Abstract
Diabetic peripheral neuropathy (DPN) affects over half of type 2 diabetes mellitus (T2DM) patients, with an urgent need for effective pharmacotherapies. While many rat and mouse models of T2DM exist, the phenotyping of DPN has been challenging with inconsistencies across laboratories. To better characterize DPN in rodents, a consensus guideline was published in 2014 to accelerate the translation of preclinical findings. Here we review DPN phenotyping in rat models of T2DM against the ‘Neurodiab’ criteria to identify uptake of the guidelines and discuss how DPN phenotypes differ between models and according to diabetes duration and sex. A search of PubMed, Scopus and Web of Science databases identified 125 studies, categorised as either diet and/or chemically induced models or transgenic/spontaneous models of T2DM. The use of diet and chemically induced T2DM models has exceeded that of transgenic models in recent years, and the introduction of the Neurodiab guidelines has not appreciably increased the number of studies assessing all key DPN endpoints. Combined high-fat diet and low dose streptozotocin rat models are the most frequently used and well characterised. Overall, we recommend adherence to Neurodiab guidelines for creating better animal models of DPN to accelerate translation and drug development.
Keywords: Diabetes mellitus, type 2; Diabetic neuropathies; Diet, high-fat; Models, animal; Models, genetic; Peripheral nerves; Rats; Streptozotocin
INTRODUCTION
Diabetes is a global health concern that cuts across socioeconomic status and national boundaries. In 2019, the International Diabetes Federation estimated that 463 million adults are living with diabetes and 374 million with impaired glucose tolerance, predicted to rise to 700 million and 548 million, respectively, by 2045 [1]. Diabetic peripheral neuropathy (DPN) is the most prevalent complication in diabetes, affecting more than 50% of long-term type 2 patients [2]. Despite such high prevalence, the basic disease mechanisms of DPN are yet to be deciphered [3]. A consequence of the rising global prevalence of diabetes and prediabetes is a corresponding increase in DPN. DPN manifests as a distal symmetric polyneuropathy affecting the lower extremities in a length-dependent fashion [4] and is primarily a disorder of sensory dysfunction characterized by pain, allodynia, numbness, and insensate feet. These initial manifestations can progress to physical impairments (e.g., loss of balance) and an increased risk of falls, foot ulceration, amputation and mortality [4,5]. Despite dramatic effects of DPN on quality of life and healthcare costs, there are still no effective disease modifying treatments other than strict glycaemic control and pain management [6].
DPN is common in both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), though it has long been recognised they have distinct pathophysiological mechanisms [7-9]. The consequences of these distinct pathophysiological mechanisms were highlighted by a meta-analysis demonstrating that glycaemic control, the only accepted disease modifying treatment, is less effective for DPN symptoms in individuals with T2DM than T1DM [10]. Research has sought to develop preclinical models that allow for the metabolic comorbidities associated with T2DM to be studied, such as obesity, hypertension, dyslipidemia, inflammation, and insulin resistance [11-13], thus requiring modifications to the high dose streptozotocin (STZ) model of T1DM. While rat and mouse models of T1DM and T2DM have been reviewed previously [14-16], here we sought to evaluate the quality of DPN phenotyping, specifically in rat models of T2DM.
The growth and refinement in rat T2DM models over the past 2 decades have been accompanied by an increased focus on improving the consistency of protocols used to objectively measure and report DPN. To this end, in 2014, the Diabetic Neuropathy Study group (Neurodiab) of the European Association for the Study of Diabetes (EASD) established a set of consensus criteria for phenotyping DPN in rodent models with the intention of enabling collaboration between laboratories, standardising data reporting, and thus expediting the discovery of effective treatments for DPN [3]. The Neurodiab guidelines define DPN as the presence of statistically significant differences between diabetic and age-matched control animals in at least two out of three measures of behaviour, nerve conduction, and peripheral nerve structure. The Neurodiab guidelines also emphasise the importance of experimental design through randomisation of animals to groups, blinding of researchers for analyses, reporting key diabetic parameters (e.g., weight, blood glucose, glycosylated hemoglobin, blood pressure, insulin, and lipids), and reporting both positive and negative results. These guidelines focus on somatic nerves as there was insufficient data to include evaluation of autonomic manifestations of DPN in rodent models.
Here we review the evolution of rat T2DM models in the study of DPN over time. We evaluated whether the publication of the Neurodiab guidelines in 2014 influenced the reporting of DPN in the literature, as indicated by use of all three key endpoints as recommended. To achieve this, we conducted a systematic search of rat models of T2DM that studied DPN and compared measures reported using an interrupted time series method. Measures of autonomic neuropathy were not among the key assessments of the Neurodiab guidelines and thus were outside the scope of this review. Mouse models of DPN have been reviewed elsewhere [15] and are not considered here.
CRITERIA FOR PHENOTYPING DPN IN RODENT MODELS ACCORDING TO ‘NEURODIAB’
Behavioural tests
Currently, there is no single gold standard behavioural test to assess neuropathy in rodent models. The behavioural test of choice for small fiber neuropathy is the Hargreaves test, which measures hindpaw withdrawal response latency when the plantar surface of a single paw is exposed to escalating heat [17]. Alternative behavioural measures assess tactile allodynia by recording hindpaw withdrawal responses in response to the application of von Frey monofilaments (or automated pressure filaments) to the plantar surface, thought to provide information about large fiber dysfunction [18,19]. Including more than one behavioural test is recommended for studying disease mechanisms and evaluating drug effects.
Nerve conduction studies
Nerve conduction studies (NCS) are the current gold standard for clinical drug trials measuring large fiber function and should be included as an endpoint in phenotyping and screening for potential therapies in diabetic rodents [3]. The Neurodiab guidelines recommend assessing nerve conduction velocity (NCV) in both motor and sensory nerves, while controlling core and near-nerve temperatures, as NCVs are temperaturesensitive [3]. Assessing NCV in the caudal nerve is not strongly recommended as this is a unique anatomical feature of rodents with less translational relevance [3].
Peripheral nerve structure
Quantifying intraepidermal nerve fiber density (IENFD) in the foot pad of diabetic rodents is considered a reliable and sensitive marker of small sensory nerve fiber loss [20-22]. Surrogate markers of peripheral nerve structure include measures of nerve trunk morphometry (large and small fibers) and corneal nerve fiber analysis [23].
METHODS: LITERATURE SEARCH AND STUDY SELECTION
A scoping review was undertaken up to 3rd November 2020 using the following terms: ((T2DM OR Non-insulin dependent diabetes mellitus OR diabetes mellitus type-II) AND (Neuropath* OR diabetic neuropathies OR polyneuropathies) AND (rat OR rats)). The search terms were entered into PubMed, Web of Science and Scopus, yielding 1,625 results. The screening and review procedure for these articles is shown in the flow diagram (Fig. 1). After removal of duplicates and screening, 125 articles were included in this review for qualitative synthesis. Criteria for inclusion were that the articles were in a rat model of T2DM and assessed at least one of the three key DPN endpoints (i.e., behavioural assessment, electrophysiology, and nerve structure). Exclusion criteria were studies on T1DM, clinical studies, reviews, book chapters, non-rat models, nondiabetic models, neo-natal models of T2DM, studies with no DPN endpoints, full text unavailable or not in English, conference abstracts, and in vitro studies. Articles that fulfilled the inclusion criteria were separated into (1) diet and chemically induced models, and (2) transgenic/spontaneous models.
Fig. 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram showing search results and study selection. T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; DPN, diabetic peripheral neuropathy.
RESULTS
Summary of included studies and evaluation of studies pre- and post-publication of the Neurodiab guidelines
Table 1 summarises the 125 articles included in this review, which were categorized into diet and chemically induced models (n=63) or transgenic/spontaneous models (n=62) of T2DM. We identified three diet and chemically induced T2DM models, the most common of which was high-fat diet (HFD) plus low dose STZ in Sprague-Dawley (SD) rats (51/63 studies). Of the eight transgenic/spontaneous models identified, Zucker diabetic fatty (ZDF) rats were most frequently studied (36/62 studies). Overall, studies employing diet and chemically induced T2DM models tended to assess all three DPN endpoints (19/63, 30%) more frequently than transgenic models (10/62, 16%) (χ2 (1, 125)=3.45, P=0.063) (Fig. 2). Similarly, significant changes in at least two endpoints—meeting the Neurodiab criteria for DPN—were detected more often in diet and chemically induced models (32/63, 50.8%) than in transgenic models (24/62, 38.7%), although this difference was not statistically significant (χ2 (1, 125)=1.85, P=0.174).
Table 1.
Rat models of type 2 diabetes mellitus assessing DPN endpoints
| Model type | No. of articles included | Proportion of articles assessing three DPN endpoints |
Articles observing significant change in ≥2 DPN endpoints |
|||
|---|---|---|---|---|---|---|
| Pre-Neurodiab | Post-Neurodiab | Pre-Neurodiab | Post-Neurodiab | |||
| Diet and chemically induced models | ||||||
| SD rat (HFD+low dose STZ) | 51 | 7/18 | 12/33 | 10/18 | 13/33 | |
| Wistar rat (HFD+low dose STZ) | 9 | - | 0/9 | - | 6/9 | |
| Wistar rat (STZ-NA model) | 3 | 0/1 | 0/2 | 1/1 | 2/2 | |
| Total | 63 | 7/19 | 12/44 | 11/19 | 21/44 | |
| Transgenic/spontaneous models | ||||||
| ZDF rat | 36 | 4/20 | 1/16 | 8/20 | 4/16 | |
| BBZDR/Wor rat | 3 | 1/3 | - | 2/3 | - | |
| GK rat | 10 | 0/8 | 1/ 2 | 2/8 | 1/2 | |
| SDT fatty rat | 5 | 0/3 | 0/2 | 2/3 | 0/2 | |
| OLETF rat | 6 | 0/5 | 1/1 | 2/5 | 1/1 | |
| ZDSD-Pco | 1 | 1/1 | - | 1/1 | - | |
| NGR | 1 | - | 1/1 | - | 1/1 | |
| WDF | 1 | 0/1 | - | 0/1 | - | |
| Total | 63-1a | 6/41-1a | 4/22 | 17/41-1a | 7/22 | |
| Grand total | 125 | 13/59 | 16/66 | 28/59 | 28/66 | |
The Neurodiab guidelines were published on the 17th of June, 2014. A 12-month window was included, and thus ‘pre-Neurodiab’ refers to articles published on or before 16th June 2015, and ‘post-Neurodiab’ refers to articles published from 17th June, 2015, to 3rd November 2020.
