Abstract
Objective
To describe and analysed the functional outcome (FO) after spinal meningioma (SM) surgery.
Methods
We processed the système national des données de santé (SNDS) i.e. , the French national administrative medical database to retrieve appropriate cases. We analysed the International Classification of Diseases 10 codes to assess the FO. Logistic models were implemented to search for variables associated with a favourable FO i.e. , a patient being independent at home without disabling symptom.
Results
A total of 2,844 patients were identified of which 79.1% were female. Median age at surgery was 66 years, interquartile range (IQR) (56–75). Ninety-five point nine percent of the SMs were removed through a posterior ± lateral approach and 0.7% need an associated stabilisation. Benign meningioma represented 92.9% and malignant 2.1%. Median follow-up was 5.5 years, IQR (2.1–8), and at data collection 9% had died. The FO was good and increased along the follow-up: 84.3% of the patients were alive and had not associated symptoms at one year, 85.9% at 2 and 86.8% at 3 years. Nonetheless, 3 years after the surgery 9.8% of the alive patients still presented at least one disabling symptom of which 2.7% motor deficit, 3.3% bladder control problem, and 2.5% gait disturbance. One point seven percent were care-provider dependent and 2.1% chair or bedfast. In the multivariable logistic regression an older age at surgery (odds ratio [OR], 0.37; 95% confidence interval [CI], 0.29–0.47, p<0.001), a high level of comorbidities (OR, 0.71; 95% CI, 0.66–0.75, p<0.001), and an aggressive tumor (OR, 0.49; 95% CI, 0.33–0.73; p<0.001) were associated with a worse FO.
Conclusion
FO after meningioma surgery is favourable but, may be impaired for older patients with a high level of comorbidities and aggressive tumor.
Keywords: Spinal meningioma, Epidemiology, Functional outcome, Predictors, Healthcare database
INTRODUCTION
Thought to arise from the meningothelial cells of the arachnoid, meningiomas are the most common primary intracranial extracerebral tumors accounting for 36.8%–37.6% in the Central Brain Tumor Registry of the United States [1]. Those developed in the vertebral canal are less frequent representing about 5% to 10% of all meningiomas [2-4]. Nonetheless, meningioma are the most common intradural tumor of the spine with 30.7%, the main differential diagnosis being schwannoma [4]. Spinal meningiomas (SM) are usually sporadic but few genetic diseases such as type 2 neurofibromatosis are identified risk factors [5]. The 2016 World Health Organization (WHO) classification of tumors affecting the central nervous system (CNS) recognises 3 grades of meningiomas [6]. WHO grade I or benign meningiomas are the most common and have usually a good outcome [7-9]. Management options include regular monitoring especially for incidental tumors, symptom control, surgical excision, radiotherapy (RT) and occasionally chemotherapy but, tailored maximal resection is the treatment of choice for SM. Further optimal management is difficult to establish; the role of RT as standard adjuvant treatment remaining controversial apart for the rare malignant forms [10-12]. Administrative medical databases (AMDB) are massive repositories of collected healthcare data for various purposes. AMDB provide a variety of already stored data with a constant and often increasing on-going collection process [13]. Available data on functional outcome (FO) after spinal meningioma surgery are restricted by the limited number of patients assessed, usually below 100 cases, short follow-up times as well as by the diversity of scales utilised. Several different classifications developed for others pathologies of the spine or its content were used by the authors who reported on FO after spinal meningioma surgery such as the modified McCormick score [14,15]. Such studied are usually made on selected population ensuring low statistical power with biases related to the representativity of a sample [2]. In addition, a number of previous studies reported contradictory data on the effects of histopathological grade and preoperative neurological impairment on outcome following spinal meningioma surgery [16]. Furthermore, even though the incidence of SM is greater in the elderly population, there may be a reluctance to operate on these patients due to an expected higher risk of adverse events and poorer outcome [17]. Studies on this topic are limited by lack of a younger control group.
The aim of this study was to describe the FO after SM surgery and search for associated prognostic factors: in this nationwide population-based cohort study, we retrospectively analysed cases of surgically treated SM order to assess baseline data, long-term clinical outcomes, predictors of neurological improvement and potential differences between elderly and non-elderly patients.
MATERIALS AND METHODS
1. Clinical Material
We performed a cross-sectional nationwide descriptive observational and analytic retrospective study using the système national des données de santé (SNDS), the national French medico-administrative database. Incidental SM never operated are not considered in this study; only surgically treated SMs were taken into account. Patients who underwent the surgical resection of a meningioma between the first of January 2008 and to the 31 December 2017 were included. Cases were selected using an algorithm combining 2 variables as described previously: the type of the surgical procedure identified by the Common Classification of Medical Acts and the primary diagnosis according to the International Classification of Diseases (ICD-10) [3,5,18,19]. Benign meningiomas were considered as corresponding to the D32 ICD-10 codes, atypical to D42 and malignant to C70. We defined the first recorded date of SM surgery as the index date. Age was classified into elderly (≥ 70 years) and non-elderly (18–69 years). Patients below 18 years were excluded (n=22). The mortality-related morbidity index (MRMI) predictive of all-cause mortality and the expenditure-related morbidity index predictive of health care expenditure were used to assess the global health-state severity [20]. These 2 weighted morbidity indices summarize the association between a set of conditions identified through algorithms using SNDS data and each outcome. They have been validated in the French context against the most used morbidity indices and offer the possibility to choose a morbidity measure adapted to the outcome under study. Further details about the conditions included as predictors, corresponding weights as well as prevalence among the study population are available as Supplementary materials (https://assurance-maladie.ameli.fr/sites/default/files/2021_indices-morbidite_cartographie.pdf).
2. Assessment for FO
We analysed the ICD-10 codes to assess the FO: 135 codes such as G82.0 flaccid paraplegia or N31.2 flaccid neuropathic bladder were classified in 7 distinct categories of symptoms or diagnostics as follows: (1) bladder disturbance, (2) care-provider dependence, (3) gait disturbance, (4) motor impairment and muscle weakness, (5) sensory alteration, (6) unspecified symptoms of spinal cord compression, (7) rehabilitation care. Unspecified symptoms of spinal cord compression include both radiological findings and unspecific or undisclosed signs. Each dates related to a specific ICD code were worked out to obtain a timeline for each patient. A total of 111,925 different observations were processed by a complex program to get a 3-dimensional-like picture of diagnostics’ evolution across the time, before, at and after the spinal meningioma surgery. A good FO was assessed by a composite variable as a patient being independent at home, excluding thus, dead patients, patients being still in rehabilitation, patients hospitalised or patients still having physiotherapy at the time considered for symptoms in relation with the SM.
