Abstract
Background
Internet gaming disorder (IGD) is a type of behavioral addiction characterized by poorly controlled and interfering patterns of game playing. Studies have suggested that the IGD is usually accompanied by increased desire or craving for gaming, suggesting that secondary rewards related to gaming may become more salient than those for primary rewards like food. However, this hypothesis has not been formally tested and potential neural mechanisms remain unclear.
Methods
This is a functional magnetic resonance imaging (fMRI) study. Twenty-one IGD subjects and 23 matched individuals with recreational game use (RGU) were scanned when exposed to gaming (secondary rewards), food (primary rewards) and neutral cues. Group-by-cue-type interaction analyses and subsequent within-group analyses for fMRI data were performed and seed-based functional connectivity (FC) analyses explored further potential neural features.
Results
IGD subjects’ subjective craving responses to gaming cues were higher than to food cues, while the opposite was observed in RGU subjects. Group-by-cue interaction effects implicated the precuneus and precuneus-caudate FC. Simple effect analysis showed that for IGD subjects, gaming-related cues elicited higher FC in precuneus-caudate relationships than did food-related cues. In the RGU subjects, the opposite was observed. Significant correlations were found between brain features and craving scores.
Conclusions
These results support the hypothesis regarding imbalances in sensitivities to different types of reward in IGD, and suggest neural mechanisms by which craving for gaming may make secondary rewards more salient than primary ones, thus promoting participation in addictive patterns of gaming.
Keywords: internet gaming disorder, addictive behaviors, craving, cue, internet addiction, video games, fMRI
Introduction
Internet gaming disorder (IGD) is a psychiatric disorder characterized by difficulties in controlling excessive and interfering patterns of gaming (Association, 2013). IGD is associated with negative consequences, such as troubled interpersonal relationships, depressed mental states, and poor academic achievement (Liu et al., 2021; Zhang, Zhou, Geng, Song, & Hu, 2020; Zhou et al., 2021b). Whereas IGD has been included as a potential disorder in the Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (Association, 2013), gaming disorder has been included as a “disorder due to addictive behaviors” in the 11th revision of the International Classification of Diseases (ICD-11) (WHO, 2017).
Rewards can motivate people’s desire to engage in behaviors and contribute importantly to addictions. Rewards may be classified into two types. Primary rewards may be considered those necessary for survival of species (Fiallos et al., 2017). For example, food is a primary reward and craving for food may motivate eating behaviors (Blanco-Gandía, Miñarro, & Rodríguez-Arias, 2020). Secondary rewards are not directly related to survival and only gain value through learned associations with lower-level rewards (Schultz, 2000). Secondary rewards include things of values learned during socialization, such as money in general and particularly relevant for people with IGD, gaming (Rizvi, Pizzagalli, Sproule, & Kennedy, 2016).
After acquiring salience, both primary and secondary rewards may have powerful reinforcing effects. Under normal circumstances, motivations or cravings for primary rewards are usually stronger than those for secondary rewards (Sescousse, Caldú, Segura, & Dreher, 2013). However, under certain circumstances, secondary rewards may acquire greater salience and potentially lead to addictions (Volkow, Wang, Fowler, Tomasi, & Baler, 2012). For example, in gambling disorder, chronic gambling may result in diminished responses of reward circuits to sexual cues, suggesting that neural mechanism initially sensitive to primary rewards may undergo changes related to secondary rewards (Sescousse et al., 2013), consistent with incentive salience models of addiction (Berridge & Robinson, 2016) and neural adaptations involving specific forms of problematic use of the internet (Brand, Young, Laier, Wölfling, & Potenza, 2016). A meta-analysis revealed that money (secondary reward) may recruit more anterior orbitofrontal cortex (OFC) regions than primary rewards such as food and sex (Kringelbach & Rolls, 2004; Sescousse, Redouté, & Dreher, 2010).
Previous studies have shown that the cue-craving tasks can effectively induce subjective and neural craving responses in IGD subjects (Zhou, Wang, et al., 2021). When exposed to gaming-related cues, IGD subjects report increased craving and demonstrate increased activation of the dorsolateral/ventromedial prefrontal cortex (DLPFC, VMPFC), striatum, and precuneus (Dong, Wang, Du, & Potenza, 2018; Liu et al., 2017). These brain areas have been related to craving for primary rewards (e.g., food) in people without addictions (Sescousse et al., 2013). Studying responses to gaming and food cues in individuals with and without IGD may provide insight into mechanisms related to imbalances in response to primary and secondary rewards that may underlie IGD. As craving has been proposed and observed to be an important treatment target in IGD (Dong & Potenza, 2014) and increasing responses to rewards that are not the focus of addiction has been observed following treatment more generally (Garrison et al., 2017), understanding potential imbalances in subjective and neural responses to primary and secondary rewards is clinically important in treatment development efforts.
Reward systems typically respond to both primary and secondary rewards (Hikosaka, Bromberg-Martin, Hong, & Matsumoto, 2008). For example, in a meta-analysis, the striatum was found to be activated by monetary, food and erotic stimuli (Sescousse et al., 2013). Ventral striatal activity has been correlated with magnitudes of rewards, supporting a role in hedonic value representation (Smith et al., 2010). When facing gaming cues, lower left-hemispheric modulatory effects in anterior cingulate cortex (ACC)-to-lentiform connectivity as implicated in IGD relative to individuals with recreational game use (RGU) (Dong, Wang, Zheng, et al., 2020). Furthermore, IGD subjects have exhibited higher cue-induced activations within both the ventral and dorsal striatum when compared with RGU subjects (Liu et al., 2017). Activity in the striatum has been linked to transitions between RGU and IGD and vice-versa (Dong et al., 2020a), with data suggesting that striatal responses to gaming cues and striatal-related patterns are linked to more patterns of gaming both cross-sectional and longitudinally (Dong, Liu, Zheng, Du, & Potenza, 2019).
In IGD, cravings represent an important element that may motivate gaming and development and maintenance of IGD (Zhou, Wang, et al., 2021). However, prior studies have not directly compared subjective and neural responses to cues related to primary and secondary rewards in IGD. In the current study, we used a cue-craving task to examine cued responses relating to primary rewards (food) or secondary rewards (games) between IGD and RGU subjects. First, given the above data, we hypothesized that IGD subjects would have stronger subjective craving responses and reward-related brain activations (e.g., in the striatum) when facing gaming cues than would RGU subjects. Second, the brain areas related to the reward system would respond to both food and gaming cues, while addiction-related response patterns, with IGD subjects showing relatively greater responses to gaming cues and RGU subjects to food cues. Finally, given that a meta-analysis found that the core regions of the default mode network (DMN) such as the caudate and ACC of IGD play an important role in the FC related to craving (Yan, Li, Yu, & Zhao, 2021), we explored such FC relationships and hypothesized that the core area of the DMN network (such as the caudate) plays an important role in the form of FC in the response of IGD to primary/secondary rewards.
Method
Participants
All participants were selected based on a modified Internet addiction test (IAT) (Young, 1996), and the nine-item diagnostic criteria in the DSM-5 (Petry, Rehbein, Ko, & O’Brien, 2015), and time and frequency of gaming (minimum 14 h per week during the last 2 years). IGD subjects scored higher than 50 on the modified IAT and concurrently met five or more DSM-5 criteria. RGU scored lower than 50 on modified IAT and met less than five DSM-5 criteria. RGU subjects also reported no cravings or urge to play online games. All subjects were familiar with gaming scenes from League of Legends (LOL).
All subjects met the following criteria: be right-handed; have normal or corrected-to-normal vision; and, have no Axis-I psychiatric disorders as per assessment from a structured psychiatric interview (MINI) (Sheehan et al., 1998). Prior to scanning, all participants were medication-free and not using any substances (e.g., alcohol, nicotine/tobacco, and coffee).
