuitable for scalable mass production [132]. In addition, they can form large aggregates that adversely affect cell proliferation and differentiation and may lead to cell death due to the hindrance of mass transport. To address these issues, engineered well platforms with various microstructures have been proposed for efficiently controlling spheroid formation. Microwell platforms with microstructures fabricated by microengineering can be developed of various structures that can finely control the spheroid size and cell composition. Microwells, inspired by lotus seedpod-structured hydrogels with temperature-response and cell-interactivity, have been developed for culture and delivery of hADMSC spheroids (Fig. 3b) [133]. The bio-inspired square microwells spontaneously formed spheroids with high viability, and fibronectins conjugated to the hydrogel successfully gripped the spheroids, similar to the funiculus gripping seeds in the lotus seedpod. Dissolvable microwell scaffolds generated using alginate hydrogels were utilized to allow cells to form self-organized clusters and then be gently released, resulting in highly reproducible multicellular structures that can be produced on a large scale [134]. The PEG hydrogel-based microwell array, containing 1225 wells (200 μm in diameter) composed of cylindrical microwells with inverted-pyramidal openings, was fabricated to prevent cell loss during the mass-production of hMSC-spheroids [135]. Agarose hydrogel-based round-bottom microwells having low cell attachment, fabricated using Teflon stamps, were used to host the dissociated single-cell suspension of hiPSCs [132]. Shape-tunable, densely packed, soft micro-honeycomb structures created using natural ECM hydrogel (i.e., collagen and Matrigel) as the microwell array were developed to fabricate a 3D liver model consisting of compact cell spheroids [20].
5.3. Spiner and rotational methods
Levitation culture methods involve using stirring and rotating mechanisms to prevent cell adhesion onto the surface; continued agitation promotes spheroid formation in suspension [136]. The spinner spheroid culture method involves continuous mixing of a cell suspension in a spinner flask bioreactor vessel [137]. In rotation culture, reconstruction of microgravity is achieved via a constant circular rotation. In levitation culture, stem cell expression factors can be manipulated by altering the fluidic flow, mass, and rotation speed in the containers [10]. Especially, the spinner and rotation systems have the advantage of being able to produce a large amount of spheroid at the same time as the relatively uniform shape and can produce spheroids of various sizes by controlling rotational speed and cell concentration of the cell suspension. However, if the rotational speed of the rotating culture vessel becomes too fast, the shear force becomes stronger, which can affect the physiological response of the cell. In addition, the spinner and rotation systems have limitations in providing an appropriate environment for producing spheroids of a very uniform size or containing various types of cells [138]. Multi-trap acoustic levitation culture platforms, fabricated using polydimethylsiloxane (PDMS), combined with an ultrasonic transducer were used to generate a controlled cell culture environment (Fig. 3c) [139]. The acoustic forces that act on the suspended cells by clustering and trapping them in multiple disk-like layers in acoustic levitation are positioned at the different pressure nodes of a resonant cylindrical cavity. An hiPSC-specific suspension culture unit consisting of a fully monitored continuous stirred tank reactor system integrated into a custom-designed and fully automated incubator, primarily constructed using 3D-printed polylactic acid (PLA) filament, was developed [140].
5.4. Hydrogel matrix-based formation methods
Hydrogel is a complex network of physically or chemically crosslinked polymer molecules that expand in aqueous media [141]. Hydrogel matrices are designed with a wide range of compositions, biophysical properties, and biological functions in order to resemble various features of native ECM to induce ECM-cell interaction [141]. The hydrogel matrix can mimic the microenvironments of the native ECM which include ECM-like viscoelasticity, interstitial flow, high-water content, and diffusive transport characteristics [142]. Spheroids are formed in the 3D environment in which cells are embedded in a porous hydrogel that serves as unique properties of the native extracellular matrix [143]. The cells can migrate and proliferate within the hydrogels and specific proximity to adjacent cells can spontaneously form the multicellular aggregates to spherical culture. The single cells proliferated and incubated in the porous hydrogel matrix are formed to the relatively loose aggregates due to the action of the integrins of cells [144]. And then, the epithelial cadherin is expressed and accumulated, and the aggregates enter the delayed phase of compression arrest [145]. Consequently, the cell aggregates form the multicellular spheroids under the strong hemophilic interaction of epithelial cadherin by forming cadherin-cadherin binding. For these reasons, hydrogel matrices are frequently used in 3D culture environments that mimic the scaffolding provided by the ECM [24]. An engineered biomaterial-based hydrogel matrix for spheroid formation provides physical support for self-assembly of stem cells [146]. Various natural polymers [e.g., alginate, collagen, gelatin, fibrin, hyaluronic acid (HA), Matrigel, and chitosan] and synthetic polymers [e.g., PEG, poly(l-lactic acid) (PLLA), PLGA, and polyvinyl alcohol (PVA)] are commonly used for 3D stem cell spheroid generation because of their specific biophysical and cell-adhesive properties. Spheroids after cell aggregation are cultured on the hydrogel matrix or cells are mixed with the liquefied hydrogel and then embedded through gelation to form spheroids. Although the natural polymer contains the biological components of ECM from natural tissues in vivo, the natural polymer-based hydrogel matrix has weak mechanical properties, can be rapidly degraded, and can elicit an immune response. On the other hand, hydrogels fabricated using synthetic polymers can control their biochemical and mechanical properties relatively easily, thus improving the mimicry of ECM. The hydrogel matrix system has disadvantages in producing large amounts of spheroids, controlling the uniform size of spheroids, and scaling up. Self-healable and cross-linked hydrogel microparticles composed of chitosan methacrylate (CHMA) and PVA hybrid hydrogels were developed and used as bioink for fabricating a 3D bioprinted hydrogel matrix with high fidelity and biocompatibility (Fig. 3d) [147]. The 3D bioprinted hydrogel matrix facilitates the fabrication of constructs with precise structures, high aspect ratios, and shapes mimicking the blood vessel, ear, and bone. A hydrogel matrix with an embossed surface formed by a replica molding mimicking commercially available sandpaper surfaces was proposed as a platform for hADSC spheroid formation that can enable the rapid formation and culture of a large quantity of size-controllable stem cell spheroids [148]. A PLGA-based porous hydrogel matrix was developed and cross-linked with cystamine (which contains disulfide bonds) to form a porous hydrogel that could realize “gel–sol” transition via the reduction effect of glutathione [149]. A gellan gum and silk fibroin-based hydrogel matrix with sponge-like structure was created by regulating the blending ratios of polymers; the resulting device acts as a scaffold for stem cell spheroid formation [150]. The thermoreversible hydrogels composed of PNIPAAm-PEG were utilized to confirm the improved expansion, differentiation, and 3D-directed differentiation of the human iPSC into multiple lineages [151]. This 3D thermoreversible hydrogel system demonstrated that the single cells in this 3D environment showed greatly increased viability, pluripotency, and uniform cell proliferation than cells cultured in 2D. In addition, the PNIPAAm-PEG hydrogel culture systems for fabricating hPSC spheroids showed a quick and efficient approach to achieve the rapid fusion capability of tissue spheroids [152]. Using this approach, the authors demonstrated that the PNIPAAm-PEG hydrogel induced a rapid fusion rate of PSC spheroids and matured differentiation of the cortical neural tissue spheroids.
5.5. Magnet-based formation methods
Magnet-manipulated stem cell spheroids are formed by rapidly and compactly aggregating magnetic-labeled stem cells using magnetic nanoparticles [153]. A magnet can control the position of the magnetized stem cell aggregate and move stem cells to the upper (i.e., levitation) or lower part of the culture plate containing the culture medium [154]. The spatial positions of stem cell aggregates or spheroids with magnetic particles, such as biocompatible iron oxide nanoparticles, are maintained because the magnetic force applied overcomes the gravitational force [155]. This spheroid formation system facilitates the induction of rapid cell aggregation, maintains good control over spheroid size, and requires minimal handling. In addition, this method enables the co-culturing of various cell types and the promotion of cell-cell connections. The formation rate of aggregate and growth rate of spheroid is high compared to the more commonly used methods for spheroid formation [156]. The aggregates and spheroids by formed magnetic force can be transferred to the desired location using magnetic tools [154]. However, this method is not very suitable for generating large amounts of aggregates or spheroids. Magnetic patterns with 100 μm diameter microtips composed of 30 × 30 nickel dots were developed using photolithography to guide cardiomyogenesis of embryonic stem cells (Fig. 3e) [19]. The embryonic stem cells incorporated with iron magnetic nanoparticles were aggregated into embryoid bodies, and the spheroids were exposed to a magnet for 5 min per day for 3 d via a cyclically stimulated electromagnet on the cylindrical magnetic patterns. The magnetic nanoparticles having a 200-nm diameter, composed of an iron oxide core coated with polydimethylamine, were used for magnetic levitation culture of human BMSC spheroids within type I collagen gel [19]. The magnetic 3D bioprinting biofabrication system was proposed for generation of spheroids of neural crest-derived mesenchymal stem cells and hDPSCs labeled with NanoShuttle (commercial magnetic nanoparticles) [157].
5.6. Microfluidic-based formation methods
Microfluidic-based engineering has evolved rapidly and has a variety of applications in cell-based analysis, tissue engineering, molecular diagnosis, and drug screening [158]. Microfluidic technology allows for precisely controlling the fluids in micro-scaled channels, which can be applied to 3D culture models [159]. In addition, microfluidic-based platforms enable the formation of a high number of uniform and size-controlled multicellular spheroids. The important functions of a microfluidic-based chip for stem cell spheroid formation include efficiently supplying nutrients and removing waste, providing a suitable chemical concentration gradient, positioning and trapping the cells, having low reagent consumption, and maintaining continuous perfusion and precise control of the pressure and shear stress on the cells [160]. Microfluidic technology also enables accurate replication of cell-cell contact, substrate properties, biochemical and mechanical signals, and stimuli. Microfluidic systems by including miniaturized size and array via microengineering can be used for high-throughput production of spheroids. Microfluidic-based engineering devices for spheroid formation and culture can have continuous-flow with single (e.g., perfusion) and multiple phases (e.g., T-junction, flow-focusing, and double emulsion) [161]. A droplet microfluidic chip based on modulating droplet confinement was proposed for trapping MSCs and guiding spheroid formation into grooves and holes (i.e., anchors) patterned in the device surface (Fig. 3f) [18]. Chips with 500 anchored droplet arrays within 2 cm2 were used to perform quantitative characterization on the population scale; they contained thousands of individual spheroids with hundreds of thousands of cells within each spheroid, correlating functionality with cellular location within the spheroid. The use of PVA-based microgels fabricated via a high-throughput microfluidic technique has been proposed to co-encapsulate stem cells and growth factors for spheroid formation and culture [162].
6. Therapeutic applications of stem cell spheroids
As previously explained, stem cells have been widely applied for various tissue repair and regeneration applications, owing to their ability to differentiate into different types of cells, growth factor secretion ability, and homing effect. However, stem cells implanted into target tissues or organs using local delivery methods exhibit low transplant efficiency, limited survival rate, and rapid loss of transplanted stem cells. Consequently, efficient platforms for integrating the transplanted stem cells into host tissues are needed in order to achieve the goal of tissue repair and regeneration. Although various scaffolds, encapsulation materials, particles, and sheet forms have been proposed as delivery platforms, the construction of cells bound to the platforms has limitations that make it difficult to mimic the in vivo 3D cell-cell and cell-ECM interactions [163]. In contrast, 3D structures of stem cell spheroids showed enhanced biological properties including cell viability, stable morphology, polarization, increased proliferative activity, and physiological metabolic function compared to 3D cell culture systems [21]. Thus, stem cell-based spheroids are used in the field of tissue engineering because of their ability to differentiate along chondrogenic, osteogenic, adipogenic, and neurogenic lineages. In this section, we summarize the applications of stem cell-based spheroids in various tissues for regeneration and repair (e.g., bone, cartilage, tendon, nerve, blood vessel, tooth, and skin) (Table 3 and Fig. 4).
Table 3.
Therapeutic applications of stem cell spheroids for tissue repair and regeneration.
