Abstract
Objective:
The coronavirus disease 2019 (COVID-19) pandemic has caused a global health crisis and may have affected healthcare-associated infection (HAI) prevention strategies. We evaluated the impact of the COVID-19 pandemic on HAI incidence in Brazilian intensive care units (ICUs).
Methods:
In this ecological study, we compared adult patients admitted to the ICU from April through June 2020 (pandemic period) with the same period in 2019 (prepandemic period) in 21 Brazilian hospitals. We used the Wilcoxon signed rank-sum test in a pairwise analysis to compare the following differences between the pandemic and the prepandemic periods: microbiologically confirmed central-line–associated bloodstream infection (CLABSI) and ventilator-associated pneumonia (VAP) incidence density (cases per 1,000 central line and ventilator days, respectively), the proportion of organisms that caused HAI, and antibiotic consumption (DDD).
Results:
We detected a significant increase in median CLABSI incidence during the pandemic: 1.60 (IQR, 0.44–4.20) vs 2.81 (IQR, 1.35–6.89) (P = .002). We did not detect a significant difference in VAP incidence between the 2 periods. In addition, we detected a significant increase in the proportion of CLABSI caused by Enterococcus faecalis and Candida spp during the pandemic, although only the latter retained statistical significance after correction for multiple comparisons. We did not detect a significant change in ceftriaxone, piperacillin–tazobactam, meropenem, or vancomycin consumption between the studied periods.
Conclusions:
There was an increase in CLABSI incidence in Brazilian ICUs during the first months of COVID-19 pandemic. Additionally, we detected an increase in the proportion of CLABSI caused by E. faecalis and Candida spp during this period. CLABSI prevention strategies must be reinforced in ICUs during the COVID-19 pandemic.
The coronavirus disease 2019 (COVID-19) pandemic has caused a health crisis around the globe. Resources routinely used to prevent healthcare-associated infection (HAI) have been redirected toward the management of the pandemic. An informal query conducted in April 2020 found that infection prevention and control (IPC) workers spent >75% of their time responding to the pandemic. The diversion of traditional HAI prevention duties may have compromised HAI monitoring and prevention. 1 Another challenge was the shortage of personal protective equipment (PPE), leading to the need for rationing its use, which in turn may have affected the spread of multidrug-resistant organisms. 2 In addition, the high rates of empirical antibiotic use in COVID-19 cases and the reduced time dedicated to antimicrobial stewardship during the pandemic may have had an effect on antimicrobial resistance. 3
Since Brazil’s first case of COVID-19 was reported on February 26, 2020, Brazil rapidly became the epicenter of the disease in Latin America and figured among the most affected countries in the world. 4 The high rates of HAI before the pandemic as well as the high rate of antimicrobial resistance in Brazil have posed additional challenges to this scenario because the high number of COVID-19 cases can be aggravated by healthcare-
associated complications. 5
In this study, we evaluated the impact of the COVID-19 pandemic on the incidence of central-line–associated bloodstream infection (CLABSI) and ventilator-associated pneumonia (VAP) in Brazilian intensive care units (ICUs).
Methods
Data sources
In this ecological study, we evaluated adult patients admitted to intensive care units (ICUs) from April through June 2020 (during the COVID-19 pandemic) compared to the same period in 2019 (before the pandemic). We collected data from 21 Brazilian hospitals from 6 different states (São Paulo, Espírito Santo, Paraná, Ceará, Pernambuco, and Maranhão) that comprise the 3 most populated Brazilian regions. The IPC team of each hospital collected clinical and laboratory data.
We collected the following data: the number of COVID-19 patients admitted to the ICUs (suspected or laboratory-confirmed cases), the total number of ward and ICU beds of each hospital, and the total number of patients admitted to adult ICUs. We also collected data on the number of patient days, central-line days, ventilator days, CLABSI, VAP, and antibiotic consumption in all adult ICUs. We classified the participating hospitals as public or private. The study protocol (no. 77243517.8) was approved by the ethics committee of each participating hospital. No patient identifiers were recorded to ensure anonymity. There was no contact with patients, and patient consent was not required.
