Abstract
A pancreatic pseudoaneurysm is a rare but life-threatening clinical entity. Prompt diagnosis and appropriate treatment are of great clinical importance. We herein present an unusual case of a pseudoaneurysm of the posterior inferior pancreaticoduodenal artery that developed as a complication of chronic pancreatitis. It was detected in a timely manner and successfully treated with minimally invasive endovascular therapy.
Keywords: Pancreatic pseudoaneurysm, chronic pancreatitis, pancreas, sandwich technique, angiography, minimally invasive treatment
Introduction
Chronic pancreatitis is characterized by repetitive episodes of pancreatic inflammation that lead to extensive fibrotic tissue replacement, resulting in permanent structural and functional damage to the pancreas. A visceral artery pseudoaneurysm is a known but relatively rare complication of chronic pancreatitis. 1 Surrounding inflammation and leakage of pancreatic proteolytic enzymes may cause erosion of the walls of the peripancreatic vessels, leading to pseudoaneurysm formation. 1 A pseudoaneurysm wall is formed from a hematoma and fibrous tissue, making it vulnerable and prone to rupture. 2 Bleeding from a ruptured pseudoaneurysm can be potentially lethal with a mortality rate of up to 40%. 3 Thus, prompt diagnosis and appropriate treatment are of great clinical importance. We herein present an unusual case of a pseudoaneurysm of the posterior inferior pancreaticoduodenal artery that developed as a complication of chronic pancreatitis. It was detected in a timely manner and successfully treated with minimally invasive endovascular treatment (the sandwich technique). Written informed consent was obtained from the patient. In accordance with the practice of our ethics committee, study approval was not required (approval is only required when patient consent has not been obtained).
Case report
This study conforms to the CARE guidelines. 4 A 55-year-old woman with a history of chronic pancreatitis due to alcohol abuse was referred to our surgical facility with acute exacerbation of abdominal pain radiating to the back as well as nausea and fatigue that had lasted for 2 months prior to admission. Five years previously, the patient had undergone distal pancreatectomy with splenectomy for treatment of chronic pancreatitis and pseudocyst formation.
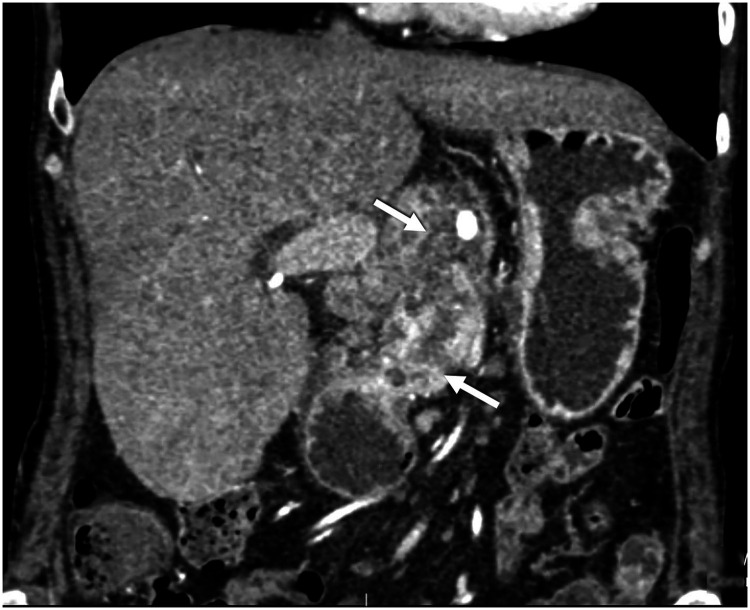
At the time of admission, abdominal examination revealed a soft abdomen with epigastric tenderness. Abdominal contrast-enhanced computed tomography (CT) revealed acute inflammation in the pancreatic head, which had developed as a complication of chronic pancreatitis and presented as necrotic pancreatitis on the CT scan. The pancreatic head was enlarged and heterogeneously enhanced, with a small area of necrosis extending into the peripancreatic fatty tissue around the celiac trunk (Figure 1). The patient was treated conservatively with antibiotic prophylaxis, high-volume crystalloid infusion therapy containing Ringer’s lactate solution, analgesics, and a proton pump inhibitor. Abdominal ultrasound (US) examination prior to discharge from the hospital revealed no worsening of the inflammation and no new liquid collections or free fluid. Therefore, with normal inflammatory marker levels, the patient’s condition was considered stable and she was dismissed from the hospital.