3. Statistical Methods
For the cohort description, continuous variables are reported as medians and interquartile ranges (IQRs); categorical variables are reported as frequencies and proportions. For continuous variables, the Mann-Whitney-Wilcoxon rank-sum test was used for between-group comparisons and for categorical data, the chi-square test was applied. A univariable and stepwise multivariable logistic regression model was employed to identify predictors of a good FO defined above. Variables were included in the multivariable regression if they had a p-value<0.15 in the univariable model. In essence, there is no lost to follow-up patient in the SNDS are those who died are automatically registered as such in the database. All tests were 2-sided and statistical significance was defined with an alpha level of 0.05 (p<0.05). Analysis was performed using the SAS Enterprise Guide (ver. 7.15 HF8, SAS Institute Inc., Cary, NC, USA) and the R programming language and software environment for statistical computing and graphics (R ver. 4.1.2 [2021-11-01]) [21]. The statistical programme and workflow were written in R Markdown v2 with RStudio for dynamic and reproducible research [22].
4. Compliance With Ethical Standards
This study was conducted according to the ethical guidelines for epidemiological research in accordance with the ethical standards of the Helsinki Declaration (2008), to the French data protection authority (CNIL) an independent national ethical committee, authorisation number: 2008538; to the RECORD guidelines for studies conducted using routinely-collected health data and, according to the SAMPL Guidelines [23,24]. Informed consent was not required due to the retrospective nature of the study and the use of anonymised data, in accordance with the European General Data Protection Regulation (GRPD EU 2016/679).
RESULTS
1. Population Description
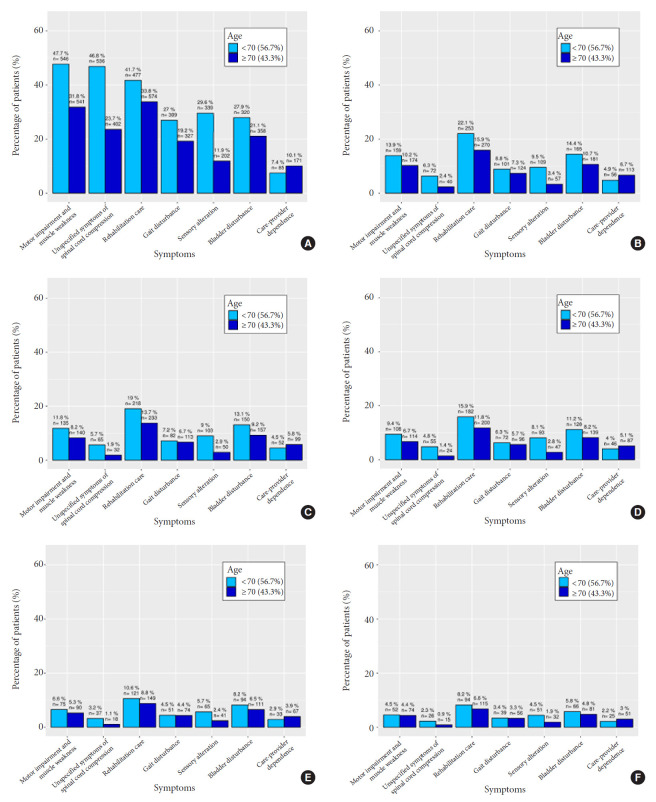
Within the SNDS, we identified 2,844 patients who had SM surgery between 2008 and 2017. 79.1% of the patients were female and the median age surgery was 66 years, IQR (56–75) (Table 1). The level of comorbidity sccording to the MRMI index increased with the age (p<0.001). Nine point four percent of the patients already had or were in rehabilitation before the SM removal, and this was more likely for elderly (16.2%) versus “young” patients (4.8%) (p<0.001) but, the probability of needing rehabilitation care before the SM surgery was also greater for patients with a high level of comorbidities (p<0.001). Motor impairment and muscle weakness was the most common symptom at SM diagnosis (38.2%), followed by the need of rehabilitation care (37%) and unspecified symptoms of spinal cord compression (33%) (Table 1, Fig. 1A). At SM diagnosis, young patients had significantly more sensory symptoms compared to elderly patients which on contrary, were more likely having rehabilitation cares (37%) and were also significantly more care-providers dependent (9%) (p<0.001 and p<0.001) (Table 1, Fig. 1A). Time between symptoms onset and surgery was significantly longer in elderly 239 days, IQR (670.5–67) compared to young patients with 181.5 days, IQR (710.6–36) (p=0.264). Ninety-five nine percent of the SM were removed through a posterior or posterior lateral approach, 7% were epidural and 0.7% need an associated stabilisation of the spine. Benign meningioma represented 92.9%, atypical 5% and malignant 2.1% (Table 1). Median hospital stay was 8 days, IQR (7–13). Fifty-nine point one percent of the patients were discharged at home, 24.8% to a rehabilitation unit and the others transferred to another hospital ward. Unsurprisingly, patients’ hospital stay was longer for those needed a rehabilitation (12 days vs. 7 days, p<0.001), also for the patients with a higher level of comorbidities (11 days vs. 8 days, p<0.001) and for elderly (10 days vs. 8 days, p<0.001). The patients discharged home were significantly younger compared to those transferred to a rehabilitation unit: 62 years vs. 72 years (p<0.001) and had significantly less comorbidities (p<0.001). Median follow-up was 5.5 years, IQR (2.1–8).
Table 1.
