Abstract
Objective
Postoperative heart block is a significant problem in congenital heart surgery because of the unpredictability and variability of conduction tissue location in complex congenital heart defects. A novel technique for intraoperative conduction system mapping during complex congenital heart surgery is described.
Methods
Intraoperative conduction system mapping was performed utilizing a high-density multielectrode grid catheter to collect intracardiac electrograms on open, beating hearts during repair of complex congenital heart defects. Electrograms were interpreted by electrophysiologists, and conduction tissue location was communicated in real time to the surgeon. After localizing conduction tissue, the heart was arrested and the repair was completed taking care to avoid injury to the mapped conduction system.
Results
Two patients with complex heterotaxy syndrome underwent intraoperative conduction mapping during biventricular repair. Mapping accurately identified the location of conduction tissue thereby enabling avoidance of conduction system injury during surgery. Notably, conduction was unexpectedly found to be located inferiorly in a patient with L-looped ventricles. Successful biventricular repair was accomplished in both patients without injury to the conduction system.
Conclusions
Intraoperative conduction mapping can effectively localize the conduction system during surgery and enable the surgeon to avoid its injury. This can lower the risk of heart block requiring pacemaker in children undergoing complex congenital heart surgery.
Abbreviations and Acronyms: AVB, atrioventricular block; BiV, biventricular; CAVC, complete atrioventricular canal; HBE, His bundle electrogram; HTX, heterotaxy syndrome; IOM, intraoperative His bundle electrogram mapping; LV, left ventricle

Mapping catheter on inferior canal (green) in a complex HTX patient. HBE on electrogram.
Central Message.
Heart block requiring permanent pacemaker remains a challenging problem in complex congenital heart surgery. Intraoperative EP mapping can identify conduction tissue to mitigate the risk of injury.
See Commentary on page 164.
Postoperative atrioventricular block (AVB) requiring permanent pacemaker remains a challenge in complex biventricular (BiV) repairs. For patients with heterotaxy syndrome (HTX), unbalanced complete atrioventricular canal (CAVC), double-outlet right ventricle (DORV), and complex transposition of the great arteries, the rate of AVB complicating BiV repair approaches 25%, resulting in substantial morbidity.1, 2, 3 To avoid AVB, we now incorporate intraoperative His bundle electrogram (HBE) mapping (IOM) into our BiV repairs.
Surgical Techniques
Patients are cooled to 34 °C on cardiopulmonary bypass. The multielectrode mapping catheter (Advisor HD Grid, Abbott Cardiovascular) has 16 electrodes in a 4 × 4 grid, comprising vertical splines (A-D) and horizontal rows (1-4). Each electrode has a letter-number coordinate (Figure 1, A and B). The catheter is laid flat with the dial pointing upward; the upward-pointing D spline is marked for catheter orientation. The catheter is connected to a recording system, which also records electrocardiogram limb leads.
Figure 1.
Intraoperative conduction mapping equipment, setup, and data acquisition. A, The Advisor HD Grid Mapping Catheter (Abbott Cardiovascular) is 105 cm in overall length and designed for percutaneous use. There is a handle and dial (arrow) to enable shaft deflection. B, Close-up view of the 4 × 4 electrode grid demonstrates 4 splines (A through D) and 4 rows (1 through 4), giving each electrode a letter-number label (eg, A3). Electrodes are equidistantly spaced 3 mm apart to create a 13 × 13 mm2 grid. Wavefront propagation can be captured in orthogonal vectors (red and blue arrows). C, Operating room setup with the Advisor Grid catheter (red arrow) on the operative field. Real-time electrograms (EGMs) are collected and displayed on the recording system (green arrow) and interpreted by the staff electrophysiologist in the room. D, Real-time EGMs are collected when the catheter is placed on the endocardial surface of the beating heart. Top tracings (white) represent surface electrocardiogram signals. Blue tracings indicate D spline. Green tracings indicate C spline. Yellow tracings indicate B spline. Pink tracings indicate A spline. Each EGM represents a bipolar signal between 2 electrodes (ie, A1A2 indicates electrical signal detected between the A1 and A2 electrodes). Atrial and ventricular signals are denoted, corresponding to the P and QRS waves on the surface electrocardiogram, respectively. The His bundle electrograms (HBEs) lie between the atrial and ventricular signals. This EGM shows HBEs in the A3A4, A2A3, and B2B3 regions. Inset, diagram of the grid demonstrates how the EGMs translate to the HBE location (pink and yellow circles) and the direction of wavefront propagation (green arrow), which are determined by the electrophysiologist in real-time as the surgeon positions the catheter in the heart. Images in panels A and B are reproduced with permission of Abbott, © 2021.
