Abstract
Snakebite envenoming (SBE) is a neglected public health problem, especially in Asia, Latin America and Africa. There is inadequate knowledge of venom toxicokinetics especially from African snakes. To mimic a likely scenario of a snakebite envenoming, we used an enzyme-linked immunosorbent assay (ELISA) approach to study the toxicokinetic parameters in rabbits, following a single intramuscular (IM) administration of Northern Nigeria Naja nigricollis venom. We used a developed and validated non-compartmental approach in the R package PK to determine the toxicokinetic parameters of the venom and subsequently used pharmacometrics modelling to predict the movement of the toxin within biological systems. We found that N. nigricollis venom contained sixteen venom protein families following a mass spectrometric analysis of the whole venom. Most of these proteins belong to the three-finger toxins family (3FTx) and venom phospholipase A2 (PLA2) with molecular weight ranging from 3 to 16 kDa. Other venom protein families were in small proportions with higher molecular weights. The N. nigricollis venom was rapidly absorbed at 0.5 h, increased after 1 h and continued to decrease until the 16th hour (Tmax), where maximum concentration (Cmax) was observed. This was followed by a decrease in concentration at the 32nd hour. The venom of N. nigricollis was found to have high volume of distribution (1250 ± 245 mL) and low clearance (29.0 ± 2.5 mL/h) with an elimination half-life of 29 h. The area under the curve (AUC) showed that the venom remaining in the plasma over 32 h was 0.0392 ± 0.0025 mg h.L−1, and the mean residence time was 43.17 ± 8.04 h. The pharmacometrics simulation suggests that the venom toxins were instantly and rapidly absorbed into the extravascular compartment and slowly moved into the central compartment. Our study demonstrates that Nigerian N. nigricollis venom contains low molecular weight toxins that are well absorbed into the blood and deep tissues. The venom could be detected in rabbit blood 48 h after intramuscular envenoming.
Keywords: Snake venom, Antisnake venom, Toxicokinetics, ELISA, Naja nigricollis, Pharmacometrics
Graphical abstract
Highlights
-
•
Toxicokinetics of Naja nigricollis venom were determined in rabbits.
-
•
A non-compartmental pharmacokinetics approach was used.
-
•
Venomics analysis of Naja nigricollis major toxins families.
-
•
Our study suggests distribution of toxins into deep tissues.
1. Introduction
Snakebite envenoming (SBE) is a neglected public health problem, especially in Asia, Latin America and Africa (WHO, 2017) and is on the rise in Nigeria and other West African countries mainly due to paucity of anti-snake venom, poor treatment knowledge, and inadequate treatment centres (Michael et al., 2018; Bala et al., 2021; Gutiérrez et al., 2021). The SBE affects poor communities in Asia and Africa, leading to compounded disadvantages with negative impacts on the economy and the healthcare sectors of the countries in the regions (Habib and Brown, 2018). A recent study estimated the burden of SBE at 1.03 million disability-adjusted life years per annum in sub-Saharan African, out of which Nigeria has the highest-burden with 43% of the total burden in West Africa (Halilu et al., 2019).
Indigenous to Africa, the cobras, belonging to the Elapidae family, represent the most medically essential snakes (WHO, 2017). Generally, spitting cobras have active venom that causes ulceration and necrosis around the bite site, with systemic neurotoxic effects. If there is significant tissue damage in the bitten limb, secondary fluid shifts may result in shock, and its sequelae and secondary infection and long-term scarring are common (Warrell, 1999; Chippaux et al., 2016). Hospital records have shown that the black-necked spitting cobra (Naja nigricollis) is the most urbanized and medically important elapid snake in northern Nigeria (Habib, 2003; Yusuf et al., 2015).
Despite the availability of institutional SBE treatment protocols in some medical centres, many centres do not have well-optimized SBE treatment protocols due in part to inadequate knowledge of the toxicokinetics of medically important snake venoms, especially in some affected communities of sub-Saharan Africa (Chippaux, 1998; Yap et al., 2014). To fully understand the pathophysiology and improve the treatment protocol of cobra envenoming, it is imperative to understand the toxicokinetics of the medically important snake venoms (Yap et al., 2013; Sanhajariya et al., 2018). There is an urgent need to improve the determination of antisnake venom dosing and timing of administration because, in many cases, the dose administered is determined based on clinical symptoms, laboratory results, and sometimes animal studies (Maduwage et al., 2016). The study of the time course of venom in biological systems will provide important information about the time course of the patients envenoming (Sanhajariya et al., 2018).
Theakston et al. (1977) first developed the use of enzyme-linked immunosorbent assay.
(ELISA) in detecting venom in biological samples, and it was found to have good sensitivity and specificity (Selvanayagam and Gopalakrishnakone (1999); Theakston, 1983). Recently, the sandwich ELISA approach represented a good approximation of the true picture of the toxicokinetics of snake venom using primary antibodies that binds to major toxins (Tan et al., 1993; Yap et al., 2013). Toxicokinetic analyses have been reported using the ELISA technique for some Asian cobras, including Naja sumatrana, Naja suputatrix, Pseudechis australis, Naja atra (Tseng et al., 1968; Yap et al., 2013; Hart et al., 2014). Another study in Asia used radioimmunoassay and reported a three compartmental toxicokinetic analysis for some cobra venoms, including Naja melanoleuca, Naja nivea, Naja nigricollis and Naja haje (Ismail et al., 1996). Thus, our study employed a non-compartmental analysis (NCA) because it is faster, cost efficient and most importantly requires fewer or no assumptions compared with compartmental-based approaches as previously described (Gabrielsson and Weiner 2012; Jaki and Wolfsegger 2012). To mimic a likely scenario of a snakebite envenoming, we used an ELISA approach to study the toxicokinetics of N. nigricollis venom captured from Northern Nigeria following a single intramuscular administration in rabbits. We used a developed and validated non-compartmental approach in the R package PK to determine the toxicokinetic parameters of the venom and subsequently used pharmacometrics modelling to predict the movement of the toxins within the biological systems.
2. Materials and methods
2.1. Ethics statement
The Ethics Committee of the College of Health Sciences, Bayero University Kano approved the study (BUK/CHS/HREC/VII/66). All animals were treated according to the WHO's ethical code for animal experimentation (Howard-Jones 1985).
2.2. Animals
Swiss albino mice weighing 18–20 g and New Zealand rabbits weighing 2.5–2.7 kg were obtained from Bayero University Kano and maintained according to Bayero University guidelines for the care and use of laboratory animals at the Animal House Facility, Department of Pharmacology and Therapeutics, Bayero University Kano, Nigeria.
2.3. Snake specie
Five N. nigricollis were captured from the wild in northern Nigeria. They were identified and housed at the Faculty of Veterinary Medicine Herpeterium, Ahmadu Bello University, Zaria, Kaduna, Nigeria.
