Abstract
Introduction
Maternal near miss (MNM) is a useful means to examine quality of obstetric care. Since the introduction of the WHO MNM criteria in 2011, it has been tested and validated, and is being used globally. We sought to systematically review all available studies using the WHO MNM criteria to develop global and regional estimates of MNM frequency and examine its application across settings.
Methods
We conducted a systematic review by implementing a comprehensive literature search from 2011 to 2018 in six databases with no language restrictions. The predefined data collection tool included sections on study characteristics, frequency of near-miss cases and study quality. Meta-analysis was performed by regional groupings. Reported adaptations, modifications and remarks about application were extracted.
Results
7292 articles were screened by title and abstract, and 264 articles were retrieved for full text review for the meta-analysis. An additional 230 articles were screened for experiences with application of the WHO MNM criteria. Sixty studies with near-miss data from 56 countries were included in the meta-analysis. The pooled global near-miss estimate was 1.4% (95% CI 0.4% to 2.5%) with regional variation in MNM frequency. Of the 20 studies that made adaptations to the criteria, 19 were from low-resource settings where lab-based criteria were adapted due to resource limitations.
Conclusions
The WHO MNM criteria have enabled the comparison of global and sub-national estimates of MNM frequency. There has been good uptake in low-resource countries but contextual adaptations are necessary.
Keywords: maternal health, maternal mortality, quality of care
Key questions.
What is already known?
In recent years, there has been growing interest in the application of the maternal nearmiss (MNM) concept as an adjunct to maternal mortality to improve quality of care.
⁃A previously conducted systematic review in 2012 on the prevalence of MNM found that there were variations in the criteria used to identify MNM cases and it was limited in its assessment of the WHO MNM criteria as it was newly introduced.
What are the new findings?
Using the WHO MNM criteria, the pooled global near-miss estimate was 1.4% (95% CI 0.4% to 2.5%) and there was no substantial between-study heterogeneity.
In low-resource countries, the WHO MNM criteria were modified and excluded a number of laboratory tests and interventions due to resource constraints.
The most commonly modified intervention was the threshold for blood transfusion.
What do the new findings imply?
The WHO MNM criteria have enabled standardised case identification and allowed for the comparison of global and subnational estimates of MNM frequency and inform quality improvement efforts.
There has been good uptake of the WHO MNM criteria in low-resource settings.
Due to resource constraints in low-resource settings, there might be a need for local adaptation, where appropriate.
Background
During the last two decades, there has been a substantial and worldwide reduction of maternal mortality.1 As maternal deaths have dropped significantly over the past two decades, measurement of maternal morbidity is crucial to the ongoing elaboration of the post-2015 Sustainable Development Goals (SDGs). Studying maternal near miss (MNM), women who nearly died but survived a complication during pregnancy, childbirth or post partum, is increasingly recognised as a useful means to examine quality of obstetric care.2
As the frequency of MNM cases at the facility level are generally higher than maternal deaths, a sufficient number of cases can generate consistent and actionable information to improve quality of care. MNM also allows to facilities to work on cases with a survival outcome, enabling open discussions and reducing the fear of blame. However, routine implementation and wider application of the near-miss approach in reviewing clinical care has been limited due to the lack of a standard definition and uniform case-identification criteria. During the 1990s and early 2000s, there was a multitude of operational definitions of MNM that made it difficult to obtain overall estimates based on those definitions.
In 2009, a World Health Organization (WHO) technical working group was established, and published an identification criteria for near miss which aims to capture standardised data that allows for comparability of the quality of care between settings, geographical areas and across time.3
WHO has previously conducted two systematic reviews in 2004 and 2012 to report on the prevalence of near miss.4 5 The previous systematic reviews included all available studies on near miss with an emphasis on the different definitions of near miss and the criteria for identification of the cases. The updated systematic review in 2012 found that there were variations in the criteria used to identify MNM cases especially in the literature published before 2011.5 Since 2011, the WHO NM criteria have been tested and validated and therefore, the first objective of our study was to determine the global and regional frequency ofMNM using studies that employed the WHO MNM criteria. Our second objective was to examine adaptions and modifications of the WHO MNM criteria especially in resource- limited settings where the uptake of the WHO MNM criteria has been high6 and the WHO has advocated for using context-relevant definitions in these settings.
Methods
Systematic review and meta-analysis
Search strategy
We performed a literature search to identify peer-reviewed articles published between 1 January 2011 and 31 December 2018. The databases that were searched include: PubMed, Embase, Lilacs and Popline. We also searched the following Regional Indexes Medici, coordinated by each WHO Regional Office: African Index Medicus, Index Medicus for Eastern Mediterranean Region Western Pacific Region Index Medicus (Index Medicus for South-East Asia Region. Reference lists of the included articles were also reviewed in order to further identify eligible studies. The search strategy in table 1 reflects the main framework and key search terms. The main framework of the search strategy was adapted for each of the databases.
Table 1.
Search strategy
| 1 | WHO near miss.mp. |
| 2 | near miss.mp. |
| 3 | 2 not 1 |
| 4 | near miss*.mp. |
| 5 | exp Pregnancy Complications/ |
| 6 | exp Pregnancy Outcome/ |
| 7 | exp Maternal Mortality/ |
| 8 | exp Maternal Health/ |
| 9 | exp Maternal Health Services/ |
| 10 | exp Postpartum Period/ |
| 11 | exp Pregnancy/ |
| 12 | exp Maternal Welfare/ |
| 13 | (matern* or mother* or pregnan* or obstetric* or postpartum or post-partum).mp. |
| 14 | 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 |
Selection criteria
Studies were eligible for inclusion for the meta-analysis if they: (1) contained near-miss incidence or prevalence data; (2) included data from 2011 onwards; (3) had a sample size of ≥200 subjects; (4) were published between 1 January 2011 and 31 December 2018; and (5) used the WHO MNM criteria. There were no language restrictions in place. All authors reviewed publications in English. Publications in Spanish and Portuguese were translated in full by JPS and CLTR.