According to the above criteria, we recruited 21 IGD subjects (10 males, 11 females) and 23 RGU subjects (15 males, 8 females) through posters and online advertisements. Two participants were excluded from analysis because of head motion, and neither neural nor behavioral data were considered in analyses. Demographic information of the remaining subjects is shown in Table 1. Age, education and other measures were matched between the two groups.
Table 1.
Demographic information and group differences
| IGD (n = 21) | RGU (n = 23) | t/χ 2 | P | Effect Size | |
| Gender | Male = 10, Female = 11 | Male = 15, Female = 8 | 1.38 | 0.239 | 0.18 |
| Age (mean ± SD) | 21.29 ± 1.52 | 21.61 ± 1.95 | 0.61 | 0.546 | 0.18 |
| DSM-5 score | 6.24 ± 1.22 | 2.35 ± 1.33 | 10.05** | <0.001 | 3.10 |
| IAT score (mean ± SD) | 70.48 ± 10.64 | 35.91 ± 13.88 | 6.54** | <0.001 | 2.01 |
| Education (years) | 14.29 ± 1.52 | 14.61 ± 1.95 | 0.61 | 0.541 | 0.19 |
IGD: Internet gaming disorder; RGU: recreational game use; Craving: self-reported craving.
IAT: Internet addiction test; DSM-5: Diagnostic and Statistical manual of Mental Disorders-5.
SD, standard deviation.
*P < 0.05, **P < 0.001.
Tasks
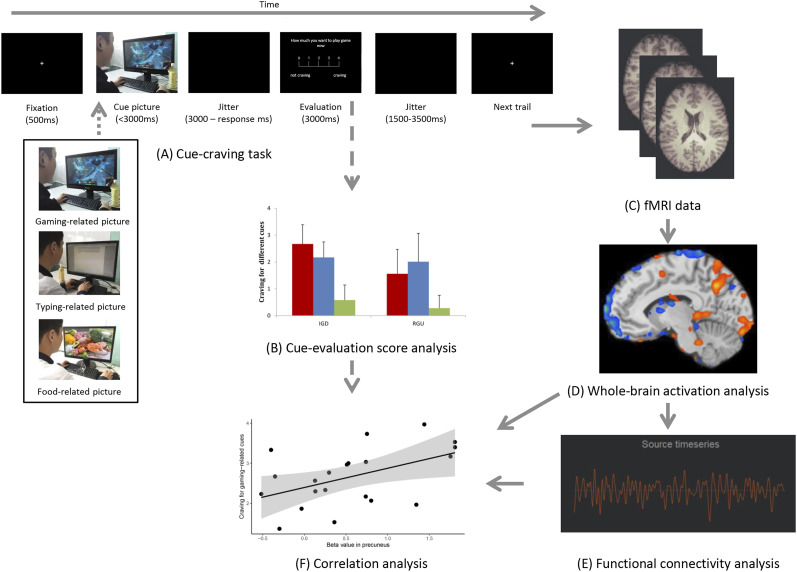
Subjects performed a cue-craving task during fMRI. Figure 1A depicts task procedures and the timeline for one trial of the task.
Fig. 1.
The analytical pipeline of this study
The structure of this study is shown in figure. (A) An example of one trial of the event-related cue-craving task and different stimulus types in the task. (B) Cue-induced craving evaluation. (C) fMRI images obtained through the task. (D) Whole-brain activation analysis. (E) Functional connectivity (FC) analysis. (F) The correlation between the whole-brain activation/FC results and the craving for gaming-/food-related cues.
First, subjects were asked to fixate their sight on a cross in the center of the screen for 500 ms. Then, cues were presented for up to 3,000 ms and will be terminated by a button-pressing. During this period, subjects need to respond according to whether there is a face in each picture by pressing button ‘1’ (refers to ‘yes’) or ‘2’ (refer to ‘no’). The cues turned black after the button press and lasted for (3,000 - response time) ms and if the response time is over 3,000, which will be took as ‘missed’ trials in GLM model. The average response time for IGD participants was 1.972 ± 0.082 s and for RGU was 1.945 ± 0.064 s, there is no difference between these two groups (t = 1.21, P = 0.23). During the subsequent craving evaluation stage, subjects were asked to evaluate the level of craving for each stimulus on a 5-point likert scale (ranging from ‘1’ (no craving) to ‘5’ (extremely high craving)). This stage lasted 3,000 ms and was terminated by button press. Finally, a black screen appeared for 1,500–3,500 ms before the next trial was presented. The task included 90 trials and lasted approximately 15 min.
The 90 pictures in the task were divided into three categories: 30 gaming-related, 30 typing-related (neutral baseline) and 30 food-related. In each category, half contained a face, and the other half contained a hand. In gaming-related pictures, a person is displayed playing the online game League of Legends (Riot Games, Inc) on a computer (Fig. 1A). Typing-related or food-related pictures were similar to the gaming-related pictures except that people were typing an article or looking at pictures of food (see Appendix).
Image acquisition
Structural images were collected using a T1-weighted three-dimensional spoiled gradient-recalled sequence covering the whole brain (196 slices, repetition time = 1,700 ms, echo time TE = 3.93 ms, slice thickness = 1.0 mm, skip = 0 mm, flip angle = 15, inversion time = 1,100 ms, field of view = 240*240 mm, in-plane resolution = 256*256). Functional MRI was performed on a 3T scanner (Siemens Trio) with a gradient-echo EPI T2* weighted-sensitive pulse sequence in 33 slices (interleaved sequence, 3 mm thickness, TR = 2,000 ms, TE = 30 ms, flip angle 90°, field of view 220 × 220 mm2, matrix 64 × 64) (Dong, Wang, Zheng, et al., 2020; Wang et al., 2017). Stimuli were presented using an Invivo synchronous system (www.invivocorp.com) through a screen in the head coil, enabling participants to view stimuli (Fig. 1C).
Preprocessing
Functional volumes were slice time-corrected and realigned using the Statistical Parametric Mapping (SPM) 12 package (http://www.fil.ion.ucl.ac.uk/spm). Images were slice timed, reoriented, and realigned to the first volume, with T1-coregistered volumes being used to correct for head movements. Images were then normalized to Montreal Neurological Institute (MNI) space and spatially smoothed using a 4-mm3 full width at half maximum Gaussian kernel. Two subjects were removed from analysis because of head motion (the exclusion criteria were 3 mm in directional movement or 2° in rotational movement).
First-level analyses
Individual participant general linear model (GLM) analysis was performed by NeuroElf v1.1 (http://neuroelf.net). A GLM was applied to identify blood-oxygen-level–dependent (BOLD) activation in relation to brain activities. Different types of trials (gaming-related, typing-related, or food-related) were separately convolved with a canonical hemodynamic response function to form task regression. The duration of each trial was 3,000 ms, and the GLMs included a constant term per run. Head motion parameters and a high-pass filter (0.01–0.1 Hz) for 128 s were included as regressions of no interest. A GLM approach was used to identify voxels that were significantly activated for each event during the response stage.
Second-level analyses
Second-level analyses were conducted at the group level. First, we identified voxels that showed a main effect in the gaming-related trials versus the food-related trials among each group (IGD, RGU). Second, we determined voxels that were significantly different in BOLD signal between the two groups [(IGDgaming – IGDneutral) – (RGUgaming – RGUneutral)] [(IGDfood – IGDneutral) – (RGUfood – RGUneutral)]. Meanwhile, a voxel-wise 2 × 2 (group: IGD, RGU; cues: gaming-neutral, food-neutral) ANOVA was administered to examine interaction effects. We then identified clusters of contiguous significantly different voxels at an uncorrected threshold P < 0.001. Finally, these clusters were tested for cluster-level FWE (family-wise-error) correction at P < 0.05. Specially, the AlphaSim estimation indicated that clusters extent of 26 adjoining voxels would achieve the FWE threshold P < 0.05 effectively. The smoothing kernel applied in simulating false-positive (noise) maps using AlphaSim software was 8.4 mm and was estimated from residual fields of the contrast maps being pooled into the one-sample t-test (Fig. 1D).