Figure 1.
Coronal section contrast-enhanced abdominal computed tomography showing an enlarged pancreatic head and heterogeneously enhanced parenchyma with a small area of necrosis extending into the peripancreatic fat plane around the celiac trunk. The intrapancreatic common bile duct is narrowed with consequent biliary stasis.
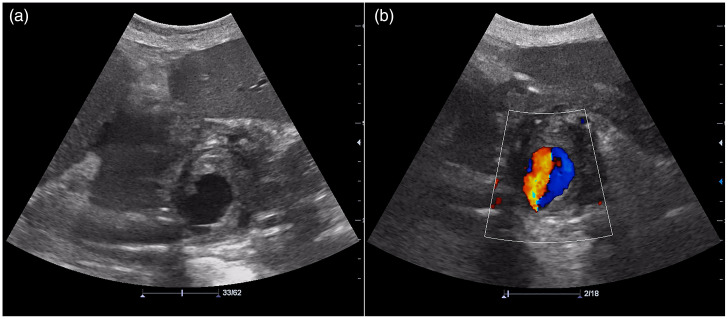
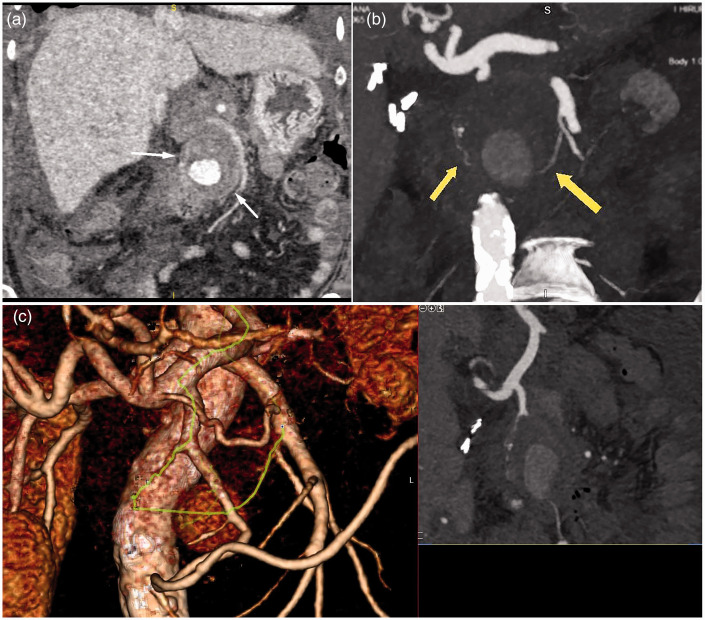
The patient returned for her scheduled follow-up 2 months after hospitalization. Abdominal US with color Doppler showed a cystic lesion with internal turbulent blood flow in the pancreatic head (Figure 2). Follow-up contrast-enhanced CT and angiography revealed an encapsulated, round necrotic mass corresponding to walled-off pancreatic necrosis (WOPN), with a maximum diameter of 6 cm in the region of the pancreatic head and a centrally enhanced component measuring approximately 3.5 cm (Figure 3). This area of contrast extravasation inside the WOPN had the characteristics of a pseudoaneurysm of the posterior inferior pancreaticoduodenal artery.
Figure 2.
