Abstract
Focal cortical dysplasia is a malformation of cortical development in which there are abnormalities with cortical lamination, neuronal maturation, and neuronal differentiation. It is the most common cause of medically refractory epilepsy in the pediatric population and the second/third most common etiology of medically intractable seizures in adults. Herein, we present the case of 23-years-old female patient, presenting with loss of consciousness, and convulsions. A MRI revealed a 5mm cortical thickening on either side of the posterior aspect of the right superior temporal gyrus without transmantle extension towards ventricle. This abnormal area is measured about 24x16mm and there was no evidence for mesial temporal sclerosis. Both hippocampi are normal is size, morphology and signal. These features are consistent with cortical dysplasia type 1. This case report emphasizes the importance of MRI in the detection of FCD. MRI can show no abnormalities in type 1 FCD, but when the changes are apparent, they are on the temporal lobe, and seizures presents most commonly in adults.
Keywords: Focal cortical dysplasia, cortical development, neuronal maturation, hippocampus
Introduction
Epilepsy is a clinical condition manifested with seizures.
The seizures can be partial or complex and they can affect young patients as well as older ones.
Generally, the diagnosis of epilepsy is clinical, especially if it has started at a young age or is a generalized seizure.
However, the diagnosis of epilepsy should be provided with etiology determination.
The causes of epilepsy can be a stroke, severe head injury, brain tumor, brain infection, alcohol, and drug abuse, or vascular malformations.
Therefore, imaging radiology plays an essential role in etiology determination for epilepsy.
MRI is the modality of choice for epilepsy, most often investigating for an underlying cause, especially in adults.
MRI is usually performed with and without contrast to examine the brain tissue and detect possible structural changes associated with the underlying seizure.
Specifically, Type I and Type IIa FCDs have a much higher association with MRI-negative epilepsy due to their difficulty in detection compared with Type IIb FCD, which more commonly manifests with greater gray matter thickening, subcortical white matter signal abnormality, and blurring of the GM-WM boundary [1].
Despite major improvement and resolution in MRI of epilepsy, detection of type I FCD remains quite challenging.
Treatment depends on the etiology, but in most cases is primarily with antiepileptic medications.
In refractory cases, especially when a causative lesion can be identified, temporal lobectomy can be effective, provided cases are carefully selected.
Case Report
A young woman, 23 years of age, presents at the neurologic clinic with complaints of loss of consciousness and seizures.
The neurologist suspects epilepsy and refers the patient to undergo an MRI examination.
The techniques used with the patient: Pre-contrast MRI images of the brain were obtained using TSE/T2W sequence in the axial plane; TSE/T1W sequence in the sagittal plane; FLAIR/T2W sequence in the axial plane; thin slice TSE/T2W, FLAIR/T2W, and Flash/T2W sequences in the oblique coronal plane (perpendicular to temporal lobes).
Post-contrast images were acquired using TSE/T1W sequence in axial, coronal, and sagittal planes.
Diffusion-weighted and ADC mapping MRI images were acquired using EPI sequence in the axial plane.
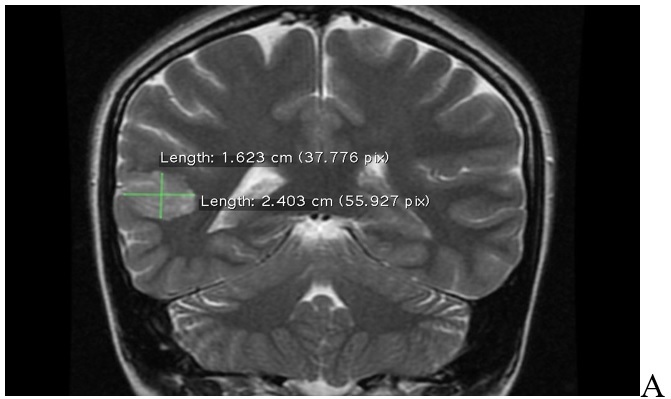
The non-contrast MRI revealed a 5mm thickening on either side of the posterior aspect of the right superior temporal sulcus (involving the posterior aspects of both superior temporal gyrus and middle temporal gyrus).
This abnormal area of possible cortical dysplasia was measured about 24x16mm.
MRI characteristics were consistent with Blumcke Type I (Taylor's type) cortical dysplasia, "without transmantle extension" towards the ventricle.
The thickening on the posterior aspect of the right superior temporal sulcus detected in the axial plane of MRI.
