Abstract
Background
Metabolic syndrome (MetS) is a group of factors associated with increased risks of cardiovascular disease and overall mortality. Nonalcoholic fatty liver disease (NAFLD) is a common disorder that has been shown to cause hepatic steatosis and fibrosis. The relationship between NAFLD and MetS appears to be bidirectional, but very few studies have examined the role of MetS in hepatic steatosis and fibrosis. The present study investigated the relationships between MetS and its components and the severity of hepatic fibrosis and steatosis, and fibrosis independent of steatosis.
Methods
The study was a cross-sectional population-based survey of 4,678 National Health and Nutrition Examination Survey participants from 2017 to 2018 in the United States. Hepatic fibrosis and steatosis were measured using liver elastography. The MetS components were assessed using demographic, examination, laboratory, and self-reported data.
Results
Using survey-weighted population estimates, 26% of the population had steatosis, 7.5% had fibrosis, and 3.3% had fibrosis without steatosis. The adjusted odds ratio for any level of steatosis was 4.12 times higher (95% confidence interval [CI], 3.16–5.37) and any level of fibrosis was 3.34 times higher (95% CI, 2.26–4.94) among participants with MetS than those without. The adjusted odds ratio for fibrosis without steatosis is 2.67 times higher (95% CI, 1.47–4.87) among participants with MetS than those without.
Conclusion
The presence of MetS significantly increases the risk of hepatic fibrosis and steatosis, providing evidence for MetS to be considered an additional independent risk factor for hepatic fibrosis together with other known etiologies.
Keywords: Metabolic syndrome, Hepatic fibrosis, Hepatic steatosis
INTRODUCTION
Chronic liver disease and cirrhosis cause significant morbidity and mortality worldwide. Recently, it was estimated that two million deaths are attributed to liver disease, while one million deaths are attributed to cirrhosis.1 In 2018, approximately 4.5 million adults in the United States (U.S.) were diagnosed with liver disease, and it contributed to 42,838 deaths.2 One of the most common liver disorders in the U.S. is nonalcoholic fatty liver disease (NAFLD), a condition affecting about 25% of U.S. adults in which excess fat accumulates in the liver of people who do not drink alcohol.3 Given the high prevalence and negative health effects of liver disease, there is a critical need to understand the factors unrelated to alcohol intake associated with these conditions that could be targets of early intervention.
Metabolic syndrome (MetS) is one such potentially modifiable factor and consists of a group of metabolic and cardiovascular factors associated with increased risks of cardiovascular disease, type 2 diabetes, and overall mortality. The components of MetS include obesity, insulin resistance, hypertension, and dyslipidemia.4 There appears to be a bidirectional relationship between NAFLD and MetS, with evidence that NAFLD contributes to the development of MetS.5 The presence of MetS is also associated with the development of nonalcoholic steatohepatitis and hepatic fibrosis, conditions which are known to contribute to NAFLD.6,7 Although the association between hepatic fibrosis and NAFLD has been well established, very few studies have investigated the direct role of MetS in liver fibrosis.8 As a result, additional research is needed to investigate the association between MetS and hepatic fibrosis. The purpose of this study was to investigate the relationships between MetS and its components and the severity of hepatic fibrosis and steatosis, and fibrosis independent of steatosis.
