Abstract
Regarding the influence of obesity on general health of children, scientific literature showed the importance of early management of children's overweight. In terms of oral health, overweight and obese children were shown to have accelerated dental development and increased prevalence of caries. Objectives. The aim of the present study is to evaluate the influence of Body Mass Index (BMI) and dietary behaviors on the oral status, eruptions and hygiene in children. Material and Methods. Ninety-two children aged 6-12 years were investigated about the oral hygiene habits, nutritional related behaviors, presence of systemic diseases. Values of BMI and oral status were noted. Statistical analysis was performed. Results. Significant moderate correlations between the values of BMI with tooth eruption and weak but significantly with dental lesions were determined. Conclusions. The dietary behaviors and BMI could influence the oral status, eruptions and oral hygiene in children.
Keywords: Oral health, body mass index
Introduction
There are systemic conditions that can be associated with oral and periodontal changes such as diabetes, obesity, cardio-vascular and hepatic diseases, rheumatoid arthritis [1].
Regarding the influence of obesity on general health of children, results showed the importance of early management of children's overweight, and parental education should start from the management of weight gain during pregnancy [2].
Most obese children have one or more obesity-related systemic conditions, such as diabetes, high blood pressure, dyslipidemia, hepatic steatosis, obstructive sleep apnea syndrome, and mental disorders [3].
Childhood obesity is also a well-known behavioral risk factor in terms of the general health of the adult, being associated with many chronic diseases of the adult, such as diabetes, cardiovascular disease, neoplasms [4,5].
For the diagnosis of obesity, there are no ideal measurements indicators, often clinical trials cannot benefit from the usefulness of the measurements used by practitioners.
These inconsistencies make it difficult to implement data from specialized studies and their use in diagnostic and management strategies for overweight and childhood obesity [6].
Body Mass Index (BMI) is the most widely used indicator of obesity (National Institute for Clinical Excellence, 2006) [7].
BMI can overestimate the degree of overweight, especially in underweight and tall children.
Alternative or complementary weight assessment methods, such as waist/hip ratio, dual energy X-ray absorptiometry (DEXA), and the estimation of the percentage of adipose tissue calculated at the folds of the skin may be more accurate, but more complex and difficult to use [6].
Although many studies have reported a positive correlation between childhood obesity and gingival inflammation, some studies have shown no association in the growing patient, with inaccurate results due to insufficient data analyzed [8].
There are results showing that BMI displayed a modest but statistically significant correlation with the number of affected teeth. This finding, although preliminary, is consistent with the current understanding that obesity is associated with the development of a systemic inflammatory state in recent reports on a significant correlation between periodontitis and BMI in adults [9].
Overweight and obese children were shown to have accelerated dental development that may also affect their orthodontic and pediatric treatment planning that is highly affected by timing [10].
It was investigated, analyzing the panoramic radiographies, the prevalence of dental anomalies in different body mass index (BMI) child and adolescent subjects.
The prevalence of root dilaceration was significantly greater in obese and overweight subjects than in normal-weight subjects [11].
BMI could be related to the diabetes in adult and children patients [12,13,14,15,16].
It is stated that type 1 diabetes is not only a disorder in cases of poor glycemic control, but it is an important factor to consider in children in planning of interceptive and periodontal orthodontic therapy, due to its influence on the formation process of the dental and periodontal structures, the moment of the dental eruption and the duration of the treatment [17].
The aim of the present study is to evaluate the influence of BMI and dietary behavior on the oral status, eruptions and oral hygiene in children.
Material and Method
In this study, 92 patients were included, presented for various dental complaints in a dental office from Galati, Galati County, Romania, 51 women and 41 males, age range 6-12 years.
The patients were oral-periodontal examined and the teeth in the process of eruption, with carious lesions, the presence of debris and extractions were noted.
Presence of dental plaque and calculus was assessed.
The Body Mass Index was calculated dividing the weight in kilograms by the square of height in meters.
The tutors’ patients filled a chart with relevant information about the oral hygiene habits, nutritional-related pattern of children, systemic diseases: diabetes, respiratory diseases, anemia, vitamin D deficiency.
Following parameters were used in the statistical analysis:
• age,
• gender,
• number of erupting teeth,
• number of dental lesions,
• consumption of fruits/vegetables/cereals /milk and dairy products/meat and dairy products/sweets: 1-daily, 2-at least twice a week, 3-occasional
• food rhythm: increased food intake in the first-1/second part of the day-2,
• number of meals per day-1, 2, 3
• snacks between meals.