DPN, diabetic peripheral neuropathy; SD, Sprague-Dawley; HFD, high-fat diet; STZ, streptozotocin; NA, nicotinamide; ZDF, Zucker diabetic fatty; BBZDR, Bio-Breeding Zucker diabetic rat; GK, Goto-Kakizaki; SDT, spontaneously diabetic Torii; OLETF, Otsuka Long-Evans Tokushima Fatty; ZDSD, Zucker diabetic Sprague-Dawley; NGR, Nile grass rat; WDF, Wistar diabetic fatty.
One study was counted as both ZDF and WDF model and then subtracted from the total numbers. A dash (-) indicates no studies available. Note that in most cases, more studies reported neuropathy (at least 2 endpoints significantly different) than number of studies testing all 3 endpoints.
Fig. 2.
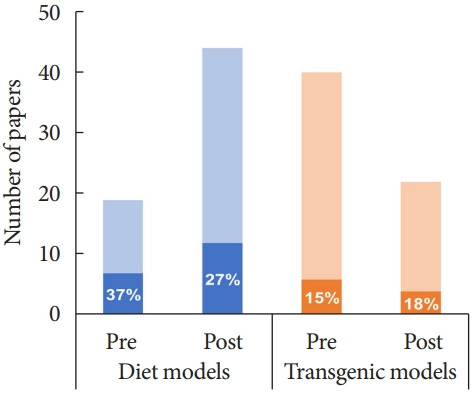
Graphical representation of publications examining diabetic peripheral neuropathy (DPN) endpoints in diet+chemically induced (blue) or transgenic (orange) rat models of type 2 diabetes mellitus before (pre) and after (post) the publication of the Neurodiab guidelines for phenotyping DPN in 2014. Dark shading and percentage values indicate the number of studies that assessed 3 DPN endpoints as recommended.
Comparing studies published pre and post the Neurodiab guidelines captured a shift toward use of diet and chemically induced models (19 studies pre-guidelines, 44 studies postguidelines) and away from transgenic/spontaneous models (40 pre-guidelines vs. 22 post-guidelines) (Fig. 2). Taken together, the proportion of studies assessing all three DPN endpoints has not increased appreciably since publication of the Neurodiab guidelines (pre: 13/59, 22%; post: 16/66, 24%). Likewise, the proportion of studies reporting DPN as per the Neurodiab definition (significant differences in at least two endpoints) was similar pre-publication (28/59 studies) and post-publication (28/66 studies) of the guidelines. This was also true when diet and chemically induced models and transgenic models were considered separately.
Diet and chemically induced models
Purely chemically induced rodent models of diabetes have primarily used alloxan and STZ, compounds toxic to pancreatic β-cells. While early studies modelled T1DM by administering high doses, leading to almost complete insulin deficiency and severe hyperglycemia, subsequent work administered STZ at lower doses alongside HFD exposure to model T2DM [24]. This approach seeks to model the aetiology of human T2DM, albeit more rapidly [25], by first inducing insulin resistance, obesity and inflammation through HFD exposure, followed by the partial destruction of pancreatic β-cells by low dose STZ to induce persistent hyperglycemia [14,26].
The present literature search identified 63 studies (2009 to 2020) testing for a DPN phenotype in rat models of T2DM induced by diet and chemical treatment. Most studies modelled T2DM through diet plus low-dose STZ (60/63 studies; 51 in SD rats and nine in Wistar rats); three combined high dose STZ and nicotinamide (STZ/NA) administration in Wistar rats with no diet manipulation (Table 2) [27-89]. Overall, behavioural tests were the most frequently assessed DPN endpoints (56/63 studies), and 31 studies measured more than one behavior. Nerve conduction was measured in 27/63 studies, with 19 studies assessing both motor nerve conduction velocity (MNCV) and sensory nerve conduction velocity (SNCV). Peripheral nerve structural analyses were reported in 33/63 studies of which 18 studies assessed IENFD, 11 studies assessed both IENFD and corneal nerve fiber length (CNFL) and 16 studies analysed peripheral nerve histology or morphometry. Below we describe the influence of diets and diabetes induction, duration of diabetes and sex differences on DPN endpoints, methods used for behavioural tests, dichotomy in thermal sensitivity results, and directions for future studies.
Table 2.
Neuropathy phenotyping in diet and chemically induced rat models of type 2 diabetes mellitus
| Rat strain/sex | Diabetes induction paradigm |
Neuropathy phenotype (wk post-STZ) |
|||||
|---|---|---|---|---|---|---|---|
| Diet description | STZ Injection No. & dose, mg/kg | Diabetes duration, wk | Pain related behaviours | Nerve conduction | Peripheral nerve structure | ||
| HFD | |||||||
| SD/male & female [60] | HFD (58% fat) | 1×35 | 2 | m-von Frey (↔0.5, 1, 2) | |||
| Needle-prick (↓0.5, 1, 2) | |||||||
| PWL (radiant heat) (↔0.5, 1, 2) | |||||||
| Cold latency (acetone test) (↔0.5, 1, 2) | |||||||
| Wistar/male [48] | HFD (lard 310 g/kg) | 1×35 | 3 | e-von Frey (↓0, 1, 2, 3) | MNCV (↓3) | ||
| PWL (Hargreaves) (↓0, 1, 2, 3) | |||||||
| Cold water latency (↓0, 1, 2, 3) | |||||||
| SD/male [62] | HFD (22% g fat) | 1×30 | 4 | m-von Frey (↓3, 4) | |||
| PWL (radiant heat) (↓3, 4) | |||||||
| SD/male [63] | HFD (22% g fat) | 1×30 | 5 | m-von Frey (↓3–5) | |||
| PWL (radiant heat) (↓3–5) | |||||||
| SD/male [30] | HFD (24% g fat) | 4×30 (wkly 5–8 wk) | 4 | R/S (↓4) | |||
| PWL (hot plate) (↑4) | |||||||
| SD/male [64] | HFD (24% g fat, lard) | 1×30 | 4 | PWL (Hargreaves) (↑4) | MNCV (↓4) | IENFD (↓4) | |
| SNCV (↓4) | CNFL (↓4) | ||||||
| SD/male [65] | HFD (22% g fat) | 1×30 | 4 | m-von Frey (↓1, 2, 3) | |||
| PWL (radiant heat) (↓1, 2, 3) | |||||||
| SD/male [66] | HFD (22% g fat) | 1×30 | 4 | m-von Frey (↓2, 3) | |||
| PWL (radiant heat) (↓2, 3) | |||||||
| SD/male [50] | HFD (45% kcal fat) | 1×30 | 4 | PWL (Hargreaves) (↑4) | MNCV (↓4) | IENFD (↓4) | |
| SNCV (↓4) | CNFL (↓4) | ||||||
| SD/male [67] | HFD (22% g fat) | 1×30 | 5 | R/S (↓1, 2, 3, 4, 5) | |||
| PWL (radiant heat) (↓1, 2, 3, 4, 5) | |||||||
| SD/male [68] | HFD (60% kcal fat) | 1×45 | 5 | m-von Frey (↓2, 3, 4, 5) | |||
| Wistar/male [69] | HFD (58% fat) | 1×35 | 6 | TFL (radiant heat) (↓6) | |||
| Wistar/male [70] | HFD (30% fat) | 1×30 | 7 | m-von Frey (↓5, 7) | Sciatic-axonal, myelin and Schwann cell injury (7) | ||
| PWL (hot plate) (↓1, 5, 7) | |||||||
| SD/male [49] | HFD (60% kcal fat) | 1×35 | 8 | PWL (hot plate) (↓5, 6, 7, 8) | |||
| Cold water latency (tail-flick) (↓5, 6, 7, 8) | |||||||
| Walking function test performance (↓8) | |||||||
| SD/NR [27] | HFD (lard 310 g/kg) | 2×25 (1 wk apart) | 9 | R/S (↓5, ↑9) | MNCV (↓6) | Sciatic-axonal injury and increased Schwann cells (9) | |
| TFL (hot water immersion) (↓5, ↑9) | |||||||
| SD/male [71] | HFD (58% fat) | 1×35 | 9 | ↓ IENFD (8.5) | |||
| SD/male [72] | HFD (58% fat) | 1×35 | 9 | m-von Frey (↔2, 4, 6.5, 8.5) | ↓ IENFD (8.5) | ||
| Needle-prick (↔2, 4, 6.5, 8.5) | |||||||
| PWL (radiant heat) (↔2, 4, 6.5, 8.5) | |||||||
| Cold latency (acetone test) (↔2, 4, 6.5, 8.5) | |||||||
| SD/female [40] | HFD (60% kcal fat) | 1×30 | 12 | m-von Frey (↓12) | MNCV (↓12) | IENFD (↓12) | |
| PWL (Hargreaves) (↑12) | SNCV (↓12) | CNFL (↓12) | |||||
| SD/male [61] | HFD (45% kcal fat) | 1×30 | 16 | PWL (Hargreaves) (↓15) | MNCV (↓16) | IENFD (↓16) | |