METHODS
Study population
The National Health and Nutrition Examination Survey (NHANES) is an ongoing cross-sectional survey conducted by the National Center for Health Statistics (NCHS). Each year, the NHANES collects health and nutrition-related information data from a nationally representative sample of the civilian, non-institutionalized U.S. population through in-home personal interviews and standardized physical examinations in mobile examination centers. Since 1999, continuous NHANES data have been released as public-use data files every 2 years. Liver fibrosis was measured using ultrasound and vibration controlled transient elastography for the 2017–2018 cycle only; for these special studies, NHANES staff obtained informed consent from each participant, and the NCHS Research Ethics Review Board reviewed and approved the protocols for conducting the surveys. A detailed description of the survey design and response rate is available on the NHANES website.9
Study sample
For the current study, data were pooled from the NHANES 2017 to 2018 cycle. Based on the availability of the data and the purpose of the study, we limited the study sample to participants at least 18 years old with available non-zero sampling weights who completed a liver ultrasound transient elastography exam (FibroScan, Waltham, MA, USA). Because of possible confounding of liver fibrosis by hepatitis B or hepatitis C infection, participants who had active hepatitis B (positive hepatitis B surface antigen and negative hepatitis B surface antibody) or active hepatitis C (positive hepatitis C RNA) infections were excluded from the study.10
Hepatic fibrosis and steatosis
Our study’s primary outcomes were hepatic fibrosis (i.e., scarring in the liver) and hepatic steatosis (fat in the liver). To obtain objective measurements of these two factors, we extracted data from the liver ultrasound elastography exam (first included in the NHANES 2017–2018 physical examinations) to assess liver stiffness and fibrosis in the liver. We also used the ultrasound control attenuation parameter, an indicator of the steatosis in the liver, that the device recorded simultaneously to measure fat content in the liver (NHANES 2017–2018 Liver Ultrasound Transient Elastography).11 Given the close association of NAFLD with MetS, we classified hepatic fibrosis and steatosis using NAFLD criteria.12 We categorized fibrosis into four stages: no fibrosis (≤8.2 kPa), mild fibrosis (8.3–9.7 kPa), moderate fibrosis (9.8–13.6 kPa), and severe fibrosis (>13.6 kPa). Similarly, hepatic steatosis was classified into four stages: no steatosis (≤302 dB/m), mild steatosis (303–331 dB/m), moderate steatosis (332–337 dB/m), and severe steatosis (>337 dB/m). We created two dichotomous variables, one for fibrosis status and another for steatosis status, by combining participants with mild, moderate, and severe stages of each condition into “yes” categories for hepatic fibrosis and hepatic steatosis. We coded “no fibrosis” or “no steatosis” as “no” for not having fibrosis or steatosis, respectively. Given the significant complications associated with advanced/severe fibrosis and steatosis, we also created “fibrosis severity” and “steatosis severity” variables to compare the “severe fibrosis or steatosis” stage to the “no fibrosis or steatosis” stage.10,13
MetS components
According to the National Heart, Lung, and Blood Institute, there are five components of MetS, which include (1) waist circumference above goal, (2) high triglyceride level, (3) low high-density lipoprotein (HDL) cholesterol level, (4) high blood pressure, and (5) high fasting blood sugar. Having at least three out of the five components meets the diagnostic criteria for MetS.14 For our study, we defined the components of MetS as (1) waist circumference above goal (≥102 cm for men or ≥88 cm for women); (2) high triglycerides (≥150 mg/dL), giving an affirmative answer to the question of whether a doctor had advised them to take prescription medication to lower cholesterol, or taking prescription medication for high cholesterol; (3) low HDL cholesterol level (<40 mg/dL for men or <50 mg/dL for women); (4) high blood pressure (≥130/85 mmHg), taking prescription medication for blood pressure, or affirming a doctor had advised them to take prescribed medicines for hypertension; and (5) high blood sugar (fasting blood sugar ≥100 mg/dL or glycosylated hemoglobin ≥6.5%), taking prescription medication for blood sugar, or affirming they either were taking insulin or taking “diabetic pills” to lower blood sugar at the time of assessment. We classified participants as having MetS if they had at least three out of five of the above MetS components.
Other covariates
In addition to MetS and its components, we extracted demographic and related covariates from the data, including age, sex, race, depression symptoms in the last two weeks, socioeconomic status (education and health insurance), physical activity level, depression status, and alcohol use.15-17 We combined races to create four categories: non-Hispanic White, non-Hispanic Black, Hispanic, and other race. Four categories for education level were identified: less than high school, high school graduate/general educational development, some college or associate of arts degree, and college graduate or above. Health insurance status was grouped into two categories: “yes” for having health insurance and “no” for not having health insurance.