• brushing frequency-0, 1, 2, 3/day
• presence of calculus
• BMI value
The Ethical Research Committee of University of Medicine and Pharmacy of Craiova approved the study (no.14/21.01.2019) and the patients’ tutors signed the informed consent.
Statistical Analysis
Statistical analyses were performed using the GraphPad version 9.3 (San Diego, CA, USA) Graph Pad Software The mean, standard deviation (SD), and median were used to represent continuous variables (interquartile range, IQR).
The discrete variables were presented as number and percentage.
Spearman correlations between patient characteristics (continuous variables) were analyzed.
Checking for normality was done using Kolmogorov-Smirnov test.
Two-sided p-value of less than 0.05 was considered statistically significant.
Results
The mean age was 9.33±3.52, 55.4% was female and 44.6% male and the environment of living was predominantly urban.
The mean weight was 36.64±14.20 and BMI 18.25±3.34.
The habits of eating, represented by the consumption of fruits, vegetables and snacks between meals were highlighted in the patients included in our study (Table 1).
Table 1.
Demographic, clinical and nutritional-related pattern characteristics in patients
|
Characteristics |
Total (n=92) |
|
Age, years Mean±SD Median, SQRT |
9.33±2.52 9 (6-12) |
|
Gender, n (%) Female Male |
51 (55.4%) 41 (44.6%) |
|
Environment, n (%) Urban Rural |
72 (82.6%) 20 (17.4%) |
|
Weight, kg Median±SD Median, SQRT |
36.64±14.20 35.6 (25-44) |
|
Hight, cm Median±SD Median, SQRT |
138.5± 15.58 140.5 (125.3-150.8) |
|
BMI Median±SD Median, SQRT |
18.25± 3.34 18.10 (16.7-19.98) |
|
Brushing 0 1 2 3 |
3 (3.3%) 44 (47.8%) 37 (40.2%) 8 (8.7%) |
|
Calculus |
74 (80.4%) |
|
Intake of food Fruits Vegetables Cereals Milk Meat Sweats |
1=39 (42.4%), 2=44 (47.8%), 3=9 (9.8%) 1=38 (41.3%), 2=41 (44.6%), 3=13 (14.1%) 1=23 (25%), 2=40 (41.5%), 3=29 (31.5%) 1=29 (31.5%), 2=47 (51.1%), 3=15 (16.3%) 1=44 (47.8%), 2=40 (43.5%), 3=7 (7.6%) 1=41 (44.6%), 2=31 (33.7%), 3=12 (13%) |
|
Food rhythm 1, yes |
42 (45.7%) |
|
Food rhythm 2, yes |
50 (54.3%) |
|
Meals number per day 1 2 3 |
6 (6.5%) 39 (42.4%) 47 (51.1%) |
|
Snacks, yes |
63 (68.5%) |
|
Eruptions |
0.96± 1.22 |
|
Systemic diseases Diabetes |
23(25%) 1(1.08%) |
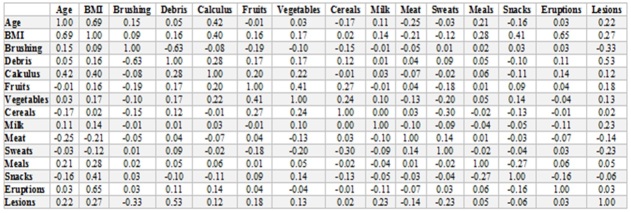
There were strong and significant correlations between the values of BMI with age (ρ=0.69, p<0.0001) and moderate and significant with calculus (ρ=0.40, p=0.0002).
BMI values have also been moderate and significantly correlated with snacks and tooth eruption (ρ=0.65, p=0.043) and weak but significantly with dental lesions (ρ=0.27, p=0.007).
A significant correlation (p=0.043) was recorded between BMI and food rhythm 2, and between food rhythm 2 and lesions (p=0.005).
The general status was not found to be correlated neither with the number of eruptions (ρ=-0.102, p=0.335) nor the number of lesions (ρ=-0.091, p=0.331).
These data are detailed in the Table 2 and 3.
Tabel 2 .
Correlations coefficients between the parameters analyzed
Table 3.
