Abstract
Background
There are a limited number of treatment options for people with corticosteroid‐refractory ulcerative colitis. Animal models of inflammatory bowel disease and uncontrolled studies in humans suggest that tacrolimus may be an effective treatment for ulcerative colitis.
Objectives
To evaluate the efficacy and safety of tacrolimus for induction of remission in people with corticosteroid‐refractory ulcerative colitis.
Search methods
We searched the Cochrane Gut group specialised register, CENTRAL, MEDLINE (PubMed), Embase, Clinicaltrials.gov and WHO ICTRP from inception to October 2021 to identify relevant randomised controlled trials (RCT).
Selection criteria
Two review authors independently selected potentially relevant studies to determine eligibility based on the prespecified criteria.
Data collection and analysis
Two review authors independently extracted data and analysed them using Review Manager Web. The primary outcomes were induction of remission and clinical improvement, as defined by the studies and expressed as a percentage of the participants randomised (intention‐to‐treat analysis).
Main results
This review included five RCTs with 347 participants who had active ulcerative colitis or ulcerative proctitis. The duration of intervention varied between two weeks and eight weeks.
Tacrolimus versus placebo
Tacrolimus (oral and rectal) may be superior in achieving clinical remission compared to placebo (oral and rectal) (14/87 participants with tacrolimus versus 1/61 participants with placebo; risk ratio (RR) 3.76, 95% confidence interval (CI) 1.03 to 13.73; 3 studies). These results are of low certainty due to imprecision and risk of bias.
Tacrolimus (oral and rectal) may be superior for clinical improvement compared to placebo (oral and rectal) (45/87 participants with tacrolimus versus 7/61 participants with placebo; RR 4.47, 95% CI 2.15 to 9.29; 3 studies). These results are of low certainty due to imprecision and risk of bias.
The evidence is very uncertain about the effects of tacrolimus (oral and rectal) on serious adverse events compared to placebo (oral and rectal) (2/87 participants with tacrolimus versus 0/61 participants with placebo; RR 2.44, 95% CI 0.12 to 48.77; 3 studies). These results are of very low certainty due to high imprecision and risk of bias.
Tacrolimus versus ciclosporin
One study compared oral tacrolimus to intravenous ciclosporin, with an intervention lasting two weeks and 113 randomised participants. The evidence is very uncertain about the effect of tacrolimus on achievement of clinical remission compared to ciclosporin (15/33 participants with tacrolimus versus 24/80 participants with ciclosporin; RR 1.52, 95% CI 0.92 to 2.50). The results are of very low certainty due to risk of bias and high imprecision.
The evidence is very uncertain about the effect of tacrolimus on clinical improvement compared to intravenous ciclosporin (23/33 participants with tacrolimus versus 62/80 participants with ciclosporin; RR 0.90, 95% CI 0.70 to 1.16). The results are of very low certainty due to risk of bias and imprecision.
Tacrolimus versus beclometasone
One study compared tacrolimus suppositories with beclometasone suppositories in an intervention lasting four weeks with 88 randomised participants. There may be little to no difference in achievement of clinical remission (16/44 participants with tacrolimus versus 15/44 participants with beclometasone; RR 1.07, 95% CI 0.60 to 1.88). The results are of low certainty due to high imprecision.
There may be little to no difference in clinical improvement when comparing tacrolimus suppositories to beclometasone suppositories (22/44 participants with tacrolimus versus 22/44 with beclometasone; RR 1.00, 95% CI 0.66 to 1.52). The results are of low certainty due to high imprecision.
There may be little to no difference in serious adverse events when comparing tacrolimus suppositories to beclometasone suppositories (1/44 participants with tacrolimus versus 0/44 with beclometasone; RR 3.00, 95% CI 0.13 to 71.70). These results are of low certainty due to high imprecision.
There may be little to no difference in total adverse events when comparing tacrolimus suppositories to beclometasone suppositories (21/44 participants with tacrolimus versus 14/44 participants with beclometasone; RR 1.50, 95% CI 0.88 to 2.55). These results are of low certainty due to high imprecision.
No secondary outcomes were reported for people requiring rescue medication or to undergo surgery.
Authors' conclusions
There is low‐certainty evidence that tacrolimus may be superior to placebo for achievement of clinical remission and clinical improvement in corticosteroid‐refractory colitis or corticosteroid‐refractory proctitis. The evidence is very uncertain about the effect of tacrolimus compared to ciclosporin for achievement of clinical remission or clinical improvement. There may be no difference between tacrolimus and beclometasone for inducing clinical remission or clinical improvement.
The cohorts studied to date were small, with missing data sets, offered short follow‐up and the clinical endpoints used were not in line with those suggested by regulatory bodies. Therefore, no clinical practice conclusions can be made.
This review highlights the need for further research that targets the relevant clinical questions, uses appropriate trial methodology and reports key findings in a systematic manner that facilitates future integration of findings with current evidence to better inform clinicians and patients. Future studies need to be adequately powered and of pertinent duration so as to capture the efficacy and effectiveness of tacrolimus in the medium to long term. Well‐structured efficacy studies need to be followed up by long‐term phase 4 extensions to provide key outputs and inform in a real‐world setting.
Plain language summary
A review about a drug called tacrolimus for the treatment of difficult‐to‐treat ulcerative colitis
What was the aim of this review?
We aimed to find out whether tacrolimus is an effective and safe treatment in people with ulcerative colitis that are difficult to treat in any other way.
Background
Ulcerative colitis is a chronic inflammatory bowel disease characterised by recurrent episodes of active disease, which commonly affect the rectum or colon or both. People with active disease may experience abdominal cramping, urgency to pass stools, and bloody diarrhoea. People with ulcerative colitis can find standard treatments for active disease are not effective. Tacrolimus is a medicine that reduces the activity of the immune system. We wanted to find out whether tacrolimus can help people with ulcerative colitis for whom other treatments do not work.
Several types of therapies have been used to try to manage difficult cases of ulcerative colitis and there is currently no agreement between clinicians as to which therapy is more helpful.
What did the review study?
In this review, we examined data from five studies that compared tacrolimus to placebo (dummy treatment) and two other medicines called beclometasone and ciclosporin.
We wanted to see if tacrolimus is better in stopping the symptoms of ulcerative colitis (achieving remission) or improving them, and if it is safe to use.
Key messages
Tacrolimus may be better than placebo for stopping the symptoms or improving them.
Tacrolimus may be no different to beclometasone for stopping the symptoms or improving them.
There are few data comparing tacrolimus to ciclosporin.
It is difficult to tell if tacrolimus causes more or fewer side effects compared to placebo or the other two medicines because of the very limited data.
What were the main results of the review?
We searched for randomised controlled trials (clinical studies in which participants are assigned to one of two or more treatment groups using a random method) comparing tacrolimus with any other treatment (such as placebo treatments) in people with difficult cases of ulcerative colitis. We found five trials including 344 participants and made the following conclusions.
There was low‐quality evidence that tacrolimus may be better than placebo for stopping or improving the symptoms of ulcerative colitis.
There was low‐quality evidence that tacrolimus may be no different to beclometasone for stopping or improving the symptoms of ulcerative colitis.
The evidence was of very low quality on whether tacrolimus is different to ciclosporin for stopping or improving the symptoms of ulcerative colitis.
The evidence was of very low quality on whether tacrolimus causes more or fewer side effects compared to placebo or the other two drugs, because of the very limited data.
How up‐to‐date is this review?
This review is up‐to‐date as of October 2021.
Summary of findings
Background
Description of the condition
Ulcerative colitis (UC) is a relapsing and remitting inflammation of the colon, which commences from the rectum and possibly extends to the proximal colon (Ungaro 2017). Depending on the anatomic extent of involvement, patients can be classified as having proctitis, left‐sided colitis (sigmoid and descending colon) or pancolitis. Inflammation limited to the rectum is referred to as ulcerative proctitis (UP). People typically present with bloody diarrhoea, rectal excretion of mucous or pus, and abdominal pain during bowel movements. Toxic megacolon is one of the serious complications associated with UC; it happens when inflammation hinders bowel movements, which induces extensive bowel extension, and can be a surgical emergency (Neurath 2019).
Disease activity for UC can be assessed as mild, moderates or severe based on the Mayo scoring system, and disease severity can be assessed using the Truelove and Witts scoring system (Magro 2017). Corticosteroid‐refractory UC is defined as "patients who have active disease despite prednisolone up to 1 mg/kg/ day for a period of 4 weeks" in the European Crohn's and Colitis Organisation (ECCO) guidelines (Gomollón 2017).
The incidence of UC has been increasing, with peak onset of the disease occurring between 15 and 25 years (Ha 2010; Ng 2017). A combination of history, clinical, radiological and histological findings are needed to confirm diagnosis, with colonoscopies being the most important diagnostic tool and source of histological samples. The cause of UC remains unclear; however, research suggests the possible links to genetics and environmental factors (Da silva 2014).
Many people with UC can be managed successfully with corticosteroids or 5‐aminosalicylic acid (5‐ASA) alone. However, people with severely active UC and those failing to achieve clinical improvements might benefit from rescue therapy such as ciclosporin and tacrolimus (Aoki 2012; Collins 2006; Ogata 2006).
Description of the intervention
Tacrolimus is a macrolide agent isolated from the bacterium Streptomyces tsukubaensis, inhibiting the activity and proliferation of T‐lymphocytes (Matsuoka 2015). Tacrolimus has been widely recognised as anti‐rejection medication, as it possesses immunosuppressive characteristics by binding to immunophilin FK binding protein (FKBP), consequently binding to calcineurin and inhibiting its activity (Scalea 2016). Research has shown its efficacy in corticosteroid‐refractory UC (Jaeger 2019; Lawrance 2017; Ogata 2006; Ogata 2012).
Other relevant comparator treatments for corticosteroid‐refractory UC include ciclosporin, steroids and biologicals.
How the intervention might work
Tacrolimus is currently approved for, and one of the most important medications to prevent, transplant rejection (Scalea 2016). Many studies have examined the efficacy of tacrolimus in people with corticosteroid‐refractory UC. Unfortunately, the most studies were open label, with few randomised controlled trials (RCT) (Benson 2008; Schmidt 2013; Yamamoto 2008).
Tacrolimus is available in oral, rectal and intravenous formulas. It is effectively absorbed in the intestine when taken orally; however, research suggests that this could potentially carry serious risks of adverse events (Lawrance 2017). The recommendation for the use of rectal tacrolimus was based on two studies (Jaeger 2019; Lawrance 2017). These studies concluded that the direct application of tacrolimus on the inflamed tissue could minimise systemic adverse effects and achieve potential clinical improvement. However, those studies focused on people with UP only.
Why it is important to do this review
The number of people with corticosteroid‐refractory UC is rising (Hoffmann 2019). More RCTs are needed to determine which treatments are preferred in this population and which patients benefit the most from rescue treatments such as tacrolimus. Effectiveness and safety need to be assessed systematically, along with the ideal dosage regimen and administration route, in order to resolve the controversy that exists around its use by professional societies and recommendations bodies (Lichtenstein 2006).
Tacrolimus is currently unlicensed in the UK for UC. The National Institute for Health and Care Excellence (NICE) state that first‐line therapy for induction of remission in UC is 5‐ASA (NICE 2019). However, several potential issues may arise such as adverse effects and treatment resistance. We conducted this review to assess the evidence supporting the use of tacrolimus in inducing remission. This systematic review is an update of a previously published Cochrane Review (Baumgart 2008).
Objectives
To evaluate the efficacy and safety of tacrolimus for induction of remission in people with corticosteroid‐refractory ulcerative colitis.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs comparing tacrolimus with standard treatment or placebo in people with active UC. Trials were included irrespective of publication status, language and blinding.
Cluster‐randomised and cross‐over trials were eligible for inclusion.
Types of participants
Children and adults with active UC.
Types of interventions
We included trials comparing tacrolimus, regardless of the mode of administration (intravenous, oral, suppositories) with placebo or any other possible pharmacological treatment. Co‐interventions were allowed if given to both groups.
Types of outcome measures
We considered both dichotomous and continuous data for inclusion.
Primary outcomes
Number of participants achieving clinical remission at study end, as defined by the primary studies and expressed as a percentage of participants randomised (intention‐to‐treat (ITT) analysis).
Number of participants achieving clinical improvement of symptoms of UC at study end, as defined by the primary studies and expressed as a percentage of participants randomised (ITT analysis).
Secondary outcomes
Number of participants who required any other rescue medication at study end.
Number of participants who underwent surgery (proctocolectomy) at study end.
Adverse event outcomes
Serious adverse events.
Withdrawals due to adverse events.
Total number of participants affected by adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases from inception to 7 October 2021.
Cochrane Gut Group Specialised Register.
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 9, via Ovid) (Appendix 1).
MEDLINE (1946 to 7 October 2021, via Ovid) (Appendix 2).
Embase (1974 to 7 October 2021) (Appendix 3).
ClinicalTrials.gov (www.clinicaltrials.gov) (Appendix 4).
World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch/) (Appendix 5).
Searching other resources
We searched the reference lists of relevant studies and review articles for additional citations not identified in the search, and contacted experts in the field.
We did not handsearch conference proceeding in this updated version, as Embase includes abstracts from these conferences since 2009.
Data collection and analysis
Selection of studies
Two review authors (GM and MP) independently screened the search results for eligible studies based on the inclusion criteria. We resolved disagreements by discussion and, if needed, sought the opinion of a third review author (MG).
Data extraction and management
Two review author (MP and RG) independently extracted data from study reports. We resolved disagreements by discussion and, if needed, sought the opinion of a other review authors (MG or VS, or both).
We extracted data about study and participant characteristics; intervention details including regimen, dosage, route and duration; outcomes; conflicts of interest and author contact information. Our consensus extractions for all studies are shown in the Characteristics of included studies table.
Assessment of risk of bias in included studies
Two review authors (MP and RG) independently assessed all studies meeting the inclusion criteria for their risk of bias using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). The domains were:
sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
other bias, such as imbalance in participants' baseline characteristics.
We judged the studies to be at low, high or unclear risk of bias for each domain assessed, based on the guidance in Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We classified overall risk of bias in the trials at low risk of bias if all the bias domains were classified at low risk of bias and high risk of bias if one or more of the bias domains described in the above paragraphs were classified at unclear or high risk of bias.
After data extraction, two review authors (VS and MG) compared the extracted data and discussed and resolved discrepancies before the data were transferred into the Characteristics of included studies table. For cluster RCTs, we intended to judge risk of bias as prescribed in Section 16.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Measures of treatment effect
We used Review Manager Web to analyse the data on an ITT basis (RevMan Web 2020). We used risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CI). For continuous outcomes, we calculated the mean difference (MD) if all studies reported their outcomes using the same scale, and standardised mean difference (SMD) if the studies used different scales to report their outcomes, both with 95% CIs.