| SNCV (↓16) | CNFL (↓16) | ||||||
| SD/male [52] | HFD (45% kcal fat) | 1×30 | 16 | PWL (Hargreaves) (↑16) | MNCV (↓16) | IENFD (↓16) | |
| SNCV (↓16) | |||||||
| SD/male [42] | HFD (45% kcal fat) | 1×30 | 16 | PWL (Hargreaves) (↑16) | MNCV (↓16) | IENFD (↓16) | |
| SNCV (↓16) | |||||||
| SD/male [43] | HFD (24% g fat, soybean oil and lard) | 1×30 | 16 | ↑ PWL (Hargreaves) (16) | MNCV (↓16) | IENFD (↓16) | |
| SNCV (↓16) | |||||||
| SD/male [41] | HFD (58% kcal fat) | 1×35 | 17 | m-von Frey and R/S (↓17) | ↓ MNCV (17) | Sciatic-axonal injury, lymphocyte infiltration (17) | |
| PWL (hot plate) (↓17) | |||||||
| TFL (hot water immersion) (↓17) | |||||||
| SD/male [56] | HFD (45% kcal fat) | 1×30 | 18 | m-von Frey (↓18) | MNCV (↓18) | IENFD (↓18) | |
| PWL (Hargreaves) (↑18) | SNCV (↓18) | CNFL (↓18) | |||||
| SD/male [57] | HFD (45% kcal fat) | 1×30 | 20 | PWL (Hargreaves) (↑8) | MNCV (↓8) | IENFD (↓20) | |
| SNCV (↓8) | CNFL (↓20) | ||||||
| SD/male [59] | HFD (45% kcal fat) | 1×30 | 20 | PWL (Hargreaves) (↑20) | MNCV (↓20) | IENFD (↓20) | |
| SNCV (↓20) | |||||||
| SD/male [55] | HFD (45% kcal fat) | 1×30 | 28 | PWL (Hargreaves) (↑16, 28) | MNCV (↓16, 28) | IENFD (↓16, 28) | |
| SNCV (↓16, 28) | CNFL (↓16, 28) | ||||||
| HFD measured at multiple time points, in different cohorts | |||||||
| SD/male [51] | HFD (45% kcal fat) | 1×30 | 2-8-16 | PWL (Hargreaves) (↑8, 16) | MNCV (↓2, 8, 16) | IENFD (↓2, 8, 16) | |
| SNCV (↓2, 8, 16) | CNFL (↓2, 8, 16) | ||||||
| SD/male [73] | HFD (45% kcal fat) | 1×30 | 4/16 | PWL (Hargreaves) (↑4, 16) | MNCV (↓4, 16) | IENFD (↓4, 16) | |
| SNCV (↓4, 16) | CNFL (↓4, 16) | ||||||
| SD/male [74] | HFD (45% kcal fat) | 1×30 | 4/16 | PWL (Hargreaves) (↑16) | MNCV (↓16) | IENFD (↓16) | |
| SNCV (↓16) | |||||||
| SD/male [58] | HFD (45% kcal fat) | 1×30 | 8-18-32 | PWL (Hargreaves) (↑18, 32) | MNCV (↓8, 18, 32) | IENFD (↓8, 18, 32) | |
| SNCV (↓8, 18, 32) | CNFL (↓8, 18, 32) | ||||||
| SD/male [54] | HFD (45% kcal fat) | 1×30 | 24/36 | PWL (Hargreaves) (↑8, 24, 36) | MNCV (↓8, 24, 36) | IENFD (↓8, 24, 36) | |
| SNCV (↓8, 24, 36) | CNFL (↓8, 24, 36) | ||||||
| SD/male [75] | HFD (58% fat) | 1×35 | 2 wk/2 mo | m-von Frey (↔2, 4, 6.5, 8.5) | |||
| Needle-prick (↔2, 4, 6.5, 8.5) | |||||||
| PWL (radiant heat) (↔2, 4, 6.5, 8.5) | |||||||
| Cold latency (acetone test) (↔2, 4, 6.5, 8.5) | |||||||
| Wistar/male [76] | HFD | 1×40 | NR | MNCV (↓NR) | |||
| HFHS | |||||||
| SD/male [77] | HFHS (10% oil, 7% sugar) | 1×30 | 3 | m-von Frey (↓3) | |||
| PWL (Hargreaves) (↓3) | |||||||
| SD/male [45] | HFHS (10% lard, 10% sucrose) | 1×35 | 3 | e-von Frey (↓2) | Sciatic-axonal and myelin injury (3) | ||
| SD/male [47] | HFHS (10% lard, 10% sucrose) | 1×35 | 3 | PWL (Hargreaves) (↓2, 3) | |||
| SD/male [44] | HFHS (10% lard, 20% sucrose) | 1×35 | 4 | e-von Frey (↓2, 2.5, 3, 4) | |||
| PWL (radiant heat) (↓2, 2.5, 3, 4) | |||||||
| SD/male [31] | HFHS (10% lard, 20% sucrose) | 1× (30, 35, 40) | 4 | e-von Frey (↓2, 4) | |||
| PWL (radiant heat) (↓2, 4) (only in 35 mg/kg group) | |||||||
| SD/male [46] | HFHS (10% oil, 7% sugar) | 1×30 | 4 | m-von Frey (↓2, 3) | |||
| PWL (Hargreaves) (↓1, 2, 3, 4) | |||||||
| SD/male [78] | HFHS (20% sucrose) | 1×25 | 5 | m-von Frey (↓1, 2, 3, 4, 5) | |||
| Albino/male [79] | HFHS (10% lard, 20% sucrose) | 1×30 | 5 | TFL (radiant heat) (↓5) | Sciatic-myelin injury, increased macrophage and mast cells (5) | ||
| SD/male [37] | HFHS (10% lard, 20% sucrose) | 1×35 | 6 | MNCV (↓6) | |||
| SNCV (↓6) | |||||||
| Wistar/male [38] | HFHS (10% lard, 20% sucrose) | 1×30 | 6 | MNCV (↓6) | Sciatic-axonal and myelin injury (6) | ||
| SNCV (↓6) | |||||||
| SD/male [80] | HFHS (15% lard, 25% sucrose) | 1×25 | 8 | Sciatic-myelin injury (8) | |||
| SD/male [81] | HFHS (10% fat, 70% carb) | 1×40 | 8 | ||||
| SD/male [35] | HFHS (10% lard, 10% sucrose) | 1×30 | 11 | PWL (radiant heat) (↑8) | MNCV (↓11) | ||
| SD/male [36] | HFHS (10% lard, 20% sucrose) | 1×35 | 12 | MNCV (↓12) | |||
| SNCV (↓12) | |||||||
| Wistar/female [82] | HFHS (10% lard, 20% sucrose) | 1×30 | 12 | m-von Frey (↓6) | |||
| SD/male [28] | HFHS (15% lard, 10% white sugar) | 2×25 (1 wk apart) | 16 | MNCV (↓16) | Sciatic-myelinated fiber abnormality (16) | ||
| SD/male [29] | HFHS (15% lard, 10% white sugar) | 2×25 (1 wk apart) | 16 | m-von Frey (↓16) | MNCV (↓16) | Sciatic-myelinated fiber abnormality (16) | |
| PWL (hot plate) (↔16) | |||||||
| SD/male [39] | HFHS (45% kcal as fat) | 1×35 | 2.5 | PWL (hot plate) (↓2.5) | NCV (↔2.5) | ||
| HFHFr | |||||||
| SD/male [83] | HFHFr (660 g fructose; 80 g fat/kg) | 1×35 | 8 | PWL (hot plate) (↓1–8) | Sciatic-axonal injury, reduced Schwann cells (8) | ||
| TFL (hot water immersion) (↓1–8) | |||||||
| SD/male [84] | HFHFr | 1×35 | 4 | e-von Frey (↓2, 3, 4) | |||
| PWL (radiant heat) (↓2, 3, 4) | |||||||
| SD/male [85] | HFHFr (660 g fruc- tose; 80 g fat/kg) | 1×35 | 8 | TFL (hot water immersion) (↓1–8) | Sciatic-axonal injury, reduced Schwann cells (8) | ||
| Formalin-evoked hyperalgesia (tail-flinch) (↑8) | |||||||
| Wistar/male & female [86] | HFHFr (10% fructose solution) | 1×35 | 12 | PWL (hot plate) (↓12) | Sciatic- (12) | ||
| TFL (hot water immersion) (↓12) | |||||||
| Cold water latency (↓12) | |||||||
| Walking function test performance (↓12) | |||||||
| Wistar/male [53] | HFHFr (25.7% g lard, 46.5% g fructose) | 4×25 (2, 8, 14, 20 wk diet) | 36 | m-von Frey (↓–2 to 36) | Sciatic- loss of small and large myelinated fibers (36) | ||
| R/S (↓10–34) | |||||||
| PWL (Hargreaves) (↔0–36) | |||||||
| High caloric diet | |||||||
| SD/male [87] | High-caloric (NR) | 1×30 | 2 | R/S (↓1) | |||
| PWL (radiant heat) (↓1) | |||||||
| SD/male [88] | High-caloric (NR) | 1×30 | 2 | m-von Frey (↓1) | |||
| PWL (radiant heat) (↓1) | |||||||
| STZ/NA models | |||||||
| Wistar/male [33] | Nicotinamide: 1×50 mg/kg IP 15 min | 2 | TFL (radiant heat) (↓2) | Sciatic- axonal and myelin injury, haemorrhage, collagen deposition, vascular degeneration (2) | |||
| Later STZ: 1×52.5 mg/mg IP | Hindpaw cold allodynia (cold water) (↓2) | ||||||
| Tail cold allodynia (cold water) (↓2) | |||||||
| Rotarod (latency to fall) (↓2) | |||||||
| Wistar/male [34] | Nicotinamide: 1×50 mg/kg IP 15 min | 2 | TFL (radiant heat) (↓2) | Sciatic- axonal and myelin injury, haemorrhage, collagen deposition, vascular degeneration (2) | |||
| Later STZ: 1×52.5 mg/kg IP | Hindpaw cold allodynia (cold water) (↓2) | ||||||
| Tail cold allodynia (cold water) (↓2) | |||||||
| Rotarod (latency to fall) (↓2) | |||||||
| Wistar albino [32] | Nicotinamide: 1×110 mg/kg IP 15 min | 5 | PWL (hot plate) (↑1, 2, 3, 4, 5) | Sciatic-nerve cell degeneration (5) | |||
| Later STZ: 1×65 mg/kg IP | Rotarod (latency to fall) (↓1, 2, 3, 4, 5) | ||||||
↑=significant increase, ↓=significant decrease, ↔=no change.