Characteristics of the 2,844 patients for the whole cohort, for patients below 70 years, and for those above 70 years
| Characteristic | Whole cohort | Nonelderly < 70 years (n = 1,699) | Elderly ≥ 70 years (n = 1,145) | p-value | |
|---|---|---|---|---|---|
| Sex female | 2,251 (79.1) | 1,334 (78.5) | 917 (80.1) | 0.335 | |
| Symptoms at surgery | |||||
| Motor impairment and muscle weakness | 1,087 (38.2) | 519 (30.5) | 568 (49.6) | < 0.001* | |
| Rehabilitation care | 1,051 (37) | 449 (26.4) | 602 (52.6) | < 0.001* | |
| Unspecified symptoms of spinal cord compression | 938 (33) | 517 (30.4) | 421 (36.8) | < 0.001* | |
| Bladder disturbance | 678 (23.8) | 301 (17.7) | 377 (32.9) | < 0.001* | |
| Gait disturbance | 636 (22.4) | 289 (17) | 347 (30.3) | < 0.001* | |
| Sensory alteration | 541 (19) | 321 (18.9) | 220 (19.2) | 0.869 | |
| Care-provider dependence | 256 (9) | 77 (4.5) | 179 (15.6) | < 0.001* | |
| Symptom severity | 1 (0–3) | 1 (0–3) | 2 (0–3) | < 0.001* | |
| Previous in hospital admission | 623 (21.9) | 260 (15.3) | 363 (31.7) | < 0.001* | |
| Physiotherapy before the surgery | 1,950 (68.6) | 1,108 (65.2) | 842 (73.5) | < 0.001* | |
| Prior rehabilitation | 266 (9.4) | 81 (4.8) | 185 (16.2) | < 0.001* | |
| Need of rehabilitation 30 days prior the surgery | 316 (11.1) | 106 (6.2) | 210 (18.3) | < 0.001* | |
| Need of rehabilitation 90 days prior the surgery | 277 (9.7) | 94 (5.5) | 183 (16) | < 0.001* | |
| Surgical delay more than 30 days | 147 (5.2) | 62 (3.6) | 85 (7.4) | < 0.001* | |
| Surgical delay more than 90 days | 38 (1.3) | 22 (1.3) | 16 (1.4) | 0.947 | |
| Age at surgery (yr) | 66 (56–75) | 58 (49–75) | 77 (73–75) | NA | |
| Age at surgery (yr) | |||||
| < 50 | NA | NA | NA | NA | |
| > 50 & < 59 | 539 (19) | 539 (31.7) | NA | NA | |
| > 60 & < 69 | 765 (26.9) | 705 (41.5) | NA | NA | |
| ≥ 70 | 1,085 (38.2) | NA | 60 (5.2) | NA | |
| Neurofibromatosis (NF2) | 25 (0.9) | NA | NA | < 0.001* | |
| Mortality-related morbidity index | 1 (0–2) | 0 (0–2) | 1 (0–2) | < 0.001* | |
| Expenditure-related morbidity index | 2 (0–9) | 0 (0–9) | 5 (0–9) | < 0.001* | |
| Surgical approach or technique | |||||
| Posterior approach | 2,728 (95.9) | 1,627 (95.8) | 1,101 (96.2) | 0.670 | |
| Anterior approach | 116 (4.1) | 72 (4.2) | 44 (3.8) | 0.670 | |
| Epidural meningioma | 198 (7) | 131 (7.7) | 67 (5.9) | 0.067 | |
| Spinal fixation | 20 (0.7) | 16 (0.9) | 4 (0.3) | 0.104 | |
| Tumor grading | |||||
| Benign | 2,641 (92.9) | 1,561 (91.9) | 1,080 (94.3) | - | |
| Atypical | 143 (5) | 98 (5.8) | 45 (3.9) | - | |
| Malignant | 60 (2.1) | 40 (2.4) | 20 (1.7) | 0.044* | |
| Length of hospital stay (day) | 8 (7–13) | 8 (6–13) | 10 (8–13) | < 0.001* | |
| Transfer to rehabilitation unit after SM surgery | 706 (24.8) | 294 (17.3) | 412 (36) | < 0.001* | |
| Need of physiotherapy after the surgery | |||||
| Any time | 859 (30.2) | 489 (28.8) | 370 (32.3) | 0.049* | |
| At 1 year | 631 (22.2) | 365 (21.5) | 266 (23.2) | 0.292 | |
| At 2 years | 523 (18.4) | 296 (17.4) | 227 (19.8) | 0.116 | |
| At 3 years | 443 (15.6) | 243 (14.3) | 200 (17.5) | 0.026* | |
| Good functional outcome | |||||
| At 1 year | 2,398 (84.3) | 1,534 (90.3) | 864 (75.5) | < 0.001* | |
| At 2 years | 2,442 (85.9) | 1,559 (91.8) | 883 (77.1) | < 0.001* | |
| At 3 years | 2,468 (86.8) | 1,574 (92.6) | 894 (78.1) | < 0.001* | |
| Death | |||||
| Within the postoperative month | 8 (0.3) | 2 (0.1) | 6 (0.5) | 0.0999 | |
| Within the 3 postoperative months | 16 (0.6) | 6 (0.4) | 10 (0.9) | 0.118 | |
| At 1 year | 37 (1.3) | 15 (0.9) | 22 (1.9) | 0.0260* | |
| At last follow-up | 257 (9) | 70 (4.1) | 187 (16.3) | < 0.001* | |
| Follow-up (yr) | 5.5 (2.1–8) | 5 (1.5–7.7) | 6.2 (3.2–8.3) | < 0.001* | |
Values are presented as number (%) or median (interquartile range).
NA, not applicable; SM, spinal meningioma.
p<0.05, statistically significant difference.
Fig. 1.
Distribution of the patients’ symptoms with spinal meningioma at surgery (A), 3 months (B), 6 months (C), 1 year (D), 2 years (E), and 3 years (F) after the surgery for “young” patients below 70 years versus elderly above 70 years old.