The contrast MRI revealed no pathologic contrast enhancement in the area (Figure 1A,1B,1C,1D).
Figure 1.
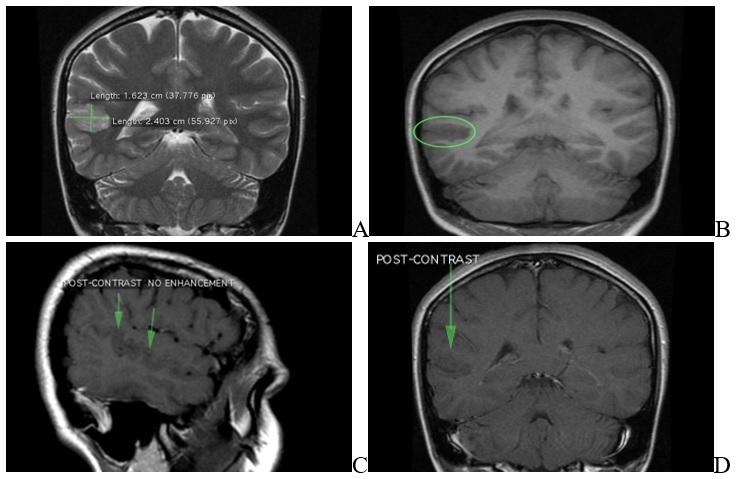
MRI of brain sequence. A: FLAIR axial plane results: cortical thickening up to 5mm on either side of posterior aspect of right superior temporal sulcus (involving the posterior aspects of both superior temporal gyrus and middle temporal gyrus). This abnormal area of cortical dysplasia is measured about 24x16mm. B: FLAIR axial plane results: cortical thickening up to 5mm on either side of posterior aspect of right superior temporal sulcus (involving the posterior aspects of both superior temporal gyrus and middle temporal gyrus). This abnormal area of cortical dysplasia is measured about 24x16mm. C: FLAIR sagital plane results: cortical thickening up to 5mm on either side of posterior aspect of right superior temporal sulcus (involving the posterior aspects of both superior temporal gyrus and middle temporal gyrus). This abnormal area of cortical dysplasia is measured about 24x16mm. D: Sequence T2 coronal plane results: cortical thickening up to 5mm on either side of posterior aspect of right superior temporal sulcus (involving the posterior aspects of both superior temporal gyrus and middle temporal gyrus). This abnormal area of cortical dysplasia is measured about 24x16mm
In diffusion-weighted and ADC mapping images, there was no restricted diffusion area that suggested acute infarction (Figure 2A,2B,2C,2D).
Figure 2.
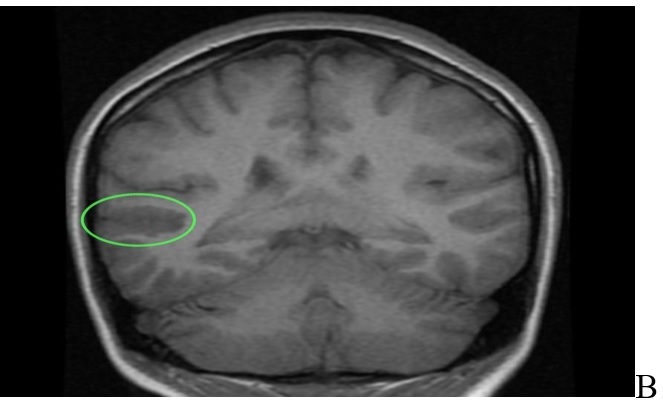
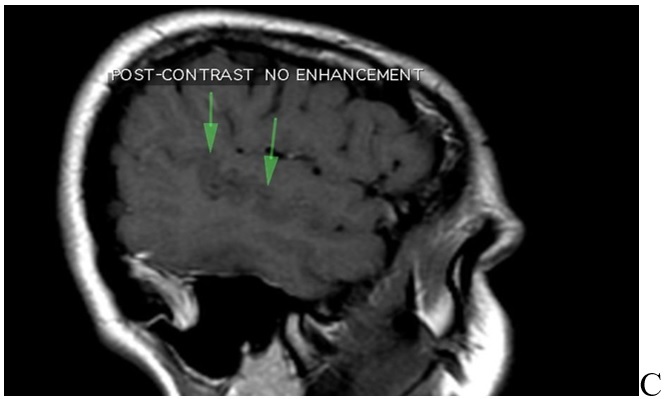
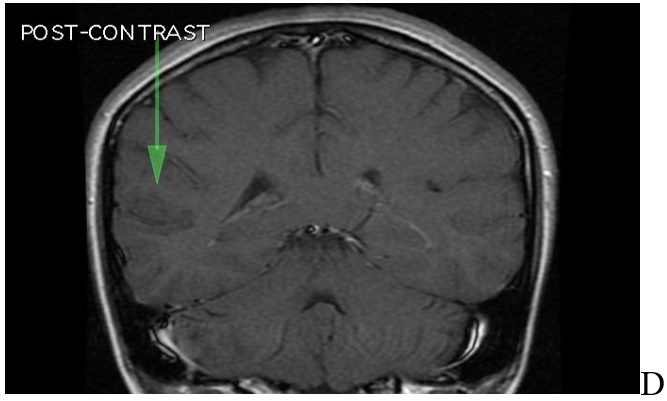
MRI of brain. A: T2 sequence coronal plane results: abnormal area of cortical dysplasia is measured about 24x16mm, consistent with Blumcke Type I (Taylor’s type) cortical dysplasia, “without transmantle extension” towards ventricle. B: Sequence T1 axial plane without contrast results: cortical thickening up to 5mm on either side of posterior aspect of right superior temporal sulcus (involving the posterior aspects of both superior temporal gyrus and middle temporal gyrus). This abnormal area of cortical dysplasia is measured about 24x16mm. C: T1 sequence sagital plane post contrast, no pathologic contrast enhancement is identified. D: T1 sequence coronal plane post contrast, no pathologic contrast enhancement is identified
Both hippocampi were normal in size, morphology, and signal.
There was no evidence for mesial temporal sclerosis.
Discussion
In 2011, the Task Force on Focal Cortical Dysplasia proposed a three-tiered classification scheme for patients with Palmini Type 1 FCDs.
The proposed criteria included the designation of subtypes 1 and 3, respectively [1].