STZ, streptozotocin; HFD, high-fat diet; m-von Frey, manual von Frey; SD, Sprague-Dawley; PWL, paw withdrawal latency; e-von Frey, electronic von Frey; MNCV, motor nerve conduction velocity; R/S, Randal-Selitto; SNCV, sensory nerve conduction velocity; IENFD, intraepidermal nerve fiber density; CNFL, corneal nerve fiber length; TFL, tail flick latency; HFHS, high-fat high-sugar; NR, not reported; NCV, nerve conduction velocity; HFHFr, high-fat high-fructose; NA, nicotinamide; IP, intra-peritoneal.
Diabetes induction approaches
Of the 60 studies that combined diet manipulation and STZ to induce T2DM, 42 were published after the Neurodiab guidelines (Table 1), reflecting increasing interest in this model. Most of the studies (50/63) used young adult rats, identified by either starting age (8 to 12 weeks, 20 studies) or weight (160 to 300 g, 28 studies). Twelve studies used younger rats (<8 weeks or <160 g). Three studies did not report starting age or body weights but two of them mentioned using adult rats. Notably, almost all studies used exclusively male rats (57/63), two studies used exclusively female rats, two studies used both male and female rats and two studies did not report sex.
Diet composition, diet duration and STZ doses varied across studies. Most studies have used HFD (34 studies), followed by high-fat high-sugar (HFHS; 18 studies) diet, high-fat high-fructose (HFHFr; five studies) diet, and unspecified diets (three studies) (Table 2). The energy derived from fat varied between 45% and 60%, and at least 18/60 studies mentioned lard as the source of fat in the diet. The duration of HFD feeding prior to STZ injection varied from 2 to 8 weeks (55/60 studies), and the dose of STZ varied from 25 to 45 mg/kg with the most common combination (18 studies) being 8 weeks of HFD feeding followed by a single dose of 30 mg/kg STZ. Most studies utilized a single STZ injection (55/60 studies). Three studies administered weekly STZ injections of 25 mg/kg, for 2 weeks [27-29], one study used weekly STZ injections of 30 mg/kg, for 4 weeks (weeks 5 to 8) [30] and another study used STZ injections of 25 mg/kg, repeated every 6 weeks from 2 to 20 weeks (total four injections) [53]. One study tested three different STZ doses (30, 35, 40 mg/kg) with a single injection and concluded that 35 mg/kg was ideal for inducing T2DM as it led to moderate and stable hyperglycemia with low rate of mortality [31]. Studies using two injections of 25 mg/kg reported hyperglycemia (250 to 400 mg/dL) [27-29] comparable to studies using a single dose, while one study using four injections of 30 mg/kg reported higher blood glucose levels (>500 mg/dL) [30]. However, one study administering four injections of 25 mg/kg at 6 weekly intervals showed a progression from moderate glucose intolerance and insulin resistance (weeks 2 to 18) to frank hyperglycemia, obesity, dyslipidemia and insulin (weeks 18 to 42) and severe organ damage to pancreas, liver and heart by weeks 42 to 56 [53].
The final mode of T2DM induction involved injecting NA shortly followed by high dose STZ (STZ/NA model) [32,90]. NA, a poly (ADP-ribose) polymerase (PARP) inhibitor, is added to mitigate the effects of STZ on pancreatic β-cells. NA prevents β-cell death caused by STZ-induced nicotinamide adenine dinucleotide (NAD+) depletion in order to better mimic the development of T2DM, and differentiating it from high dose STZ induced T1DM models [90]. The STZ-NA rat model of T2DM has been reported to lead to moderate hyperglycemia without the need for insulin support, depending on the doses of STZ (typically 45 to 65 mg/kg) and NA (100 to 120 mg/kg) [90]. However, all three studies that examined neuropathy and were included in this review demonstrated significant weight loss and two induced severe hyperglycemia (blood glucose >31 mmol/L) and hypoinsulinemia [33,34].
Effects of different T2DM induction methods on DPN phenotypes
1) HFD plus STZ
Thirty-four studies utilized HFD and low dose STZ to induce T2DM (30 in SD rat and four in Wistar rats) (Table 2). In these studies, behavioural tests were the most frequently reported DPN endpoint (32 studies) followed by peripheral nerve structure analyses (21 studies) and NCS (20 studies). Eighteen studies assessed all three DPN endpoints, as recommended by the Neurodiab guidelines, and 20 studies reported significant changes in at least two DPN endpoints. Among behavioural tests, mechanical sensitivity was tested in 16 studies, with 14 of these reporting mechanical hyperalgesia or decreased paw withdrawal threshold (PWT) and two studies reporting no change in diabetic animals.
Effects of diabetes on thermal sensitivity, which was tested in 31 out of 34 HFD/STZ studies were more varied. Of these, 11 studies reported thermal hyperalgesia (decreased paw withdrawal latency [PWL] or tail flick latency [TFL]), 17 studies observed thermal hypoalgesia (increased paw/tail withdrawal latency) and three studies reported no change (Table 2). Cold sensitivity was tested in five studies of which only two reported cold hyperalgesia (Table 2).
In line with the Neurodiab recommendation, 16 studies reported both motor and sensory nerve conduction deficits (MNCV and SNCV), while four reported only MNCV. Of the 20 studies to report peripheral nerve structural changes, 18 reported a decline in IENFD, of which 11 showed simultaneous reductions in CNFL (Table 2). Three studies examined sciatic nerve histopathology or morphometry which demonstrated axonal swelling/degeneration, lymphocyte infiltration as well as Schwann cell injury and demyelination (Table 2).
2) HFHS plus STZ
Eighteen studies utilised a HFHS diet plus a low dose of STZ to induce T2DM of which 15 were in SD rats and three were in Wistar rats (Table 2). These diets typically comprised 10%– 15% lard and 7%–20% sucrose. Like HFD models, behavioural tests were the most frequently reported DPN endpoint (13 studies), followed by NCS (seven studies) and peripheral nerve structure analyses (six studies). Notably, only one study assessed all three DPN endpoints [29] and six studies reported significant changes in at least two DPN endpoints (Table 2). Within the behavioural measures, nine studies assessed mechanical sensitivity, and all reported mechanical hyperalgesia (decreased PWT). In contrast to the HFD models, nine studies assessed thermal sensitivity and seven found thermal hyperalgesia (Table 2) while one found thermal hypoalgesia [35], and one found no change [29]. Out of seven studies that assessed nerve conduction, three reported a decline in both MNCV and SNCV [36-38] while three studies reported a decline in only MNCV [28,29,35] and one study reported no change in sciatic NCV without specifying motor or sensory nerve tested [39]. Unlike studies using the HFD/STZ model, none of the studies with HFHS diet assessed IENFD or CNFL, though six studies examined sciatic nerve histopathology or morphometry and reported features such as axonal atrophy, increased macrophage and mast cells, demyelination or myelin abnormalities (Table 2).
3) HFHFr plus STZ
Five studies utilised a HFHFr diet plus low dose STZ paradigm to induce T2DM of which three were in SD rats and two were in Wistar rats (Table 2). Fructose content in the diets varied widely (10% to 66%). Behavioural tests were reported in all five studies. None of these studies assessed nerve conduction nor IENFD/CNFL. Three studies reported peripheral nerve structural analyses with altered sciatic nerve histopathology and morphometry, which included axonal degeneration, demyelination and loss of both small and large myelinated nerve fibers. While no study assessed all three DPN endpoints, three studies found significant changes in two DPN endpoints. Among behavioural tests, two studies assessed mechanical sensitivity, and all reported mechanical hyperalgesia (decreased PWT), five studies assessed thermal sensitivity and four of them found thermal hyperalgesia while one study found no change. Nerve structural injuries were reported in four studies all investigating sciatic nerve and reported axonal degeneration, loss of large, and small myelinated fibers, reduced Schwann cell numbers, demyelination, etc.
4) STZ/NA model
None of the three studies using the STZ-NA model assessed all three DPN endpoints, but all three found significant differences in two DPN endpoints (Table 2), albeit only 2 to 5 weeks post-STZ. Two studies reported hyperalgesia [33,34] and one reported hypoalgesia [32] in tests of thermal sensitivity. Additionally, two studies reported increased cold sensitivity (Table 2). Nerve conduction and IENFD were not investigated in these studies, but all three studies documented irregularities in sciatic nerve histopathology and decreased fall latency on the rotarod test of motor function (Table 2). Sciatic nerve histopathological features included nerve cell degeneration, haemorrhage and collagen deposition within the nerve, myelin ballooning and vascular degeneration. Further work is needed to characterise DPN endpoints like mechanical sensitivity, NCS, IENFD, or CNFL along with studies of longer duration.