(a) Abdominal ultrasound examination showing a large hypoechogenic cystic lesion in the pancreatic head. (b) Doppler ultrasound showing bidirectional blood flow due to the swirling of blood within the aneurysm, representing the “yin and yang” sign.
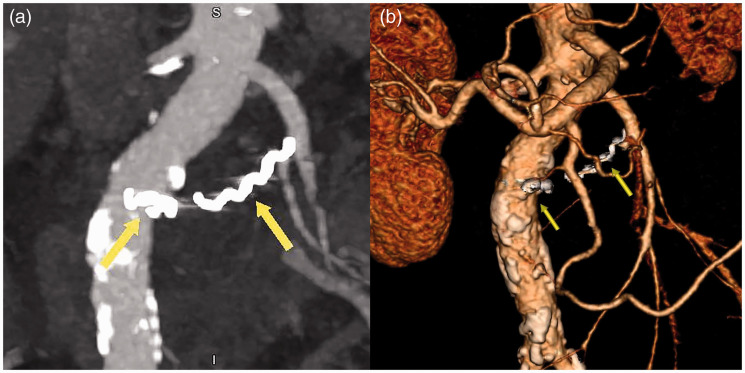
Figure 3.
(a, b) Coronal section contrast-enhanced abdominal computed tomography showing a central enhancing component measuring approximately 3.5 cm in an encapsuled necrotic mass (walled-off pancreatic necrosis) with a maximal diameter of 6 cm in the region of the pancreatic head, indicating a pseudoaneurysm of the jejunal artery. (c) Three-dimensional volume-rendering computed tomography showing a high-density area (arrows) suggesting a formed pseudoaneurysm of the pancreatic-duodenal arcade (green line).
Considering the location and size of the pseudoaneurysm as well as the patient’s clinical condition, we opted for digital subtraction angiography (DSA) together with endovascular coil embolization.
Selective catheterization of the celiac trunk and superior mesenteric artery was performed with a 5-Fr Cobra 3 catheter (Optitorque; Terumo, Leuven, Belgium), and DSA confirmed the location of the pseudoaneurysm at the inferior duodenal pancreatic artery and superior duodenal pancreatic artery as a collateral vessel to the gastroduodenal artery.
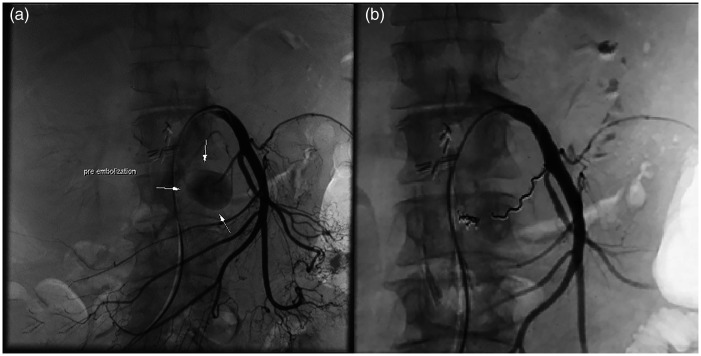
Sub-selective catheterization of the posterior inferior pancreaticoduodenal artery was also possible with the same catheter and enabled us to visualize the exact location of the arterial lesion. A microcatheter (Rebar TM14; ev3 Inc., Covidien, Irvine, CA, USA) was inserted into the distal part of the artery to facilitate utilization of the sandwich technique, and both the inflow and outflow of the pseudoaneurysm were embolized with 0.014-inch detachable three-dimensional coils (2 × 8 mm and 4 × 8 mm) (Concerto; Covidien) (Figure 4). Control DSA of the SMA and celiac trunk showed complete exclusion of the pseudoaneurysm with coils and regular flow through the gastroduodenal artery and SMA, which was confirmed on follow-up CT (Figure 5).
Figure 4.