The histopathological characteristic of this type of cortical layering includes an abnormal radial cortical layer with microcolumnar structure, abnormal tangential layering, and horizontal dyslamination.
For FCD type 1, the histopathological evaluation of specimens is usually focused on the missing cellular or cytoarchitectural features.
This type of FCD is prone to over-diagnosis.
The histopathology of focal lesions in epileptic patients is not considered a variant of normal.
In some cases, hematopathology is required to show structural defect despite the absence of any abnormality [2].
FCD 1 is a physiological state that occurs during the first half of gestation.
It has been hypothesized that this process is a mechanism of pathogenesis.
FCD 1A exhibits a micro-columnar architecture in the cerebral cortex, which is often linked to the development of metabolic and genetic diseases.
The cortex adjacent to a porencephalic cyst shows a foetal micro-columnar structure.
It is believed that this feature is secondary to chronic ischemia [3,4].
The FCD Type 1 subgroup has been severely limited by the existence of other entities that can diagnose the condition.
This group has been re-examined through the reevaluation of histopathological examinations and clinical reports.
Mild malformation of the cortical development is a major differential diagnosis of FCD Type 1.
It is characterized by the presence of oligodendroglioma (MOGHE). Both are known to cause childhood-onset epilepsy [5,6].
To summarize, FCD 1A is a deformity of cortical development, lamination, and maturation, and DNA methylation analyses able to identify FCD 1A from other FCD variations [5] FCD could be caused by both a germline and a somatic mutation affecting DEPDC5 in a subpopulation of brain cells, implying a biallelic 2-hit mutational mechanism [7,8].
The most common null variations in FCD patients were nonsense and frameshift [1,2,3], while missense variants have also been found [9,10].
Because loss-of-function mutations in DEPDC5 cause mTORC1 hyperactivation, as indicated by increased phosphorylated S6 immunostaining in resected FCD tissue [8], mTORC1 inhibitors, such as rapamycin, could be used as a targeted treatment.
Electrophysiologic recordings may indicate distant secondary zones of ictal activity (intraictal activation) capable of self-sustaining epileptogenesis even in MRI-positive dysplastic lesions [11].
If these abnormal electrophysiologic networks are not properly comprehended and removed simultaneously with the primary focus, surgical failure can occur [11].
Intraictally activated zones might be contiguous, neighboring, extra lobar, or contralateral to the central epileptogenic zone.