Insulin and lipids in diet and chemically induced models
Circulating insulin levels were assessed in 27% (17/63) of studies using diet and chemically induced models. It is difficult to generalise the insulin response across the diverse range of dose, frequency and timelines used. In HFD/STZ models, insulin was reported to be decreased [40], increased [41], and unchanged [42,43] at 16 weeks with no clear relationship to DPN phenotype (Table 2). In HFHS/STZ models, four studies [44-47] demonstrated an increase in insulin that corresponded with mechanical or thermal hyperalgesia. However, all these studies were of short duration (<5 weeks) where hyperalgesia is common (Fig. 3). On balance, insulin was infrequently reported, and no clear link could be made with DPN endpoints.
Fig. 3.
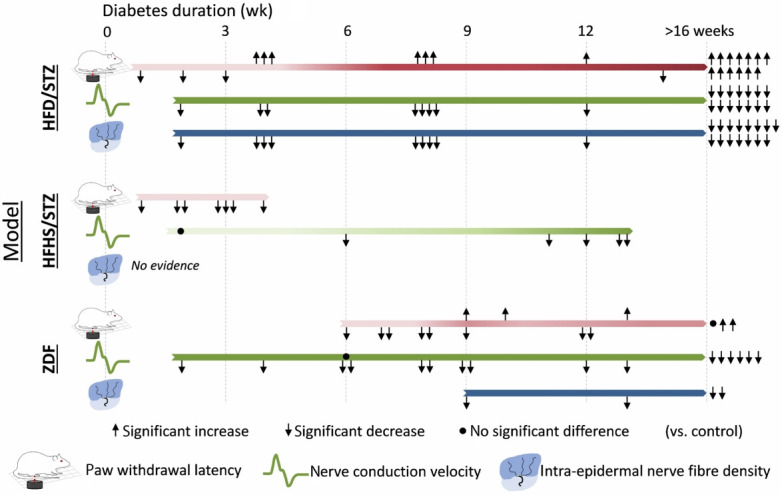
Schematic representation comparing evidence for significant changes in key diabetic peripheral neuropathy (DPN) endpoints between diabetes models across disease duration. Arrows indicate the direction of significant change, while dots represent no change. Each data point represents the findings of papers that reported the timepoint at which the assessments were collected using the recommended DPN measures (behaviour assessed by Hargreaves paw withdrawal latency, electrophysiology assessed by motor and sensory nerve conduction velocity and peripheral nerve structure assessed by intraepidermal nerve fibre density). The darkness of the bar indicates the strength of evidence for impairment in the measure vs. control. High-fat high-fructose/streptozotocin (STZ), STZ/nicotinamide, Zucker diabetic Sprague-Dawley, and Nile grass rat were not included as they had ≤1 data point for each of the requisite DPN measures (Tables 2 and 3). Diabetes duration for Zucker diabetic fatty (ZDF) models was calculated as age minus 6 weeks. HFD, high-fat diet; HFHS, high-fat high-sugar.
Circulating lipid profiles were assessed in 35% (22/63) of studies using diet and chemically induced models. Most of these studies measured free fatty acid, total cholesterol and triglycerides which were reported to be elevated at both early (3 to 12 weeks of diabetes) [27,48-50] and late-stages (≥16 weeks of diabetes) [28,41-43,51-59]. Only three studies reported high-density lipoprotein cholesterol [27,28,48] and three studies reported low-density lipoprotein cholesterol [28,35,48]. Studies showing evidence of dyslipidemia tended to observe changes in the key neuropathy endpoints (mechanical/thermal sensitivity, nerve conduction, and IENFD) at both early and late timepoints post-STZ (Table 2). The presence of dyslipidemia compared to the onset of DPN phenotypes was unclear, with several studies assessing lipids at a single time, typically at endpoint.
Overall, there is insufficient clarity to confidently determine whether there is any relationship between circulating insulin and lipids and the neuropathic phenotype across models. Moreover, as only a minority of studies report such metabolic measures, further work is needed to characterise these changes systematically across disease progression.
Effects of diabetes duration on DPN phenotypes
The studies reviewed here tested for DPN phenotypes between 2 and 36 weeks after the administration of STZ. Since diabetes duration is an important determinant of the severity of DPN, we compared DPN phenotypes between studies with short and long duration of diabetes, by classifying studies that assessed DPN between 0 and 6 weeks post-STZ as short-term diabetes and studies that assessed DPN beyond 6 weeks post-STZ as long duration diabetes. As shown in Fig. 3, studies using the HFD/STZ model have found consistent impairments in NCV and IENFD across short to long-term diabetes duration, whereas a change in PWL (measured using Hargreaves test) from hyper- to hypoalgesia was evident at approximately 4 weeks for this model. HFHS/STZ models also show hyperalgesia over short disease durations, with longer periods not studied; the reviewed studies using this model did not assess IENFD. For the ZDF model (see section 4.3.1), NCV and IENFD results concur with diet+STZ models, whereas PWL outcomes were more varied and not assessed at earlier timepoints.
1) Behavioural tests
Irrespective of the diet, mechanical sensitivity reported consistently as hyperalgesia was observed in 23/30 studies between 0 and 6 weeks post-STZ (short diabetes) while only 7/30 studies reported mechanical hyperalgesia between 7 and 36 weeks post-STZ (long diabetes). Only one study reported mechanical hypoalgesia at 9 weeks post-STZ [27].
Thermal sensitivity tests yielded dichotomous results with diabetes models producing hyperalgesic and hypoalgesic responses in a time-dependent fashion. Overall, 27 studies reported thermal hyperalgesia of which 26 studies were 0 to 6 weeks post-STZ while only five of them detected thermal hyperalgesia with long diabetes duration (>6 weeks post-STZ). By contrast, thermal hypoalgesia was found in 20 studies of which 16 were in long diabetes (7 to 32 weeks post-STZ) while only five of them found thermal hypoalgesia in short diabetes (0 to 6 weeks). These data suggest that diabetes duration has dynamic effects on behavioural measures, with hyperalgesia more often detected early and hypoalgesia observed late in diabetes progression. Notably, one study supporting this notion reported mechanical and thermal hyperalgesia at 5 weeks post-STZ whereas hypoalgesia was reported at 9 weeks post-STZ [27].
2) Nerve conduction studies
Twenty-six studies reported a decline in MNCV, the majority of which were in long duration diabetes (19 studies, 7 to 36 weeks post-STZ) while eight studies found reduced MNCV in short duration diabetes (0 to 6 weeks post-STZ). Nineteen studies reported a decline in SNCV, with most of these in long duration diabetes models (15 studies, 7 to 36 weeks) while only six studies reported reduced SNCV in short duration diabetes (0 to 6 weeks post-STZ).
3) Peripheral nerve structural analyses
As a measure of anatomical nerve injury, 18 studies reported a reduction in IENFD (all in HFD model) of which 16 reports were in long duration diabetes with only four reports in short duration diabetes. Eleven studies reported reduced CNFL (all in HFD model) of which nine studies were in long duration diabetes while four studies were in short duration diabetes. There were 15 studies that reported abnormal sciatic nerve histopathology or morphometric features both at short and long duration diabetes (seven and eight studies, respectively). Notably, 15 studies that reported sciatic nerve histopathology did not study IENFD or CNFL.
Influence of sex on DPN phenotypes
There were only four studies that used female rats, and only a single study directly compared pain-related behaviours between male and female T2DM rats, which found no sex differences [60]. One study in female rats used HFD with a higher fat content than most studies in males (60% vs. 45% kcal as fat) and longer duration of feeding (12 weeks vs. 8 weeks) and observed mechanical hyperalgesia and thermal hypoalgesia, reduced MNCV and SNCV and loss of IENFD and CNFL [40,61]. Another study in female rats used HFHS diet to induce T2DM but only reported altered mechanical sensitivity, with no other endpoints assessed [82].
Methods used for mechanical and thermal sensitivity tests
The methods used for mechanical and thermal sensitivity tests are an important consideration. Since animals cannot vocalize pain severity, detecting pain in animals requires indirect measurement of pain-like behaviours [91]. Of the studies in this review that tested mechanical sensitivity, 66.7% (20/30) utilised manual von-Frey, 16.7% (5/30) used electronic von-Frey, 23.3% (7/30) used Randal-Selitto tests, and three used needle-prick tests. For thermal sensitivity tests, 45 studies reported PWL (seconds) while nine studies reported TFL (seconds). 41.2% (21/51) studies used Hargreaves apparatus, 37.2% (19/51) studies used radiant heat stimulation system (either commercial or custom-made apparatus), 17.6% (9/51) studies used hot plate, and 9.8% (5/51) studies used hot water immersion test.
Transgenic/spontaneous rat models of T2DM
Transgenic rat models of T2DM have been used to study DPN since the 1990s. Most transgenic models involve a recessive homozygous mutation that results in the loss of function in the leptin receptor (fa) gene, triggering severe hyperphagia, obesity, and eventually T2DM [92]. As shown in Table 1, we identified 61 transgenic/spontaneous rat T2DM studies that studied DPN from 1994 to 2020, encompassing eight transgenic/spontaneous models, including obese genetic models such as the ZDF rat (36 studies), Bio-Breeding Zucker diabetic rat (BBZDR)/Wor rat (three studies), spontaneously diabetic Torii (SDT) fatty rat (fa/fa) (five studies), sucrose fed Otsuka Long-Evans Tokushima Fatty (OLETF) rat (six studies), Wistar diabetic fatty (WDF) rat (one study), Zucker diabetic Sprague-Dawley (ZDSD) rat (one study), Nile grass rat (NGR) (one study) and the non-obese genetic models such as the Goto-Kakizaki (GK) rat (10 studies). As demonstrated in Table 1, aside from the ZDF strain, the use of transgenic T2DM models has waned, with too few studies to meaningfully evaluate the impact of the Neurodiab guidelines. Aside from ZDF rats, 27 studies used seven other transgenic/spontaneous rat T2DM models, which have been reviewed previously [14] and of which only six (22%) were published after the Neurodiab guidelines. Therefore, here we summarise DPN phenotyping in ZDF rats and two recent transgenic/spontaneous models that have evaluated DPN with all key endpoints.
