Abstract
Evaluating multimorbidity combinations, racial/ethnic background, educational attainment, and sex associations with age-related cognitive changes is critical to clarifying the health, sociodemographic, and socioeconomic mechanisms associated with cognitive function in later life. Data from the 2011–2018 National Health and Aging Trends Study for respondents aged 65 years and older (N = 10,548, mean age = 77.5) were analyzed using linear mixed effect models. Racial/ethnic differences (mutually-exclusive groups: non-Latino White, non-Latino Black, and Latino) in cognitive trajectories and significant interactions with sex and education (<high school, high school, some college, and ≥ college degree) were evaluated. Models included sex, education, ever covered by Medicaid, coupled status, waist-height ratio, study cohort, and chronic disease category (no diseases; one disease; advanced cardiovascular multimorbidity; metabolic multimorbidity; advanced cardiovascular-metabolic multimorbidity; and neither advanced cardiovascular nor metabolic multimorbidity). In covariate-adjusted models, Black (b = −1.31, 95% CI: 1.74,-0.89) and Latino (b = −0.83, 95% CI: 1.58,-0.07) respondents had lower cognitive scores at age 65 and steeper declines with age (b = −0.08, 95% CI: −0.15,-0.01; b = −0.20, 95% CI: 0.34,-0.05, respectively) compared with White respondents. Cognitive scores were lower among respondents with advanced cardiovascular (b = −0.28, 95% CI: 0.54,-0.01) and advanced cardiovascular-metabolic (b = −0.56, 95% CI: 0.86,-0.27) multimorbidity compared with respondents with none of the chronic diseases of interest. In interaction models, protective associations by female sex and higher education were not observed among minority racial/ethnic groups. It is important to develop interventions to postpone cognitive decline among older Black and Latino adults.
Keywords: Cognitive function, Race/ethnicity, Disparities, Multimorbidity
Highlights
-
•
Black and Latino respondents had lower initial cognitive scores and steeper declines with age.
-
•
Respondents with advanced cardiovascular-metabolic multimorbidity had lower cognitive scores.
-
•
Protective associations by female sex and higher education were not seen in minoritized groups.
1. Introduction
Alzheimer's disease and related dementias (ADRD) affects more than 6 million older adults, and represents extensive costs to individuals, their families, and public programs (Alzheimer's Association., 2020; Hurd et al., 2013; Aranda et al., 2021). ADRD disproportionately affects minoritized racial and ethnic populations in the United States (Aranda et al., 2021; Jutkowitz et al., 2017). Older Black Americans experience roughly double the incidence, and Latino Americans have 1.5 times the incidence of ADRD observed among older White Americans (Alzheimer's Association., 2020). High incidence is also evident among American Indian/Alaska Native populations (Mayeda et al., 2016). Black Americans are even more likely to be diagnosed later in the disease course, and to present with more severe dementia, which limits access to a greater range of services and supports, and also shortens the available time for potentially therapeutic treatment options. (Jutkowitz et al., 2017; Prince et al., 2011).
A number of studies have identified lower educational attainment, and specifically, lower quality of education (Avila et al., 2021; Sisco et al., 2015), as a strong risk factor for poor cognitive function and ADRD (Díaz-Venegas et al., 2016). High levels of education and lifelong learning may afford greater cognitive reserve—the ability to engage alternate brain network pathways or cognitive strategies to cope with pathological cognitive changes involved in AD-dementia progression (Gurland et al., 1999; Sperling et al., 2011). This cognitive reserve hypothesis is thought to be one mechanism underlying racial and ethnic disparities in dementia prevalence (Gurland et al., 1999). Alternately, higher education may be associated with behavioral or environmental factors that diminish the risk of cognitive impairment (Langa et al., 2008; Sattler et al., 2012).
Nevertheless, various studies have found racial/ethnic differences in cognitive function that persist even after adjusting for education (Castora-Binkley et al., 2015; Vásquez et al., 2016). Cumulative inequality theory (Ferraro & Shippee, 2009) provides a useful framework for assessing and understanding ways in which social systems and embedded structural processes generate and perpetuate inequality in health outcomes ocurring throughout and over the life course via demographic and developmental processes. As such, individual cognitive trajectories are shaped by the accumulation of risks as well as the availability and deployment of protective resources (e.g., education, cognitive reserve, compensatory skills). In this vein, the lifelong and lasting consequences of structural educational inequities and cumulative stressors such as exposure to racial discrimination throughout the life course, are increasingly recognized as important social factors that confer substantial risk for low and/or declining cognitive function (Avila et al., 2021; Zuelsdorff et al., 2020). These studies highlight the importance of disentangling the various contributors to ADRD. In particular, the interconnections between sociodeomographic and socioeconomic factors—sex, race, ethnicity, and education—and higher co-existing chronic morbidity observed among minoritized racial/ethnic groups may cumulatively disadvantage these groups through additional risks for onset and worsening cognitive function.
Preexisting health issues, such as high chronic disease and multimorbidity burden observed among racial/ethnic groups earlier in the life course (Johnson-Lawrence et al., 2017; Quiñones et al., 2019) may provide yet another pathway for cognitive declines later in life. Recent work documents accelerated cognitive declines among older adults with multimorbidity (Wei et al., 2019). In the United States, Black and Latino adults are burdened with higher multimorbidity levels and more accelerated accumulation of chronic disease with age compared with White adults (Quiñones et al., 2019). Still, the role that multimorbidity and specific multimorbidity profiles play in modifying or accelerating the trajectory of cognitive decline on the ADRD continuum remains unclear (Heun et al., 2013). While cardiovascular disease and metabolic syndrome have been consistently linked with cognitive impairment, several other factors have not been systematically evaluated, including vascular dementia, the progression from mild cognitive impairment to dementia (Atti et al., 2019; Yaffe et al., 2004), particularly among minority racial/ethnic groups (Karlamangla et al., 2009; Osei & Gaillard, 2017), their role in the context of multimorbidity, and the impact of social factors.