2. Functional Outcomes
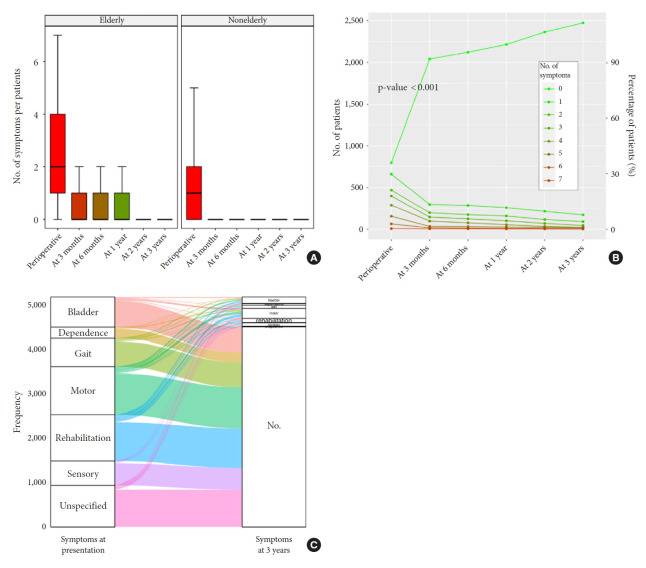
The FO was good and increased along the follow-up: 82.5% of the patients were alive and had recovered 3 months after the surgery. Eighty-three point six percent of the patients were symptoms free and were considered as having a good FO at 6 months, 84.3% at 1 year, 85.9% at 2 years, and 86.8% at 2 years and Fig. 1 shows the distribution of the patients’ symptoms at surgery, 3 months, 6 months, 1 year, 2 years, and 3 years after the surgery. A 3-dimensional scatter plot of the symptoms distribution over the time after SM surgery with the display of the associated regression plane shows a highly significant decreased of all symptoms over the time (p<0.001) (Fig. 2). Multiple pairwise-comparisons analysis over the time showed a dramatic improvement of all symptoms and deficit after SM resection (Friedman test, p<0.001; Mann-Whitney-Wilcoxon test, p<0.001) (Fig. 3). Nonetheless, 3 years after the surgery 9.8% of the alive patients still presented at least one disabling symptom of which 2.7% motor deficit, 3.3% bladder control problem, 2.5% gait disturbance. 1.7% were care-provider dependent, and 2.1% chair or bedfast. The patients who had poor functional status preoperatively i.e., chair or bedfast at surgery (30.1%) had a worse FO after SM resection whether at 1, 2 or 3 years (p<0.001, p<0.001, and p<0.001, respectively). Likewise, the patients who were care-provider dependent at surgery (9%) had a worse FO whether at 1, 2, or 3 years (p<0.001, p<0.001, and p<0.001, respectively). Elderly patients (40.3 %) demonstrate a worse FO after SM resection whether at 1 (75.5% vs. 90.3%), 2 (77.1% vs. 91.8%), or 3 years (78.1% vs. 92.6%) (p<0.001, p<0.001, and p<0.001, respectively). Additionally, Fig. 3A shows that elderly patients remain significantly more symptomatic over the time or at least that the functional recovery took more time which is in essence quite obvious. Our data showed that the functional recuperation is maximal within the first 3 or 6 months after the surgery and that the probability to recover decreased over the time (Fig. 3B). An alluvial or Sankey diagram which represents the outcome of each symptom at 2 years. It indicates that of all preoperative symptoms, the need of rehabilitation is the most commonly persistent at 3 years (Fig. 3C).
Fig. 2.
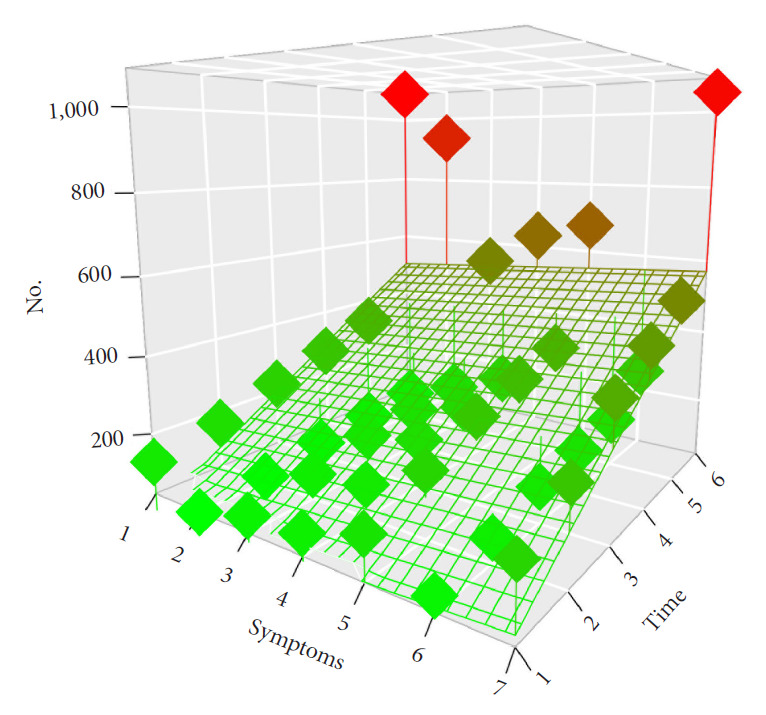
Three-dimensional scatter plot of the symptoms distribution over the time after spinal meningioma surgery with the display of the associated regression plane indicating a highly significant decreased of all symptoms over the time (p<0.001).
Fig. 3.
(A) Evolution of the number of symptoms per patients over the time for the elderly and for the nonelderly patients. (B) Variation of the number of symptoms over the time, for whole enrolled patients. (C) Alluvial or Sankey diagram which represents the outcome of each symptom at 3 years.
3. Predictors of the FO
In univariable logistic regression, many variables were predictors of the FO (Table 2). In multivariable analysis regression, the age at surgery, the level of comorbidities, and an aggressive tumor remained associated with a favourable FO (Table 3). The surgical technique nor the need for a spinal osteosynthesis did not influence the FO. Likewise, the severity of symptoms was not associated with a worse outcome in the multivariable regression.
Table 2.