Outcomes
The primary end points were the incidence density of CLABSI and of VAP in each hospital. The definition of these infections is shown in Supplementary Table S1 (online); they were based on the National Health Surveillance Agency (ANVISA) surveillance that was adapted from the CDC/NHSN surveillance definitions and criteria for HAI. 6
Statistical analysis
Overall, data from April through June 2019 (prepandemic period) were compared with data from April through June 2020 (pandemic period). A single incidence density measurement of CLABSI (cases per 1,000 central-line days) or VAP (cases per 1,000 ventilator days) was made in the 3-month periods before the pandemic and after the pandemic started. Categorical variables are presented as absolute numbers and percentages; continuous variables are presented as median (interquartile range, IQR) due to their nonnormal distributions. The comparison of the number of patients admitted to the ICU, patient days, central-line days, total CLABSI, CLABSI incidence density, ventilator days, total VAP, and VAP incidence density of each period were compared in a pairwise analysis using the Wilcoxon signed rank-sum test. The pairwise comparisons of the CLABSI and VAP incidence densities were also stratified by public and private hospitals. The effect of total number of patient days in April–June 2020 and proportion of ICU admissions by COVID-19 patients in April–June 2020 on the change of the incidence density of HAI between the prepandemic and the pandemic periods was evaluated by entering each of these 2 independent variables separately in a linear regression model with the change in incidence density of CLABSI or VAP as the dependent variable. The organisms that caused CLABSI and VAP from the 2 periods were compared using the Wilcoxon signed rank-sum test. Because the total number of CLABSIs and VAP cases might differ from one year to another, the proportions of each organism causing HAI in each hospital in each period were compared. Because several causative organisms were evaluated, we adjusted this analysis for multiple comparisons according to the Hochberg procedure. 7 We also compared the proportion of drug-resistant bacteria between the prepandemic and pandemic periods in a pairwise analysis using the Wilcoxon signed rank-sum test. We evaluated the proportion of carbapenem-resistance among Enterobacteriaceae, carbapenem-resistance among Pseudomonas aeruginosa and Acinetobacter baumannii (nonfermentative gram-negative bacilli), methicillin-resistance among Staphylococcus aureus, and vancomycin-resistance among Enterococcus faecalis and Enterococcus faecium. The association of antibiotic consumption (estimated by the defined daily doses (DDDs) per 1,000 patient days of selected antimicrobials) with the proportion of specific organisms causing HAI in each hospital was compared using a linear regression model. The change in the proportion of specific organisms causing HAI between the pandemic and the prepandemic periods was the dependent variable, and the changes in DDD between the 2 periods were compared as the independent variable. The linear regression models used to evaluate factors associated with changes in the proportion of organisms causing HAI and to evaluate changes in incidence density of HAI presented residuals that were approximately normally distributed and the data showed homoscedasticity. Statistical tests were 2-tailed with a significance level of .05. The SPSS version 17.0 software (IBM, Armonk, NY) and R version 4.1.0 software (R Foundation for Statistical Computing, Vienna, Austria) were used for the analyses.
Results
The 21 hospitals had a median of 164 beds (IQR, 127–275) in patient wards and 50 ICU beds (IQR, 32–85). Among them, 11 hospitals (52%) were private (Supplementary Table S2 online). Although the first hospitalizations for COVID-19 were recorded in February 2020, the number of COVID-19 hospitalizations showed a substantial increase in April 2020 and stabilized between April and June 2020 (Fig. 1). Therefore, the analyses in our study are centered on the period of April through June. We evaluated 118,704 patient days from April through June in 2019 and 2020. We could not obtain data regarding the number of COVID-19 admissions from one of the hospitals. Thus, during the study period, 4,563 patients with COVID-19 were admitted to an ICU in 1 of 20 hospitals from April through June 2020. All other analyses evaluated data from the 21 hospitals unless stated otherwise.
Fig. 1.
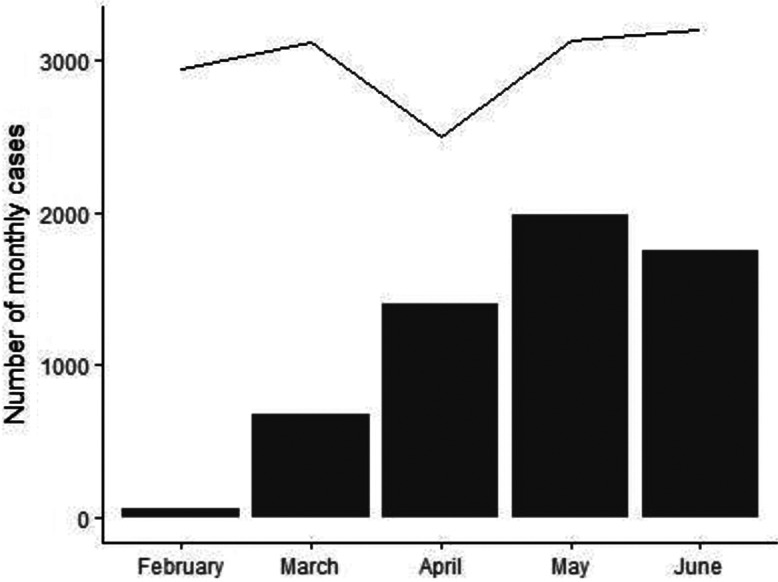
Total number of admissions (line) and number of COVID-19 admissions (bars) to the intensive care unit of 20 Brazilian hospitals from February 2020 through June 2020.