Functional connectivity (FC) analyses
Since the precuneus was identified in the interaction analysis, we explored the precuneus further. Specifically, we extracted the obtained precuneus cluster as the region-of-interest (ROI) for further seed-based FC analysis. Task-related FC was assessed using the CONN toolbox (https://www.nitrc.org/projects/conn) in MATLAB (Whitfield-Gabrieli, Alfonso, & connectivity, 2012). BOLD signal was extracted from ROI during the cue-craving task (onset equal to the appearance time of the gaming-related/food-related/neutral cues). A correlation map was obtained by computing the correlation coefficients between the reference time series and the time series of the whole-brain voxels. Correlation coefficients were then converted to Z-values using Fisher Z-transformation to improve the normality of the distribution. Finally, a voxel-wise 2 × 2 (group: IGD, RGU; cues: gaming-neutral, food-neutral) ANOVA was administered to examine statistically significant interaction effects of group and cues in FC (Fig. 1E).
In this study, the family-wise error (FWE) correction with a Gaussian random field was used to control multiple comparisons, and the threshold was set as voxel P-value = 0.005, cluster P-value = 0.05. The FC results were identified for threshold regions with statistical significance masked on MNI brain templates.
Statistical analyses
At first, for group comparisons of demographic information and cue-induced cravings, two-sample t-tests, mixed-designed ANOVA and post-hoc tests were performed using SPSS version 24.0 (IBM Corporation, Armonk, NY, USA), and a chi-square test was used to compare data between men and women given previous reports of gender-related differences in cue responsivity in IGD (Fig. 1B) (Dong et al., 2018).
Next, to identify the correlation between brain activities and behavioral performances, we first extracted BOLD signal from the mean value of the remaining clusters (beta value) that showed an interaction effect in the gaming-related trials and the food-related trials among each group. Then, the BOLD data for all subjects were submitted to correlation analyses with the craving scores. Next, correlation analysis was performed on FC strength and craving scores (Fig. 1F).
Ethics
The experiment conforms to The Code of Ethics of the World Medical Association (Declaration of Helsinki). The Human Investigations Committee of Hangzhou Normal University approved this research. Participants were college students recruited through advertisements.
Results
Cue-induced cravings
A mixed-design ANOVA of cue-craving score with factors Group (IGD vs. RGU) and Cue (Game vs. Food vs. Neutral) showed a significant main effect of Group (F (1, 42) = 10.256, P = 0.003), and a significant effect of Cue (F (2, 84) = 130.793, P < 0.001). The interaction between Group and Cue was significant (F (2, 84) = 6.251, P = 0.003).
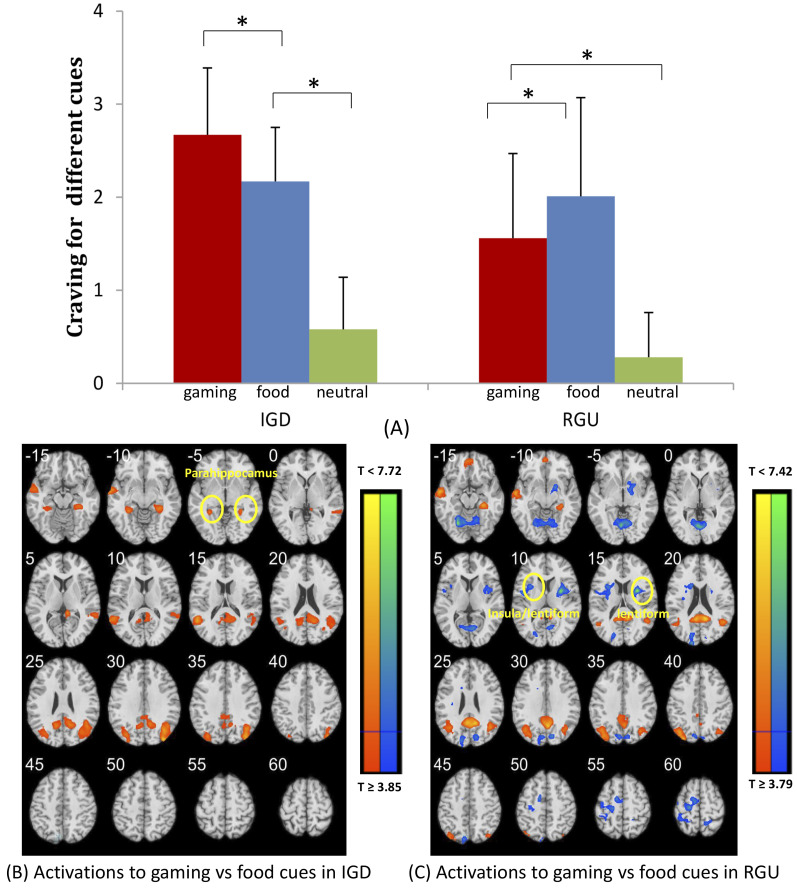
Post hoc analysis showed that IGD subjects reported higher gaming craving during viewing of gaming pictures (t = 10.47, P < 0.001; gaming cues: mean ± SD = 2.7 ± 0.72, neutral cues: 0.58 ± 0.56) as well as food craving during viewing of food pictures (t = 9.04, P < 0.001; food cues: mean ± SD = 2.1 ± 0.5, neutral cues: 0.58 ± 0.56) compared with viewing of neutral pictures each during the cue-craving task (Fig. 2A). IGD subjects showed significant lower food craving scores during viewing of food pictures as compared with gaming craving during viewing of gaming pictures (t = 2.45, P = 0.019; mean ± SD = 2.7 ± 0.72 for gaming craving and 2.1 ± 0.5 for food craving).
Fig. 2.
Cue-induced craving evaluation results and main effect in gaming and food conditions
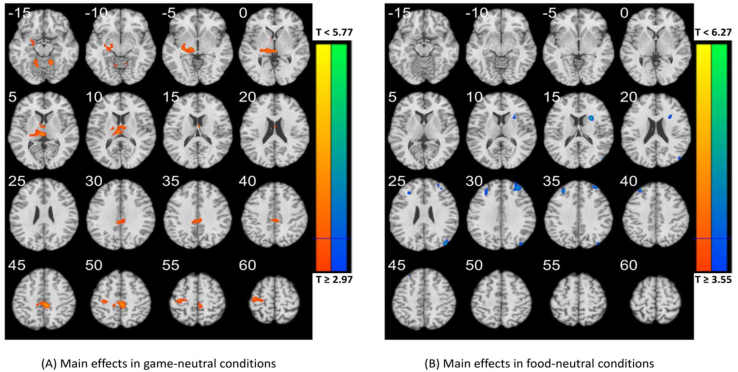
The subplot (A) shows the results of cue-induced craving evaluation. IGD and RGU subjects both reported higher game craving as well as food craving as compared with viewing of neutral pictures. Furthermore, IGD subjects showed lower food craving scores as compared with gaming craving scores. In addition, compared with RGU subjects, IGD subjects reported higher gaming craving during viewing of gaming pictures. The subplot (B) shows the main effect in gaming-food conditions in IGD. For the IGD group, compared with food cues, gaming cues elicited higher activations in the bilateral parahippocampus, left middle temporal gyrus (MTG) and right precuneus (voxel P < 0.001, uncorrected, in combination with cluster-level P < 0.05, FWE corrected). The subplot (C) shows main effect in gaming-food conditions in RGU subjects. For the RGU group, bilateral insula, bilateral cuneus, bilateral lentiform nucleus and left PCG showed lower activation during exposure to gaming as compared with food cues.
* indicates a significant difference.
RGU subjects reported higher food craving during exposure to food vs neutral cues (t = 7.11, P < 0.001; food cues: mean ± SD = 2.0 ± 1.1, neutral cues: 0.3 ± 0.5). Compared with RGU subjects, IGD subjects reported greater difference in gaming craving score during viewing of gaming pictures (t = 2.05, P < 0.001; mean ± SD = 2.7 ± 0.72 for IGD and 1.6 ± 0.9 for RGU).
