Abstract
Background
We aimed to build mouse models of small for gestational age (SGA), recapitulating failure of catch-up growth and dysregulated metabolic outcomes in adulthood.
Methods
Pregnant C57BL/6 mice were given a protein-restricted diet (PRD; 6% kcal from protein) during pregnancy without (model 1) or with cross-fostering (model 2). Model 3 extended the PRD to the end of the lactation period. Model 4 changed to a 9% PRD without cross-fostering.
Results
Model 1 yielded a reduced size of offspring with a poor survival rate. Model 2 improved survival but offspring showed early catch-up growth. Model 3 maintained a reduced size of offspring after weaning with a higher body mass index and blood glucose levels in adult stages. Model 4 increased the survival of the offspring while maintaining a reduced size and dysregulated glucose metabolism.
Conclusion
Models 3 and 4 are suitable for studying SGA accompanying adulthood short stature and metabolic disorders.
Keywords: Small for gestational age, Growth failure, Glucose metabolism disorders, Obesity
INTRODUCTION
Birth weight is a surrogate marker for an adequate intrauterine environment during pregnancy and both extremes could cause various metabolic consequences.1,2 Small for gestational age (SGA) is defined as having a weight at birth below the bottom 10th percentile of the weight standard, a common complication of pregnancy. Maternal undernutrition is a major problem in underdeveloped countries and pregnancy at an advanced maternal age has become more common over the last decades, which all increase the burden of SGA.1 Subjects with SGA are vulnerable to several metabolic diseases in adulthood, including type 2 diabetes and obesity.3 More than 10% of all individuals born SGA do not complete postnatal catch-up growth and retain a short stature. Therefore, they are commonly recommended to receive growth hormone (GH) treatment to improve their adult height.4 As GH acts as a counterregulatory hormone on insulin, a long-term GH treatment in SGA subjects might worsen the risk of diabetes.3
A proper animal model is required for the study of SGA-related adult complications including catch-up growth failure and dysregulated metabolic outcome. In the present study, we attempted to establish a good SGA mouse model by restricting the amount of protein in the maternal diet during pregnancy and the lactation period.
METHODS
Animal care
The C57BL/6 mice (8–9 weeks of age) used in this study were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were housed in a temperature- and humidity-controlled environment. Food and water were available ad libitum unless otherwise noted. All experimental procedures were approved by and performed in accordance with the standards of the Animal Care and Use Committee of Seoul National University (No. SNU-190225-1).
Protein restriction studies
Female mice were allowed to mate during the dark cycle with males. Upon detection of a vaginal plug on the next day, females were placed on either a normal chow diet (NCD; control group, 24.5% kcal from protein with 63.1% kcal from carbohydrate and 12.4% kcal from fat; #38057, Purina Korea, Seoul, Korea) or a protein-restricted diet (PRD; SGA group; 6.0% or 9.0% kcal from protein with 83.8% or 80.8% kcal from carbohydrate, respectively, and with 10.2% kcal from fat; based on #D02041001, Research Diets, New Brunswick, NJ, USA) during pregnancy and/or lactation periods, as described for each model. Afterwards, NCD was provided to both groups and all offspring. If needed, cross-fostering was conducted between 0 and 6 hours after both biological and adoptive dams had given birth. The procedure consisted of removing the biological mother, placing the litter in a clean cage containing bedding of the adoptive mother, and finally placing the adoptive mother in the cage. Pups were weaned at four weeks of age.
Glucose tolerance test
In overnight-fasted mice, following an intraperitoneal injection of 2 g of glucose/kg body mass in 20% glucose solution, the blood glucose level was measured at each time point (0, 15, 30, 60, 120 minutes) through a glucometer (Accu-CHEK Performa; Roche Diagnostics, Mannheim, Germany).
Insulin tolerance test
In 4-hour-fasted mice, following an intraperitoneal injection of 0.75 IU of Humulin R (Eli Lilly, Indianapolis, IN, USA) per kg body mass, the blood glucose level was measured at each time point (0, 15, 30, 60, 120 minutes).
Statistical analysis
Data are expressed as the mean±standard error of the mean. The Student t-test was used to compare the groups. Two-way repeated-measures analysis of variance was used for glucose tolerance test (GTT) and insulin tolerance test (ITT) followed by Bonferroni correction. The log-rank test was used for a survival analysis. P<0.05 was considered significant. IBM SPSS version 25.0 (IBM Corp., Armonk, NY, USA) was used to assist in the analysis.