Their anatomical placement is unusual, and it is possible that they do not correspond to fascicular routes [12].
Intraictal activation happens instead as a result of abnormal neural networks being activated directly in the cortex [13].
As a result, dysplastic cortex, functional engagement in the epileptogenic network.
There is a substantial challenge in precisely characterizing functional areas because there is no single “gold standard” test; each test has limitations.
Noninvasive fMRI and magneto-encephalography (MEG) reveal classical and atypical distributed functional networks; however, they may be suppressed incorrectly in the postictal period.
Transcranial magnetic stimulation is becoming more popular, however its accuracy in identifying interhemispheric eloquent regions is unknown.
It is critical to understand these limitations in order to get the most out of tests.
For example, null activation or activation patterns that are not consistent with classical language processing or motor areas should be considered unreliable, and a Wada test or electrocortical stimulation mapping for either lateralization or localization should be required prior to resection [14].
As a result, surgical resection techniques in FCD should be individually adjusted for each patient.
Although neuronal plasticity in the very young leads to bigger resections with the goal of optimizing seizure control, older children tactics tend to favor smaller, more restricted resections to lower the danger of new impairments, albeit at the expense of seizure freedom.
Awake surgery combined with clinical testing, or cortical and subcortical electrocortical stimulation mapping combined with tractography, can help maximize resection and safety while minimizing eloquent function loss.
Resecting the subcortical transmantle tail in people with FCD type IIb is rarely necessary since it puts their function at risk without improving their chances of seizure independence [15].
Conclusion
Cortical dysplasia’s are potent epileptic foci responsible for intractable or recalcitrant seizures.
It should be suspected when loss of consciousness or convulsions happens in children or young adults.
For these cases should be ordered an MRI, which can detect the characteristics of FCD such as cortical thickening or thinning, blurring of white matter-gray matter junction with abnormal architecture of subcortical layer, transmantle sign, altered signal from gray matter and so on. MRI may show no abnormalities in type 1 dysplasia.
But when the changes are on the temporal lobe without extension in other parts of the brain and the symptoms occur in an adult age, it is highly suggestive of type 1 FCD.
Conflict of interests
None to declare.
References
- 1.Blümcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, Jacques TS, Avanzini G, Barkovich AJ, Battaglia G, Becker A, Cepeda C, Cendes F, Colombo N, Crino P, Cross JH, Delalande O, Dubeau F, Duncan J, Guerrini R, Kahane P, Mathern G, Najm I, Ozkara C, Raybaud C, Represa A, Roper SN, Salamon N, Schulze-Bonhage A, Tassi L, Vezzani A, Spreafico R. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52(1):158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120(Pt 4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- 3.Sarnat HB, Flores-Sarnat L. Radial microcolumnar cortical architecture: maturational arrest or cortical dysplasia. Pediatr Neurol. 2013;48(4):259–270. doi: 10.1016/j.pediatrneurol.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Blümcke I, Sarnat HB, Coras R. Surgical neuropathology of focal epilepsies: textbook and atlas. Montrouge: John Libbey Eurotext. 2015 [Google Scholar]