Cue-induced brain activations
Main effect of group
Gaming-neutral conditions
Compared with RGU subjects, IGD subjects showed higher activations to gaming vs neutral cues in left thalamus, right post cingulate cortex (PCC) and left precentral gyrus (PCG) (at voxel P < 0.001, uncorrected, in combination with cluster-level P < 0.05, FWE corrected) (see Appendix, Figure S2A, Table S1).
Food-neutral conditions
Compared with RGU subjects, IGD subjects showed lower activations to food vs neutral cues in left middle frontal gyrus (MFG), right superior frontal gyrus (SFG) and right superior occipital gyrus (SOG) (at voxel P < 0.001, uncorrected, in combination with cluster-level P < 0.05, FWE corrected) (see Appendix, Figure S2B, Table S2).
Gaming-food conditions
Compared with RGU subjects, IGD subjects showed higher activations to gaming vs food cues in the left precuneus, lentiform, and culmen (at voxel P < 0.001, uncorrected, in combination with cluster-level P < 0.05, FWE corrected). Among IGD subjects, as comparing to food cues, gaming cues elicited higher activations in the bilateral parahippocampus/lentiform, bilateral superior occipital gyrus (SOG), left middle temporal gyrus (MTG) and right precuneus (voxel P < 0.001, uncorrected, in combination with cluster-level P < 0.05, FWE corrected) (Fig. 2B, Table 2). Among RGU subjects, the left PCG and precuneus showed higher activation during exposure to gaming cues as compared with food cues. Conversely, the bilateral insula, bilateral cuneus, right lentiform nucleus and left PCG showed relatively lower activation during exposure to gaming as compared with food cues (Fig. 2C, Table 2).
Table 2.
Regions showing differences in gaming-as compared with food-cue-induced activations in IGD and RGU subjects
| Condition | Region | Number of voxels | x, y, z a | Max F | Bab | Hemispherec |
| IGD: Gaming > Food | ||||||
| Parahippocampus | 75 | 30,−43,−1 | 5.32 | 19 | R | |
| 53 | −31,−45,−2 | 5.24 | 19 | L | ||
| Superior occipital gyrus | 126 | −33,−77,29 | 5.41 | 19 | L | |
| 424 | 36,−85,25 | 6.70 | 19 | R | ||
| Middle temporal gyrus | 99 | −55,−52,14 | 5.87 | 39 | L | |
| Superior temporal gyrus | 98 | −65,−9,−2 | 5.45 | 21 | L | |
| Precuneus | 420 | 16,−61,22 | 5.30 | 31 | R | |
| RGU: Gaming > Food | ||||||
| Posterior Cingulate | 430 | 12,−54,20 | 6.60 | 31 | R | |
| Middle temporal gyrus | 206 | −59,−8,−6 | 6.06 | 21 | L | |
| 185 | 45,−64,20 | 5.24 | 39 | R | ||
| Precuneus | 336 | −33,−82,34 | 5.68 | 19 | L | |
| Medial frontal gyrus | 64 | −3,48,−13 | 5.02 | 11 | L | |
| RGU: Gaming < Food | ||||||
| Insula | 144 | 32,−4,15 | −7.97 | 13 | R | |
| 160 | −33,7,15 | −5.16 | 13 | L | ||
| Lentiform Nucleus | 65 | 22,0,−2 | −5.44 | R | ||
| Cuneus | 168 | −16,−93,19 | −5.56 | 18 | L | |
| 55 | 12,−79,26 | −5.27 | 18 | R | ||
| Precentral Gyrus | 282 | −23,−21,53 | −5.35 | 4 | L | |
| Postcentral Gyrus | 67 | 28,−43,65 | −5.11 | 5 | R | |
| Inferior parietal lobule | 81 | −38,−42,54 | −4.38 | 40 | L | |
| Lingual gyrus | 679 | −3,−70,2 | −6.86 | 18 | L | |
The surviving clusters at P < 0.05, voxel P < 0.001, and voxel cluster threshold >26.
IGD: Internet gaming disorder; RGU: recreational game use.
a Peak Montreal Neurological Institute (MNI) coordinates.
b Brodmann's area.
c The activation area was on the right side (R) or the left side (L).
Group-by-cue interaction effects
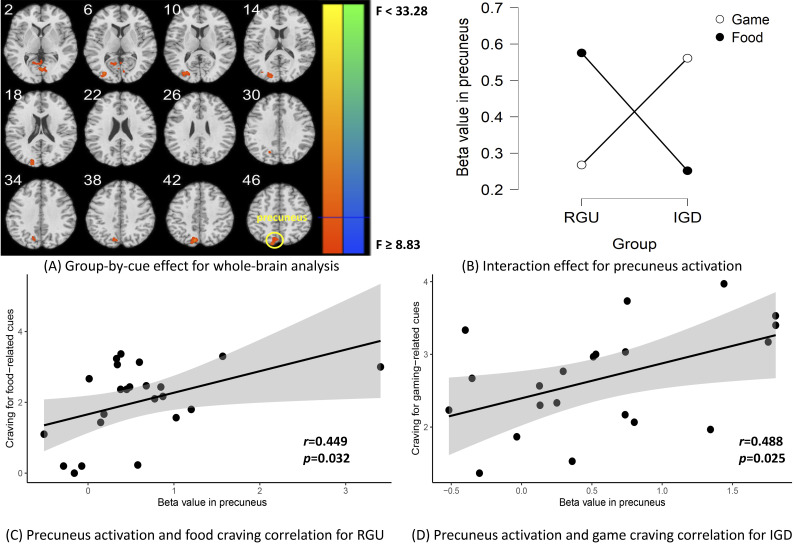
Group-by-cue interaction effects implicated the left precuneus, culmen and middle occipital gyrus (voxel P < 0.001, uncorrected, in combination with cluster-level P < 0.05, FWE corrected) (Fig. 3A, Table 3). Since the precuneus and IGD cravings were linked in previous studies (Dong, Wang, Wang, et al., 2020), we extracted beta values of the precuneus cluster for further analysis. Simple effect analysis showed that in the IGD group, gaming-related cues elicited higher brain activities in the precuneus than food-related cues. In the RGU group, the results were opposite (Fig. 3B).
Fig. 3.
Group-by-cue effect for whole-brain analysis and correlation analysis
The subplot (A) shows the group-by-cue interaction. The whole-brain analysis implicated the left precuneus, culmen and middle occipital gyrus (voxel P < 0.001, uncorrected, in combination with cluster-level P < 0.05, FWE corrected). The subplot (B) shows the simple effect analysis results that in the IGD group, gaming-related cues elicited higher brain activities in the precuneus than did food-related cues. In the RGU group, food-related cues elicited higher brain activities in the precuneus than did gaming-related cues. The subplot (C) shows for RGU subjects, the correlation analysis showed that precuneus activation to food vs neutral cues showed a significant positive correlation with food-craving scores. The subplot (D) shows for IGD, the correlation analysis found that precuneus activation to gaming vs neutral cues showed a significant positive correlation with gaming-craving scores.
Table 3.
Group-by-cue-type interactions
| Region | Number of voxels | x, y, z a | Max F | Bab | Hemispherec |
| Precuneus | 68 | −10,−75,37 | 19.38 | 7 | L |
| Middle Occipital Gyrus | 67 | −31,−80,9 | 16.50 | 19 | L |
| Lingual Gyrus | 183 | 3,−73,4 | 22.42 | 18 | R |
The surviving clusters at P < 0.05, voxel P < 0.001, and voxel cluster threshold >26.
a Peak Montreal Neurological Institute (MNI) coordinates.
b Brodmann's area.
c The activation area was on the right side (R) or the left side (L).
In the subsequent correlation analysis, we found that for IGD subjects, precuneus activation to gaming cues were positively correlated with gaming-craving scores (r = 0.49, P uncorrected = 0.025) (Fig. 3D). For RGU subjects, precuneus activation to food cues were positive correlated with food-craving scores (r = 0.45, P uncorrected = 0.032) (Fig. 3C).