Unit of analysis issues
The unit of analysis was the participant. We included cross‐over trials when data were available for the first phase of the trial prior to cross‐over. To deal with events that may have re‐occurred (e.g. adverse events), we reported on the proportion of participants who experienced at least one event. We performed separate comparisons for studies that compared tacrolimus to placebo and studies that compared tacrolimus to other active therapies. If we encountered multiple treatment groups (e.g. different dose groups of tacrolimus), we divided the placebo group across the treatment groups or combined groups to create a single pairwise comparison as appropriate.
Dealing with missing data
We used an ITT analysis for dichotomous outcomes whereby participants with missing treatment outcomes were assumed to be treatment failures. We performed sensitivity analyses to assess the impact of this assumption on the effect estimate.
In the case of missing outcome data, we contacted study authors to request them.
Assessment of heterogeneity
We assessed heterogeneity using the Chi2 test (P < 0.10 was considered statistically significant) and the I2 statistic. We considered an I2 statistic of 75% or greater to indicate high heterogeneity among study data, 50% or greater to indicate moderate heterogeneity and 25% or greater to indicate low heterogeneity (Higgins 2003). We planned sensitivity analyses to explore possible explanations for heterogeneity, by closely examining the appropriate forest plots for significant outliers and exploring underlying causes for heterogeneity, such as clinical, methodological or risk of bias sources of heterogeneity.
Assessment of reporting biases
We initially compared outcomes listed in the protocol to those reported in the published manuscript. If we did not have access to the protocol, we used the outcomes listed in the methods sections of the published manuscript compared to what was reported in the results section. If any pooled analyses included 10 or more studies, we planned to investigate potential publication bias using funnel plots. In case of funnel plot asymmetry, we intended to use the test of linear regression of intervention effect estimate against its standard error, weighted by the inverse of the variance of the intervention effect estimate (Egger 1997).
Data synthesis
We combined data for meta‐analysis from individual trials when the interventions, participant groups and outcomes were similar, as deemed by author consensus. We calculated the pooled RR and corresponding 95% CI for dichotomous outcomes and the pooled MD and corresponding 95% CI for continuous outcomes. We used the SMD and 95% CI when studies used different scales to measure the same outcome.
We used a random‐effects model for all analyses as this does not assume that the effects estimates from individual studies are identical (Chapter 9; Higgins 2021). We also undertook fixed‐effect analyses to further explore the presence of unexplained heterogeneity, with an intention to present the random‐effects results if there was no major difference between the results of the two analyses models, or indication of funnel plot asymmetry. We did not pool data for meta‐analysis if there was a high degree of statistical heterogeneity (I2 of 75% or greater). We also did not undertake pooling in meta‐analyses if there were clear sources of clinical or methodological heterogeneity informed by the data extraction and key characteristics of studies as seen in Table 4 and Table 5. In the case where a meta‐analysis was not possible, we decided to present a narrative synthesis of the results.
1. Summary of interventions of included studies.
| Study ID | Intervention (tacrolimus agent, form and route) | Number of randomised participants in intervention group | Control | Number of randomised participants in control group | Length of therapy | Length of follow‐up | Time of outcomes measurement |
| Aoki 2012 | IV tacrolimus 0.05–0.15 mg/kg bodyweight/day | 33 | IV ciclosporin 2 mg/kg bodyweight/day | 80 | 2 weeks followed by 12 months |
NR | 12 months |
| Lawrance 2017 | Rectal tacrolimus ointment 0.5 mg/mL administered as 3 mL twice daily | 11 | Rectal placebo ointment, identical preparation method to the intervention group, without the addition of the tacrolimus powder | 10 | 8 weeks | 2 weeks, 4 weeks and 8 weeks | 8 weeks |
| Lie 2020 | Tacrolimus suppositories 2 mg, once daily, for 28 days | 44 | Beclometasone suppositories 3 mg, once daily, for 28 days | 44 | 4 weeks | 2 weeks and 4 weeks | 4 weeks |
| Ogata 2006 | Oral tacrolimus 5–10 ng/mL (low trough concentration) | 21 | Placebo: pseudo‐dose adjusted | 21 | 2 weeks | 0 weeks and 2 weeks followed by an open‐label 10‐week extension |
2 weeks |
| Oral tacrolimus 10–15 ng/mL (high trough concentration) | 23 | ||||||
| Ogata 2012 | Oral tacrolimus, capsules used contained 0.5 mg or 1 mg to achieve blood trough concentration of 10–15 ng/mL | 32 | Oral placebo, pseudo‐dose adjusted | 30 | 2 weeks | 0 weeks and 2 weeks, followed by an open‐label 10‐week extension | 2 weeks |
IV: intravenous; NR: not reported.
2. Summary of clinical characteristics and conflicts of interest of included studies.
| Study ID | Disease type | Definition of refractory | Definition of clinical remission/improvement | Conflicts of interest |
| Aoki 2012 | UC | NR |
Clinical remission: participants who achieved a CAI score of ≥ 3 were considered to have achieved remission. Clinical improvement: a decrease in CAI by ≥ 4 points was considered clinical improvement. |
NR |
| Lawrance 2017 | UC | People who failed conventional therapies of oral or rectal (or both) 5‐aminosalicylate or oral and rectal steroids (or both), or were intolerant of these medications. |
Clinical remission: observed by a Mayo score ≤ 2 with no subscore > 1. In addition to endoscopic score of 0 or 1 indicating mucosal healing. Clinical improvement: a reduction in Mayo score ≥ 3 points and a decrease of > 30% from the baseline score. In addition, a reduction of ≥ 1 on the rectal bleeding subscale, or alternatively an absolute rectal score of 0 or 1. |
Authors declared no conflicts of interest. |
| Lie 2020 | UP | Mesalamine‐refractory UP (defined as a failure to at least the use of mesalamine suppositories of a maximum of 1 g for ≥ 21 days) or recurring UP (defined as a relapse within 3 months after stopping adequate local mesalamine therapy). |
Clinical remission: defined as a Mayo score ≤ 2, and endoscopic remission as no visible inflammation (i.e. Mayo subscore 0). Clinical response: defined as an absolute decrease in Mayo score of 3 points, with a relative decrease of 30% of the total score and ≥ 1 point decrease in the rectal bleeding subscore or an absolute rectal bleeding subscore of 0 or 1. |
Authors declared no conflicts of interest. |
| Ogata 2006 | UC | Steroid resistance defined as unresponsiveness to oral or intravenous corticosteroid therapy. Steroid dependency was defined as either chronic active UC for > 6 months or frequent recurrence (> once a year, or ≥ 3 times every 2 years regardless of intensive medical therapy). |
Clinical remission: defined as a DAI score ≤ 2, with no individual subscore > 1, and mucosal healing was defined as an endoscopy subscore (≥ 2 at entry) of 0 or 1. Clinical improvement: defined as combination of partial and complete response. Partial response was defined as a reduction of > 4 points on DAI with improvement in all categories. Complete response was defined as resolution of all symptoms (all assessment scores 0). |
Authors declared no conflicts of interest. |
| Ogata 2012 | UC | Steroid resistance was when the disease failed to respond to a systemic daily dose of 1 mg/kg bodyweight, or ≥ 40 mg of prednisolone given over ≥ 7 days, or the equivalent of a daily dose of prednisolone of ≥ 30 mg over ≥ 2 weeks. Steroid‐dependent participants were defined as people with active UC in whom attempts to taper steroids had been unsuccessful. |
Clinical remission: defined as a DAI score ≤ 2, with an individual subscore 0 or 1, and mucosal healing defined as an endoscopy subscore of 0 or 1. Clinical response: defined as a reduction in DAI by ≥ 4 points and improvements in all categories (stool frequency, rectal bleeding, mucosal appearance, and physician’s overall assessment). |
Authors declared no conflicts of interest. |
CAI: Clinical Activity Index; DAI: Disease Activity Index; NR: not reported; UC: ulcerative colitis; UP: ulcerative proctitis.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analysis (data allowing) for our primary outcomes included:
age of participant (children versus adults);
different doses.
To carry out a statistical assessment of the disagreement between estimates within each pairwise comparison, we used the I2 statistic. We also visually assessed the overlap of the CIs with the prediction interval and the variability in the point estimates. We interpreted the I2 statistic thresholds as follows (Higgins 2021):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Sensitivity analysis
Where possible, we undertook a sensitivity analysis on the primary outcomes of achievement of clinical remission and clinical improvement, to assess whether the findings of the review were robust to the decisions made during the review process. In particular, we excluded studies at high or unclear risk of selection bias due to allocation bias and performance bias, from analyses that had a mix of studies with different risk of bias judgements. Where data analyses included studies with reported and estimated standard deviations (SD), we planned to exclude those with estimated SDs to assess whether this affected the findings of the review. We investigated whether the choice of model (fixed‐effect versus random‐effects) may have affected the results.
Summary of findings and assessment of the certainty of the evidence
We presented the main results in summary of findings tables including an overall grading of the evidence using the GRADE approach (Schünemann 2021). Based on risk of bias, inconsistency, imprecision, indirectness and publication bias, two review authors graded the certainty of the evidence for each outcome as high, moderate, low or very low. These ratings were defined as follows:
high: further research is very unlikely to change our confidence in the estimate of effect;
moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate;
low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate;
very low: any estimate of effect is very uncertain.
We provided justification for all decisions to downgrade the certainty of studies in the footnotes of the summary of findings tables and we made comments to aid the reader's understanding of the review where necessary.
We included the following in the tables.
Clinical remission.
Clinical improvement.
Serious adverse events.
Total adverse events.
Results
Description of studies
The search identified four new RCTs (nine records) (Aoki 2012; Lawrance 2017; Lie 2020; Ogata 2012). Ogata 2006 was the only included study in the previous version of this review (Baumgart 2008). The review includes five RCTs (11 records).
Results of the search
Our electronic search, conducted up to October 2021, identified 836 records. After removing duplicates, 706 records underwent title and abstract screening to assess eligibility, of which 682 were excluded. The remaining 24 records underwent full‐text review, of which we excluded six full‐text articles because they were not RCTs (Barrio 2008; Fellermann 2002; Hisamatsu 2000; JPRN‐UMIN000003785; JPRN‐UMIN000005033; Touchefeu 2007) (see Characteristics of excluded studies table), and included four new studies (nine records) (Aoki 2012; Lawrance 2017; Lie 2020; Ogata 2012). The review includes five RCTs (Aoki 2012; Lawrance 2017; Lie 2020; Ogata 2006; Ogata 2012) (see Characteristics of included studies table).
We classed seven records from trial registries as awaiting classification. We contacted the authors of five records by email on the 23 November 2020 to request clarification and received no responses (CTRI/2015/10/006252; CTRI/2019/04/018626; JPRN‐UMIN000003952; JPRN‐UMIN000004201; JPRN‐UMIN000010776). Two trial registrations had no contact email address (JPRN‐UMIN000007406; NCT00347048) (see Characteristics of studies awaiting classification table).
There were no ongoing studies.
The results of the search are presented in the PRISMA flow diagram (Figure 1).
1.
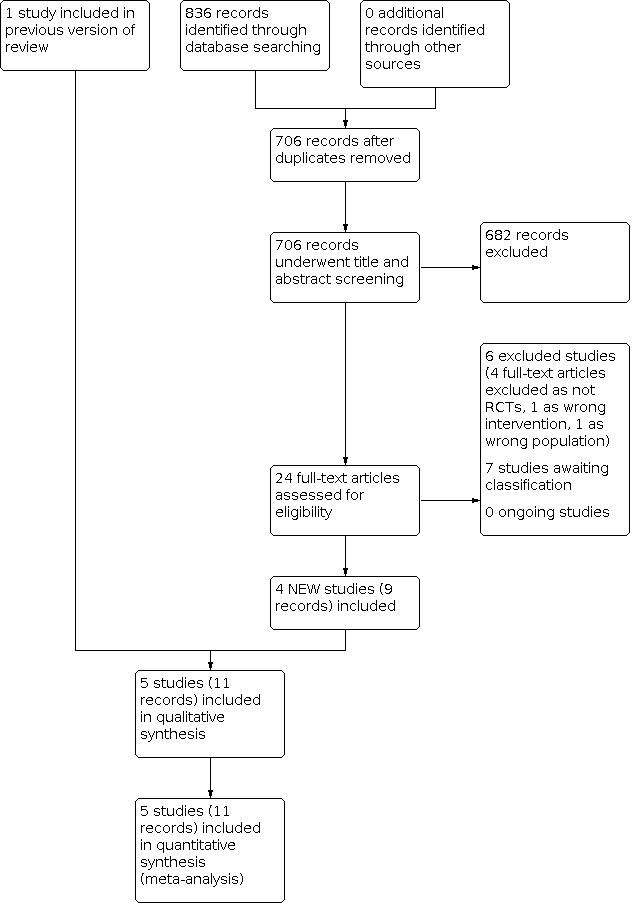
Study flow diagram. RCT: randomised controlled trial.
Included studies
See Characteristics of included studies table.
A summary of key characteristics across the included studies is shown in Table 4 and Table 5.
Study design
Four studies were conducted across multicentre hospitals in Australia (Lawrance 2017); Belgium and the Netherlands (Lie 2020); and Japan (Ogata 2006; Ogata 2012). The setting of Aoki 2012 was not mentioned. Aoki 2012 only reported their results in abstract form and no full report was available. We found no contact information for the authors in order to request a full report.
Participants
The studies included 347 participants who had active UC (Aoki 2012; Lawrance 2017; Ogata 2006; Ogata 2012) or UP (Lie 2020).
Interventions
Aoki 2012 compared oral tacrolimus to intravenous ciclosporin administered for 14 days and followed up for 12 months.
Lawrance 2017 compared rectal tacrolimus ointment to placebo for eight weeks.
Lie 2020 compared tacrolimus suppositories to beclometasone suppositories for 28 days.
Ogata 2006 compared oral tacrolimus to achieve low trough concentration (5 ng/mL to 10 ng/mL), oral tacrolimus to achieve high trough concentration (10 ng/mL to 15 ng/mL) and placebo for 14 days followed by an open‐label extension for 10 weeks.
Ogata 2012 compared oral tacrolimus to placebo for 14 days followed by an open‐label extension for 10 weeks.
Control/comparisons
Three studies used identical non‐active placebo as a control (Lawrance 2017; Ogata 2006; Ogata 2012), while the other two used ciclosporin (Aoki 2012) and beclometasone (Lie 2020). Four studies had two study arms (Aoki 2012; Lawrance 2017; Lie 2020; Ogata 2012), while one study had three study arms (Ogata 2006).
Concurrent therapies
Lawrance 2017 allowed participants to use 5‐ASA oral or topical; glucocorticoids oral or topical and immunosuppressants.
Lie 2020 allowed participants to use oral mesalamine, immunomodulators and biologicals.
Ogata 2006 allowed participants to use 5‐ASA) oral or topical and prednisolone.