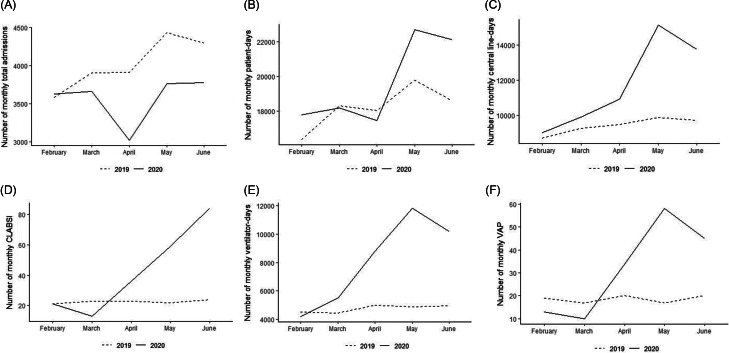
During the pandemic compared to the prepandemic period, the overall number of ICU admissions decreased and the number of patient days remained stable in the ICUs. In contrast, central-line days, CLABSIs, ventilator days, and VAPs all increased during the pandemic (Fig. 2 and Table 1).
Fig. 2.
Total number of patients admitted (A), patient days (B), central-line days (C), CLABSIs (D), ventilator days (E), and VAP cases (F) in the intensive care units of 21 Brazilian hospitals between February–June 2019 and February–June 2020.
Table 1.
Comparison of ICU Admissions, Patient Days, CLABSIs, and VAP Cases in the ICUs of 21 Brazilian Hospitals Between the Prepandemic and Early Pandemic Periods
| Variable | Median (IQR) | ||
|---|---|---|---|
| Apr–Jun 2019 | Apr–Jun 2020 |
P
Value a |
|
| No. of patients admitted to the ICU | 531 (243–889) | 357 (238–716) | .012 |
| Patient days | 2,199 (1,339–3,629) | 2,114 (1,432–3,772) | .297 |
| Central-line days | 1,137 (631–1,992) | 1,424 (934–2,311) | .001 |
| CLABSIs, no. | 2 (1–6) | 4 (2–14) | .001 |
| CLABSI incidence density (per 1,000 central-line days) | |||
| Overall (n=21) | 1.60 (0.44–4,20) | 2.81 (1.35–6.89) | .002 |
| Public hospitals (n=10) | 1.69 (0–4.14) | 4.67 (1.39–7.54) | .008 |
| Private hospitals (n=11) | 1.60 (0.99–4.60) | 2.69 (0.98–5.21) | .075 |
| Ventilator days | 674 (361–1,057) | 1,078 (725–1,881) | <.001 |
| VAP cases, no. | 2 (1–5) | 4 (1–10) | .002 |
| VAP incidence density (per 1,000 ventilator days) | |||
| Overall (n=21) | 2.99 (0.72–4.80) | 3.65 (1.38–6.46) | .167 |
| Public hospitals (n=10) | 4.65 (2.27–7.25) | 3.65 (1.14–6.43) | .767 |
| Private hospitals (n=11) | 2.54 (0–3.98) | 3.65 (1.42–6.57) | .033 |
Note. ICU, intensive care unit; IQR, interquartile range; CLABSI, central-line–associated bloodstream infection; VAP, ventilator-associated pneumonia.
Pairwise comparison of medians by the Wilcoxon signed rank-sum test.