| Tissue | Stem cell | Formation method | Spheroid size | Seeding density | In vivo model (Functional evaluation) | Paracrine factors | Ref. |
|---|---|---|---|---|---|---|---|
| Bone | hADSC | Microfibers, centrifugation | 1.2 mm2 | 2 × 104 cells/100 μL | Calvarial bone defect (Bone regeneration: 42.48 ± 10.84%) | VEGF | [169] |
| hADSC | Nanofibers, centrifugation | 0.425 mm2 | 4 × 104 cells | Calvarial bone defect(Bone regeneration: 59.97 ± 18.33%) | Non-analysis | [168] | |
| BMSC | Ultra-Low Attachment plates | 530 μm | 1 × 104 cells | Calvarial bone defect(Bone regeneration: 77.1 ± 4.1%) | Non-analysis | [170] | |
| BMSC | Nonadherent microwell plates | 300 μm | 1 × 104 cells | Segmental bone defect(Bone volume: 58 mm3) | VEGF | [171] | |
| Cartilage | ADMSC | Non-fouling scaffold | 80–110 μm | 5 × 107 cells/mL | Articular cartilage defect(Collagen type II: 92%GAG: 89%) | Non-analysis | [175] |
| hADSC | Porous scaffold | 100–200 μm | 5 × 107 cells/mL | Articular cartilage defect(Collagen type II: 83%GAGs: 82%) | TGF-β1 | [176] | |
| MSC | Ultra-Low Attachment plates | 291.3 μm | 1 × 104 cells/well | Osteochondraldefect(Cartilage tissue regeneration: 74.0 ± 5.9%) | Non-analysis | [178] | |
| ASC | Porous scaffold | 80–110 μm | 5 × 107 cells/mL | Osteochondraldefect(Collagen type II: 81%,GAGs: 91%) | – | [177] | |
| ADSC | Membrane | ∼90 μm | 8 × 104 cells/well | Articular cartilage defect(High GAG expression) | SDF-1 | [179] | |
| Nerve | hGMSC | Ultra-Low Attachment plates | ∼500 μm | 4 × 104 cells/well | Facial nerve(High β-tubulin IIIand S-100 β expression) | Non-analysis | [188] |
| ADMSC | Sulfonated chitosan well plate | – | 2.8 × 104 cells/cm2 | Nerve conduits(90% differentiation into immature Schwann cells; great peak amplitude and nerve conduction velocity) | HGF, NGF, BDNF, GDNF, and SDF-1 | [117] | |
| Blood vessel | hAMSC | Porous hydrogel | 200–300 μm | 5 × 106 cells/mL | Ischemic limb(Promoted angiogenesis, healthy myofiber, and regeneration of myofiber) | VEGF and IGF-1 | [192] |
| hAMSC | Porous scaffold | 220 μm | 5 × 107 cells/mL | Subcutaneous vascular ingrowth, and adipose tissue regeneration | VEGF and FGF-2 | [190] | |
| Tooth | hDPSC | Agarose microwell | ∼300 μm | 2.6 × 105 cells/190 μL | Regenerated and vascularized dental pulp-like tissue | Non-analysis | [194] |
| hDPSC | Ultra-Low Attachment plates | ∼60 μm | 3.3 × 105 cells in 3 mL of pellet | Regenerative dentin and neurovascular-like structures that mimicked the native teeth | VEGF and TGF-β | [195] | |
| Skin | hADSC | Hanging drop | 200–300 μm | 8000−10000 cells/25 μL | Skin wounds(Wound regeneration: 100%epithelium thickness: 150 μmcollagen density: 40%CD31 expression: 35%) | IL-10 and TGF-β1 | [200] |
| hADSC | Ultra-Low Attachment plates | 1.2–1.5 mm | 7.5 × 104 cells/cm2 | Skin wounds(Rapid wound closure andhigher histological score) | VEGF, FGF, and HGF | [199] | |
| Human placenta-derived MSCs | Hanging drop | 100 μm | 5000 cells/droplet | Skin wound(Fast wound reepithelization and closure,enhanced microvessel density, andhigher expression of paracrine factors) | VEGFa, SDF-1, and Ang1 | [197] | |
| Heart | hBMSC | Hanging drop | 260 μm | 3 × 105 cells | Myocardial infarction(Enhanced vascularization, enhanced Cx43 expression, and improved cardiac function | VEGF, FGF, and HGF | [250] |
| Human cardiac stem cell | Ultra-Low Attachment plates | 95 μm | 5 × 105 | Myocardial infarction(Enhanced neovascularization, decreased fibrotic area, increased left ventricle thickness, and improved cardiac function) | FGF1, FGF2, FGF4, FGF5, FGF7, FGF16, IGF1, NGFβ, NRG4, ZEP91, ANGPT4, EFNA1, EFNA5, EFNB3, PDGFC, VEGFC, GH1, IFNA1, and IFNE1 | [112] | |
| Human cardiac stem cell | 6-well tissue culture plates coated with poly-2-hydroxyethyl methacrylate | 75 μm | 1052 cells | Myocardial infarction(Improved cardiac function and decreased fibrotic area) | SDF-1α and MCP-1 | [201] | |
| Kidney | hMSC | Ultra-Low Attachment plates | 241 μm | 1500 cells | Hypertensive kidney disease(Reduced proteinuria, improved glomerular permselective function) | VEGFA, HGF, IGF, and SDF-1 | [204] |
| hADMSC | Hanging drop | 200 μm | 25,000 cells | Ischemic kidney(Decreased creatinine and BUN levels, reduced tissue apoptosis and damage, and enhanced vascularization) | VEGF, EGF, IGF, bFGF, HGF, and TSG-6 | [113] | |
| Liver | hADMSC | Hanging drop | 500 μm | 1 × 106 cells | Hepatic fibrosis(Reduced hepatic fibrosis, decreased collagen I and collagen III expression, and improved liver function | IGF-1, HGF, and IL-6 | [114] |
| Human teeth-derived Stem cells | Ultra-Low Attachment plates | – | 1 × 105 cells | Hepatic fibrosis(Reduced hepatic fibrosis and reduced bleeding) | TGF-β1 | [206] | |
| human umbilical cord-derived -MSC | Rocker system | 111 μm | 1 × 106 cells | Acute liver failure(Activated liver regeneration, reduced necrosis, and improved regeneration of hepatocytes | IFN-γ, IL-6, and TNF-α | [251] | |
| Lung | hADMSC | PDMS-based microwell | 150 μm | 1 × 105 cells | Emphysema(Recovered alveolar damage and enhanced growth factor production) | FGF-2, VEGF, and HGF | [115] |
Fig. 4.
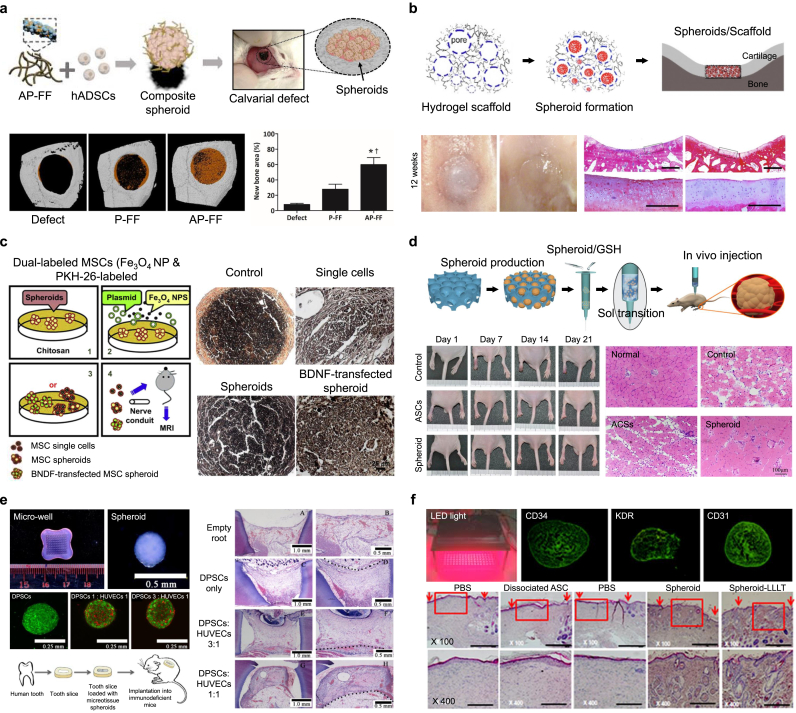
Therapeutic applications of stem cell-based spheroids for tissue regeneration and repair. (a) Bone tissue regeneration and repair using human adipose-derived stem cell (hADSC) spheroids formed by adenosine/polydopamine-coated electrospun nanofiber fragments. Transplantation of spheroids into the calvarial defect promoted significant bone regeneration (image of micro-CT). Reproduced with permission from Ref. [168]. (b) Cartilage tissue regeneration and repair using ADSC spheroids formed by PLGA/chitosan scaffolds with porous structures and surfaces. The defect was repaired with the ADSC spheroids/scaffold (H&E staining). Reproduced with permission from Ref. [175]. (c) Nerve tissue regeneration and repair using BDNF-transfected ADMSC spheroids formed by Fe3O4 nanoparticles and chitosan scaffolds. BDNF-transfected spheroids enhanced the middle portion of the nerve conduit (H&E staining). Reproduced with permission from Ref. [117]. (d) Blood vessel tissue regeneration and repair using ADSC spheroids formed by PLGA-cystamine-PEG monomethyl ether porous hydrogels. Injection of spheroids/hydrogel solved by glutathione promoted angiogenesis and muscle regeneration (H&E staining). Reproduced with permission from Ref. [192]. (e) Dental tissue regeneration and repair using dental pulp stem cell (DPSC) spheroids formed by engineered gelled agarose microplate. Pulp-like tissue regenerated by DPSC spheroids (H&E staining). Reproduced with permission from Ref. [194]. (f) Skin tissue regeneration and repair using ADSC spheroids irradiated by an LED light source. The spheroids enhanced re-epithelialization and granulation (H&E staining). Reproduced with permission from Ref. [199].
6.1. Bone tissue repair and regeneration
Bone is a dynamic and multifunctional tissue that exhibits control of mineral homeostasis, blood formation, and mechanical structure in response to changing physical stress and physiological needs [164]. In the process of bone formation, mechanical and structural stimulation, cell differentiation, and the generation of mineralized organic matrix are synchronized to create hybrid hierarchical structures [165]. In the process of active bone formation and remodeling, the cells (e.g., stem cells and osteoblasts) produce the collagen-based ECM structures and then mineralize them to form new layered bone tissues according to the differentiation of the cells [166,167]. Therefore, when generating functional spheroid implants for bone defects, it is important to improve cell-to-cell interactions and the regeneration capacity for efficient remodeling of bone ECM.
PLLA electrospun nanofiber fragments coated with adenosine and polydopamine were developed to create a 3D stem cell instructive microenvironment for generating stable spheroids using hADSCs (Fig. 4a) [168]. The adenosine-coated nanofibers combined with spheroids significantly improved the expression of adenosine 2b receptor; osteogenic genes such as Runx2, OPN, OCN, and OSX; and mineral deposition. Implantation of engineered spheroids enhanced bone formation in a calvarial defect mouse model. These results indicate stem cells promote differentiation into osteoblasts and the role of A2bR receptors in regulating bone inflammation. Platelet-derived growth factor (PDGF)-coated and bio-mineral-coated nanofibers were proposed as tools for forming stable stem cell spheroids for bone and blood vessel formation in mouse calvarial defects [169]. Implanted spheroids showed improved regeneration capacity by promoting the expression of VEGF and von Willebrand factor. The bone marrow-derived MSC spheroids generated using low attachment round-bottom culture plates were implanted into rat calvarial bone defects and significantly induced the regeneration of bone filled with matured collagenous fibers [170]. Upregulated expression of RUNX-2, OSX, OPN, and BSP genes in spheroids induced accelerated bone formation. Spheroids formed by bone marrow-derived MSCs preconditioned in short-term hypoxic culture were used to increase the cell survival, trophic factor secretion, and bone tissue formation in vivo. The stem cells were preconditioned in 1% O2 in monolayer culture for 3 d before spheroid generation, and hypoxia-preconditioned stem cell spheroids were generated via entrapment in alginate hydrogels [171]. The preconditioned spheroids were transplanted into rat critical-sized femoral segmental bone defects and were shown to promote an increase in angiogenesis, bone volume, bone mineral density, and stiffness compared to the control groups. The enhanced expression of VEGF induced by hypoxia had an effect on the regeneration effect.
6.2. Cartilage tissue repair and regeneration
Cartilage tissue has the limited self-healing capacity, and the damaged cartilage is difficult to repair because of poor blood supply and neural control [172]. Despite advances in the use of stem cells for the repair of the structure and function of damaged cartilage, the regeneration of fibrocartilage and hyaline cartilage expressing collagen type II still remains a challenge [173,174]. Zhang et al. generated rabbit adipose-derived stem cell spheroids using a non-fouling scaffold with porous structures and surfaces using amide-bonded PLGA and chitosan (Fig. 4b) [175]. The spheroids formed in scaffolds promoted chondrogenic differentiation by increasing the expression of glycosaminoglycans (GAGs) and collagen type II. The spheroids implanted into full-thickness articular cartilage defects in rabbits promoted enhanced expression of GAGs and collagen type II and suppression of collagen type I; the tissue had Young's modulus equal to 95% of that of native cartilage. The PLGA-based porous scaffolds were developed with tunable inner surfaces that could sequentially realize cell-scaffold attachment and detachment, as well as facilitate in situ spheroid formation [176]. The porous scaffolds with the spheroids were implanted into full-thickness articular cartilage defects in rabbits and enhanced the expression of GAGs and collagen type II and suppressed the collagen type I; the resulting tissue had a compressive modulus that reached 80% of that of normal cartilage tissue. Another porous scaffold possessing two different regions for forming ADSC spheroids was fabricated, and the porous scaffolds with spheroids resulted in significantly increased formation of cartilage and subchondral bone tissue by expressing TGF-β1 and IGF-1 [177]. Magnetic-responsive spheroids co-cultured with MSCs and magnetic nanoparticles were generated to target cartilage defects with high efficiency [178]. The spheroids showed the upregulated expression of collagen type II, SOX9, and Aggrecan resulted in the regeneration of cartilage tissue. Magnetic-responsive spheroids target-delivered into ex vivo cartilage defects of porcine femur osteochondral discs using an electromagnetic actuation system promoted regeneration of the defect area. ADSC spheroids formed by membrane-type scaffolds with chitosan and hyaluronan have been utilized for repairing cartilage defects through enhanced secretion of SDF-1 [179]. Recently, autologous chondrocyte implantation (ACI) using 3D cell spheroid form developed by CO.DON AG (Berlin, Germany) as a cell therapy is being utilized for the treatment of focal cartilage defects in the European Union [180,181]. These chondrocyte spheroids were prepared by culturing 1 × 105 and/or 2 × 105 cells in agarose hydrogel-coated 96 well plates [182]. Patients Implanted with less than 70 spheroids showed substantial improvement in various clinical outcomes [180,[183], [184], [185]]. Based on these clinical results, it is expected that the treatment efficiency of limited cartilage can be improved if stem cell-based spheroids are used as cell therapy by replacing chondrocytes in this treatment technology with stem cells.
6.3. Nerve tissue repair and regeneration
Because neural networks form complex 3D interwoven structures, mimicking the 3D microenvironment is critical, and this involves cellular, biochemical, mechanical, and topographical cues for the regeneration of target tissues [186]. Recently, it was reported that 3D self-assembled spheroids exhibited mature neuronal electrophysiology and expressed markers of multiple neural cell types [187]. The transplantation of stem cell spheroids improved the induction of nerve tissue regeneration while maintaining neural functions within the complex neural networks. For peripheral nerve regeneration, spheroids of ADMSCs labeled with superparamagnetic nanoparticles or transfected with the brain-derived neurotrophic factor gene were developed on chitosan scaffolds (Fig. 4c) [117]. The spheroids induced the axonal phase of the nerve repair by secretion of SDF-1, NGF, BDNF, and GDNF. Implantation of the engineered spheroids into rats resulted in short gap bridging time, largely regenerated nerves, and a thick myelin sheath. In addition, spheroids of human gingiva-derived mesenchymal stem cells were transplantable into bridge facial nerve defects in rats and improved regenerated nerve diameter, compound muscle action potential recovery, and aligned axonal regeneration by expressing β-tubulin III and β-tubulin III [188].
6.4. Blood vessel repair and regeneration
The development of new vessels is a dynamic process requiring strict regulation by pro- and anti-vascular factors and relies on the close interaction of endothelial cells with perivascular cells and ECM compounds [189]. Stem cell spheroids improved angiogenic efficacy in vivo, according to the hypoxia-induced paracrine secretion of high amounts of VEGF and FGF-2 [190]. Therefore, the implantation of spheroids with cells that have been preconditioned in ischemic environments could be a powerful tool to improve angiogenic efficacy [191]. Hong et al. formed ADSC spheroids using PLGA-based porous hydrogels with gel-sol transition properties for easy injection to treat hind limb ischemia (Fig. 4d) [192]. The injected spheroids improved paracrine secretion, resulting in the promotion of angiogenesis and muscle regeneration. The significant therapeutic effect on limb ischemia is due to the secretion of VEGF and IGF-1 related to pro-angiogenic and immunomodulatory factors by stem cells to support angiogenesis. In addition, porous hydrogel scaffolds based on PLGA activated by EDC and cross-linked by adipic dihydrazide were proposed for inducing the formation of ADSC spheroids. The scaffolds combined with spheroids were implanted into the dorsum of nude mice and significantly promoted subcutaneous vascular ingrowth and adipose tissue regeneration due to enhanced [190]. Spheroids of increasing size are described to induce hypoxia and upregulate VEGF and FGF-2 expression. Therefore, the induction of hypoxia condition in stem cell spheroids is effective to enhance the secretion of angiogenic factors.