Univariable logistic regression of good functional outcome after spinal meningioma surgery
| Variable | At 3 months |
At 6 months |
At 1 year |
At 2 years |
At 3 years |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95 % CI) | p-value | OR (95 % CI) | p-value | OR (95 % CI) | p-value | OR (95 % CI) | p-value | OR (95 % CI) | p-value | ||
| Male sex | 1.06 (0.84–1.36) | 0.623 | 1.09 (0.85–1.4) | 0.520 | 1.14 (0.89–1.48) | 0.310 | 1.01 (0.79–1.32) | 0.913 | 0.97 (0.75–1.27) | 0.827 | |
| Symptoms at surgery | |||||||||||
| Sensory alteration | 1.43 (1.1–1.88) | 0.009* | 1.61 (1.22–2.15) | 0.001* | 1.61 (1.22–2.17) | 0.0012* | 1.5 (1.12–2.03) | 0.008* | 1.54 (1.14–2.12) | 0.006* | |
| Motor impairment and muscle weakness | 0.55 (0.45–0.67) | < 0.001* | 0.58 (0.47–0.71) | < 0.001* | 0.58 (0.47–0.71) | < 0.001* | 0.58 (0.47–0.71) | < 0.001* | 0.56 (0.45–0.7) | < 0.001* | |
| Bladder disturbance | 0.63 (0.51–0.78) | < 0.001* | 0.68 (0.55–0.85) | < 0.001* | 0.67 (0.54–0.85) | < 0.001* | 0.66 (0.52–0.83) | < 0.001* | 0.65 (0.51–0.83) | < 0.001* | |
| Gait disturbance | 0.75 (0.6–0.94) | 0.012* | 0.8 (0.64–1.01) | 0.055 | 0.8 (0.63–1.01) | 0.059 | 0.77 (0.61–0.99) | 0.038* | 0.78 (0.61–1) | 0.048* | |
| Unspecified symptoms of spinal cord compression | 0.8 (0.66–0.98) | 0.033* | 0.86 (0.7–1.06) | 0.152 | 0.83 (0.67–1.03) | 0.081 | 0.82 (0.66–1.02) | 0.078 | 0.83 (0.66–1.04) | 0.0999 | |
| Rehabilitation care | 0.51 (0.42–0.62) | < 0.001* | 0.54 (0.44–0.66) | < 0.001* | 0.57 (0.46–0.7) | < 0.001* | 0.57 (0.46–0.71) | < 0.001* | 0.57 (0.45–0.7) | < 0.001* | |
| Care-provider dependence | 0.42 (0.32–0.57) | < 0.001* | 0.46 (0.34–0.62) | < 0.001* | 0.48 (0.36–0.66) | < 0.001* | 0.46 (0.34–0.63) | < 0.001* | 0.45 (0.33–0.62) | < 0.001* | |
| No. of symptoms | 0.84 (0.79–0.88) | < 0.001* | 0.86 (0.81–0.91) | < 0.001* | 0.86 (0.82–0.92) | < 0.001* | 0.86 (0.81–0.91) | < 0.001* | 0.85 (0.8–0.91) | < 0.001* | |
| Previous in-hospital admission within 3 months | 0.47 (0.38–0.58) | < 0.001* | 0.49 (0.39–0.6) | < 0.001* | 0.46 (0.37–0.58) | < 0.001* | 0.46 (0.36–0.57) | < 0.001* | 0.85 (0.8–0.91) | < 0.001* | |
| Prior rehabilitation | 0.49 (0.37–0.66) | < 0.001* | 0.56 (0.42–0.75) | < 0.001* | 0.54 (0.4–0.73) | < 0.001* | 0.53 (0.39–0.72) | < 0.001* | 0.52 (0.38–0.72) | < 0.001* | |
| Need of rehabilitation 30 days prior the SM surgery | 0.48 (0.37–0.62) | < 0.001* | 0.52 (0.4–0.69) | < 0.001* | 0.5 (0.38–0.66) | < 0.001* | 0.48 (0.36–0.64) | < 0.001* | 0.47 (0.36–0.64) | < 0.001* | |
| Need of rehabilitation 90 days prior the SM surgery | 0.5 (0.38–0.67) | < 0.001* | 0.55 (0.41–0.74) | < 0.001* | 0.53 (0.4–0.72) | < 0.001* | 0.52 (0.38–0.7) | < 0.001* | 0.51 (0.37–0.7) | < 0.001* | |
| Surgical delay more than 30 days | 0.48 (0.33–0.69) | < 0.001* | 0.47 (0.32–0.68) | < 0.001* | 0.49 (0.34–0.73) | < 0.001* | 0.55 (0.37–0.83) | 0.003* | 0.57 (0.38–0.88) | 0.009 | |
| Surgical delay more than 90 days | 0.4 (0.21–0.82) | 0.009* | 0.42 (0.21–0.86) | 0.014* | 0.45 (0.23–0.95) | 0.027 | 0.52 (0.26–1.18) | 0.094 | 0.57 (0.27–1.33) | 0.157 | |
| Age at surgery | 0.96 (0.95–0.97) | < 0.001* | 0.96 (0.95–0.97) | < 0.001* | 0.96 (0.95–0.97) | < 0.001* | 0.95 (0.95–0.96) | < 0.001* | 0.95 (0.94–0.96) | < 0.001* | |
| Age at surgery (4 categories) (ref. <50 yr) (yr) | |||||||||||
| > 50 & < 60 | 1 (0.66–1.53) | 0.990 | 0.96 (0.62–1.49) | 0.869 | 0.87 (0.55–1.37) | 0.558 | 0.9 (0.54–1.48) | 0.672 | 1.08 (0.64–1.81) | 0.775 | |
| > 60 & < 70 | 0.71 (0.49–1.03) | 0.076 | 0.7 (0.47–1.02) | 0.069 | 0.63 (0.42–0.94) | 0.029* | 0.59 (0.37–0.91) | 0.0200* | 0.68 (0.43–1.05) | 0.088 | |
| ≥ 70 | 0.28 (0.19–0.38) | < 0.001* | 0.27 (0.19–0.38) | < 0.001* | 0.25 (0.17–0.35) | < 0.001* | 0.22 (0.14–0.32) | < 0.001* | 0.23 (0.15–0.34) | < 0.001* | |
| Elderly (≥ 70 yr) vs. nonelderly (< 70 yr) | 0.34 (0.28–0.42) | < 0.001* | 0.34 (0.28–0.42) | < 0.001* | 0.33 (0.27–0.41) | < 0.001* | 0.3 (0.24–0.38) | < 0.001* | 0.28 (0.22–0.36) | < 0.001* | |
| Mortality-related morbidity index | 0.67 (0.63–0.71) | < 0.001* | 0.68 (0.63–0.72) | < 0.001* | 0.67 (0.63–0.72) | < 0.001* | 0.65 (0.61–0.7) | < 0.001* | 0.65 (0.61–0.69) | < 0.001* | |
| Expenditure-related morbidity index | 0.89 (0.88–0.91) | < 0.001* | 0.9 (0.88–0.91) | < 0.001* | 0.89 (0.88–0.91) | < 0.001* | 0.89 (0.87–0.91) | < 0.001* | 0.89 (0.87–0.9) | < 0.001* | |
| Surgical technique (ref. posterior or posterior lateral approach) | |||||||||||
| Anterior or anterior lateral approach | 0.77 (0.49–1.23) | 0.248 | 0.74 (0.48–1.2) | 0.203 | 0.7 (0.45–1.14) | 0.132 | 0.69 (0.44–1.14) | 0.129 | 0.64 (0.4–1.05) | 0.064 | |
| Epidural meningioma | 0.83 (0.58–1.2) | 0.309 | 0.84 (0.59–1.24) | 0.365 | 0.83 (0.57–1.22) | 0.317 | 0.75 (0.52–1.11) | 0.139 | 0.74 (0.51–1.12) | 0.139 | |