DPN phenotyping in ZDF rats
Zucker fatty rats have a leptin receptor mutation that induces dramatic hyperphagia, leading to obesity and metabolic impairments without overt hyperglycemia [93,94]. A second mutation and selective inbreeding led to the development of ZDF rats with a diabetogenic phenotype characterized by hyperglycemia [95]. ZDF rats are less obese than the Zucker fatty rats but show more severe insulin resistance resulting from increased apoptosis of the insulin secreting β-cells [96]. This leads to hyperinsulinemia at around 8 weeks of age followed by a decline in insulin levels, ultimately causing hyperglycemia or diabetes at 8 to 10 weeks in male rats, but not in females [93,97].
The ZDF rat has been extensively used to study T2DM-related complications including DPN [97-99]. Of the 36 studies included here, 20 were published pre-publication and 16 were published post-publication of the Neurodiab guidelines (Table 3) [97,99-135]. Only 5/36 studies (14%) assessed all three endpoints in this model however, all key DPN endpoints have been well characterized, and one third of the studies reported significant differences in at least two endpoints (12/26, 33%). Behavioural tests were the most commonly assessed DPN endpoint, examined in 75% of studies (27/36). Among the behavioural tests, mechanical sensitivity was assessed in 25 studies, thermal sensitivity was assessed in 20 studies and 18 studies tested both mechanical and thermal sensitivity. One study reported impaired motor performance on rotarod and grip-strength tests, spontaneous locomotor activity was assessed in two studies (one reported an impairment, and one reported no change) and one study reported cold hyperalgesia. Nerve conduction was assessed in 56% (20/36) studies of which 17 studies reported a decline in MNCV, 12 reported a decline in SNCV, and 10 studies reported decline in both MNCV and SNCV. Two studies reported NCV in tail nerve without specifying motor or sensory. Peripheral nerve structural features were analysed in 22% (8/36) studies of which five reported reduced IENFD and seven studies analysed peripheral nerve histopathologies of which five studies reported features such as sciatic-tibial nerve demyelination, sciatic nerve DNA damage and neuronal injury, sural nerve axonal atrophy, tibial nerve reduced myelin thickness, and reduced myelination in peroneal nerve. Two studies found no change in sciatic and sural nerve morphometric features. Corneal nerve analysis has not been evaluated in this model.
Table 3.
Neuropathy phenotyping in transgenic/spontaneous rat models of type 2 diabetes mellitus, in order of earliest neuropathic assessment
| Rat strain/sex | Diabetic animal model details |
Neuropathy phenotype (age in wk) |
||||
|---|---|---|---|---|---|---|
| Age (start-end), wk | Diabetes established, wk | Behavioural assessment of nerve function | Nerve conduction | Peripheral nerve structure | ||
| ZDF | ||||||
| ZDF/male [105] | 5–16 | BGL measured, not diagnostic | m-von Frey (↓6, 12, 14, 16) | |||
| PWL (radiant heat) (↓6, 12, 14, 16) | ||||||
| ZDF/male [106] | 6–17 | BGL measured, not diagnostic | e-von Frey (↓7–8, 12–13, 16–17) | |||
| PWL (Hargreaves) (↑16–17) | ||||||
| ZDF/male [107] | 5–10 | Yes | R/S (↓8–9) | |||
| ZDF/male [100] | 8–39 | BGL measured, not diagnostic | R/S (↓18, 24, 32, 36) | |||
| TFL (↓8, ↑16, 20, 24, 28, 36) | ||||||
| ZDF/male [108] | 8–14 | Yes | e-von Frey (↓10, 14) | MNCV (↓8, 10, 12, 14) | ||
| PWL (radiant heat) (↓8, 14) | SNCV (↓8, 10, 12, 14) | |||||
| ZDF/male [109] | 7–29 | Yes | m-von Frey (↔9, 11, 13, 15, 17, 19, 21, 23, 25, 27, 29) | |||
| ZDF/male [110] | 4–30a | Yes | e-von Frey (↓14a) | MNCV (↓26a) | IENFD (↓30a) | |
| PWL (Hargreaves) (↓18a) Grip-strength (↓16a) | SNCV (↓12.5a) | Sural nerve axonal atrophy (30) | ||||
| Rotarod (latency to fall) (↓14a) | ||||||
| ZDF/male [111] | 5–13 | NR | m-von Frey (↓11, 12, 13) | |||
| PWL (Hargreaves) (↓13) | ||||||
| ZDF/male [101] | 6–40 | BGL measured, not diagnostic | MNCV (↓12, 14, 16, 20, 24, 28, 32, 36, 40) | |||
| ZDF/male [112] | 7–13 | Yes | R/S (↓12, 13) | |||
| e-von Frey (↓12, 13) | ||||||
| ZDF/male [113] | 8–NR | BGL measured, not diagnostic | MNCV (↓12a) | |||
| ZDF/NR [114] | 8–16 | Yes | m-von Frey (↓14, 15) | SNCV (↓12, 14, 15) | ||
| PWL (Hargreaves) (↓12, 14, 15) | ||||||
| ZDF/male [115] | 6–14 | Yes | m-von Frey (↓13, 14) | |||
| PWL (hot plate) (↓13, 14) | ||||||
| ZDF/male [116] | 6–15 | BGL measured, not diagnostic | m-von Frey (↓13, 14, 15) | Spontaneous locomotor activity (↔13, 14, 15) | ||
| PWL (Hargreaves) (↓13, 14) | ||||||
| ZDF/male [117] | 7–16 | Yes | m-von Frey (↓13, 14, 15, 16) | |||
| PWL (hot plate) (↓13, 14, 15, 16) | ||||||
| ZDF/NR [118] | 8–16 | Yes | m-von Frey (↓13, 16) | SNCV (↓13, 16) | ||
| PWL (radiant heat) (↓13, 16) | ||||||
| ZDF/male [119] | NR | BGL measured, not diagnostic | R/S (↓13) | |||
| ZDF/male [120] | 13 | NR | Sciatic-tibial nerve demyelination (↑13) | |||
| ZDF/male [121] | 14–23 | BGL measured, not diagnostic | R/S (↓18, 20, 22) | MNCV (↓23) | IENFD (↓23) | |
| TFL (↑14, 16,18, 20, 22) | SNCV (↓23) | Peroneal nerve myelinated fiber density (↑23) | ||||
| Myelin area (↓23) | ||||||
| ZDF/NR [122] | 6–38 | Yes | R/S (↓15) | |||
| m-von Frey (↓15) | ||||||
| Spontaneous locomotor activity (↓7, 15, 31) | ||||||
| ZDF/male [123] | 4–19 | Yes | R/S (↓15, 16, 17, 18) | |||
| PWL (hot plate) (↓15, 17, 18) | ||||||
| Cold hyperalgesia (↑18) | ||||||
| ZDF/male [97] | 5–20 | BGL measured, not diagnostic | MNCV (↓19) | |||
| ZDF/male [124] | 9–19 | BGL measured, not diagnostic | m-von Frey (↓15, 19) | MNCV (↓15, 19) | IENFD (↓15/19) | |
| R/S (↑15, 19) | SNCV (↓15, 19) | Tibial nerve myelin thickness (↓19) | ||||
| PWL (Hargreaves) (↑15, 19) | G-ratio and axon diameter/myelin thickness (↑19) | |||||
| ZDF/male [125] | 14–20 | BGL measured, not diagnostic | m-von Frey (↔16, 18, 20) | |||
| ZDF/male [126] | 6–25 | BGL measured, not diagnostic | m-von Frey (↓25a) | MNCV (↔12a, ↓25a) | IENFD (↓25a) | |
| PWL (Hargreaves) (↔25a) | SNCV (↔12a, ↓25a) | Sural nerve morphometry (↔25a) | ||||
| ZDF/male [127] | 6–40 | Yes | R/S (↓17, 33) | MNCV (↓38–40) | IENFD (↓35) | |
| m-von Frey (↑31, 38) | SNCV (↓38–40) | |||||
| ZDF/male [128] | 7–17 | BGL measured, not diagnostic | MNCV (↓17) | |||
| ZDF/female [129] | 8–18 | BGL measured, not diagnostic | MNCV (↓18) | Sciatic nerve DNA damage and neuronal injury (↑18) | ||
| SNCV (↓18) | ||||||
| ZDF/male [102] | 9–19 | NR | MNCV (↓18, 19) | Sciatic nerve histopathology (↔19) | ||
| NCV (↓18, 19) | ||||||
| ZDF/male [130] | 10–18 | Yes | R/S (↓18) | |||
| PWL (Hargreaves) (↓18) | ||||||
| ZDF/male [131] | 14–22 | BGL measured, not diagnostic | MNCV (↓21, 22) | |||
| ZDF/male [132] | NR | BGL measured, not diagnostic | m-von Frey (↓18, 32) | MNCV (↓NR) | ||
| PWL (Hargreaves) (↑NR) | SNCV (↓NR) | |||||
| ZDF/male [133] | 7–21/27 | BGL measured, not diagnostic | MNCV (↓21/27) | |||
| ZDF/male [134] | 6–24 | NR | PWL (Hargreaves) (↑24) | MNCV (↓24) | ||
| SNCV (↓24) | ||||||
| ZDF/male [135] | 6–28 | NR | PWL (Hargreaves) (↑28) | MNCV (↓28) | ||
| SNCV (↓28) | ||||||
| ZDF/male [99] | 6–35 | BGL measured, not diagnostic | e-von Frey (↔ up to 35) | NCV (↔up to 35) | ||
| TFL (↓35) | ||||||
| ZDSD | ||||||
| ZDSD/male [103] | 16–34 | BGL measured, not diagnostic | PWL (Hargreaves) (↑34) | MNCV (↓34) | IENFD (↔34) | |
| SNCV (↓34) | CNFL (↓34) | |||||
| NGR | ||||||
| African NGR/male [104] | 52–104 | Yes | m-von Frey ↑ | MNCV ↓ | IENFD ↓ | |
| PWL (Hargreaves) ↑ | ||||||
For each rat strain, studies are listed according to age of earliest standardised neuropathic assessment. Number(s) in parentheses refer to the age of rats in weeks, except for where diabetes duration is used and clearly mentioned. ↑=significant increase, ↓=significant decrease, ↔=no change.