6.5. Tooth repair and regeneration
Tooth regeneration has evolved with the aim of regenerating pulp-dentin complexes and repairing damaged functions due to pulp injury and/or inflammation [193]. Establishing an appropriate microenvironment that enables angiogenesis and vasculogenesis development and innervation is a challenge because of the unique anatomical limitations of dental structures. To mimic the microenvironment of the ECM of natural dental pulp tissues, microtissue-concepted spheroids of DPSCs pre-vascularized by human umbilical vein endothelial cells (HUVECs) were established for the regeneration of dental pulp tissues (Fig. 4e) [194]. The spheroids inserted into the canal space of tooth-root slices in vitro were implanted under the skin of immunodeficient mice. The spheroids enhanced the well-vascularized and cellular pulp-like tissues of tooth-root slices, which contained odontoblast-like cells organized along with the dentin. Histological observation demonstrated the expression of human mitochondria and containing odontoblast-like cells organized along with the dentin, as assessed by immunostaining for nestin and dentin sialoprotein. Chen et al. identified multipotent dental pulp regenerative stem cells from mouse dental papilla and showed that the stem cell-based spheroids promoted the osteogenic and odontogenic differentiation for regenerating the pulp-dentinal complex-like tissue and neurovascular-like structures in vivo [195]. Expression of Sp7 as a key transcription factor influenced the formation of dentin, blood vessels and nerve tissue caused the Self-renewal of the stem cells.
6.6. Skin tissue repair and regeneration
Skin wound healing is a complex physiological process involving multiple cell types and bioactive factors, such as chemokines and cytokines, leading to cell proliferation, neovascularization, and granulation tissue generation, followed by epidermal formation [196,197]. However, chronic wounds caused by diabetes, burns, and inflammation can induce serious damage to skin tissue due to improper wound healing processes [198]. Treatment with stem cell-based spheroids has been proposed for stimulating granulation and epithelialization and improving collagen fiber deposition, organization, and thickness. In the implantation of matured spheroids for skin tissue regeneration, ADSC-based spheroids were treated with low-level light irradiation using an LED light source (Fig. 4f) [199]. The LED light-exposed spheroids exhibited up-regulated expression of VEGF, basic fibroblast growth factor (FGF), and hepatocyte growth factor (HGF). Implantation of the spheroids into excisional wound splinting model mice resulted in differentiated endothelial cells and enhanced density of vascular formation and skin tissue regeneration at the lesion site. Enhanced expression of VEGF and FGF induced by the hypoxia condition accelerated the re-epithelialization and neovascularization to promote the wound healing capacity. To efficiently deliver the spheroids into ear skin tissue, ADSC spheroids were encapsulated into microscale alginate beads fabricated using an electrospray system. After injection of hydrogel combined with alginate beads encapsulating the spheroids, it was confirmed that wound healing effectiveness was improved, as demonstrated by granulation, re-epithelialization, well-organized collagen fibrils, increased expression of α-smooth muscle actin (α-SMA), and high expression of angiogenesis [200]. IL-10 and TGF-β1 expression of the spheroids provides the environment for re-epithelization by improving fibroblast proliferation, secretion of matrix proteins, tissue granulation, and α-SMA.
6.7. Organ function repair and regeneration
Stem cell-based spheroids are increasingly being employed In vivo therapeutic applications for recovery of organ functions beyond the specific tissue repair and regeneration. Transplantation of spheroids formed with stem cells is a promising strategy for the repair of cardiac function with infarcted myocardium [14]. Park et al. reported the MSCs in spheroids incorporated with reduced graphene oxide nanomaterials showed improved expression of connexin 43, a gap junction protein, which can attenuate postinfarct arrhythmia [14]. In addition, spheroids engineered with the graphene nanomaterials highly expressed the VEGF, FGF-2, and HGF which are major growth factor that induces angiogenesis, which plays an important role in heart repair. The spheroids transplanted into mouse myocardial infarction models improved the vascularization in the infarcted myocardium, expression of connexin 43 in repaired cardiac area, and cardiac function with reduction of fibrosis. The existence of the nanomaterials in the spheroids enhanced cardiac repair and cardiac function restoration by promoting the expression of angiogenic growth factor and Cx43 of MSC. Cardiac stem cells designed in cell spheroid formulations improved the therapeutic efficacy of ischemic heart disease [112]. The cardiac stem cell spheroids significantly increased expression of the growth-related genes, angiogenic factors, anti-inflammatory factors, cardiopoietic factors, and cardiomyocyte markers after cardiomyocyte differentiation compared to the cardiac stem cell group. Transplanted cardiac stem cell spheroids in the infarcted myocardium induced significant decreases in the infarction size and functional improvement of the infarcted heart by promoting neovascularization. Another group reported the genetically engineered cardiac stem cell spheroids improved cardiac function and decreased fibrotic area [201]. The authors described that the genetically engineered cardiac stem cell spheroids improved cardiac function after acute myocardial infarction by increasing the mRNA expression of SDF-1α and CXCR4.
Stem cell-based therapy has been reported to be effective in reducing glomerular fibrosis and restoring kidney function [202,203]. However, Spheroid-assisted transplantation is promising due to the lack of a three-dimensional (3D) context that mimics the microenvironment of the native glomerulus. Yang et al. reported that the hybrid cell spheroids composed of podocytes, MSCs, and HUVECs promoted the repair of damaged kidneys by confirming the effective reduction of proteinuria in mice with hypertensive nephropathy [204]. MSCs in the hybrid spheroid contributed generation of environment for uniform spheroid constructs because it promotes cell-cell and cell-ECM interactions. In addition, MSCs increased the secretion of laminin and fibronectin and promoted the function of podocytes by highly expressing VEGFA, HGF. Another group reported that spheroids composed of MSCs improved therapeutic effects of acute kidney injury [113]. Spheroids produced higher levels of collagen I, fibronectin, and laminin, and exhibited stronger anti-apoptotic and anti-oxidative capacities. In addition, the spheroids improved reduced cell apoptosis, less tissue damage, increased vascularization in the kidney tissue by promoting secretion of VEGF, FGF, EGF, HGF, IGF, and TSG-6.
MSC-based therapy has been reported to be a promising treatment approach for liver regenerations and functions [205]. Zhang et al. demonstrated the treatment effect of hepatic fibrosis the spheroids derived ADMSCs [114]. The authors described that the enhanced expression of insulin growth factor 1 (IGF-1), interleukin-6 (IL-6), and hepatocyte growth factor (HGF) of spheroids protected the hepatocytes from cell injury and apoptosis. The spheroids improved liver functions by inhibiting hepatic fibrosis due to the reduction of expression levels of collagen I and collagen III. It is undisputed that 3D spheroids composed of stem cells promote the paracrine secretion of beneficial cytokines compared to 2D cultured MSCs. This effect suggested an important role in protecting hepatocytes from cell damage and fibrosis formation as well as liver function and fibrosis. Takahashi et al. developed spheroids using stem cells derived from human exfoliated deciduous teeth for improving liver function due to fibrosis effects [206]. The spheroids transplanted into chronic liver fibrosis of mouse model suppressed the progression of liver fibrosis and structural disarrangement by reducing Acta 2, collagen type I, matrix metalloproteinase 2, Mmp9, tissue inhibitors of matrix metalloproteinase 1, and transforming growth factor beta 1. In addition, the spheroids showed significant anti-hemorrhagic. These studies suggest that they show improved results not only for small-scale tissue regeneration but also for organ tissue regeneration and functional recovery.
7. Engineering techniques for maturation of stem cell organoids
Organoids are formed according to a self-organizing process in which early homogeneous populations of stem cells voluntarily break symmetry and undergo in vivo-like environment formation [207]. However, controlling the maturation of organoids in order to generate higher levels of tissue organization and functionality compared to other commonly used tissue engineering strategies, such as scaffolds, is challenging [208,209]. Developing mature stem cell organoids that possess the functions and physiological properties of native organs is a critical challenge for therapeutic applications, drug screening, and basic biology studies. In this section, we introduce recently developed engineering tools to enhance the maturation of stem cell-based organoids (Table 4).
Table 4.
Engineering tools for maturation of organoids.
| Engineering tool | Materials | Features | Stem cell (Derived species) | Organoid type | Ref |
|---|---|---|---|---|---|
| Microwell | PEG | 400 μm (diameter) | Intestinal stem cells (mouse) | Gastrointestinal organoids | [212] |
| Polystyrene | 500 μm (diameter), 400 μm (depth), and 30 μm (dimple) | iPSCs (human) | Liver bud organoids | [213] | |
| PDMS | 800 μm (diameter) | Embryonic stem cell (human) | Cerebral organoids | [211] | |
| Alginate | 800 μm (diameter) | Adipose-derived MSC (human) | Vascularized organoids | [134] | |
| Microfluidic | PDMS, collagen type 1, Matrigel, | Two external medium reservoirs and two inlet and outlet reservoirs | Intestinal stem cells (human) | Intestinal organoids | [216] |
| PDMS | Pump-free microfluidic device | iPSCs (human) | Brain organoids | [215] | |
| PDMS and hyaluronic acid | Vasculature-like perfusion channel | iPSCs (human) | Retinal organoids | [217] | |
| PDMS | Five parallel functional channels | iPSCs (human) | Brain organoids | [252] | |
| Matrix | Allyl sulfide photodegradable hydrogel | Improved rate of degradation | Intestinal stem cells (mouse) | Intestinal organoids | [227] |
| RGD-functionalized PEG | Mechanically dynamic matrices | Intestinal stem cells (mouse and human) | Intestinal organoids | [226] | |
| Decellularized porcine small intestine mucosa | Biochemical signature of tissue-specific ECM | Intestinal stem cells (mouse) | Endodermal organoids | [118] | |
| PEG-4MAL hydrogels | Well-defined structure, stoichiometric incorporation of biofunctional groups, and tunable reaction timescales for in situ gelation | ESCs and iPSCs (human) | Intestinal organoids | [228] | |
| Alginate | Minimally supportive hydrogel with no inherent cell instructive properties | ESCs and iPSCs (human) | Intestinal organoids | [229] | |
| Amikagels | Improved stiffness, protein adsorption, and surface biochemistry/amine content | ESCs (human) | Islet organoids | [230] | |
| Bioreactor | Miniaturized spinning bioreactor | 12-well size | iPSCs (human) | Brain organoids | [221] |
| NASA-designed rotating-well vessel bioreactors | Rotation of 4, 24.3, 44.5 rpm | ESCs (mouse) | Retinal organoids | [222] | |
| Simple spinner-flask bioreactor | 125 mL spinner flask and rotation of 90 rpm | iPSCs (human) | Kidney organoid | [223] |
7.1. Microwell-based maturation methods
The microwell array contains concave portions with diameters on the microscale, which allows cells to form aggregates with defined shapes and sizes [210]. Microwell-based 3D multicellular formation plates, commonly fabricated using lithography or micropatterning, provide a method for controlling the formation of cell aggregates, such as spheroids and organoids [207]. Microwell array-based platforms are high-throughput with the properties of high-scalability, cost-effectiveness, easy operation, and ability to generate cell aggregates with uniform size and structure [211]. To control the quality of organoid production, Brandenberg et al. developed microwell arrays with a U shape using a PEG hydrogel via soft-lithography-based methods in combination with replica PDMS molding (Fig. 5a) [212]. The developed microwell arrays were fabricated on conventional multi-well plates with 121 microwells, with a diameter of 400 μm per well. The microwell arrays promoted homogeneity and reproducibility of organoids and enabled high-throughput and high-content organoid-based drug screening. The omni-well-array with a U-bottom-shaped microwell plate (500 μm diameter and 400 μm depth of each dimple) was fabricated by employing a molding machine process using resin molds [213]. The omni-well-array plates enabled scalable clinical-scale production of liver bud organoids, efficient differentiation into liver-bud-forming triple progenitors from human iPSCs, and differentiation by stimulating human iPSCs. The mPEG-coated PDMS microwell arrays with highly curved bottoms for human cerebral organoid culture were fabricated by 3D printing reverse molds [211]. The microwell arrays were used to form mature human cerebral organoids with the desired sphericity, organoid size, wrinkling index, lumen size and thickness, and neuronal layer thickness. To form and gently harvest the well-organized organoids, Rossen et al. developed dissolving alginate-based hydrogel microwell arrays [134]. The dissolving hydrogel microwells were used to produce a large number of vascularized organoids with reproducible size and structure. The generated organoids promoted the restoration of perfusion and muscle fiber regeneration upon injection into an ischemic hindlimb.
Fig. 5.
Various engineering techniques for maturation of specific stem cell-induced organoids. (a) Microwell plate maturation method using microwell arrays with U shape using poly(ethylene glycol) (PEG) hydrogel for generating the intestinal organoids. Reproduced with permission from Ref. [212]. (b) Microfluidic-based maturation method using perfusion-based pump-free microfluidic culture devices for generating cerebral organoids. Microfluidic devices reproducibly improved the organization of progenitor zones and cortical layers in brain organoids. Reproduced with permission from Ref. [215]. (c) Bioreactor and spinner platform-based maturation method using miniaturized multiwell spinning bioreactor systems of 12 well size for generating cerebral organoids including the forebrain, midbrain, and Hypothalamic organoids. Reproduced with permission from Ref. [221]. (d) Hydrogel matrix-based maturation method using synthetic hydrogel networks and mechanically dynamic PEG polymer matrix for generating intestinal organoids. Organoids formed in PEG hydrogels contain differentiated intestinal cells. Reproduced with permission from Ref. [226].
7.2. Microfluidic-based maturation methods
Microfluidic-based platforms provide an efficient system for formation and maturation of organoids because they enable the continuous infusion of nutrients and growth factors, as well as precise replication of cell-cell contacts, matrix characteristics, biochemical and mechanical cues, and stimuli [124]. Microfluidics enabled the creation of organoids associated with early human development on a scale that is not possible using conventional cell culture methods [214]. To achieve considerable advances in organoid models, organoid-on-a-chip combined with microfluidic systems can be used for more advanced and accurate drug and treatment studies by combining multiple organs on the same platform. Pump-free perfusion-based microfluidic culture devices were developed for generating matured human cerebral organoids from human iPSCs (Fig. 5b) [215]. Cerebral organoids were developed by reconstructing 3D brain-imitating microenvironments with decellularized human brain tissue-derived extracellular substrates and dynamic microfluidic systems. As a culture platform for intestine organoids, hybrid microchip systems were developed by combining tubular hydrogel scaffolds in a microchannel with an in vivo-like anatomical intestine structure [216]. The hybrid microchip promotes the self-organization of intestinal stem cells into a functional intestinal epithelium and induces the formation of organoids with the diverse cell types observed in intestinal systems in vivo. A retina organoid-on-a-chip was proposed as a microphysiological model of the human retina, integrating more than seven different essential retinal cell types derived from hiPSCs [217]. The developed organoid chips consisted of four identical micro-tissues connected through microchannels, two PDMS layers, and porous PET membranes with transparency and biocompatibility. The retina organoid-on-a-chip improved the formation of outer segment-like structures and the establishment of in vivo-like physiological processes, such as outer segment phagocytosis and calcium dynamics. Multilayer micro fluidic devices with a through-hole PDMS layer and polycarbonate porous membrane were developed for generating the islet organoids [218]. The microchip devices provided the easy integration of controlled embryonic body formation, in situ differentiation, and generation of heterogeneous islet organoids and enabled long-term 3D culture of islet organoids with cell viability- and islet-specific functions, owing to the continuous perfusion system.