This study aims to evaluate the interplay between cognitive trajectories in aging, multimorbidity combinations, racial/ethnic background, educational attainment, and sex. Thus, this study clarifies the health, sociodemographic, and socioeconomic mechanisms associated with cognitive function in later life.
2. Methods
2.1. Data source
The National Health and Aging Trends Study (NHATS) is a publicly available, population-based longitudinal study, which investigates a nationally representative sample of Medicare beneficiaries ages 65 and older. The initial sample was enrolled for interviews in 2011, with a replenishment of the sample enrolled in 2015. Interviews were conducted annually and full details of NHATS have been published (Freedman & Kasper, 2019). The NHATS protocol was approved by the Johns Hopkins University Institutional Review Board (IRB) and the study protocol was approved by our institutional IRB (STUDY00019414).
2.2. Study population
There were 7609 respondents in the 2011 original cohort and 3949 respondents in the 2015 refreshment cohort who participated in at least one eligible interview. We excluded 568 respondents from the 2011 cohort and 389 respondents from the 2015 cohort who reported “other” race, with no information on education or race, or who did not provide a cognitive test at first eligible interview. We also excluded those with inconsistent chronic disease patterns (clinically-inconsistent patterns of somatic chronic disease self-report in subsequent interview years) (Cigolle et al., 2018). As a result, the final analytic study population consisted of 10,548 respondents.
2.3. Measures
2.3.1. Primary outcome: cognitive function
The cognitive function battery in the NHATS questionnaire (Kasper et al., 2013) is assessed annually and is designed to evaluate several aspects of cognitive function. This includes: (1) memory: an immediate and delayed 10-word recall with a subscale from 0 to 20; (2) orientation: querying the date, month, year, and day of the week and naming the President and Vice President with a subscale from 0 to 8; and (3) executive function: a clock drawing test with a subscale from 0 to 5. The total cognitive function score sums across the composite subscales (range: 0–33), with higher scores indicating better cognitive function. If the sampled person could not respond, a proxy interview was conducted, and an attempt was made to administer the cognitive test to the sample person. Proxy respondents are familiar with the sample person's health, daily routine, and were most often persons who lived with the sample person. Those who refused, answered “don't know” or were unable to answer received a score of 0. Additional details are described elsewhere (Kasper & Freedman, 2021).
2.3.2. Multimorbidity
Information on eight self- and proxy-reported, physician-diagnosed conditions were collected at each interview: heart disease (myocardial infarction or heart disease including angina or congestive heart failure), stroke, diabetes, hypertension, arthritis, lung disease, osteoporosis, cancer. Depression was assessed using the Patient Health Questionnaire 2 (PHQ-2), a validated screening instrument for depression in older adults (Kroenke et al., 2003), with a PHQ-2 score of ≥3 indicating a positive screen for depression (Levis et al., 2020).
Chronic disease multimorbidity categories were defined at each round. Respondents with none or a single chronic disease were categorized in the no or one disease group. To assess the role of cardiovascular and metabolic combinations, respondents with ≥2 chronic conditions were categorized as having the following specific multimorbidity combinations: (1) advanced cardiovascular consisting of respondents with multimorbidity including heart disease and/or stroke and excluding diabetes; (2) metabolic consisting of respondents with multimorbidity including diabetes and excluding heart disease and stroke; (3) advanced cardiovascular-metabolic consisting of respondents with multimorbidity including heart disease and/or stroke, and diabetes; and (4) neither advanced cardiovascular nor metabolic consisting of respondents with multimorbidity excluding heart disease, stroke, or diabetes. These configurations ensured that all respondents would be categorized into mutually-exclusive groups at each round.
2.3.3. Covariates
Baseline sociodemographic and socioeconomic characteristics were collected, including race/ethnicity (mutually-exclusive categories: non-Latino White, non-Latino Black, Latino), age, sex, highest education level attained (less than high school, high school or equivalent certificates, some college without obtaining a degree, and obtaining a college degree or greater), coupled status (binary: married/partnered = 1), and an indicator of whether the respondent has ever been covered by Medicaid during the study period as a proxy for low socioeconomic status. High waist-height ratio was calculated with the established formula, waist measurement divided by self-reported height at each round. Prior studies have indicated that WHtR is an effective anthropometric index and an appropriate measure for central adiposity (Ashwell & Gibson, 2016). In accordance with these prior studies, we included the indicator for high waist-height ratio in the analysis operationalized as WHtR ≥0.5. A cohort variable was included to indicate whether the respondent was from 2011 or 2015 NHATS entry cohort.
2.4. Statistical analysis
The descriptive characteristics of the study population at their first eligible round are reported. The relationship between race/ethnicity, multimorbidity, and cognitive function (evaluated via cognitive test score) over time was assessed using linear mixed effect models with respondent-level random intercepts and slopes. Age was treated as the time effect in the longitudinal format because it is more compatible with our study hypothesis and specific purpose to study cognitive function change over aging. We centered age at 65 years to facilitate interpretation of model trajectories. A quadratic function for age was added to the models because the coefficient was statistically significant and its inclusion improved model fit (chi-square, AIC) compared with a linear function.