2.4. Venom collection
Venoms from the five captured N. nigricollis were manually pooled at Veterinary Medicine Herpetarium, Ahmadu Bello University Zaria, Kaduna, Nigeria, using the method described by Markfarlane (1967).
2.5. Proteomic analysis of N. nigricollis venom
2.5.1. Mass spectrometric analysis of N. nigricollis whole venom
3.5.1.1. Sample preparation
One microgram of snake venom was denatured in 0.1% RapiGest (Waters, Milford, MA, USA) and reduced with 5 mM dithiothreitol for 30 min at 50 °C. Proteins were alkylated in 15 mM iodoacetamide for 1 h in the dark at room temperature and then digested with proteomics-grade trypsin at a 1:100 ratio overnight at 37 °C. The pH was adjusted below 3, and the sample was incubated for 45 min at 37 °C. The sample was centrifuged at 16,000×g to remove RapiGest. The supernatant was collected, and peptides were dissolved in 5 μL of 0.25% formic acid (FA) with 3% CAN (Willard et al., 2021).
3.5.1.2. Liquid chromatography-tandem mass spectrometry (LC–MS/MS)
The LC-MS-MS analysis was conducted as described by Willard et al. (2021). Samples were dried completely in a vacuum centrifuge and stored at −80 °C. One microgram of each dried peptide sample was dissolved in 10.5 μL of 0.05% trifluoroacetic acid with 3% (vol/vol) acetonitrile. In total, 10 μL of each sample was injected into an Ultimate 3000 nano UHPLC system (Thermo Fisher Scientific, Vantaa, Finland). Peptides were captured on a 2 cm Acclaim PepMap trap column and separated on a heated 50 cm column packed with ReproSil Saphir 1.8 μm C18 beads (Dr Maisch GmbH, Ammerbuch, Germany). The mobile phase buffer consisted of 0.1% formic acid in ultrapure water (buffer A) with an eluting buffer of 0.1% formic acid in 80% (vol/vol) acetonitrile (buffer B) ran with a linear 60 min gradient of 6–30% buffer B at a flow rate of 300 nL/min. The UHPLC was coupled with a Q Exactive HF-X mass spectrometer (Thermo Fisher Scientific). The mass spectrometer was operated in the data-dependent mode, in which a full-scan MS (from m/z 375 to 1500 with the resolution of 60,000) was followed by MS/MS of the 15 most intense ions (30,000 resolution; normalized collision energy—28%; automatic gain control target (AGC)—2E4: maximum injection time—200 ms; 60 s exclusion). The raw files were searched directly against the Naja/Echis (384/74) in UniProt with no redundant entries, using Byonic (Protein Metrics) and SEQUEST search engines loaded into Proteome Discoverer 2.3 software (Thermo Fisher Scientific). MS1 precursor mass tolerance was set at 10 ppm, and MS2 tolerance was set at 20 ppm. Search criteria included a static carbamidomethylation of cysteines (+57.0214 Da) and variable modifications of oxidation (+15.9949 Da) on methionine residues and acetylation (+42.011 Da) at the N-terminus of proteins. A search was performed with full trypsin/P digestion and allowed a maximum of two missed cleavages on the peptides analyzed from the sequence database. The false-discovery rates of proteins and peptides were set at 0.01. All protein and peptide identifications were grouped, and any redundant entries were removed. Unique peptides and unique master proteins were reported.
2.5.2. Separation of N. nigricollis major toxins
Fresh, lyophilized crude venom of N. nigricollis was dissolved in 1 mL Phosphate-buffered saline (PBS) to a final concentration of 10 mg/mL. The venom's size exclusion chromatography was carried out using fast protein liquid chromatography (FPLC) with ÄKTA pure 25MT. The column (24 mL Superose 6 column 10/300 GL {GE Healthcare} and a 0.5 mL loop was slowly equilibrated at 0.2 mL/min to remove the storage buffer (20% ethanol) with MilliQ water (2 column volume) then at 0.5 mL/min with PBS (2 column volume). The loop was also equilibrated with MilliQ water followed by PBS. Pump washes were carried out using MilliQ water and PBS. During size-exclusion chromatography, the sample injection volume was 250 μL (50% of the loop volume), and the elution buffer was PBS pH 7.4 at a flow rate of 0.5 mL/min. Fractions were collected into tubes and were stored at −20 °C in aliquots before molecular weight determination using 15% Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE).
3.5.2.1. Molecular weight determination of N. nigricollis venom toxins
The molecular weight of the separated fractions of N. nigricollis venom and the purified anti- N. nigricollis IgG were checked using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli (1970). A broad range SDS-PAGE standard (250–10 kDa; Bio-Rad™) and stained with Coomassie Brilliant Blue G 250 for 4 h. Destaining was conducted with a destaining solution as described by the manufacturer (Solarbio ™).
2.6. Toxicokinetics studies of N. nigricollis venom
2.6.1. Production and purification of polyclonal IgG against Naja nigricollis venom
Three New Zealand rabbits were immunized with the N. nigricollis venom as described by Yap et al. (2013). Fifty micrograms (50 μg) of the venom in 0.2 mL of PBS was mixed with an equal volume of Complete Freund's adjuvant (Sigma), and the three rabbits were immunized with the whole volume by intramuscular (IM) injection through the quadriceps muscles. The second and third immunizations were performed on days 14 and 28 with Freund's incomplete adjuvant (Sigma), and finally on day 35. After the last immunization, 20 mL of blood was collected from the rabbit with the highest IgG titer through the jugular vein. The collected blood was allowed to clot by leaving it undisturbed at room temperature for about 15 min and then centrifuged at 3000×g for 5 min. The resulting serum was immediately transferred into a clean container tube using a Pasteur pipette and maintained at −20 °C for further use.
3.6.1.1. Precipitation of rabbit polyclonal IgG (ammonium sulfate)
The collected serum was precipitated using a 40% ammonium sulfate and loaded onto an affinity chromatography column as described by Mariam et al. (2015). The IgG containing serum was transferred to a beaker and stirred. Saturated ammonium sulfate was slowly added to stir the sample to bring the final concentration to 50% saturation. The sample was kept at 4 °C overnight, after which it was centrifuged at 3000 g for 30 min. The supernatant was carefully removed and discarded. The residue (pellet) was suspended in 50% of the starting volume in 1X PBS.
3.6.1.2. Dialysis and affinity chromatography
The precipitated antibody solution was transferred into an 8000–14,000 wt dialysis tube (Solarbio ™) and dialyzed against three changes of tris - phosphate buffer (pH 8.1). The dialyzed anti - N. nigricollis venom IgG was further purified using affinity chromatography as described by Hudson and Hay (1980). Two millilitres (2 mL) of protein A-Sepharose was packed into a small chromatography column (Solarbio ™), then 5 mL serum was diluted with an equal volume of PBS and filtered through the column at a flow rate of 6 mL/h for 30 min. The column (unbound protein) was then washed with PBS and the bound IgG was eluted with glycine–HCl buffer (pH 2.8). The concentration was checked using a spectrophotometer and the purity was checked with SDS PAGE under reducing condition.