7.3. Bioreactor system-based maturation methods
Suspension culture with a rotating bioreactor provides enhanced diffusion of oxygen and nutrients to support the growth and expansion of 3D tissues despite the absence of vascular structures [219]. Rotating-wall vessel culture is a relatively easy method for generating and maintaining organoids compared with other methods because improved nutrient exchange allows for consistent formation of organoids with superior morphology and unique organotypic gene expression [220]. The bioreactor system slowly rotates the liquid-filled cylinder on the axis and gently drags the fluid and all the particles in a perfectly circular path. Bioreactor systems facilitate the rapid generation of large and highly viable organoids and allow for high-throughput organoid generation for drug discovery, personalized medicine, and tissue therapy [220]. Miniaturized spinning bioreactor systems using 12-well cell culture plates were applied to generate cerebral organoids from human iPSCs (Fig. 5c) [221]. The organoids generated using the proposed bioreactor system improved human cortical development, including multi-layer progenitor zone organization, neurogenesis, gene expression, and a distinct human-specific outer radial glia cell layer. NASA-designed rotating-wall vessel bioreactors were utilized for generating retinal organoids using mouse-derived pluripotent stem cells. The rotating wall vessel promoted a well-defined morphology and increased proliferation and differentiation capacity of neurons including ganglion cells and S-cone photoreceptors [222]. Przepiorski et al. generated numerous kidney organoids from human iPSCs using 125-mL spinner flask bioreactors, which allowed for large-scale organoid production using a cost-effective protocol. The generated organoids formed nephrons containing podocytes, proximal and distal tubule segments, presumptive collecting ducts, and endothelial cells [223].
7.4. Hydrogel matrix-based maturation methods
Development of the hydrogel matrix and integration with microscale technology and stem cell biology has provided great opportunities for model systems of tissues and organs to be more realistic [224]. The organoids generated using other common methods are typically formed in the absence of essential components of in vivo microenvironments, such as the ECM [225]. The hydrogel matrix, with high biocompatibility and tunable properties, such as permeability, elasticity, stiffness, and chemical reactivity, exhibits similar properties to the microenvironment of native ECM. Various natural and synthetic hydrogel-base materials have been extensively used as alternatives to Matrigel, a hydrogel commonly used for guiding the formation of matured organoids. The modular synthetic hydrogel networks were developed using a mechanically dynamic PEG polymer matrix for the well-defined intestinal organoid formation systems from mouse intestinal stem cells (Fig. 5e) [226]. Furthermore, in the case of switching intestinal stem cell colonies to intestinal organoids, the mechanically dynamic PEG polymer matrix exhibited stronger induction of differentiation of intestinal cells, including high YAP activation, compared with the stable PEG matrix. The allyl sulfide photodegradable hydrogel based on PEG macromer was used to achieve rapid degradation through radical addition-fragmentation chain transfer (AFCT) reactions, to support routine passaging of intestinal organoids [227]. The synthetic hydrogel matrix based on a four-arm PEG macromer with maleimide groups at each terminus was developed for growth and expansion of human intestinal organoids [228]. The developed hydrogel matrix induced the matured intestinal tissue structure and was used as the delivery platform of intestine organoids via injection into murine intestinal mucosal wounds. A natural polymer, decellularized ECM, provides structural support and delivers biochemical signals that are fundamental for assisting the regeneration process for organoid formation. ECM hydrogel matrix derived from decellularized porcine small intestine mucosa was used for forming gastric, hepatic, pancreatic, and small intestinal tissue [118]. Unmodified native alginate polymer crosslinked by calcium was utilized as a simple hydrogel system to induce human intestinal organoids for transplantation into immunocompromised mice [229]. Intestinal organoids formed by the alginate hydrogel systems exhibited a level of engraftment and maturation similar to that of organoids grown under the Matrigel matrix. As a novel hydrogel matrix for 3D cell culture systems, a hydrogel platform based on amine-glycidylether coupled hydroxyl linkages was used for generating mature pancreatic organoids [230].
8. Therapeutic applications of stem cell organoids
The need for organ development research models and the limitations of donor sources for organ transplantation have triggered the study of the development of functional substitutes for organs [231]. Organoid systems have spatially organized biomimetic cellular structures on both microscopic and macroscopic scales, as well as diverse cell phenotypes and extracellular environments [232]. There is a growing interest in using various organoids developed and matured by advanced engineering in regenerative medicine [233]. The use of organoids can advance regenerative medicine because transplants with organ-specific tissues could be more successfully integrated into the patient's own tissues compared with stem cells [234,235]. For regenerative medicine, obtaining mature organoids is crucial to achieve vascularization, minimize the chances of unwanted differentiation after transplantation, and promote function. Organoids contain stem cells and are likely to develop into an entire organ; hence, organoids can provide a source of autologous cells and tissues for transplantation. In this section, we introduce the recent therapeutic applications of various organoids generated from stem cells for organ therapy (Table 5 and Fig. 6).
Table 5.
Stem cell-derived organoid-based therapies for regeneration of tissues and restoration of organ function.
| Target organ | Stem cell type (co-culture cell) | Formation method | Seeding density | In vivo model (Functional evaluation) | Ref. |
|---|---|---|---|---|---|
| Bone | Human periosteum-derived stem cells | Agarose microwell | 5 × 105 cells | Segmental bone defect (Bone regeneration: 83.3%/8 weeks) | [236] |
| Blood-vessel | Mouse MSCs (HUVEC) | Alginate microwell | 0.5 × 106 cells | Ischemic hindlimb mouse model (Rapid restoration of vascular perfusion and muscle fiber regeneration) | [134] |
| Hair bearing skin | PSC | Defined protocol | 3500 cells | 2–5-mm hairs growing out, rete-ridge-like structures, vasculature, and sebaceous glands and bulge | [60] |
| IntestineIntestine | ESC and iPSC | Synthetic polymer hydrogel matrix | – | Improved engraftment and colonic wound healing | [228] |
| Intestinal stem cell | Matrigel, non-adherent plates plate | – | Epithelial regeneration with crypt and villus structures; differentiation into various intestinal epithelial cells | [237] | |
| Liver | iPSC | Microwell | 900 cells | Efficient delivery and attachment of organoid into liver | [238] |
| Brain | ESC | Ultra-low attachment 96-well plate | 1.35 × 104 cells | Enhanced neurogenesis, synaptic reconstruction, axonal regeneration, and angiogenesis, and decreased neural apoptosis | [239] |
Fig. 6.
Therapeutic applications of stem cell-induced organoids for regeneration of tissue and restoration of organ function. (a) Bone tissue repair and regeneration using human periosteum-derived stem cells-derived multiple callus organoids developed by agarose microwell. Reproduced with permission from Ref. [236]. Callus organoids were assembled to form larger constructs in agarose well. Assembled callus organoids enhanced regeneration of critical-sized long bone defects. (b) Blood vessel and muscle tissue repair and regeneration using vascularized organoids. Mouse MSCs and HUVECs-derived vascularized organoids developed by sacrificial alginate microwells. Prevascularized organoids regained perfusion of the ischemic limb, had more viable myofibers, and exhibited regenerating myofibers. Reproduced with permission from Ref. [134]. (c) Skin tissue repair and regeneration using cyst-like skin organoids. Cyst-like skin organoids derived from human ESCs were used for reconstructing appendage-bearing skin tissue. The developed organoids enhanced the formation of planar hair-bearing skin, including hair follicles and sweat glands. Reproduced with permission from Ref. [60]. (d) Intestinal tissue repair and regeneration using intestinal organoids. Intestinal organoids generated by encapsulation of hPSCs into a synthetic four-armed maleimide-terminated PEG hydrogel. The matured organoids improved the mucosal colonic wound closure. Reproduced with permission from Ref. [228].
Stem cells derived from human periosteum have been used for the scalable production of microspheroids that are differentiated into callus organoids (Fig. 6a) [236]. The assembly of multiple callus organoids into an easy-to-handle scaffold-free implant resulted in the complete bridging of a critical-sized long bone defect via the formation of cortical-like bone tissue with a medullary cavity containing bone marrow with the absence of fibrous tissue. In addition, the structure of the regenerated defect was similar to that of the native tibia. The effectiveness of vascularized organoids in the treatment of ischemic conditions observed in peripheral artery disease was evaluated (Fig. 6b) [134]. The vascularized organoids formed by dissolvable alginate microwells, which enable the formation of highly reproducible, scalable, and easy-to-harvest organoids, improved rapid restoration of vascular perfusion and muscle fiber regeneration. Cyst-like skin organoids derived from human embryonic stem cells were used for reconstructing appendage-bearing skin tissue (Fig. 6c) [60]. The developed organoids had cranial epithelial cells and neural crest cells that enhanced the formation of planar hair-bearing skin, including hair follicles and sweat glands, when grafted onto nude mice. Intestinal organoids generated by encapsulation of hPSCs into a synthetic hydrogel matrix based on the four-armed maleimide-terminated PEG hydrogel were delivered to repair colonic mucosal intestine wounds (Fig. 6d) [228]. Intestinal organoids developed using the PEG hydrogel system allowed for differentiated intestinal epithelium, including an intestine with crypt–villus architecture and underlying lamina propria, muscularis mucosae, and submucosa with structured collagen fibers and differentiated goblet cells. The matured organoids exhibited the enhanced engraftment into host intestinal epithelial tissue and significantly improved the mucosal colonic wound closure. Jee et al. evaluated the ability of colon organoids to treat radiation proctitis. After induction of radiation proctitis by irradiating the recta, the colon organoids generated from colonic crypts were transplanted into mice [237]. The transplanted colon organoids were found to successfully engraft onto the damaged rectal mucosa of the irradiated mice, owing to which they reconstituted epithelial structure and integrity. The liver organoids derived from human iPSCs were transplanted for treatment of chronic liver damage [238]. The liver organoids promoted significant reconstruction of hepatocytes and replacement of 70% of the damaged liver. Cerebral organoids induced from human embryonic stem cells were transplanted into model rats with middle cerebral artery occlusion to investigate stroke treatment capacity. The transplanted cerebral organoids significantly reduced brain infarct volume, improved neurological motor function, showed multilineage differentiation potential, supported motor cortex region-specific reconstruction, formed neurotransmitter-related neurons, and achieved synaptic connection [239].
9. Limitations, challenges, and prospects
9.1. Limitations
Since the emergence of tissue engineering and regenerative medicine field, the technologies and applications of stem cells have been actively studied to restore damaged tissues or organs. A variety of engineering (e.g., biomaterial, genetic modification, external stimuli, molecule, and ECM) have been proposed to control engraftment, viability, renewal, proliferation, differentiation, and paracrine effect of stem cells. Nevertheless, the limitations for increasing treatment efficiency of the viability and loss of stem cells transplanted into tissues or organs still remain as the task to be researched. If the damaged area of tissues or organs is large or continuous treatment is required, transplantation of a sufficient amount of stem cells can maximize the treatment efficiency. Since the area, disease mechanisms, and biological properties of damaged human tissues are diverse, a guideline on the appropriate amount of stem cells for maximizing therapeutic efficacy is needed. Currently, in the European Union, spheroids composed of autologous chondrocytes are used for the treatment of focal human cartilage defects [181]. This spheroid therapy contains 1 × 105 or 2 × 105 cells, and in clinical application studies, less than 70 spheroids are implanted in the focal cartilage defect (>2 cm2) of the patients. If it is expanded to spheroids using stem cells based on these clinical trials, it has the potential to provide information on stem cell density and spheroid size in clinical studies using stem cell-based spheroids. In addition, it is important to establish a standardized system that can secure a large number of stem cells for the treatment of the large area. Stem cell therapy consumes a lot of time and money. Therefore, technologies to maximize survival and to prevent cell loss for efficiently delivering a large number of stem cells should be actively developed to maximize treatment efficiency without complicated procedures. 3D cultured stem cells could be a good alternative to the transplantation of 2D cultured stem cells.
9.2. Challenges
3D stem cell culture systems, such as spheroids and organoids, are promising therapeutic strategies that can overcome the limitations of stem cell therapy. However, there are several limitations and challenges to be addressed. Spheroids and organoids have some limitations in mass production for high-throughput screening and therapeutic application. Commonly used cell culture plates with round bottom and low attachment are difficult to control the shape of spheroids and organoids and lacks flexibility and controllability in terms of size. In addition, there is a limit to mass production as expanding the culture surface area is not possible. These limitations can be overcome by the technological realization of automated high-throughput production and directed fusion into large tissues. Recently, the formation of 1500 spheroids/ml was achieved using a bioreactor-based 3D cell culture system [240]. Based on this point, the combination of general 3D culture methods and micro/nanoengineering can produce a large number of spheroids and organoids, and at the same time, it is possible to produce a suitable treatment. Micro-fluidic platforms and 3D bioprinting allow the mass production and fused organization of spheroids and organoids of uniformly sized spheroids. In addition, nanoscale biomaterials (e.g., nanoparticles, nanocapsules, and nanofibers) are promising as functional additive materials to modulate the spheroid formation process and therapeutic potential. Furthermore, functional nanomaterials facilitate improving the engraftment, viability, renewal, proliferation, differentiation, and paracrine effect of stem cell spheroids. Recently, with the advancement of 3D bioprinting, it has become possible to construct a tissue body maintained in a desired position by printing and fusing spheroids and organoids as bio-inks [241]. In addition, this approach improves the production and efficiency of various cellular structures [242]. However, Spheroids and organoids are required to be mass-produced in a standardized and uniform size and to construct tissues with similar biological properties to apply clinical trials. Therefore, functional biomaterials, physical/structural modulation, and tissue organization technology combined with the existing 3D cell culture system can lead to the development and production of spheroids and organoids suitable for clinical therapeutic applications.
9.3. Prospects
In the near future, organoids may serve as a promising strategy as regenerative therapies for the regeneration of damaged tissues and the replacement of parts in damaged organs. Despite considerable success in culturing physiologically relevant organoids, research on regenerative therapy through transplantation of organoids is still in its infancy and challenges to achieve practical applications remain. Since organoids have already differentiated with functions similar to those of organs, there is a problem of engraftment and fusion at the transplant organ site. In addition, organoids are limited in reproducing the function of a fully mature organ with current technology and cannot be expanded to the scale of native organs. In addition, the organoids currently developed have limitations in completely mimicking the functions of the vascular system, the immune system, and the nervous system. Therefore, it is a challenge to reach full maturity using organ on a chip, co-culture, and vascularization based on the current organoid culture methodologies through temporal, spatial, and chemical control. After the establishment of fully matured organoids, technology development for mass-producing organoids of uniform size should be actively conducted. Organoid technology can be extended to personalized cancer research and biotherapy using patients' autologous stem cells and the application of artificial organ transplantation. Recently, research for drug screening by developing organoids using patients’ adult stem cells, ESCs, and iPSCs are emerging, enabling the realization of personalized medicine [[243], [244], [245]]. Personalized stem cell organoids can overcome the transplant immune rejection of artificial organ transplantation. The fusion of micro/nanoengineering-based maturation, biological factors, and mass production systems will enable regenerative therapy through personalized organoid transplantation.