The NHANES uses the Patient Health Questionnaire-9 (PHQ-9), a nine-item depression screening instrument, to evaluate the participants’ depression symptoms over the previous 2 weeks.18 Symptoms on the PHQ-9 receive a score of 0 to 3 points, corresponding to the categories of “not at all,” “several days,” “more than half the days,” and “nearly every day.” Scores on the PHQ-9 ranged from 0 to 27 points, with higher scores corresponding to more depression symptoms. We created a dichotomous variable for depression status: “yes” for scores greater than nine points, and “no” for scores of nine or fewer points.19
The NHANES uses the Alcohol Use Questionnaire to assess alcohol use. Participants who had not drunk alcohol, or who indicated they had drunk zero alcoholic drinks per day during the previous 12 months were classified as “no alcohol use.” Participants who reported any amount of alcohol consumption during the previous 12 months were classified as “alcohol use.” Supplementary Tables 1 and 2 provide additional demographic and covariate details for the participants.
The NHANES includes questions regarding moderate and vigorous recreational activity. Based on World Health Organization, Centers for Disease Controland Prevention , and American Heart Association guidelines recommend a minimum of 150 minutes of moderate activity per week: participants who met this goal were classified as “≥150 minutes per week,” and if they did not meet this goal they were classified as “<150 minutes per week.”
Statistical analyses
All data analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC, USA). We incorporated appropriate sampling weights and SAS survey procedures to account for complex design, including planned oversampling, survey non-response, and poststratification following the NHANES analytic and reporting guidelines.20 Demographic characteristics, components of MetS, MetS classification by fibrosis and steatosis status were compared using chi-square analyses for categorical variables and regression analyses for continuous variables. We conducted logistic regression to assess the associations between (1) MetS and fibrosis status adjusted for demographic variables, (2) MetS and steatosis status adjusted for demographic variables, (3) MetS and fibrosis severity status adjusted for demographic variables, and (4) MetS and steatosis severity status adjusted for demographic variables. Additionally, we estimated adjusted odds ratios of fibrosis status by MetS using data without steatosis participants. All statistical tests were two-tailed, and a P-value less than 0.05 was considered statistically significant.
RESULTS
Using survey-weighted population estimates, 26% of study participants had steatosis and 7.5% had fibrosis. Demographic characteristics of participants by fibrosis and steatosis status are presented in Table 1 (unweighted) and Table 2 (survey-weighted). Participants with steatosis and fibrosis were older, more likely to be male, less likely to meet weekly physical activity guidelines, and had lower levels of education. Participants with steatosis were more likely to be Hispanic or non-Hispanic white. There were no differences in race among participants with and without fibrosis. Alcohol use, health insurance status, and diagnosis of depression were not significantly different between those with and without steatosis or fibrosis. Of those without steatosis, 3.3% had fibrosis.
Table 1.
Unweighted samples sizes for demographic characteristics by steatosis and fibrosis status (n= 4,678)
| Variable | Steatosis status* | Fibrosis status† | ||
|---|---|---|---|---|
|
|
|
|||
| Yes (n= 1,280) | No (n= 3,397) | Yes (n= 414) | No (n= 4,264) | |
| Severity | ||||
| Severe | 620 | - | 111 | - |
| Not severe | 660 | - | 303 | - |
| Sex | ||||
| Male | 748 | 1,559 | 248 | 2,060 |
| Female | 532 | 1,838 | 166 | 2,204 |
| Race | ||||
| Hispanic | 366 | 739 | 118 | 987 |
| Non-Hispanic White | 468 | 1,118 | 146 | 1,440 |
| Non-Hispanic Black | 213 | 847 | 85 | 976 |
| Other race | 233 | 693 | 65 | 861 |
| Education | ||||
| Less than high school | 263 | 653 | 97 | 819 |
| High school/GED | 335 | 825 | 115 | 1,045 |
| Some college or AA degree | 431 | 1,064 | 138 | 1,358 |
| College graduate and beyond | 247 | 851 | 64 | 1,034 |
| Health insurance | ||||
| Yes | 1,092 | 2,857 | 353 | 3,597 |
| No | 185 | 530 | 59 | 656 |
| Depression | ||||
| Yes | 120 | 253 | 38 | 335 |
| No | 1,089 | 2,924 | 351 | 3,663 |
| Alcohol use | ||||
| Yes | 814 | 2,223 | 262 | 2,776 |
| No | 466 | 1,174 | 152 | 1,488 |
| Physical activity | ||||
| ≥ 150 minutes per week | 317 | 1,177 | 90 | 1,405 |
| < 150 minutes per week | 963 | 2,220 | 324 | 2,859 |
*1 missing in the steatosis group; †159 had fibrosis without steatosis.