Data extraction
The titles and abstracts of all identified citations for the meta-analysis were reviewed independently by at least two assessors (CLTR, JPS or OT). Three reviewers (TF, CL and CLTR) independently screened titles and abstracts of the studies for the narrative review. Full text copies of any studies that were considered to be potentially eligible for inclusion by at least one assessor were retrieved. The full text articles were assessed for inclusion independently by two reviewers (CLTR and JPS), and any discrepancies were resolved by discussion, or by consulting the third reviewer (OT) if consensus could not be reached. The reviewers were not blinded to the authorship or results of the included studies. Any discrepancies were again resolved by discussion, or by consulting the third reviewer.
Data were extracted from the full text articles independently by two reviewers (CLTR and JPS), using a predefined and piloted data collection form that was initially developed for the 2012 review, and updated accordingly for the current meta-analysis. Data were extracted on the general study characteristics (eg, study design, population, setting), and the prevalence/incidence of MNM and the definition/identification criteria used. The authors of the original studies were contacted if additional information or clarification was required. We adapted the National Institute of Health (NIH) Quality Assessment Tool Observational Cohort and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) for the purposes of this study and rated studies as good, fair or poor quality.
Data analysis
The characteristics of each study were described, along with the reported measures of MNM frequency. The incidence or prevalence measures were calculated by dividing the number of identified MNM cases by the number of deliveries or live births (whichever was reported in the study) that occurred during the study period. We aggregated MNM incidence and prevalence to determine frequency. NM frequency was reported as ranges and by SDG regional groupings (https://unstats.un.org/sdgs/report/2019/regional-groups/).
We performed a meta-analysis as we only included studies that applied the WHO MNM criteria, and therefore, would not have variable definitions of near miss. Estimates were reported as percentages with 95% CIs. Between study heterogeneity was assessed using the I2 statistic. All of the analyses were performed using STATA V.16 (StataCorp).
Experiences with application of WHO NM criteria
We reviewed papers included in the meta-analysis to understand the experiences with application by examining adaptions or modifications that were made to the WHO MNM criteria. As we hypothesised that the WHO MNM criteria would be highly used in low-resource settings and possibly require contextual adaptations, we conducted an additional search to identify further papers from these settings. We defined low-resource settings as low-income and lower-middle-income countries as defined by the World Bank Country and Lending Groups (https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups). We searched the same databases as the meta-analysis and used the same search terms as the systematic review with the addition of ‘low’ and ‘lower middle-income countries’. We handsearched references of pertinent papers to identify further eligible papers. A grey literature search using Google was also performed.
The methods section of each study was carefully reviewed to determine whether adaptations or modifications were made to one or more aspects of the WHO MNM criteria (severe maternal complications, critical interventions or intensive care unit (ICU) use and life-threatening conditions). The discussion section of studies was also reviewed for remarks on challenges to applying the WHO MNM criteria in the study setting. Data were extracted on general study characteristics (eg, study design, setting and country) and adaptations made to each aspect of the MNM criteria Relevant text on challenges to applicability were also extracted. The data are reported in a narrative fashion.
Patient and public involvement
As this is a systematic review, there was no patient or public involvement
Results
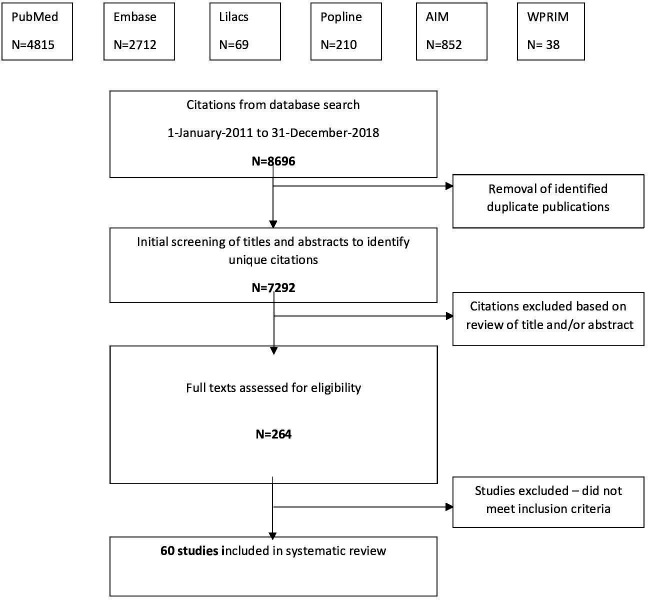
A total of 7292 articles underwent initial screening by title and abstract, 264 had a full text evaluation, and a total of 60 studies met all of the criteria for inclusion for the meta-analysis (figure 1).7–66 Four of these studies were multi-country studies.18 27 39 55 The remaining 56 studies were from 24 countries, with 15 studies (27%) from Sub-Saharan Africa, 3 studies (5%) from Northern Africa and Western Asia, 15 studies (27%) from Central and Southern Asia, 3 studies (5%) from Eastern and South-Eastern Asia, 14 studies (25%) from Latin America and the Caribbean, 5 studies (9%) from Oceania and one study (2%) from Europe and Northern America. Only four studies were from high-income countries and 19 studies were from upper-middle-income countries. The majority were cross sectional studies (57%) and all studies used data from facilities, mainly tertiary care hospitals. Only 10 studies used deliveries as the denominator to report MNM rate while the other 50 studies used live births as the denominator. Study characteristics are detailed in online supplemental table S1. Seventy-three percent of the studies were rated as good quality, 25% were of fair quality and only one study (4%) was found to be of poor quality (online supplemental table S2).