2.7. Determination of the antigenic reactivity of the purified IgG used in ELISA
The affinity of the purified IgG against N. nigricollis crude venom and the separated fractions were observed using indirect ELISA. The ELISA microplate was coated with sodium carbonate/bicarbonate buffer pH 9.6 (solarbioMT) in six serial dilutions of 1000 ng/mL of crude venom and each fraction, and were incubated overnight at 4 °C. The plate was blocked with 5% BSA (Solarbio™) and washed with PBS–Tween 20. Anti-N. nigricollis IgG (dilution of 1:500) was added and allowed to incubate at room temperature for 1 h. It was followed by incubation with 100 μL goat anti-rabbit IgG horseradish peroxidase conjugate and 100 μL of TMB substrate for 1 h. The reaction was then terminated by adding 50 μL sulfuric acid (12.5%). The absorbance at 492 nm was determined using an EnSight multimode plate reader (PerkinElmer, USA).
2.8. Determination of serum venom (antigen) using sandwich ELISA
Three rabbits were injected with 0.5 mg/kg of venom intramuscularly through the quadriceps muscles and blood samples were collected through the marginal ear vein at 0.5, 1, 2, 4, 8, 16 and 32 h. The serum antigen concentrations at specific times were measured using sandwich ELISA as described by Tan et al. (1993). ELISA microplates were coated overnight at 4 °C with 100 μL of the anti- N. nigricollis IgG (4 mg/mL) using sodium carbonate/bicarbonate buffer pH 9.6 (SolarbioMT). The plates were blocked with 5% BSA (Solarbio™) and subsequently incubated with 100 μL of diluted serum samples (1:50) collected at different time intervals. The plates were washed with PBS–Tween and subsequently anti-N. nigricollis IgG (dilution of 1:500) was added and allowed to incubate at room temperature for 1 h. It was followed by incubation with 100 μL goat anti-rabbit IgG horseradish peroxidase conjugate (1:1000) for 2 h, and 100 μL of 3,3′5,5′- Tetramethylbenzidine (TMB) substrate was added. The reaction was terminated 1 h later by adding 50 μL sulfuric acid (12.5%), and the absorbance was determined at 492 nm using a microplate reader. A standard curve was constructed using serial dilutions of venom in pre-envenomed sera (1000, 500, 250, 125, 62.5, 31.25, 15.63, and 7.81 ng/mL).
2.9. Pharmacokinetic and pharmacometrics analysis
Non-compartmental pharmacokinetics (PK) analysis was conducted using the R Core Team (2020), and data were presented as mean ± standard error of the mean (SEM). PK parameters of interest were estimated as described by Jaki and Wolfsegger (2011). The mrgsolve package in R was also used to evaluate the venom movement following a simulation approach. The Vd and CL estimated from the non-compartmental approach were used to simulate the distribution in the First extravascular compartment (EV1), Central compartment (CENT, Mass) and Plasma concentration (CP, mass/volume) as described by Elmokadem et al. (2019).
4. Results
4.1. Median lethal dose (LD50)
The median lethal dose (LD50) of N. nigricollis venom estimated intraperitoneolly (IP) using probit analysis was found to be 1.0 mg/kg in mice (see Fig. 1).
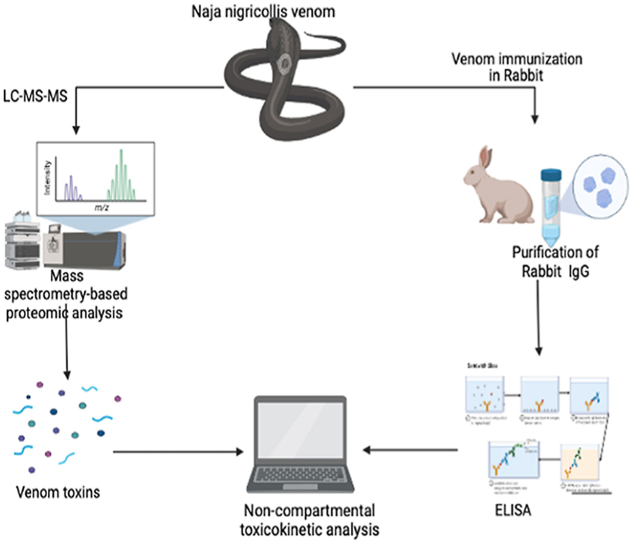
Fig. 1.
Graphical illustration of the study.
4.2. Development of polyclonal IgG against N. nigricollis venom
The concentration of the developed and purified polyclonal IgG against N. nigricollis venom was 13 mg/mL and the purity was demonstrated using SDS PAGE as shown on Fig. 2.
Fig. 2.
SDS PAGE of purified rabbit IgG.
4.3. Proteome of N. nigricollis venom
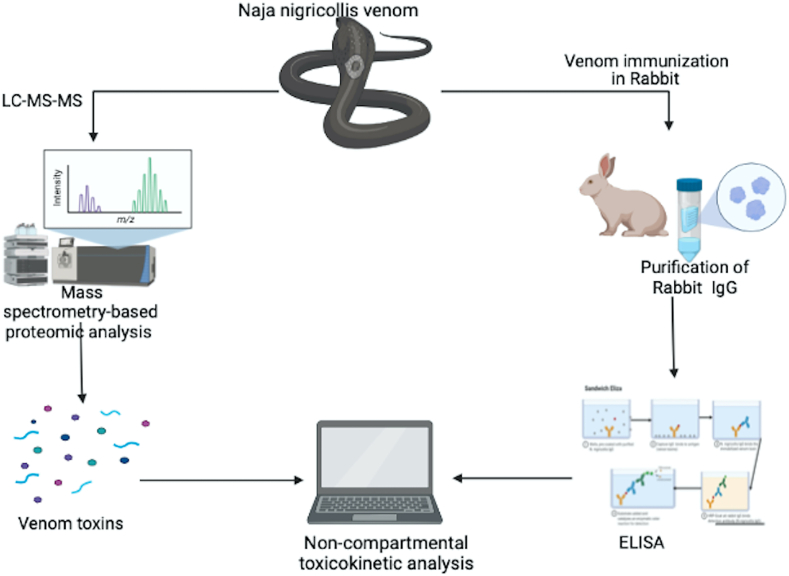
Proteomic analysis of N. nigricollis venom revealed sixteen (16) venom protein families with a total number of seventy-seven (77) proteins. Most of these proteins belong to the three-finger toxins family (3 TFx) and Venom phospholipase A2 (PLA2) with molecular weight ranging from 3 to 16 kDa. Other families are in small proportions with higher molecular weights, as shown in Fig. 3.