10. Conclusion
Spheroids and organoids have been developed for advanced therapeutic applications. In this review, we summarized the therapeutic applications of functional 3D stem cell culture using state-of-the-art engineering technologies for tissue regeneration and repair. Compared with stem cell therapy, which is limited by low transplant efficiency, limited survival rate, and rapid loss of transplanted stem cells in the host tissue, the spheroids and organoids enable efficient tissue regeneration and repair, owing to their more in vivo-like structure. The use of advanced engineering technologies could improve the production, size, and maturity of spheroids and organoids to achieve the goals of efficient tissue repair and regeneration and enhanced organ function. Organoids have started to gain attention for their ability to promote tissue regeneration, especially considering the limitations of donor sources for organ transplantation. We envisage that spheroids and organoids generated using 3D stem cell culture systems will overcome the limitations of current stem cell therapy and will be used in the regeneration and repair of tissues or organs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (NRF-2021R1A4A3025206, NRF-2019M3A9H1103737, NRF-2021M3E5E7026407, and NRF-2019R1I1A3A0106345).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Pampaloni F., Reynaud E.G., Stelzer E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 2.Huh D., Hamilton G.A., Ingber D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21:745–754. doi: 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrila J., Radtke A.L., Crabbé A., Sarker S.F., Herbst-Kralovetz M.M., Ott C.M., et al. Organotypic 3D cell culture models: using the rotating wall vessel to study host–pathogen interactions. Nat. Rev. Microbiol. 2010;8:791–801. doi: 10.1038/nrmicro2423. [DOI] [PubMed] [Google Scholar]
- 4.Wendt D., Riboldi S.A., Cioffi M., Martin I. Potential and bottlenecks of bioreactors in 3D cell culture and tissue manufacturing. Adv. Mater. 2009;21:3352–3367. doi: 10.1002/adma.200802748. [DOI] [PubMed] [Google Scholar]
- 5.Ong C.S., Zhou X., Han J.N., Huang C.Y., Nashed A., Khatri S., et al. In vivo therapeutic applications of cell spheroids. Biotechnol. Adv. 2018;36:494–505. doi: 10.1016/j.biotechadv.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang P., Sun A.X., An J., Chua C.K., Chew S.Y. 3D neural tissue models: from spheroids to bioprinting. Biomaterials. 2018;154:113–133. doi: 10.1016/j.biomaterials.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Breslin S., O'Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov. Today. 2013;18:240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Cui X., Hartanto Y., Zhang H. Advances in multicellular spheroids formation. J. R. Soc. Interface. 2017;14 doi: 10.1098/rsif.2016.0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duval K., Grover H., Han L.-H., Mou Y., Pegoraro A.F., Fredberg J., et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology. 2017;32:266–277. doi: 10.1152/physiol.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 10.Fennema E., Rivron N., Rouwkema J., van Blitterswijk C., De Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013;31:108–115. doi: 10.1016/j.tibtech.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Laird D.J., von Andrian U.H., Wagers A.J. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 12.Bianco P., Robey P.G. Stem cells in tissue engineering. Nature. 2001;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 13.Lane S.W., Williams D.A., Watt F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014;32:795–803. doi: 10.1038/nbt.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ark J., Kim Y.S., Ryu S., Kang W.S., Park S., Han J., et al. Graphene potentiates the myocardial repair efficacy of mesenchymal stem cells by stimulating the expression of angiogenic growth factors and gap junction protein. Adv. Funct. Mater. 2015;25:2590–2600. doi: 10.1002/adfm.201500365. [DOI] [Google Scholar]
- 15.Lewis E.E.L., Wheadon H., Lewis N., Yang J., Mullin M., Hursthouse A., et al. A quiescent, regeneration-responsive tissue engineered mesenchymal stem cell bone marrow niche model via magnetic levitation. ACS Nano. 2016;10:8346–8354. doi: 10.1021/acsnano.6b02841. [DOI] [PubMed] [Google Scholar]
- 16.Richards D.J., Tan Y., Coyle R., Li Y., Xu R.Y., Yeung N., et al. Nanowires and electrical stimulation synergistically improve functions of hiPSC cardiac spheroids. Nano Lett. 2016;16:4670–4678. doi: 10.1021/acs.nanolett.6b02093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo J., Lee J.S., Lee K., Kim D., Yang K., Shin S., et al. Switchable water‐adhesive, superhydrophobic palladium‐layered silicon nanowires potentiate the angiogenic efficacy of human stem cell spheroids. Adv. Mater. 2014;26:7043–7050. doi: 10.1002/adma.201402273. [DOI] [PubMed] [Google Scholar]
- 18.Sart S., Tomasi R.F.-X., Amselem G., Baroud C.N. Multiscale cytometry and regulation of 3D cell cultures on a chip. Nat. Commun. 2017;8:1–13. doi: 10.1038/s41467-017-00475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mary G., Van de Walle A., Perez J.E., Ukai T., Maekawa T., Luciani N., et al. High‐throughput differentiation of embryonic stem cells into cardiomyocytes with a microfabricated magnetic pattern and cyclic stimulation. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 20.Jeong G.S., Lee J., Yoon J., Chung S., Lee S.-H. Viscoelastic lithography for fabricating self-organizing soft micro-honeycomb structures with ultra-high aspect ratios. Nat. Commun. 2016;7:1–9. doi: 10.1038/ncomms11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laschke M.W., Menger M.D. Life is 3D: boosting spheroid function for tissue engineering. Trends Biotechnol. 2017;35:133–144. doi: 10.1016/j.tibtech.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y.B., Kim E.M., Byun H., Chang H.K., Jeong K., Aman Z.M., et al. Engineering spheroids potentiating cell-cell and cell-ECM interactions by self-assembly of stem cell microlayer. Biomaterials. 2018;165:105–120. doi: 10.1016/j.biomaterials.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 23.Kim S., Cho A.N., Min S., Kim S., Cho S.W. Organoids for advanced therapeutics and disease models. Adv. Therap. 2019;2 [Google Scholar]
- 24.Kratochvil M.J., Seymour A.J., Li T.L., Paşca S.P., Kuo C.J., Heilshorn S.C. Engineered materials for organoid systems. Nat. Rev. Mater. 2019;4:606–622. doi: 10.1038/s41578-019-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roper J., Tammela T., Cetinbas N.M., Akkad A., Roghanian A., Rickelt S., et al. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat. Biotechnol. 2017;35:569–576. doi: 10.1038/nbt.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huch M., Gehart H., Van Boxtel R., Hamer K., Blokzijl F., Verstegen M.M., et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garreta E., Kamm R.D., de Sousa Lopes S.M.C., Lancaster M.A., Weiss R., Trepat X., et al. Rethinking organoid technology through bioengineering. Nat. Mater. 2020:1–11. doi: 10.1038/s41563-020-00804-4. [DOI] [PubMed] [Google Scholar]
- 28.Wilson H.V. A new method BY WHICH sponges may be artificially reared. Science. 1907;25:912–915. doi: 10.1126/science.25.649.912. [DOI] [PubMed] [Google Scholar]
- 29.Harrison R.G., Greenman M., Mall F.P., Jackson C. Observations of the living developing nerve fiber. Anat. Rec. 1907;1:116–128. [Google Scholar]
- 30.Holtfreter J. Experimental studies on the development of the pronephros. Rev. can biol. 1943;3:220–250. [Google Scholar]
- 31.Weiss P., Taylor A. Reconstitution of complete organs from single-cell suspensions of chick embryos in advanced stages of differentiation. Proc. Natl. Acad. Sci. U. S. A. 1960;46:1177. doi: 10.1073/pnas.46.9.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutherland R.M., McCredie J.A., Inch W.R. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J. Natl. Cancer Inst. 1971;46:113–120. [PubMed] [Google Scholar]
- 33.Rheinwatd J.G., Green H. Seria cultivation of strains of human epidemal keratinocytes: the formation keratinizin colonies from single cell is. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 34.Rheinwald J.G., Green H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell. 1975;6:317–330. doi: 10.1016/0092-8674(75)90183-x. [DOI] [PubMed] [Google Scholar]
- 35.Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. Unit. States Am. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 39.Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M., et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Sato T., Vries R.G., Snippert H.J., Van De Wetering M., Barker N., Stange D.E., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 41.Spence J.R., Mayhew C.N., Rankin S.A., Kuhar M.F., Vallance J.E., Tolle K., et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Lancaster M.A., Renner M., Martin C.-A., Wenzel D., Bicknell L.S., Hurles M.E., et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia Y., Nivet E., Sancho-Martinez I., Gallegos T., Suzuki K., Okamura D., et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat. Cell Biol. 2013;15:1507–1515. doi: 10.1038/ncb2872. [DOI] [PubMed] [Google Scholar]
- 45.Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 46.Dye B.R., Hill D.R., Ferguson M.A., Tsai Y.-H., Nagy M.S., Dyal R., et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 2015;4 doi: 10.7554/eLife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang L., Holtzinger A., Jagan I., BeGora M., Lohse I., Ngai N., et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell–and patient-derived tumor organoids. Nat. Med. 2015;21:1364–1371. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurmann A.A., Serra M., Hawkins F., Rankin S.A., Mori M., Astapova I., et al. Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell. 2015;17:527–542. doi: 10.1016/j.stem.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoang P., Wang J., Conklin B.R., Healy K.E., Ma Z. Generation of spatial-patterned early-developing cardiac organoids using human pluripotent stem cells. Nat. Protoc. 2018;13:723. doi: 10.1038/nprot.2018.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wimmer R.A., Leopoldi A., Aichinger M., Wick N., Hantusch B., Novatchkova M., et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019;565:505–510. doi: 10.1038/s41586-018-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCracken K.W., Catá E.M., Crawford C.M., Sinagoga K.L., Schumacher M., Rockich B.E., et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barker N., Huch M., Kujala P., van de Wetering M., Snippert H.J., van Es J.H., et al. Lgr5+ ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 53.Huch M., Dorrell C., Boj S.F., Van Es J.H., Li V.S., Van De Wetering M., et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huch M., Bonfanti P., Boj S.F., Sato T., Loomans C.J., Van De Wetering M., et al. Unlimited in vitro expansion of adult bi‐potent pancreas progenitors through the Lgr5/R‐spondin axis. EMBO J. 2013;32:2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J.-H., Bhang D.H., Beede A., Huang T.L., Stripp B.R., Bloch K.D., et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams K.E., Lemieux G.A., Hassis M.E., Olshen A.B., Fisher S.J., Werb Z. Quantitative proteomic analyses of mammary organoids reveals distinct signatures after exposure to environmental chemicals. Proc. Natl. Acad. Sci. Unit. States Am. 2016;113:E1343–E1351. doi: 10.1073/pnas.1600645113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren W., Lewandowski B.C., Watson J., Aihara E., Iwatsuki K., Bachmanov A.A., et al. Single Lgr5-or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc. Natl. Acad. Sci. Unit. States Am. 2014;111:16401–16406. doi: 10.1073/pnas.1409064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koehler K.R., Mikosz A.M., Molosh A.I., Patel D., Hashino E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature. 2013;500:217–221. doi: 10.1038/nature12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hisha H., Tanaka T., Kanno S., Tokuyama Y., Komai Y., Ohe S., et al. Establishment of a novel lingual organoid culture system: generation of organoids having mature keratinized epithelium from adult epithelial stem cells. Sci. Rep. 2013;3:1–10. doi: 10.1038/srep03224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J., Rabbani C.C., Gao H., Steinhart M.R., Woodruff B.M., Pflum Z.E., et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature. 2020;582:399–404. doi: 10.1038/s41586-020-2352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J., Bae W.-G., Choung H.-W., Lim K.T., Seonwoo H., Jeong H.E., et al. Multiscale patterned transplantable stem cell patches for bone tissue regeneration. Biomaterials. 2014;35:9058–9067. doi: 10.1016/j.biomaterials.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 62.Raghunath J., Salacinski H.J., Sales K.M., Butler P.E., Seifalian A.M. Advancing cartilage tissue engineering: the application of stem cell technology. Curr. Opin. Biotechnol. 2005;16:503–509. doi: 10.1016/j.copbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 63.Park S., Park H.-H., Sun K., Gwon Y., Seong M., Kim S., et al. Hydrogel nanospike patch as a flexible anti-pathogenic scaffold for regulating stem cell behavior. ACS Nano. 2019;13:11181–11193. doi: 10.1021/acsnano.9b04109. [DOI] [PubMed] [Google Scholar]
- 64.Go G., Jeong S.-G., Yoo A., Han J., Kang B., Kim S., et al. Human adipose–derived mesenchymal stem cell–based medical microrobot system for knee cartilage regeneration in vivo. Sci. Robot. 2020;5 doi: 10.1126/scirobotics.aay6626. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y., Jin S., Luo D., He D., Shi C., Zhu L., et al. Functional regeneration and repair of tendons using biomimetic scaffolds loaded with recombinant periostin. Nat. Commun. 2021;12:1–19. doi: 10.1038/s41467-021-21545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J., Li Z., Chu D., Jin L., Zhang X. Fabrication and biocompatibility of core–shell structured magnetic fibrous scaffold. J. Biomed. Nanotechnol. 2019;15:500–506. doi: 10.1166/jbn.2019.2701. [DOI] [PubMed] [Google Scholar]
- 67.Ouyang J., Ji X., Zhang X., Feng C., Tang Z., Kong N., et al. In situ sprayed NIR-responsive, analgesic black phosphorus-based gel for diabetic ulcer treatment. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117:28667–28677. doi: 10.1073/pnas.2016268117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Z., Zhang X., Guo Z., Shi L., Jin L., Zhu L., et al. Nature-derived bionanomaterials for sustained release of 5-fluorouracil to inhibit subconjunctival fibrosis. Mater. Today Adv. 2021;11 [Google Scholar]