ZDF, Zucker diabetic fatty; BGL, blood glucose level; m-von Frey, manual von Frey; PWL, paw withdrawal latency; e-von Frey, electronic von Frey; R/S, Randal-Selitto; TFL, tail flick latency; MNCV, motor nerve conduction velocity; SNCV, sensory nerve conduction velocity; IENFD, intraepidermal nerve fiber density; NR, not reported; NCV, nerve conduction velocity; ZDSD, Zucker diabetic Sprague-Dawley; CNFL, corneal nerve fiber length; NGR, Nile grass rat.
Values were converted to weeks of age, from months or weeks of diabetes, as reported in original study.
1) Effect of age on DPN phenotyping
Most studies (21/25) reported mechanical hyperalgesia (a decrease in PWT) between 6 and 36 weeks of age. Two studies reported mechanical hypoalgesia (an increase in PWT), one at 31 and 38 weeks of age, and another at 15 and 19 weeks of age. Three studies reported no change in mechanical sensitivity (up to 35 weeks of age). As seen for diet-induced models, thermal sensitivity tests showed both hyperalgesia (13 studies) and hypoalgesia (seven studies) in ZDF rats, with one study showing no change. Also consistent with the diet-induced models, thermal sensitivity appeared to be increased at early ages (12 studies reported hyperalgesia between 6 and 18 weeks of age) and reduced at later ages (seven studies reported hypoalgesia between 14 and 36 weeks) (Table 3, Fig. 3). One study examining the time course of thermal sensation in ZDF rats reported hyperalgesia at 8 weeks of age (coupled with insulin resistance without frank hyperglycemia), and hypoalgesia at 16 to 36 weeks of age (coinciding with loss of insulin and severe hyperglycemia) [100]. Sixteen studies reported a decline in MNCV in diabetic rats between the age of 8 to 40 weeks, and 12 studies reported a decline in SNCV between the age of 8 to 40 weeks (Table 3). One study showed a progressive and steady decline in MNCV from 12 to 40 weeks of age [101]. Two studies tested NCV in the tail nerve, one of them found no change [99] but another reported reduction in mixed NCV (motor and sensory) [102]. Five observed reduced IENFD in 15 to 35-week-old ZDF rats. Five studies reported changes in nerve morphometry or demyelination or axonal atrophy in sciatic/sural/tibial/peroneal nerves between 18 weeks and 6 months of age, while two studies reported no change in sciatic and sural nerves of ZDF rats at 4 to 5 months of age (Table 3).
Effect of sex on DPN: Nearly all studies in this model used male rats (32/36), three studies did not report sex while only one study used female rats which reported decline in MNCV and SNCV and altered sciatic nerve histopathology (increased DNA damage and neuronal injury). No study made a direct comparison in DPN between male and female rats.
Methods used for mechanical and thermal sensitivity tests: The manual von Frey method was used in 12 studies, six studies utilized electronic von Frey, and 10 studies utilized RandallSelitto test to determine mechanical sensitivity. For thermal sensitivity tests, 17 studies assessed PWL, and three studies assessed TFL. Eleven studies utilized Hargreaves apparatus, six studies heat stimulation apparatus with radiant heat source and three studies used hot plates.
The ZDSD rat model of T2DM
The ZDSD rat was created by crossbreeding the Charles River Laboratory diet-induced obese rat (SD-derived) with lean ZDF–/– rats to generate a model of polygenic obesity and T2DM with an intact leptin pathway [136-138]. ZDSD rats are predisposed to obesity and develop overt diabetes between 15 to 21 weeks of age with dietary manipulations, accruing less adiposity than ZDF rats [137]. The only study using a ZDSD model characterized DPN using all key endpoints (Table 3), reporting significant impairments in small and large nerve fiber function (thermal hypoalgesia and decreased MNCV, SNCV), decreased CNFL but no loss of IENFD in 34-week-old rats that were hyperglycemic for 12 weeks [103]. The strength of this model lies in its intact leptin signalling pathway and some evidence for changes in DPN endpoints; thus, future work should contrast this model with other diet and chemically induced models.
African NGR model of T2DM
A recently developed rat T2DM model is the African NGR, which spontaneously develops T2DM when fed a standard laboratory chow diet without the need for any dietary modification, chemical agent or leptin receptor mutation [139]. The NGR T2DM model produces moderate hyperglycemia (approximately 10 mmol/L) [104,139] with a gradual progression from hyperinsulinemia (2 months) to hyperglycemia (6 to 12 months), to pancreatic β-cell dysfunction, dyslipidemia, weight gain, microvascular pathology, hypertension, and ketonuria after 12 months [139,140].
The one study to examine DPN in this model assessed all three endpoints recommended by Neurodiab, and observed mechanical and thermal hyposensitivity, loss of IENFD, and reduced MNCV in aged rats that were diabetic from 12 to 24 months of age (Tables 1 and 2) [104]. Since these rats usually become hyperglycemic at 6 to 12 months of age and this study used older rats, the evidence of hyposensitivity parallels the loss of sensation in late-stage human DPN. The slow onset of T2DM and, in turn DPN in this model, may provide unique opportunities to conduct comprehensive longitudinal phenotyping and evaluate preventative treatment.
METHODOLOGICAL CONSIDERATIONS FOR CHOICE OF BEHAVIOURAL TESTS
Careful considerations are needed in choosing specific methods or apparatus for behavioural tests, keeping in mind type of nerve fibers being stimulated. Although manual-von-Frey is criticised as subjective and time consuming, it remains the most frequently used approach to detect mechanical sensitivity. Electronic von-Frey is preferred by some researchers mainly because it is less time consuming and PWT and pressure applied is objectively recorded automatically. Manual von-Frey filaments exert a non-noxious fixed pressure once the filament is bent against the hindpaw while electronic von-Frey and Randal-Selitto tests exert a gradual ramp of pressure which is often noxious and elicit painful responses. It should be noted that studies in mice found that the PWT was considerably higher with electronic than manual von-Frey leading to speculation that electronic von-Frey activates high-threshold mechanoreceptor while manual von-Frey filaments activate low threshold mechanoreceptors [91]. On the other hand, the Randal-Selitto test requires the animal to be heavily restrained during the procedure while manual and electronic von-Frey are applied in unrestrained animals [141]. The Randal-Selitto test mainly activates nociceptors but can also stimulate low threshold mechanoreceptors [141]. Mechanical sensitivity is reported as PWT (grams) equivalent to the amount of force or pressure needed to cause the animal to withdraw the hindpaw from the filament or probe.
In thermal tests, irrespective of the heat source, tail flick testing requires the animal to be restrained and tail-flick response is primarily due to spinal reflex and associated with motor processing rather than pain although the supraspinal processing to the tail-flick response can be partially mediated by heat stimuli [142-144]. The hot plate test also done in unrestrained animals but it is subject to criticism as the whole body (forepaws, hindpaws, and tail) of the animal is exposed to heat with the potential to cause unintended tissue damage as well as well as learned behavioural responses which can lead to quick withdrawal in subsequent tests [91]. However, in Hargreaves or Radiant-heat stimulation, the heat source is applied specifically underneath the hindpaw and the animal need not be restrained [91]. While these studies only assessed stimulus-evoked pain behaviours, there are concerns regarding the clinical translatability of these approaches. Therefore, in recent years, there has been interest in the development and implementation of spontaneous or non-stimulus evoked methods, such as grimace scales, burrowing, weight bearing and gait analysis. Future studies in rodent models of DPN should also consider such spontaneous or non-stimulus evoked pain assessment.
DISCUSSION
This review has evaluated DPN phenotypes in diet-induced and transgenic models of T2DM rats with reference to the publication of the Neurodiab guidelines in 2014. Our analysis shows a shift from transgenic to diet-induced models of T2DM to study DPN, except for the ZDF rat, which remains a popular choice. Among the diet and chemically induced models, all key DPN endpoints have been fully characterized only in the HFD/STZ model. Further work is needed to better characterize DPN in HFHS diet or HFHFr diet plus STZ models and STZ/NA. Studies with female rats are scarce and thus more studies should be conducted to characterise sex differences in DPN pathology. Evidence to date indicates no difference in DPN phenotypes between male and female rats [40,60] and a recent study in a mouse model of HFD induced prediabetes found no sex difference in DPN phenotypes, though the onset of insulin resistance was delayed in females [145]. A recent review female mice and rats appear less susceptible to HFD-induced obesity and metabolic insults compared to males despite similar PN phenotypes [146]. More studies are needed in female rats or head-to-head comparison between male and female rats utilizing different diets to induce T2DM and study DPN.