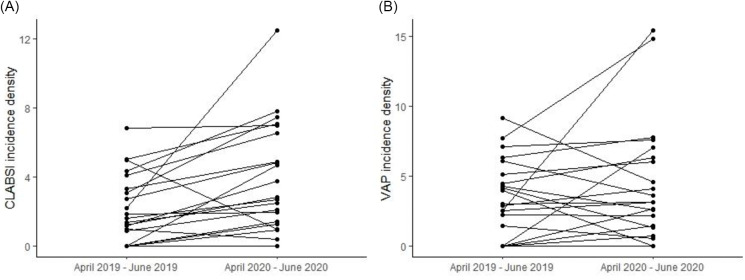
Compared to the prepandemic period, there was an increase in CLABSI incidence density during the pandemic in 18 hospitals, whereas 2 hospitals showed a decrease in CLABSI and 1 hospital did not report any CLABSI in either period (Fig. 3 and Supplementary Table S2 online). We detected an overall increase in CLABSI incidence density during the pandemic compared to the prepandemic period: 2.81 (IQR, 1.35–6.89) versus 1.60 (IQR, 0.44–4.20; P = .002 in the pairwise comparison) (Table 1). The stratified analyses showed that the increase in CLABSI reached statistical significance among public hospitals but not private hospitals (Table 1). A sensitivity analysis excluding one outlier (ie, a public hospital with an important increase in CLABSI incidence) confirmed that there was a significant increase in CLABSI incidence overall (2.75 [IQR, 1.31–6.71] vs 1.46 [IQR, 0.22–4.27]; P = .003) and among public hospitals (2.81 [IQR, 1.35–7.23] vs 1.20 [IQR, 0–4.20]; P = .012) during the pandemic compared to the prepandemic period. There was no significant effect of total number of patient days (β = 0.001; P = .635) or the proportion of ICU admissions by COVID-19 patients (β = 1.844; P = .558) on the change in CLABSI incidence density between the 2 periods.
Fig. 3.
Variation in CLABSI incidence density (A) and in VAP incidence density (B) in the ICUs of 21 Brazilian hospitals between April–June 2019 and April–June 2020.
We detected an increase in VAP incidence density in 15 hospitals during the pandemic compared to the prepandemic period. However, 5 hospitals demonstrated a decrease and 1 hospital did not have any VAP in either period (Fig. 3 and Supplementary Table S2 online). Overall, the pairwise comparison showed no significant change in VAP incidence density during the pandemic compared to the prepandemic period: 3.65 (IQR, 1.38–6.46) versus 2.99 (IQR, 0.72–4.80; P = .167) (Table 1). Private hospitals showed a significant increase in VAP incidence density, while there was no significant change in VAP incidence among public hospitals (Table 1). A sensitivity analysis excluding 2 outliers (ie, 1 private hospital and 1 public hospital with major increases in VAP incidence) between the pandemic and prepandemic periods showed no significant change in VAP incidence overall (3.64 [IQR, 1.34–6.05] vs 2.99 [IQR, 0–4.45]; P = .446) nor among private hospitals (3.14 [IQR, 1.25–6.41] vs 2.25 [IQR, 0–4.04]; P = .059) nor public hospitals (3.64 [IQR, 0.94–5.32] vs 4.14 [IQR, 1.99–6.60]; P = .327). We did not detect a significant effect of total number of patient days (β = 0.001; P = .879) nor the proportion of ICU admissions by COVID-19 patients (β = −3.618; P = .417) on the change in VAP incidence density between the 2 periods.
During the pandemic, the most frequent causes of CLABSI were Candida spp (n = 38) and coagulase negative Staphylococcus (n = 38). During the prepandemic period, Klebsiella pneumoniae (n = 16) and Candida spp (n = 10) were the most frequent causes of CLABSI (Tables 2 and Supplementary Table S3 online). We detected an increase in the proportion of CLABSI caused by E. faecalis and Candida spp as well as a decrease in the proportion of CLABSI caused by S. aureus during the pandemic. However, after adjusting for multiple comparisons in the pairwise analysis, the only significant change was the increase in E. faecalis: 0% (IQR, 0–0) versus 8% (IQR, 0–15%; P = .028).
Table 2.