Significances of correlations (*=p<0.05 statistically significant)
|
|
BMI correlations |
p-value |
|
Age |
0.69 |
<0.0001* |
|
Brushing |
0.09 |
0.03* |
|
Debris |
0.16 |
0.179 |
|
Calculus |
0.40 |
0.0002* |
|
Fruits |
0.16 |
0.038* |
|
Vegetables |
0.17 |
0.13 |
|
Cereals |
0.02 |
0.09 |
|
Milk |
0.14 |
0.87 |
|
Meat |
-0.21 |
0.19 |
|
Sweats |
0.12 |
0.04 |
|
Meals |
0.28 |
0.26 |
|
Snacks |
0.41 |
0.043* |
|
Eruptions |
0.65 |
0.043* |
|
Lesions |
0.27 |
0.007* |
|
Food rhythm1 |
0.247 |
0.431 |
|
Food rhythm2 |
0.653 |
0.043* |
The presence of calculus has been significantly correlated with age (ρ=0.42, p=0.002) and BMI.
We could not establish correlations between BMI values and systemic diseases.
Matrix correlation between eruptions, lesions and general status of the patients included in our study are presented in Figure 1.
Figure 1.
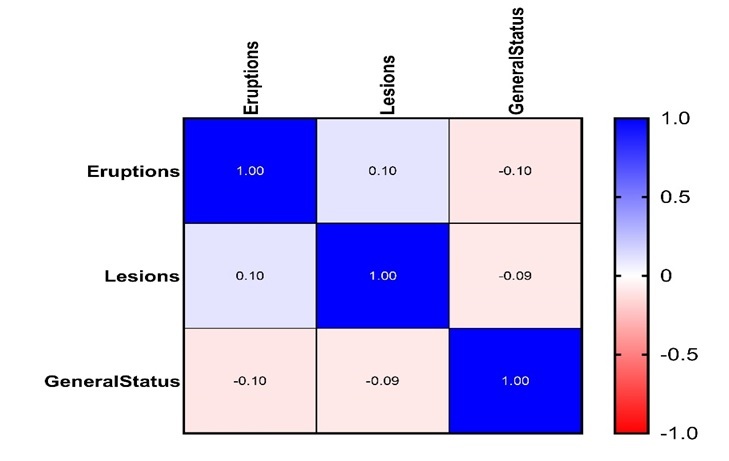
Heatmap of the correlation matrix between general status of patients and the number of dental lesions and eruptions
The positive and negative correlations are represented by the color range from blue (strong positive correlations; i.e. r=1.0) to red (strong negative correlation; i.e. r=-1.0).
Discussions
In the present study, we could not establish correlations between the assessed parameters and systemic diseases, even 25% of patients had an association with a systemic pathology.
We find only one children suffers from diabetes, even is one of the most prevalent recognized association with obesity and with oral and periodontal changes [15].
The association between diabetes mellitus and periodontal attachment and bone loss were studied by Lalla E. in 2007, authors founding higher plaque index for patients with diabetes mellitus than non-diabetic and higher percentage of sites with plaque [18] and the number of affected teeth was significantly higher in diabetic children versus control children in both age-subgroups, <12 years of age or >12 year of age.
The percentage of children with poor metabolic control was significantly higher and BMI-for-age were similar in the two groups, but BMI was significantly higher in the older group compared with the younger one [19].
A weak association was seen between accelerated eruption and increased BMI percentile in diabetic children [20].
In our study, we obtained similar data in systemic healthy children with significant correlation between BMI and eruptions and food rhythm and highly significant between age and BMI, regardless a systemic diagnostic.
In a study published in 2012 the relationship between the numbers of permanent teeth erupted and weight status was examined by using three categories of children: normal weight, overweight, and obese.
Obesity status, gender (female), and age were all shown to be substantially linked with the number of permanent teeth erupting.
After adjusting for age, sex, and race/ethnicity, obese patients erupted with 1.44 teeth more than non-obese patients [21].
Accelerated eruption and increased BMI were associated in children, these findings support the claim that obese children had an accelerated dental development in terms of erupted permanent tooth number at 12 years of age [22].
A three-year study was undertaken on digital panoramic radiographs of patients aged 6-12 years and found significantly higher values for the mean age of patients with at least one dental anomaly compared to patients with no anomaly who presented with a mean age of 7.90 years.
Permanent teeth had a prevalence of 60.5%, which was significantly higher than primary teeth, which had a prevalence of 3.3% [23].