GED, general educational development; AA, associate of arts.
Table 2.
Survey-weighted prevalence of demographic characteristics by steatosis and fibrosis status
| Variable | Steatosis status | Fibrosis status | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Yes | No | P | Yes | No | P | |
| Sample size | - | - | ||||
| Unweighted | 1,280 | 3,397 | 414 | 4,264 | ||
| Weighted | 56,180,078 | 156,487,049 | 15,905,392 | 196,795,225 | ||
| Age (yr), mean (95% CI) | 51.0 (49.5–52.5) | 45.3 (43.9–46.7) | < 0.001* | 51.8 (48.8–54.8) | 46.4 (45.1–47.7) | < 0.001* |
| Sex | < 0.001* | 0.005* | ||||
| Male | 59.5 (55.0–64.0) | 45.3 (43.1–47.6) | 59.8 (51.5–68.2) | 48.2 (46.2–50.2) | ||
| Female | 40.5 (36.0–45.0) | 54.7 (52.4–56.9) | 40.2 (31.8–48.5) | 51.8 (49.8–53.8) | ||
| Race | 0.001* | 0.332 | ||||
| Hispanic | 19.2 (13.1–25.3) | 15.3 (11.4–19.2) | 19.9 (14.1–25.8) | 16.1 (11.6–20.5) | ||
| Non-Hispanic White | 63.4 (56.1–70.7) | 61.7 (56.2–67.3) | 61.1 (52.3–69.9) | 62.2 (56.9–67.6) | ||
| Non-Hispanic Black | 7.7 (4.8–10.6) | 12.2 (8.7–15.7) | 9.9 (4.9–15.0) | 11.1 (7.9–14.4) | ||
| Other race | 9.7 (6.6–12.8) | 10.7 (7.8-13.6) | 9.0 (4.7–13.4) | 10.6 (7.9–13.2) | ||
| Education | < 0.001* | < 0.001* | ||||
| Less than high school | 11.2 (8.7–13.7) | 11.3 (9.4–13.1) | 13.0 (9.1–16.9) | 11.1 (9.2–13.0) | ||
| High school/GED | 31.7 (27.2–36.1) | 26.0 (22.3–29.6) | 37.6 (30.7–44.4) | 26.6 (23.3–30.0) | ||
| Some college or AA degree | 33.0 (30.1–35.9) | 29.8 (26.4–33.3) | 31.6 (26.1–37.2) | 30.6 (27.6–33.6) | ||
| College graduate and beyond | 24.2 (18.4–30.0) | 33.0 (27.1–38.9) | 17.8 (10.4–25.2) | 31.7 (25.9–37.4) | ||
| Health insurance | 0.250 | 0.838 | ||||
| Yes | 87.4 (83.2–91.6) | 85.8 (81.6–90.0) | 86.2 (82.2–90.2) | 86.8 (79.9–93.6) | ||
| No | 12.6 (8.4–16.8) | 14.2 (10.0–18.4) | 13.8 (9.8–17.8) | 13.2 (6.4–20.1) | ||
| Alcohol use | 0.190 | 0.086 | ||||
| Yes | 70.5 (66.3–74.7) | 73.6 (71.2–76.0) | 69.0 (63.9–74.0) | 73.1 (71.1–75.1) | ||
| No | 29.5 (25.3–33.7) | 26.4 (24.0–28.8) | 31.0 (26.0–36.1) | 26.9 (24.9–28.9) | ||
| Depression | 0.440 | 0.845 | ||||
| Yes | 8.9 (6.6–11.1) | 7.9 (6.7–9.1) | 8.4 (4.9–12.0) | 8.1 (7.1–9.1) | ||
| No | 91.1 (88.9–93.4) | 92.1 (90.0–93.3) | 91.6 (88.0–95.1) | 91.9 (90.9–92.9) | ||
| Physical activity | < 0.001* | < 0.001* | ||||
| ≥ 150 minutes per week | 26.3 (22.8–29.9) | 40.8 (37.0–44.5) | 21.3 (13.6–28.9) | 38.2 (34.8–41.7) | ||
| < 150 minutes per week | 73.7 (70.1–77.2) | 59.2 (55.5–63.0) | 78.7 (71.1–86.4) | 61.8 (58.3–65.2) | ||
Values are presented as percent (95% CI) unless otherwise indicated.