(a) Selective catheterization the superior mesenteric artery showing a pancreaticoduodenal artery pseudoaneurysm. (b) Post-embolization digital subtraction angiography showing that the pseudoaneurysm is excluded from the circulation by the sandwich technique. The image shows two 2- × 8-mm coils in the outflow part of the artery (Concerto; Covidien, Irvine, CA, USA) and one 4- × 8-mm coil in the inflow part of the artery (Concerto; Covidien).
Figure 5.
Follow-up (a) coronal section contrast-enhanced computed tomography and (b) three-dimensional volume-rendering computed tomography showing complete exclusion of the pseudoaneurysm with coils.
The day after the procedure, a blood test revealed mild leukocytosis (white blood cell count of 10.6 × 109/L) and a mildly elevated C-reactive protein level (22.2 mg/L); the serum amylase and lipase concentrations were normal. The patient was discharged from the hospital 2 days later. One month after embolization, she was symptom-free and doing well.
Discussion
Chronic pancreatitis is characterized by repeated, progressive inflammation of the pancreatic parenchyma and fibrosis. Irreversible destruction of the pancreas results in the loss of exocrine and endocrine function. Pancreatic fluid collection, pseudocysts, and WOPN are the result of exacerbation of this chronic inflammatory process.5,6 Acute or chronic pancreatitis complicated by pancreatic or peripancreatic necrosis leads to the formation of acute necrotic collections. Such collections gradually become organized and, after 4 weeks, are termed WOPN according to the Revised Atlanta Classification. 7 Throughout the process of its formation and expansion, WOPN incorporates nearby vessels into its wall. Thick-walled arteries, unlike veins (which mainly thrombose), remain exposed to enzymatic activity. 1 Protein-lysing enzymes erode the arterial wall, leading to pseudoaneurysm formation. 1 The main clinical significance of a pseudoaneurysm lies in its potential to rupture. Unlike the wall of a true aneurysm, the wall of a pseudoaneurysm does not contain arterial tissue but instead consists of fibrous tissue and contains a hematoma, which can enlarge or bleed. The most commonly involved artery is the splenic artery, followed by the gastroduodenal, pancreaticoduodenal, and hepatic arteries.8,9
Many non-bleeding pseudoaneurysms present with nonspecific symptoms such as abdominal discomfort or pain. 1 In cases of hemorrhage, pseudoaneurysms typically manifest as anemia, melena, bleeding in the pancreatic or bile duct, or massive hemorrhage in the peritoneal cavity with acute abdominal pain and shock. 1 Bearing this in mind, a patient in whom peripancreatic fluid collections have formed after an episode of acute or chronic pancreatitis should be monitored more intensively in the weeks following the detection of these collections. 10
Diagnostic modalities applied for pseudoaneurysms include US, Doppler US, CT, magnetic resonance imaging, and angiography. Although Doppler US can reveal a pseudoaneurysm, contrast-enhanced CT should be the diagnostic tool of choice because it can properly identify the pancreatic pseudoaneurysm as well as its location. 11 Several studies have shown that angiography is extremely valuable in localizing bleeding pseudoaneurysms.12,13
The management of pseudoaneurysms depends on their dimensions, location, and complications and the patient’s condition. Open abdominal surgery of pancreatic pseudoaneurysms has been linked to a high mortality rate. 14 Hence, surgery is reserved only for hemodynamically unstable patients whose life is in danger. In contrast, endovascular treatment with angioembolization is being increasingly used because of its improved technical capabilities and low morbidity rates.13,14 Angioembolization offers exceptional diagnostic and therapeutic possibilities for precise identification and localization of blood vessels involved in the formation of pancreatic pseudoaneurysms. Furthermore, exclusion of blood vessels from the circulation by