To examine racial/ethnic differences in trajectories of cognitive function, we began by fitting a minimally adjusted model of cognitive score as a function of race/ethnicity, age, and their interaction (Model 1). We then extended this model with the inclusion of multimorbidity category (Model 2). Next, we adjusted for sex, education, history of Medicaid coverage, coupled status, waist-height ratio, and cohort (Model 3). Finally, we conducted tests of interactions between race and multimorbidity category and model covariates. The fully-adjusted model (Model 4) included all covariates and statistically significant interactions. Notably, the interaction of race/ethnicity with multimorbidity category was not statistically significant and not included in the final model. Marginal estimates of mean cognitive score by age were derived from fitted models and used to construct plots of cognitive trajectories by racial/ethnic group and interaction plots. Mixed effects modeling using maximum likelihood estimation was performed in STATA/SE 16.1. We accounted for intermittently missing data (round-missingness) and cohort attrition using random-forest imputation and inverse probability weighting (IPW) in a two-step process (Seaman et al., 2012). Further details regarding IPW development are included in the Appendix.
3. Results
The analytic sample consisted of 10,548 respondents who contributed a total of 43,990 repeated observations over the study period. The mean age of respondents at baseline was 77 years (SD = 7.8) and the mean baseline cognitive score was 17.8 (SD = 5.5). Non-Latino White respondents formed 71.1% of the analytic sample, while 22.4% were non-Latino Black, and 6.5% were Latino. Baseline prevalence of multimorbidity was 73.2% among Non-Latino White, 79.2% among non-Latino Black, and 74% among Latino respondents. Baseline descriptive characteristics of the respondents are presented in Table 1.
Table 1.
General characteristics of the study population at baseline (N = 10,548).
| NL-White | NL-Black | Latino | TOTAL | p value# | |
|---|---|---|---|---|---|
| N (%) | 7504(71.1) | 2362(22.4) | 682(6.5) | 10,548(100.0) | |
| Age, mean (SD) | 77.4(7.9) | 76.1(7.5) | 76.4(7.7) | 77.0(7.8) | <0.001 |
| Sex, n (%) | <0.05 | ||||
| Male | 3236 (43.1) | 939 (39.8) | 295 (43.3) | 4470 (42.4) | |
| Female | 4268 (56.9) | 1423 (60.2) | 387 (56.7) | 6078 (57.6) | |
| Education, n (%) | <0.001 | ||||
| < High School | 1266 (16.9) | 923 (39.1) | 418 (61.3) | 2607 (24.7) | |
| High School/Certificates | 2825 (37.6) | 730 (30.9) | 138 (20.2) | 3693 (35.0) | |
| Some College | 1143 (15.2) | 260 (11.0) | 48 (7.0) | 1451 (13.8) | |
| ≥ College Degree | 2270 (30.3) | 449 (19.0) | 78 (11.4) | 2797 (26.5) | |
| Cohort, n (%) | 0.123 | ||||
| Entry year 2011 | 4990 (66.5) | 1577 (66.8) | 428 (62.8) | 6995 (66.3) | |
| Entry year 2015 | 2514 (33.5) | 785 (33.2) | 254 (37.2) | 3553 (33.7) | |
| Medicaid, n (%) | <0.001 | ||||
| No | 6978 (93.0) | 1623 (68.7) | 405 (59.4) | 9006 (85.4) | |
| Yes | 526 (7.0) | 739 (31.3) | 277 (40.6) | 1542 (14.6) | |
| Coupled, n (%) | <0.001 | ||||
| No | 3325 (44.3) | 1527 (64.6) | 344 (50.4) | 5196 (49.3) | |
| Yes | 4179 (55.7) | 835 (35.4) | 338 (49.6) | 5352 (50.7) | |
| High WHtR, n (%) | <0.001 | ||||
| No | 595 (7.9) | 217 (9.2) | 22 (3.2) | 834 (7.9) | |
| Yes | 6909 (92.1) | 2145 (90.8) | 660 (96.8) | 9714 (92.1) | |
| Multimorbidity Group, n (%) | <0.001 | ||||
| No Multimorbidity—Zero Disease | 664 (8.8) | 158 (6.7) | 54 (7.9) | 876 (8.3) | |
| No Multimorbidity— One Disease | 1359 (18.1) | 335 (14.2) | 123 (18.0) | 1817 (17.2) | |
| Advanced Cardiovascular | 1558 (20.8) | 394 (16.7) | 87 (12.8) | 2039 (19.3) | |
| Metabolic | 823 (11.0) | 507 (21.5) | 146 (21.4) | 1476 (14.0) | |
| Advanced Cardiovascular-Metabolic | 717 (9.6) | 328 (13.9) | 91 (13.3) | 1136 (10.8) | |
| Neither Advanced Cardiovascular Nor Metabolic | 2383 (31.8) | 640 (27.1) | 181 (26.5) | 3204 (30.4) | |
| Cognitive Score, mean (SD) | 17.8(5.5) | 15.1(5.7) | 14.1(5.6) | 17.0(5.7) | <0.001 |
| Follow-up years, mean (SD) | 4.3(2.6) | 4.0(2.5) | 3.8(2.4) | 4.2(2.5) | <0.001 |
| Follow-up Status | |||||
| Deceased* | 1560(20.8) | 487(20.6) | 108(15.8) | 2155(20.4) | <0.01 |
| Refusal* | 1952(26.0) | 685(29.0) | 192(28.2) | 2829(26.8) | <0.05 |
| Lost (other reasons)* | 577(7.7) | 194(8.2) | 107(15.7) | 878(8.3) | <0.001 |
Abbreviations: NL = non-Latino, SD = standard deviation; WHtR = waist-height ratio; MM = multimorbidity.
Notes: High WHtR ≥0.5. #ANOVA test was used to compare continuous variables between groups. Chi-square test was used for categorical variables; *Event during follow-up period (8 years for 2011 cohort and 4 years for 2015 cohort).