Functional connectivity results
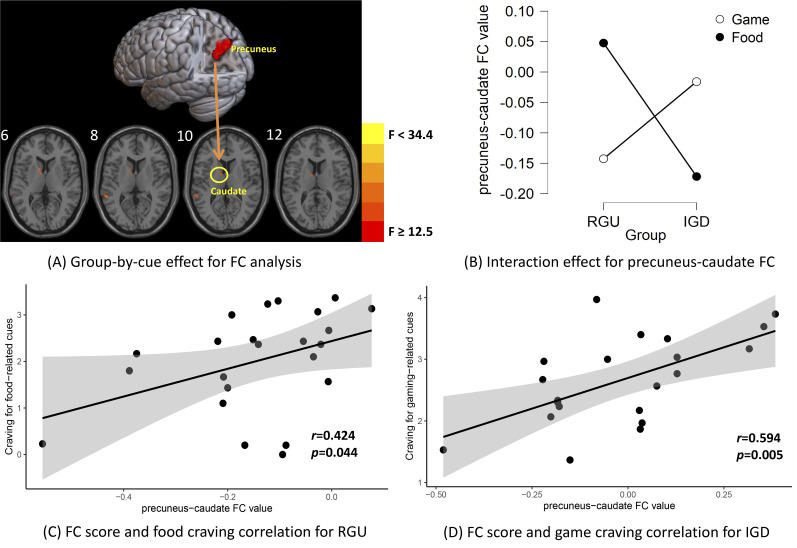
We next took the precuneus as ROI and performed FC analyses, identifying a significant interaction effect involving precuneus-caudate FC (x = −12, y = 8, z = 6, F =17.08) (Gaussian Random Field corrected, voxel P < 0.005, cluster P < 0.05), implicating the reward system (Fig. 4A, Table 4). The simple effect analysis findings are similar to those involving precuneus activation; in the IGD group, gaming-related cues showed higher precuneus-caudate FC strength than food-related cues, whereas in the RGU group, the results showed an opposite relationship (Fig. 4B).
Fig. 4.
Group-by-cue effect for functional connectivity (FC) analysis and correlation analysis
The subplot (A) shows the group-by-cue interaction effect. The FC analysis implicated the left caudate and superior temporal gyrus (Gaussian Random Field corrected, voxel P < 0.005, cluster P <0.05). The subplot (B) shows the simple effect analysis results. In the IGD group, gaming-related cues elicited higher precuneus-caudate FC strength than did food-related cues. In the RGU group, food-related cues elicited higher precuneus-caudate FC strength than did gaming-related cues. The subplot (C) shows for RGU subjects, the correlation analysis showed that precuneus-caudate FC strength to food vs neutral cues showed a significant positive correlation with food-craving scores. The subplot (D) shows for IGD subjects, the correlation analysis found that precuneus-caudate FC strength to gaming vs neutral cues showed a significant positive correlation with gaming-craving scores.
Table 4.
Precuneus functional connectivity interaction effects (Group*cue)
| Connected regions | Number of voxels | x, y, z a | Max F | Bab | Hemispherec |
| Precuneus to: | |||||
| Caudate | 61 | −12,8,6 | 17.08 | L | |
| Superior Temporal Gyrus | 24 | −58,42,8 | 18.62 | 22 | L |
GRF corrected: Gaussian Random Field corrected, voxel P < 0.005, cluster P < 0.05.
a Peak voxel coordinates in Montreal Neurological Institute space.
b Brodmann area.
c The activation area was on the right side (R) or the left side (L).
In the correlation analysis, we found similar results as with precuneus activation. Specifically, precuneus-caudate FC during exposure to gaming cues positively correlated with gaming-craving scores in IGD subjects (r = 0.59, P uncorrected = 0.005) (Fig. 4D). In RGU subjects, precuneus-caudate FC during exposure to food cues positively correlated with food-craving scores (r = 0.42, P uncorrected = 0.044) (Fig. 4C).
Discussion
This study examined subjective and neural responses to primary and secondary rewards cues among IGD and RGU subjects. The results largely supported our hypotheses and provide additional insight into neural mechanisms of potential imbalances regarding responses to primary and secondary rewards in addictive disorders like IGD.
IGD subjects report stronger subjective cravings to gaming cues than to food cues
Cue-elicited gaming and food craving responses differed in IGD and RGU groups. In the latter, craving responses to gaming cues were lower than those to food cues, suggestive of natural responses to primary and secondary rewards. However, in IGD, subjective craving responses to gaming cues were higher than those to food cues, suggesting that for IGD subjects gaming cues have taken priority, consistent with incentive salience models of addictions. The strong motivations elicited by gaming cues could drive IGD subjects to spend more time in pursuing gaming behaviors to the point of neglecting other important activities and despite negative consequences. In sum, the current results supported our hypothesis that among IGD subjects cravings for gaming may be stronger than those for primary rewards like food, consistent with real world accounts of people neglecting their children, bodily needs and other important domains of functioning relating to survival (Potenza, 2018).
Neural correlates of processing of primary and secondary rewards in IGD and RGU groups
For the IGD group, compared to food cues, gaming cues elicited higher activations in the bilateral parahippocampus, a region implicated in providing contextual representation functions (Rudy, 2009), including during cue-induced craving for gaming (Ko et al., 2013). The parahippocampus is part of the limbic system, which is involved in the storage of conditioned emotional memories (Pezze & Feldon, 2004). The parahippocampus helps evaluate the meaning of information based on inputs received from the amygdala, and helps process emotions and motivations based on the meaning of information (Salzmann, Vidyasagar, & Creutzfeldt, 1993). Gaming cues triggered stronger craving responses in IGD subjects than did food cues, consistent with the increased activation of the parahippocampus.
For the RGU group, brain responses to gaming cues were less robust than to food cues in the insula and lentiform nucleus. The lentiform nucleus includes the ventral striatum (VS), and a previous meta-analysis found that this region was activated by food and erotic stimuli (Sescousse et al., 2013). The lentiform nucleus receives projections from the OFC, amygdala and midbrain. It is thus well positioned to integrate cognitive, motor and affective information and influence goal-directed behavior (Haber & Knutson, 2010). Lentiform nucleus activity has been correlated with magnitudes of rewards, supporting its involvement in hedonic value representation (Izuma, Saito, & Sadato, 2008). Thus, food cues may elicit greater lentiform responses in a manner linked to hedonic value in RGU subjects than gaming cues.
In sum, the results suggest that, relative to cues related to the primary reward of food, those related to the secondary reward of gaming (the focus of the addiction) carry more salience subjectively in individuals with IGD, and this is reflected in neural correlates involving regions implicated previously in reward processing and craving. Among individuals with RGU, the patterns were different and suggested greater responses to cues related to the primary reward of food.
Precuneus activation for gaming and food cues in IGD and RGU groups
A group-by-cue interaction implicated the precuneus. Post-hoc analyses revealed that this interaction effect was related to opposing patterns of precuneus activation to the two cue types in IGD and RGU subjects. Specifically, for the IGD group, gaming-related cues elicited higher activation in the precuneus than did food-related cues. In the RGU group, the results were reversed. In the IGD subjects, precuneus activation to gaming cues positively correlated with gaming-craving scores, whereas in the RGU group, precuneus activation to food cues correlated positively with food-craving scores.
The precuneus is an important component of the DMN, and has been implicated in self-referential processing and episodic memory retrieval (Utevsky, Smith, & Huettel, 2014). The activation of the precuneus during cue-craving tasks may represent processes occurring further upstream from the experience of subjective craving (Cavanna & Trimble, 2006). Furthermore, the precuneus may contribute to habitual behaviors through cue-related responses (Courtney, Ghahremani, London, & Ray, 2014). In IGD, the precuneus may act as a hub for information originating from executive control and motivational drive networks (Dong, Wang, Wang, et al., 2020), with a similar role for precuneus suggested in executive control in addictions like cocaine use disorder (Yang et al., 2021). Given the role for the precuneus in mindfulness-based processes, the findings suggest a possible strategy for intervention in IGD, consistent with findings in IGD that suggest that a craving behavioral intervention strategy alters functional connectivity involving the precuneus during exposure to gaming cues (Zhang et al., 2016).