Fig. 3.
Percentage of venom families found in Naja nigricollis venom.
3FTX = Three Finger Toxins,PLA2 = Phospholipase A2,SVMP = Snake Venom MetalloproteinasesCVF = Cobra Venom Factor,CRISPs = Cysteine-Rich Secretory Proteins,KTSPIs = Kunitz-Type Serine Proteise Inhibitor,HF = Hydrolase Family,SWF = Snake Waprin Family,SF = Serine Family,CTLF = C-Type Lectin Like Family,L-AAO = L-amino acid oxidase,VC = Venom Cystatin,PF = Phosphodiesterase Family,VOF = Venom Ohanin Family,VC = Venom Cathelcidin
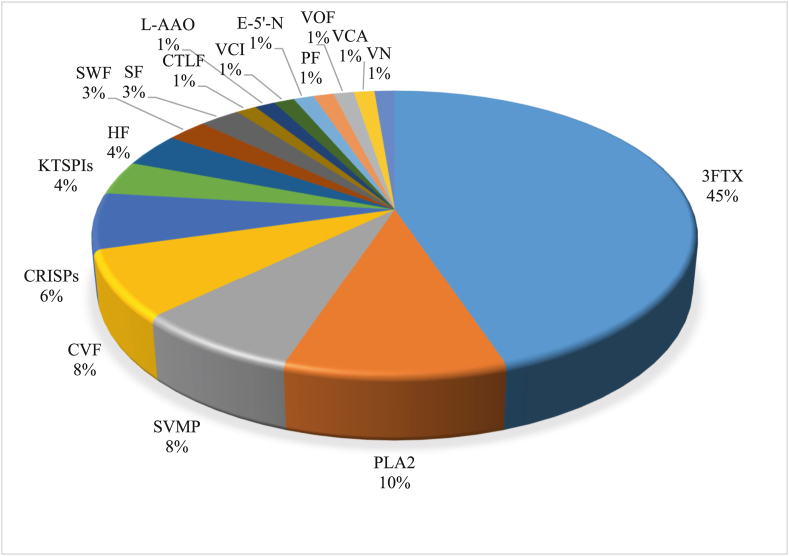
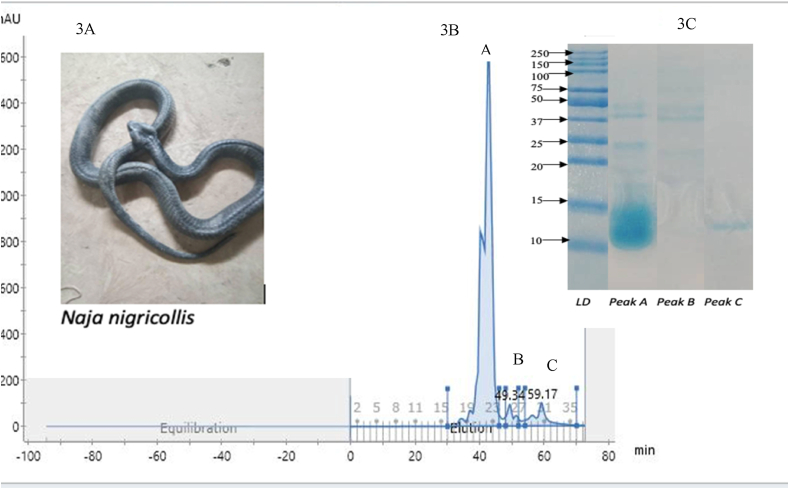
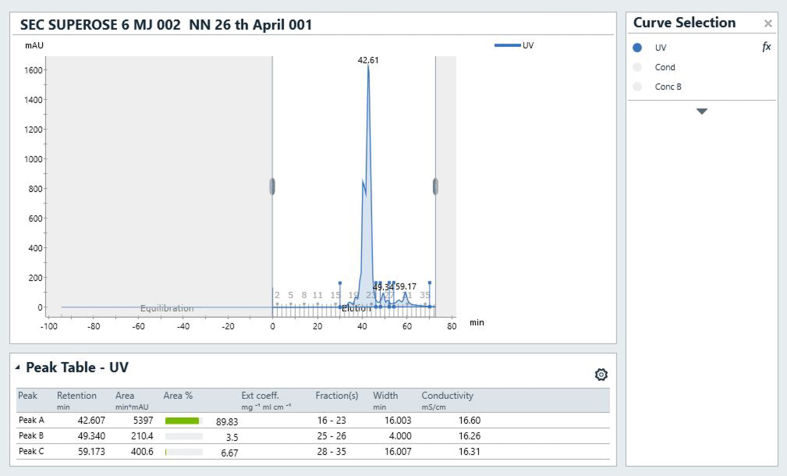
4.4. Separation of N. nigricollis toxins for indirect ELISA
Three peaks (fractions) were collected from the Fast Protein Lipid Chromatography. The SDS PAGE analysis of these peaks revealed proteins with lower molecular weights of 7–15 kDa and a few with higher molecular weights proteins 28–37 kDa, as shown in (Fig. 4). The molecular weights of proteins in Peak A were found to be similar to the 3FTx, PLA2 and CRISP, Peak B were within the serine & cathelcidin family as revealed in the mass spectrometric analysis, while Peak C contains 3FTx and PLA2.
Fig. 4.
Size exclusion fractionation of N. nigricollis venom and SDS PAGE analysis of its fractions. 3 A) Naja nigricollis in image. 3 B) size exclusion chromatogram 3C) SDS-PAGE of the three fractions.
4.5. Indirect ELISA of Naja nigricollis whole venom and its fractions
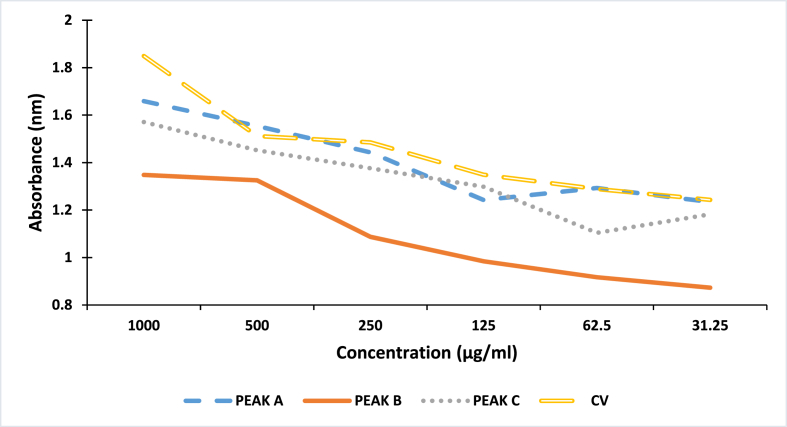
Indirect ELISA demonstrated the affinity of the Purified N. nigricollis IgG against venom and the separated fractions (Fig. 5). We also found higher affinity of the IgG against the crude venom followed by fraction A, fraction C and fraction B.