Aoki 2012 and Ogata 2012 did not mention the use of concurrent therapies.
Disease activity
Four studies reported disease activity at the beginning of the study. In Lawrance 2017, the mean Mayo score was 8.4 for the tacrolimus group and 9.6 for the control group. In Lie 2020, the median Mayo score for both the tacrolimus and control groups was 7. In Ogata 2006, disease activity was a mean Disease Activity Index (DAI) score of 9.2 for the tacrolimus group and 9.4 for the control group. In Ogata 2012, disease activity was a mean DAI score of 9.8 for the tacrolimus group and 9.1 for the control group.
Disease duration
Three studies reported disease duration. In Lawrance 2017, mean duration was 9.2 years for the tacrolimus group and 7.2 years for the control group. In Lie 2020, median disease duration was 5.8 years for the tacrolimus group and 7.4 for the control group. In Ogata 2006, disease duration was between 4.8 years and 7 years for the tacrolimus groups and 6 years for the control group.
Extent of disease
Two studies reported extent of disease. In Lie 2020, the median extent of the disease was 10 cm for the tacrolimus group and 13 cm for the control group. In Ogata 2006, 26 participants had pancolitis and 14 had left‐sided colitis in the tacrolimus group compared to 10 had pancolitis and 10 had left‐sided colitis in the control group.
Age
Four studies reported mean or median participant age, which ranged from 30 years to 48 years (Aoki 2012; Lawrance 2017; Lie 2020; Ogata 2006).
Funding and conflicts of interest
None of the authors declared conflict of interest. Astellas pharmaceutical company provided funding for Ogata 2006 and Ogata 2012. The University of Australia provided funding for Lawrance 2017 and ZonMW provided funding for Lie 2020. There was no funding information for Aoki 2012.
Excluded studies
We excluded six records. Four studies were not RCTs (Barrio 2008; Fellermann 2002; Hisamatsu 2000; Touchefeu 2007), one had a wrong patient population (JPRN‐UMIN000003785), and one was a wrong intervention (JPRN‐UMIN000005033).
Risk of bias in included studies
The risk of bias of included studies is displayed in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The five included studies were described as randomised, two of which had sufficient information about randomisation to be judged at low risk (Lawrance 2017; Lie 2020). Three studies did not mention the randomisation method and so were judged at unclear risk (Aoki 2012; Ogata 2006; Ogata 2012). We wrote to the authors and received no response with clarification.
Two studies had adequate evidence of allocation concealment and were judged at low risk. Lawrance 2017 was at low risk due to a response received by the author confirming allocation achieved through the use of a research nurse not involved in any other part of the trial. Lie 2020 was also at low risk of bias after receiving a response from the author stating that the hospital pharmacy of each participating centre possessed their own allocation list. The allocation list showed which participant was to receive tacrolimus or beclometasone, based on their assigned study number/identifier. This identifier was known to the investigators and the participants, but the assigned intervention was not. Aoki 2012, Ogata 2006, and Ogata 2012 were at unclear risk due to not providing sufficient information for judgement. The authors for Ogata 2006 and Ogata 2012 did not respond to our email. There were no email contact details for Aoki 2012.
Blinding
Four included studies were described as double blind and were at low risk for performance and detection bias (Lawrance 2017; Lie 2020; Ogata 2006; Ogata 2012). Aoki 2012 was at unclear risk both performance and detection bias due to the lack of details. We could not contact the authors to clarify.
Incomplete outcome data
We judged all five studies at low risk for attrition bias as they had low attrition and it was balanced between groups.
Selective reporting
All five studies were at low risk of reporting bias as their results as reflected the outcomes outlined in the methods section. However, only Lawrance 2017 and Lie 2020 registered their trials prospectively and the reported results matched their registered outcomes. The other studies provided no protocol or trial registration information (Aoki 2012; Ogata 2006; Ogata 2012).
Other potential sources of bias
We judged three studies at low risk of other bias (Lawrance 2017; Lie 2020; Ogata 2006), one study at unclear risk due to insufficient information on the baseline characteristics of both groups (Ogata 2012), and one study at high risk due to major imbalances in the characteristics of the intervention and control groups (Aoki 2012). We received no response from Ogata 2012 and could not contact Aoki 2012.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Tacrolimus compared to placebo for induction of clinical remission in refractory ulcerative colitis.
| Tacrolimus compared to placebo for induction of remission in refractory ulcerative colitis | |||||
| Patient or population: adults with refractory, moderate‐to‐severe ulcerative colitis Settings: multicentre across Japan and Australia Intervention: tacrolimus (oral, rectal) Comparison: placebo (oral, rectal) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with placebo | Risk with tacrolimus | ||||
| Clinical remission | Study population |
RR 3.76 (1.03 to 13.73) |
148 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| 16 per 1000 | 62 per 1000 (16 to 220) | ||||
| Clinical improvement | Study population |
RR 4.47 (2.15 to 9.29) |
148 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| 115 per 1000 | 513 per 1000 (247 to 1000) | ||||
| Serious adverse events | Study population |
RR 2.44 (0.12 to 48.77) |
148 (3 RCTs) |
⊕⊝⊝⊝ Very lowa,d |
|
| 8 per 1000c | 23 per 1000 (1 to 400) | ||||
| Total adverse events | Study population |
RR 1.18 (0.91 to 1.54) |
148 (3 RCTs) |
⊕⊕⊝⊝ Lowa,b |
|
| 476 per 1000 |
561 per 1000 (433 to 733) |
||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||
aDowngraded one level due to imprecision. bDowngraded one level due to risk of bias. cThe risks with placebo were calculated by dividing the number of participants with events to the number of randomised participants. If the total events were zero, as in this case, a token small number was used (i.e. 0.5) so that a range could be calculated. dDowngraded two levels due to imprecision from very sparse data.
Summary of findings 2. Tacrolimus compared with ciclosporin for induction of remission in refractory ulcerative colitis.
| Tacrolimus compared with ciclosporin for induction of remission in refractory ulcerative colitis | ||||||
|
Patient or population: adults with refractory, moderate‐to‐severe ulcerative colitis Settings: not reported Intervention: tacrolimus (oral) Comparison: ciclosporin (intravenous) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ciclosporin | Risk with tacrolimus | |||||
| Clinical remission | Study population |
RR 1.52 (0.92 to 2.50) |
113 (1 RCT) |
⊕⊝⊝⊝ Very lowb,c | — | |
| 300 per 1000a |
456 per 1000 (276 to 750) |
|||||
| Clinical improvement | Study population |
RR 0.90 (0.70 to 1.16) |
113 (1 RCT) |
⊕⊝⊝⊝ Very lowb,c | — | |
| 775 per 1000 |
697 per 1000 (540 to 899) |
|||||
| Serious adverse events | — | — | — | — | — | Not reported |
| Total adverse events | — | — | — | — | — | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aThe risks with placebo were calculated by dividing the number of participants with events by the number of randomised participants. bDowngraded two levels due to risk of bias. cDowngraded one level due to imprecision from sparse data.
Summary of findings 3. Tacrolimus compared with beclometasone for induction of remission in refractory ulcerative colitis.
| Tacrolimus compared with beclometasone for induction of remission in refractory ulcerative colitis | |||||
|
Patient or population: adults with refractory, moderate‐to‐severe ulcerative colitis Settings: hospitals across Belgium and the Netherlands Intervention: tacrolimus (rectal) Comparison: beclometasone (rectal) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with beclometasone | Risk with tacrolimus | ||||
| Clinical remission | Study population |
RR 1.07 (0.60 to 1.88) |
88 (1 RCT) |
⊕⊕⊝⊝ Lowa | |
| 341 per 1000 | 365 per 1000 (205 to 641) | ||||
| Clinical improvement | 500 per 1000 |
500 per 1000 (330 to 760) |
RR 1.00 (0.66 to 1.52) |
88 (1 RCT) |
⊕⊕⊝⊝ Lowa |
| Serious adverse events | Study population |
RR 3.00 (0.13 to 71.70) |
88 (1 RCT) |
⊕⊕⊝⊝ Lowa |
|
| 11 per 1000b |
34 per 1000 (1 to 789) |
||||
| Total adverse events | Study population |
RR 1.50 (0.88 to 2.55) |
88 (1 RCT) |
⊕⊕⊝⊝ Lowa |
|
| 318 per 1000 |
477 per 1000 (280 to 811) |
||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | |||||
aDowngraded two levels due to imprecision from very sparse data. bThe risks with placebo were calculated by dividing the number of participants with events to the number of randomised participants. If the total events were zero, as in this case, a token small number was used (i.e. 0.5) so that a range could be calculated.
Tacrolimus versus placebo
Three studies compared tacrolimus to placebo, with interventions lasting from two weeks to eight weeks and 148 randomised participants (Lawrance 2017; Ogata 2006; Ogata 2012).
Primary outcome
Clinical remission
Tacrolimus may be superior in achieving clinical remission compared to placebo (14/87 participants with tacrolimus versus 1/61 participants with placebo; RR 3.76, 95% CI 1.03 to 13.73; P = 0.05, I² = 0%; 3 studies, 148 participants; low‐certainty evidence; Analysis 1.1). These results are of low certainty due to imprecision and risk of bias (Table 1).
1.1. Analysis.

Comparison 1: Tacrolimus versus placebo, Outcome 1: Achievement of clinical remission
We conducted an unplanned exploratory subgroup analysis for achievement of clinical remission when comparing oral tacrolimus versus placebo. Oral tacrolimus may increase clinical remission, but the result is uncertain (9/76 participants with oral tacrolimus versus 1/51 participants with placebo; RR 2.85, 95% CI 0.66 to 12.35; P = 0.16, I² = 0%; 2 studies, 127 participants; low‐certainty evidence; Analysis 1.2). These results are of low certainty due to imprecision and risk of bias. The results of the subgroup analysis are consistent with the main analysis.
1.2. Analysis.

Comparison 1: Tacrolimus versus placebo, Outcome 2: Achievement of clinical remission for oral tacrolimus vs placebo (subgroup analysis)
For oral administration only, we performed a subgroup analysis comparing high and low concentrations of oral tacrolimus for clinical remission. The RR appeared consistent across the two subgroups (low concentration: RR 2.29, 95% CI 0.12 to 43.84; P = 0.58; high concentration: RR 3.07, 95% CI 0.57 to 16.58; P = 0.19; Analysis 1.1).
We conducted an unplanned exploratory subgroup analysis for achievement of clinical remission comparing rectal tacrolimus with placebo. Rectal tacrolimus may increase clinical remission, but the result is uncertain (5/11 participants with rectal tacrolimus versus 0/10 participants with placebo; RR 10.08, 95% CI 0.63 to 162.06; P = 0.10; 1 study, 21 participants; low‐certainty evidence; Analysis 1.3). These results are of very low certainty due to imprecision from very sparse data. The results of the subgroup analysis are consistent with the main analysis.
1.3. Analysis.

Comparison 1: Tacrolimus versus placebo, Outcome 3: Achievement of clinical remission for rectal tacrolimus vs placebo (subgroup analysis)
We found no trials of children and, therefore, could not perform the planned subgroup analysis of adults versus children.
When we used a fixed‐effect method of analysis and removed studies from analysis for risk of bias our conclusions remained the same. We found no trials for which we had to estimate SDs and, therefore, did not perform the planned sensitivity analysis.
Clinical improvement
Tacrolimus may be superior for clinical improvement compared to placebo (45/87 with tacrolimus versus 7/61 with placebo; RR 4.47, 95% CI 2.15 to 9.29; P < 0.0001, I² = 0%; 3 studies, 148 participants; low‐certainty evidence; Analysis 1.4). These results are of low certainty due to imprecision and risk of bias (Table 1).
1.4. Analysis.

Comparison 1: Tacrolimus versus placebo, Outcome 4: Clinical improvement
We conducted an unplanned exploratory subgroup analysis for achievement of clinical improvement comparing oral tacrolimus to placebo. Tacrolimus may be superior for clinical improvement (37/76 participants with oral tacrolimus versus 6/51 participants with placebo; RR 4.11, 95% CI 1.86 to 9.08; P = 0.0005, I² = 0%; 2 studies, 127 participants; low‐certainty evidence; Analysis 1.5). These results are of low certainty due to imprecision from very sparse data. The results of the subgroup analysis are consistent with the main analysis.
1.5. Analysis.

Comparison 1: Tacrolimus versus placebo, Outcome 5: Clinical improvement for oral tacrolimus vs placebo (subgroup analysis)
For oral administration only, we performed a subgroup analysis comparing high and low concentrations of oral tacrolimus for clinical improvement. The RR appeared consistent across the two subgroups (low concentration: RR 3.48, 95% CI 0.50 to 24.25; P = 0.21; high concentration: RR 4.25, 95% CI 1.78 to 10.12; P = 0.001; Analysis 1.4).
We conducted an unplanned exploratory subgroup analysis for achievement of clinical improvement comparing rectal tacrolimus to placebo. Tacrolimus may be superior for clinical improvement (8/11 participants with rectal tacrolimus versus 1/10 participants with placebo; RR 7.27, 95% CI 1.09 to 48.35; P = 0.04; 1 study, 21 participants; low‐certainty evidence; Analysis 1.6). These results are of very low certainty due to imprecision from very sparse data. The results of the subgroup analysis are consistent with the main analysis.
1.6. Analysis.

Comparison 1: Tacrolimus versus placebo, Outcome 6: Clinical improvement for rectal tacrolimus vs placebo (subgroup analysis)
When we used a fixed‐effect method of analysis and removed studies from analysis for risk of bias our conclusions remained the same. We did not perform other preplanned subgroup or sensitivity analyses.
Secondary outcomes
Any other rescue medication
No studies reported use of any other rescue medication.
Surgery (proctocolectomy)
Ogata 2006 reported that none of the randomised participants underwent surgery during the study period. None of the other studies mentioned surgery.
Serious adverse events
The evidence is very uncertain about the effects of tacrolimus on serious adverse events (2/87 participants with tacrolimus versus 0/61 participants with placebo; RR 2.44, 95% CI 0.12 to 48.77; P =0.56, I² = 0%; 3 studies, 148 participants; very low‐certainty evidence; Analysis 1.7). These results are of very low certainty due to high imprecision and risk of bias (Table 1).
1.7. Analysis.

Comparison 1: Tacrolimus versus placebo, Outcome 7: Serious adverse events
Withdrawals due to adverse events
All studies reported no withdrawals due to adverse events; therefore, it was not possible to estimate an effect.
Total number of participants affected by adverse events
There may be little to no difference about the effects of tacrolimus on total adverse events compared to placebo (45/87 participants with tacrolimus versus 29/61 participants with placebo; RR 1.18, 95% CI 0.91 to 1.54; P = 0.22, I² = 0%; 3 studies, 148 participants; low‐certainty evidence; Analysis 1.8). These results are of low certainty due to imprecision and risk of bias (Table 1).