| Spinal fixation | 1.21 (0.4–5.19) | 0.764 | 1.11 (0.37–4.77) | 0.867 | 1.05 (0.35–4.53) | 0.933 | 0.93 (0.31–4.01) | 0.911 | 0.86 (0.29–3.71) | 0.814 | |
| Tumour grading (ref. benign) | |||||||||||
| Atypical | 0.61 (0.42–0.92) | 0.015* | 0.64 (0.43–0.97) | 0.029* | 0.63 (0.42–0.96) | 0.026* | 0.54 (0.36–0.84) | 0.004* | 0.6 (0.4–0.95) | 0.023* | |
| Malignant | 0.26 (0.15–0.44) | < 0.001* | 0.26 (0.15–0.44) | < 0.001* | 0.24 (0.14–0.41) | < 0.001* | 0.21 (0.13–0.36) | < 0.001* | 0.21 (0.12–0.36) | < 0.001* | |
| Length of hospital stay | 0.97 (0.96–0.98) | < 0.001* | 0.97 (0.96–0.98) | < 0.001* | 0.97 (0.96–0.98) | < 0.001* | 0.97 (0.96–0.98) | < 0.001* | 0.97 (0.96–0.98) | < 0.001* | |
| Transfer to rehabilitation after SM surgery | 0.54 (0.44–0.66) | < 0.001* | 0.56 (0.45–0.69) | < 0.001* | 0.57 (0.46–0.71) | < 0.001* | 0.6 (0.48–0.75) | < 0.001* | 0.59 (0.47–0.75) | < 0.001* | |
OR, odds ratio; CI, confidence interval; SM, spinal meningioma.
p<0.05.
Table 3.
Multivariable logistic regression of good functional outcome after spinal meningioma surgery
| Variable | At 1 year |
At 2 years |
At 3 years |
|||
|---|---|---|---|---|---|---|
| OR (95 % CI) | p-value | OR (95 % CI) | p-value | OR (95 % CI) | p-value | |
| Elderly (≥ 70 yr) vs. nonelderly (< 70 yr) | 0.37 (0.29–0.47) | < 0.001* | 0.34 (0.26–0.43) | < 0.001* | 0.32 (0.25–0.42) | < 0.001* |
| Mortality-related morbidity index | 0.71 (0.66–0.75) | < 0.001* | 0.69 (0.64–0.74) | < 0.001* | 0.68 (0.64–0.73) | < 0.001* |
| Aggressive meningioma (WHO grade II & III) | 0.49 (0.33–0.73) | < 0.001* | 0.42 (0.28–0.62) | < 0.001* | 0.47 (0.31–0.71) | < 0.001* |
OR, odds ratio; CI, confidence interval; WHO, World Health Organization.
p<0.05.
DISCUSSION
SM are intradural extramedullary tumors originating from the meningothelial cells of the vertebral canal leptomeninges. The goal of surgery is to achieve complete tumor removal whilst avoiding additional neurological damage. SM resection is usually a relatively simple neurosurgical intervention usually performed via a hemilaminectomy approach with reported rates of complete resection usually above 90% [25–27]. Such procedure is associated with a low morbidity, a rare mortality; 3-month death of 0.6% in our cohort. Compared to intracranial meningioma, aggressive SMs are infrequent; once removed recurrence is uncommon. It is therefore of great importance to focus on the functional rather than on the oncological outcome: complete excision should be the goal of the surgery providing thus usually a cure to the patient and its symptoms improvement. SM are usually slowly growing tumor and thus, they produce a variety of symptoms due to cord and roots compression only once they have reached a significant volume: pain, sensory and sphincter disturbance, motor weakness up to paraplegia. Clinical presentation is rather unspecific with back and radiating pain being often the leading symptoms and usually misinterpreted until the diagnosis is suspected on a magnetic resonance imaging requested. In most patients, the diagnosis is thus not confirmed until motor deficit or gait disturbance arise, witnessing a long-lasting spinal cord compression. Previous studies on FO after SM surgery have been somewhat contradictory and in need of validation from larger cohorts such as the present one. The aim of this study was to assess baseline data and long-term FO with its predictors of SM patients.
Our study presents a unique modern nationwide population-based analysis of SM patients. Thanks to the SNDS, we managed to gather the largest ever published series assessing the FO after SM surgery. The strengths of the SNDS reside both in the high number of patients and in the exhaustive data available from every hospital in France, private and public. The SNDS includes many information such as demographic data, medical and surgical procedure with associated diagnoses and date of death. The database representativeness is nearly perfect, since it includes the whole country’s population of nearly 68 million of inhabitants constituting one of the largest AMDB in the world [13]. Compiled from a number of institutions, the SNDS accuracy is nonetheless limited by inconstancies in data collection and recording. The retrospective nature of this study, together with the lack of clarity regarding treatment rationales and nonhomogeneous management strategies without random assignment, needs to be considered when evaluating the results. Moreover, gathering information on the patient status using electronic medical records software, is associated with a risk of imprecision regarding the preoperative clinical state and the outcome.