In the study conducted by Wong et al. and published in 2017, on a group of patients of which 20% of children were considered overweight, according to the WHO’s BMI for age criteria, they found significant correlations between erupted permanent tooth number and three types of anthropometric measurements, including BMI [22], while our results revealed also that the children with a higher BMI had an accelerated eruption.
It was demonstrated a significant association between caries frequency and obesity in school children.
The importance of obesity should not only be emphasised with respect to general diseases but also with regard to carious lesions [24].
Poor oral hygiene and low frequency of brushing is associated with dental lesions and in our study, poor oral status and hygiene manifested by the presence of debris and calculus deposits has been significantly correlated with the presence of dental lesions.
Obesity causes a faster maturation in children who develop teeth faster, and experience cases of tooth eruption more often than normal-weight children of the same age [25].
It was also showed that the underweight could be a risk factor for delayed tooth eruption in children of 8-11years [26].
In a study of Abdullatif et al. about the prevalence of overweight and dietary behaviors among adolescents, the prevalence of unhealthy dietary habits from 30 days prior to the survey was common, 21.3% of adolescents reported not eating fruits and 19.7% not eating vegetables.
Regarding the dairy consumption, 3.3% of adolescents did not drink milk or consume dairy products in the 7 days prior to the survey, 31% consumed a carbonated drink daily, and 18.4% did not eat breakfast. Eating fast food was high as 78.9% reported eating fast food one or more days [27].
Aiming to find out associations between eruption times of permanent teeth and consumption of meat, rice, vegetables and milk in children, it was shown that more frequent use of meat showed a trend of early eruption of permanent teeth [28].
The results of our study showed that the most children eat meat daily, while fruits and vegetables, more rarely, at least twice at weak, but no correlation was found with any of parameters. Sixty-three percent of our patients consume snacks between meals.
Significant correlation was found between food rhythm (food intake in the second part of the day) and dental lesions.
Conclusions
The dietary behaviors and BMI could influence the oral health in children.
Conflict of interests
None to declare.
References
- 1.Surlin P, Foia L, Solomon S, Popescu DM, Gheorghe DN, Camen A, Martu MA, Rauten AM, Olteanu M, Pitru A, Toma V, Popa S, Boldeanu MV, Martu S, Rogoveanu I. In: Cytokines. Behzadi P, editor. Zagreb Croatia: IntechOpen; 2020. Cytokines’ involvement in periodontal changes. [Google Scholar]
- 2.Brown CL, Halvorson EE, Cohen GM, Lazorick S, Skelton JA. Addressing childhood obesity: opportunities for prevention. Pediatric Clinics. 2015;62(5):1241–1261. doi: 10.1016/j.pcl.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. J Family Med Prim Care. 2015;4(2):187–187. doi: 10.4103/2249-4863.154628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Prev Med. 1993;22(2):167–177. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- 5.Boyer BP, Nelson JA, Holub SC. Childhood body mass index trajectories predicting cardiovascular risk in adolescence. J Adolesc Health. 2015;56(6):599–605. doi: 10.1016/j.jadohealth.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyson N. Childhood and adolescent obesity definitions as related to bmi, evaluation and management options. Best Pract Res Clin Obstet Gynaecol. 2018;48:158–164. doi: 10.1016/j.bpobgyn.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Tosh JC, Longworth LG, George E. Utility values in National Institute for Health and Clinical Excellence (NICE) Technology Appraisals. Value Health. 2011;14(1):102–109. doi: 10.1016/j.jval.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Nascimento GG, Seerig LM, Vargas‐Ferreira F, Correa FO, Leite FR, Demarco FF. Are obesity and overweight associated with gingivitis occurrence in brazilian schoolchildren. J Clin Periodontol. 2013;40(12):1072–1078. doi: 10.1111/jcpe.12163. [DOI] [PubMed] [Google Scholar]