1.8. Analysis.

Comparison 1: Tacrolimus versus placebo, Outcome 8: Total adverse events
Tacrolimus versus ciclosporin
Aoki 2012 compared oral tacrolimus to intravenous ciclosporin, with an intervention lasting two weeks and 113 randomised participants.
Primary outcomes
Clinical remission
The evidence is very uncertain about the effect of tacrolimus on achievement of clinical remission compared to ciclosporin (15/33 participants with tacrolimus versus 24/80 participants with ciclosporin; RR 1.52, 95% CI 0.92 to 2.50; P = 0.10; 1 study, 113 participants; very low‐certainty evidence; Analysis 2.1). The results are of very low certainty due to risk of bias and imprecision (Table 2).
2.1. Analysis.

Comparison 2: Tacrolimus versus ciclosporin, Outcome 1: Achievement of clinical remission
Clinical improvement
The evidence is very uncertain about the effect of tacrolimus on clinical improvement compared to ciclosporin (23/33 participants with tacrolimus versus 62/80 participants with ciclosporin; RR 0.90, 95% CI 0.70 to 1.16; P = 0.41; 1 study, 113 participants; very low‐certainty evidence; Analysis 2.2). The results are of very low certainty due to risk of bias and imprecision (Table 2).
2.2. Analysis.

Comparison 2: Tacrolimus versus ciclosporin, Outcome 2: Clinical improvement
Secondary outcomes
Any other rescue medication
The study did not report use of any other rescue medication.
Surgery (proctocolectomy)
The study did not report need for surgery.
Serious adverse events
The study did not report serious adverse events.
Withdrawals due to adverse events
The study did not report withdrawals due to adverse events.
Total number of participants affected by adverse events
The study did not report the number of participants affected by adverse events.
Tacrolimus versus beclometasone
Lie 2020 compared tacrolimus suppositories to beclometasone suppositories with an intervention lasting four weeks and 88 randomised participants.
Primary outcomes
Clinical remission
There may be little to no difference in achievement of clinical remission when comparing tacrolimus to beclometasone (16/44 participants with tacrolimus versus 15/44 participants with beclometasone; RR 1.07, 95% CI 0.60 to 1.88; P = 0.82; 1 study, 88 participants; low‐certainty evidence; Analysis 3.1). The results are of low certainty due to high imprecision (Table 3).
3.1. Analysis.

Comparison 3: Tacrolimus versus beclometasone, Outcome 1: Achievement of clinical remission
Clinical improvement
There may be little to no difference in clinical improvement when comparing tacrolimus to beclometasone (22/44 participants with tacrolimus versus 22/44 participants with beclometasone; RR 1.00, 95% CI 0.66 to 1.52; P = 1.00; 1 study, 88 participants; low‐certainty evidence; Analysis 3.1). The results are of low certainty due to high imprecision (Table 3).
Secondary outcomes
Use of any other rescue medication
The study did not report use of any other rescue medication.
Surgery (proctocolectomy)
The study did not report need for surgery.
Serious adverse events
There may be little to no difference in serious adverse events when comparing tacrolimus to beclometasone (1/44 participants with tacrolimus versus 0/44 participants with beclometasone; RR 3.00, 95% CI 0.13 to 71.70; P = 0.50; 1 study, 88 participants; low‐certainty evidence; Analysis 3.3). These results are of low certainty due to high imprecision (Table 3).
3.3. Analysis.

Comparison 3: Tacrolimus versus beclometasone, Outcome 3: Serious adverse events
Withdrawals due to adverse events
All studies reported no withdrawals due to adverse events, therefore, it was not possible to estimate an effect.
Total number of participants affected by adverse events
Tacrolimus may lead to more total adverse events when compared with beclometasone (21/44 participants with tacrolimus versus 14/44 participants with beclometasone; RR 1.50, 95% CI 0.88 to 2.55; P = 0.14; 1 study, 88 participants; low‐certainty evidence; Analysis 3.4). These results are of low certainty due to high imprecision (Table 3).
3.4. Analysis.

Comparison 3: Tacrolimus versus beclometasone, Outcome 4: Total adverse events
Discussion
Summary of main results
This review included five studies assessing the efficacy of tacrolimus in inducing clinical remission or clinical improvement in people with UC.
We analysed and summarised data from 347 participants.
Tacrolimus may be superior in achieving clinical remission when compared to placebo, based on evidence from three studies using oral and rectal preparations. However, the results are of low certainty due to imprecision and risk of bias. Exploratory sensitivity analyses indicate that there may be little to no difference in achieving clinical remission when comparing oral tacrolimus to placebo and similarly there may be little to no difference in achieving clinical remission when comparing rectal tacrolimus to placebo.
Tacrolimus may be superior for clinical improvement when compared to placebo, based on evidence from three studies using oral and rectal preparations. However, the results are of low certainty due to imprecision and risk of bias. Exploratory sensitivity analyses indicated that oral tacrolimus may be superior to placebo for clinical improvement and similarly rectal tacrolimus may be superior to placebo.
The evidence is very uncertain about the effect of tacrolimus (in oral form) on achievement of clinical remission or clinical improvement when compared to ciclosporin (in intravenous form) based on very low‐certainty results due to imprecision from very sparse data and risk of bias from a single study.
There may be little to no difference in achievement of clinical remission or clinical improvement when comparing tacrolimus (in rectal form) to beclometasone (in rectal form), however the results are of low certainty due to imprecision from very sparse data from a single study.
The evidence is very uncertain about the effects of tacrolimus (oral and rectal) on serious adverse events when compared to placebo based on very low‐certainty data due to imprecision from very sparse data and risk of bias. There may be little to no difference on total adverse events, based on low‐certainty data due to imprecision and risk of bias.
There may be little to no difference in serious and total adverse events when comparing tacrolimus (rectal) to beclometasone (rectal). This is based on low‐certainty evidence due to imprecision from very sparse data.
There were no data for serious or total adverse events when comparing tacrolimus (oral) to ciclosporin (intravenous).
All studies reported that no participants withdrew from the studies due to adverse events.
None of the studies reported data on our secondary outcomes of participants requiring rescue medications or surgery.
Overall completeness and applicability of evidence
The heterogeneity of the studies analysed severely limit any attempt at generalising the findings. The age and gender of participants recruited in these studies were comparable and reflective of real‐life situations. The first study was published in 2006 while the most recent study was published in 2020, spanning an extended period over which clinical practice in inflammatory bowel disease has changed (Harbord 2017; Lamb 2019; Rubin 2019).
The clinical characteristics of the participants were diverse with two studies recruiting a corticosteroid‐dependent or resistant cohort (Ogata 2006; Ogata 2012), one study recruiting participants with mesalamine refractory disease (Lie 2020), one study recruiting participants refractory to both mesalamine and corticosteroids (Lawrance 2017), and one study provided no specific participant characteristics (Aoki 2012). While this may represent true clinical disparity in what is considered 'refractory', it limits applicability.
Most studies allowed concomitant mesalamine use (Lawrance 2017; Lie 2020; Ogata 2006), two studies allowed topical or oral glucocorticoid use (Lawrance 2017; Ogata 2006), two studies allowed immunosuppressant use (Lawrance 2017; Lie 2020), with only the latest study allowing concomitant biological therapy use.
Although all five studies were in UC, two studies were specifically in UP using topical therapy (Lawrance 2017; Lie 2020), while the rest of the studies were in more extensive disease using oral or intravenous formulations of tacrolimus (Aoki 2012; Ogata 2006; Ogata 2012). Similarly, studies using topical therapy used a standard tacrolimus dose, while other studies administering tacrolimus orally or parenterally used a dosing regimen, thus precluding any conclusions related to the best dose or formulation of tacrolimus to be used in clinical practice.
Similarly, the comparator arms were diverse and thus was not possible to consecutively use the findings from these studies to build the certainty of evidence related to tacrolimus. The comparators used were non‐active placebo (Lawrance 2017; Ogata 2006; Ogata 2012), beclometasone (Lie 2020), and ciclosporin (Aoki 2012). The treatment durations were two weeks (Aoki 2012; Ogata 2006; Ogata 2012), four weeks (Lie 2020), and eight weeks (Lawrance 2017). All studies measured the primary efficacy endpoint at the end of treatment while in the Aoki 2012 study measured the outcome (number of participants achieving remission or clinical improvement) at 12 months. Thus, it is not possible to identify what is the best duration of tacrolimus treatment.
Most recent guidance from licencing bodies related to trial design in UC specifically identify the need to use patient‐reported outcomes as a clinical symptom measure of disease activity together with a rigorous endoscopic measure (EMA 2018; FDA 2016). The clinical utility of histological remission is gaining momentum among the clinical and academic irritable bowel disease community with a variety of histological scoring systems being validated (Geboes 2000; Marchal‐Bressenot 2017; Mosli 2014; Mosli 2015; Mosli 2017). This was another area where the evidence presented is limited in its applicability to people with corticosteroid‐refractory UC.
Quality of the evidence
The certainty of evidence ranged from very low to low. The main reason for downgrading pertained to imprecision due to sparse events and total event numbers, and due to serious risk of bias.
All studies analysed had relatively small cohorts ranging from 21 participants to 148 participants. Adequately powered and sample‐sized studies are needed to assess the efficacy and safety of tacrolimus. Larger cohorts leading to more event numbers may narrow the CIs allowing a higher certainty of evidence.
Only two studies were at low risk of bias (Lawrance 2017; Lie 2020). Ogata 2006 and Ogata 2012 had issues with the reporting of the randomisation and allocation concealment process and Ogata 2012 did not have enough baseline information to judge the 'other' risks of bias. Aoki 2012 had very serious risk of bias issues, where no clear process for randomisation, allocation concealment or blinding for participants, personnel and assessors was described. It also had high risk of 'other' risk of bias due to great imbalances at baseline between the intervention and control groups.
Most of these sources of bias could be easily managed through following the international guidance on trial design.
Another limitation was that due to very small numbers of serious adverse events we could not reach any conclusions on serious and rare adverse events.
Potential biases in the review process
The review authors contacted the study authors for clarification or additional information, however not all authors responded. We aim to include the data that may become available in future updates, but this could represent a source of bias in the review.
One study was only published in abstract format and did not provide sufficient information, while we were unable to find any contact information for the author group, so this may lead to a reporting bias (Aoki 2012).
We are aware of the possibility of industry funding on the validity of the results. Funding from manufacturing companies or any conflicts of interests were declared by the authors.
Agreements and disagreements with other studies or reviews
The previous version of this Cochrane Review on the efficacy of tacrolimus in UC was conducted in 2008 (Baumgart 2008), and it included only one study (Ogata 2006, also included in this review). It had concluded that "tacrolimus may be effective for short‐term clinical improvement in patients with steroid‐refractory ulcerative colitis". We found that this may be the case when tacrolimus is compared to placebo, but there may be no difference when tacrolimus is compared to beclometasone. We also found insufficient evidence to draw any conclusions on serious adverse events when tacrolimus was compared to placebo and that there may be little difference in serious adverse events, based on low‐certainty evidence, when compared to beclometasone. Also based on low‐certainty evidence, we found that tacrolimus may lead to more total adverse events compared to beclometasone, while there may be little to no difference compared to placebo.
Since the previous version of this review (Baumgart 2008), three more relevant clinical trials have been published. The British Society of Gastroenterology guidelines mention the use of rectal tacrolimus (0.5 mg/mL three times daily) in chronic steroid‐refractory proctitis, albeit it is recognised that the evidence originates from small trials or case series (Lamb 2019). Similarly, the European Crohn's and Colitis Organisation mentions tacrolimus as a possible treatment option in steroid‐refractory chronic active colitis, acute severe colitis or steroid‐refractory proctitis (Harbord 2017). The American College of Gastroenterology UC guidelines mention the use of tacrolimus as a possible therapy to chronic active colitis or acute severe colitis (Rubin 2019).
Authors' conclusions
Implications for practice.
There is low‐certainty evidence that tacrolimus may be effective at inducing clinical remission and clinical improvement in corticosteroid‐refractory chronic active ulcerative colitis or corticosteroid‐refractory ulcerative proctitis when compared to placebo, while there may be no difference when compared to beclometasone. The cohorts studied to date are small, with missing data sets, offer short follow‐up and the clinical endpoints used are not in line with those suggested by regulatory bodies. The certainty of the evidence is primarily impacted by imprecision and risk of bias, as well as heterogeneity across the studies. Therefore, no clinical practice conclusions can be made.
Implications for research.
This review highlights the need for further research that targets the relevant clinical questions, uses appropriate trial methodology and reports key findings systematically that facilitates future integration of findings with current evidence to better inform clinicians and patients alike.
Key stakeholders including people with the relevant clinical condition need to be involved in future studies both to inform the trial design and refine outcomes to make them more clinically pertinent. Commonly used objective patient‐reported outcomes in ulcerative colitis assess stool frequency and rectal bleeding (Jairath 2015). People with ulcerative proctitis are not commonly affected by diarrhoea but more often experience urgency, tenesmus and proximal constipation. These symptoms are not routinely captured in patient‐reported outcomes. Proctitis‐specific key outcome sets might need to be developed with essential psychometric validation undertaken to develop a sophisticated objective tool that accurately reflects gold‐standard disease activity measures.
Future studies need to be adequately powered and of pertinent duration to capture the efficacy and effectiveness of tacrolimus in the medium‐to‐long term, and compared to other pharmacological agents. In the majority, present studies only provided two weeks to eight weeks of follow‐up, which may not be reflective of real‐life scenarios. Larger cohorts will allow adequate minimisation by key variables of interest and will be appropriately powered to allow relevant subanalyses. Nested early‐phase studies investigating the pharmacodynamics effect of tacrolimus are also warranted.
The pharmacological arsenal at the clinician's disposal now includes anti‐tumour necrosis factor agents, anti‐integrins, antibodies to interleukin‐12/23 and small molecules (Feagan 2013; Hanauer 2002; Sandborn 2012; Sandborn 2014; Sandborn 2017; Sands 2019). Although the efficacy of all these agents is well documented in ulcerative colitis, all pivotal studies excluded ulcerative proctitis as a condition of interest and thus the efficacy of these agents in this patient population is not well described. Moreover, in present financially restricted world‐wide healthcare systems, solid data relating to clinical effectiveness is key to help inform the clinician and patient alike in biological sequencing in steroid‐refractory disease.
Mucosal healing remains a key outcome of interest that is not studied. This will facilitate better comparisons with other licenced medications through future reviews. Adverse events were poorly reported among the studies. Given the immunosuppressive nature of tacrolimus, it is important that adverse events are graded as minor and severe, and defined in studies to aid future systematic reviews and clinical guideline generation, and allow clinicians and patients to make informed decisions.
Well‐structured efficacy studies need to be followed up by long‐term phase 4 extensions to provide key outputs and inform in a real‐world setting.