The heart is fibrillated, an atriotomy is made, and the heart is drained with drop suckers across the AV valves to prevent air embolism. After defibrillating, IOM commences with the surgeon placing the catheter along the endocardial surface of the empty, beating heart. IOM can also be performed via ventriculotomy. Bipolar electrograms are viewed at 100 to 200 mm/sec sweep speeds with band-pass filtering at 30 to 500 Hz and interpreted by electrophysiologists to identify the HBE (Figure 1, C and D). Three-dimensional heart models are used for reference while mapping. The HBE location is marked on the heart with a marking pen. The patient is then cooled to 28 °C, and the heart is arrested to perform the repair (Video 1).
Video 1.
Intraoperative conduction mapping workflow. Recording system is positioned adjacent to the operating room table. Surgeon head camera (monitor 1) shows the grid catheter placed inside the open, beating heart. Mapping requires close communication between the electrophysiologist and surgeon as the electrograms are interpreted in real-time and the location/path of conduction is ascertained. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00066-9/fulltext.
The Boston Children's Hospital Institutional Review Board approved the protocol (No. P00036219; August 13, 2020) and publication of data. The institutional review board waived patient written consent for the publication of study data because the protocol qualified as exempt from the 45 CFR 46 requirements.
Results
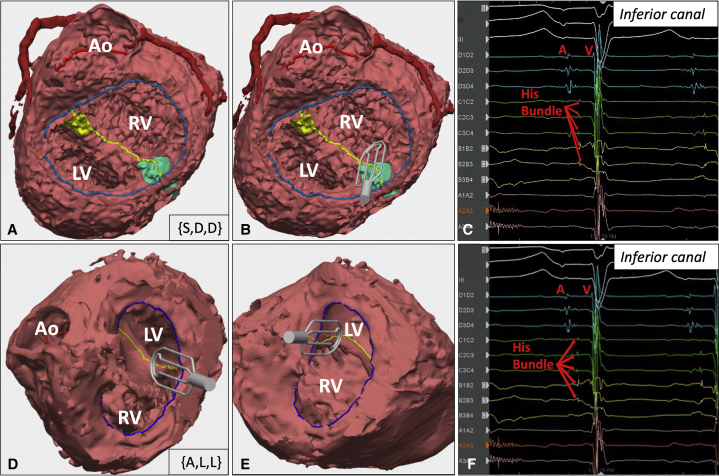
Two BiV repair patients with complex HTX underwent IOM. The first was a 14-year-old with HTX/polysplenia, levocardia, {S,D,D}, right-dominant CAVC, transposition of the great arteries, and pulmonary atresia who had undergone Fontan palliation. BiV repair involved Fontan takedown, CAVC repair baffling the left ventricle (LV) to aorta, and right ventricle to pulmonary artery conduit (Figure 2, A). Conduction was predicted to be inferior. IOM confirmed conduction located inferiorly and ruled out conduction superiorly (Figure 2, B and C). This enabled muscle resection superiorly to enlarge the baffle pathway. The child underwent successful BiV repair without AVB (Video 2).
Video 2.
Case 1 is a 14-year-old with heterotaxy/polysplenia, levocardia, {S,D,D}, right-dominant complete atrioventricular canal (CAVC), transposition of the great arteries, and pulmonary atresia undergoing biventricular repair. Intraoperative mapping identified conduction along the inferior aspect of the CAVC, thereby enabling muscle bundle division/resection superiorly to optimize the baffle pathway. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00066-9/fulltext.
Figure 2.