Fig. 5.
Affinity of purified IgG against Naja nigricollis venom and separated toxins.CV = Crude venom. A = Fraction A, B = Fraction B, C = Fraction C.
4.6. Sandwich ELISA for quantification of venom serum concentration
Venom serum concentration was detected 30 min after venom administration (1364 ± 167 ng/mL), and it increases until it reaches the maximum plasma concentration (1813 ± 175 ng/mL) at the 16th hour (Table 1).
Table 1.
Serum venom concentration of N. nigricollis using sandwich ELISA.
| Time (hours) | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 |
| Conc (ng/ml) | 1364 ± 167 | 1448 ± 180 | 1253 ± 230 | 1134 ± 75 | 1105 ± 215 | 1813 ± 175 | 614 ± 22 |
Time = time of measurement post administration, Con. = concentration of serum venom, ng/ml = nanogram per millimeter ± standard error of mean, rabbit weight (n = 3), 2.2–2.3 kg, Dose of venom injected = 0.5 mg/kg (intramuscular).
4.7. Toxicokinetic parameters of Naja nigricollis venom
The venom AUCt32 and (AUC∞) were determined to be 0.0392 ± 0.0025 and 0.0691 ± 0.0059 mg/h/L−1, respectively. The elimination half-life (29.92 ± 5.57 h), clearance (28.95 ± 2.45 mL/h), volume of distribution (1249.64 ± 245.33 mL), and mean residence (43.17 ± 8.04 h) following the non-compartmental pharmacokinetic analysis as shown in Table 2.
Table 2.
Toxicokinetic parameters of Naja nigricollis venom.
| Parameter | Value |
|---|---|
| AUCt32 (mg/hr/L−1) | 0.0392 ± 0.0025 |
| AUC∞ (mg/hr/L−1) | 0.0691 ± 0.0059 |
| Mean residence time (hr) | 43.17 ± 8.04 |
| Elimination Half-life (hr) | 29.92 ± 5.57 |
| Clearance (mL/hr) | 28.95 ± 2.45 |
| Volume of distribution (mL) | 1249.64 ± 245.33 |
| Cmax (ng/mL) | 1813 ± 175 |
| Tmax (hr) | 16 |
| Elimination rate constant (K10) (hr−1) | 0.0232 |
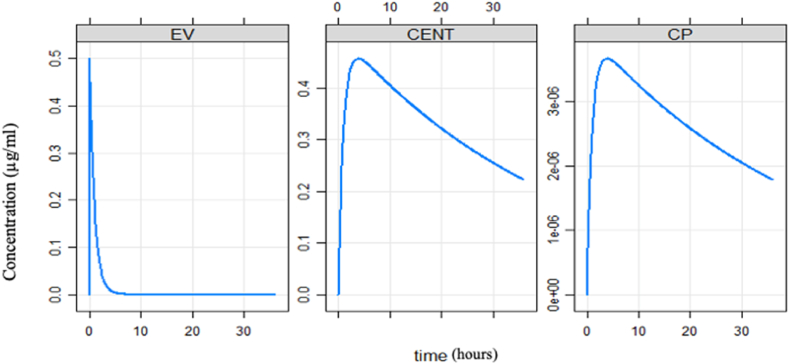
4.8. Pharmacometrics simulation
The pharmacometrics simulation shows that the venom was instantaneously distributed into the extravascular compartment compared to the central compartment (Fig. 6).
Fig. 6.
Simulated redistribution of toxin between compartments.
EV = extravascular compartments, CENT = Central compartments, CP = Plasma concentration.
5. Discussion
The LC-MS-MS analysis of the crude venom revealed 16 protein families, with the majority belonging to 3FTx (45%), PLA2 (10%) followed by SVMP (8%), CVF (8%) and CRISPs (6%). The venom protein families in our study are similar to what has been reported by previous studies (Petras et al., 2011; Tasoulis and Isbister, 2017; Adamude et al., 2021). Clinically, CRISPs & PLA2 have been reported to penetrate into the deep tissues and induce necrosis while 3FTx & PLA2 have been implicated in neurotoxicity and cytotoxicity (Linda and David 1975; AdinaChaim-Matyas and Ovadia, 1987; Kuzun et al., 1994; Conlon et al., 2020). To confirm that our purified primary anti- N. nigricollis IgG binds to the important toxins in the crude venom, we fractionated some of the major and clinically implicated toxins (3FTx, PLA2 & CRISPs) using size exclusion chromatography and confirmed their molecular weights using SDS PAGE. The indirect ELISA conducted against these fractionated toxins has made it possible to monitor the serum venom concentration post administration of the venom. The developed and purified IgG shows binding affinity to the fractions in a similar way to the crude venom.
5.1. Toxicokinetics
In clinical settings, the victim's venom blood level is rarely studied. Still, these data have been reported in studies involving laboratory animals using immunoassays such as ELISA and radioimmunoassay (Theakston et al., 1977; Theakston and Laing, 2014; Sanhajariya et al., 2020). The compartmental approach represent the body as a system of compartments, even though these compartments usually have no absolute physiologic or anatomic reality, this approach make certain assumptions such as; the body is made up of one, two or multiple compartments, they also assume that the rate of transfer between compartments and the rate of drug elimination from compartments follow first-order or linear kinetics (Gabrielsson and Weiner, 2012; Qusai et al., 2016). In our study, we employed a non-compartmental analysis (NCA) because it is faster, cost efficient and most importantly requires fewer or no assumptions compared with compartmental-based approaches as previously described (Gabrielsson and Weiner 2012; Jaki and Wolfsegger 2012). Based on our study, the volume of distribution (Vd) of the crude venom was found to be higher (1250 ± 245 mL) compared with the average blood of rabbit 57–65 mL/kg. It therefore, suggests that the majority of the toxins can penetrate deep into the extravascular tissues, as reported in some cobra venom from Asia (Tseng et al., 1968; Guo et al., 1993; Ismail et al., 1996; Yap et al., 2014). This could be as a result of the low molecular weight (7–37 kDa) of the toxins (Tasoulis and Isbister, 2017; Conlon et al., 2020; Adamude et al., 2021). The knowledge of venom Vd could play a vital role in the timing and selection of appropriate antisnake venom (ASV), because of difference in formulations of ASV which can either contain fragmented [F (ab')2], Fab’ or intact IgG (Ismail et al., 1998; WHO 2017). The fragmented IgGs have smaller molecular weights and larger Vds than the intact IgGs, implying that N. nigricollis venom might be better neutralized in the deep tissues with ASV that contains IgG fragments (F (ab')2s or Fab's). The terminal half-life (t1/2) of the N. nigricollis venom (29.9 h) was found to be higher than those reported from Asian Cobras (12–22 h) even though those studies used either two or multiple compartmental approaches in determining the toxicokinetic parameters (Tseng et al., 1968; Guo et al., 1993; Yap et al., 2014). The of high Vd, t1/2 and low clearance (29 ± 246 mL/h) is an indication that the slow absorption and deep tissue distribution might be the cause of the slow elimination of cobra venom. Similar elimination t1/2 in both intravenous and intermuscular injection were reported in Asian cobras (Yap et al., 2014).