There are only a handful studies reporting on SM using AMDB of which all take advantage of American database such as the Surveillance, Epidemiology, and End Results (SEER) and/or the CBTRUS (Central Brain Tumor Registry of the United States) [4,28-30]. However, they do not report on the FO but mainly on descriptive epidemiology and overall survival. Our findings are alike Cao et al. [28] who analysed the SEER and found among 4,204 SM patients of which 80.8% were removed an age-adjusted incidence rate of 0.37 cases per 1,000,000 person-years; a greatest incidence in the 60–69 years group and also a female preponderance with a sex ratio of 4 versus 3.8 in our study. With the SEER, Westwick and Shamji [4] found an age-adjusted incidence of 0.193 per 100,000 population (95% CI, 0.183–0.202) and Kshettry et al. [29] an overall age-adjusted incidence of 0.33 per 100,000 population which was relatively stable over the study period; a highest incidence in the 75- to 84 year-old age group and a slightly lower sex ratio of 3.37. Compared to their intracranial counterparts, SM occurred even more frequently in women (79.1% vs. 74.6%, p<0.001) and at a much older age (66 years, IQR [56–75] vs. 58 years, IQR [48–67], p<0.001). Yet, no satisfactory reason has been provided to explain these contrasts even if differences in hormone responsiveness and genomic make-up have been suggested [4,31]. All studies on meningioma regardless their insertion have showed a female prevalence around 3/4 which suggests the influence of sexual hormones as meningiomas are known to be hormone-sensitive and usually express progesterone receptors [3]. Despite their abundant expression which are found in 88% of the meningioma, it is unknown, however, how their expression is regulated, especially since oestrogen receptors are virtually absent in these tumors [32,33].
Compared to the preoperative assessment, our results show that SM surgery was followed by significant improvement of motor deficits, sensory deficits, gait disturbances, bladder dysfunction and pain. A severe preoperative functional status is not a limiting factor for complete recovery and favourable FO also occurs in the majority of elderly patients [34,35]. All studies report on functional improvement after SM removal, however, it is uneasy to summarize and compare these data regarding the variety of classification used to assess pre- and post-operative functional status. In Sandalcioglu et al. [2] series the outcome was improved or unchanged in 96.2% at the time of last follow-up and 12% of their patients were unable to walk independently. In Maiti et al. [16] literature review, improved or unchanged FO is reported for at least 90% of the patients across all the 14 studies assessed. Even patients with severe preoperative state may experience an excellent neurological recovery after careful surgery and appropriate rehabilitation as we found in our study. However, our results show a significant worse FO for older patients with a high level of comorbidities.
The elderly cohort also had a higher degree of preoperative neurological impairment and comorbidities compared to the non-elderly as reported previously [15]. This may result in delayed diagnosis due to symptoms being attributed to other age-related diseases. Even though the incidence of SM is greater in the elderly population, there may be a reluctance to operate on these patients due to an expected higher risk of adverse events and poor outcomes. However, in a previous study we found that the 5-year survival relative to the expected survival of an age-, and sex-matched French standard population was 100.6% 95% CI (98.6–102.5) suggesting that SM surgery did not contributed to overall mortality with an absolute excess risk of death being null and a related standardised mortality ratio of 1, 95% CI (0.9–1.2) (p=0.565) [36]. If indicated, SM surgery should thus be performed regardless the age and these patients should be referred for surgery as early as possible asserting the concept of “time is spinal cord.” [15]
This work highlights the great value of this unique database to evaluate the FO after SM surgery and its predictors. Further inclusion and prolonged follow-up are required to assess other prognostic factors such as the histopathological subtypes, best after SNDS, and the French Brain Tumour DataBase merging [9].
CONCLUSION
FO after meningioma surgery is favourable but, may be impaired for older patients with a high level of comorbidities and aggressive tumor.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contribution
Conceptualization: CCD, JW, VJ; Data curation: CCD, JW; Formal analysis: CCD; Methodology: CCD, JW; Project administration: CCD, NP, VJ; Visualization: CCD, NP; Writing - original draft: CCD; Writing - review & editing: CCD, NP, JW, VJ.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1 and 2 can be found via https://doi.org/10.14245/ns.2143186.593.
Summary of predictors and corresponding weights for computation of the mortality-related morbidity index (MRMI) and expenditure-related morbidity index (ERMI) of each predictor in the study cohort
Summary of predictors and corresponding weights for computation of the mortality-related morbidity index (MRMI) and expenditure-related morbidity index (ERMI) of each predictor in the study cohort
REFERENCES
- 1.Ostrom QT, Patil N, Cioffi G, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro Oncol. 2020;22(12 Suppl 2):iv1–96. doi: 10.1093/neuonc/noaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandalcioglu IE, Hunold A, Müller O, et al. Spinal meningiomas: critical review of 131 surgically treated patients. Eur Spine J. 2008;17:1035–41. doi: 10.1007/s00586-008-0685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champeaux C, Weller J, Katsahian S. Epidemiology of meningiomas. A nationwide study of surgically treated tumours on french medico-administrative data. Cancer Epidemiol. 2019;58:63–70. doi: 10.1016/j.canep.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Westwick HJ, Shamji MF. Effects of sex on the incidence and prognosis of spinal meningiomas: a surveillance, epidemiology, and end results study. J Neurosurg Spine. 2015;23:368–73. doi: 10.3171/2014.12.SPINE14974. [DOI] [PubMed] [Google Scholar]
- 5.Champeaux-Depond C, Weller J, Resche-Rigon M. Neurofibromatosis type 2: a nationwide population-based study focused on survival after meningioma surgery. Clin Neurol Neurosurg. 2020;198:106236. doi: 10.1016/j.clineuro.2020.106236. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 7.Champeaux C, Weller J, Katsahian S. Epidemiology of meningiomas. A nationwide study of surgically treated tumours on French medico-administrative data. Cancer Epidemiol. 2018;58:63–70. doi: 10.1016/j.canep.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Champeaux C, Houston D, Dunn L. Intracranial WHO grade I meningioma: a competing risk analysis of progression and disease-specific survival. Acta Neurochir (Wien) 2019 Nov 9; doi: 10.1007/s00701-019-04096-9. [Epub]. [DOI] [PubMed] [Google Scholar]