stenting or embolization techniques is also possible. In addition to endovascular coil embolization and covered stent implantation, other embolic agents are also available. Liquid embolic agents such as N-butyl 2-cyanoacrylate (NBCA) and ethylene vinyl alcohol copolymer can achieve permanent occlusion of small vascular branches as well as sources of collateral circulation. Complications associated with the use of these agents include catheter adherence to the material from early polymerization and non-target embolization. NBCA can reportedly cause vascular cytotoxicity by damaging the vascular intima and triggering inflammatory and foreign body responses. 15 Another option is the use of gelatinous foam, which is a relatively safe but temporary solution. For occlusion of larger vessels, placement of a self-expandable vascular plug is preferred. After the intervention, control angiography is advised to ensure that there are no other collateral vessels supplying the pseudoaneurysm. In our case, control angiography from the superior mesenteric artery and gastroduodenal artery confirmed exclusion of the pseudoaneurysm from the circulation. If the pseudoaneurysm has a narrow neck and if angioembolization is not possible for anatomical reasons, thrombin injection is advised. This is a less expensive but more invasive procedure. 16
Intraprocedural rupture of the pseudoaneurysm and delayed reconstitution of arterial flow are very rare complications of embolization but are usually unrelated to the angiographic technique. Technique-related complications include non-target coil embolization and maldeployment of the stent-graft. Further arterial embolization may be complicated by solid organ ischemia or development of an organic abscess. Exacerbation of pancreatitis and enlargement of pancreatic necrosis can also occur, but very rarely. 16
Although the pancreas has a rich collateral blood supply, pancreatic tissue is susceptible to ischemia, which can induce the development of acute pancreatitis. 17 It can be assumed that embolization of peripancreatic vascular structures may lead to tissue ischemia. In practice, however, acute pancreatitis due to pancreatic ischemia is rare. Only a few cases of pancreatitis after embolization have been reported in the literature.18–20 In such circumstances, acute pancreatitis may have different clinical presentations. It can present as a prolonged increase in the serum lipase and amylase concentrations with minimal symptomatology, which in most cases resolves spontaneously. Rarely, complications such as pancreatic necrosis or abscess formation may occur. We firmly believe that every patient who undergoes embolization of peripancreatic vessels should be observed for signs of acute pancreatitis. Because of the unpredictable course of this potentially fatal disease, discharge must be delayed until normalization of inflammatory parameters and serum pancreatic enzymes.
Conclusion
A pseudoaneurysm is a rare but life-threatening complication of chronic pancreatitis, and its main clinical significance is its potential to rupture. It is important to keep in mind that a pseudoaneurysm may form at any time secondary to acute infection or the formation of necrotic collections. Thus, it is very important to follow the guidelines for diagnostic monitoring of these patients, ensuring that life-threatening complications are detected in a timely manner and treated with available minimally invasive therapeutic procedures.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Milica Mitrovic Jovanovic https://orcid.org/0000-0002-7525-7615
Boris Tadic https://orcid.org/0000-0001-5400-1015
References
- 1.Hoilat GJ, Mathew G and Ahmad H. Pancreatic pseudoaneurysm. Treasure Island (FL): StatPearls Publishing, 2021. [PubMed]
- 2.Pang TC, Maher R, Gananadha S, et al. Peripancreatic pseudoaneurysms: a management-based classification system. Surg Endosc 2014; 28: 2027–2038. 2014/02/13. DOI: 10.1007/s00464-014-3434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novacic K, Vidjak V, Suknaic S, et al. Embolization of a large pancreatic pseudoaneurysm converted from pseudocyst (hemorrhagic pseudocyst). JOP 2008; 9: 317–321. 2008/05/13. [PubMed] [Google Scholar]
