Abstract
Objective. This study aimed to discover a new index for disease activity by reviewing the relationship between the Systemic Immune-Inflammation Index and Systemic Inflammation Response Index in rheumatoid arthritis. Method. A total of 109 patients with rheumatoid arthritis and 31 healthy controls were involved in the study. Based on disease activity score (DAS-28) calculated by the erythrocyte sedimentation rate, rheumatoid arthritis patients were divided into two groups: Group 1 comprised patients in remission (DAS-28<2.6); Group 2 was the active patient group (DAS-28>2.6). The Systemic Immune Inflammation Index and the Systemic Inflammation Response Index compared between the groups. Results. The Systemic Immune-Inflammation Index is 666.415±33.00 in the patient group and 596.71±57.64 in the control group, and the difference between the groups is statistically significant (p=0.002). The Systemic Immune-Inflammation Index was 574.69±34.72 in group 1 and 702.25±39.56 in group 2. There was a significant statistical difference between the active and remission patients (p=0.030). The Systemic Inflammation Response Index was not statistically significant between the groups. Different cut-off points were compared to detect the optimal cut-off value for SII. Based on the ROC curve analysis, SII cut-off point of 574.20 showed 56.3% sensitivity and 45.5% specificity and with the Area Under Curve (AUC) 95% was the optimal cut-off point for active RA. Conclusion. This is the first study to review the Systemic Immune-Inflammation Index in rheumatoid arthritis. The obtained conclusion verified that the Systemic Immune-Inflammation Index could be used as a new tool, showing disease activity.
Keywords: Systemic Immune-Inflammation Index, Systemic Inflammation Response Index, rheumatoid arthritis, disease activity score
Introduction
Rheumatoid arthritis (RA) is a disease with an unknown etiology and with a course including articular and extra-articular symptoms [1].
The prevalence of incidence in the world is 0.5-1% [2].
The disease causes loss of functions since it causes progressive destruction, primarily in joints. Systemic involvements being the most common cause of mortality. Early diagnosis and treatment are essential for preventing morbidity and mortality. Various laboratory tests are used to verify the diagnosis, determine the prognosis, detect the disease activity, and follow the treatment response. The most frequently used lab tests are for rheumatoid factor, anti-cyclic citrullinated peptide for disease diagnosis, and C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) for the determination of disease activity. The disease activity guides the treatment plan. The most frequent disease activity score (DAS-28) is used for evaluating the activity. DAS-28 is obtained by calculating the number of swollen joints, the number of tender joints, the visual analog scale of global health, and the CRP or ESR ratio. A DAS-28 value above 5.1 shows high disease activity; a value between 3.2-5.1 shows medium disease activity; a value between 2.6-3.2 shows low disease activity; and values lower than 2.6 are considered remission [3].
Complete blood count is simple, cheap and includes important follow-up parameters for many diseases. Neutrophils, lymphocytes, and monocytes play a crucial role in inflammation. The Systemic Immune-Inflammation Index (SII) and Systemic Inflammation Response Index (SIRI), obtained from those parameters, were found to be significant in terms of disease activity and prognosis, especially in patients with COVID-19 infection, inflammatory and cardiovascular diseases, and cancer [4,5,6].
There was no SII study of RA in the literatures which we searched. This shall be the first study to be conducted in RA. Only one study has been conducted on SIRI, and it displayed a significant relation to systemic inflammation; however, it was not related to RA disease activity [7].
The study reviewed the relationship between the SII, SIRI, and disease activity in RA patients and found a new index for the disease activity.
Material and Method
The retrospective study included 109 patients diagnosed with RA based on the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) diagnostic criteria [8] and 31 healthy controls. The healthy control group whose no inflammatory disease was formed of people with complete blood count, ESR and CRP records and was obtained from the hospital records. The RA group was formed of people with complete blood count, ESR, CRP records and global health score were obtained from the hospital records. The DAS-28 is obtained by calculating 0.56*sqrt (tender 28) +0.28*sqrt (swollen 28)+0.70*Ln (ESR)+0.014*global health;The SII is obtained by calculating (neutrophil*thrombocyte)/ lymphocyte; The SIRI: is obtained by calculating (neutrophil*monocyte)/lymphocyte.
Based on DAS-28 scores, RA patients were divided into two groups: group 1, the patients in remission (DAS-28<2.6); group 2, the active patient group (DAS-28>2.6). Those with cardiovascular disease, liver or kidney failure, malignancy, and other inflammatory and infectious diseases were excluded from the study.