Comparison of the Most Important Causative Organisms of CLABSI and VAP in the ICUs of 21 Brazilian Hospitals Between the Prepandemic and Early Pandemic Periods a
| Organism | Total Among All Hospitals, No. (%) b |
Proportion of Cases Identified in Each Hospital, Mean % (SD) |
Proportion of Cases Identified in Each Hospital Median % (IQR) | |||||
|---|---|---|---|---|---|---|---|---|
| Apr–Jun 2019 | Apr–Jun 2020 | Apr–Jun 2019 | Apr–Jun 2020 | Apr–Jun 2019 | Apr–Jun 2020 | P Value c | Adjusted. P Value d |
|
| CLABSI causative organism | ||||||||
| Candida spp | 10 (15) e | 38 (21) f | 6 (12) | 27 (33) | 0 (0–7) | 14 (0–50) | .017 | .221 |
| Enterococcus faecalis | 2 (3) | 21 (12) | 1 (4) | 14 (17) | 0 (0–0) | 8 (0–15) | .002 | .028 |
| Klebsiella pneumoniae | 16 (24) | 26 (15) | 15 (21) | 12 (23) | 0 (0–33) | 0 (0–20) | .209 | 1.000 |
| Staphylococcus aureus | 8 (12) | 10 (6) | 7 (13) | 2 (4) | 0 (0–13) | 0 (0–4) | .046 | .552 |
| Coagulase negative Staphylococcus | 10 (15) | 38 (21) | 20 (37) | 19 (26) | 0 (0–35) | 13 (0–29) | .865 | 1.000 |
| VAP causative organism | ||||||||
| Acinetobacter baumannii | 7 (12) | 24 (18) | 6 (13) | 16 (30) | 0 (0–0) | 0 (0–25) | .155 | 1.000 |
| Pseudomonas aeruginosa | 21 (37) | 44 (32) | 29 (36) | 33 (33) | 11 (0–50) | 27 (0–44) | .983 | 1.000 |
| Staphylococcus aureus | 11 (19) | 21 (15) | 19 (31) | 10 (15) | 0 (0–24) | 0 (0–20) | .650 | 1.000 |
Note. ICU, intensive care unit; IQR, interquartile range; CLABSI, central-line–associated bloodstream infection; VAP, ventilator-associated pneumonia.
The complete list of causative organisms of CLABSI and VAP (see Supplementary Table S3 online).
Some healthcare-associated infections had >1 causative organism.
Pairwise comparison of medians by the Wilcoxon signed rank-sum test (P value unadjusted for multiple comparisons).
P values adjusted for multiple comparisons according to the Hochberg procedure.
Candida spp: 2 C. albicans, 2 C. glabrata, 1 C. krusei, 4 C. parapsilosis, and 1 C. tropicalis.
Candida spp: 13 C. albicans, 8 C. glabrata, 8 C. parapsilosis, 8 C. tropicalis and 1 non-albicans Candida spp.
The most frequent causative agents of VAP were P. aeruginosa (n = 44) and Acinetobacter baumannii (n = 24) during the pandemic, and P. aeruginosa (n = 21) and S. aureus (n = 11) in the prepandemic period. We did not detect significant changes in the frequency of VAP causative agents between the 2 periods (Tables 2 and Supplementary Table S3 online).
Among the causative agents of CLABSI and VAP evaluated together, there was a non–statistically significant increase in the rate of carbapenem resistance among Enterobacteriaceae isolates and methicillin resistance among Staphylococcus aureus isolates between the 2 periods (Table 3).
Table 3.
Comparison of the Rate of Drug-Resistant Organisms Causing HAIs in the ICUs of 21 Brazilian Hospitals Between the Prepandemic and Early Pandemic Periods
| HAI Causative Organism | Total Isolates With Antimicrobial Resistance Among All Hospitals, No. (%) a,b |
Proportion of Isolates with Antimicrobial Resistance in Each Hospital, Mean % (SD) b |
Proportion of Isolates with Antimicrobial Resistance in Each Hospital, Median % (IQR) b |
||||
|---|---|---|---|---|---|---|---|
| Apr–Jun 2019 | Apr–Jun 2020 | Apr–Jun 2019 | Apr–Jun 2020 | Apr–Jun 2019 | Apr–Jun 2020 | P Value c |
|
| Carbapenem-resistant Enterobacteriaceae | 15 (32) | 34 (44) | 18 (36) | 22 (35) | 0 (0–13) | 0 (0–46) | .878 |
| Carbapenem-resistant nonfermentative gram-negative bacilli | 28 (68) | 67 (67) | 26 (44) | 35 (44) | 0 (0–75) | 0 (0–88) | .397 |
| Oxacillin-resistant Staphylococcus aureus | 10 (53) | 21 (68) | 14 (32) | 17 (34) | 0 (0–0) | 0 (0–25) | .450 |
| Vancomycin-resistant Enterococcus | 1 (33) | 7 (23) | 5 (22) | 15 (32) | 0 (0–0) | 0 (0–10) | .066 |
Note. HAI, healthcare-associated infection; ICU, intensive care unit; SD, standard deviation; IQR, interquartile range.
Some healthcare-associated infections had >1 causative organism.
Proportions were calculated among each group of organisms (eg, proportion of oxacillin-resistant Staphylococcus aureus was calculated dividing the number of oxacillin-resistant Staphylococcus aureus by the number of Staphylococcus aureus identified).
Pairwise comparison of medians by the Wilcoxon signed rank-sum test.