- 69.Sui B.-D., Hu C.-H., Liu A.-Q., Zheng C.-X., Xuan K., Jin Y. Stem cell-based bone regeneration in diseased microenvironments: challenges and solutions. Biomaterials. 2019;196:18–30. doi: 10.1016/j.biomaterials.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 70.Clark A.Y., Martin K.E., García J.R., Johnson C.T., Theriault H.S., Han W.M., et al. Integrin-specific hydrogels modulate transplanted human bone marrow-derived mesenchymal stem cell survival, engraftment, and reparative activities. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-019-14000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phelps E.A., Enemchukwu N.O., Fiore V.F., Sy J.C., Murthy N., Sulchek T.A., et al. Maleimide cross‐linked bioactive peg hydrogel exhibits improved reaction kinetics and cross‐linking for cell encapsulation and in situ delivery. Adv. Mater. 2012;24:64–70. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu J., Li G., Ye T., Lu G., Li R., Deng L., et al. Stem cell-laden injectable hydrogel microspheres for cancellous bone regeneration. Chem. Eng. J. 2020;393 [Google Scholar]
- 73.Watanabe J., Yamada M., Niibe K., Zhang M., Kondo T., Ishibashi M., et al. Preconditioning of bone marrow-derived mesenchymal stem cells with N-acetyl-L-cysteine enhances bone regeneration via reinforced resistance to oxidative stress. Biomaterials. 2018;185:25–38. doi: 10.1016/j.biomaterials.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 74.Debnath S., Yallowitz A.R., McCormick J., Lalani S., Zhang T., Xu R., et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562:133–139. doi: 10.1038/s41586-018-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Z., Zhang X., Ouyang J., Chu D., Han F., Shi L., et al. Ca2+-supplying black phosphorus-based scaffolds fabricated with microfluidic technology for osteogenesis. Bioact. Mater. 2021;6:4053–4064. doi: 10.1016/j.bioactmat.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang R., Chen X., Dong Y., Zhang X., Wei Y., Yang Z., et al. MXene composite nanofibers for cell culture and tissue engineering. ACS Appl. Bio Mater. 2020;3:2125–2131. doi: 10.1021/acsabm.0c00007. [DOI] [PubMed] [Google Scholar]
- 77.Li Z., Chu D., Chen G., Shi L., Jin L., Zhang X., et al. Biocompatible and biodegradable 3D double-network fibrous scaffold for excellent cell growth. J. Biomed. Nanotechnol. 2019;15:2209–2215. doi: 10.1166/jbn.2019.2846. [DOI] [PubMed] [Google Scholar]
- 78.Barba M., Di Taranto G., Lattanzi W. Adipose-derived stem cell therapies for bone regeneration. Expet Opin. Biol. Ther. 2017;17:677–689. doi: 10.1080/14712598.2017.1315403. [DOI] [PubMed] [Google Scholar]
- 79.Cui D., Li H., Xu X., Ye L., Zhou X., Zheng L., et al. Mesenchymal stem cells for cartilage regeneration of TMJ osteoarthritis. Stem Cell. Int. 2017;2017 doi: 10.1155/2017/5979741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu H., Cheng Y., Chen J., Chang F., Wang J., Ding J., et al. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018;73:103–111. doi: 10.1016/j.actbio.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 81.Li J., Huang Y., Song J., Li X., Zhang X., Zhou Z., et al. Cartilage regeneration using arthroscopic flushing fluid-derived mesenchymal stem cells encapsulated in a one-step rapid cross-linked hydrogel. Acta Biomater. 2018;79:202–215. doi: 10.1016/j.actbio.2018.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yin H., Wang Y., Sun X., Cui G., Sun Z., Chen P., et al. Functional tissue-engineered microtissue derived from cartilage extracellular matrix for articular cartilage regeneration. Acta Biomater. 2018;77:127–141. doi: 10.1016/j.actbio.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 83.Kim W., Gwon Y., Kim Y.-K., Park S., Kang S.-J., Park H.-K., et al. Plasma-assisted multiscale topographic scaffolds for soft and hard tissue regeneration. NPJ Regener. Med. 2021;6:1–13. doi: 10.1038/s41536-021-00162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim W., Kim G.-E., Attia Abdou M., Kim S., Kim D., Park S., et al. Tendon-inspired nanotopographic scaffold for tissue regeneration in rotator cuff injuries. ACS Omega. 2020;5:13913–13925. doi: 10.1021/acsomega.0c01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lui P.P.Y. Stem cell technology for tendon regeneration: current status, challenges, and future research directions. Stem Cell. Clon Adv. Appl. 2015;8:163. doi: 10.2147/SCCAA.S60832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Q., Yu Y., Reisdorf R.L., Qi J., Lu C.-K., Berglund L.J., et al. Engineered tendon-fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model. Biomaterials. 2019;192:189–198. doi: 10.1016/j.biomaterials.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 87.Tang Y., Chen C., Liu F., Xie S., Qu J., Li M., et al. Structure and ingredient-based biomimetic scaffolds combining with autologous bone marrow-derived mesenchymal stem cell sheets for bone-tendon healing. Biomaterials. 2020;241 doi: 10.1016/j.biomaterials.2020.119837. [DOI] [PubMed] [Google Scholar]
- 88.Yang S., Shi X., Li X., Wang J., Wang Y., Luo Y. Oriented collagen fiber membranes formed through counter-rotating extrusion and their application in tendon regeneration. Biomaterials. 2019;207:61–75. doi: 10.1016/j.biomaterials.2019.03.041. [DOI] [PubMed] [Google Scholar]
- 89.Wang X., Jin J., Hou R., Zhou M., Mou X., Xu K., et al. Differentiation of bMSCs on biocompatible, biodegradable, and biomimetic scaffolds for largely defected tissue repair. ACS Appl. Bio Mater. 2019;3:735–746. doi: 10.1021/acsabm.9b01063. [DOI] [PubMed] [Google Scholar]
- 90.Quarta M., Cromie M., Chacon R., Blonigan J., Garcia V., Akimenko I., et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat. Commun. 2017;8:1–17. doi: 10.1038/ncomms15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park S.-J., Kim R.Y., Park B.-W., Lee S., Choi S.W., Park J.-H., et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat. Commun. 2019;10:1–12. doi: 10.1038/s41467-019-11091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leong D.J., Sun H.B. Mesenchymal stem cells in tendon repair and regeneration: basic understanding and translational challenges. Ann. N. Y. Acad. Sci. 2016;1383:88–96. doi: 10.1111/nyas.13262. [DOI] [PubMed] [Google Scholar]
- 93.Sullivan R., Dailey T., Duncan K., Abel N., Borlongan C.V. Peripheral nerve injury: stem cell therapy and peripheral nerve transfer. Int. J. Mol. Sci. 2016;17:2101. doi: 10.3390/ijms17122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsu M.-N., Liao H.-T., Li K.-C., Chen H.-H., Yen T.-C., Makarevich P., et al. Adipose-derived stem cell sheets functionalized by hybrid baculovirus for prolonged GDNF expression and improved nerve regeneration. Biomaterials. 2017;140:189–200. doi: 10.1016/j.biomaterials.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 95.Du J., Zhen G., Chen H., Zhang S., Qing L., Yang X., et al. Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials. 2018;181:347–359. doi: 10.1016/j.biomaterials.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ansari S., Diniz I.M., Chen C., Sarrion P., Tamayol A., Wu B.M., et al. Human periodontal ligament‐and gingiva‐derived mesenchymal stem cells promote nerve regeneration when encapsulated in alginate/hyaluronic acid 3D scaffold. Adv. Healthc. Mater. 2017;6 doi: 10.1002/adhm.201700670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fan L., Liu C., Chen X., Zou Y., Zhou Z., Lin C., et al. Directing induced pluripotent stem cell derived neural stem cell fate with a three-dimensional biomimetic hydrogel for spinal cord injury repair. ACS Appl. Mater. Interfaces. 2018;10:17742–17755. doi: 10.1021/acsami.8b05293. [DOI] [PubMed] [Google Scholar]
- 98.Faroni A., Mobasseri S.A., Kingham P.J., Reid A.J. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015;82–83:160–167. doi: 10.1016/j.addr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 99.Galler K.M., D'Souza R.N., Hartgerink J.D. Biomaterials and their potential applications for dental tissue engineering. J. Mater. Chem. 2010;20:8730–8746. [Google Scholar]
- 100.Zhang R., Xie L., Wu H., Yang T., Zhang Q., Tian Y., et al. Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration. Acta Biomater. 2020;113:305–316. doi: 10.1016/j.actbio.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 101.Piva E., Tarlé S.A., Nör J.E., Zou D., Hatfield E., Guinn T., et al. Dental pulp tissue regeneration using dental pulp stem cells isolated and expanded in human serum. J. Endod. 2017;43:568–574. doi: 10.1016/j.joen.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Itoh Y., Sasaki J., Hashimoto M., Katata C., Hayashi M., Imazato S. Pulp regeneration by 3-dimensional dental pulp stem cell constructs. J. Dent. Res. 2018;97:1137–1143. doi: 10.1177/0022034518772260. [DOI] [PubMed] [Google Scholar]
- 103.Xuan K., Li B., Guo H., Sun W., Kou X., He X., et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aaf3227. [DOI] [PubMed] [Google Scholar]
- 104.Zheng C., Chen J., Liu S., Jin Y. Stem cell-based bone and dental regeneration: a view of microenvironmental modulation. Int. J. Oral Sci. 2019;11:1–15. doi: 10.1038/s41368-019-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dong Y., Rodrigues M., Li X., Kwon S.H., Kosaric N., Khong S., et al. Injectable and tunable gelatin hydrogels enhance stem cell retention and improve cutaneous wound healing. Adv. Funct. Mater. 2017;27 [Google Scholar]
- 106.Shafiee A., Cavalcanti A.S., Saidy N.T., Schneidereit D., Friedrich O., Ravichandran A., et al. Convergence of 3D printed biomimetic wound dressings and adult stem cell therapy. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120558. [DOI] [PubMed] [Google Scholar]
- 107.Xu Q., Sigen A., Gao Y., Guo L., Creagh-Flynn J., Zhou D., et al. A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing. Acta Biomater. 2018;75:63–74. doi: 10.1016/j.actbio.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 108.Chen L., Xing Q., Zhai Q., Tahtinen M., Zhou F., Chen L., et al. Pre-vascularization enhances therapeutic effects of human mesenchymal stem cell sheets in full thickness skin wound repair. Theranostics. 2017;7:117. doi: 10.7150/thno.17031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu J., Wang M.-Y., Tai H.-C., Cheng N.-C. Cell sheet composed of adipose-derived stem cells demonstrates enhanced skin wound healing with reduced scar formation. Acta Biomater. 2018;77:191–200. doi: 10.1016/j.actbio.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 110.Shi C., Zhu Y., Su Y., Cheng T. Stem cells and their applications in skin-cell therapy. Trends Biotechnol. 2006;24:48–52. doi: 10.1016/j.tibtech.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 111.Jeong H.S., Park C.-Y., Kim J.-H., Joo H.J., Choi S.-C., Choi J.-H., et al. Cardioprotective effects of genetically engineered cardiac stem cells by spheroid formation on ischemic cardiomyocytes. Mol. Med. 2020;26:1–14. doi: 10.1186/s10020-019-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Han M.A., Jeon J.H., Shin J.Y., Kim H.J., Lee J.S., Seo C.W., et al. Intramyocardial delivery of human cardiac stem cell spheroids with enhanced cell engraftment ability and cardiomyogenic potential for myocardial infarct repair. J. Contr. Release. 2021;336:499–509. doi: 10.1016/j.jconrel.2021.06.040. [DOI] [PubMed] [Google Scholar]
- 113.Xu Y., Shi T., Xu A., Zhang L. 3D spheroid culture enhances survival and therapeutic capacities of MSC s injected into ischemic kidney. J. Cell Mol. Med. 2016;20:1203–1213. doi: 10.1111/jcmm.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang X., Hu M.-G., Pan K., Li C.-H., Liu R. 3D spheroid culture enhances the expression of antifibrotic factors in human adipose-derived MSCs and improves their therapeutic effects on hepatic fibrosis. Stem Cell. Int. 2016;2016 doi: 10.1155/2016/4626073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cho R.J., Kim Y.S., Kim J.Y., Oh Y.M. Human adipose-derived mesenchymal stem cell spheroids improve recovery in a mouse model of elastase-induced emphysema. BMB Rep. 2017;50:79–84. doi: 10.5483/BMBRep.2017.50.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heo D.N., Hospodiuk M., Ozbolat I.T. Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater. 2019;95:348–356. doi: 10.1016/j.actbio.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 117.Tseng T.C., Hsu S.H. Substrate-mediated nanoparticle/gene delivery to MSC spheroids and their applications in peripheral nerve regeneration. Biomaterials. 2014;35:2630–2641. doi: 10.1016/j.biomaterials.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 118.Giobbe G.G., Crowley C., Luni C., Campinoti S., Khedr M., Kretzschmar K., et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019;10:1–14. doi: 10.1038/s41467-019-13605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kang S.M., Kim D., Lee J.H., Takayama S., Park J.Y. Engineered microsystems for spheroid and organoid studies. Adv. Healthc. Mater. 2021;10 doi: 10.1002/adhm.202001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Velasco V., Shariati S.A., Esfandyarpour R. Microtechnology-based methods for organoid models. Microsyst. & Nanoeng. 2020;6:76. doi: 10.1038/s41378-020-00185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yin X., Mead B.E., Safaee H., Langer R., Karp J.M., Levy O. Engineering stem cell organoids. Cell Stem Cell. 2016;18:25–38. doi: 10.1016/j.stem.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kretzschmar K., Clevers H. Organoids: modeling development and the stem cell niche in a dish. Dev. Cell. 2016;38:590–600. doi: 10.1016/j.devcel.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 123.Liu D., Chen S., Naing M.W. A review of manufacturing capabilities of cell spheroid generation technologies and future development. Biotechnol. Bioeng. 2020 doi: 10.1002/bit.27620. [DOI] [PubMed] [Google Scholar]
- 124.Velasco V., Shariati S.A., Esfandyarpour R. Microtechnology-based methods for organoid models. Microsyst. & Nanoeng. 2020;6:1–13. doi: 10.1038/s41378-020-00185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tung Y.-C., Hsiao A.Y., Allen S.G., Torisawa Y-s, Ho M., Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;136:473–478. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oliveira N.M., Martins-Cruz C., Oliveira M.B., Reis R.L., Mano J.F. Coculture of spheroids/2D cell layers using a miniaturized patterned platform as a versatile method to produce scaffold-free tissue engineering building blocks. Adv. Biosyst. 2018;2:8. doi: 10.1002/adbi.201700069. [DOI] [Google Scholar]
- 127.Aijian A.P., Garrell R.L. Digital microfluidics for automated hanging drop cell spheroid culture. J. Lab. Autom. 2015;20:283–295. doi: 10.1177/2211068214562002. [DOI] [PubMed] [Google Scholar]
- 128.Cho C.-Y., Chiang T.-H., Hsieh L.-H., Yang W.-Y., Hsu H.-H., Yeh C.-K., et al. Development of a novel hanging drop platform for engineering controllable 3D microenvironments. Front. Cell Dev. Biol. 2020;8:327. doi: 10.3389/fcell.2020.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Faulkner-Jones A., Greenhough S., King J.A., Gardner J., Courtney A., Shu W. Development of a valve-based cell printer for the formation of human embryonic stem cell spheroid aggregates. Biofabrication. 2013;5 doi: 10.1088/1758-5082/5/1/015013. [DOI] [PubMed] [Google Scholar]
- 130.Kimlin L., Kassis J., Virador V. 3D in vitro tissue models and their potential for drug screening. Expet Opin. Drug Discov. 2013;8:1455–1466. doi: 10.1517/17460441.2013.852181. [DOI] [PubMed] [Google Scholar]