The study protocol was approved by the Ethics Committee of Clinical Research of Harran University (HRU/21.07.25). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Statistical Analysis
SPSS 20.0 for Windows suit was used. Kolmogorov-Smirnov test was conducted for normality distribution analysis. Unpaired T-test was conducted for the normally distributed parameters, and the Mann-Whitney U test was conducted for the parameters that were not conforming to the distribution. The data are given as mean±standard deviation. Receiver operating characteristics (ROC) curve analysis was used to obtain the optimum cutoff value of the SII. Those with a P<0.05 value were accepted as significant as statistical.
Results
The average age of the patients were 46.69±1.11 years, and the average age of the controls were 46.74±1.16 years. The gender was 78% females and 22% males in the patient group and 64.5% females and 35.5% males in the control group. There were no significant difference age and gender between patients and control groups (p=0.070, p=0.100). The SII was 666.415±33.00 in the patient group and 596.71±57.64 in the control group, and the difference between the groups was statistically significant (p=0.002). SIRI value was determined as 1.39±0.06 in the patient group and as 1.33±0.10 in the control group. A statistical significance was not found between patients and controls groups (p=0.758). The SII, SIRI, ESR, CRP, and other hemogram parameters are summarized in Table 1.
Table 1.
Laboratory parameters of patients and control groups
|
|
Patients gruop (n=109) |
Control group (n=31) |
p |
|
Age |
49.69±1.11 |
46.74±1.16 |
0.070 |
|
Platelets, x109\L |
291.88±6.58 |
266.00±13.26 |
0.046 |
|
Leukocytes, x109\L |
8.20±0.22 |
8.20±0.35 |
0.874 |
|
Neutrophils, x109\L |
4.96±0.17 |
4.76±0.22 |
0.950 |
|
Lymhocytes, x109\L |
2.33±0.69 |
2.35±0.12 |
0.725 |
|
Monocytes, x109\L |
0.62±0.02 |
0.61±0.02 |
0.819 |
|
CRP, mg\dl |
1.22±0.18 |
0.38±0.05 |
0.000 |
|
ESR, mm\h |
24.75±1.69 |
14.29±1.44 |
0.002 |
|
SII, % |
666.415±33.00 |
596.71±57.64 |
0.002 |
|
SIRI, % |
1.39±0.06 |
1.33±0.10 |
0.758 |
|
CRP C reaktive protein, ESR erythrocyte sedimentation rate, SII Systemic Immune-Inflammation Index, SIRI Systemic Inflammation Response Index | |||
There was no statistically significant difference between remission and active patients groups in terms of age and sex (p=0.850, p=0.343). The SII was 574.69±34.72 in the remission and 702.25±39.56 in the active patients; this difference was significant (p=0.030). The SIRI was 1.19±0.11 and 1.43±0.70, respectively, and the difference was not statistically significant between the groups (p=0.850). The data between groups 1 and 2 are summarized in Table 2.
Table 2.
Laboratory parameters of patients groups
|
| |||
|
|
Group 1(n=22) |
Group 2 (87) |
p |
|
Age |
49.81±2.22 |
49.66±1.33 |
0.850 |
|
Gender |
16(72.7%)\6(27.3%) |
69(79.3%)\18(20.7%) |
0.343 |
|
CRP |
0.46±0.08 |
1.41±0.22 |
0.000 |
|
ESR |
11.50±1.55 |
28.10±1.93 |
0.000 |
|
SII |
574.69±34.72 |
702.25±39.56 |
0.030 |
|
SIRI |
1.19±0.11 |
1.43±0.70 |
0.850 |
|
CRP C reactive protein, ESR erythrocyte sedimentation rate, SII Systemic Immune-Inflammation Index, SIRI Systemic Inflammation Response Index | |||
Different cut-off points were compared to detect the optimal cut-off value for SII. Based on the ROC curve analysis, SII cut-off point of 574.20 showed 56.3% sensitivity and 45.5% specificity and with the Area Under Curve (AUC) 95 % was the optimal cut-off point for active RA (Table 3 and Figure 1).
Table 3.
ROC curve for SII (Systemic Immune-Inflammation Index)
|
|
AUC (95%) |
Cutt Off |
p |
Sensitivity% |
Specificity% |
|
SII |
0.643(0.534-0.753) |
574.20 |
0.039 |
56.3 |
45.5 |
Figure 1.
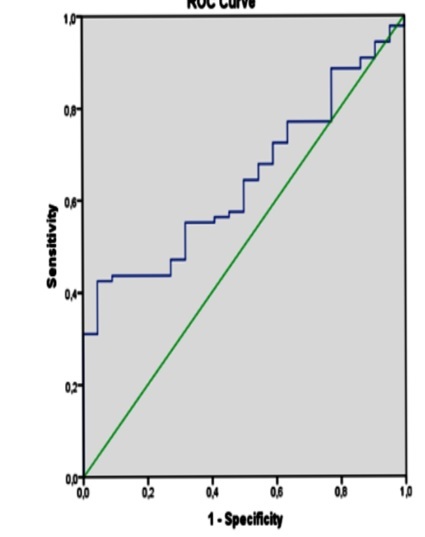
ROC curve for SII (Systemic Immune-Inflammation Index)
Discussion
SII and SIRI were evaluated with the disease activity in the RA patients in this study. The SII was higher in active RA patients than in remission RA patients. There was no difference in SIRI.