Table 2 presents the results of mixed effects regression models of change in cognitive score with age. In the model unadjusted for multimorbidity status or other covariates (Model 1), non-Latino Black respondents had lower cognitive scores at age 65 (b = −2.63, 95% CI: 3.08,-2.17) and exhibited greater linear decline with age (b = −0.10, 95% CI: −0.17,-0.01) compared with non-Latino Whites. Latino respondents also demonstrated lower cognitive scores at age 65 (b = −2.84, 95% CI: −3.66,-2.03) and greater linear decline (b = −0.23, 95% CI: 0.38,-0.08).
Table 2.
Unadjusted and adjusted mixed effect models of cognitive function trajectories with age (N = 10,548).
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Linear and Quadratic Age | ||||
| Age | 0.05**(0.01,0.08) | 0.05**(0.02,0.09) | 0.07***(0.03,0.10) | 0.09***(0.05,0.13) |
| Age2 | −0.013***(-0.015,-0.012) | −0.013***(-0.015,-0.012) | −0.013***(-0.014,-0.012) | −0.014***(-0.015,-0.012) |
| Race/ethnicity | ||||
| NL-White | Reference | Reference | Reference | Reference |
| NL-Black | −2.63***(-3.08,-2.17) | −2.60***(-3.05,-2.14) | −1.31***(-1.74,-0.89) | −0.82**(-1.38,-0.26) |
| Latino | −2.84***(-3.66,-2.03) | −2.81***(-3.62,-1.99) | −0.83*(-1.58,-0.07) | −0.06(-1.07,0.96) |
| NL-White*age | Reference | Reference | Reference | Reference |
| NL-Black*age | −0.10*(-0.17,-0.01) | −0.09*(-0.17,-0.02) | −0.08*(-0.15,-0.01) | −0.08*(-0.15,-0.01) |
| Latino*age | −0.23**(-0.38,-0.08) | −0.23**(-0.38,-0.08) | −0.20**(-0.34,-0.05) | −0.19**(-0.34,-0.05) |
| NL-White*age2 | Reference | Reference | Reference | Reference |
| NL-Black*age2 | 0.0027(-0.0001,0.0056) | 0.0027(-0.0002,0.0055) | 0.0025(-0.0003,0.0053) | 0.0025(-0.0003,0.0052) |
| Latino*age2 | 0.0066*(0.0008,0.0123) | 0.0067*(0.0010,0.0124) | 0.0057*(0.0001,0.0113) | 0.0057*(0.0001,0.0113) |
| Multimorbidity Group | ||||
| No Multimorbidity—Zero Disease | Reference | Reference | Reference | |
| No Multimorbidity— One Disease | 0.06(-0.17,0.29) | 0.06(-0.16, 0.29) | 0.07(-0.15,0.29) | |
| Advanced Cardiovascular | −0.42**(-0.69,-0.14) | −0.29*(-0.55,-0.03) | −0.28*(-0.54,-0.01) | |
| Metabolic | −0.06(-0.36,0.23) | 0.02(-0.26,0.31) | 0.04(-0.24,0.32) | |
| Advanced Cardiovascular-Metabolic | −0.83***(-1.14,-0.52) | −0.58***(-0.88,-0.29) | −0.56***(-0.86,-0.27) | |
| Neither Advanced Cardiovascular Nor Metabolic | 0.37**(0.11,0.62) | 0.34**(0.10,0.58) | 0.35**(0.12,0.59) | |
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.98***(0.82,1.15) | 1.16***(0.97,1.35) | ||
| Sex*Race | ||||
| Male*NL-White | Reference | |||
| Female*NL-Black | −0.41*(-0.80,-0.01) | |||
| Female*Latino | −1.13***(-1.79,-0.46) | |||
| Education | ||||
| High School/Certificates | Reference | Reference | ||
| <High School | −2.18***(-2.42,-1.94) | −2.15***(-2.46,-1.84) | ||
| Some College | 1.15***(0.91,1.39) | 1.18***(0.92,1.44) | ||
| ≥College Degree | 2.21***(2.02,2.41) | 2.39***(2.17,2.61) | ||
| Education*Race | ||||
| High School/Certificates*NL-White | Reference | |||
| <High School*NL-Black | −0.21(-0.74,0.32) | |||
| <High School*Latino | 0.001(-0.82,0.82) | |||
| Some College*NL-Black | −0.13(-0.75,0.51) | |||
| Some College*Latino | 0.14(-0.99,1.26) | |||
| ≥College Degree*NL-Black | −0.75**(-1.27,-0.23) | |||
| ≥College Degree*Latino | −1.12(-2.30,0.05) | |||
| Ever Medicaid | ||||
| No | Reference | Reference | ||
| Yes | −1.71***(-1.95,-1.48) | −1.75***(-1.98,-1.51) | ||
| Coupled | ||||
| No | Reference | Reference | ||
| Yes | 0.18*(0.04,0.33) | 0.18*(0.04,0.33) | ||
| High WHtR | ||||
| No | Reference | Reference | ||
| Yes | 0.16(-0.001,0.31) | 0.17*(0.01,0.33) | ||
Abbreviations: IPW = inverse probability weight; NL = non-Latino; WHtR = waist-height ratio; MM = multimorbidity. Note: High WHtR ≥0.5.
Model 1: includes age (as time effect; linear and quadratic terms), race/ethnicity, and their interactions.
Model 2: Model 1 + multimorbidity group.
Model 3: Model 2 + sex, education, ever covered by Medicaid, coupled status, high WHR, entry cohort (not shown).
Model 4: Model 3 + sex*race, education*race, cohort*age, cohort*age2 (not shown).
*p < 0.05, **p < 0.01, ***p < 0.001.