We had data on antimicrobial consumption from 16 hospitals. Although we detected an increase in median ceftriaxone and meropenem DDD during the pandemic, there was no significant change in median ceftriaxone, piperacillin–tazobactam, meropenem, or vancomycin DDD between the prepandemic and the pandemic periods (Supplementary Table S4 online). We did not detect an association between the change in ceftriaxone consumption and the difference in the proportion of CLABSI caused by Enterococcus faecalis between the 2 periods (β = 0.001; P = .726). Similarly, we did not detect an association between the change in piperacillin–tazobactam (β = 0.001; P = .942), meropenem (β = −0.002; P = .260), or vancomycin (β = .001; P = .704) consumption, nor for the difference in the proportion of CLABSI caused by Candida spp between the 2 periods.
Discussion
Our study demonstrated that there was a substantial increase in CLABSI incidence in Brazilian ICUs during the early months of the COVID-19 pandemic. On the other hand, we did not detect significant variation in VAP incidence. We also observedan increase in the proportion of CLABSI caused by Enterococcus faecalis and Candida spp during this period.
The increase in CLABSI in Brazilian ICUs during the pandemic in our study corroborates the increase in CLABSI demonstrated in a multicenter study evaluating 78 ICUs in the United States during the pandemic compared to the prepandemic period. 8 Additionally, COVID-19 patients are at higher risk of CLABSI acquisition than non–COVID-19 patients. 8 In contrast, another study did not find a significant variation in CLABSI incidence in the ICU during the COVID-19 pandemic compared to the 2 preceding years. 9 This difference might be related to distinct impacts of the pandemic on HAI prevention strategies in different hospitals.
The absence of significant variation in VAP incidence during the pandemic in our study corroborates the finding of another investigation. 9 However, other studies have already demonstrated an increase in VAP incidence during the pandemic, suggesting a higher risk of VAP in COVID-19 patients compared to non–COVID-19 patients on invasive mechanical ventilation. 10–12 The contrast between the findings of those studies and our study might be related to differences in IPC practices among the different hospitals.
Several factors could explain the increase in HAIs during the pandemic. The increase in central-line days and ventilator days during the pandemic in our study suggest an increased need of invasive procedures and an increased severity of ICU patients during this period. The increased severity of ICU patients could explain at least in part the higher risk of HAI during the pandemic. 13 The increase in healthcare workers workload has also been demonstrated to increase the risk for HAI in ICUs. 14 Therefore, the high workload of healthcare workers during the pandemic might also have influenced the incidence of HAI. However, the number of patient days and the proportion of COVID-19 patients admitted to the ICU, which could be considered proxies of workload during the pandemic, did not show a significant association with HAI incidence in our study. In addition to these factors, we hypothesize that the IPC teams from different hospitals might have been overwhelmed with the increased workload during the pandemic, and this may have negatively influenced basic infection control measures and favored the occurrence of HAI. Nevertheless, the confirmation of the association between IPC team workload and HAI incidence needs to be explored in future studies.
Our study results also demonstrated changes in the causative agents of CLABSI during the pandemic. We detected an increase in the proportion of CLABSI caused by E. faecalis and Candida spp, although the increase in the latter did not retain statistical significance after correction for multiple comparisons. The increase in hospital infection caused by these 2 microorganisms during the pandemic have been demonstrated in several studies. 8,15,16 Prolonged use of central lines might have contributed to the increase in CLABSI caused by Candida spp. 17 We had hypothesized that the use of antimicrobials could have contributed to the increase in CLABSI caused by E. faecalis 18 and Candida spp. 19,20 However, we did not find an association between ceftriaxone use and enterococcal CLABSI or between broad-spectrum antibiotic use and CLABSI caused by Candida spp in our study.
Although there was no significant variation in the proportion of HAI caused by carbapenem-resistant gram-negative bacilli infection and methicillin-resistant S. aureus on the comparison between the pandemic and prepandemic periods in our study, we observed a trend toward the increase in HAI caused by drug-resistant organisms during the pandemic. A single-center study also demonstrated an increase in hospital infections caused by drug-resistant organisms during the pandemic. 16 On the other hand, other studies have shown stability or decreases in HAIs caused by multidrug-resistant organisms during the pandemic. 9,21,22 The differences in the rates of multidrug-resistant HAI among distinct hospitals during the pandemic may be due to differences in empiric antimicrobial treatments practices and in preexisting drug-resistant microorganisms colonization patterns.
Our study had several limitations. Data from a longer period and from hospitals of other Brazilian regions, would have better represented the pandemic across the country. In addition, it was not possible to evaluate the use of immunosuppressant medications, which could have influenced HAIs. However, we evaluated the early months of the pandemic, when the use of immunosuppressant medications was not standard of care for COVID-19 management in Brazil. Thus, we did not expect a marked increase in immunosuppressant use during the pandemic period evaluated.