The studies confirme that platelets play an active role in the inflammation. Inflammation is a phenomenon generated by living tissue against pathological factors, detracting the pathogen, thereby and starting and accelerating the recovery period. Platelets are produced from megakaryocytes in the bone marrow and have no cores. They have a vital role in stopping bleeding and inflammation. Chemotaxis, proteolysis, and adhesion are formed by platelet activation in inflammation [9].
TNF-α, IL-1β, IL6, IL-8, which are important proinflammatory cytokines during inflammation, are released with platelet activation [10].
Monocytes and macrophages play critical roles in RA pathogenesis by increasing the release of proinflammatory cytokines such as TNF-α, IL-1, and IL-6 [11].
The presence of activate neutrophils in chronic inflammatory diseases (e.g., RA) induces cells that contribute to chronic inflammation. Many factors secreted by macrophages, lymphocytes, and fibroblasts influence this process. As a result, direct or indirect tissue damage occurs [12].
SII studies concerning bone, breast, kidney, and gynecologic cancers and coronary artery diseases, primarily gastrointestinal cancers, are available [13,14]. It was concluded that the same index could be a marker for the severity and survival in COVID-19 infection [15].
SII was observed to be high in adult-onset Still’s disease [16]. Similarly, emphasis was put on it can being used a biomarker for osteoporosis and osteoporotic fracture [17,18,19].
The studies have shown that SII can be an important marker to evaluate antineutrophil cytoplasmic antibody vasculitis and Behçet's disease [20,21].
It was concluded to be the determinant for the prognosis of psoriatic arthritis [22]. In our literature search, we did not find any study investigating SII in RA, therefore, this is the first study conducted in RA.It was observed that SIRI was significant as a prognostic factor in various cancers [23,24].
Only one study concerning RA was encountered, and this study was observed to be significantly related to systemic inflammation; however, it did not relate to RA disease activity [7].
At the end of the study, we concluded that there was no correlation to the disease activity.
The small number of patients and the failure to evaluated the DMARDs or biological agents used by the patients are the most important limitation of this study. Large number of patients who first diagnosed and used drugs and multicenter studies are needed.
Conclusions
This study is the first study in which the SII was evaluated in RA, and the obtained findings show that the SII may be used as a new index capable of displaying disease activity but SIRI not.
Conflict of interests
Conflict of interests
The author disclose no potential conflicts of interest.
Compliance with ethical standards
The study protocol was approved by the Ethics Committee of Clinical Research of Harran University (HRU/21.07.25). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Acknowledgments
Funding
The author received no financial and material support.
References
- 1.Erre GL, Buscetta G, Paliogiannis P, Mangoni AA, Carru C, Passiu G, Zinellu A. Coronary flow reserve in systemic rheumatic diseases: a systematic review and meta-analysis. Rheumatol Int. 2018;38(7):1179–1190. doi: 10.1007/s00296-018-4039-8. [DOI] [PubMed] [Google Scholar]
- 2.Tobón GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J Autoimmun. 2010;35(1):10–14. doi: 10.1016/j.jaut.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 3.van Riel PL. The development of the disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28) Clin Exp Rheumatol. 2014;32(5 Suppl 85):S65–S74. [PubMed] [Google Scholar]
- 4.Huang H, Liu L, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y. Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Patients with Cervical Cancer. Sci Rep. 2019;9(1):3284–3284. doi: 10.1038/s41598-019-39150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fois AG, Paliogiannis P, Scano V, Cau S, Babudieri S, Perra R, Ruzzittu G, Zinellu E, Pirina P, Carru C, Arru LB, Fancellu A, Mondoni M, Mangoni AA, Zinellu A. The Systemic Inflammation Index on Admission Predicts In-Hospital Mortality in COVID-19 Patients. Molecules. 