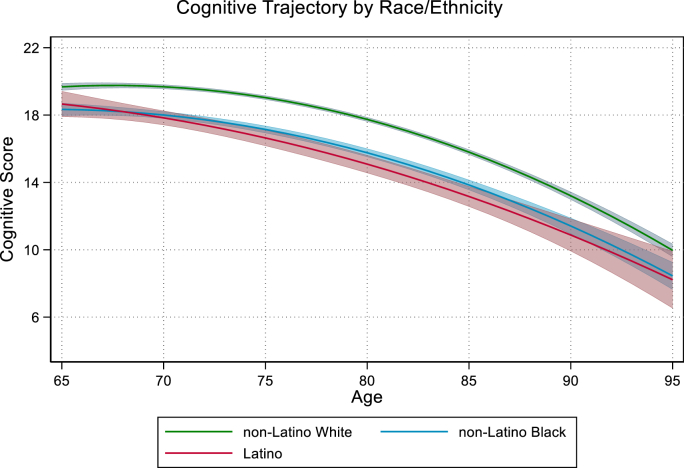
The observed differences in cognitive score were slightly attenuated in the covariate-adjusted model accounting for multimorbidity group, sex, education, coupled status, history of Medicaid coverage, waist-height ratio, and entry cohort (Model 3). Compared with Non-Latino Whites, both Non-Latino Black (b = −1.31, 95% CI: 1.74,-0.89) and Latino (b = −0.83, 95% CI: 1.58,-0.07) respondents had lower cognitive scores at age 65 and steeper declines with age (b = −0.08, 95% CI: −0.15,-0.01; b = −0.20, 95% CI: 0.34,-0.05, respectively) after controlling for covariates (Fig. 1).
Fig. 1.
Note: Bands around each trajectory represent 95% CI.
In the fully-adjusted model that included race/ethnic interactions by sex and education (Model 4), cognitive scores were lower among respondents with advanced cardiovascular (b = −0.28, 95% CI: 0.54,-0.01) and advanced cardiovascular-metabolic (b = −0.56, 95% CI: −0.86,-0.27) multimorbidity. There were no observed differences in cognitive score between respondents with no chronic conditions and a single chronic condition, although persons with neither advanced cardiovascular nor metabolic multimorbidity had higher cognitive scores than respondents without any of the reported chronic conditions of interest (b = 0.35, 95% CI: 0.12, 0.59).
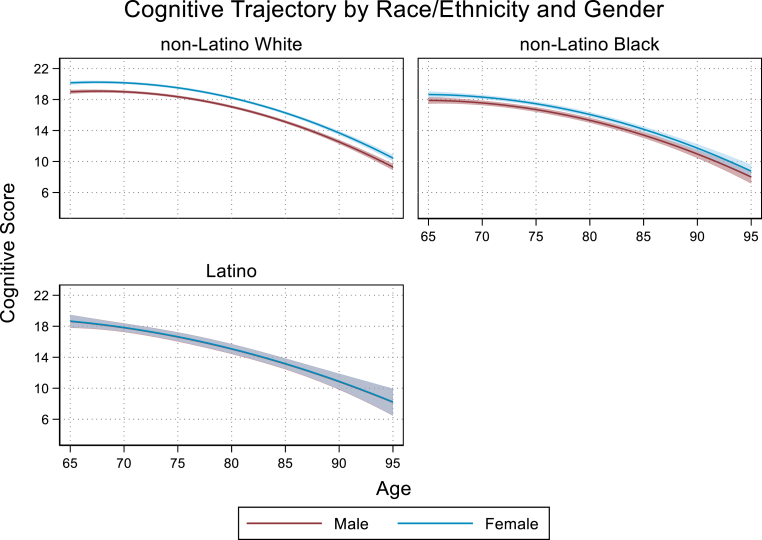
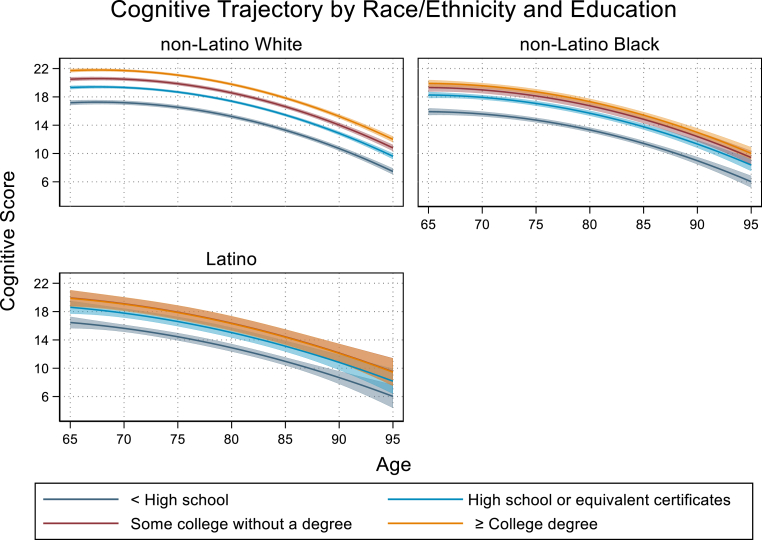
The race/ethnic group by sex interaction indicated that the magnitude of the observed difference in cognitive scores among female compared to male respondents differed significnantly between racial ethnic groups. Among non-Latino White females, cognitive score at age 65 was an average of 1.16 points higher (95% CI: 0.97,1.35) than non-Latino White males. This observed increase among females relative to males was reduced by 35% among non-Latino Black respondents (b = −0.41, 95% CI: 0.80,-0.01) and by 97% among Latino respondents (b = −1.13, 95% CI: 1.79,-0.46) (Fig. 2). Similarly, the increase in cognitive score at age 65 observed among college graduates compared to high school graduates was highest among non-Latino White respondents (b = 2.39, 95% CI: 2.17,2.61). Among non-Latino Black respondents, this observed difference in cognitive score was reduced by 31% relative to non-Latino whites (b = −0.75, 95% CI: 1.27,-0.23). Among Latino repondents we observed a similar trend in the cognitive benefit of higher education being attenuated relative to that observed among non-Latino White respondents but it did not reach statistical significance (b = −1.12, 95% CI: −2.30,0.05) (Fig. 3). Results from sensitivity analyses indicated that these findings were robust to changes in model specification and alternative approaches to intermittent missingness and loss to follow-up (see Appendix).