Figure 1.
Review and selection of articles.
bmjgh-2021-007077supp001.pdf (297.8KB, pdf)
Table 2 summarizes the MNM frequencies and meta-analysis results by region.
Table 2.
Overview of near miss frequency and meta-analysis by region
| Region | Near miss frequency (range) [N=60 studies] |
Near miss meta-analysis ES [95% CI] [N= 57 studies] |
| Central and Southern Asia | 1.67–120 | 0.022 [95% CI −0.012 to 0.055] |
| Eastern and South Eastern Asia | 2.1–12.7 | 0.006 [95% CI −0.090 to 0.102] |
| Sub Saharan Africa | 0.39–198.5 | 0.024 [95% CI −0.004 to 0.052] |
| Latin America and the Caribbean | 2.2–77.2 | 0.011 [95% CI −0.001 to 0.023] |
| Northern Africa and Western Asia | 3.1–12.9 | 0.007 [95% CI −0.087 to 0.100] |
| Oceania | 4.8–25.4 | 0.016 [95% CI −0.093 to 0.126] |
| Europe and Northern America | 47.9 | 0.048 [95% CI −0.031 to 0.127] |
Near miss by SDG regional groupings
Online supplemental table 3 shows the near-miss ratios in each region. Sub-Saharan Africa had the largest variation, with the lower near-miss ratio being 0.39/1000 live births and the upper near-miss ratio being 198.5/ 1000 live births. Latin America and the Caribbean also had a variable range, 2.2–77. 2/1000 live births. In Central and Southern Asia, the near-miss ratio ranged from 1.67–120/ 1000 live births. NM ratios in Eastern and South-Eastern Asia (2.1–12.7/1000 live births) and Northern Africa and Western Asia (3.1–12.9/ 1000 live births) were similar. Studies from Oceania had a reported range of 4.8–25.4/ 1000 live births. There was only one study from Europe and Northern America, from the USA, which had a near-miss rate of 47.9/ 1000 live births.7 This study specifically looked at a multi-ethnic population and sought to determine differences in near-miss rates between different ethnic groups.
Meta-analysis of near miss using WHO approach
Three studies were excluded from the meta-analysis. Using the same approach as the 2012 review, two studies were deemed to be outliers as the data points were out of range when compared with other regional data points.25 63 One multicountry study was excluded because we could not determine the data for individual countries included in the study.55
The pooled near-miss estimate using the WHO criteria was 1.4% (95% CI 0.4% to 2.5%). Confidence intervals (CI) in the analysis were generally narrow (Online supplemental table S4a). Our data do not show substantial between-study heterogeneity (online supplemental table S4b).
Regional meta-analysis found a pooled near-miss estimate of 0.6% in Eastern and South-Eastern Asia, 0.7% in Northern Africa and Western Asia, 1.1% in Latin America and the Caribbean, 1.6% in Oceania, 2.2% in Central and Southern Asia, 2.4% in sub-Saharan Africa and 4.8% in Europe and Northern America.
Experiences of application of WHO NM criteria
In addition to reviewing each of the papers included in the meta-analysis, we screened an additional 230 articles for adaptations, modifications and remarks on application of the WHO NM criteria. A total of 20 studies from 14 countries met our inclusion criteria of modifying or adapting the WHO MNM criteria (online supplemental table S5).13 17 23–25 30 43 44 49–52 54 60 64 67–71
Fifteen studies from the meta-analysis included adaptations to the WHO MNM criteria.13 17 23–25 30 43 44 49–52 54 60 64 Fourteen of these studies were from low-resource settings and only one study was from an upper-middle-income country.17 The additional search yielded five more papers, all of which were from low-resource settings.67–71
Twelve of the included studies were from sub-Saharan Africa,13 23 43 44 52 54 64 67–71 one study each from Northern Africa and Western Asia,50 three studies from Central and Southern Asia,16 25 30 two studies from Eastern and Southeastern Asia17 24 and two studies from Oceania.49 51 All were observational studies with one study being a population-based study24 and another being a mixed-methods descriptive study.16 Almost all were set in tertiary, teaching or referral hospitals. Five studies were set in district hospitals16 49 51 68–70 including one study which was set in rural district hospitals.69 Two studies adapted the WHO MNM criteria for use at the community level.16 24
At the level of tertiary or district hospitals, nine studies expanded the criteria for severe maternal complications to include conditions such as obstructed labour, uterine infection, complications of caesarean section and malaria.23 43 50 54 60 64 67 68 71 Four studies included additional criteria for life threatening conditions.30 44 50 68 Three of these studies included anaemia but with varying definitions.30 50 68 The most commonly excluded laboratory criteria due to unavailability were lactate (10 studies), pH (11 studies), arterial oxygen tension (pAO2)/fractional inspired oxygen (FiO2) (10 studies), urine ketoacids (6 studies), creatinine (3 studies), bilirubin (four studies) and platelets (1 study). Interventions excluded due to unavailability were dialysis (five studies) and continuous use of vasoactive drugs (six studies). The threshold for blood transfusion was modified in 63% of the studies (n=12).13 23 44 49–51 64 67–71 One study included all types of blood products whereas one study modified it to ≥4 units, four studies modified it to ≥3 units and 3 studies each to ≥2 units and ≥1 unit, respectively.