Precuneus-caudate FC relative to primary/secondary rewards in IGD and RGU
A group-by-cue interaction effect implicated precuneus-caudate FC. Post-hoc analyses suggested that this interaction effect was related to opposing patterns of responses to the two cue types in IGD and RGU subjects. Specifically, in the IGD group, gaming-related cues elicited higher precuneus-caudate FC than did food-related cues. In the RGU group, food-related cues elicited higher precuneus-caudate FC than did gaming-related cues. Interestingly, the precuneus-caudate FC strength related to craving scores in a similar manner as did precuneus activation.
The results suggest that the precuneus may operate as a hub for reward system functioning and craving. The caudate has been described as a component of reward circuitry (Dong, Wang, & Potenza, 2016; Grahn, Parkinson, & Owen, 2008) and individuals with cocaine use disorder show higher caudate activation and more dopamine release to cocaine cues relative to food cues in a manner that links to craving (Volkow et al., 2002, 2006). Anatomically, the caudate and precuneus have direct anatomical neural connections (Selemon & Goldman-Rakic, 1985). Precuneus-caudate FC has been related to changes in pleasure and craving (Brody et al., 2007; Dong, Wang, Wang, et al., 2020), which may promote maintenance of addiction behaviors or relapses (Wang et al., 2021). Precuneus-caudate FC has also been related to habitual behaviors (Wan et al., 2011) and reward processing with respect to integrating contextual information related to episodic memories and reward valuation (Nakamura & Ikuta, 2017).
Limitations
Several limitations warrant mention. First, the study included a small sample size and slightly unbalanced male to female ratio, but our results were significant with full consideration of multiple comparisons. Second, participants were instructed to fast before the scan, and all reported not eating or drinking anything but water before scans. However, we did not have an objective measure (for example, blood test) to confirm their reports. Third, the study was cross-sectional, limiting causal inferences. Future longitudinal studies should investigate the extent to which changes in responses to primary and secondary rewards (subjective and neural) change with respect to IGD status, including dining treatment. Fourth, one type of primary reward (food) was investigated. The extent to which the findings may extend to other primary rewards deserves additional study.
Conclusion
Our finding supports the notion of an imbalance in primary/secondary reward sensitivities in individuals with IGD and the neural mechanisms (precuneus and striatal activations, precuneus-caudate FC) underlying craving and cue-reactivity in IGD. These findings provide insight into mechanisms underlying craving in IGD. Future longitudinal studies should investigate changes over time and the extent to which the relationships might represent vulnerability factors, changes brought about by excessive and interfering patterns and/or subjective and neural responses that may be amenable to interventions.
Funding sources
This research was supported by The Cultivation Project of Province levelled Preponderant Characteristic Discipline of Hangzhou Normal University (20JYXK008), and Zhejiang Provincial Natural Science Foundation (LY20C090005). The funding agencies did not contribute to the experimental design or conclusions, and the views presented in the manuscript are those of the authors and may not reflect those of the funding agencies.
Authors’ contribution
WR-Z analyzed the study data and wrote the initial draft (including substantive translation). HH-D, XX-D and ZJ-Z conducted the research and investigations, specifically performing the experiments and data collection. MJ-W helped revise the manuscript. MNP, GH-D, participated in editing, interpretation and revision. All authors contributed to and have approved the final manuscript.
Conflict of interest
The authors report that they have no financial conflicts of interest with respect to the content of this manuscript.
APPENDIX Controlling for stimuli pictures

(The picture shows a male player, there are also female players)
All pictures were taken in the same place (white wall background and a white desk with a black screen). The genders of individuals in the pictures were also balanced within each category and type. Subjects cannot distinguish emotions as only single sides of faces were included, which is to control for potentially confounding factors (e.g., emotions and accessories). In each category, half contained a face (like right picture), and the other half only contained a hand (like left picture).
General group effects in food-neutral and gaming-neutral conditions
Our research found that in the case of food-neutral and game-neutral comparisons, IGD and RGU subjects showed different brain activation patterns. Compared with RGU subjects, IGD subjects showed higher activations to gaming vs neutral cues in the left thalamus, right post cingulate cortex (PCC) and left precentral gyrus (PCG). The findings replicated those from previous studies (Dong, Wang, Wang, Du, & Potenza, 2019b; Wang et al., 2017). These results suggested that tendencies to regulate game cravings may be compromised. The thalamus has been previously implicated in motivational drives including those related to rewarding behaviors including gaming (Dong et al., 2019).
In addition, compared with RGU subjects, IGD subjects showed lower activations to food vs neutral cues in the left middle frontal gyrus (MFG), right superior frontal gyrus (SFG) and right superior occipital gyrus (SOG). This result is consistent with the results of previous cocaine studies (Zhang, Zhornitsky, Le, & Li, 2019) and those exposed prenatally to cocaine (Dong, Wang, Du, & Potenza, 2018), that the secondary rewards were related to blunted responses to primary rewards. These areas with blunted responses to food cues have been implicated in executive control (Wesley & Bickel, 2014), with related networks underlying effective regulation of craving. Prior studies have shown that prefrontal brain regions (engaged during regulation of craving) and fronto-striatal pathways contribute to the regulation of appetitive desires (Kober et al., 2010; Volkow, Fowler, & Wang, 2003). In short, these two main effects provide support that our task can effectively induce craving, consistent with the subjective report data.
The subplot (A) shows for gaming-neutral conditions, compared with the recreational game use (RGU) group, the internet gaming disorder (IGD) group showed higher activations to gaming vs neutral cues in left thalamus, right PCC and left precentral gyrus (PCG). The subplot (B) shows for food-neutral conditions, compared with the RGU group, the IGD group showed lower activations to food vs neutral cues in left middle frontal gyrus (MFG), right superior frontal gyrus (SFG) and right superior occipital gyrus (SOG) Figure S1.
After viewing the pictures, the subjects were asked to assess their craving for pictures. In this stage, subjects were asked to evaluate the level of the craving (How much you want to play game now) for each stimulus on a 5-point Likert scale (ranging from ‘1’ (no craving) to ‘5’ (extremely high craving)). This stage lasted 3,000 ms and was terminated by button press. Finally, a black screen appeared for 1,500–3,500 ms before the next trial was presented Figure S2.
Fig. S1.
Main effects in different cue conditions
Fig. S2.
Depiction of the cue-craving evaluation stage of this study
Table S1.
Regions showing differences in gaming vs neutral cue-induced activations between IGD and RGU subjects
| Condition | Region | Number of voxels | x, y, za | Max F | Bab | Hemispherec |
| Gaming - Neutral: IGD > RGU | ||||||
| Precentral gyrus | 139 | −41,−16,57 | 4.35 | 4 | L | |
| Posterior cingulate gyrus | 162 | 7,−30,41 | 4.24 | 31 | R | |
| Thalamus | 246 | −3,−22,9 | 4.26 | L | ||
| Culmen | 270 | 11,−48,−18 | 4.85 | R | ||
| Gaming - Neutral: IGD < RGU | ||||||
| none | ||||||
The surviving clusters at P < 0.05, at voxel P < 0.005, and with voxel cluster number >51.
a Peak Montreal Neurological Institute (MNI) coordinates.
b Brodmann's area.
c The activation area was on the right side (R) or the left side (L).
Table S2.