*Indicates statistical significance with P-value less than 0.05.
CI, confidence interval; GED, general educational development; AA, associate of arts.
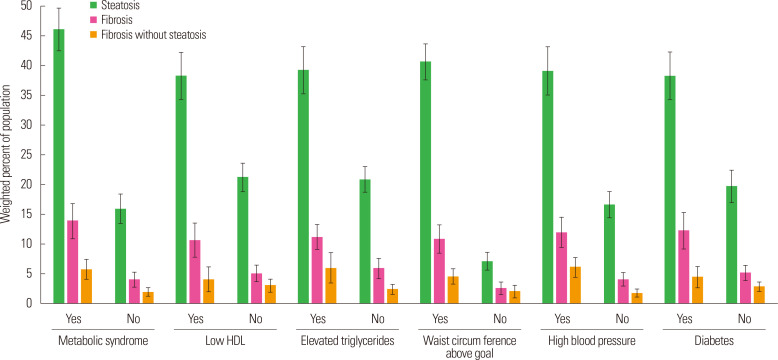
The proportion of participants with steatosis or fibrosis with respect to MetS and its components are presented in Fig. 1. The results indicated that steatosis was more common than fibrosis. Further, steatosis and fibrosis were also more common among participants with MetS. The results were similar for components of MetS as well as for participants with fibrosis without steatosis.
Figure 1.
Hepatic fibrosis and steatosis in metabolic syndrome. HDL, high-density lipoprotein.
Odds ratios from logistic regression analyses for steatosis and fibrosis category and MetS are presented in Table 3. The odds of having any level of steatosis or fibrosis were higher among participants with MetS. In models adjusted for demographic factors, the odds of any steatosis were 4.12 times higher in those with MetS than in those without (95% confidence interval [CI], 3.16–5.37), and odds of any fibrosis were 3.34 times higher (95% CI, 2.26–4.94). The odds of having fibrosis without steatosis were 2.67 times higher in those with MetS than in those without (95% CI, 1.47–4.87).
Table 3.
Logistic regression models for steatosis and fibrosis by metabolic syndrome and adjusted for demographic covariates
| Variable | Any steatosis | Any fibrosis | Severe steatosis | Severe fibrosis | Fibrosis without steatosis |
|---|---|---|---|---|---|
| Metabolic syndrome | |||||
| Yes | 4.12 (3.16–5.37)* | 3.34 (2.26–4.94)* | 5.20 (3.56–8.12)* | 3.57 (1.80–7.10)* | 2.67 (1.47–4.87)* |
| No | - | - | - | - | - |
| Age | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 1.00 (0.99–1.02) | 1.01 (0.99–1.04) |
| Sex | |||||
| Male | 1.95 (1.49–2.56)* | 1.68 (1.14–2.49)* | 2.30 (1.67–3.15)* | 1.52 (0.78–2.95) | 1.18 (0.70–1.98) |
| Female | - | - | - | - | - |
| Race | |||||
| Non-Hispanic White | - | - | - | - | - |
| Non-Hispanic Black | 0.62 (0.47–0.81)* | 0.90 (0.58–1.41) | 0.58 (0.40–0.83)* | 0.45 (0.19–1.07) | 1.39 (0.78–2.48) |
| Hispanic | 1.40 (1.04–1.90)* | 1.29 (0.81–2.08) | 1.16 (0.80–1.69) | 1.04 (0.49–2.22) | 1.28 (0.68–2.43) |
| Other | 0.97 (0.59–1.58) | 0.87 (0.51–1.50) | 1.03 (0.53–1.98) | 1.26 (0.54–2.94) | 0.87 (0.34–2.25) |
| Education | |||||
| Less than high school | - | - | - | - | - |
| High school/GED | 1.48 (1.09–2.02)* | 1.32 (0.85–2.05) | 1.71 (1.24–2.35)* | 1.24 (0.41–3.71) | 0.63 (0.31–1.30) |
| Some college or AA | 1.38 (1.09–1.74)* | 1.08 (0.69–1.68) | 1.43 (1.05–1.94)* | 0.82 (0.32–2.11) | 0.71 (0.34–1.46) |
| College graduate or above | 1.12 (0.80–1.55) | 0.69 (0.39–1.22) | 1.16 (0.69–1.96) | 0.36 (0.09–1.43) | 0.56 (0.23–1.34) |
| Health insurance | |||||
| Yes | 1.16 (0.82–1.66) | 1.13 (0.57–2.21) | 1.10 (0.67–1.82) | 1.24 (0.44–3.52) | 1.47 (0.59–3.65) |
| No | - | - | - | - | - |
| Depression | |||||
| Yes | 1.07 (0.74–1.56) | 0.96 (0.58–1.59) | 1.10 (0.73–1.65) | 1.05 (0.49–2.24) | 0.75 (0.32–1.77) |
| No | - | - | - | - | - |
| Alcohol use | |||||
| Yes | 0.91 (0.71–1.17) | 0.93 (0.70–1.23) | 0.96 (0.70–1.30) | 0.83 (0.38–1.80) | 1.10 (0.69–1.74) |
| No | - | - | - | - | - |
| Physical activity | |||||
| ≥ 150 minutes per week | 0.59 (0.49–0.72)* | 0.53 (0.31–0.92)* | 0.49 (0.35–0.70)* | 0.77 (0.29–2.02) | 0.55 (0.27–1.12) |
| < 150 minutes per week | - | - | - | - | - |
Values are presented as odds ratio (95% confidence interval).
*Indicates statistical significance with P-value less than 0.05.
GED, general educational development; AA, associate of arts.
DISCUSSION