5.2. Pharmacometric simulation
Previous studies showed that snake venom toxins distribute extensively to the peripheral or extravascular tissues, which seems to be a general phenomenon for venom toxins (Barral-Netto and von Sohsten, 1991; Guo et al., 1993; Audebert et al., 1994). The simulation results also indicated that N. nigricollis venom does not follow a single compartmental model of kinetics, especially when we look at serum venom concentration profile, the rapid absorption within 0.5 h (1364 ± 167 ng/mL) which increased after 30 min (1448 ± 180 ng/mL) and subsequently started reducing until the 16th hour where we observed the sharp increase to the maximun concentarrion (1813 ± 175 ng/mL), this was followed by a decrease in concentration (614 ± 22 ng/mL) up to the 32nd hour. The increase in concentration at the 16th hour may explained by the redistribution of the low molecular weight toxins back into the blood circulation from the extravascular system. This redistribution of venom toxins may be related to the rebound phenomenon that sometimes occurs during ASV therapy (Gutierrez et al., 2003; Chippaux 2006). Experimental studies in rabbits have indicated that ASV therapy can cause a redistribution of venom from the extravascular to the vascular compartment. A good antisnake venom will be immediately sequestered by venom antibodies (Choumet et al., 1996; Rivière et al., 1997).
5.3. Limitation
We only used a single intramuscular (IM) administration of venom, which limited us in determining distribution half-life and actual bioavailability of the venom after administration.
5.4. Conclusion
We found that Nigerian N. nigricollis venom contains low molecular weight toxins that are well absorbed into rabbit blood and deep tissues and were detected 48 h after intramuscular envenoming.
Statement of authorship
We declared that this work was conducted by the authors named in this article, and all liabilities relating to the content of this article were borne by them.
Auwal A. Bala conceived the original idea, developed the theory and co-developed the methods. Sani Malami, Murtala Jibril, Binta Kurfi, Jacob A. Galan, Elda E. Sanchez and Basheer A.Z. Chedi, developed the study methods and co-supervised the work. Auwal A. Bala and Yusuf Abubakar, performed laboratory work and co-wrote the manuscript. George Oche Ambrose and Mustapha Mohammed designed and conducted the formal statistical analysis. Ismaila Raji and Sunusi Muhammad conducted literature review and co-wrote the introduction. Jacob A. Galan performed the proteomic analysis and reviewd the manuscript. Elda E. Sanchez edited and critically reviewed the manuscript for intellectual content, Basheer AZ. Chedi., gave the supervisory approval and finally revised the manuscript for intellectual content.
Funding
This research was funded by a grant from the NIH/ORIP, Viper Resource Grant #P40OD01960-18, Elda E. Sánchez, NIH/SCGM136606-02, Jacob Galan and the Robert A.Welch Foundation, grant # AC-0006 (TAMUK—Department of Chemistry).
Statement of authorship
We declared that this work was conducted by the authors named in this article. Auwal A. Bala and Basheer A.Z. Chedi conceived the original idea, developed the theory and co-developed the methods. Sani Malami, Murtala Jibril, Binta Kurfi, Jacob A. Galan, Elda E. Sanchez and Basheer A.Z. Chedi developed the study methods and co-supervised the work. Auwal A. Bala and Yusuf Abubakar performed laboratory work and co-wrote the manuscript. George Oche Ambrose and Mustapha Mohammed designed and conducted a formal statistical analysis. Ismaila Raji and Sunusi Muhammad conducted the literature review and co-wrote the introduction. Jacob A. Galan performed the proteomic analysis and reviewed the manuscript. Elda E. Sanchez edited and critically reviewed the manuscript for intellectual content, Basheer AZ. Chedi gave the supervisory approval and finally revised the manuscript for intellectual content.
Ethical statement
The study was approved by the Ethics Committee of the College of Health Sciences, Bayero University Kano (BUK/CHS/HREC/VII/66). All animals were treated according to the WHO's ethical code for animal experimentation (Howard-Jones 1985).
Declaration of competing interest
The authors declare no competing interest.
Acknowledgements
The authors would like to thank and appreciate Mr Aliyu Ahmed, Mr Abdusabur Imam, Mr Nasiru Shehu and Mr Abdullahi (Department of Pharmacology and Therapeutics, Bayero University Kano) for providing technical support. We also wish to thank and appreciate Mr Aliyu Abba, Mr Ibrahim and Mr Shamsudden (Center for Biotechnology Research, Bayero University Kano) for their technical support. Special appreciation to all Young Pharmacists Scholars (YPS) members for their support and guidance. We also wish to appreciate the Nigerian Snakebite Research and Intervention Centre (NSRIC) and National Natural Toxin Research Center, Texas A&M, Kingsville, USA, for providing training and support.