Regions showing differences in food vs neutral cue-induced activations between IGD and RGU subjects
| Condition | Region | Number of voxels | x, y, za | Max F | Bab | Hemispherec |
| Food - Neutral: IGD > RGU | ||||||
| none | ||||||
| Food - Neutral: IGD < RGU | ||||||
| Superior Occipital Gyrus | 42 | 44,−85,23 | −5.10 | 19 | R | |
| Middle Frontal Gyrus | 33 | −35,37,34 | −4.76 | 9 | L | |
| Superior Frontal Gyrus | 50 | 39,42,48 | −4.63 | 9 | R | |
The surviving clusters at P < 0.05, at voxel P < 0.001, and with voxel cluster number >26.
a Peak Montreal Neurological Institute (MNI) coordinates.
b Brodmann's area.
c The activation area was on the right side (R) or the left side (L).
References
- Association, A. P. (2013). Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.). Arlington, VA, US: American Psychiatric Publishing, Inc. [Google Scholar]
- Berridge, K. C. , & Robinson, T. E. (2016). Liking, wanting, and the incentive-sensitization theory of addiction. The American Psychologist , 71(8), 670–679. 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Gandía, M. C. , Miñarro, J. , & Rodríguez-Arias, M. (2020). Common neural mechanisms of palatable food intake and drug abuse: Knowledge obtained with animal models. Current Pharmaceutical Design , 26(20), 2372–2384. 10.2174/1381612826666200213123608. [DOI] [PubMed] [Google Scholar]
- Brand, M. , Young, K. S. , Laier, C. , Wölfling, K. , & Potenza, M. N. (2016). Integrating psychological and neurobiological considerations regarding the development and maintenance of specific Internet-use disorders: An Interaction of Person-Affect-Cognition-Execution (I-PACE) model. Neuroscience and Biobehavioral Reviews , 71, 252–266. 10.1016/j.neubiorev.2016.08.033. [DOI] [PubMed] [Google Scholar]
- Brody, A. L. , Mandelkern, M. A. , Olmstead, R. E. , Jou, J. , Tiongson, E. , Allen, V. , … Cohen, M. S. (2007). Neural substrates of resisting craving during cigarette cue exposure. Biological Psychiatry , 62(6), 642–651. 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna, A. E. , & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain , 129(Pt 3), 564–583. 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Courtney, K. E. , Ghahremani, D. G. , London, E. D. , & Ray, L. A. (2014). The association between cue-reactivity in the precuneus and level of dependence on nicotine and alcohol. Drug and Alcohol Dependence , 141, 21–26. 10.1016/j.drugalcdep.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G. , Liu, X. , Zheng, H. , Du, X. , & Potenza, M. N. (2019a). Brain response features during forced break could predict subsequent recovery in internet gaming disorder: A longitudinal study. Journal of Psychiatric Research , 113, 17–26. 10.1016/j.jpsychires.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Dong, G. , Wang, Z. , Wang, Y. , Du, X. , & Potenza, M. N. (2019b). Gender-related functional connectivity and craving during gaming and immediate abstinence during a mandatory break: Implications for development and progression of internet gaming disorder. Progress in Neuro-psychopharmacology & Biological Psychiatry , 88, 1–10. 10.1016/j.pnpbp.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Dong, G. , & Potenza, M. N. (2014). A cognitive-behavioral model of Internet gaming disorder: Theoretical underpinnings and clinical implications. Journal of Psychiatric Research , 58, 7–11. 10.1016/j.jpsychires.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G. , Wang, L. , Du, X. , & Potenza, M. N. (2018). Gender-related differences in neural responses to gaming cues before and after gaming: implications for gender-specific vulnerabilities to Internet gaming disorder. Social Cognitive and Affective Neuroscience , 13(11), 1203–1214. 10.1093/scan/nsy084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G. , Wang, Z. , Wang, Y. , Du, X. , & Potenza, M. N. (2019). Gender-related functional connectivity and craving during gaming and immediate abstinence during a mandatory break: Implications for development and progression of internet gaming disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry , 88, 1–10. 10.1016/j.pnpbp.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Dong, G. , Wang, M. , Liu, X. , Liang, Q. , Du, X. , & Potenza, M. N. (2020a). Cue-elicited craving-related lentiform activation during gaming deprivation is associated with the emergence of Internet gaming disorder. Addiction Biology , 25(1), e12713. 10.1111/adb.12713. [DOI] [PubMed] [Google Scholar]
- Dong, G. , Wang, Y. , & Potenza, M. N. (2016). The activation of the caudate is associated with correct recollections in a reward-based recollection task. Human Brain Mapping , 37(11), 3999–4005. 10.1002/hbm.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G. H. , Wang, M. , Wang, Z. , Zheng, H. , Du, X. , & Potenza, M. N. (2020b). Addiction severity modulates the precuneus involvement in internet gaming disorder: Functionality, morphology and effective connectivity. Progress in Neuro-Psychopharmacology & Biological Psychiatry , 98, 109829. 10.1016/j.pnpbp.2019.109829. [DOI] [PubMed] [Google Scholar]
- Dong, G. H. , Wang, M. , Zheng, H. , Wang, Z. , Du, X. , & Potenza, M. N. (2020c). Disrupted prefrontal regulation of striatum-related craving in Internet gaming disorder revealed by dynamic causal modeling: Results from a cue-reactivity task. Psychological Medicine , 1–13. 10.1017/s003329172000032x. [DOI] [PubMed] [Google Scholar]
- Fiallos, A. M. , Bricault, S. J. , Cai, L. X. , Worku, H. A. , Colonnese, M. T. , Westmeyer, G. G. , & Jasanoff, A. (2017). Reward magnitude tracking by neural populations in ventral striatum. Neuroimage , 146, 1003–1015. 10.1016/j.neuroimage.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison, K. A. , Yip, S. W. , Balodis, I. M. , Carroll, K. M. , Potenza, M. N. , & Krishnan-Sarin, S. (2017). Reward-related frontostriatal activity and smoking behavior among adolescents in treatment for smoking cessation. Drug and Alcohol Dependence , 177, 268–276. 10.1016/j.drugalcdep.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn, J. A. , Parkinson, J. A. , & Owen, A. M. (2008). The cognitive functions of the caudate nucleus. Progress in Neurobiology , 86(3), 141–155. 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Haber, S. N. , & Knutson, B. (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology , 35(1), 4–26. 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka, O. , Bromberg-Martin, E. , Hong, S. , & Matsumoto, M. (2008). New insights on the subcortical representation of reward. Current Opinion in Neurobiology , 18(2), 203–208. 10.1016/j.conb.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma, K. , Saito, D. N. , & Sadato, N. (2008). Processing of social and monetary rewards in the human striatum. Neuron , 58(2), 284–294. 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Ko, C. H. , Liu, G. C. , Yen, J. Y. , Yen, C. F. , Chen, C. S. , & Lin, W. C. (2013). The brain activations for both cue-induced gaming urge and smoking craving among subjects comorbid with Internet gaming addiction and nicotine dependence. Journal of Psychiatric Research , 47(4), 486–493. 10.1016/j.jpsychires.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Kober, H. , Mende-Siedlecki, P. , Kross, E. F. , Weber, J. , Mischel, W. , Hart, C. L. , & Ochsner, K. N. (2010). Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America , 107(33), 14811–14816. 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach, M. L. , & Rolls, E. T. (2004). The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology , 72(5), 341–372. 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Liu, S. , Wang, S. , Zhang, M. , Xu, Y. , Shao, Z. , Chen, L. , … Yuan, K. (2021). Brain responses to drug cues predict craving changes in abstinent heroin users: A preliminary study. Neuroimage , 237, 118169. 10.1016/j.neuroimage.2021.118169. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Yip, S. W. , Zhang, J. T. , Wang, L. J. , Shen, Z. J. , Liu, B. , … Fang, X. Y. (2017). Activation of the ventral and dorsal striatum during cue reactivity in Internet gaming disorder. Addiction Biology , 22(3), 791–801. 10.1111/adb.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, Y. , & Ikuta, T. (2017). Caudate-precuneus functional connectivity is associated with obesity preventive eating tendency. Brain Connectivity , 7(3), 211–217. 10.1089/brain.2016.0424. [DOI] [PubMed] [Google Scholar]