Patient models and electrograms. A, Three-dimensional model of patient #1 (14-year-old with heterotaxy/polysplenia, levocardia, {S,D,D}, right-dominant complete atrioventricular canal (CAVC), transposition of the great arteries (TGA) and pulmonary atresia) looking through the common atrioventricular (AV) valve shows the posterior left ventricle (LV) with the aorta (Ao) arising off the anterior right ventricle (RV). Blue line indicates common AV valve annulus. Yellow line indicates ventricular septal defect crest. Yellow region indicates superior aspect of CAVC. Green region indicates inferior aspect of CAVC. B, Three-dimensional model with digital rendering of the grid catheter lying along the inferior aspect of the CAVC. C, Electrogram obtained with the mapping catheter in this position demonstrates His bundle potentials between the atrial (A) and ventricular (V) signals, confirming the presence of conduction along the inferior aspect of the CAVC. D, Three-dimensional model of patient #2 (3-year-old with heterotaxy/asplenia, dextrocardia, {A,L,L}, right-dominant CAVC, DORV, and pulmonary atresia) looking through the common AV valve shows the anterior LV with the Ao arising off the RV. The grid catheter is lying along the inferior aspect of the CAVC. E, Grid catheter lying along the superior aspect of the CAVC. F, Electrogram obtained with the mapping catheter along the inferior CAVC shows His bundle potentials in this location despite L-looped ventricles.
The second patient was a 3-year-old with HTX/asplenia, dextrocardia, {A,L,L}, right-dominant CAVC, DORV, and pulmonary atresia who had undergone bilateral bidirectional Glenns. BiV repair involved Glenn takedown, atrial switch, CAVC repair baffling the LV to aorta, and right ventricle to pulmonary artery conduit. Conduction was anticipated to be superiorly located given L-looped ventricles. However, conduction was mapped inferiorly in the canal. No HBE was identified superiorly, enabling muscle resection in this region to enlarge the LV-aorta pathway (Figure 2, D-F). Successful BiV repair was completed without AVB (Video 3).
Video 3.
Case 2 is a 3-year-old with heterotaxy/asplenia, dextrocardia, {A,L,L}, right-dominant complete atrioventricular canal (CAVC), double-outlet right ventricle, and pulmonary atresia undergoing biventricular repair. Intraoperative mapping identified conduction along the inferior aspect of the CAVC despite L-looped ventricles. Mapping enabled muscle bundle division/resection superiorly to create an unobstructed baffle pathway. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00066-9/fulltext.
Discussion
AVB requiring permanent pacemaker remains a significant complication of complex BiV repairs, with attendant pacemaker/lead-related reinterventions, increased healthcare costs, and mortality.2,3 IOM enables avoidance of conduction tissue injury by precise localization in these patients with highly variable anatomy.
IOM has been described previously, but was limited by available catheter technology.4 This catheter's grid design is favorable for IOM because orthogonal bipolar electrograms make precise alignment along wavefront propagation vectors less critical. HBE localization is quick and precise, with a median mapping time of 8 minutes. Compared with preoperative mapping, IOM offers a closer correlation of mapped and anatomic location at the time of the surgery, with the ability to mark the HBE site for reference.
To date we have observed decreased AVB rates, with 13% experiencing AVB despite IOM.5 We have found conduction in an unexpected location (based on ventricular looping) in nearly 20% of patients, demonstrating the difficulty in predicting conduction tissue course by anatomical principles alone. Some of our failures were early; that is, preceding technical refinements (eg, marking the endocardial surface after IOM and gaining familiarity with catheter manipulation). Although IOM helps localize conduction, improvements to the resolution of IOM are still needed. The catheter is placed along the beating/moving heart; even subtle movements over the endocardial surface limit spatial and temporal resolution. Moreover, the catheter's size and stiffness limit our ability to map in certain regions, such as underneath valve tissue, especially in smaller hearts, where catheter manipulation can be more challenging.
There is the potential for air embolism if open, beating-heart bypass is conducted improperly. Notably, other surgical procedures involve open, beating-heart bypass, including LV apical ventricular assist device implantation. By keeping the beating heart fully decompressed we have not observed any embolic phenomena and believe this technique can be employed safely.
Conclusions
The novel application of modern high-density mapping to the long-standing challenge of postoperative AVB in congenital heart disease shows promise in early applications. Larger studies are underway to establish efficacy in reducing postoperative AVB. Future work is critical to optimize technology for intraoperative use.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://aats.blob.core.windows.net/media/21%20AM/AM21_C04/AM21_C04_02.mp4.

Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest
Footnotes
Drs Feins and O'Leary contributed equally to this article as co-first authors.
Drs Emani and DeWitt contributed equally to this article co-senior authors.
Supplementary Data
Intraoperative conduction mapping workflow. Recording system is positioned adjacent to the operating room table. Surgeon head camera (monitor 1) shows the grid catheter placed inside the open, beating heart. Mapping requires close communication between the electrophysiologist and surgeon as the electrograms are interpreted in real-time and the location/path of conduction is ascertained. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00066-9/fulltext.
Case 1 is a 14-year-old with heterotaxy/polysplenia, levocardia, {S,D,D}, right-dominant complete atrioventricular canal (CAVC), transposition of the great arteries, and pulmonary atresia undergoing biventricular repair. Intraoperative mapping identified conduction along the inferior aspect of the CAVC, thereby enabling muscle bundle division/resection superiorly to optimize the baffle pathway. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00066-9/fulltext.
Case 2 is a 3-year-old with heterotaxy/asplenia, dextrocardia, {A,L,L}, right-dominant complete atrioventricular canal (CAVC), double-outlet right ventricle, and pulmonary atresia undergoing biventricular repair. Intraoperative mapping identified conduction along the inferior aspect of the CAVC despite L-looped ventricles. Mapping enabled muscle bundle division/resection superiorly to create an unobstructed baffle pathway. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00066-9/fulltext.
References
- 1.Makhija Z., Marwah A., Mishra S., Kumar J., Goel A., Sharma R. Biventricular repair in heterotaxy patients. World J Pediatr Congenit Heart Surg. 2015;6:195–202. doi: 10.1177/2150135114563772. [DOI] [PubMed] [Google Scholar]
- 2.Fortescue E.B., Berul C.I., Cecchin F., Walsh E.P., Triedman J.K., Alexander M.E. Patient, procedural, and hardware factors associated with pacemaker lead failures in pediatrics and congenital heart disease. Heart Rhythm. 2004;1:150–159. doi: 10.1016/j.hrthm.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Liberman L., Silver E.S., Chai P.J., Anderson B.R. Incidence and characteristics of heart block after heart surgery in pediatric patients: a multicenter study. J Thorac Cardiovasc Surg. 2016;152:197–202. doi: 10.1016/j.jtcvs.2016.03.081. [DOI] [PubMed] [Google Scholar]
- 4.Dick M., II, Krongrad E., Antar R.E., Ross S., Bowman F.O., Malm J.R., et al. Intraoperative recording of the His bundle electrogram in man. An assessment of its precision. Circulation. 1976;53:224–229. doi: 10.1161/01.cir.53.2.224. [DOI] [PubMed] [Google Scholar]
- 5.O’Leary E.T., Feins E.N., Triedman J.K., Walsh E.P., Schulz N., Eickhoff E., et al. Intraoperative high density conduction system mapping during complex congenital heart surgery: a pilot initiative to reduce postoperative heart block. J Am Coll Cardiol. 2021;77(18 Suppl):504. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraoperative conduction mapping workflow. Recording system is positioned adjacent to the operating room table. Surgeon head camera (monitor 1) shows the grid catheter placed inside the open, beating heart. Mapping requires close communication between the electrophysiologist and surgeon as the electrograms are interpreted in real-time and the location/path of conduction is ascertained. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00066-9/fulltext.
Case 1 is a 14-year-old with heterotaxy/polysplenia, levocardia, {S,D,D}, right-dominant complete atrioventricular canal (CAVC), transposition of the great arteries, and pulmonary atresia undergoing biventricular repair. Intraoperative mapping identified conduction along the inferior aspect of the CAVC, thereby enabling muscle bundle division/resection superiorly to optimize the baffle pathway. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00066-9/fulltext.
Case 2 is a 3-year-old with heterotaxy/asplenia, dextrocardia, {A,L,L}, right-dominant complete atrioventricular canal (CAVC), double-outlet right ventricle, and pulmonary atresia undergoing biventricular repair. Intraoperative mapping identified conduction along the inferior aspect of the CAVC despite L-looped ventricles. Mapping enabled muscle bundle division/resection superiorly to create an unobstructed baffle pathway. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00066-9/fulltext.