Handling Editor: Dr. Glenn King
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2022.100122.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- Adamude F.A., Dingwoke E.J., Abubakar M.S., Ibrahim S., Mohamed G., Klein A., Sallau A.B. Proteomic analysis of three medically important Nigerian Naja (Naja haje, Naja katiensis and Naja nigricollis) snake venoms. Toxicon. 2021;15(197):24–32. doi: 10.1016/j.toxicon.2021.03.014. Epub 2021 Mar 26. PMID: 33775665. [DOI] [PubMed] [Google Scholar]
- Chaim-Matyas Adina, Ovadia Michael. Cytotoxic activity of various snake venoms on melanoma, B16F10 and chondrosarcoma. Life Sci. 1987;40(16):1601–1607. doi: 10.1016/0024-3205(87)90126-3. 10.1016/0024-3205(87)90126-3. PMID 3561167. [DOI] [PubMed] [Google Scholar]
- Audebert F., Urtizberea M., Sabouraud A., Scherrmann J.M., Bon C. Pharmacokinetics of Vipera aspis venom after experimental envenomation in rabbits. J. Pharmacol. Exp. Therapeut. 1994;268(3):15121517. [PubMed] [Google Scholar]
- Bala A.A., Jatau A.I., Yunusa I., Mohammed M., Mohammed A.H., Isa A.M., Wada A.S., Gulma K.A., Bello I., Malami S., Michael G.C., Chedi B.A. Knowledge assessment of anti-snake venom among healthcare practitioners in northern Nigeria. Ther Adv Infect Dis. 2021 doi: 10.1177/20499361211039379. 2021 Aug 19;8:20499361211039379. 10.1177/20499361211039379. PMID: 34434552; PMCID: PMC8381460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral-Netto M., von Sohsten R.L. Serum kinetics of crotoxin from Crotalus durissus terrificus venom in mice: evidence for a rapid clearance. Toxicon. 1991;29(4–5):527–531. doi: 10.1016/0041-0101(91)90028-p. [DOI] [PubMed] [Google Scholar]
- Chippaux J.P.P. Snake-bites: appraisal of the global situation. Bull. World Health Organ. 1998;76(55):515–524. [PMC free article] [PubMed] [Google Scholar]
- Chippaux J.P. In: Huchzermeyer F.W., Orig French, editors. Krieger Publishing Company; Florida: 2006. Snake Venoms and Envenomations; p. 300. Translated by. 2002, First English Ed. 2006. [Google Scholar]
- Chippaux J., White J., Habib A.G. African snakes. 2016. [DOI]
- Choumet V., Audebert F., Rivière g., Sorkine M., Urtizberea M., Sabouraud A., Scherrmann J.-M., Bon C. In: Envenomings and Their Treatments. BON C., GOYFFON M., editors. Fondation Marcel Merieux; Lyon: 1996. Toxicokinetics of Vipera aspis envenoming and antivenom therapy; pp. 127–133. [Google Scholar]
- Conlon J.M., Attoub S., Musale V., Leprince J., Casewell N.R., Sanz L., Calvete J.J. Isolation and characterization of cytotoxic and insulin-releasing components from the venom of the black-necked spitting cobra Naja nigricollis (Elapidae) Toxicon X. 2020;18(6):100030. doi: 10.1016/j.toxcx.2020.100030. 10.1016/j.toxcx.2020.100030. PMID: 32550585; PMCID: PMC7285909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmokadem A., Riggs M.M., Baron K.T. Quantitative systems Pharmacology and physiologically-based pharmacokinetic modeling with mrgsolve: a hands-on tutorial. CPT Pharmacometrics Syst. Pharmacol. 2019;8(12):883–893. doi: 10.1002/psp4.12467. 10.1002/psp4.12467. Epub 2019 Nov 14. PMID: 31652028; PMCID: PMC6930861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsson J., Weiner D. Non-compartmental analysis. Methods Mol. Biol. 2012;929:377–389. doi: 10.1007/978-1-62703-050-2_16. PMID: 23007438. [DOI] [PubMed] [Google Scholar]
- Guo M.P., Wang Q.C., Liu G.F. Pharmacokinetics of cytotoxin from Chinese cobra (Naja naja atra) venom. Toxicon. 1993;31:339–343. doi: 10.1016/0041-0101(93)90151-8. [DOI] [PubMed] [Google Scholar]
- Gutierrez J.M., Leo'n G., Lomonte B. Pharmacokinetic-pharmacodynamic relationships of immunoglobulin therapy for envenomations. Clin. Pharmacokinet. 2003;42:721–741. doi: 10.2165/00003088-200342080-00002. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J.M., Maduwage K., Iliyasu G., Habib A. Snakebite envenoming in different national contexts: Costa Rica, Sri Lanka, and Nigeria. Toxicon X. May. 2021;25:9–10. doi: 10.1016/j.toxcx.2021.100066. 100066. 10.1016/j.toxcx.2021.100066. PMID: 34124644; PMCID: PMC8175406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib A.G. Tetanus complicating snake bite in northern Nigeria: clinical presentation and public health implications. Acta Trop. 2003;85(1):87–91. doi: 10.1016/S0001-706X(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Habib A.G., Brown N.I. The snakebite problem and antivenom crisis from a health-economic perspective. Toxicon. 2018;150(February):115–123. doi: 10.1016/j.toxicon.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Halilu S., Iliyasu G., Hamza M., Chippaux J.P., Kuznik A., Habib A.G. Snakebite burden in Sub-Saharan Africa: estimates from 41 countries. Toxicon. 2019;159:1–4. doi: 10.1016/j.toxicon.2018.12.002. pmid:30594637. [DOI] [PubMed] [Google Scholar]
- Hart A.J., Hodgson W.C., O'Leary M., Isbister G.K. Pharmacokinetics and pharmacodynamics of the myotoxic venom of pseudechis australis (mulga snake) in the anaesthetized rat. Clin. Toxicol. 2014;52:604–610. doi: 10.3109/15563650.2014.914526. [DOI] [PubMed] [Google Scholar]
- Howard-Jones N.A. A CIOMS ethical code for animal experimentation. WHO (World Health Organ.) Chron. 1985;39:51–56. [PubMed] [Google Scholar]
- Hudson L., Hay F.C. second ed. Blackwell Scientific Publications; Oxford: 1980. Practical Immunology; p. 222. [Google Scholar]
- Ismail M., Aly M.H.M., Abd-Elsalam M.A., Morad A.M. A three-compartment open pharmacokinetic model can explain variable toxicities of cobra venoms and their alpha toxins. Toxicon. 1996;34:1011–1026. doi: 10.1016/0041-0101(96)00055-4. [DOI] [PubMed] [Google Scholar]
- Ismail M., Abd-Elsalam M.A., Al-Ahaidib M.S. Pharmacokinetics of 125I-labelled Walterinnesia aegyptia venom and its specific antivenins: flash absorption and distribution of the venom and its toxin versus slow absorption and distribution of IgG, F(ab')2 and F(ab) of the antivenin. Toxicon. 1998;36(1):93–114. doi: 10.1016/s0041-0101(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Jaki T., Wolfsegger M.J. Estimation of pharmacokinetic parameters with the R package PK. Pharmaceut. Stat. 2011;10:284–288. doi: 10.1002/pst.449. [DOI] [Google Scholar]
- Jaki T., Wolfsegger M.J. Non-compartmental estimation of pharmacokinetic parameters for flexible sampling designs. Stat. Med. 2012;31:1059–1073. doi: 10.1002/sim.4386. [DOI] [PubMed] [Google Scholar]