- 9.Zouaoui S, Darlix A, Rigau V, et al. Descriptive epidemiology of 13,038 newly diagnosed and histologically confirmed meningiomas in France: 2006-2010. Neurochirurgie. 2018;64:15–21. doi: 10.1016/j.neuchi.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Champeaux C, Wilson E, Brandner S, et al. World Health Organization grade III meningiomas. A retrospective study for outcome and prognostic factors assessment. Br J Neurosurg. 2015;29:693–8. doi: 10.3109/02688697.2015.1054350. [DOI] [PubMed] [Google Scholar]
- 11.Champeaux C, Dunn L. World health organization grade II meningioma: a 10-year retrospective study for recurrence and prognostic factor assessment. World Neurosurg. 2016;89:180–6. doi: 10.1016/j.wneu.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 12.Champeaux C, Houston D, Dunn L. Atypical meningioma. A study on recurrence and disease-specific survival. Neurochirurgie. 2017;63:273–81. doi: 10.1016/j.neuchi.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65 Suppl 4:S149–67. doi: 10.1016/j.respe.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 14.McCormick PC, Torres R, Post KD, et al. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523–32. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- 15.Pettersson-Segerlind J, Fletcher-Sandersjöö A, Tatter C, et al. Long-term follow-up and predictors of functional outcome after surgery for spinal meningiomas: a population-based cohort study. Cancers (Basel) 2021;13:3244. doi: 10.3390/cancers13133244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiti TK, Bir SC, Patra DP, et al. Spinal meningiomas: clinicoradiological factors predicting recurrence and functional outcome. Neurosurg Focus. 2016;41:E6. doi: 10.3171/2016.5.FOCUS16163. [DOI] [PubMed] [Google Scholar]
- 17.Engel DC, Gawellek L, Peraio S, et al. Spinal meningioma surgery in the elderly: who can benefit from it? J Neurosurg Sci. 2021;65:408–13. doi: 10.23736/S0390-5616.18.04582-4. [DOI] [PubMed] [Google Scholar]
- 18.Champeaux-Depond C, Constantinou P, Weller J. Cause-specific survival after meningioma surgery: a nationwide population-based competing risk study. World Neurosurg. 2021;146:e67–75. doi: 10.1016/j.wneu.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Champeaux-Depond C, Weller J, Froelich S, et al. A nationwide population-based study on overall survival after meningioma surgery. Cancer Epidemiol. 2021;70:101875. doi: 10.1016/j.canep.2020.101875. [DOI] [PubMed] [Google Scholar]
- 20.Constantinou P, Tuppin P, Fagot-Campagna A, et al. Two morbidity indices developed in a nationwide population permitted performant outcome-specific severity adjustment. J Clin Epidemiol. 2018;103:60–70. doi: 10.1016/j.jclinepi.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 21.R Core Team . Vienna (Austria): R Foundation for Statistical Computing; 2014. R: A language and environment for statistical computing [Internet] [cited 2022 Jan 16]. Available from: http://www.R-project.org/ [Google Scholar]
- 22.RStudio Team . Boston (MA): RStudio; Inc.; 2015. RStudio: Integrated development environment for r [Internet] [cited 2022 Jan 16]. Available from: http://www.rstudio.com/ [Google Scholar]
- 23.Lang TA, Altman DG. Basic statistical reporting for articles published in biomedical journals: the “Statistical Analyses and Methods in the Published Literature” or the SAMPL Guidelines. Int J Nurs Stud. 2015;52:5–9. doi: 10.1016/j.ijnurstu.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls SG, Quach P, von Elm E, et al. The REporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) statement: methods for arriving at consensus and developing reporting guidelines. PLoS One. 2015;10:e0125620. doi: 10.1371/journal.pone.0125620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua L, Zhu H, Deng J, et al. Clinical and prognostic features of spinal meningioma: a thorough analysis from a single neurosurgical center. J Neurooncol. 2018;140:639–47. doi: 10.1007/s11060-018-2993-3. [DOI] [PubMed] [Google Scholar]
- 26.Jamilson Araújo Pereira B, Nogueira de Almeida A, Silva Paiva W, et al. Neuro-oncological features of spinal meningiomas: systematic review. Neurochirurgie. 2020;66:41–4. doi: 10.1016/j.neuchi.2019.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Turel MK, D’Souza WP, Rajshekhar V. Hemilaminectomy approach for intradural extramedullary spinal tumors: an analysis of 164 patients. Neurosurg Focus. 2015;39:E9. doi: 10.3171/2015.5.FOCUS15170. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Jiang Y, Liu C, et al. Epidemiology and survival of patients with spinal meningiomas: a SEER analysis. Eur J Surg Oncol. 2021;47:2340–5. doi: 10.1016/j.ejso.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Kshettry VR, Hsieh JK, Ostrom QT, et al. Descriptive epidemiology of spinal meningiomas in the united states. Spine (Phila Pa 1976) 2015;40:E886–9. doi: 10.1097/BRS.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 30.Dibas M, Rajab AM, Atiah MJ, et al. Racial disparities in the incidence and survival of spinal meningioma. Asian J Neurosurg. 2020;15:877–81. doi: 10.4103/ajns.AJNS_306_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodbelt AR, Barclay ME, Greenberg D, et al. The outcome of patients with surgically treated meningioma in england: 1999-2013. A cancer registry data analysis. Br J Neurosurg. 2019;33:641–7. doi: 10.1080/02688697.2019.1661965. [DOI] [PubMed] [Google Scholar]
- 32.Blankenstein MA, Verheijen FM, Jacobs JM, et al. Occurrence, regulation, and significance of progesterone receptors in human meningioma. Steroids. 2000;65:795–800. doi: 10.1016/s0039-128x(00)00193-8. [DOI] [PubMed] [Google Scholar]
- 33.Goffin J. Estrogen- and progesterone-receptors in meningiomas. Review article. Clin Neurol Neurosurg. 1986;88:169–75. doi: 10.1016/s0303-8467(86)80024-5. [DOI] [PubMed] [Google Scholar]
- 34.Sacko O, Haegelen C, Mendes V, et al. Spinal meningioma surgery in elderly patients with paraplegia or severe paraparesis: a multicenter study. Neurosurgery. 2009;64:503–9. doi: 10.1227/01.NEU.0000338427.44471.1D. discussion 509-10. [DOI] [PubMed] [Google Scholar]
- 35.Morandi X, Haegelen C, Riffaud L, et al. Results in the operative treatment of elderly patients with spinal meningiomas. Spine (Phila Pa 1976) 2004;29:2191–4. doi: 10.1097/01.brs.0000141173.79572.40. [DOI] [PubMed] [Google Scholar]
- 36.Jecko V, Weller J, Houston D, et al. Epidemiology and survival after spinal meningioma surgery: a nationwide population-based study. Asian Spine J. 2022 Jan 24; doi: 10.31616/asj.2021.0213. . [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of predictors and corresponding weights for computation of the mortality-related morbidity index (MRMI) and expenditure-related morbidity index (ERMI) of each predictor in the study cohort
Summary of predictors and corresponding weights for computation of the mortality-related morbidity index (MRMI) and expenditure-related morbidity index (ERMI) of each predictor in the study cohort