In conclusion, our study showed an important increase in CLABSI incidence in Brazilian ICUs during the early months of COVID-19 pandemic. However, there was no significant change in VAP incidence in the same period. Additionally, during the first months of the pandemic, we detected an increase in the proportion of CLABSI caused by E. faecalis and Candida spp. Therefore, HAI prevention strategies, especially regarding prevention of CLABSI, must be reinforced in adult ICUs during the COVID-19 pandemic.
Acknowledgments
We thank the HAI/COVID-19 Brazilian task force: Ana Carina Serfaty (Hospital Santa Paula, São Paulo/SP); Ana Carolina D’Ettorres (Hospital Unimed Vitória, Vitória/ES); Ariane Melare Ramos dos Santos (Hospital Estadual de Vila Alpina, São Paulo/SP); Chayenne Mika Matsumoto Pinto Tonheiro (Hospital Paulistano, São Paulo, SP); Christianne Fernandes Valente Takeda (Hospital São José, Fortaleza, CE); Cibelle Soares Saturnino (Real Hospital Portugues de Beneficência, Recife, PE); Cibelly Bono (Hospital Universitário de Londrina, Londrina, PR); Clenia Vanusa Cavalcanti de Siqueira (Real Hospital Portugues de Beneficência, Recife, PE); Demétrius Montenegro (Real Hospital Portugues de Beneficência, Recife, PE); Diana Hilda Teixeira de França (Hospital Santa Catarina, São Paulo, SP); Eduarda Beraldo (Hospital Universitário de Londrina, Londrina, PR); Elaine Maria de Freitas (Hospital Estadual de Diadema Governador Orestes Quercia, Diadema, SP); Fabiana Veríssimo dos Santos (Real Hospital Portugues de Beneficência, Recife, PE); Fernanda Karoline Macedo Nascimento Farias (Real Hospital Portugues de Beneficência, Recife, PE); Fernando Lazzaro Rodrigues (Hospital Estadual de Sapopemba, São Paulo, SP); Geiza Karla Barcellos Oliveira (Hospital Unimed Vitória, Vitória, ES); Gerlany Gisely Bezerra da Silva (Real Hospital Portugues de Beneficência, Recife, PE); Jorge Luiz Nobre Rodrigues (Hospital Universitário Walter Cantídio, Fortaleza, CE); Jacqueline Roque Ferrari (Hospital Estadual de Sapopemba, São Paulo, SP); Jéssica Lieto Campos (Hospital São Luiz Unidade São Caetano, São Caetano do Sul, SP); José Maria Francisco dos Santos Cardoso (Hospital Estadual de Sapopemba, São Paulo/SP); Joseane Pascual (Hospital Universitário de Londrina, Londrina, PR); Juliana Salles de Carvalho (Hospital Estadual de Vila Alpina, São Paulo, SP); Lauro Vieira Perdigão Neto (Hospital Paulistano, São Paulo, SP); Lícia Borges Pontes (Hospital Regional da Unimed, Fortaleza, CE); Lumena Vaz (Hospital Santa Catarina, São Paulo, SP); Marcelle Gonçalves da Rocha (Hospital Santa Catarina, São Paulo, SP); Maysa Bonfleur Alves (Hospital Santa Catarina, São Paulo, SP); Michelli França Evaristo (Real Hospital Portugues de Beneficência, Recife, PE); Mirela Lucia Gigek Lopes (Hospital Estadual de Sapopemba, São Paulo, SP); Nataly Tiago Santos (Hospital Santa Paula, São Paulo, SP); Nathalia Gabriella Catão Ferreira Verçosa Leite (Real Hospital Portugues de Beneficência, Recife, PE); Neuza Figueira (Hospital Universitário de Londrina, Londrina, PR); Priscila Maia de Souza Carvalho (Real Hospital Portugues de Beneficência, Recife, PE); Renata Belei (Hospital Universitário de Londrina, Londrina, PR); Rosangela Cipriano de Souza (Hospital São Domingos (São Luis, MA); Sandra Maria Dias (Hospital Universitário de Londrina, Londrina, PR); Sandra Nascimento dos Anjos (Hospital Paulistano, São Paulo, SP); Vivian Biazon Feijó (Hospital Universitário de Londrina, Londrina, PR); Zuleide Nunes Honorato (Hospital Estadual de Diadema Governador Orestes Quercia, Diadema, SP).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/ice.2022.65.
click here to view supplementary material
Financial support
No financial support was acquired for this article.