The current study investigated the associations between MetS and the severity of hepatic fibrosis and steatosis, and between MetS and fibrosis independent of steatosis using nationally representative data from the NHANES 2017–2018 cycle. Results indicated that MetS is an independent risk factor for hepatic fibrosis, even in the participants who do not have hepatic steatosis. In addition, compared to those without MetS, individuals with MetS were 3.3 times more likely to have hepatic fibrosis and 4.1 more likely to have steatosis. For those with MetS without steatosis, the odds of developing hepatic fibrosis were 2.7 times greater than for those without MetS.
The estimated prevalence of MetS in our study was 35%, and this rate corresponds to previously reported estimates of approximately 34%.21,22 Additionally, with respect to the five components of MetS, our prevalence estimates in the current study were as follows: waist circumference above goal, 58.0%; high blood pressure, 43.7%; high triglycerides, 30.3%; low HDL, 30.5%; and high blood sugar, 36.3%. In comparison, other recent studies have estimated the prevalence of the components of MetS as follows: waist circumference above goal, 56.1%; high blood pressure, 24%; high triglycerides, 24%; high cholesterol, 30%; and high glucose, 19.9%.23 The strongest clustering effect occurred with hypertriglyceridemia; hyperglycemia and hypertension for males; and hypertriglyceridemia, low HDL, and abdominal obesity for females.24
The pathophysiology of hepatic fibrosis in MetS appears to be multifactorial. Hypothesized pathways include adiposity, NAFLD, hypertension, hyperglycemia, and dyslipidemia.25 While NAFLD has been shown to be a strong risk factor for hepatic fibrosis, other potential risk factors have not been independently studied. Given the close association of NAFLD with insulin resistance, obesity, and hyperlipidemia, NAFLD is considered a component of MetS.6,26,27 There is growing evidence that the two diseases are different but have similar risk factors.7,28 In a study comparing subjects with NAFLD only, MetS only, NAFLD with MetS and Non-NAFLD with MetS, subjects with NAFLD and MetS had increased risks of type 2 diabetes, cardiovascular disease, and left ventricular mass index in comparison to those with NAFLD without MetS.29 In another study, reciprocal associations between MetS and NAFLD were also found, but the effect of MetS on NAFLD was greater than the effect of NAFLD on MetS.5
The results from the current study provide evidence that individuals with MetS and hypertension can develop hepatic fibrosis with or without steatosis. While up to 50% of the patients with hypertension were noted to have NAFLD, the role of hypertension in hepatic fibrosis without steatosis is not well understood.30 Our findings might suggest that fibrosis in MetS can occur via both steatosis and non-steatosis pathways. Further research into various potential mechanisms of fibrosis may have clinical implications in understanding and managing fibrosis in this population. Studies in mice have shown that hypertension could cause hepatic fibrosis without hepatic steatosis via decreased Interleukin 10 or heme oxygenase-1 pathways.31 The role of hypertension in cirrhotic patients is an area of much debate. The difference between hypotensive cirrhotic patients and normotensive cirrhotic patients appears to be subtle. There is a hypothesized complex mechanism between systemic circulation and hepatosplenic circulation that is unique to the stage of liver disease, shifting from hypertensive cirrhotic patients to normotensive cirrhotic patients.