- 5.Kobow K, Ziemann M, Kaipananickal H, Khurana I, Mühlebner A, Feucht M, Hainfellner JA, Czech T, Aronica E, Pieper T, Holthausen H, Kudernatsch M, Hamer H, Kasper BS, Rössler K, Conti V, Guerrini R, Coras R, Blümcke I, El-Osta A, Kaspi A. Genomic DNA methylation distinguishes subtypes of human focal cortical dysplasia. Epilepsia. 2019;60(6):1091–1103. doi: 10.1111/epi.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonduelle T, Hartlieb T, Baldassari S, Sim NS, Kim SH, Kang HC, Kobow K, Coras R, Chipaux M, Dorfmüller G, Adle-Biassette H, Aronica E, Lee JH, Blumcke I, Baulac S. Frequent SLC35A2 brain mosaicism in mild malformation of cortical development with oligodendroglial hyperplasia in epilepsy (MOGHE) Acta Neuropathol Commun. 2021;9(1):3–3. doi: 10.1186/s40478-020-01085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baulac S, Ishida S, Marsan E, Miquel C, Biraben A, Nguyen DK, Nordli D, Cossette P, Nguyen S, Lambrecq V, Vlaicu M, Daniau M, Bielle F, Andermann E, Andermann F, Leguern E, Chassoux F, Picard F. Familial focal epilepsy with focal cortical dysplasia due to DEPDC5 mutations. Ann Neurol. 2015;77(4):675–683. doi: 10.1002/ana.24368. [DOI] [PubMed] [Google Scholar]
- 8.Ribierre T, Deleuze C, Bacq A, Baldassari S, Marsan E, Chipaux M, Muraca G, Roussel D, Navarro V, Leguern E, Miles R, Baulac S. Second-hit mosaic mutation in mTORC1 repressor DEPDC5 causes focal cortical dysplasia-associated epilepsy. J Clin Invest. 2018;128(6):2452–2458. doi: 10.1172/JCI99384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldassari S, Picard F, Verbeek NE, van Kempen M, Brilstra EH, Lesca G, Conti V, Guerrini R, Bisulli F, Licchetta L, Pippucci T, Tinuper P, Hirsch E, de Saint Martin A, Chelly J, Rudolf G, Chipaux M, Ferrand-Sorbets S, Dorfmüller G, Sisodiya S, Balestrini S, Schoeler N, Hernandez-Hernandez L, Krithika S, Oegema R, Hagebeuk E, Gunning B, Deckers C, Berghuis B, Wegner I, Niks E, Jansen FE, Braun K, de Jong D, Rubboli G, Talvik I, Sander V, Uldall P, Jacquemont ML, Nava C, Leguern E, Julia S, Gambardella A, d'Orsi G, Crichiutti G, Faivre L, Darmency V, Benova B, Krsek P, Biraben A, Lebre AS, Jennesson M, Sattar S, Marchal C, Nordli DR, Lindstrom K, Striano P, Lomax LB, Kiss C, Bartolomei F, Lepine AF, Schoonjans AS, Stouffs K, Jansen A, Panagiotakaki E, Ricard-Mousnier B, Thevenon J, de Bellescize J, Catenoix H, Dorn T, Zenker M, Müller-Schlüter K, Brandt C, Krey I, Polster T, Wolff M, Balci M, Rostasy K, Achaz G, Zacher P, Becher T, Cloppenborg T, Yuskaitis CJ, Weckhuysen S, Poduri A, Lemke JR, Møller RS, Baulac S. The landscape of epilepsy-related GATOR1 variants. Genet Med. 2019;21(2):398–408. doi: 10.1038/s41436-018-0060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Chen ZR, Xu HQ, Liu DT, Mao Y, Liu HK, Liu XR, Zhou P, Lin SM, Li B, He N, Su T, Zhai QX, Meng H, Liao WP, Yi YH. DEPDC5 Variants Associated Malformations of Cortical Development and Focal Epilepsy With Febrile Seizure Plus/Febrile Seizures: The Role of Molecular Sub-Regional Effect. Front Neurosci. 2020;14:821–821. doi: 10.3389/fnins.2020.00821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayakar P, Duchowny M, Resnick TJ. Subdural monitoring in the evaluation of children for epilepsy surgery. J Child Neurol. 1994;9(Suppl 2):61–66. [PubMed] [Google Scholar]
- 12.Thiebaut de Schotten M, Kinkingnéhun S, Delmaire C, Lehéricy S, Duffau H, Thivard L, Volle E, Levy R, Dubois B, Bartolomeo P. Visualization of disconnection syndromes in humans. Cortex. 2008;44(8):1097–1103. doi: 10.1016/j.cortex.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Duchowny M, Jayakar P, Levin B. Aberrant neural circuits in malformations of cortical development and focal epilepsy. Neurology. 2000;55(3):423–428. doi: 10.1212/wnl.55.3.423. [DOI] [PubMed] [Google Scholar]
- 14.Jayakar P, Gaillard WD, Tripathi M, Libenson MH, Mathern GW, Cross JH, Task Force for Paediatric Epilepsy Surgery. Commission for Paediatrics, and the Diagnostic Commission of the International League Against Epilepsy Diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia. 2014;55(4):507–518. doi: 10.1111/epi.12544. [DOI] [PubMed] [Google Scholar]
- 15.Wagner J, Urbach H, Niehusmann P, von Lehe M, Elger CE, Wellmer J. Focal cortical dysplasia type IIb: completeness of cortical, not subcortical, resection is necessary for seizure freedom. Epilepsia. 2011;52(8):1418–1424. doi: 10.1111/j.1528-1167.2011.03158.x. [DOI] [PubMed] [Google Scholar]