The two studies that modified the WHO MNM criteria for use in the community applied them at community level birthing centres in Nepal16 and in villages in four districts in Lao People’s Democratic Republic.24 Both of these studies predominantly used clinical markers for organ dysfunction. The study from Nepal also expanded the criteria for severe maternal complications to include severe postpartum hemorrhage, placenta previa and obstructed/prolonged labour.16
Although regional comparisons cannot not be made, the Haydom criteria was used in two studies in Tanzania13 and Rwanda,69 and the Papua New Guinea (PNG) criteria were used in the two studies from Oceania.49 51 The Haydom criteria excluded six laboratory parameters (PaO2/FiO2, creatinine, bilirubin, pH, lactate and urine ketoacids) and two management interventions (vasoactive drugs and dialysis). The PNG criteria only excluded four laboratory parameters (PaO2/FiO2, pH, lactate and urine ketoacids).
Fifteen studies made qualitative remarks about challenges, of which five studies72–76 did not make adaptations to the MNM criteria. The most commonly cited challenges were the absence of an ICU (four studies), lack of laboratory testing (four studies) and unavailability of blood products (two studies). Three studies also commented on the applicability of the MNM criteria in private facilities and lower level facilities due to limited facilities, the need to separate MNM cases on arrival hospital vs those that develop in the hospital setting and a need for MNM criteria at the community level.
Discussion
We have included 60 studies from 56 countries in this meta-analysis with a pooled near-miss estimate using the WHO MNM criteria of 1.4% (95% CI 0.4% to 2.5%). We found that the near-miss rates differed between the SDG regions, with sub-Saharan Africa having the largest variation in range. While we expected a high degree of heterogeneity between the studies given the observational nature of the included studies and the fact that the studies were drawn from different hospital settings and from numerous countries around the world, our analysis of MNM rates by regional groupings did not find substantial in between study heterogeneity.
While the utilisation of the WHO MNM approach has increased over time, with 52.2% of papers on MNM published in 2017 using this approach, the WHO MNM criteria is predominantly used in low-resource countries.6 Of the 60 studies we included in our review, only 4 studies were conducted in high-income countries (USA, Australia and New Zealand) and 19 studies were from upper-middle income countries. Of the 15 papers from the meta-analysis that made adaptations to the WHO MNM criteria, only one was from an upper middle income country. We found that a number of adaptations and modifications were made to the WHO MNM criteria in low-resource settings, particularly to laboratory investigations. Interventions such as dialysis and the continuous use of vasoactive drugs were excluded and almost two-thirds of the studies reduced the threshold for blood transfusion due to resource limitations. Our findings are in keeping with other reviews which have also found that the WHO MNM approach has mainly been used in Asian and African countries and rarely in North American and European countries.6
Our study builds on the 2012 WHO systematic review.6 At that time, a meta‐analysis of near miss could not be conducted because of the variety of identification criteria and as the WHO MNM criteria was only newly introduced, the previous review was limited in its assessment of the WHO MNM criteria. To our knowledge, there is one previous systematic review and meta-analysis that has looked at the global prevalence of MNM using the WHO MNM criteria.77 Our study is more comprehensive in its scope as it includes a larger number of studies and did not have language or population restrictions while the other review included 49 studies, English language only and excluded studies that did not include a generalisable population. The review had a similar weighted pooled MNM prevalence (1.867% (95% CI 16.23% to 21.06%)) to ours but unlike us, the review found significant heterogeneity between studies in their regional analysis
Our paper is the first to bring together a meta-analysis and an examination of applications across different settings. We included a large number of papers in the meta-analysis as we did not have language restrictions. We analysed adaptations globally across all settings while previously published literature have focused on adaptations in sub-Saharan Africa only.78 As we hypothesised that contextual adaptations to the WHO MNM criteria would be needed in low-resource settings, we conducted an additional search to capture more papers from these settings to fully understand the types of adaptations that are needed to operationalise the criteria with resource limitations.
One of the limitations of our analysis is that the denominators for MNM rate included both deliveries and live births. Using deliveries instead of live births in the denominator may result in underestimation of the MNM frequency. We did not examine the variation in the reported rates of MNM, which could also reflect differences in monitoring and reporting due to different practices or patient populations.6 We purposefully included studies prior to the COVID-19 pandemic in order to avoid bias from the impact of the pandemic on maternal health outcomes. The COVID-19 pandemic has had profound impact on healthcare systems and during this time, global maternal and fetal outcomes have worsened with an increase in maternal deaths and morbidity, particularly in low-resource settings.79 MNM events could be another key indicator to study the impact of the pandemic.
As we continue to use MNM as an indicator to monitor the quality of obstetric care, there is a need to be aware of adaptations and modifications that are required in low-resource settings and interpret data accordingly. Some studies have also found that the application of the WHO MNM tool in low-resource settings has resulted in under-reporting of life-threatening events, which are felt to be due to lack of blood for transfusion and lack of laboratory and diagnostic resources.80 Although several of the WHO MNM parameters may not be applicable to low-resource settings, there is a lack of well-founded alternative parameters. In 2017, a Delphi consensus group was convened to develop an adapted set of criteria for Sub-Saharan Africa that focus more on clinical criteria rather than lab parameters.80 While we have not identified any studies using this adapted tool, several studies have evaluated modified versions with a lower threshold for blood transfusion in low-resource settings and have found that lowering the threshold for blood transfusion leads to a higher detection rate of MNM and can provide a more consistent estimate of MNM incidence and mortality index.70 81 Future directions could include integrating the use of ICD codes into WHO MNM criteria to help with standardisation, developing an integrative module for poor-resource health facilities and assessing the specificity, sensitivity, and predictive value of these adapted tools.