What's new
| Date | Event | Description |
|---|---|---|
| 1 November 2020 | New search has been performed | This review is the first update of a previously published review. The study includes four new randomised controlled trials and 279 new participants. It uses GRADE for the assessment of the certainty of the evidence. |
| 1 November 2020 | New citation required and conclusions have changed | There is low‐certainty evidence that tacrolimus may be superior to placebo and slightly or not different to beclomethasone for achievement of clinical remission and clinical improvement in refractory chronic active colitis or refractory proctitis. The evidence is uncertain about serious adverse events. No clinical practice conclusions could be made. |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 3, 2008
Acknowledgements
Current update
Cochrane Gut supported the authors in the development of this review update.
The following people conducted the editorial process for this article.
Sign‐off Editor: Paul Moayyedi, Department of Medicine, McMaster University.
Managing Editor: Teo Quay, Department of Medicine, McMaster University.
Information Specialist: Yuhong (Cathy) Yuan, Department of Medicine, McMaster University.
Statistical Editor: Sarah Rhodes, Centre for Biostatistics, University of Manchester.
Assistant Managing Editor: Yasamin Farbod, Department of Medicine, McMaster University.
Copy Editor: Anne Lawson, Cochrane Copy Edit Support.
Peer‐reviewers: Mohammed Kareem Shariff, (clinical/content reviewer), Rujan Shrestha (clinical/content reviewer). The listed peer‐reviewers provided peer‐review comments but were not otherwise involved in the editorial process or decision‐making for this article.
In this updated version, the search strategies were designed and run by Yuhong Yuan (Information Specialist at the Cochrane Gut Group).
Previous version of the review
Funding for the IBD/FBD Review Group (1 October 2005 to 30 September 2010) was provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch; the Canadian Agency for Drugs and Technologies in Health (CADTH); and the CIHR Institutes of Health Services and Policy Research; Musculoskeletal Health and Arthritis; Gender and Health; Human Development, Child and Youth Health; Nutrition, Metabolism and Diabetes; and Infection and Immunity.
Miss Ila Stewart provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Appendices
Appendix 1. CENTRAL search strategy (via Ovid Evidence‐Based Medicine Reviews Database (EBMR))
Inflammatory bowel diseases/
exp colitis, ulcerative/
(colitis or proctocolitis or proctosigmoiditis or proctitis or rectosigmoiditis or rectocolitis or colorectitis or coloproctitis).tw,kw.
(inflammatory bowel disease or IBD or UC).tw,kw.
or/1‐4
exp Tacrolimus/
Tacrolimus.mp.
(109581‐93‐3 or fk 506 or fk506 or fr 900506 or fr900506 or prograf or prograft or Protopic or wm0haq4wnm or y5l2157c4j).tw,kw.
(advagraf or astagraf or envarsus or fujimycin or hecoria or modigraf or mustopic oint or protopy or tacforius or tacrolimus hydrate).tw,kw.
Calcineurin Inhibitors/
(calcineurin adj2 (antagonist* or blocker* or inhibitor*)).tw,kw.
(Adoport or Capexion or Graceptor or Pangraf or Panraf or Prohraf or Regraf or T‐inmun).tw,kw.
or/6‐12
5 and 13
Appendix 2. MEDLINE search strategy (via Ovid)
Inflammatory bowel diseases/
exp colitis, ulcerative/
(colitis or proctocolitis or proctosigmoiditis or proctitis or rectosigmoiditis or rectocolitis or colorectitis or coloproctitis).tw,kw.
(inflammatory bowel disease or IBD or UC).tw,kw.
or/1‐4
exp Tacrolimus/
Tacrolimus.mp.
(109581‐93‐3 or fk 506 or fk506 or fr 900506 or fr900506 or prograf or prograft or Protopic or wm0haq4wnm or y5l2157c4j).tw,kw.
(advagraf or astagraf or envarsus or fujimycin or hecoria or modigraf or mustopic oint or protopy or tacforius or tacrolimus hydrate).tw,kw.
Calcineurin Inhibitors/
(calcineurin adj2 (antagonist* or blocker* or inhibitor*)).tw,kw.
(Adoport or Capexion or Graceptor or Pangraf or Panraf or Prohraf or Regraf or T‐inmun).tw,kw.
or/6‐12
5 and 13
randomized controlled trial.pt.
controlled clinical trial.pt.
randomi?ed.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/15‐22
exp animals/ not humans.sh.
23 not 24
14 and 25
Lines 15‐25. RCT filter, Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format.
Appendix 3. Embase search strategy (via Ovid)
inflammatory bowel disease/
exp ulcerative colitis/
(colitis or proctocolitis or proctosigmoiditis or proctitis or rectosigmoiditis or rectocolitis or colorectitis or coloproctitis).tw,kw.
(inflammatory bowel disease or IBD or UC).tw,kw.
or/1‐4
exp tacrolimus/
Tacrolimus.mp.
(109581‐93‐3 or fk 506 or fk506 or fr 900506 or fr900506 or prograf or prograft or Protopic or wm0haq4wnm or y5l2157c4j).tw,kw.
(advagraf or astagraf or envarsus or fujimycin or hecoria or modigraf or mustopic oint or protopy or tacforius or tacrolimus hydrate).tw,kw.
calcineurin inhibitor/
(calcineurin adj2 (antagonist* or blocker* or inhibitor*)).tw,kw.
(Adoport or Capexion or Graceptor or Pangraf or Panraf or Prohraf or Regraf or T‐inmun).tw,kw.
or/6‐12
5 and 13
random:.tw.
placebo:.mp.
double‐blind:.tw.
or/15‐17
exp animal/ not human/
18 not 19
14 and 20
Lines 15‐18. Hedge Best balance of sensitivity and specificity filter for identifying "therapy studies" in Embase. hiru.mcmaster.ca/hiru/HIRU_Hedges_EMBASE_Strategies.aspx
Appendix 4. Clinicaltrials.gov search strategy
Advanced search:
Condition or disease: Inflammatory bowel disease OR IBD OR ulcerative colitis
Intervention/ treatment: Tacrolimus OR FK506 OR FK‐506
Study type: Interventional Studies (Clinical Trials)
Study results: All studies
Appendix 5. WHO ICTRP search strategy
Advanced search:
Condition: Inflammatory bowel disease OR IBD OR ulcerative colitis
Intervention: Tacrolimus OR FK506 OR FK‐506
Recruitment status: All
Data and analyses
Comparison 1. Tacrolimus versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Achievement of clinical remission | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 3.76 [1.03, 13.73] |
| 1.1.1 Oral tacrolimus – low target serum concentration of tacrolimus: 5–10 ng/mL | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [0.12, 43.84] |
| 1.1.2 Oral tacrolimus – high target serum concentration of tacrolimus: 10–15 ng/mL | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 3.07 [0.57, 16.58] |
| 1.1.3 Rectal tacrolimus vs placebo | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 10.08 [0.63, 162.06] |
| 1.2 Achievement of clinical remission for oral tacrolimus vs placebo (subgroup analysis) | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 2.85 [0.66, 12.35] |
| 1.2.1 Oral tacrolimus – low target serum concentration of tacrolimus: 5–10 ng/mL | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [0.12, 43.84] |
| 1.2.2 Oral tacrolimus – high target serum concentration of tacrolimus: 10–15 ng/mL | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 3.07 [0.57, 16.58] |
| 1.3 Achievement of clinical remission for rectal tacrolimus vs placebo (subgroup analysis) | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 10.08 [0.63, 162.06] |
| 1.3.1 Rectal tacrolimus versus placebo | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 10.08 [0.63, 162.06] |
| 1.4 Clinical improvement | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 4.47 [2.15, 9.29] |
| 1.4.1 Low target serum concentration of tacrolimus: 5–10 ng/mL | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 3.48 [0.50, 24.25] |
| 1.4.2 High target serum concentration of tacrolimus: 10–15 ng/mL | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 4.25 [1.78, 10.12] |
| 1.4.3 Rectal tacrolimus vs placebo | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 7.27 [1.09, 48.35] |
| 1.5 Clinical improvement for oral tacrolimus vs placebo (subgroup analysis) | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 4.11 [1.86, 9.08] |
| 1.5.1 Low target serum concentration of tacrolimus: 5–10 ng/mL | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 3.48 [0.50, 24.25] |
| 1.5.2 High target serum concentration of tacrolimus: 10–15 ng/mL | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 4.25 [1.78, 10.12] |
| 1.6 Clinical improvement for rectal tacrolimus vs placebo (subgroup analysis) | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 7.27 [1.09, 48.35] |
| 1.6.1 Rectal tacrolimus versus placebo | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 7.27 [1.09, 48.35] |
| 1.7 Serious adverse events | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.12, 48.77] |
| 1.8 Total adverse events | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.91, 1.54] |
Comparison 2. Tacrolimus versus ciclosporin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Achievement of clinical remission | 1 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.92, 2.50] |
| 2.2 Clinical improvement | 1 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.70, 1.16] |
Comparison 3. Tacrolimus versus beclometasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Achievement of clinical remission | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.60, 1.88] |
| 3.2 Clinical improvement | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.66, 1.52] |
| 3.3 Serious adverse events | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 71.70] |
| 3.4 Total adverse events | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.88, 2.55] |
3.2. Analysis.

Comparison 3: Tacrolimus versus beclometasone, Outcome 2: Clinical improvement
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aoki 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Settings: NR Study period: January 2006 to January 2011 Trial/protocol registration and availability: not available |
|
| Participants |
Inclusion criteria: active UC, refractory to corticosteroids Exclusion criteria: NR Disease activity: NR Disease duration: NR Extent of disease: NR Age: mean: IG: 41.4 (SD 15.7) years; CG: 34.7 (SD 13.9) years Sex (M/F): IG: 23/10; CG: 40/40 Concurrent therapies: NR Number randomised: IG: 33; CG: 80 Number reaching end of study: 113 |
|
| Interventions |
IG: tacrolimus 0.05–0.15 mg/kg bodyweight/day, IV CG: ciclosporin 2 mg/kg bodyweight/day, IV |
|
| Outcomes |
Length of intervention and follow‐up points: 14 days of remission induction therapy, followed up for 12 months Primary outcomes Definition of remission or clinical improvement by study authors: participants who achieved a CAI score ≥ 3 were considered to have achieved remission. A decrease in CAI by ≥ 4 points was considered clinical improvement Number of participants who achieved remission: IG: 15; CG: 24 Number of participants who achieved clinical improvement: IG: 23; CG: 62 Secondary outcomes Number of participants who required other rescue medication: NR Number of participants who underwent surgery: NR Adverse events: NR Withdrawal due to adverse events: NR Serious adverse events: NR Time to adverse events from beginning of study: NR |
|
| Notes |
Author contact details: no details found Conflict of interest: NR Sponsor: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | RCT but randomisation method not specified. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided. Likely high as tacrolimus was provided orally and ciclosporin IV. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants reached end of study. |
| Selective reporting (reporting bias) | Low risk | Authors stated outcomes in their methods were reflected in their results. |
| Other bias | High risk | Major differences in numbers randomised between groups. Also, the ratio of M:F in the CG was 1:1 (40 M and 40 F); however, in the IG, the ratio was about 2:1 (23 M and 10 F). |
Lawrance 2017.
| Study characteristics | ||
| Methods | RCT Setting: multicentre specialist irritable bowel disease centres in Australia (Fremantle Hospital, Western Australian; Royal Brisbane and Women's Hospital, Queensland; Royal Adelaide Hospital, South Australia; and Liverpool Hospital, New South Wales) Study period: October 2012 to November 2016 Trial/protocol registration and availability: NCT01418131 |
|
| Participants |
Inclusion criteria: adults aged ≥ 18 years; able to provide informed consent; diagnosis of UC; inflammation limited to 25 cm proximal to the anal verge; failed to achieve remission or intolerant to oral or rectal (or both) 5‐ASA or oral and rectal corticosteroids (or both); an active UC with a Mayo score 6–12; receiving ≥ 1 of: oral 5‐ASA/oral corticosteroids (minimum of 4 weeks and participant on a stable dose 2 weeks prior to the screening visit), oral AZA/6‐MP or MTX (minimum of 12 weeks and participant is on a stable dose 4 weeks prior to screening), and rectal preparations: must have been ceased before commencing on the trial; normal serum potassium range 3.4–5.0 mmol/L; GFR > 60 mL/minute; free from other significant health conditions and willing to comply with the study instructions Exclusion criteria: Crohn's disease; colitis extending > 25 cm from the anal verge; pregnant/breastfeeding; known allergy to tacrolimus; uncontrolled hypertension; abnormal potassium levels outside range of 3.4–5.0 mmol/L; currently receiving a potassium‐sparing diuretic medication; abnormal eGFR (< 60 mL/minute); HIV infection, malignancy, alcoholic liver disease; dementia or inability to provide consent or understand study requirements; drug abuse or alcohol dependence Disease duration: IG: 9.2 (SD 1.9) years; CG: 7.2 (SD 1.3) years Disease activity: mean Mayo score: IG: 8.6 (SEM 0.4); G: 9.6 (SEM 0.5) Extent of disease: inflammation limited to 25 cm proximal to the anal verge Age: IG: 48.4 (SD 4.9) years; CG: 39.0 (SD 4.8) years Sex (M/F): IG: 8/3; CG: 4/6 Concurrent therapies: 5‐ASA oral/ topical: IG: 8; CG: 8 Glucocorticoids oral/topical: IG: 2/4; CG: 3/2 Immunosuppressants AZA–6‐MP/MTX: IG: 5/1; CG: 5/0 Number randomised: IG: 11; CG: 10 Number reaching end of study (8 weeks): IG: 10; CG: 10 |
|
| Interventions |
IG: rectal tacrolimus ointment 0.5 mg/mL administered as 3 mL twice daily. Prepared by adding 5 mL of propylene glycol to tacrolimus powder (amount NR). Then 70 mL of white paraffin liquid BP added until the preparation was evenly mixed. Process was repeated using 125 mL of white paraffin liquid. CG: placebo ointment, identical preparation method to the IG, without the addition of the tacrolimus powder. |
|
| Outcomes |
Length of intervention: 8 weeks Follow‐up points: 2 weeks, 4 weeks and 8 weeks during the study. In these follow‐ups, calculated Mayo score and IBDQ, noted any adverse effects. Primary outcomes: Definition of clinical improvement by study authors: reduction in Mayo score ≥ 3 points and decrease of > 30% from the baseline score. In addition, a reduction of ≥ 1 on the rectal bleeding subscale, or alternatively an absolute rectal score of 0 or 1. Number of participants who achieved clinical improvement: IG: 8/11; CG: 1/10 Definition of remission by study authors: clinical remission at week 8 observed by a Mayo score ≤ 2 with no subscore > 1. In addition to endoscopic score of 0 or 1 indicating mucosal healing Number of participants who achieved remission: clinical remission: IG: 5/11; CG: 0/10 Secondary outcomes: Number of participants who required other rescue medication: NR Number of participants who underwent surgery: NR Adverse events: IG: 4 participants; CG: 2 participants Fine tremor: IG: 1; CG: 0 Upper respiratory tract infection: IG: 1; CG: 0 Self‐limiting dizziness: IG: 1; CG: 0 Headache required paracetamol: IG: 1; CG: 0 Burning feet and wrist pain: IG: 0; CG: 1 Throat infection requiring antibiotics: IG: 0; CG: 1 Withdrawal due to adverse events: IG: 0; CG: 0 Serious adverse events: IG: 0; CG: 0 |
|
| Notes |
Author contact details: Ian.Lawrance@uwa.edu.au Conflict of interest: authors declared there are no conflicts of interest. Sponsor: The University of Western Australia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated selection randomisation schedule. |
| Allocation concealment (selection bias) | Low risk | Email received 6 December 2020 from Ian Lawrence confirming allocation achieved through use of a research nurse not involved in any other part of the trial ensuring concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo, double‐blind study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo, double‐blind study. Assessment carried out by a blinded independent statistician and gastroenterological clinicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition. An interim analysis was undertaken, as per the protocol, after 20 participants had completed the 8‐week study. Due to the highly significant differences identified between the groups, across multiple endpoints, it was decided that ethically the study should be closed with any participants already commenced on the study allowed to complete the study. |
| Selective reporting (reporting bias) | Low risk | Authors stated outcomes in their methods and trial registration were reflected in their results. |
| Other bias | Low risk | No significant differences observed between both groups. |
Lie 2020.