- 131.Gionet-Gonzales M.A., Leach J.K. Engineering principles for guiding spheroid function in the regeneration of bone, cartilage, and skin. Biomed. Mater. 2018;13:15. doi: 10.1088/1748-605X/aab0b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pettinato G., Wen X., Zhang N. Formation of well-defined embryoid bodies from dissociated human induced pluripotent stem cells using microfabricated cell-repellent microwell arrays. Sci. Rep. 2014;4:1–11. doi: 10.1038/srep07402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim S.J., Park J., Kim E.M., Choi J.J., Kim H.N., Chin I.L., et al. Lotus seedpod-inspired hydrogels as an all-in-one platform for culture and delivery of stem cell spheroids. Biomaterials. 2019;225:14. doi: 10.1016/j.biomaterials.2019.119534. [DOI] [PubMed] [Google Scholar]
- 134.Rossen N.S., Anandakumaran P.N., Zur Nieden R., Lo K., Luo W., Park C., et al. Injectable therapeutic organoids using sacrificial hydrogels. iScience. 2020;23 doi: 10.1016/j.isci.2020.101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cha J.M., Shin E.K., Sung J.H., Moon G.J., Kim E.H., Cho Y.H., et al. Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci. Rep. 2018;8:1–16. doi: 10.1038/s41598-018-19211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ryu N.-E., Lee S.-H., Park H. Spheroid culture system methods and applications for mesenchymal stem cells. Cells. 2019;8:1620. doi: 10.3390/cells8121620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Benien P., Swami A. 3D tumor models: history, advances and future perspectives. Future Oncol. 2014;10:1311–1327. doi: 10.2217/fon.13.274. [DOI] [PubMed] [Google Scholar]
- 138.Katt M.E., Placone A.L., Wong A.D., Xu Z.S., Searson P.C. In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Front. Bioeng. Biotechnol. 2016;4:12. doi: 10.3389/fbioe.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jeger-Madiot N., Arakelian L., Setterblad N., Bruneval P., Hoyos M., Larghero J., et al. Self-organization and culture of Mesenchymal Stem Cell spheroids in acoustic levitation. Sci. Rep. 2021;11:1–8. doi: 10.1038/s41598-021-87459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schwedhelm I., Zdzieblo D., Appelt-Menzel A., Berger C., Schmitz T., Schuldt B., et al. Automated real-time monitoring of human pluripotent stem cell aggregation in stirred tank reactors. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-48814-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li Y., Kumacheva E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aas8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shin S., Ikram M., Subhan F., Kang H.Y., Lim Y., Lee R., et al. Alginate–marine collagen–agarose composite hydrogels as matrices for biomimetic 3D cell spheroid formation. RSC Adv. 2016;6:46952–46965. [Google Scholar]
- 143.Shen H., Cai S., Wu C., Yang W., Yu H., Liu L. Recent advances in three-dimensional multicellular spheroid culture and future development. Micromachines. 2021;12:96. doi: 10.3390/mi12010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Akther F., Little P., Li Z., Nguyen N.-T., Ta H.T. Hydrogels as artificial matrices for cell seeding in microfluidic devices. RSC Adv. 2020;10:43682–43703. doi: 10.1039/d0ra08566a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kouroupis D., Correa D. Increased mesenchymal stem cell functionalization in three-dimensional manufacturing settings for enhanced therapeutic applications. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.621748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin R.Z., Chang H.Y. Recent advances in three‐dimensional multicellular spheroid culture for biomedical research. Biotechnol. J.: Healthc. Nutr. Technol. 2008;3:1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 147.Zhang H., Cong Y., Osi A.R., Zhou Y., Huang F., Zaccaria R.P., et al. Direct 3D printed biomimetic scaffolds based on hydrogel microparticles for cell spheroid growth. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 148.Kim S-j, Park J., Byun H., Park Y.-W., Major L.G., Lee D.Y., et al. Hydrogels with an embossed surface: an all-in-one platform for mass production and culture of human adipose-derived stem cell spheroids. Biomaterials. 2019;188:198–212. doi: 10.1016/j.biomaterials.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 149.Hong Y., Chen J., Fang H., Li G., Yan S., Zhang K., et al. All-in-One hydrogel realizing adipose-derived stem cell spheroid production and in vivo injection via “gel–sol” transition for angiogenesis in hind limb ischemia. ACS Appl. Mater. Interfaces. 2020;12:11375–11387. doi: 10.1021/acsami.9b23534. [DOI] [PubMed] [Google Scholar]
- 150.Kundu B., Bastos A.R.F., Brancato V., Cerqueira M.T., Oliveira J.M., Correlo V.M., et al. Mechanical property of hydrogels and the presence of adipose stem cells in tumor stroma affect spheroid formation in the 3D osteosarcoma model. ACS Appl. Mater. Interfaces. 2019;11:14548–14559. doi: 10.1021/acsami.8b22724. [DOI] [PubMed] [Google Scholar]
- 151.Lei Y., Schaffer D.V. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc. Natl. Acad. Sci. Unit. States Am. 2013;110:E5039–E5048. doi: 10.1073/pnas.1309408110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin H., Li Q., Lei Y. Three-dimensional tissues using human pluripotent stem cell spheroids as biofabrication building blocks. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa663b. [DOI] [PubMed] [Google Scholar]
- 153.Liu D., Chen S., Win Naing M. A review of manufacturing capabilities of cell spheroid generation technologies and future development. Biotechnol. Bioeng. 2021;118:542–554. doi: 10.1002/bit.27620. [DOI] [PubMed] [Google Scholar]
- 154.Souza G.R., Molina J.R., Raphael R.M., Ozawa M.G., Stark D.J., Levin C.S., et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 2010;5:291–296. doi: 10.1038/nnano.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Türker E., Demirçak N., Arslan-Yildiz A. Scaffold-free three-dimensional cell culturing using magnetic levitation. Biomater. Sci. 2018;6:1745–1753. doi: 10.1039/c8bm00122g. [DOI] [PubMed] [Google Scholar]
- 156.Lv D., Hu Z., Lu L., Lu H., Xu X. Three-dimensional cell culture: a powerful tool in tumor research and drug discovery. Oncol. Lett. 2017;14:6999–7010. doi: 10.3892/ol.2017.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Adine C., Ng K.K., Rungarunlert S., Souza G.R., Ferreira J.N. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials. 2018;180:52–66. doi: 10.1016/j.biomaterials.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 158.Vadivelu R.K., Kamble H., Shiddiky M.J., Nguyen N.-T. Microfluidic technology for the generation of cell spheroids and their applications. Micromachines. 2017;8:94. [Google Scholar]
- 159.Van Duinen V., Trietsch S.J., Joore J., Vulto P., Hankemeier T. Microfluidic 3D cell culture: from tools to tissue models. Curr. Opin. Biotechnol. 2015;35:118–126. doi: 10.1016/j.copbio.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 160.Moshksayan K., Kashaninejad N., Warkiani M.E., Lock J.G., Moghadas H., Firoozabadi B., et al. Spheroids-on-a-chip: recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sensor. Actuator. B Chem. 2018;263:151–176. [Google Scholar]
- 161.Shao C., Chi J., Zhang H., Fan Q., Zhao Y., Ye F. Development of cell spheroids by advanced technologies. Adv. Mater. Technol. 2020;5 [Google Scholar]
- 162.Hou Y., Xie W., Achazi K., Cuellar-Camacho J.L., Melzig M.F., Chen W., et al. Injectable degradable PVA microgels prepared by microfluidic technology for controlled osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018;77:28–37. doi: 10.1016/j.actbio.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 163.Antoni D., Burckel H., Josset E., Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int. J. Mol. Sci. 2015;16:5517–5527. doi: 10.3390/ijms16035517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Park Y., Cheong E., Kwak J.-G., Carpenter R., Shim J.-H., Lee J. Trabecular bone organoid model for studying the regulation of localized bone remodeling. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abd6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Akiva A., Melke J., Ansari S., Liv N., van der Meijden R., van Erp M., et al. An organoid for woven bone. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 166.Pievani A., Sacchetti B., Corsi A., Rambaldi B., Donsante S., Scagliotti V., et al. Human umbilical cord blood-borne fibroblasts contain marrow niche precursors that form a bone/marrow organoid in vivo. Development. 2017;144:1035–1044. doi: 10.1242/dev.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shirure V.S., Hughes C.C., George S.C. Engineering vascularized organoid-on-a-chip models. Annu. Rev. Biomed. Eng. 2021;23 doi: 10.1146/annurev-bioeng-090120-094330. [DOI] [PubMed] [Google Scholar]
- 168.Ahmad T., Byun H., Lee J., Perikamana S.K.M., Shin Y.M., Kim E.M., et al. Stem cell spheroids incorporating fibers coated with adenosine and polydopamine as a modular building blocks for bone tissue engineering. Biomaterials. 2020;230:14. doi: 10.1016/j.biomaterials.2019.119652. [DOI] [PubMed] [Google Scholar]
- 169.Lee J., Lee S., Ahmad T., Perikamana S.K.M., Lee J., Kim E.M., et al. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials. 2020;255:16. doi: 10.1016/j.biomaterials.2020.120192. [DOI] [PubMed] [Google Scholar]
- 170.Yamaguchi Y., Ohno J., Sato A., Kido H., Fukushima T. Mesenchymal stem cell spheroids exhibit enhanced in-vitro and in-vivo osteoregenerative potential. BMC Biotechnol. 2014;14 doi: 10.1186/s12896-014-0105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ho S.S., Hung B., Heyrani N., Lee M.A., Leach J.K. Hypoxic preconditioning of mesenchymal stem cells with subsequent spheroid formation accelerates repair of segmental bone defects. Stem Cell. 2018;36:1393–1403. doi: 10.1002/stem.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang K., Yan S., Li G., Cui L., Yin J. In-situ birth of MSCs multicellular spheroids in poly (L-glutamic acid)/chitosan scaffold for hyaline-like cartilage regeneration. Biomaterials. 2015;71:24–34. doi: 10.1016/j.biomaterials.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 173.Huey D.J., Hu J.C., Athanasiou K.A. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 175.Zhang K.X., Yan S.F., Li G.F., Cui L., Yin J.B. In-situ birth of MSCs multicellular spheroids in poly(L-glutamic acid)/chitosan scaffold for hyaline-like cartilage regeneration. Biomaterials. 2015;71:24–34. doi: 10.1016/j.biomaterials.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 176.Zhang K.X., Fang H.W., Qin Y.C., Zhang L.L., Yin J.B. Functionalized scaffold for in situ efficient gene transfection of mesenchymal stem cells spheroids toward chondrogenesis. ACS Appl. Mater. Interfaces. 2018;10:33993–34004. doi: 10.1021/acsami.8b12268. [DOI] [PubMed] [Google Scholar]
- 177.Zhang K.X., He S.M., Yan S.F., Li G.F., Zhang D.Q., Cui L., et al. Regeneration of hyaline-like cartilage and subchondral bone simultaneously by poly(L-glutamic acid) based osteochondral scaffolds with induced autologous adipose derived stem cells. J. Mater. Chem. B. 2016;4:2628–2645. doi: 10.1039/c5tb02113h. [DOI] [PubMed] [Google Scholar]
- 178.Yoo A., Go G., Nguyen K.T., Lee K., Min H.K., Kang B., et al. Magnetoresponsive stem cell spheroid-based cartilage recovery platform utilizing electromagnetic fields. Sensor. Actuator. B Chem. 2020;307:11. doi: 10.1016/j.snb.2019.127569. [DOI] [Google Scholar]
- 179.Huang G.S., Hsieh P.S., Tseng C.S., Hsu S.H. The substrate-dependent regeneration capacity of mesenchymal stem cell spheroids derived on various biomaterial surfaces. Biomater. Sci. 2014;2:1652–1660. doi: 10.1039/c4bm00053f. [DOI] [PubMed] [Google Scholar]
- 180.Niemeyer P., Laute V., Zinser W., John T., Becher C., Diehl P., et al. Safety and efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology is independent of spheroid dose after 4 years. Knee Surg. Sports Traumatol. Arthrosc. 2020;28:1130–1143. doi: 10.1007/s00167-019-05786-8. [DOI] [PubMed] [Google Scholar]
- 181.Vonk L.A., Roël G., Hernigou J., Kaps C., Hernigou P. Role of matrix-associated autologous chondrocyte implantation with spheroids in the treatment of large chondral defects in the knee: a systematic review. Int. J. Mol. Sci. 2021;22:7149. doi: 10.3390/ijms22137149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Anderer U., Libera J. In vitro engineering of human autogenous cartilage. J. Bone Miner. Res. 2002;17:1420–1429. doi: 10.1359/jbmr.2002.17.8.1420. [DOI] [PubMed] [Google Scholar]
- 183.Grevenstein D., Mamilos A., Schmitt V.H., Niedermair T., Wagner W., Kirkpatrick C.J., et al. Excellent histological results in terms of articular cartilage regeneration after spheroid-based autologous chondrocyte implantation (ACI) Knee Surg. Sports Traumatol. Arthrosc. 2021;29:417–421. doi: 10.1007/s00167-020-05976-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sumida Y., Nakamura K., Feil S., Siebold M., Kirsch J., Siebold R. Good healing potential of patellar chondral defects after all-arthroscopic autologous chondrocyte implantation with spheroids: a second-look arthroscopic assessment, Knee Surgery, Sports Traumatology. Arthroscopy. 2021:1–8. doi: 10.1007/s00167-021-06584-x. [DOI] [PubMed] [Google Scholar]
- 185.Hoburg A., Niemeyer P., Laute V., Zinser W., Becher C., Kolombe T., et al. Matrix-associated autologous chondrocyte implantation with spheroid technology is superior to arthroscopic microfracture at 36 months regarding activities of daily living and sporting activities after treatment. Cartilage. 2021;13:437S–448S. doi: 10.1177/1947603519897290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Song L., Tsai A.-C., Yuan X., Bejoy J., Sart S., Ma T., et al. Neural differentiation of spheroids derived from human induced pluripotent stem cells–mesenchymal stem cells coculture. Tissue Eng Part A. 2018;24:915–929. doi: 10.1089/ten.tea.2017.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Boutin M.E., Kramer L.L., Livi L.L., Brown T., Moore C., Hoffman-Kim D. A three-dimensional neural spheroid model for capillary-like network formation. J. Neurosci. Methods. 2018;299:55–63. doi: 10.1016/j.jneumeth.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 188.Zhang Q.Z., Nguyen P.D., Shi S.H., Burrell J.C., Cullen D.K., Le A.D. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci. Rep. 2018;8:11. doi: 10.1038/s41598-018-24888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Laschke M.W., Menger M.D. Spheroids as vascularization units: from angiogenesis research to tissue engineering applications. Biotechnol. Adv. 2017;35:782–791. doi: 10.1016/j.biotechadv.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 190.Zhang K.X., Song L., Wang J., Yan S.F., Li G.F., Cui L., et al. Strategy for constructing vascularized adipose units in poly(L-glutamic acid) hydrogel porous scaffold through inducing in-situ formation of ASCs spheroids. Acta Biomater. 2017;51:246–257. doi: 10.1016/j.actbio.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 191.Bhang S.H., Lee S., Shin J.-Y., Lee T.-J., Kim B.-S. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng Part A. 2012;18:2138–2147. doi: 10.1089/ten.tea.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hong Y.H., Chen J.L., Fang H.W., Li G.F., Yan S.F., Zhang K.X., et al. All-in-One hydrogel realizing adipose-derived stem cell spheroid production and in vivo injection via "Gel-Sol" transition for angiogenesis in hind limb ischemia. ACS Appl. Mater. Interfaces. 2020;12:11375–11387. doi: 10.1021/acsami.9b23534. [DOI] [PubMed] [Google Scholar]