Conflicts of interest
The authors declare no conflict of interest relevant to this article.
References
- 1. Stevens MP, Doll M, Pryor R, et al. Impact of COVID-19 on traditional healthcare-associated infection prevention efforts. Infect Control Hosp Epidemiol 2020;41:946–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ranney M, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the COVID-19 pandemic. N Engl J Med 2020;382:e41. [DOI] [PubMed] [Google Scholar]
- 3. Stevens MP, Patel PK, Nori P. Involving antimicrobial stewardship programs in COVID-19 response efforts: all hands on deck. Infect Control Hosp Epidemiol 2020;41:744–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodriguez-Morales AJ, Gallego V, Escalera-Antezana JP, et al. COVID-19 in Latin America: the implications of the first confirmed case in Brazil. Travel Med Infect Dis 2020;35:101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ribas RM, Campos PA, Brito CS, et al. Coronavirus disease 2019 (COVID-19) and healthcare-associated infections: emerging and future challenges for public health in Brazil. Travel Med Infect Dis 2020;37:101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Critérios diagnósticos de infecções relacionadas à assistência à saúde/agência nacional de vigilância sanitária Brasília. ANVISA website. http://portal.anvisa.gov.br/documents/33852/3507912/Caderno+2+-+Critérios+Diagnósticos+de+Infecção+Relacionada+à+Assistência+à+Saúde/7485b45a-074f-4b34-8868-61f1e5724501. Published 2017. Accessed February 22, 2021.
- 7. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988;75:800–802. [Google Scholar]
- 8. Fakih MG, Bufalino A, Sturm L, et al. COVID-19 pandemic, CLABSI, and CAUTI: the urgent need to refocus on hardwiring prevention efforts. Infect Control Hosp Epidemiol 2022;43:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wee LEI, Conceicao EP, Tan JY, et al. Unintended consequences of infection prevention and control measures during COVID-19 pandemic. Am J Infect Control 2021;49:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maes M, Higginson E, Pereira-Dias J, et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care 2021;25:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Razazi K, Arrestier R, Haudebourg AF, et al. Risks of ventilator-associated pneumonia and invasive pulmonary aspergillosis in patients with viral acute respiratory distress syndrome related or not to coronavirus-19 disease. Crit Care 2020;24:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rouzé A, Martin-Loeches I, Povoa P, et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med 2021;47:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of healthcare-associated infections. N Engl J Med 2014;370:1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daud-Gallotti RM, Costa SF, Guimarães T, et al. Nursing workload as a risk factor for healthcare associated infections in ICU: a prospective study. PLoS One 2012;7:e52342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamidi AA, Yilmaz S. Antibiotic consumption in the hospital during COVID-19 pandemic, distribution of bacterial agents and antimicrobial resistance: a single-center study. J Surg Med 2021;5:124–127. [Google Scholar]
- 16. Cultrera R, Barozzi A, Libanore M, et al. Coinfections in critically ill patients with or without COVID-19: a comparison of clinical microbial culture findings. Int J Environ Res Public Health 2021;18:4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poissy J, Damonti L, Bignon A, et al. Risk factors for candidemia: a prospective matched case–control study. Crit Care 2020;24:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pallares R, Pujol M, Peña C, et al. Cephalosporins as risk factor for nosocomial Enterococcus faecalis bacteremia: a matched case-control study. Arch Intern Med 1993;153:1581–1586. [PubMed] [Google Scholar]
- 19. Hebert C, Villaran R, Tolentino J, et al. Prior antimicrobial exposure and the risk for bloodstream infection with fluconazole-nonsusceptible Candida strains. Scand J Infect Dis 2010;42:506–509. [DOI] [PubMed] [Google Scholar]
- 20. Barberino MG, Silva N, Rebouças C, Barreiro K, et al. Evaluation of bloodstream infections by Candida in three tertiary hospitals in Salvador, Brazil: a case-control study. Braz J Infect Dis 2006;10:36–40. [DOI] [PubMed] [Google Scholar]
- 21. Lo SH, Lin CY, Hung CT, et al. The impact of universal face masking and enhanced hand hygiene for COVID-19 disease prevention on the incidence of hospital-acquired infections in a Taiwanese hospital. Int J Infect Dis 2020;104:15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guisado-Gil AB, Infante-Domínguez C, Peñalva G, et al. Impact of the COVID-19 pandemic on antimicrobial consumption and hospital-acquired candidemia and multidrug-resistant bloodstream infections. Antibiotics 2020;9:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/ice.2022.65.
click here to view supplementary material