| Study characteristics | ||
| Methods | RCT Setting: 8 hospitals across Belgium and the Netherlands Study period: February 2014 to November 2017 Trial/protocol registration and availability: NL4205, NTR4416 |
|
| Participants |
Inclusion criteria: adults aged 18–70 years; diagnosis of active UP; inflammation limited to 20 cm proximal to the anal verge; failed to achieve remission on oral or rectal (or both) 5‐ASA (maximum of 1 g for ≥ 21 days); recurrent UP (relapse within 3 months after stopping of local adequate 5‐ASA treatment); written informed consent; permitted concomitant therapy: aminosalicylates, AZA, 6‐MP and MTX at stable dose for 12 weeks Exclusion criteria: use of enemas within 14 days prior to randomisation; infliximab or other anti‐TNF treatment within 12 weeks prior to randomisation; treatment with tacrolimus prior to randomisation; treatment with any investigational drug within 12 weeks of randomisation; treatment with any form of corticosteroids within 4 weeks of randomisation; abnormal renal function (eGFR < 30 mL/minute); presence of ova, parasites, toxins or other signs of infectious agents in stool sample; pre‐existent leukopenia or thrombopenia (neutrophil count < 1800/mm3 or platelets < 90,000/mm3); liver function tests abnormalities (> 2 upper limits of normal); other significant medical illness that might interfere with this study (current malignancy, immunodeficiency syndromes, pre‐existing psychiatric condition, central nervous system trauma or active seizure disorders requiring medication, significant cardiovascular dysfunction within the past 6 months (e.g. angina, congestive heart failure, recent myocardial infarction, severe hypertension or significant arrhythmia), poorly controlled diabetes mellitus, significant pulmonary dysfunction/chronic disease (e.g. chronic obstructive pulmonary disease), renal insufficiency (elevated serum creatinine)); pregnancy, lactation; substance abuse; receiving methadone within the past 2 years Disease duration: median: IG: 5.8 (range 0.3–36.7) years; CG: 7.4 (range 0.3–47.8) years Disease activity: total Mayo score median: IG: 7 (range 3–12); CG: 7 (range 3–12) Extent of disease: median: IG: 10 (range 2–20) cm; CG: 13 (range 1–20) cm Age: mean: IG: 39.6 (range 18.3–75.1) years; CG: 43.2 (range 18.6–76.4) years Sex (M/F): IG: 16/27; CG: 14/28 Concurrent therapies: Oral mesalamine: IG: 15/43; CG: 24/42 Immunomodulators: IG: 10/43; CG: 6/42 Biologicals (anti‐TNF 8; vedolizumab 1): IG: 4/43; CG: 5/42 Number randomised: IG: 44; CG: 44 Number reaching end of study: IG: 43; CG: 42 |
|
| Interventions |
IG: tacrolimus suppositories 2 mg, once daily, for 28 days CG: beclometasone suppositories 3 mg, once daily, for 28 days |
|
| Outcomes |
Length of intervention: 4 weeks Follow‐up points: 2 weeks and 4 weeks Primary outcomes: Definition of clinical improvement by study authors: clinical response after 4 weeks of treatment, defined as an absolute decrease in Mayo score of 3 points, with a relative decrease of 30% of the total score and ≥ 1 point decrease in the rectal bleeding subscore or an absolute rectal bleeding subscore of 0 or 1 Clinical remission defined as a Mayo score ≤ 2, and endoscopic remission as no visible inflammation (i.e. Mayo subscore 0) Number of participants who achieved clinical response: IG: 22; CG: 22 Number of participants who achieved remission: IG: 16; CG: 15 Secondary outcomes: Number of participants who required other rescue medication: NR Number of participants who underwent surgery: NR Adverse events: total IG: 21 participants (29 events); CG: 14 participants (18 events) Abdominal pain: IG: 3; CG: 3 Arthritis: IG: 0; CG: 1 Perianal effects: IG: 9; CG: 3 Clostridium infection: IG: 1; CG: 0 Cytomegalovirus: IG: 1; CG: 0 Nausea/dizziness/weakness: IG: 2; CG: 1 Skin reaction: IG: 3; CG: 4 Flatulence: IG: 5; CG: 2 Headache: IG: 2; CG: 1 Rectal urgency: IG: 1; CG: 0 Night sweats: IG: 1; CG: 0 Palpitations: IG: 1; CG: 1 Upper airway infection: IG: 0; CG: 2 Withdrawal due to adverse events: IG: 1 (clostridium infection); CG: 0 Serious adverse events: IG: 0; CG: 0 |
|
| Notes |
Author contact details: c.vanderwoude@erasmusmc.nl Conflict of interest: authors declared there were no conflicts of interest. Sponsor: financed by ZonMW, grant number 836011003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed centrally by an independent clinical research bureau. |
| Allocation concealment (selection bias) | Low risk | An email received from Dr Lie on 7 December 2020 stated that the hospital pharmacy of each participating centre possessed their own allocation list. The allocation list showed which participant was to receive tacrolimus or beclometasone, based on their assigned study number/identifier. This identifier was known to the investigators and the participants, but the assigned intervention was not. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The intervention medications were custom made for this trial and were of identical appearance and weight. Participants, treating physicians, endoscopists and investigators remained blinded throughout the study. Tacrolimus serum levels were centrally measured during the study and were thus unavailable to the investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, treating physicians, endoscopists and investigators remained blinded throughout the study. Tacrolimus serum levels were centrally measured during the study and were thus unavailable to the investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition very low and balanced. |
| Selective reporting (reporting bias) | Low risk | Authors stated outcomes in their trial registration and methods were reflected in their results. |
| Other bias | Low risk | No major imbalance baseline characteristics. |
Ogata 2006.
| Study characteristics | ||
| Methods | RCT Setting: 17 hospitals in Japan Study period: June 2002 to September 2003 Trial/protocol registration and availability: protocol or trial registration not available |
|
| Participants |
Inclusion criteria: active moderate/severe UC; left‐sided colitis and pancolitis (except proctosigmoiditis); steroid resistance (unresponsiveness to oral or IV corticosteroid therapy ≥ 2 weeks); permitted medications (5‐ASA, steroids), without adjustments in the previous 2 weeks or throughout the study Exclusion criteria: positive Clostridium difficile on stool culture; hepatic/renal failure; pregnant or lactating women; AZA or 6‐MP was prohibited for concomitant use after initiation; cytapheresis within 28 days Disease duration: IG H: 7 years; IG L: 4.8 years; CG: 6.0 years Disease activity: DAI total score mean IG H: 9.2 (SD 1.2) (ranges DAI 6: 0; DAI 7–9: 13; DAI 10–12: 6) IG L: 9.2 (SD 1.8) (ranges DAI 6: 2; DAI 7–9: 9; DAI 10–12: 10) CG: 9.4 (SD 1.5) (ranges DAI 6: 1; DAI 7–9: 8; DAI 10–12: 11) Extent of disease: Pancolitis: IG H: 12; IG L: 14; CG: 10 Left‐sided: IG H: 7; IG L: 7; CG: 10 Age: IG H: mean 33.3 years; IG L: mean 31.2 years; CG: 30.0 years Sex (M/F): IG H: 9/10; IG L: 11/10; CG: 9/11 Concurrent therapies: Prednisolone (> 10 mg/day): IG H: 19/19; IG L: 21/21; CG: 20/20 5‐ASA: IG H: 19/19; IG L: 21/21; CG: 18/20 Immunosuppressants and cytapheresis: 0 Number randomised: IG H: 21; IG L: 23; CG: 21 Number reaching end of study: IG H: 19; IG L: 20; CG: 14 |
|
| Interventions |
IG H: high trough concentration: 2 capsules daily × tacrolimus 1 mg (dose adjusted to receive and maintain trough levels of 10–15 ng/mL) IG L: low trough concentration 2 capsules daily × tacrolimus 0.5 mg (dose adjusted to receive and maintain trough levels of 5–10 ng/mL) CG: placebo: pseudo‐dose adjusted The initial oral dose of tacrolimus was 0.025 mg/kg twice daily (as stated in the correction article). Blood was taken to assess trough concentration at 12 hours or 24 hours after initial dose and dosage was adjusted to maintain concentrations within the assigned target range. In the open‐label extension, all participants received tacrolimus. The trough level was adjusted to 5–15 ng/mL during the initial stage of tacrolimus administration, and 5–10 ng/mL after attaining remission at the treating physician's discretion. Dosage reduction was allowed when adverse drug reactions were observed. |
|
| Outcomes |
Length of intervention: 2 weeks followed by an open‐label 10‐week extension Follow‐up points: week 0, week 2 and week 10 Primary outcomes: Definition of clinical improvement by study authors: proportion of participants with improvement (defined as combination of partial and complete response). Partial response defined as a reduction of > 4 points on DAI with improvement in all categories. Complete response was defined as resolution of all symptoms (all assessment scores 0) Number of participants who achieved clinical improvement: IG H: 13; IG L: 8; CG: 2 Definition of remission by study authors: clinical remission was defined as a DAI score ≤ 2, with no individual subscore > 1, and mucosal healing was defined as an endoscopy subscore (≥ 2 at entry) of 0 or 1 Number of participants who achieved remission: clinical remission: IG H: 4; IG L: 2; CG: 1 Secondary outcomes: Number of participants who required other rescue medication: NR Number of participants who underwent surgery: NR Adverse events: Tremor finger: IG H: 4; IG L: 2; CG: 0 (9 total in the open‐label phase) Sleepiness: IG H: 2; IG L: 0; CG: 1 (0 total in the open‐label phase) Hot flushes: IG H: 2; IG L: 1; CG: 0 (0 total in the open‐label phase) Headache: IG H: 0; IG L: 0; CG: 2 (5 total in the open‐label phase) Queasy: IG H: 0; IG L: 2; CG: 2 (3 total in the open‐label phase) Abdominal discomfort: IG H: 2; IG L: 0; CG: 1 (1 total in the open‐label phase) Withdrawal due to adverse events: IG H: 0; IG L: 0; CG: 0 (1 participant withdrew during the open‐label phase due to headache and fever) Serious adverse events: IG H: 1 (viral gastroenteritis); IG L: 1 (acinetobacter sepsis); CG: 0 |
|
| Notes |
Author contact details: NR Conflict of interest: authors declared there were no conflicts of interest. Sponsor: Industry funding (Astellas Pharma Inc., Japan) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR and no clarification given when author was contacted. |
| Allocation concealment (selection bias) | Unclear risk | NR and no clarification given when author was contacted. Quote: "to preserve the blindness of the study, blood trough levels were measured by SRL Inc. (a third party organisation independent of the study physicians or sponsor) and values were forwarded to Control Center (a third‐party organisation independent of the study physicians or sponsor)". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo, double‐blind study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo, double‐blind study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low numbers of attrition and equal between groups. All exclusions and withdrawals were accounted for. |
| Selective reporting (reporting bias) | Low risk | Authors stated outcomes in their methods were reflected in their results. |
| Other bias | Low risk | No major differences between groups at baseline. |
Ogata 2012.