- 9.Kubo M, Iida M, Yamashita Y. Relationshipbetween obesity, glucose tolerance, and periodontal disease in Japanese women:the Hisayama study. J Periodontal Res. 2005;40:346–353. doi: 10.1111/j.1600-0765.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 10.Hilgers KK, Akridge M, Scheetz JP, Kinane DF. Childhood obesity and dental development. Pediatr Dent. 2006;28:18–22. [PubMed] [Google Scholar]
- 11.Simsek H, Korkmaz YN, Buyuk SK. Relationship between obesity and prevalence of dental anomalies: Does body mass index play a role. Eur J Paediatr Dent. 2019;20(2):95–99. doi: 10.23804/ejpd.2019.20.02.02. [DOI] [PubMed] [Google Scholar]
- 12.Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diab Rep. 2014;14(8):508–508. doi: 10.1007/s11892-014-0508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbasi A, Juszczyk D, van Jaarsveld CHM, Gulliford MC. Body mass index and incident type 1 and type 2 diabetes in children and young adults: a retrospective cohort study. J Endocr Soc. 2017;1(5):524–537. doi: 10.1210/js.2017-00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray N, Picone G, Sloan F, Yashkin A. Relation between BMI and diabetes mellitus and its complications among US older adults. South Med J. 2015;108(1):29–36. doi: 10.14423/SMJ.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of comorbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88–107. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 17.Almadih A, Al-Zayer M, Dabel S, Alkhalaf A, Al Mayyad A, Bardisi W, Alsihati Z. Orthodontic Treatment Consideration In Diabetic Patients. J Clin Med Res. 2018;10(2):77–77. doi: 10.14740/jocmr3285w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalla E, Cheng B, Lal S, Kaplan S, Softness B, Greenberg E, Goland RS, Lamster IB. Diabetes Mellitus Promotes Periodontal Destruction In Children. J Clin Periodontol. 2007;34:294–298. doi: 10.1111/j.1600-051X.2007.01054.x. [DOI] [PubMed] [Google Scholar]
- 19.Lalla E, Cheng B, Lal S, Tucker S, Greenberg E, Goland R, Lamster IB. Periodontal Changes in Children and Adolescents with Diabetes. Diabetes Care. 2006;29(2):295–299. doi: 10.2337/diacare.29.02.06.dc05-1355. [DOI] [PubMed] [Google Scholar]
- 20.Lal S, Cheng B, Kaplan S, Softness B, Greenberg E, Goland RS, Lalla E, Lamster IB. Accelerated tooth eruption in children with diabetes mellitus. Pediatrics. 2008;121(5):e1139–43. doi: 10.1542/peds.2007-1486. [DOI] [PubMed] [Google Scholar]
- 21.Must A, Phillips SM, Tybor DJ, Lividini K, Hayes C. The Association between Childhood Obesity and Tooth Eruption. Obesity (Silver Spring) 2012;20(10):2070–2074. doi: 10.1038/oby.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong HM, Peng SM, Yang Y, King NM, McGrath CPJ. Tooth eruption and obesity in 12-year-old children. J Dent Sci. 2017;12(2):126–132. doi: 10.1016/j.jds.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner VP, Arrué T, Hilgert E, Arús NA, da Silveira HLD, Martins MD, Rodrigues JA. Prevalence and distribution of dental anomalies in a pediatric population based on panoramic radiographs analysis. Eur J Paediatr Dent. 2020;21(4):292–298. doi: 10.23804/ejpd.2020.21.04.7. [DOI] [PubMed] [Google Scholar]
- 24.Sakeenabi B, Swamy HS, Mohammed RN. Association between obesity, dental caries and socioeconomic status in 6- and 13-year-old school children. Oral Health Prev Dent. 2012;10(3):231–41. [PubMed] [Google Scholar]
- 25.Boston University Theses & Dissertations, 2017, Alsulaiman AT. The effect of obesity on tooth development in children and adolescents. Available from: http://open.bu.edu[Accessed 09.11.2021]
- 26.Reis CLB, Barbosa MCF, Henklein S, Madalena IR, de Lima DC, Oliveira M, Kuchler EC, de Oliveira DSB. Nutritional Status is Associated with Permanent Tooth Eruption in a Group of Brazilian School Children. Glob Ped Health. 2021;8:2333794X211034088–2333794X211034088. doi: 10.1177/2333794X211034088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdullatif M, AlAbady K, Altheeb A, Rishmawi F, Jaradat H, Farooq S. Prevalence of overweight, obesity, and dietary behaviors among adolescents in Dubai schools: a complex design survey 2019. Dubai Med J. 2021:1–9. [Google Scholar]
- 28.Khan H, Khan N, Baloch MR, Abbasi SA. Effect of diet on eruption times for permanent teeth of children in Peshawar. Pak Oral Dent J. 2020;40(1):24–30. [Google Scholar]