The strengths of the current study include the use of NHANES data, which is collected nationwide by the NCHS using both interviews and physical examinations, providing greater accuracy in reporting.32 Additionally, the utilization of weight estimates representing the U.S. population allows extrapolation from our study to the larger U.S. population.
The current study has several known limitations. First, FibroScan was used to diagnose hepatic fibrosis and steatosis. Although it is regularly used for this purpose, it is estimated to be between 80% and 90% accurate in diagnosing fibrosis and steatosis compared to liver biopsy.33 Liver biopsy is the gold standard for these diagnoses; however, given the non-invasive nature of FibroScan, it is now widely accepted as the initial test of choice to evaluate liver fibrosis.34 Second, we used NAFLD criteria to determine steatosis and fibrosis in MetS because of previously reported close associations between the two. The FibroScan criteria for MetS have not been defined, highlighting the importance of establishing guidelines for these criteria to investigate this disease. Third, some of the study constructs were measured using self-reporting methods which are susceptible to retrospective self-reporting bias. We attempted to limit the influence of retrospective self-reporting biases by using complementary information from the dietary, physical examination, and laboratory data wherever possible—hence, creating a robust and more accurate dataset. Finally, although efforts were made to identify and account for confounding factors like hepatitis C virus, hepatitis B virus infection, and alcohol-induced liver disease, other etiologies of liver disease that can cause hepatic fibrosis could not be completely excluded.
Overall, the results of the current study indicate that MetS significantly increases the risk of hepatic fibrosis and steatosis, providing evidence for MetS as an independent risk factor for hepatic fibrosis, together with other known etiologies such as NAFLD. Additional research to investigate non-steatosis pathways in fibrosis development should be conducted given the therapeutic implications in preventing hepatic fibrosis and cirrhosis from MetS.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1 and 2 can be found via https://doi.org/10.7570/jomes.21062.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: VGRG, CP; acquisition of data: JX; analysis and interpretation of data: JX, CP; drafting of the manuscript: VGRG, JX, CP, AA; critical revision of the manuscript: AA, RT, JX; statistical analysis: JX; administrative, technical, or material support: VGRG, CP, AA; and study supervision: CP, AA, RT.
REFERENCES
- 1.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–71. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, author. Chronic liver disease and cirrhosis [Internet] Centers for Disease Control and Prevention; Atlanta (GA): 2018. [cited 2022 Feb 25]. Available from: https://www.cdc.gov/nchs/fastats/liver-disease.htm . [Google Scholar]
- 3.Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323:1175–83. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 4.Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43:1–23. doi: 10.1016/j.ecl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Zhang T, Zhang C, Tang F, Zhong N, Li H, et al. Identification of reciprocal causality between non-alcoholic fatty liver disease and metabolic syndrome by a simplified Bayesian network in a Chinese population. BMJ Open. 2015;5:e008204. doi: 10.1136/bmjopen-2015-008204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Järvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010;42:320–30. doi: 10.1016/j.dld.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Wainwright P, Byrne CD. Bidirectional relationships and disconnects between NAFLD and features of the metabolic syndrome. Int J Mol Sci. 2016;17:367. doi: 10.3390/ijms17030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158:1611–25. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics, author. NHANES survey methods and analytic guidelines [Internet] National Center for Health Statistics; Hyattsville (MA): 2020. [cited 2022 Feb 25]. Available from: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx . [Google Scholar]