2020;25(23):5725–5725. doi: 10.3390/molecules25235725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei L, Xie H, Yan P. Prognostic value of the systemic inflammation response index in human malignancy: A meta-analysis. Medicine (Baltimore) 2020;99(50):e23486–e23486. doi: 10.1097/MD.0000000000023486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erre GL, Buscetta G, Mangoni AA, Castagna F, Paliogiannis P, Oggiano M, Carru C, Passiu G, Zinellu A. Diagnostic accuracy of different blood cells-derived indexes in rheumatoid arthritis: A cross-sectional study. Medicine (Baltimore) 2020;99(44):e22557–e22557. doi: 10.1097/MD.0000000000022557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 9.Gawaz M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc Res. 2004;61(3):498–511. doi: 10.1016/j.cardiores.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 10.Kaplanski G, Farnarier C, Kaplanski S, Porat R, Shapiro L, Bongrand P, Dinarello CA. Interleukin-1 induces interleukin-8 secretion from endothelial cells by a juxtacrine mechanism. Blood. 1994;84(12):4242–4248. [PubMed] [Google Scholar]
- 11.Cooles FA, Isaacs JD. Pathophysiology of rheumatoid arthritis. Curr Opin Rheumatol. 2011;23(3):233–240. doi: 10.1097/BOR.0b013e32834518a3. [DOI] [PubMed] [Google Scholar]
- 12.Haynes J, Garcia B, Stollar EJ, Rath A, Andrews BJ, Davidson AR. The biologically relevant targets and binding affinity requirements for the function of the yeast actin-binding protein 1 Src-homology 3 domain vary with genetic context. Genetics. 2007;176(1):193–208. doi: 10.1534/genetics.106.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J Cancer. 2018;9(18):3295–3302. doi: 10.7150/jca.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Çirakoğlu ÖF, Yılmaz AS. Systemic immune-inflammation index is associated with increased carotid intima-media thickness in hypertensive patients. Clin Exp Hypertens. 2021;15(1):1–7. doi: 10.1080/10641963.2021.1916944. [DOI] [PubMed] [Google Scholar]
- 15.Muhammad S, Fischer I, Naderi S, Faghih Jouibari M, Abdolreza S, Karimialavijeh E, Aslzadeh S, Mashayekhi M, Zojaji M, Kahlert UD, Hänggi D. Systemic Inflammatory Index Is a Novel Predictor of Intubation Requirement and Mortality after SARS-CoV-2 Infection. Pathogens. 2021;10(1):58–58. doi: 10.3390/pathogens10010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JW, Jung JY, Suh CH, Kim HA. Systemic immune-inflammation index combined with ferritin can serve as a reliable assessment score for adult-onset Still's disease. Clin Rheumatol. 2021;40(2):661–668. doi: 10.1007/s10067-020-05266-2. [DOI] [PubMed] [Google Scholar]
- 17.Fang H, Zhang H, Wang Z, Zhou Z, Li Y, Lu L. Systemic immune-inflammation index acts as a novel diagnostic biomarker for postmenopausal osteoporosis and could predict the risk of osteoporotic fracture. J Clin Lab Anal. 2020;34(1):e23016–e23016. doi: 10.1002/jcla.23016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang ZC, Jiang W, Chen X, Yang L, Wang H, Liu YH. Systemic immune-inflammation index independently predicts poor survival of older adults with hip fracture: a prospective cohort study. BMC Geriatr. 2021;21(1):155–155. doi: 10.1186/s12877-021-02102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du YN, Chen YJ, Zhang HY, Wang X, Zhang ZF. Inverse association between systemic immune-inflammation index and bone mineral density in postmenopausal women [published online ahead of print, 2021 Feb 16] Gynecol Endocrinol. 2021;2(16):1–5. doi: 10.1080/09513590.2021.1885642. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, Choi H, Jung SM, Song JJ, Park YB, Lee SW. Systemic immune-inflammation index could estimate the cross-sectional high activity and the poor outcomes in immunosuppressive drug-naïve patients with antineutrophil cytoplasmic antibody-associated vasculitis. Nephrology (Carlton) 2019;24(7):711–717. doi: 10.1111/nep.13491. [DOI] [PubMed] [Google Scholar]
- 21.Tanacan E, Dincer D, Erdogan FG, Gurler A. A cutoff value for the Systemic Immune-Inflammation Index in determining activity of Behçet disease. Clin Exp Dermatol. 2021;46(2):286–291. doi: 10.1111/ced.14432. [DOI] [PubMed] [Google Scholar]
- 22.Yorulmaz A, Hayran Y, Akpinar U, Yalcin B. Systemic Immune-Inflammation Index (SII) Predicts Increased Severity in Psoriasis and Psoriatic Arthritis. Curr Health Sci J. 2020;46(4):352–357. doi: 10.12865/CHSJ.46.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei L, Xie H, Yan P. Prognostic value of the systemic inflammation response index in human malignancy: A meta-analysis. Medicine (Baltimore) 2020;99(50):e23486–e23486. doi: 10.1097/MD.0000000000023486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Zhou Y, Xia S. Prognostic value of the systemic inflammation response index (SIRI) before and after surgery in operable breast cancer patients. Cancer Biomark. 2020;28(4):537–547. doi: 10.3233/CBM-201682. [DOI] [PubMed] [Google Scholar]