Conclusion
As countries progress through the stages of obstetric transition, and as maternal mortality decreases and women increasingly deliver in facilities, tracking and evaluating maternal morbidity, specifically, MNM is a necessary step in improving the quality of care. Strategies toward ending preventable maternal mortality (EPMM) and the Every Newborn Action Plan have been important efforts to set out agreed targets and priorities. These are now also embedded in the Global Strategy for Women’s Children’s and Adolescent’s Health, and have gained political momentum in shaping national strategies. As part of this strategy, one of the cross-cutting actions is to improve metrics, measurement systems and data quality while one of the five strategic objectives is to address all causes of maternal mortality, reproductive and maternal morbidities and related disabilities. The WHO MNM concept (pragmatically or strictly defined) is useful to generate actionable information at the health service level, improve women’s delivery experiences and outcomes and strengthenhealth systems. The WHO MNM criteria have enabled standardised case identification and allowed for the comparison of global and subnational estimates of MNM frequency and inform quality improvement efforts.
There has been good uptake of the WHO MNM criteria in low-resource settings but due to resource constraints in low-resource settings, there might be a need for local adaptation, where appropriate.
Acknowledgments
We would like to acknowledge Olha Lutsiv and Simroop Ladhar.
Footnotes
Handling editor: Seye Abimbola
Twitter: @otuncalp
Contributors: ÖT, JPS and TF conceptualised the manuscript. TF drafted the manuscript. TF, CLTR, CL and OT contributed to data extraction. All authors contributed to editing the manuscript. TF is the guarantor.
Funding: The study was funded by HRP (the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.UNICEF, UNFPA WHO, et al. Maternal mortality: levels and trends 2000 to 2017: estimates by who, UNICEF, UNFPA, world bank group and the United nations population division. Geneva: World Health Organization, 2019. [Google Scholar]
- 2.Tunçalp Ö, Souza JP. Maternal near-miss audits to improve quality of care. BJOG 2014;121(Suppl 4):102–4. 10.1111/1471-0528.12868 [DOI] [PubMed] [Google Scholar]
- 3.Say L, Souza JP, Pattinson RC, et al. Maternal near miss--towards a standard tool for monitoring quality of maternal health care.. Best Pract Res Clin Obstet Gynaecol 2009;23:287–96. 10.1016/j.bpobgyn.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 4.Say L, Pattinson RC, Gülmezoglu AM. WHO systematic review of maternal morbidity and mortality: the prevalence of severe acute maternal morbidity (near miss). Reprod Health 2004;1:3. 10.1186/1742-4755-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tunçalp O, Hindin MJ, Souza JP, et al. The prevalence of maternal near miss: a systematic review. BJOG 2012;119:653–61. 10.1111/j.1471-0528.2012.03294.x [DOI] [PubMed] [Google Scholar]
- 6.England N, Madill J, Metcalfe A, et al. Monitoring maternal near miss/severe maternal morbidity: a systematic review of global practices. PLoS One 2020;15:e0233697. 10.1371/journal.pone.0233697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown HL, Small M, Taylor YJ, et al. Near miss maternal mortality in a multiethnic population. Ann Epidemiol 2011;21:73–7. 10.1016/j.annepidem.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 8.Cecatti JG, Souza JP, Oliveira Neto AF, et al. Pre-validation of the WHO organ dysfunction based criteria for identification of maternal near miss. Reprod Health 2011;8:22. 10.1186/1742-4755-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayaratnam S, De Costa C, Howat P. Developing an assessment tool for maternal morbidity ‘near-miss’- A prospective study in a large Australian regional hospital. Aust New Zeal J Obstet Gynaecol 2011;51:421–5. 10.1111/j.1479-828X.2011.01330.x [DOI] [PubMed] [Google Scholar]
- 10.Morse ML, Fonseca SC, Gottgtroy CL, et al. Severe maternal morbidity and near misses in a regional reference Hospital. Rev Bras Epidemiol 2011;14:310–22. 10.1590/s1415-790x2011000200012 [DOI] [PubMed] [Google Scholar]
- 11.Jabir M, Abdul-Salam I, Suheil DM, et al. Maternal near miss and quality of maternal health care in Baghdad, Iraq. BMC Pregnancy Childbirth 2013;13:11. 10.1186/1471-2393-13-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lobato G, Nakamura-Pereira M, Mendes-Silva W, et al. Comparing different diagnostic approaches to severe maternal morbidity and near-miss: a pilot study in a Brazilian tertiary hospital. Eur J Obstet Gynecol Reprod Biol 2013;167:24–8. 10.1016/j.ejogrb.2012.10.030 [DOI] [PubMed] [Google Scholar]
- 13.Nelissen EJT, Mduma E, Ersdal HL, et al. Maternal near miss and mortality in a rural referral hospital in northern Tanzania: a cross-sectional study. BMC Pregnancy Childbirth 2013;13:141. 10.1186/1471-2393-13-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira LC, Costa AARda, AARd C. Fetal and neonatal deaths among cases of maternal near miss. Rev Assoc Med Bras 2013;59:487–94. 10.1016/j.ramb.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 15.Ps R, Verma S, Rai L, et al. "Near miss" obstetric events and maternal deaths in a tertiary care hospital: an audit. J Pregnancy 2013;2013:393758. 10.1155/2013/393758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rana A, Baral G, Dangal G. Maternal near-miss: a multicenter surveillance in Kathmandu Valley. JNMA J Nepal Med Assoc 2013;52:299–304. [PubMed] [Google Scholar]