- Petry, N. M. , Rehbein, F. , Ko, C. H. , & O’Brien, C. P. (2015). Internet gaming disorder in the DSM-5. Current Psychiatry Reports , 17(9). 10.1007/s11920-015-0610-0. [DOI] [PubMed] [Google Scholar]
- Pezze, M. A. , & Feldon, J. (2004). Mesolimbic dopaminergic pathways in fear conditioning. Progress in Neurobiology , 74(5), 301–320. 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Potenza, M. N. (2018). Do gaming disorder and hazardous gaming belong in the ICD-11? Considerations regarding the death of a hospitalized patient that was reported to have occurred while a care provider was gaming. Journal of Behavioral Addictions , 7(2), 206–207. 10.1556/2006.7.2018.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi, S. J. , Pizzagalli, D. A. , Sproule, B. A. , & Kennedy, S. H. (2016). Assessing anhedonia in depression: Potentials and pitfalls. Neuroscience and Biobehavioral Reviews , 65, 21–35. 10.1016/j.neubiorev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy, J. W. (2009). Context representations, context functions, and the parahippocampal-hippocampal system. Learning & Memory , 16(10), 573–585. 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann, E. , Vidyasagar, T. R. , & Creutzfeldt, O. D. (1993). Functional comparison of neuronal properties in the primate posterior hippocampus and parahippocampus (area TF/TH) during different behavioural paradigms involving memory and selective attention. Behavioural Brain Research , 53(1–2), 133–149. 10.1016/s0166-4328(05)80273-6. [DOI] [PubMed] [Google Scholar]
- Schultz, W. (2000). Multiple reward signals in the brain. Nature Reviews Neuroscience , 1(3), 199–207. 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Selemon, L. D. , & Goldman-Rakic, P. S. (1985). Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. The Journal of Neuroscience , 5(3), 776–794. 10.1523/jneurosci.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse, G. , Caldú, X. , Segura, B. , & Dreher, J. C. (2013). Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews , 37(4), 681–696. 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Sescousse, G. , Redouté, J. , & Dreher, J. C. (2010). The architecture of reward value coding in the human orbitofrontal cortex. The Journal of Neuroscience , 30(39), 13095–13104. 10.1523/jneurosci.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan, D. V. , Lecrubier, Y. , Sheehan, K. H. , Amorim, P. , Janavs, J. , Weiller, E. , … Dunbar, G. C. (1998). The mini-international neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry , 59(Suppl 20), 22–33; quiz 34-57. 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- Smith, D. V. , Hayden, B. Y. , Truong, T. K. , Song, A. W. , Platt, M. L. , & Huettel, S. A. (2010). Distinct value signals in anterior and posterior ventromedial prefrontal cortex. The Journal of Neuroscience , 30(7), 2490–2495. 10.1523/jneurosci.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky, A. V. , Smith, D. V. , & Huettel, S. A. (2014). Precuneus is a functional core of the default-mode network. The Journal of Neuroscience , 34(3), 932–940. 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow, N. D. , Wang, G. J. , Fowler, J. S. , Logan, J. , Jayne, M. , Franceschi, D. , … Pappas, N. (2002). “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse , 44(3), 175–180. 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- Volkow, N. D. , Fowler, J. S. , & Wang, G. J. (2003). The addicted human brain: insights from imaging studies. Journal of Clinical Investigation , 111(10), 1444–1451. 10.1172/jci18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow, N. D. , Wang, G. J. , Fowler, J. S. , Tomasi, D. , & Baler, R. (2012). Food and drug reward: Overlapping circuits in human obesity and addiction. Current Topics in Behavioral Neurosciences , 11, 1–24. 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Volkow, N. D. , Wang, G. J. , Telang, F. , Fowler, J. S. , Logan, J. , Childress, A. R. , …, Wong, C. (2006). Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. The Journal of Neuroscience , 26(24), 6583–6588. 10.1523/jneurosci.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Huang, P. , Shen, Z. , Qian, W. , Wang, S. , Jiaerken, Y. , … Zhang, M. (2021). Increased striatal functional connectivity is associated with improved smoking cessation outcomes: A preliminary study. Addiction Biology , 26(2), e12919. 10.1111/adb.12919. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Wu, L. , Wang, Y. , Li, H. , Liu, X. , Du, X. , & Dong, G. (2017). Altered Brain Activities Associated with Craving and Cue Reactivity in People with Internet Gaming Disorder: Evidence from the Comparison with Recreational Internet Game Users. Frontiers in Psychology , 8, 1150. 10.3389/fpsyg.2017.01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, X. , Nakatani, H. , Ueno, K. , Asamizuya, T. , Cheng, K. , & Tanaka, K. (2011). The neural basis of intuitive best next-move generation in board game experts. Science , 331(6015), 341–346. 10.1126/science.1194732. [DOI] [PubMed] [Google Scholar]
- Wesley, M. J. , & Bickel, W. K. (2014). Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biological Psychiatry , 75(6), 435–448. 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli, S. , & Nieto-Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks . [DOI] [PubMed] [Google Scholar]
- WHO (2017). ICD-11 beta draft – mortality and morbidity statistics . [Internet]. Retrieved from https://id.who.int/icd/entity/1448597234. [Google Scholar]
- Yang, B. Z. , Balodis, I. M. , Kober, H. , Worhunsky, P. D. , Lacadie, C. M. , Gelernter, J. , & Potenza, M. N. (2021). GABAergic polygenic risk for cocaine use disorder is negatively correlated with precuneus activity during cognitive control in African American individuals. Addictive Behaviors , 114, 106695. 10.1016/j.addbeh.2020.106695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, H. , Li, Q. , Yu, K. , & Zhao, G. (2021). Large-scale network dysfunction in youths with Internet gaming disorder: A meta-analysis of resting-state functional connectivity studies. Progress in Neuro-Psychopharmacology & Biological Psychiatry , 109, 110242. 10.1016/j.pnpbp.2021.110242. [DOI] [PubMed] [Google Scholar]
- Young, K. S. (1996). Psychology of computer use: XL. Addictive use of the Internet: A case that breaks the stereotype. Psychological Reports , 79(3 Pt 1), 899–902. 10.2466/pr0.1996.79.3.899. [DOI] [PubMed] [Google Scholar]
- Zhang, J. T. , Yao, Y. W. , Potenza, M. N. , Xia, C. C. , Lan, J. , Liu, L. , … Fang, X. Y. (2016). Effects of craving behavioral intervention on neural substrates of cue-induced craving in Internet gaming disorder. NeuroImage: Clinical , 12, 591–599. 10.1016/j.nicl.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Zhornitsky, S. , Le, T. M. , & Li, C. R. (2019). Hypothalamic Responses to Cocaine and Food Cues in Individuals with Cocaine Dependence. The International Journal of Neuropsychopharmacology , 22(12), 754–764. 10.1093/ijnp/pyz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Zhou, H. , Geng, F. , Song, X. , & Hu, Y. (2020). Internet gaming disorder increases mind-wandering in Young adults. Frontiers in Psychology , 11, 619072. 10.3389/fpsyg.2020.619072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W. R. , Wang, M. , Zheng, H. , Wang, M. J. , & Dong, G. H. (2021a). Altered modular segregation of brain networks during the cue-craving task contributes to the disrupted executive functions in internet gaming disorder. Progress in Neuro-psychopharmacology & Biological Psychiatry , 107, 110256. 10.1016/j.pnpbp.2021.110256. [DOI] [PubMed] [Google Scholar]
- Zhou, W. , Zheng, H. , Wang, M. , Zheng, Y. , Chen, S. , Wang, M. J. , & Dong, G. H. (2021b). The imbalance between goal-directed and habitual systems in internet gaming disorder: Results from the disturbed thalamocortical communications. Journal of Psychiatric Research , 134, 121–128. 10.1016/j.jpsychires.2020.12.058. [DOI] [PubMed] [Google Scholar]