- Kuzun W.M., Marcus J.R., Kerluke L.D., Phillips J.H. African spitting cobra (Naja nigricollis) bite of the hand. Can. J. Plast. Surg. 1994;2(2):90–92. doi: 10.1177/229255039400200201. [DOI] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linda F., David E. Complete covalent structure of a cardiotoxin from the venom of Naja nigricollis (African black-necked spitting cobra) Biochemistry. 1975;14(13):2865–2871. doi: 10.1021/bi00684a012. 10.1021/bi00684a012. PMID 1148181. [DOI] [PubMed] [Google Scholar]
- Maduwage K., Silva A., O'Leary M.A., Hodgson W.C., Isbister G.K. Efficacy of Indian polyvalent snake antivenoms against Sri Lankan snake venoms: lethality studies or clinically focussed in vitro studies. Sci. Rep. 2016;6:26778. doi: 10.1038/srep26778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariam S.H.S., Ooi C.W., Tan W.S., Janna O.A., Arbakariya A., Tey B.T. Purification of rabbit polyclonal immunoglobulin G with ammonium sulphate precipitation and mixed-mode chromatography. Separ. Purif. Technol. 2015;(144):133–138. doi: 10.1016/j.seppur.2015.02.012. [DOI] [Google Scholar]
- Markfarlane R.G. Russell's viper venoms, 1953 – 1964. Br. J. Haematol. 1967;13:437–451. doi: 10.1111/j.1365-2141.1967.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Michael G.C., Grema B.A., Aliyu I., Alhaji M.A., Lawal T.O., Ibrahim H., Fikin A.G., Gyaran F.S., Kane K.N., Thacher T.D. Knowledge of venomous snakes, snakebite first aid, treatment, and prevention among clinicians in northern Nigeria: a cross-sectional multicentre study. Trans. R. Soc. Trop. Med. Hyg. 2018;112(2):47–56. doi: 10.1093/trstmh/try028. [DOI] [PubMed] [Google Scholar]
- Petras D., Sanz L., Segura A., Herrera M., Villalta M., Solano D., Vargas M., León G., Warrell D.A., Theakston R.D., Harrison R.A., Durfa N., Nasidi A., Gutiérrez J.M., Calvete J.J. Snake venomics of African spitting cobras: toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J. Proteome Res. 2011;10(3):1266–1280. doi: 10.1021/pr101040f. 4. 10.1021/pr101040f. Epub 2011 Jan 31. PMID: 21171584. [DOI] [PubMed] [Google Scholar]
- Qusai U., Hameed A., Rasheed K. Compartmental and non-compartmental pharmacokinetic analysis of extended release diclofenac sodium tablet. Al-Nahrain Univ. Coll. Eng. J. 2016;91(1):161–165. [Google Scholar]
- Rivière G., Choumet V., Audebert f., Sabouraud A., Debray M., Scherrmann J.M., Bon C. Effect of antivenom on venom pharmacokinetics in experimentally envenomed rabbits: towards optimization of antivenom therapy. J. Pharmacol. Exp. Ther. 1997;pp281:1–8. [PubMed] [Google Scholar]
- Sanhajariya S., Duffull S.B., Isbister G.K. Pharmacokinetics of snake venom. Toxins. 2018;10(2) doi: 10.3390/toxins10020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhajariya S., Isbister G.K., Duffull S.B. The influence of the different Disposition Characteristics of snake toxins on the pharmacokinetics of snake venom. Toxins. 2020;12(3):188. doi: 10.3390/toxins12030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvanayagam Z.E., Gopalakrishnakone P. Tests for detection of snake venoms, toxins and venom antibodies: review on recent trends (1987–1997) Toxicon. 1999;37:565–586. doi: 10.1016/S0041-0101(98)00203-7. [DOI] [PubMed] [Google Scholar]
- Tan N.H., Lim K.K., Jaafar M.I. An investigation into the anti- genic cross-reactivity of Ophiophagus hannah (king cobra) venom neurotoxin, phospholipase A2, hemorrhagin and L-amino acid oxidase using enzyme linked immunosorbent assay. Toxicon. 1993;31:865–872. doi: 10.1016/0041-0101(93)90221-4. [DOI] [PubMed] [Google Scholar]
- Tasoulis T., Isbister G.K. A review and Database of snake venom Proteomes. Toxins. 2017;9(9):290. doi: 10.3390/toxins9090290. 18. 10.3390/toxins9090290. PMID: 28927001; PMCID: PMC5618223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theakston R.D.G. The application of immunoassay techniques, including enzyme-linked immunosorbent assay (ELISA), to snake venom research. Toxicon. 1983;21:3 pp341–352. doi: 10.1016/0041-0101(83)90090-9. [DOI] [PubMed] [Google Scholar]
- Theakston R.D., Laing G.D. Diagnosis of snakebite and the importance of immunological tests in venom research. Toxins. 2014;6:1667–1695. doi: 10.3390/toxins6051667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theakston R.D., Lloyd-Jones M.J., Reid H.A. Micro-ELISA for detecting and assaying snake venom and venom-antibody. Lancet. 1977;2:639–641. doi: 10.1016/s0140-6736(77)92502-8. [DOI] [PubMed] [Google Scholar]
- Tseng L.F., Chiu T.H., Lee C.Y. Absorption and distribution of 131-I- labelled cobra venom and its purified toxins. Toxicol. Appl. Pharmacol. 1968;12:526–535. [Google Scholar]
- Warrell D.A. WHO guidelines for the clinical management of snake bites in the Southeast Asian region. Southeast Asian J. Trop. Med. Public Health. 1999;30:1–84. [PubMed] [Google Scholar]
- WHO . vol. 964. WHO Technical Report Series; 2017. pp. 197–388. (Annex 5. Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins Replacement of Annex 2 of WHO Technical Report Series, No. 964). [Google Scholar]
- Willard N.K., Salazar E., Oyervides F.A., Wiebe C.S., Ocheltree J.S., Cortez M., Perez R.P., Markowitz H., Iliuk A., Sanchez E.E., Suntravat M., Galan J.A. Proteomic identification and Quantification of snake venom Biomarkers in venom and plasma Extracellular Vesicles. Toxins. 2021;13(9):654. doi: 10.3390/toxins13090654. 15. 10.3390/toxins13090654. PMID: 34564658; PMCID: PMC8473211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap M.K.K., Tan N.H., Sim S.M., Fung S.Y. Toxicokinetics of Naja sputatrix (Javan spitting cobra) venom following intramuscular and intravenous administrations of the venom into rabbits. Toxicon. 2013;68:18–23. doi: 10.1016/j.toxicon.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Yap M.K., Tan N.H., Sim S.M., Fung S.Y., Tan C.H. Pharmacokinetics of Naja sumatrana (equatorial spitting cobra) venom and its major toxins in experimentally envenomed rabbits. PLoS Negl Trop Dis. 2014;5(8) doi: 10.1371/journal.pntd.0002890. 6. Erratum in: PLoS Negl Trop Dis. 2014 Sep;8(9):e3277. PMID: 24901441; PMCID: PMC4046969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf P.O., Mamman M., Ajagun E., Suleiman M.M., Kawu M.U., Shittu M., Isa H.I., Tauheed M. Yusuf A. Snakes Responsible for bites in Norths-Eastern Nigeria – a Hospital-based Survey. IOSR Journal of Environmental Science Ver. II. 2015;9(9):2319–2399. doi: 10.9790/2402-0992118121. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.