| Study characteristics | ||
| Methods | RCT Setting: multicentre hospitals in Japan Study period: August 2006 to February 2008 Trial/protocol registration and availability: protocol or trial registration not available |
|
| Participants |
Inclusion criteria: active moderate/severe UC; left‐sided colitis and pancolitis; steroid resistance or steroid dependent; permitted medications (5‐ASA, steroids), without adjustments in the previous 2 weeks or throughout the study Exclusion criteria: positive Clostridium difficile on stool culture; hepatic/renal failure; pregnant or lactating women; AZA > 3 months before screening, and were permitted to continue taking AZA at an unchanged dose over the period beginning 3 months prior to the start of the study, until completion of the study; cytapheresis within 14 days; ciclosporin, biological therapies, 6‐MP, or other immunosuppressants Disease duration: NR Disease activity: DAI score mean: IG: 9.8 (SD 1.61); CG: 9.1 (SD 1.05) Extent of disease: NR Age: NR Sex (M/F): NR Concurrent therapies: NR Number randomised: 32/30 Number reaching end of study: 32/30 |
|
| Interventions |
IG: oral tacrolimus capsules contained 0.5 mg or 1 mg to achieve blood trough concentration of 10–15 ng/mL Tacrolimus therapy was initiated at a small dose of 1–2.5 mg per time, twice daily. Blood collection at 12 hours and 24 hours was required after the initial dose for determination of the trough concentration of tacrolimus. CG: placebo: pseudo‐dose adjusted After 2 weeks, the RCT was ended and all participants started receiving tacrolimus for the open‐label phase of the study. |
|
| Outcomes |
Length of intervention: 2 weeks followed by a 10‐week open‐label phase Follow‐up points: week 0, week 2 and week 12 Primary outcomes: Definition of clinical improvement by study authors: clinical response defined as a reduction in DAI by ≥ 4 points and improvements in all categories (stool frequency, rectal bleeding, mucosal appearance and physician's overall assessment) Number of participants who achieved clinical improvement: IG: 16/32; CG: 4/30 Definition of remission by study authors: clinical remission defined as a DAI score ≤ 2, with an individual subscore 0 or 1, and mucosal healing was defined as an endoscopy subscore of 0 or 1 Number of participants who achieved remission: IG: 3/32; CG: 0/30 Secondary outcomes: Number of participants who required other rescue medication: NR Number of participants who underwent surgery: NR Adverse events: IG: 26; CG: 21 Related adverse events occurring in > 5% of participants in ≥ 1 of the treatment groups: Nausea: IG: 4; CG: 3 Headache: IG: 4; CG: 3 Numbness: IG: 4; CG: 0 Finger tremor: IG: 3; CG: 1 Dysmenorrhoea: IG: 3; CG: 1 Hot flushes: IG: 2; CG: 1 Abdominal pain upper: IG: 2; CG: 1 Back pain: IG: 2; CG: 1 Withdrawal due to adverse events: IG: 0; CG: 0 Serious adverse events: IG: 0; CG: 0 |
|
| Notes |
Author contact details: NR Conflict of interest: authors declared there were no conflicts of interest. Sponsor: industry funding (Astellas Pharma Inc., Japan) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation performed by the Control Center (Bellsystem 24), a third‐party organisation independent of study physicians and sponsor. However, not mentioned how it was performed and we received no response from authors. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned how it was performed and we received no response from authors. Quote: "to preserve the blindness of the study, blood trough levels were measured by SRL Inc. (a third party organisation independent of the study physicians or sponsor) and values were forwarded to Control Center (a third‐party organisation independent of the study physicians or sponsor)". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo, double‐blind study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo, double‐blind study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants finished the study. |
| Selective reporting (reporting bias) | Low risk | Authors stated outcomes in their methods were reflected in their results. |
| Other bias | Unclear risk | Insufficient information provided for baseline characteristics. |
5‐ASA: 5‐aminosalicylic acid; 6‐MP: 6‐mercaptopurine; AZA: azathioprine; CAI: Clinical Activity Index; CG: control group; DAI: Disease Activity Index; eGFR: estimated glomerular filtration rate; F: female; GFR: glomerular filtration rate; IBDQ: Inflammatory Bowel Disease Questionnaire; IG: intervention group; IG H: intervention group high trough concentration; IG L: intervention group low trough concentration; IV: intravenous; M: male; MTX: methotrexate; NR: not reported; RCT: randomised controlled trial; SD: standard deviation; SEM: standard error of the mean; TNF: tumour necrosis factor; UC: ulcerative colitis; UP: ulcerative proctitis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barrio 2008 | Not a randomised trial. |
| Fellermann 2002 | Not a randomised trial. |
| Hisamatsu 2000 | Not a randomised trial. |
| JPRN‐UMIN000003785 | Wrong patient population. |
| JPRN‐UMIN000005033 | Wrong intervention. |
| Touchefeu 2007 | Not a randomised trial. |
Characteristics of studies awaiting classification [ordered by study ID]
CTRI/2015/10/006252.
| Methods |
Study design: RCT Settings: India Study period: 15 October 2015 to NR |
| Participants |
Inclusion criteria: active mild‐to‐moderate UC; DAI score 4–10; rectal bleeding score ≥ 1; baseline mucosal appearance score ≥ 1 Exclusion criteria: proximal or universal UC; evidence of signs and symptoms of fulminant colitis, bowel stricture, toxic megacolon, anticipated need for blood transfusion for gastrointestinal bleeding or demonstrated evidence of peritonitis; evidence of dysplasia on biopsy; evidence of enteric pathogens on stool sample Disease activity: NR Disease duration: NR Extent of disease: NR Age: NR Sex (M/F): NR Concurrent therapies: NR Number enrolled: 60 Number randomised: NR Number reaching end of study: NR |
| Interventions |
IG: tacrolimus 2 mg in 60 mL once daily rectally CG: tacrolimus 4 mg in 60 mL once daily rectally |
| Outcomes |
Length of intervention and follow‐up points: 4 weeks Definition of remission or clinical improvement by study authors: NR Number of participants who achieved remission: NR Number of participants who achieved clinical improvement: NR Time to onset of action for tacrolimus from beginning of study: NR Number of participants who required other rescue medication: NR Time to other rescue medication from beginning of study: NR Number of participants who underwent surgery: NR Time to surgery from beginning of study: NR Adverse events: NR Withdrawal due to adverse events: NR Serious adverse events: NR Time to adverse events from beginning of study: NR |
| Notes | Contacted author by email on 23 November 2020, awaiting response. |
CTRI/2019/04/018626.
| Methods |
Study design: randomised, parallel group trial Settings: 32 hospitals in India Study period: 15 May 2019 to present (estimated duration 2 years) |
| Participants |
Inclusion criteria: adults age 18–65 years; mild‐to‐moderate active UC involving whole of colon of left side (around 60 cm from the anal verge); DAI score 4–10; baseline rectal bleeding score ≥ 1; baseline mucosal appearance score ≥ 1; baseline stool frequency score ≥ 1; failed to achieve remission on topical or oral (or both) standard treatment regimen of mesalamine over minimum 4 weeks of duration; childbearing age females must have a negative serum pregnancy test at screening and negative urine pregnancy test at enrolment; both men and women must agree to use appropriate form of contraceptives themselves and their partners; able to understand and sign an informed consent form; acceptable biochemical markers Exclusion criteria: proximal UC, Crohn's pancolitis; DAI score 3 or ≥ 11; with signs and symptoms of fulminant colitis, bowel stricture, toxic megacolon, an anticipated need for blood transfusion for gastrointestinal bleeding or demonstrate evidence of peritonitis; high‐grade dysplasia on biopsy; known allergy to the study medications; enteric pathogens on stool culture; steroids or immunosuppressants < 4 weeks prior to screening; antibiotic, antifungal or antiparasitic < 1 week prior to screening; history of cancer; hyperkalaemia; positive pregnancy test; history of substance abuse, HIV, hepatitis B and C; receiving potassium‐sparing diuretics, pre‐existant renal failure, hypertension, liver disorders, pulmonary disease, psychiatric and metabolic disorders; participation in any clinical study < 30 days prior to screening; or other major health conditions. 303 participants Disease activity: NR Disease duration: NR Extent of disease: NR Age: NR Sex (M/F): NR Concurrent therapies: NR Number enrolled: NR Number randomised: NR Number reaching end of study: NR |
| Interventions |
IG: tacrolimus CG: hydrocortisone |
| Outcomes |
Primary outcome: evaluation of the efficacy of tacrolimus lipid suspension for enema against (hydrocortisone retention enema) in adults with mild‐to‐moderately active UC refractory to mesalamine treatment Duration: 4 weeks Secondary outcomes: Evaluation of the safety of the participants exposed to the study drugs Estimation of the blood level of tacrolimus following its local administration in participants randomised in test arm Duration: throughout the study Length of intervention and follow‐up points: NR Definition of remission or clinical improvement by study authors: NR Number of participants who achieved remission: NR Number of participants who achieved clinical improvement: NR Time to onset of action for tacrolimus from beginning of study: NR Number of participants who required other rescue medication: NR Time to other rescue medication from beginning of study: NR Number of participants who underwent surgery: NR Time to surgery from beginning of study: NR Adverse events: NR Withdrawal due to adverse events: NR Serious adverse events: NR Time to adverse events from beginning of study: NR |
| Notes | Contacted authors by email on 23 November 2020 to request further details (prashantmodi@lambda‐cro.com). Received response on 24 November 2020 stating that the trial is under confidentiality agreement with the sponsor, hence details of the methods and results cannot be shared. |
JPRN‐UMIN000003952.
| Methods | Study design: RCT |
| Participants | 40 |
| Interventions |
Fasting condition group: tacrolimus 1 hour before meal Fed condition group: tacrolimus immediately following consumption of meal |
| Outcomes |
Primary outcome: clinical response (Mayo score) Follow‐up point: 2 weeks and 12 weeks after treatment Secondary outcomes: Mean maximum tacrolimus blood concentration, mean time of maximum tacrolimus blood concentration, mean area under the blood concentration–time curve, trough level at day 1 Percentage of participants achieved trough tacrolimus whole blood levels between 10 ng/mL and 15 ng/mL within 2 weeks Dose of tacrolimus at 2 weeks Safety |
| Notes | Contact: Nobuyuki Hida at hidan@hyo‐med.ac.jp |
JPRN‐UMIN000004201.
| Methods | Study design: RCT |
| Participants | 50 |
| Interventions |
Remission maintenance therapy group: for 48 weeks with immunomodulator from 2 weeks to 4 weeks following remission induction with tacrolimus No remission maintenance therapy group: with immunomodulator following remission induction with tacrolimus |
| Outcomes | Remission rate 48 weeks after the administration of tacrolimus |
| Notes | Contact: Kenji Watanabe at kenjiw@med.osaka‐cu.ac.jp |
JPRN‐UMIN000007406.
| Methods |
Study design: randomised, parallel group trial Setting: Jichi Medical University hospital for induction of remission Study period: 1 April 2012 to NR |
| Participants |
Inclusion criteria: adults aged 20–75 years; moderate‐to‐severe active UC; people with comorbidities should be stable and no alterations to their therapeutic regimen are expected; able to understand and sign an informed consent Exclusion criteria: hypersensitivity or contraindication to tacrolimus; unable to make an informed consent due to any disorder such as dementia 20 participants Disease activity: NR Disease duration: NR Extent of disease: NR Age: NR Sex (M/F): NR Concurrent therapies: NR Number enrolled: NR Number randomised: NR Number reaching end of study: NR |
| Interventions |
IG: conventional steroid tapering (5 mg/day every 2 weeks) CG: rapidly tapering dose of steroids |
| Outcomes |
Primary outcome: Mayo score Secondary outcome: Stool frequency, rectal bleeding, Matts Score, C‐reactive protein Duration: NR Length of intervention and follow‐up points: NR Definition of remission or clinical improvement by study authors: NR Number of participants who achieved remission: NR Number of participants who achieved clinical improvement: NR Time to onset of action for tacrolimus from beginning of study: NR Number of participants who required other rescue medication: NR Time to other rescue medication from beginning of study: NR Number of participants who underwent surgery: NR Time to surgery from beginning of study: NR Adverse events: NR Withdrawal due to adverse events: NR Serious adverse events: NR Time to adverse events from beginning of study: NR |
| Notes | Contacted author by email on 23 November 2020 for further information; the email was undeliverable. |
JPRN‐UMIN000010776.
| Methods | Study design: RCT |
| Participants | 40 |
| Interventions |
IG: tacrolimus (Prograf) CG: ciclosporin (Sandimmune) |
| Outcomes | Clinical response rates at 2 weeks of treatment |
| Notes | Contact: Tatsuro Katsuno at katsuno@faculty.chiba‐u.jp |
NCT00347048.
| Methods |
Study design: randomised, parallel‐group trial Quadruple blinding (participant, care provider, investigator, outcomes assessor) Settings: multicentre in Japan Study period: September 2006 to April 2008 |
| Participants |
Inclusion criteria: moderate‐to‐severe refractory UC; disease activity: > 4 stools a day, bloody stool, moderate‐to‐severe endoscopic finding; steroid resistance or dependence to meet ≥ 1 of the following condition: no efficacy with > 40 mg/day or 1 mg/kg/day of steroid over ≥ 1 week, no efficacy with 30–40 mg/day of steroid over ≥ 2 weeks, exacerbation along with steroid reduction; age 16–64 years. Exclusion criteria: mild/fulminant UC; hepatic or renal (or both) failure (people receiving 6‐mercaptopurine, cyclosporin or other immunosuppressants within 12 weeks prior to screening); received leukocytapheresis or granulocytapheresis within 2 weeks prior to entry; receiving steroids or started new dose of steroids < 2 weeks before enrolment; changed the dose of steroid or started steroid within 1 week prior to entry in case they received > 40 mg/day or 1 mg/kg/day of steroid just before the study. 67 participants Disease activity: NR Disease duration: NR Extent of disease: NR Age: 16–64 years Sex (M/F): NR Concurrent therapies: NR Number enrolled: NR Number randomised: NR Number reaching end of study: NR |
| Interventions |
IG: tacrolimus CG: placebo |
| Outcomes |
Primary outcome: improvement of DAI score Duration: 2 weeks Secondary outcomes: Changes of DAI score in total and in each component Change in clinical severity and symptoms Endoscopic findings Participant's impression Requirement of steroid Duration: 2 weeks Length of intervention and follow‐up points: 12 weeks Definition of remission or clinical improvement by study authors: NR Number of participants who achieved remission: NR Number of participants who achieved clinical improvement: NR Time to onset of action for tacrolimus from beginning of study: NR Number of participants who required other rescue medication: NR Time to other rescue medication from beginning of study: NR Number of participants who underwent surgery: NR Time to surgery from beginning of study: NR Adverse events: NR Withdrawal due to adverse events: NR Serious adverse events: NR Time to adverse events from beginning of study: NR |
| Notes | No email address on trials webpage or on the responsible party (Astellas Pharma Inc), only telephone number provided. |
CG: control group; DAI: Disease Activity Index; F: female; IG: intervention group; M: male; NR: not reported; RCT: randomised controlled trial; UC: ulcerative colitis.
Differences between protocol and review
This updated review has amended the methodology to follow current Cochrane standards, including the use of GRADE analysis and summary of findings tables. Changes have also been made to the data collection and analysis approach (see: Methods).
The wording of the objectives has been changed to make them more specific (see: Objectives).
There are differences in the secondary and adverse events outcomes, and we have removed the economic and timing of outcome assessment outcomes (see: Types of outcome measures).
We have added the Cochrane Gut Group Specialised Register, Embase, ClinicalTrials.gov and WHO ICTRP to the search, and removed ISI Research Institute (see: Search methods for identification of studies).
Contributions of authors
MG led the team, oversaw and contributed to the search, data extraction, analysis and completed the write‐up and approved the final text.
VS contributed to searching, data extraction, analysis and write up and approved the final text.
AA gave support to the review and analysis and approved the final text.
MP contributed to searching, data extraction and approved the final text.
RG contributed on data extraction and results write up, and approved the final text.
GM completed the search and contributed to the write‐up and approved the final text.
Sources of support
Internal sources
-
None, Other
None
External sources
-
None, Other
None
Declarations of interest
MG: since February 2018, I have received travel fees to attend international scientific and training meetings from Pharma companies. These grants included no honoraria, inducement, advisory role or any other relationship and were restricted to the travel and meeting‐related costs of attending such meetings. These include: DDW May 2018, Advances in IBD December 2018 and DDW May 2019. The companies include: Biogaia (2018 to 2019), Ferring (2018), Synergy (bankrupt – 2018) and Tillots (2018 to 2019). None of these companies have had any involvement in any works completed by me and I have never had any payments for any other activities for them, as confirmed below. From these date onwards, I have made a personal undertaking to take no further funds from any pharmaceutical or formula company in any form for travel or other related activities. This is to lift the limitations such funding has on my ability to act as a first and corresponding author on reviews, in line with the Cochrane policies on such matters and is reported in line with these policies. These current declarations will expire over the next three years and this statement updated regularly to reflect this.