RESULTS
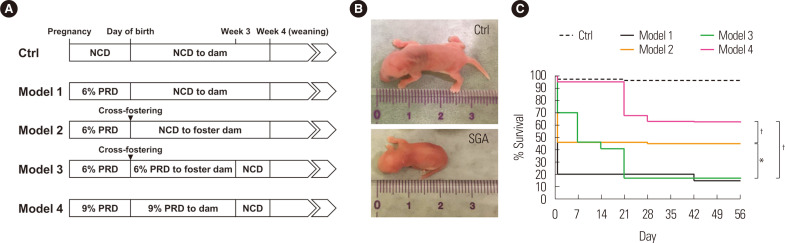
Compared to the NCD control, 6% PRD during pregnancy (model 1) yielded a smaller size of offspring, but most of them were lost due to cannibalism or neglect by the dam (Fig. 1). This problem was partially solved by cross-fostering (model 2), but due to catch-up growth, the offspring recovered their body weights and lengths to the levels of the controls during the pre-weaning period (data not shown). Therefore, model 1 and model 2 may not be suitable for the study of SGA.
Figure 1.
Protocols for mouse modeling of small for gestational age (SGA). (A) Scheme of each model. Please note that the same cross-fostering procedure at the day of birth was applied for control (Ctrl) groups of model 2 and 3. (B) Comparison of the body size at birth between a Ctrl and the SGA model. (C) Survival curve of offspring of each model. All the data of Ctrl groups were combined into one group. *P< 0.05 for model 2 vs. model 1 or 3; †P< 0.001 for model 4 vs. any of the other models. NCD, normal chow diet; PRD, protein-restricted diet.
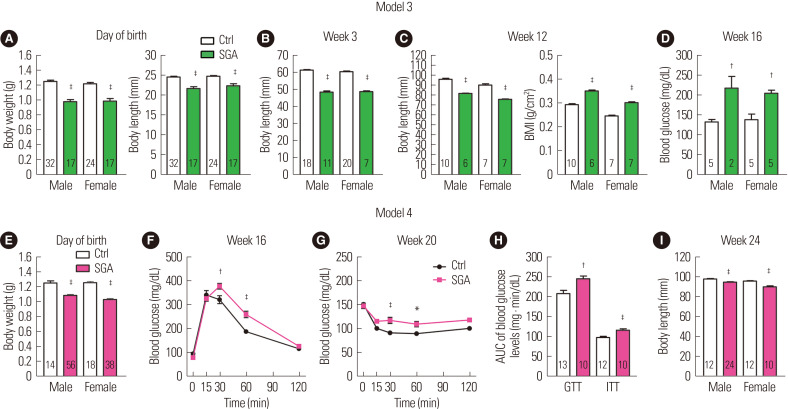
In model 3, we extended the period of 6% PRD to the lactation period with cross-fostering. The offspring had smaller body sizes at birth and at 3 weeks and 12 weeks old (Fig. 2A-C), successfully recapitulating the phenotype of failure of catch-up growth. Moreover, the offspring displayed a higher body mass index at 12 weeks and increased fasting blood glucose levels at 16 weeks (Fig. 2C and D). Thus, model 3 successfully induced dysregulated glucose metabolism in the adulthood of the offspring here. However, the extended PRD period significantly reduced the long-term survival rate compared to that in model 2 (Fig. 1C). Thus, model 3 may have a disadvantage with regard to obtaining a sufficient number of offspring. To improve the yield, we increased the protein content of PRD to 9% in model 4 and instead omitted cross-fostering. This model significantly increased the long-term survival rate of the offspring, even without cross-fostering (Fig. 1C). Although the degree of reduction in adult body lengths in model 4 was less than in model 3, the offspring were significantly smaller than the NCD controls (Fig. 2E and I). Thus, this model also recapitulated the phenotype of failure of catch-up growth. Moreover, GTT and ITT studies, performed at 16 and 20 weeks, demonstrated glucose intolerance and insulin resistance in model 4 (Fig. 2F-H).
Figure 2.
Anthropometric and metabolic parameters of mouse models of small for gestational age (SGA). Data of model 3 (A-D) and model 4 (E-I) are listed in chronological order. (A) Body weight and length at day of birth. (B) Body length of at week 3. (C) Body length and body mass index (BMI) at week 12. (D) Random blood glucose levels at week 16. (E) Body weight at day of birth. (F) Glucose tolerance test (GTT) of females at week 16. (G) Insulin tolerance test (ITT) of females at week 20. (H) Area under the curve (AUC) of GTT and ITT. (I) Body length at week 24. The value under the bar graphs indicates n for each group. Body length was determined by measuring the nasal-toanal distance. Values are presented as the mean± standard error of the mean. *P< 0.05, †P< 0.01, ‡P< 0.001 vs. control (Ctrl) within the same sex.
DISCUSSION
In this study, we developed two mouse models in order to recapitulate the human SGA phenotypes accompanying failure of catch-up growth and dysregulated metabolic outcomes in adulthood; the strength of model 3 lies in its more significant phenotypes, and that of model 4 is in the better yield without cross-fostering.
