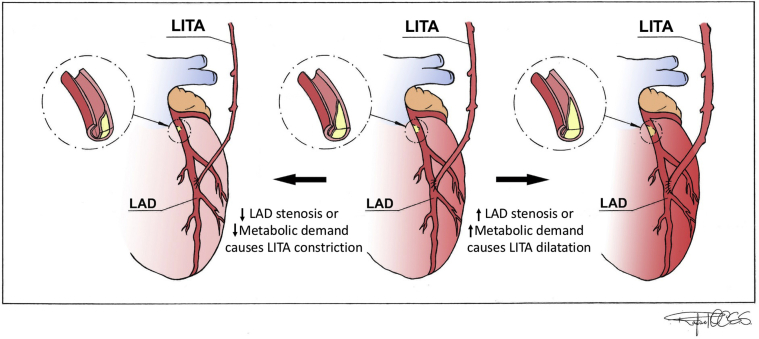
LITA's adaptation according to varying degrees of stenosis in the coronary.
Central Message.
The internal thoracic artery graft can adapt according to the degree of stenosis in the target coronary and the metabolic demands of the myocardium even years after surgery.
See Article page 72.
The string sign is a diffuse narrowing of arterial grafts used for surgical revascularization without significant stenosis of an anastomosis.1 It is an adaptation of the arterial graft as a function of myocardial demands and competitive flow. Arterial grafts can dilate and therefore increase their flow in response to increased metabolic demands of the myocardium, or when the degree of stenosis in the coronary increases and vice versa (Figure 1).2,3
Figure 1.
Illustration showing physiologic adaptation of the LITA according to varying degrees of stenosis in the coronary and to the metabolic myocardium demands. Arrow to the right shows that LITA can dilate and therefore increase its flow in response to increased metabolic demands of the myocardium, or when the degree of stenosis in the coronary increases. Arrow to the left shows that LITA can constrict and therefore decrease its flow in response to decreased metabolic demands of the myocardium, or when the degree of stenosis in the coronary decreases. LITA, Left internal thoracic artery; LAD, left anterior descending artery.
In this issue of the Journal, Yazbeck and colleagues4 report a case in which a left internal thoracic artery (LITA) string sign occurred early on, after improvement in the degree of left anterior descending artery (LAD) stenosis. Fifteen years later, there was disappearance of the string sign as the stenosis on the LAD had progressed and became more hemodynamically significant.4
Two features of this interesting report should catch our attention. First, the importance—still incompletely understood—of preoperatively assessing the degree of stenosis for the optimal function of arterial grafts. As of this writing, data on this issue inform composite arterial grafts and radial artery grafts5,6 but are largely lacking in regard to independent ITA grafts. It is plausible—albeit unproven—that independent ITA grafts may be less susceptible to competitive flow than composite grafts. Overall, this remains an important area for ongoing research.
The second feature is the constant adaptation of the LITA graft even years after the surgery, because of dynamic changes in the extent of coronary artery disease. The 2 joined arteries, the LITA and its target revascularized coronary, interact together in supplying the myocardium.2 Tsirikos Karapanos and colleagues2 have showed experimentally that as the stenosis in the coronary increases or decreases, the blood flow through each artery compensates for the other. In addition, biological signaling likely influences the LITA according to competitive flow and myocardium demands.2,3 Gaudino and colleagues7 have previously showed, in a series, that a high proportion of LITA grafts found to be malfunctioning at late angiography were due to technical issues, whereas functional LITA insufficiency only seemed to play a marginal role. It might be only in rare cases, ie, when the flow coming from the LITA is completely superfluous, that competitive flow leads to a permanently unfunctional graft. This brings back the longstanding question: with moderate LAD stenosis, should one graft it or not?
In conclusion, this case report elegantly illustrates a physiologic adaptation of the LITA to varying degrees of stenosis in the target coronary many years after the original surgery. It is because of the complex interaction between the graft, its adaptation, the degree of stenosis in the coronary, and the physiological demands for blood supply that “we are talking about a vessel and not just a pipe.” We still, however, do not have an answer to the longstanding question as to whether a moderate LAD stenosis, say during a combined valve and coronary procedure, should be left alone or grafted. The present report may suggest that there could be little intrinsic risk in going ahead and grafting it.
Acknowledgments
The Figure 1 was created by Rafael Oliveira Coutinho Santos Soares. We thank him for his work.
Footnotes
Disclosures: The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Yokoyama K., Miyauchi K., Kawamura M., Kajimoto K., Dohi T., Yamagami S., et al. String-sign in left internal thoracic artery is associated with regression in left main trunk stenosis after coronary artery bypass. Int Heart J. 2011;52:84–87. doi: 10.1536/ihj.52.84. [DOI] [PubMed] [Google Scholar]
- 2.Tsirikos Karapanos N., Suddendorf S.H., Li Z., Huebner M., Joyce L.D., Park S.J. The impact of competitive flow on distal coronary flow and on graft flow during coronary artery bypass surgery. Interact Cardiovasc Thorac Surg. 2011;12:993–997. doi: 10.1510/icvts.2010.255398. [DOI] [PubMed] [Google Scholar]
- 3.Gaudino M., Serricchio M., Tondi P., Glieca F., Giordano A., Trani C., et al. Non-invasive evaluation of mammary artery flow reserve and adequacy to increased myocardial oxygen demand. Eur J Cardiothorac Surg. 1998;13:404–409. doi: 10.1016/s1010-7940(98)00025-6. [DOI] [PubMed] [Google Scholar]
- 4.Yazbeck M.M., Jebara V.A., Azar R.R. String sign recovery of the left internal mammary artery bypass graft. J Thorac Cardiovasc Surg Tech. 2022;12:72–74. doi: 10.1016/j.xjtc.2021.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glineur D., Grau J.B., Etienne P.Y., Benedetto U., Fortier J.H., Papadatos S., et al. Impact of preoperative fractional flow reserve on arterial bypass graft anastomotic function: the IMPAG trial. Eur Heart J. 2019;40:2421–2428. doi: 10.1093/eurheartj/ehz329. [DOI] [PubMed] [Google Scholar]
- 6.Hayward P.A., Gordon I.R., Hare D.L., Matalanis G., Horrigan M.L., Rosalion A., et al. Comparable patencies of the radial artery and right internal thoracic artery or saphenous vein beyond 5 years: results from the Radial Artery Patency and Clinical Outcomes trial. J Thorac Cardiovasc Surg. 2010;139:60–65. doi: 10.1016/j.jtcvs.2009.09.043. discussion 65-7. [DOI] [PubMed] [Google Scholar]
- 7.Gaudino M., Trani C., Luciani N., Alessandrini F., Possati G. The internal mammary artery malperfusion syndrome: late angiographic verification. Ann Thorac Surg. 1997;63:1257–1261. doi: 10.1016/s0003-4975(96)01257-x. [DOI] [PubMed] [Google Scholar]