Fig. 2.
Notes: Bands around each trajectory represent 95% CI. Trajectories reflect interaction model findings that significant differences between non-Latino White women and men are not as pronounced between non-Latino Black women and men and are not evident between Latino women and men.
Fig. 3.
Notes: Bands around each trajectory represent 95% CI. Trajectories reflect interaction model findings that significant differences between non-Latino White adults by higher educational attainment categories are not as pronounced between non-Latino Black or Latino adults.
4. Discussion
This study identified substantial racial/ethnic disparities in the trajectories of cognitive function with age, as well as racial/ethnic differences in the association between social factors and cognitive function trajectories in a national sample of Medicare fee-for-service beneficiaries. Our findings demonstrated that while highest educational attainment was protective for cognitive decline, the benefit of education was not consistent across racial/ethnic group. While Black and Latino older adults generally sustained cognitive benefit related to having a high school diploma, there was less cognitive benefit to having a greater than high school diploma relative to White older adults. Similarly, while female sex was protective of cognitive decline for non-Latino White respondents, no such benefit for females was observed among the Black and Latino groups.
Our finding that the buffering association of education does not appear ubiquitous across racial/ethnic groups aligns with existing literature (Avila et al., 2021; Díaz-Venegas et al., 2016; Vásquez et al., 2016). The explicit evaluation of interactions by race/ethnicity, sex, and educational categories clarifies the nature of race/ethnic and educational inequities in the progression of cognitive function in later life. The totality of the research examining racial inequities in cognition highlights the importance of interpreting differences by race/ethnicity and educational attainment not only as derived from differences in cognitive reserve, but also as the result of pervasive structural inequalities in educational opportunities (Avila et al., 2021).
Similar to a number of studies in the extant literature (Bangen et al., 2019; Lamar et al., 2015; Yaffe et al., 2004), we found that metabolic and cardiovascular etiologies of cognitive decline were also present. However, a lack of a significant interaction between multimorbidity category and race/ethnicity suggests that the risk of cognitive decline for metabolic and cardiovascular combinations applies regardless of race/ethnic background and that race/ethnicity is associated with lower cognitive function above and beyond the type of multimorbidity diverse older adults have.
Notably, while we anticipated older adults with advanced cardiovascular and metabolic multimorbidity combinations would show more pronounced declines in cognitive function, we did not anticipate that adults with multimorbidity combinations that did not include heart disease, stroke, or diabetes would have better cognitive function. We present demographic information for each of the multimorbidity combination groups and the most frequent combinations present in the neither advanced cardiovascular nor metabolic combination in the Appendix. It is possible that having arthritis, hypertension, or a history of cancer may not be associated with the etiological pathway for cognitive decline, or may indicate greater engagement in cognitively-protective factors and behaviors. However, it is also likely that the chronic conditions discernible in the NHATS public dataset are not sufficiently discriminating and granular to assess specific associations of, for example, congestive heart failure, with cognition.
This study has several strengths. First, annually-collected data provide a robust set of longitudinal data over an extended period of time to evaluate cognitive changes as respondents age. Longitudinal self-reported data on race, sex, and educational attainment are particularly well-suited to assessing the role these sociodemographic and socioeconomic factors play in the progression of important clinical and patient-centered health outcomes. Second, this study brings meaningful findings of unique heterogeneities that underlie cognitive differences by race/ethnicity and sex and by race/ethnicity and education. These findings point to important insights into later life cognitive function disparities differ for Black and Latino women and for highly educated Black and Latino older adults relative to their White peers.
A few limitations should also be noted. First, we were unable to identify or assess other race/ethnicities with these data because of very small sample sizes or lack of representation in these data. Greater efforts to include larger representations of other races and ethnicities is needed to assess the importance of sociodemographic and chronic disease burden mechanisms for underrepresented groups, in particular American Indians and Alaska Natives, who are also disproportionately burdened with high levels of dementia. Second, chronic disease data were self-reported, which may be subject to underreporting with varying levels of experienced severity and symptomatology of diseases (Halm et al., 2006; Leventhal et al., 1997). However, prior studies show adequate concordance between self-reported and administrative or clinical data sources (Okura et al., 2004; Skinner et al., 2005). Additionally, others point out the importance of documenting patient-reported disease status, given that these are conditions individuals believe and understand they have (Giles et al., 1995; Petrie et al., 2007). Third, as mentioned previously, the NHATS public-use files did not allow for assessment of a more comprehensive set of chronic disease nor a more granular assessment of diagnoses—for example, congestive heart disease in lieu of the broad cardiovascular disease category. Future studies might assess socioeconomic differences in linked clinical or administrative datasets that permit these examinations, while retaining the ability to assess important educational and demographic factors. Finally, this study would benefit from improved measures of individual income. While NHATS does provided imputed income, these data were recorded biennially. We instead opted to control for history of Medicaid coverage because this captures the lowest socioeconomically disadvantaged study participants.