- 17.Shen F-R, Liu M, Zhang X, et al. Factors associated with maternal near-miss morbidity and mortality in Kowloon Hospital, Suzhou, China. Int J Gynaecol Obstet 2013;123:64–7. 10.1016/j.ijgo.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 18.Souza JP, Gülmezoglu AM, Vogel J, et al. Moving beyond essential interventions for reduction of maternal mortality (the who multicountry survey on maternal and newborn health): a cross-sectional study. Lancet 2013;381:1747–55. 10.1016/S0140-6736(13)60686-8 [DOI] [PubMed] [Google Scholar]
- 19.Abalos E, Cuesta C, Carroli G, et al. Pre‐eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the world Health organization multicountry survey on maternal and newborn health. BJOG 2014;121(Suppl 1):14–24. 10.1111/1471-0528.12629 [DOI] [PubMed] [Google Scholar]
- 20.Aziz N, Reddy P. Comparison of etiology of maternal near miss in a tertiary referral centre in booked and referred population: EP7. 53. BJOG 2014;121:115–6. [Google Scholar]
- 21.Dias MAB, Domingues RMSM, Schilithz AOC, et al. Incidence of maternal near miss in hospital childbirth and postpartum: data from the birth in Brazil study. Cad Saude Publica 2014;30(Suppl 1):S1–12. 10.1590/0102-311x00154213 [DOI] [PubMed] [Google Scholar]
- 22.Galvão LPL, Alvim-Pereira F, de Mendonça CMM, et al. The prevalence of severe maternal morbidity and near miss and associated factors in Sergipe, northeast Brazil. BMC Pregnancy Childbirth 2014;14:25. 10.1186/1471-2393-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litorp H, Kidanto HL, Rööst M, et al. Maternal near-miss and death and their association with caesarean section complications: a cross-sectional study at a university hospital and a regional hospital in Tanzania. BMC Pregnancy Childbirth 2014;14:244. 10.1186/1471-2393-14-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luexay P, Malinee L, Pisake L, et al. Maternal near-miss and mortality in Sayaboury Province, Lao PDR. BMC Public Health 2014;14:945. 10.1186/1471-2458-14-945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandey A, Das V, Agarwal A, et al. Evaluation of obstetric near miss and maternal deaths in a tertiary care hospital in North India: shifting focus from mortality to morbidity. J Obstet Gynaecol India 2014;64:394–9. 10.1007/s13224-014-0552-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tunçalp Özge, Hindin MJ, Adu-Bonsaffoh K, et al. Assessment of maternal near-miss and quality of care in a hospital-based study in Accra, Ghana. Int J Gynaecol Obstet 2013;123:58–63. 10.1016/j.ijgo.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 27.Bashour H, Saad-Haddad G, DeJong J, et al. A cross sectional study of maternal 'near-miss' cases in major public hospitals in Egypt, Lebanon, Palestine and Syria. BMC Pregnancy Childbirth 2015;15:296. 10.1186/s12884-015-0733-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cecatti JG, Costa ML, Haddad SM, et al. Network for surveillance of severe maternal morbidity: a powerful national collaboration generating data on maternal health outcomes and care. BJOG 2016;123:946–53. 10.1111/1471-0528.13614 [DOI] [PubMed] [Google Scholar]
- 29.Karolinski A, Mercer R, Micone P. Bases para establecer un sistema de vigilancia activa y respuesta rápida para el manejo de la morbilidad materna severa. Rev argent salud publica 2015;6:7–14. [Google Scholar]
- 30.Kulkarni R, Chauhan S, Daver R, et al. Prospective observational study of near-miss obstetric events at two tertiary hospitals in Mumbai, Maharashtra, India. Int J Gynaecol Obstet 2016;132:170–3. 10.1016/j.ijgo.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 31.Madeiro AP, Rufino AC, Lacerda Érica Zânia Gonçalves, et al. Incidence and determinants of severe maternal morbidity: a transversal study in a referral hospital in Teresina, Piaui, Brazil. BMC Pregnancy Childbirth 2015;15:210. 10.1186/s12884-015-0648-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menezes FEF, Galvão LPL, de Mendonça CMM, et al. Similarities and differences between WHO criteria and two other approaches for maternal near miss diagnosis. Trop Med Int Health 2015;20:1501–6. 10.1111/tmi.12568 [DOI] [PubMed] [Google Scholar]
- 33.Naderi T, Foroodnia S, Omidi S, et al. Incidence and correlates of maternal near miss in Southeast Iran. Int J Reprod Med 2015;2015:914713. 10.1155/2015/914713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oladapo OT, Adetoro OO, Ekele BA, et al. When getting there is not enough: a nationwide cross-sectional study of 998 maternal deaths and 1451 near-misses in public tertiary hospitals in a low-income country. BJOG 2016;123:928–38. 10.1111/1471-0528.13450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rulisa S, Umuziranenge I, Small M, et al. Maternal near miss and mortality in a tertiary care hospital in Rwanda. BMC Pregnancy Childbirth 2015;15:203. 10.1186/s12884-015-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shahid A, Rizwan S, Khawaja N. Near miss events frequency and most common causes. Pakistan J Med Heal Sci 2015;9:920–2. [Google Scholar]
- 37.Soma-Pillay P, Pattinson RC, Langa-Mlambo L, et al. Maternal near miss and maternal death in the Pretoria academic complex, South Africa: a population-based study. S Afr Med J 2015;105:578–63. 10.7196/SAMJnew.8038 [DOI] [PubMed] [Google Scholar]