- 10.Ren H, Wang J, Gao Y, Yang F, Huang W. Metabolic syndrome and liver-related events: a systematic review and meta-analysis. BMC Endocr Disord. 2019;19:40. doi: 10.1186/s12902-019-0366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Health and Nutrition Examination Survey (NHANES), author Liver ultrasound transient elastography procedures manual: Fibroscan manual [Internet] Centers for Disease Control and Prevention; Atlanta (GA): 2018. [cited 2022 Feb 25]. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/2018_liver_ultrasound_elastography_procedures_manual.pdf . [Google Scholar]
- 12.Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–30. doi: 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 13.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–73. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 15.Limon VM, Lee M, Gonzalez B, Choh AC, Czerwinski SA. The impact of metabolic syndrome on mental health-related quality of life and depressive symptoms. Qual Life Res. 2020;29:2063–72. doi: 10.1007/s11136-020-02479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanquet M, Legrand A, Pélissier A, Mourgues C. Socio-economics status and metabolic syndrome: a meta-analysis. Diabetes Metab Syndr. 2019;13:1805–12. doi: 10.1016/j.dsx.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Boyle M, Masson S, Anstee QM. The bidirectional impacts of alcohol consumption and the metabolic syndrome: cofactors for progressive fatty liver disease. J Hepatol. 2018;68:251–67. doi: 10.1016/j.jhep.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics, author. Analytic guidelines [Internet] National Center for Health Statistics; Hyattsville (MA): 2020. [cited 2022 Feb 25]. Available from: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#analytic-guidelines . [Google Scholar]
- 21.Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. 2020;323:2526–8. doi: 10.1001/jama.2020.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988-2012. Prev Chronic Dis. 2017;14:E24. doi: 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YJ, Hwang HR. Clustering effects of metabolic factors and the risk of metabolic syndrome. J Obes Metab Syndr. 2018;27:166–74. doi: 10.7570/jomes.2018.27.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanthier N, Horsmans Y, Leclercq IA. The metabolic syndrome:how it may influence hepatic stellate cell activation and hepatic fibrosis. Curr Opin Clin Nutr Metab Care. 2009;12:404–11. doi: 10.1097/MCO.0b013e32832c7819. [DOI] [PubMed] [Google Scholar]
- 26.Cortez-Pinto H, Camilo ME, Baptista A, De Oliveira AG, De Moura MC. Non-alcoholic fatty liver: another feature of the metabolic syndrome? Clin Nutr. 1999;18:353–8. doi: 10.1016/S0261-5614(99)80015-6. [DOI] [PubMed] [Google Scholar]
- 27.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 28.Qureshi K, Abrams GA. Prevalence of biopsy-proven non-alcoholic fatty liver disease in severely obese subjects without metabolic syndrome. Clin Obes. 2016;6:117–23. doi: 10.1111/cob.12132. [DOI] [PubMed] [Google Scholar]
- 29.Käräjämäki AJ, Bloigu R, Kauma H, Kesäniemi YA, Koivurova OP, Perkiömäki J, et al. Non-alcoholic fatty liver disease with and without metabolic syndrome: different long-term outcomes. Metabolism. 2017;66:55–63. doi: 10.1016/j.metabol.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68:335–52. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Arima S, Uto H, Ibusuki R, Kumamoto R, Tanoue S, Mawatari S, et al. Hypertension exacerbates liver injury and hepatic fibrosis induced by a choline-deficient L-amino acid-defined diet in rats. Int J Mol Med. 2014;33:68–76. doi: 10.3892/ijmm.2013.1544. [DOI] [PubMed] [Google Scholar]
- 32.National Center for Health Statistics, author. About the National Health and Nutrition Examination Survey [Internet] National Center for Health Statistics; Hyattsville (MA): 2017. [cited 2022 Feb 25]. Available from: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm . [Google Scholar]
- 33.Jiang W, Huang S, Teng H, Wang P, Wu M, Zhou X, et al. Diagnostic accuracy of point shear wave elastography and transient elastography for staging hepatic fibrosis in patients with non-alcoholic fatty liver disease: a meta-analysis. BMJ Open. 2018;8:e021787. doi: 10.1136/bmjopen-2018-021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roulot D, Roudot-Thoraval F, NKontchou G, Kouacou N, Costes JL, Elourimi G, et al. Concomitant screening for liver fibrosis and steatosis in French type 2 diabetic patients using Fibroscan. Liver Int. 2017;37:1897–906. doi: 10.1111/liv.13481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.