Adequate delivery of amino acids from mother to fetus throughout placenta is necessary for proper growth and development. Suggested mechanisms of PRD-induced SGA include impaired uterine secretions, impaired cell signaling in mother and fetus, reduced placental angiogenesis with reduced supply of nutrients from mother to fetus, which all contribute to a vicious cycle.5 Previous reports demonstrated other SGA models using PRD, but the body length results were omitted6,7 or strains other than C57BL/6 were evaluated.8 The final two models are relatively non-invasive and easy to perform without special equipment compared to modeling by surgical procedures during pregnancy9 or a cesarean section.6
Known underlying reasons for SGA include intrinsic fetal factors, placental insufficiency, other maternal disorders and even infection.1 Our models cannot recapitulate all of the etiologies of SGA, limiting their utility. If needed, researchers can use more relevant models for specific purposes, such as hypoxia models10 or modeling with other species.11 As the C57BL/6 mouse is the most widely used genetic strain in the field of metabolic research,12 these models can be adopted properly according to the purpose of translational study of each researcher.
ACKNOWLEDGMENTS
This study was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean Government (NRF-2020R1A4A3078962 to OK), by grant No. 16-2018-010 from the SNUBH research fund (to JHK), by the Creative-Pioneering Researchers Program through Seoul National University (to OK), and by a grant (to OK, 2019F-4) from the Korean Diabetes Association.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: JHK and OK; acquisition of data: HSM and HK; analysis and interpretation of data: HSM, HK, JHK, and OK; drafting of the manuscript: HSM, HK, JHK, and OK; critical revision of the manuscript: BK and MSK; statistical analysis: HSM and OK; obtaining funding: JHK and OK; and study supervision: JHK and OK.
REFERENCES
- 1.Blencowe H, Krasevec J, de Onis M, Black RE, An X, Stevens GA, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2019;7:e849–60. doi: 10.1016/S2214-109X(18)30565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong YH, Lee JE. Large for gestational age and obesity-related comorbidities. J Obes Metab Syndr. 2021;30:124–31. doi: 10.7570/jomes20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mericq V, Martinez-Aguayo A, Uauy R, Iñiguez G, Van der Steen M, Hokken-Koelega A. Long-term metabolic risk among children born premature or small for gestational age. Nat Rev Endocrinol. 2017;13:50–62. doi: 10.1038/nrendo.2016.127. [DOI] [PubMed] [Google Scholar]
- 4.de Zegher F, Ong KK, Ibáñez L, Dunger DB. Growth hormone therapy in short children born small for gestational age. Horm Res. 2006;65 Suppl 3:145–52. doi: 10.1159/000091520. [DOI] [PubMed] [Google Scholar]
- 5.Herring CM, Bazer FW, Johnson GA, Wu G. Impacts of maternal dietary protein intake on fetal survival, growth, and development. Exp Biol Med (Maywood) 2018;243:525–33. doi: 10.1177/1535370218758275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhasin KK, van Nas A, Martin LJ, Davis RC, Devaskar SU, Lusis AJ. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes. 2009;58:559–66. doi: 10.2337/db07-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin LJ, Meng Q, Blencowe M, Lagarrigue S, Xiao S, Pan C, et al. Maternal high-protein and low-protein diets perturb hypothalamus and liver transcriptome and metabolic homeostasis in adult mouse offspring. Front Genet. 2018;9:642. doi: 10.3389/fgene.2018.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Almeida Silva LC, de Oliveira AC, Cavalcante-Silva V, Franco M, D'Almeida V. Hyperlipidic diet affects body composition and induces anxiety-like behaviour in intrauterine growth-restricted adult mice. Exp Physiol. 2020;105:2061–72. doi: 10.1113/EP088859. [DOI] [PubMed] [Google Scholar]
- 9.Coe BL, Kirkpatrick JR, Taylor JA, vom Saal FS. A new 'crowded uterine horn' mouse model for examining the relationship between foetal growth and adult obesity. Basic Clin Pharmacol Toxicol. 2008;102:162–7. doi: 10.1111/j.1742-7843.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- 10.Jang EA, Longo LD, Goyal R. Antenatal maternal hypoxia: criterion for fetal growth restriction in rodents. Front Physiol. 2015;6:176. doi: 10.3389/fphys.2015.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanson AM, David AL. Animal models of fetal growth restriction: considerations for translational medicine. Placenta. 2015;36:623–30. doi: 10.1016/j.placenta.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Fontaine DA, Davis DB. Attention to background strain is essential for metabolic research: C57BL/6 and the International Knockout Mouse Consortium. Diabetes. 2016;65:25–33. doi: 10.2337/db15-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]