- 38.Abha S, Chandrashekhar S, Sonal D. Maternal near miss: a valuable contribution in maternal care. J Obstet Gynaecol India 2016;66:217–22. 10.1007/s13224-015-0838-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Mucio B, Abalos E, Cuesta C, et al. Maternal near miss and predictive ability of potentially life-threatening conditions at selected maternity hospitals in Latin America. Reprod Health 2016;13:134. 10.1186/s12978-016-0250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Ghardallou M, Nabli Ajmi T, Mkhazni A, et al. Maternal near miss and quality of obstetric care in a Tunisian tertiary level maternity. Afr J Reprod Health 2016;20:44–50. 10.29063/ajrh2016/v20i4.4 [DOI] [PubMed] [Google Scholar]
- 41.Ghazivakili Z, Lotfi R, Kabir K, et al. Maternal near miss approach to evaluate quality of care in Alborz Province, Iran. Midwifery 2016;41:118–24. 10.1016/j.midw.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 42.Jayaratnam S, Burton A, Connan KF, et al. Maternal 'near miss' at Royal Darwin Hospital: An analysis of severe maternal morbidity at an Australian regional tertiary maternity unit. Aust N Z J Obstet Gynaecol 2016;56:381–6. 10.1111/ajo.12436 [DOI] [PubMed] [Google Scholar]
- 43.Kalisa R, Rulisa S, van den Akker T, et al. Maternal near miss and quality of care in a rural Rwandan Hospital. BMC Pregnancy Childbirth 2016;16:324. 10.1186/s12884-016-1119-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakimuli A, Nakubulwa S, Kakaire O, et al. Maternal near misses from two referral hospitals in Uganda: a prospective cohort study on incidence, determinants and prognostic factors. BMC Pregnancy Childbirth 2016;16:24. 10.1186/s12884-016-0811-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nanda S, Yadav S. A prospective observational study of near miss events and maternal deaths in a tertiary hospital in India: EP8. 021. BJOG 2016;123. [Google Scholar]
- 46.Norhayati MN, Nik Hazlina NH, Sulaiman Z, et al. Severe maternal morbidity and near misses in tertiary hospitals, Kelantan, Malaysia: a cross-sectional study. BMC Public Health 2016;16:229. 10.1186/s12889-016-2895-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parmar NT, Parmar AG, Mazumdar VS. Incidence of Maternal "Near-Miss" Events in a Tertiary Care Hospital of Central Gujarat, India. J Obstet Gynaecol India 2016;66:315–20. 10.1007/s13224-016-0901-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rathod AD, Chavan RP, Bhagat V, et al. Analysis of near-miss and maternal mortality at tertiary referral centre of rural India. J Obstet Gynaecol India 2016;66:295–300. 10.1007/s13224-016-0902-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanimia H, Jayaratnam S, Mola GL, et al. Near-misses at the Port Moresby General Hospital: a descriptive study. Aust N Z J Obstet Gynaecol 2016;56:148–53. 10.1111/ajo.12430 [DOI] [PubMed] [Google Scholar]
- 50.Akrawi VS, Al-Hadithi TS, Al-Tawil NG. Major determinants of maternal near-miss and mortality at the maternity teaching Hospital, Erbil City, Iraq. Oman Med J 2017;32:386–95. 10.5001/omj.2017.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolnga JW, Morris M, Totona C, et al. Maternal near-misses at a provincial hospital in Papua New Guinea: a prospective observational study. Aust N Z J Obstet Gynaecol 2017;57:624–9. 10.1111/ajo.12650 [DOI] [PubMed] [Google Scholar]
- 52.Herklots T, van Acht L, Meguid T, et al. Severe maternal morbidity in Zanzibar's referral Hospital: measuring the impact of in-hospital care. PLoS One 2017;12:e0181470. 10.1371/journal.pone.0181470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liyew EF, Yalew AW, Afework MF, et al. Maternal near-miss and the risk of adverse perinatal outcomes: a prospective cohort study in selected public hospitals of Addis Ababa, Ethiopia. BMC Pregnancy Childbirth 2018;18:345. 10.1186/s12884-018-1983-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mbachu II, Ezeama C, Osuagwu K, et al. A cross sectional study of maternal near miss and mortality at a rural tertiary centre in southern Nigeria. BMC Pregnancy Childbirth 2017;17:251. 10.1186/s12884-017-1436-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serruya SJ, de Mucio B, Martinez G, et al. Exploring the concept of degrees of maternal morbidity as a tool for surveillance of maternal health in Latin American and Caribbean settings. Biomed Res Int 2017;2017:8271042. 10.1155/2017/8271042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chikadaya H, Madziyire MG, Munjanja SP. Incidence of maternal near miss in the public health sector of Harare, Zimbabwe: a prospective descriptive study. BMC Pregnancy Childbirth 2018;18:1–6. 10.1186/s12884-018-2092-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esparza-Valencia DM, Toro-Ortiz JC, Herrera-Ortega O. Prevalencia de morbilidad materna extrema en un Hospital de segundo nivel de San Luis Potosí, México. Ginecol Obstet Mex 2018;86:304–12. [Google Scholar]
- 58.Iwuh I, Fawcus S. Maternal near miss in the Metro West maternity services, Cape town. BJOG 2017;124:102. [DOI] [PubMed] [Google Scholar]
- 59.Jayaratnam S, Kua S, deCosta C, et al. Maternal ‘near miss’ collection at an Australian tertiary maternity hospital. BMC Pregnancy Childbirth 2018;18:1–6. 10.1186/s12884-018-1862-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rana HB, Banjara MR, Joshi MP, et al. Assessing maternal and neonatal near-miss reviews in rural Nepal: an implementation research study to inform scale-up. Acta Paediatr 2018;107(Suppl 471):17–23. 10.1111/apa.14300 [DOI] [PubMed] [Google Scholar]