Our results have several important research, clinical, and policy implications. Our findings that there are substantial differences in how sociodemographic and socioeconomic factors operate for different racial/ethnic groups have relevance for programs and health policy interventions focused on reducing societal burdens of ADRD on individuals, their caregivers, and health care systems. In particular, greater efforts to assess and monitor ADRD should be encouraged, but there is a critical need to increase societal investments in programs that promote and preserve cognitive function and address structural inequalities in educational opportunities. It will be imperative to identify promising points and modes of intervention, and to increase awareness around testing and maintaining cognitive function for minoritized racial groups, regardless of educational attainment. Our findings also point to the likely life-long leveling effect of better educational and vocational opportunities. Finally, these findings suggest that specific multimorbidity profiles, and in particular those including both cardiovascular and metabolic conditions should represent a focus of clinical and research interest across racial/ethnic and sociodemographic groups. It will be important to identify disease-specific modifiable risk factors and to develop interventions to postpone cognitive decline among older minority ethnic adults.
Ethical statement
This study was conducted using publicly available NHATS data. The study was reviewed and approved by the Institutional Review Board at Oregon Health & Science University.
Author statement
Ana Quiñones: Writing - Original Draft, Conceptualization, Methodology, Resources, Supervision, Project administration, Funding acquisition; Siting Chen: Data curation, Software, Visualization, Formal analysis, Writing - Review & Editing. Corey L. Nagel: Visualization, Conceptualization, Methodology, Formal analysis, Writing - Review & Editing. Anda Botoseneanu: Conceptualization, Methodology, Writing - Review & Editing. Heather G. Allore: Conceptualization, Methodology, Writing - Review & Editing. Jason T. Newsom: Conceptualization, Methodology, Writing - Review & Editing. Stephen Thielke: Conceptualization, Methodology, Writing - Review & Editing; Jeffrey Kaye: Conceptualization, Methodology, Writing - Review & Editing.
Declaration of competing interest
None.
Acknowledgements
Declaration of sources of funding: This work was supported by the National Institute on Aging at the National Institutes of Health [grant numbers RF1AG058545 to ARQ; R01AG047891 to HGA who contributed from the Yale Claude D. Pepper Older Americans Independence Center P30AG021342 and Yale Alzheimer's Disease Research Center P30AG066508; and P30AG008017, P30AG066518, and P30AG024978 to JK]. The National Health and Aging Trends Study (NHATS) is sponsored by the National Institute on Aging [grant number U01AG032947] through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health. Content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health. The funders played no role in the design, execution, analysis, or interpretation of the data or writing of the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2022.101084.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alzheimer’s Association Alzheimer's disease facts and figures. Alzheimer's and Dementia. 2020;16:391–460. 2020. [Google Scholar]
- Aranda M.P., Kremer I.N., Hinton L., et al. Impact of dementia: Health disparities, population trends, care interventions, and economic costs. Journal of the American Geriatrics Society. 2021;69:1774–1783. doi: 10.1111/jgs.17345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell M., Gibson S. Waist-to-height ratio as an indicator of ‘early health risk’: Simpler and more predictive than using a ‘matrix’ based on BMI and waist circumference. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atti A.R., Valente S., Iodice A., et al. Metabolic syndrome, mild cognitive impairment, and dementia: A meta-analysis of longitudinal studies. American Journal of Geriatric Psychiatry. 2019;27:625–637. doi: 10.1016/j.jagp.2019.01.214. [DOI] [PubMed] [Google Scholar]
- Avila J.F., Rentería M.A., Jones R.N., et al. Education differentially contributes to cognitive reserve across racial/ethnic groups. Alzheimer's and Dementia. 2021;17:70–80. doi: 10.1002/alz.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen K.J., Armstrong N.M., Au R., Gross A.L. Metabolic syndrome and cognitive trajectories in the framingham offspring study. J Alzheimers Dis. 2019;71:931–943. doi: 10.3233/JAD-190261. [DOI] [PubMed] [Google Scholar]
- Castora-Binkley M., Peronto C.L., Edwards J.D., Small B.J. A longitudinal analysis of the influence of race on cognitive performance. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2015;70:512–518. doi: 10.1093/geronb/gbt112. [DOI] [PubMed] [Google Scholar]
- Cigolle C.T., Nagel C.L., Blaum C.S., Liang J., Quiñones A.R. Inconsistency in the self-report of chronic diseases in panel surveys: Developing an adjudication method for the health and retirement study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2018;73:901–912. doi: 10.1093/geronb/gbw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Venegas C., Downer B., Langa K.M., Wong R. Racial and ethnic differences in cognitive function among older adults in the USA. International Journal of Geriatric Psychiatry. 2016;31:1004–1012. doi: 10.1002/gps.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro K.F., Shippee T.P. Aging and cumulative inequality: How does inequality get under the skin? The Gerontologist. 2009;49:333–343. doi: 10.1093/geront/gnp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman V.A., Kasper J.D. Cohort profile: The national health and aging trends study (NHATS) International Journal of Epidemiology. 2019;48:1044–1045g. doi: 10.1093/ije/dyz109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W.H., Croft J.B., Keenan N.L., Lane M.J., Wheeler F.C. The validity of self-reported hypertension and correlates of hypertension awareness among blacks and whites within the stroke belt. American Journal of Preventive Medicine. 1995;11:163–169. [PubMed] [Google Scholar]
- Gurland B.J., Wilder D.E., Lantigua R., et al. Rates of dementia in three ethnoracial groups. International Journal of Geriatric Psychiatry. 1999;14:481–493. [PubMed] [Google Scholar]