VS: none.
AA: none.
MP has received speaker honoraria from Janssen.
RG: none.
GM has received educational support from Abbvie, Janssen, NAPP, Takeda Pharmaceuticals, Merck Sharp & Dohme Ltd, Ferring and Dr Falk. He has received speaker honoraria from Merck Sharp & Dohme Ltd, Abbvie, Janssen, Pfizer, Ferring and Takeda Pharmaceuticals. He attended advisory boards for Abbvie, Celgene, Takeda Pharmaceuticals, Janssen, Medtronic, AstraZeneca, Phebra Pharmaceuticals, Servertus Associates Ltd and Dr Falk. Dr Moran is a consultant for Alimentiv.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aoki 2012 {published data only}
- Aoki H, Furukawa R, Suzuki Y.Oral tacrolimus versus cyclosporine A in patients with moderate to severe ulcerative colitis refractory to corticosteroids. Gastroenterology 2012;142(5):S-205. [Google Scholar]
Lawrance 2017 {published data only}
- Lawrance IC, Baird A, Lightower D, Radford-Smith G, Andrews JM, Connor S.A multi-centre double blind randomised placebo-controlled study of the use of rectal tacrolimus in the treatment of resistant ulcerative proctitis [ ]. Journal of Crohn's and Colitis 2017;11(1):S4-5. [Google Scholar]
- Lawrance IC, Baird A, Lightower D, Radford-Smith G, Andrews JM, Connor S.Efficacy of rectal tacrolimus for induction therapy in patients with resistant ulcerative proctitis. Clinical Gastroenterology and Hepatology 2017;15(8):1248-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT01418131.A multi-centre double blind randomised placebo-controlled study of the use of rectal tacrolimus in the treatment of resistant ulcerative proctitis. clinicaltrials.gov/ct2/show/NCT01418131 (first received 16 August 2011).
Lie 2020 {published data only}
- EUCTR2013-001259-11-NL.Tacrolimus suppositories versus beclomethason suppositories for the treatment of proctitis refractory to local 5-ASA. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013%E2%80%90001259%E2%80%9011%E2%80%90NL (first received 17 December 2013).
- Kreijne J, Lie M, Dijkstra G, Löwenberg M, Assche G, West R, et al.Tacrolimus suppositories as induction therapy for refractory ulcerative proctitis: a randomised controlled trial. Journal of Crohn's and Colitis 2019;12(1):S045. [Google Scholar]
- Lie M, Kreijne J, Dijkstra G, Löwenberg M, Assche G, West R, et al.No superiority of tacrolimus suppositories vs beclomethasone suppositories in a randomized trial of patients with refractory ulcerative proctitis. Clinical Gastroenterology and Hepatology 2020;18(8):1777-84. [DOI] [PubMed] [Google Scholar]
- NTR4416.Tacrolimus suppositories versus beclomethasone suppositories for the treatment of proctitis refractory to local 5-ASA. Summary. www.trialregister.nl/trial/4205 (first received 1 February 2014).
Ogata 2006 {published data only}
- Ogata H, Matsui T, Nakamura M, Iida M, Takazoe M, Suzuki Y, et al.A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut 2006;55(9):1255-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H, Matsui T, Nakamura M, Iida M, Takazoe M, Suzuki Y, et al.Correction: a randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut 2006;55(11):1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogata 2012 {published data only}
- Ogata H, Kato J, Hirai F, Hida N, Matsui T, Matsumoto T, et al.Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflammatory Bowel Diseases 2012;18(5):803-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barrio 2008 {published data only}
- Barrio J, Atienza R, Julián-Gómez L, Gil-Simón P, García-Pajares F, Caro-Patón A.Ulcerative colitis, tacrolimus and mucosa remission [Colitis ulcerosa, tacrolimus y curación mucosa]. Gastroenterologia y Hepatologia 2008;31(10):711-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fellermann 2002 {published data only}
- Fellermann K, Tanko Z, Herrlinger KR, Witthoeft T, Homann N, Bruening A, et al.Response of refractory colitis to intravenous or oral tacrolimus (FK506). Inflammatory Bowel Diseases 2002;8(5):317-24. [DOI] [PubMed] [Google Scholar]
Hisamatsu 2000 {published data only}
- Hisamatsu T, Watanabe M.Tacrolimus (FK506), a novel immunosuppressive drug for inflammatory bowel disease? Journal of Gastroenterology 2000;35:655-7. [DOI] [PubMed] [Google Scholar]
JPRN‐UMIN000003785 {published data only}
- JPRN-UMIN000003785.Remission maintenance treatment of ulcerative colitis with tacrolimus in azathioprine-intolerant patients: a randomized comparative trial of 5-aminosalicylic acid (5-ASA) monotherapy versus 5-ASA and tacrolimus combination therapy. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000003785 (first received 1 July 2010).
JPRN‐UMIN000005033 {published data only}
- JPRN-UMIN000005033.Open-labeled prospective randomized study between tacrolimus only and tacrolimus with intensive (twice a week) GMA (granulocyte and monocyte absorptive apheresis) for ulcerative colitis. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000005033 (first received 1 March 2011).
Touchefeu 2007 {published data only}
- Touchefeu Y.Tacrolimus in refractory ulcerative rectocolitis treatment to a steroid therapy: a randomized study versus placebo [Le tacrolimus dans le traitment des rectocolites hemorragiques refractaires a la corticotherapie: une etude randomisee versus placebo]. Hepato-Gastro & Oncologie Digestive 2007;14(1):78-9. [Google Scholar]
References to studies awaiting assessment
CTRI/2015/10/006252 {published data only}
- CTRI/2015/10/006252.A double-blind, randomized, two-arm, parallel, clinical study to evaluate efficacy, safety and tolerability of two dose strengths of tacrolimus lipid suspension for injection rectally administered in adult patients with mild to moderately active left-sided/distal ulcerative colitis including proctosigmoiditis/proctitis refractory to mesalamine treatment. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2015/10/006252 (first received 12 October 2015).
CTRI/2019/04/018626 {published data only}
- CTRI/2019/04/018626.A randomized, multi-centre, open label, two-arm, parallel, clinical study to evaluate the efficacy, safety and tolerability of tacrolimus lipid suspension for enema of Intas Pharmaceuticals Limited, India against CORTENEMA® (hydrocortisone retention enema) of Anip Acquisition Company, USA in adult patients with mild to moderately active left-sided/distal ulcerative colitis refractory to mesalamine treatment. ctri.nic.in/Clinicaltrials/showallp.php?mid1=32147&EncHid=&userName=018626 (first received 15 April 2019).
JPRN‐UMIN000003952 {published data only}
- JPRN-UMIN000003952.A multicenter, randomised, open-label study to evaluate the clinical efficacy and pharmacokinetics of oral tacrolimus (FK506) therapy under fasting and fed conditions in refractory ulcerative colitis. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000003952 (first received 1 July 2010).
JPRN‐UMIN000004201 {published data only}
- JPRN-UMIN000004201.The evaluation of efficacy for remission maintenance with immunomodulator following remission induction with tacrolimus in patients with moderate to severe active refractory ulcerative colitis: mucosal healing for prognosis. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000004201 (first received 14 September 2010).
JPRN‐UMIN000007406 {published data only}
- JPRN-UMIN000007406.Clinical and endoscopic evaluation of the therapeutic effect of tacrolimus with rapid dose reduction of steroid for ulcerative colitis. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000007406 (first received 1 April 2012).
JPRN‐UMIN000010776 {published data only}
- JPRN-UMIN000010776.An open label randomized controlled trial of tacrolimus versus cyclosporine treatment for severe ulcerative colitis. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000010776 (first received 1 June 2013).
NCT00347048 {published data only}
- NCT00347048.Tacrolimus (FK506) P-III placebo-controlled double-blind study in moderate to severe refractory ulcerative colitis patients. www.clinicaltrials.gov/ct2/show/NCT00347048 (first received 4 July 2006).
Additional references
Benson 2008
- Benson A, Barrett T, Sparberg M, Buchman AL.Efficacy and safety of tacrolimus in refractory ulcerative colitis and Crohn's disease: a single-center experience. Inflammatory bowel diseases 2008;14(1):7-12. [DOI] [PubMed] [Google Scholar]
Collins 2006
- Collins P, Rhodes J.Ulcerative colitis: diagnosis and management. BMJ 2006;333(7563):340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Da silva 2014
- Da Silva C, Lyra C, Rocha R, Santana O.Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World Journal of Gastroenterology 2014;20(28):9458-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C.Bias in meta-analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EMA 2018
- Guideline on the development of new medicinal products for the treatment of ulcerative colitis. www.ema.europa.eu/en/documents/scientific-guideline/guideline-development-new-medicinal-products-treatment-ulcerative-colitis-revision-1_en.pdf (accessed prior to 25 February 2022).
FDA 2016
- FDA.Ulcerative colitis: clinical trial endpoints guidance for industry. www.fda.gov/files/drugs/published/Ulcerative-Colitis--Clinical-Trial-Endpoints-Guidance-for-Industry.pdf (accessed prior to 25 February 2022).
Feagan 2013
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al.Vedolizumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2013;369(8):699-710. [DOI] [PubMed] [Google Scholar]
Geboes 2000
- Geboes K, Riddell R, Öst A, Jensfelt B, Persson T, Löfberg R.A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000;47(3):404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomollón 2017
- Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, et al.3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. Journal of Crohn's and Colitis 2017;11(1):3-25. [DOI] [PubMed] [Google Scholar]
Ha 2010
- Ha CY, Newberry RD, Stone CD, Ciorba MA.Patients with late-adult-onset ulcerative colitis have better outcomes than those with early onset disease. Clinical Gastroenterology and Hepatology 2010;8(8):682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanauer 2002
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al.Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002;359(9317):1541-9. [DOI] [PubMed] [Google Scholar]
Harbord 2017
- Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, et al.Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. Journal of Crohn's and Colitis 2017;11(7):769-84. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hoffmann 2019
- Hoffmann P, Wehling C, Krisam J, Pfeiffenberger J, Belling N, Gauss A.Performance of tacrolimus in hospitalized patients with steroid-refractory acute severe ulcerative colitis. World Journal of Gastroenterology 2019;25(13):1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaeger 2019
- Jaeger U, Klag T, Hoeger K, Klumpp S, Escher M, Malek N, et al.Tacrolimus suppositories in therapy-resistant ulcerative proctitis. Inflammatory Intestinal Diseases 2019;3(3):116-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jairath 2015
- Jairath V, Khanna R, Zou GY, Stitt L, Mosli M, Vandervoort MK, et al.Development of interim patient-reported outcome measures for the assessment of ulcerative colitis disease activity in clinical trials. Alimentary Pharmacology & Therapeutics 2015;42(10):1200-10. [DOI] [PubMed] [Google Scholar]
Lamb 2019
- Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al.British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lichtenstein 2006
- Lichtenstein GR, Abreu MT, Cohen R, Tremaine W.American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006;130(3):940-87. [DOI] [PubMed] [Google Scholar]
Magro 2017
- Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, et al.Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. Journal of Crohn's and Colitis 2017;11(6):649-70. [DOI] [PubMed] [Google Scholar]
Marchal‐Bressenot 2017
- Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, Bastien C, Cahn V, Cadiot G, et al.Development and validation of the Nancy histological index for UC. Gut 2017;66(1):43-9. [DOI] [PubMed] [Google Scholar]
Matsuoka 2015
- Matsuoka K, Saito E, Fujii T, Takenaka K, Kimura M, Nagahori M, et al.Tacrolimus for the treatment of ulcerative colitis. Intestinal Research 2015;13(3):219-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosli 2014
- Mosli MH, Feagan BG, Zou G, Geboes K, Langner C, Peterson MR, et al.Agreement of central readers in the evaluation of histopathological disease activity in ulcerative colitis. Gastroenterology 2014;5(1):S225. [Google Scholar]
Mosli 2015
- Mosli MH, Feagan BG, Zou G, Sandborn WJ, D'Haens G, Khanna R, et al.Reproducibility of histological assessments of disease activity in UC. Gut 2015;64(11):1765-73. [DOI] [PubMed] [Google Scholar]
Mosli 2017
- Mosli MH, Feagan BG, Zou G, Sandborn WJ, D'Haens G, Khanna R, et al.Development and validation of a histological index for UC. Gut 2017;66(1):50-8. [DOI] [PubMed] [Google Scholar]
Neurath 2019
- Neurath F, Lepkkes M.Resolution of ulcerative colitis. Seminars in Immunopathology 2019;41(6):747-56. [DOI] [PubMed] [Google Scholar]
Ng 2017
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al.Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies [ ]. Lancet 2017;390(10114):2769-78. [DOI] [PubMed] [Google Scholar]
RevMan Web 2020 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web).The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Rubin 2019
- Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD.ACG clinical guideline: ulcerative colitis in adults. American Journal of Gastroenterology 2019;114(3):384-413. [DOI] [PubMed] [Google Scholar]
Sandborn 2012
- Sandborn WJ, Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, et al.Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142(2):257-65. [DOI] [PubMed] [Google Scholar]
Sandborn 2014
- Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al.Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146(1):96-109. [DOI] [PubMed] [Google Scholar]
Sandborn 2017
- Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, et al.Tofacitinib as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2017;376(18):1723-36. [DOI] [PubMed] [Google Scholar]
Sands 2019
- Sands BE, Sandborn WJ, Panaccione R, O'Brien CD, Zhang H, Johanns J, et al.Ustekinumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2019;381(13):1201-14. [DOI] [PubMed] [Google Scholar]
Scalea 2016
- Scalea JR, Levi ST, Ally W, Brayman KL.Tacrolimus for the prevention and treatment of rejection of solid organ transplants. Expert Revie of Clinical Immunology 2016;12(3):333-42. [DOI] [PubMed] [Google Scholar]
Schmidt 2013
- Schmidt KJ, Herrlinger KR, Emmrich J, Barthel D, Koc H, Lehnert H, et al.Short-term efficacy of tacrolimus in steroid-refractory ulcerative colitis – experience in 130 patients. Alimentary Pharmacology & Therapeutics 2013;37(1):129-36. [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Ungaro 2017
- Ungaro R, Mehandru S, Allen B, Peyrin-Biroulet L, Colombel F.Ulcerative colitis. Lancet 2017;389(10080):1756-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamamoto 2008
- Yamamoto S, Nakase H, Mikami S, Inoue S, Yoshino T, Takeda Y, et al.Long-term effect of tacrolimus therapy in patients with refractory ulcerative colitis. Alimentary Pharmacology & Therapeutics 2008;28(5):589-97. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Baumgart 2008
- Baumgart DC, MacDonald JK, Feagan B.Tacrolimus (FK506) for induction of remission in refractory ulcerative colitis. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No: CD007216. [DOI: 10.1002/14651858.CD007216] [DOI] [PubMed] [Google Scholar]