- 61.Reena RP, Radha KR. Factors associated with maternal near miss: a study from Kerala. Indian J Public Health 2018;62:58. 10.4103/ijph.IJPH_20_16 [DOI] [PubMed] [Google Scholar]
- 62.Schwenck P, Santos Oliveira CA, deLima Ferreira RV. A cross sectional study of maternal near miss cases at a high-risk maternity in Brazil. Int J Gynecol Obstetrics 2018. [Google Scholar]
- 63.Sheriar Z, Patil S. Maternal near miss in a tertiary care hospital. Int J Gynaecol Obstet 2018. [Google Scholar]
- 64.Tura AK, Zwart J, van Roosmalen J, et al. Severe maternal outcomes in eastern Ethiopia: application of the adapted maternal near miss tool. PLoS One 2018;13:e0207350. 10.1371/journal.pone.0207350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verschueren KJ, Kodan LR, Paidin RR, et al. Applicability of the WHO maternal near-miss tool: a nationwide surveillance study in Suriname. J Glob Health 2020;10:020429. 10.7189/jogh.10.020429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woldeyes WS, Asefa D, Muleta G. Incidence and determinants of severe maternal outcome in Jimma university teaching Hospital, south-west Ethiopia: a prospective cross-sectional study. BMC Pregnancy Childbirth 2018;18:1–2. 10.1186/s12884-018-1879-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benimana C, Small M, Rulisa S. Preventability of maternal near miss and mortality in Rwanda: a case series from the University teaching hospital of Kigali (CHUK). PLoS One 2018;13:e0195711. 10.1371/journal.pone.0195711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Owolabi OO, Cresswell JA, Vwalika B, et al. Incidence of abortion-related near-miss complications in Zambia: cross-sectional study in central, Copperbelt and Lusaka provinces. Contraception 2017;95:167–74. 10.1016/j.contraception.2016.08.014 [DOI] [PubMed] [Google Scholar]
- 69.Sayinzoga F, Bijlmakers L, van der Velden K, et al. Severe maternal outcomes and quality of care at district hospitals in Rwanda- a multicentre prospective case-control study. BMC Pregnancy Childbirth 2017;17:394. 10.1186/s12884-017-1581-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Witteveen T, Bezstarosti H, de Koning I, et al. Validating the WHO maternal near miss tool: comparing high- and low-resource settings. BMC Pregnancy Childbirth 2017;17:194. 10.1186/s12884-017-1370-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van den Akker T, Beltman J, Leyten J, et al. The WHO maternal near miss approach: consequences at Malawian district level. PLoS One 2013;8:e54805. 10.1371/journal.pone.0054805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adamu AN, Okusanya BO, Tukur J, et al. Maternal near-miss and death among women with hypertensive disorders in pregnancy: a secondary analysis of the Nigeria near-miss and maternal death survey. BJOG 2019;126(Suppl 3):12–18. 10.1111/1471-0528.15427 [DOI] [PubMed] [Google Scholar]
- 73.Giordano JC, Parpinelli MA, Cecatti JG, et al. The burden of eclampsia: results from a multicenter study on surveillance of severe maternal morbidity in Brazil. PLoS One 2014;9:e102208. 10.1371/journal.pone.0097401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haddad SM, Cecatti JG, Souza JP, et al. Applying the maternal near miss approach for the evaluation of quality of obstetric care: a worked example from a multicenter surveillance study. Biomed Res Int 2014;2014:989815. 10.1155/2014/989815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mazhar SB, Batool A, Emanuel A, et al. Severe maternal outcomes and their predictors among Pakistani women in the who multicountry survey on maternal and newborn health. Int J Gynaecol Obstet 2015;129:30–3. 10.1016/j.ijgo.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 76.Dragoman M, Sheldon WR, Qureshi Z, et al. Overview of abortion cases with severe maternal outcomes in the WHO multicountry survey on maternal and newborn health: a descriptive analysis. BJOG 2014;121(Suppl 1):25–31. 10.1111/1471-0528.12689 [DOI] [PubMed] [Google Scholar]
- 77.Abdollahpour S, Heidarian Miri H, Khadivzadeh T. The global prevalence of maternal near miss: a systematic review and meta-analysis. Health Promot Perspect 2019;9:255. 10.15171/hpp.2019.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tura AK, Trang TL, van den Akker T, et al. Applicability of the WHO maternal near miss tool in sub-Saharan Africa: a systematic review. BMC Pregnancy Childbirth 2019;19:79. 10.1186/s12884-019-2225-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chmielewska B, Barratt I, Townsend R, et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health 2021;9:E759–77. 10.1016/S2214-109X(21)00079-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tura AK, Stekelenburg J, Scherjon SA, et al. Adaptation of the WHO maternal near miss tool for use in sub-Saharan Africa: an international Delphi study. BMC Pregnancy Childbirth 2017;17:445. 10.1186/s12884-017-1640-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pembe AB, Hirose A, Alwy Al-Beity F, et al. Rethinking the definition of maternal near-miss in low-income countries using data from 104 health facilities in Tanzania and Uganda. Int J Gynaecol Obstet 2019;147:389–96. 10.1002/ijgo.12976 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-007077supp001.pdf (297.8KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Not applicable.