- Halm E.A., Mora P., Leventhal H. No symptoms, No asthma: The acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthma. Chest. 2006;129:573–580. doi: 10.1378/chest.129.3.573. [DOI] [PubMed] [Google Scholar]
- Heun R., Schoepf D., Potluri R., Natalwala A. Alzheimer's disease and co-morbidity: Increased prevalence and possible risk factors of excess mortality in a naturalistic 7-year follow-up. European Psychiatry. 2013;28:40–48. doi: 10.1016/j.eurpsy.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Hurd M.D., Martorell P., Delavande A., Mullen K.J., Langa K.M. Monetary costs of dementia in the United States. New England Journal of Medicine. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Lawrence V., Zajacova A., Sneed R. Education, race/ethnicity, and multimorbidity among adults aged 30-64 in the National Health Interview Survey. SSM Popul Health. 2017;3:366–372. doi: 10.1016/j.ssmph.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkowitz E., MacLehose R.F., Gaugler J.E., Dowd B., Kuntz K.M., Kane R.L. Risk factors associated with cognitive, functional, and behavioral trajectories of newly diagnosed dementia patients. J Gerontol A Biol Sci Med Sci. 2017;72:251–258. doi: 10.1093/gerona/glw079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla A.S., Miller-Martinez D., Aneshensel C.S., Seeman T.E., Wight R.G., Chodosh J. Trajectories of cognitive function in late life in the United States: Demographic and socioeconomic predictors. American Journal of Epidemiology. 2009;170:331–342. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper J.D., Freedman V.A. Johns Hopkins University School of Public Health; Baltimore, Maryland: 2021. NHATS_User_Guide_R10_Final_Release.pdf. [Google Scholar]
- Kasper JD, Freedman VA, Spillman BC. Classification of persons by dementia status in the national health and aging trends study. Technical Paper #5 2013; Baltimore MD Johns Hopkins University School of Public Health.
- Kroenke K., Spitzer R.L., Williams J.B.W.D. The patient health questionnaire-2: Validity of a two-item depression screener. Medical Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- Lamar M., Rubin L H., Ajilore O., et al. What metabolic syndrome contributes to brain outcomes in african American & caucasian cohorts. Current Alzheimer Research. 2015;12:640–647. doi: 10.2174/1567205012666150701102325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa K.M., Larson E.B., Karlawish J.H., et al. Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimer's and Dementia. 2008;4:134–144. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal E.A., Crouch M. In: Perceptions of health and illness. Petrie K.J., Weinman J.A., editors. Routledge; New York, NY: 1997. Are there differences in perceptions of illness across the lifespan? pp. 77–102. [Google Scholar]
- Levis B., Sun Y., He C., et al. Accuracy of the PHQ-2 alone and in combination with the PHQ-9 for screening to detect major depression: Systematic Review and meta-analysis. JAMA. 2020;323:2290–2300. doi: 10.1001/jama.2020.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda E.R., Glymour M.M., Quesenberry C.P., Whitmer R.A. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer's and Dementia. 2016;12:216–224. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura Y., Urban L.H., Mahoney D.W., Jacobsen S.J., Rodeheffer R.J. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. Journal of Clinical Epidemiology. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Osei K., Gaillard T. Disparities in cardiovascular disease and type 2 diabetes risk factors in blacks and whites: Dissecting racial paradox of metabolic syndrome. Frontiers in Endocrinology. 2017;8(204):1–7. doi: 10.3389/fendo.2017.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie K.J., Jago L.A., Devcich D.A. The role of illness perceptions in patients with medical conditions. Current Opinion in Psychiatry. 2007;20:163–167. doi: 10.1097/YCO.0b013e328014a871. [DOI] [PubMed] [Google Scholar]
- Prince M.J., Bryce R., Ferri C.P. 2011. World Alzheimer Report 2011: The benefits of early diagnosis and intervention. [Google Scholar]
- Quiñones A.R., Botoseneanu A., Markwardt S., et al. Racial/ethnic differences in multimorbidity development and chronic disease accumulation for middle-aged adults. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler C., Toro P., Schönknecht P., Schröder J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer's disease. Psychiatry Research. 2012;196:90–95. doi: 10.1016/j.psychres.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Seaman S.R., White I.R., Copas A.J., Li L. Combining multiple imputation and inverse-probability weighting. Biometrics. 2012;68:129–137. doi: 10.1111/j.1541-0420.2011.01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisco S., Gross A.L., Shih R.A., et al. The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2015;70:557–567. doi: 10.1093/geronb/gbt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner K.M., Miller D.R.S., Lincoln E.M., Lee A., Kazis L.E.S. Concordance between respondent self-reports and medical records for chronic conditions: Experience from the veterans health study. Journal of Ambulatory Care Management Health Status Measurement and Ambulatory Space Management. 2005;28:102–110. doi: 10.1097/00004479-200504000-00002. [DOI] [PubMed] [Google Scholar]
- Sperling R.A., Aisen P.S., Beckett L.A., et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez E., Botoseneanu A., Bennett J.M., Shaw B.A. Racial/ethnic differences in trajectories of cognitive function in older adults: Role of education, smoking, and physical activity. Journal of Aging and Health. 2016;28:1382–1402. doi: 10.1177/0898264315620589. [DOI] [PubMed] [Google Scholar]
- Wei M.Y., Levine D.A., Zahodne L.B., Kabeto M.U., Langa K.M. Multimorbidity and cognitive decline over 14 years in older Americans. J Gerontol A Biol Sci Med Sci. 2019 doi: 10.1093/gerona/glz147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K., Kanaya A., Lindquist K., et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Zuelsdorff M., Okonkwo O.C., Norton D., et al. Stressful life events and racial disparities in cognition among middle-aged and older adults. J Alzheimers Dis. 2020;73:671–682. doi: 10.3233/JAD-190439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.