- 193.Dissanayaka W.L., Zhang C. Scaffold-based and scaffold-free strategies in dental pulp regeneration. J. Endod. 2020;46:S81–S89. doi: 10.1016/j.joen.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 194.Dissanayaka W.L., Zhu L., Hargreaves K.M., Jin L., Zhang C. Scaffold-free prevascularized microtissue spheroids for pulp regeneration. J. Dent. Res. 2014;93:1296–1303. doi: 10.1177/0022034514550040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen H., Fu H.C., Wu X., Duan Y.F., Zhang S.C., Hu H., et al. Regeneration of pulpo-dentinal-like complex by a group of unique multipotent CD24a(+) stem cells. Sci. Adv. 2020;6:14. doi: 10.1126/sciadv.aay1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Murphy K.C., Whitehead J., Zhou D., Ho S.S., Leach J.K. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater. 2017;64:176–186. doi: 10.1016/j.actbio.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yang M., He S., Su Z., Yang Z., Liang X., Wu Y. Thermosensitive injectable chitosan/collagen/β-glycerophosphate composite hydrogels for enhancing wound healing by encapsulating mesenchymal stem cell spheroids. ACS Omega. 2020;5:21015–21023. doi: 10.1021/acsomega.0c02580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lu T.-Y., Yu K.-F., Kuo S.-H., Cheng N.-C., Chuang E.-Y., Yu J.-S. Enzyme-crosslinked gelatin hydrogel with adipose-derived stem cell spheroid facilitating wound repair in the murine burn model. Polymers. 2020;12:2997. doi: 10.3390/polym12122997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Park I.S., Chung P.S., Ahn J.C. Enhancement of ischemic wound healing by spheroid grafting of human adipose-derived stem cells treated with low-level light irradiation. PLoS One. 2015;10:16. doi: 10.1371/journal.pone.0122776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nilforoushzadeh M.A., Yazdi M.K., Ghavami S.B., Farokhimanesh S., Amirabad L.M., Zarrintaj P., et al. Mesenchymal stem cell spheroids embedded in an injectable thermosensitive hydrogel: an in situ drug formation platform for accelerated wound healing. ACS Biomater. Sci. Eng. 2020;6:5096–5109. doi: 10.1021/acsbiomaterials.0c00988. [DOI] [PubMed] [Google Scholar]
- 201.Jeong H.S., Park C.-Y., Kim J.-H., Joo H.J., Choi S.-C., Choi J.-H., et al. Cardioprotective effects of genetically engineered cardiac stem cells by spheroid formation on ischemic cardiomyocytes. Mol. Med. 2020;26:1–14. doi: 10.1186/s10020-019-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tögel F.E., Westenfelder C. Kidney protection and regeneration following acute injury: progress through stem cell therapy. Am. J. Kidney Dis. 2012;60:1012–1022. doi: 10.1053/j.ajkd.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 203.Peired A.J., Sisti A., Romagnani P. Mesenchymal stem cell-based therapy for kidney disease: a review of clinical evidence. Stem Cell. Int. 2016;2016 doi: 10.1155/2016/4798639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang W.Y., Chen L.C., Jhuang Y.T., Lin Y.J., Hung P.Y., Ko Y.C., et al. Injection of hybrid 3D spheroids composed of podocytes, mesenchymal stem cells, and vascular endothelial cells into the renal cortex improves kidney function and replenishes glomerular podocytes. Bioeng. & Transl. Med. 2021;6 doi: 10.1002/btm2.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kuo T.K., Hung S.P., Chuang C.H., Chen C.T., Shih Y.R.V., Fang S.C.Y., et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121. doi: 10.1053/j.gastro.2008.03.015. e2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Takahashi Y., Yuniartha R., Yamaza T., Sonoda S., Yamaza H., Kirino K., et al. Therapeutic potential of spheroids of stem cells from human exfoliated deciduous teeth for chronic liver fibrosis and hemophilia a. Pediatr. Surg. Int. 2019;35:1379–1388. doi: 10.1007/s00383-019-04564-4. [DOI] [PubMed] [Google Scholar]
- 207.Brassard J.A., Lutolf M.P. Engineering stem cell self-organization to build better organoids. Cell Stem Cell. 2019;24:860–876. doi: 10.1016/j.stem.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 208.Varzideh F., Pahlavan S., Ansari H., Halvaei M., Kostin S., Feiz M.-S., et al. Human cardiomyocytes undergo enhanced maturation in embryonic stem cell-derived organoid transplants. Biomaterials. 2019;192:537–550. doi: 10.1016/j.biomaterials.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 209.Ranga A., Gjorevski N., Lutolf M.P. Drug discovery through stem cell-based organoid models. Adv. Drug Deliv. Rev. 2014;69–70:19–28. doi: 10.1016/j.addr.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 210.Murrow L.M., Weber R.J., Gartner Z.J. Dissecting the stem cell niche with organoid models: an engineering-based approach. Development. 2017;144:998–1007. doi: 10.1242/dev.140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen C., Rengarajan V., Kjar A., Huang Y. A matrigel-free method to generate matured human cerebral organoids using 3D-Printed microwell arrays. Bioact. Mater. 2021;6:1130–1139. doi: 10.1016/j.bioactmat.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Brandenberg N., Hoehnel S., Kuttler F., Homicsko K., Ceroni C., Ringel T., et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 2020;4:863–874. doi: 10.1038/s41551-020-0565-2. [DOI] [PubMed] [Google Scholar]
- 213.Takebe T., Sekine K., Kimura M., Yoshizawa E., Ayano S., Koido M., et al. Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 2017;21:2661–2670. doi: 10.1016/j.celrep.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 214.Skardal A., Shupe T., Atala A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov. Today. 2016;21:1399–1411. doi: 10.1016/j.drudis.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cho A.-N., Jin Y., An Y., Kim J., Choi Y.S., Lee J.S., et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat. Commun. 2021;12:1–23. doi: 10.1038/s41467-021-24775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nikolaev M., Mitrofanova O., Broguiere N., Geraldo S., Dutta D., Tabata Y., et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature. 2020;585:574–578. doi: 10.1038/s41586-020-2724-8. [DOI] [PubMed] [Google Scholar]
- 217.Achberger K., Probst C., Haderspeck J., Bolz S., Rogal J., Chuchuy J., et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife. 2019;8 doi: 10.7554/eLife.46188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tao T., Wang Y., Chen W., Li Z., Su W., Guo Y., et al. Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip. 2019;19:948–958. doi: 10.1039/c8lc01298a. [DOI] [PubMed] [Google Scholar]
- 219.Qian X., Jacob F., Song M.M., Nguyen H.N., Song H., Ming G-l. Generation of human brain region–specific organoids using a miniaturized spinning bioreactor. Nat. Protoc. 2018;13:565–580. doi: 10.1038/nprot.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Phelan M.A., Gianforcaro A.L., Gerstenhaber J.A., Lelkes P.I. An air bubble-isolating rotating wall vessel bioreactor for improved spheroid/organoid formation. Tissue Eng. C Methods. 2019;25:479–488. doi: 10.1089/ten.tec.2019.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Qian X., Nguyen H.N., Song M.M., Hadiono C., Ogden S.C., Hammack C., et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.DiStefano T., Chen H.Y., Panebianco C., Kaya K.D., Brooks M.J., Gieser L., et al. Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell. Rep. 2018;10:300–313. doi: 10.1016/j.stemcr.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Przepiorski A., Sander V., Tran T., Hollywood J.A., Sorrenson B., Shih J.-H., et al. A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell. Rep. 2018;11:470–484. doi: 10.1016/j.stemcr.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Liu H., Wang Y., Cui K., Guo Y., Zhang X., Qin J. Advances in hydrogels in organoids and organs‐on‐a‐chip. Adv. Mater. 2019;31 doi: 10.1002/adma.201902042. [DOI] [PubMed] [Google Scholar]
- 225.Magno V., Meinhardt A., Werner C. Polymer hydrogels to guide organotypic and organoid cultures. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 226.Gjorevski N., Sachs N., Manfrin A., Giger S., Bragina M.E., Ordóñez-Morán P., et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 227.Yavitt F.M., Brown T.E., Hushka E.A., Brown M.E., Gjorevski N., Dempsey P.J., et al. The effect of thiol structure on allyl sulfide photodegradable hydrogels and their application as a degradable scaffold for organoid passaging. Adv. Mater. 2020;32 doi: 10.1002/adma.201905366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cruz-Acuña R., Quirós M., Farkas A.E., Dedhia P.H., Huang S., Siuda D., et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 2017;19:1326–1335. doi: 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Capeling M.M., Czerwinski M., Huang S., Tsai Y.-H., Wu A., Nagy M.S., et al. Nonadhesive alginate hydrogels support growth of pluripotent stem cell-derived intestinal organoids. Stem Cell. Rep. 2019;12:381–394. doi: 10.1016/j.stemcr.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Candiello J., Grandhi T.S.P., Goh S.K., Vaidya V., Lemmon-Kishi M., Eliato K.R., et al. 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials. 2018;177:27–39. doi: 10.1016/j.biomaterials.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 231.He J., Zhang X., Xia X., Han M., Li F., Li C., et al. Organoid technology for tissue engineering. J. Mol. Cell Biol. 2020;12:569–579. doi: 10.1093/jmcb/mjaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Marti-Figueroa C.R., Ashton R.S. The case for applying tissue engineering methodologies to instruct human organoid morphogenesis. Acta Biomater. 2017;54:35–44. doi: 10.1016/j.actbio.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nakamura T., Sato T. Advancing intestinal organoid technology toward regenerative medicine. Cell. Mol. Gastroenterol. Hepatol. 2018;5:51–60. doi: 10.1016/j.jcmgh.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Akkerman N., Defize L.H. Dawn of the organoid era: 3D tissue and organ cultures revolutionize the study of development, disease, and regeneration. Bioessays. 2017;39 doi: 10.1002/bies.201600244. [DOI] [PubMed] [Google Scholar]
- 235.Olgasi C., Cucci A., Follenzi A. iPSC-derived liver organoids: a journey from drug screening, to disease modeling, arriving to regenerative medicine. Int. J. Mol. Sci. 2020;21:6215. doi: 10.3390/ijms21176215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Nilsson Hall G., Mendes L.F., Gklava C., Geris L., Luyten F.P., Papantoniou I. Developmentally engineered callus organoid bioassemblies exhibit predictive in vivo long bone healing. Adv. Sci. 2020;7 doi: 10.1002/advs.201902295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jee J., Park J.-H., Im J.H., Kim M.S., Park E., Lim T., et al. Biomaterials; 2021. Functional Recovery by Colon Organoid Transplantation in a Mouse Model of Radiation Proctitis. [DOI] [PubMed] [Google Scholar]
- 238.Tsuchida T., Murata S., Matsuki K., Mori A., Matsuo M., Mikami S., et al. The Regenerative effect of portal vein injection of liver organoids by retrorsine/partial hepatectomy in rats. Int. J. Mol. Sci. 2020;21:178. doi: 10.3390/ijms21010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang S.-N., Wang Z., Xu T.-Y., Cheng M.-H., Li W.-L., Miao C.-Y. Cerebral organoids repair ischemic stroke brain injury. Transl. Stroke Res. 2020;11:983–1000. doi: 10.1007/s12975-019-00773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Santo V.E., Estrada M.F., Rebelo S.P., Abreu S., Silva I., Pinto C., et al. Adaptable stirred-tank culture strategies for large scale production of multicellular spheroid-based tumor cell models. J. Biotechnol. 2016;221:118–129. doi: 10.1016/j.jbiotec.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 241.Murata D., Arai K., Nakayama K. Scaffold‐free bio‐3D printing using spheroids as “bio‐inks” for tissue (Re‐) construction and drug response tests. Adv. Healthc. Mater. 2020;9 doi: 10.1002/adhm.201901831. [DOI] [PubMed] [Google Scholar]
- 242.Kang H.-W., Lee S.J., Ko I.K., Kengla C., Yoo J.J., Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 243.Drost J., Boxtel Rv, Blokzijl F., Mizutani T., Sasaki N., Sasselli V., et al. Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. Science. 2017;358:234–238. doi: 10.1126/science.aao3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Huang L., Holtzinger A., Jagan I., BeGora M., Lohse I., Ngai N., et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell– and patient-derived tumor organoids. Nat. Med. 2015;21:1364–1371. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Liu H., Zhang Y., Zhang Y.-Y., Li Y.-P., Hua Z.-Q., Zhang C.-J., et al. Human embryonic stem cell-derived organoid retinoblastoma reveals a cancerous origin. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117:33628–33638. doi: 10.1073/pnas.2011780117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Go G., Jeong S.-G., Yoo A., Han J., Kang B., Kim S., et al. Human adipose–derived mesenchymal stem cell–based medical microrobot system for knee cartilage regeneration in vivo. Sci. Robot. 2020;5 doi: 10.1126/scirobotics.aay6626. [DOI] [PubMed] [Google Scholar]
- 247.Ahmad T., Shin H.J., Lee J., Shin Y.M., Perikamana S.K.M., Park S.Y., et al. Fabrication of in vitro 3D mineralized tissue by fusion of composite spheroids incorporating biomineral-coated nanofibers and human adipose-derived stem cells. Acta Biomater. 2018;74:464–477. doi: 10.1016/j.actbio.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 248.Ahmad T., Lee J., Shin Y.M., Shin H.J., Perikamana S.K.M., Park S.H., et al. Hybrid-spheroids incorporating ECM like engineered fragmented fibers potentiate stem cell function by improved cell/cell and cell/ECM interactions. Acta Biomater. 2017;64:161–175. doi: 10.1016/j.actbio.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 249.Lee J., Lee S., Ahmad T., Perikamana S.K.M., Lee J., Kim E.M., et al. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120192. [DOI] [PubMed] [Google Scholar]
- 250.Park J., Kim Y.S., Ryu S., Kang W.S., Park S., Han J., et al. Graphene potentiates the myocardial repair efficacy of mesenchymal stem cells by stimulating the expression of angiogenic growth factors and gap junction protein. Adv. Funct. Mater. 2015;25:2590–2600. [Google Scholar]
- 251.Li Y., Guo G., Li L., Chen F., Bao J., Shi Y-j, et al. Three-dimensional spheroid culture of human umbilical cord mesenchymal stem cells promotes cell yield and stemness maintenance. Cell Tissue Res. 2015;360:297–307. doi: 10.1007/s00441-014-2055-x. [DOI] [PubMed] [Google Scholar]
- 252.Wang Y., Wang L., Zhu Y., Qin J. Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip. 2018;18:851–860. doi: 10.1039/c7lc01084b. [DOI] [PubMed] [Google Scholar]