- 4.Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med 2013; 2: 38–43. 2014/01/15. DOI: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasch S, Notzel B, Phillip V, et al. Management of pancreatic pseudocysts-a retrospective analysis. PLoS One 2017; 12: e0184374. 2017/09/07. DOI: 10.1371/journal.pone.0184374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan JH, Zhou L, Cao RC, et al. Identification of risk factors for pancreatic pseudocysts formation, intervention and recurrence: a 15-year retrospective analysis in a tertiary hospital in China. BMC Gastroenterol 2018; 18: 143. 2018/10/05. DOI: 10.1186/s12876-018-0874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster BR, Jensen KK, Bakis G, et al. Revised Atlanta Classification for Acute Pancreatitis: a pictorial essay. Radiographics 2016; 36: 675–687. 2016/05/11. DOI: 10.1148/rg.2016150097. [DOI] [PubMed] [Google Scholar]
- 8.Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg 2003; 38: 969–974. 2003/11/07. DOI: 10.1016/s0741-5214(03)00710-9. [DOI] [PubMed] [Google Scholar]
- 9.Woods MS, Traverso LW, Kozarek RA, et al. Successful treatment of bleeding pseudoaneurysms of chronic pancreatitis. Pancreas 1995; 10: 22–30. 1995/01/01. DOI: 10.1097/00006676-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Udd M, Leppaniemi AK, Bidel S, et al. Treatment of bleeding pseudoaneurysms in patients with chronic pancreatitis. World J Surg 2007; 31: 504–510. 2007/02/27. DOI: 10.1007/s00268-006-0209-z. [DOI] [PubMed] [Google Scholar]
- 11.Hsu JT, Yeh CN, Hung CF, et al. Management and outcome of bleeding pseudoaneurysm associated with chronic pancreatitis. BMC Gastroenterol 2006; 6: 3. 2006/01/13. DOI: 10.1186/1471-230X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambiez LP, Ernst OJ, Merlier OA, et al. Arterial embolization for bleeding pseudocysts complicating chronic pancreatitis. Arch Surg 1997; 132: 1016–1021. 1997/09/25. DOI: 10.1001/archsurg.1997.01430330082014. [DOI] [PubMed] [Google Scholar]
- 13.Uyar İS. A giant splenic artery aneurysm: a case report. Turkish Journal of Thoracic and Cardiovascular Surgery 2013; 21: 799–802. DOI: 10.5606/tgkdc.dergisi.2013.6286. [Google Scholar]
- 14.Kiviluoto T. Pseudocysts in chronic pancreatitis. Archives of Surgery 1989; 124. DOI: 10.1001/archsurg.1989.01410020114019. [DOI] [PubMed] [Google Scholar]
- 15.Wang YM, Cheng LF, Li N. Histopathological study of vascular changes after intra-arterial and intravenous injection of N-butyl-2-cyanoacrylate. Chin J Dig Dis 2006; 7: 175–179. 2006/07/01. DOI: 10.1111/j.1443-9573.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- 16.Barge JU, Lopera JE. Vascular complications of pancreatitis: role of interventional therapy. Korean J Radiol 2012; 13: S45–S55. DOI: 10.3348/kjr.2012.13.S1.S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakorafas GH, Tsiotou AG. Etiology and pathogenesis of acute pancreatitis: current concepts. J Clin Gastroenterol 2000; 30: 343–356. 2000/06/30. DOI: 10.1097/00004836-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Matta A, Tandra PK, Cichowski E, et al. Acute necrotising pancreatitis: a late and fatal complication of pancreaticoduodenal arterial embolisation. BMJ Case Rep 2014; 2014: bcr2014204197. 2014/06/01. DOI: 10.1136/bcr-2014-204197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuyumcu G, Latich I, Hardman RL, et al. Gastrodoudenal embolization: indications, technical pearls, and outcomes. J Clin Med 2018; 7: 101. 2018/05/05. DOI: 10.3390/jcm7050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chua WM, Venkatanarasimha N, Damodharan K. Acute ischemic pancreatitis: a rare complication of empirical gastroduodenal artery embolization. Indian J Radiol Imaging 2017; 27: 338–341. 2017/11/02. DOI: 10.4103/0971-3026.215571. [DOI] [PMC free article] [PubMed] [Google Scholar]