The proportion of studies including all three key DPN endpoints has not increased appreciably since publication of the Neurodiab guidelines. Future research would benefit from supplementing behavioural measures of DPN, which were overwhelmingly most common, with nerve conduction and structural analyses, performed in about half of the studies discussed here, to enable the ontogeny of DPN to be documented systematically. Increasing use of corneal nerve fiber analysis may complement the existing DPN phenotyping criteria. It is important to note that there have been advances in the measurement of autonomic dysfunction in animal models of DPN over recent years [147]. The ongoing development of this domain is an important future direction to support the inclusion of autonomic dysfunction in studies and guidelines [148].
Our synthesis of the literature generates several novel insights for future research. First, DPN symptomatology did not appear to be dependent on disease duration, with altered mechanical and thermal sensitivity, nerve conduction and IENFD observed across a range of short (0 to 6 weeks) and chronic (7 to 36 weeks) T2DM durations. T2DM induced a biphasic effect on thermal sensitivity, with hyperalgesia more common in short duration diabetes and hypoalgesia more frequently observed after long duration diabetes (Fig. 3). The molecular basis of this time-dependent dissociation between hyper- and hypoalgesia was recently studied in a prospective observational clinical study. Here, increased serum neurofilament light chain level (indicative of axonal injury) associated with hyperalgesia in T2DM patients with DPN while a decreased level of circulatory mRNA of myelin protein zero (indicative of demyelination) was associated with hypoalgesia [149]. Moreover, neurofilament light chain level correlated with quantitative sensory tests involving small unmyelinated C-fibers (warm detection, heat, and cold pain) whereas myelin protein zero mRNA level correlated with quantitative sensory tests involving myelinated A-δ and A-β fibers (mechanical sensitivity). However, these molecular markers were not correlated with diabetes duration, directing future research to dissect the molecular mechanisms underlying this effect. In addition, one study in ZDF rats implicated declining insulin levels in the transition from thermal hyperalgesia to hypoalgesia across prolonged disease duration [100].
While some transgenic models have declined in popularity, we believe that their unique features hold value in modelling distinct aspects of DPN pathophysiology and treatment in different T2DM populations (e.g., normal-weight T2DM patients). Overall, greater adherence to Neurodiab guidelines will accelerate understanding of the mechanisms underlying DPN and will drive the development of successful, translatable therapies to reduce disease burden.
Acknowledgments
None
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This work was supported by funding to Margaret J Morris (NHMRC, Australia, Grant 1126929). Ria Arnold was supported by a NHRMC Fellowship (NHMRC, Australia, Grant 1091006). Md Jakir Hossain was supported by a Scientia PhD scholarship from UNSW Sydney.
REFERENCES
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:41. doi: 10.1038/s41572-019-0092-1. [DOI] [PubMed] [Google Scholar]
- 3.Biessels GJ, Bril V, Calcutt NA, Cameron NE, Cotter MA, Dobrowsky R, et al. Phenotyping animal models of diabetic neuropathy: a consensus statement of the diabetic neuropathy study group of the EASD (Neurodiab) J Peripher Nerv Syst. 2014;19:77–87. doi: 10.1111/jns5.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman EL, Nave KA, Jensen TS, Bennett DL. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93:1296–313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:136–54. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azmi S, Petropoulos IN, Ferdousi M, Ponirakis G, Alam U, Malik RA. An update on the diagnosis and treatment of diabetic somatic and autonomic neuropathy. F1000Res. 2019;8 (F1000 Faculty Rev):186. doi: 10.12688/f1000research.17118.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sima AA, Zhang W, Xu G, Sugimoto K, Guberski D, Yorek MA. A comparison of diabetic polyneuropathy in type II diabetic BBZDR/Wor rats and in type I diabetic BB/Wor rats. Diabetologia. 2000;43:786–93. doi: 10.1007/s001250051376. [DOI] [PubMed] [Google Scholar]
- 8.Sima AA. Diabetic neuropathy in type 1 and type 2 diabetes and the effects of C-peptide. J Neurol Sci. 2004;220:133–6. doi: 10.1016/j.jns.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Sima AA, Nathaniel V, Bril V, McEwen TA, Greene DA. Histopathological heterogeneity of neuropathy in insulin-dependent and non-insulin-dependent diabetes, and demonstration of axo-glial dysjunction in human diabetic neuropathy. J Clin Invest. 1988;81:349–64. doi: 10.1172/JCI113327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543. doi: 10.1002/14651858.CD007543.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen DH, Knudsen ST, Gylfadottir SS, Christensen LB, Nielsen JS, Beck-Nielsen H, et al. Metabolic factors, lifestyle habits, and possible polyneuropathy in early type 2 diabetes: a nationwide study of 5,249 patients in the Danish Centre for Strategic Research in type 2 diabetes (DD2) cohort. Diabetes Care. 2020;43:1266–75. doi: 10.2337/dc19-2277. [DOI] [PubMed] [Google Scholar]
- 12.Stino AM, Rumora AE, Kim B, Feldman EL. Evolving concepts on the role of dyslipidemia, bioenergetics, and inflammation in the pathogenesis and treatment of diabetic peripheral neuropathy. J Peripher Nerv Syst. 2020;25:76–84. doi: 10.1111/jns.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hossain MJ, Kendig MD, Wild BM, Issar T, Krishnan AV, Morris MJ, et al. Evidence of altered peripheral nerve function in a rodent model of diet-induced prediabetes. Biomedicines. 2020;8:313. doi: 10.3390/biomedicines8090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yorek MA. Alternatives to the streptozotocin-diabetic rodent. Int Rev Neurobiol. 2016;127:89–112. doi: 10.1016/bs.irn.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien PD, Sakowski SA, Feldman EL. Mouse models of diabetic neuropathy. ILAR J. 2014;54:259–72. doi: 10.1093/ilar/ilt052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islam MS. Animal models of diabetic neuropathy: progress since 1960s. J Diabetes Res. 2013;2013:149452. doi: 10.1155/2013/149452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 18.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 19.Ferrier J, Marchand F, Balayssac D. Assessment of mechanical allodynia in rats using the electronic von Frey test. Bio Protoc. 2016;6:e1933 [Google Scholar]
- 20.Beiswenger KK, Calcutt NA, Mizisin AP. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem. 2008;110:351–62. doi: 10.1016/j.acthis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, et al. Intraepidermal nerve fiber density in rat foot pad: neuropathologic-neurophysiologic correlation. J Peripher Nerv Syst. 2005;10:202–8. doi: 10.1111/j.1085-9489.2005.0010210.x. [DOI] [PubMed] [Google Scholar]
- 22.Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, et al. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12:747–58. doi: 10.1111/j.1468-1331.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- 23.Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56:2148–54. doi: 10.2337/db07-0285. [DOI] [PubMed] [Google Scholar]
- 24.Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, et al. A new rat model of type 2 diabetes: the fatfed, streptozotocin-treated rat. Metabolism. 2000;49:1390–4. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- 25.Skovso S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig. 2014;5:349–58. doi: 10.1111/jdi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao F, Zheng ZM. Animal models of diabetic neuropathic pain. Exp Clin Endocrinol Diabetes. 2014;122:100–6. doi: 10.1055/s-0033-1363234. [DOI] [PubMed] [Google Scholar]
- 27.Mehta BK, Nerkar D, Banerjee S. Characterization of peripheral neuropathy in rat model of type 2 diabetes. Indian J Pharm Educ Res. 2017;51:92–101. [Google Scholar]
- 28.Ding Y, Dai X, Zhang Z, Jiang Y, Ma X, Cai X, et al. Proanthocyanidins protect against early diabetic peripheral neuropathy by modulating endoplasmic reticulum stress. J Nutr Biochem. 2014;25:765–72. doi: 10.1016/j.jnutbio.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Ding Y, Dai X, Jiang Y, Zhang Z, Li Y. Functional and morphological effects of grape seed proanthocyanidins on peripheral neuropathy in rats with type 2 diabetes mellitus. Phytother Res. 2014;28:1082–7. doi: 10.1002/ptr.5104. [DOI] [PubMed] [Google Scholar]
- 30.Lu M, Yi T, Xiong Y, Wang Q, Yin N. Cortex Mori Radicis extract promotes neurite outgrowth in diabetic rats by activating PI3K/AKT signaling and inhibiting Ca2+ influx associated with the upregulation of transient receptor potential canonical channel 1. Mol Med Rep. 2020;21:320–8. doi: 10.3892/mmr.2019.10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dang JK, Wu Y, Cao H, Meng B, Huang CC, Chen G, et al. Establishment of a rat model of type II diabetic neuropathic pain. Pain Med. 2014;15:637–46. doi: 10.1111/pme.12387_1. [DOI] [PubMed] [Google Scholar]
- 32.Sharma AK, Sharma A, Kumari R, Kishore K, Sharma D, Srinivasan BP, et al. Sitagliptin, sitagliptin and metformin, or sitagliptin and amitriptyline attenuate streptozotocin-nicotinamide induced diabetic neuropathy in rats. J Biomed Res. 2012;26:200–10. doi: 10.7555/JBR.26.20110054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moustafa PE, Abdelkader NF, El Awdan SA, El-Shabrawy OA, Zaki HF. Extracellular matrix remodeling and modulation of inflammation and oxidative stress by sulforaphane in experimental diabetic peripheral neuropathy. Inflammation. 2018;41:1460–76. doi: 10.1007/s10753-018-0792-9. [DOI] [PubMed] [Google Scholar]
- 34.Moustafa PE, Abdelkader NF, El Awdan SA, El-Shabrawy OA, Zaki HF. Liraglutide ameliorated peripheral neuropathy in diabetic rats: involvement of oxidative stress, inflammation and extracellular matrix remodeling. J Neurochem. 2018;146:173–85. doi: 10.1111/jnc.14336. [DOI] [PubMed] [Google Scholar]
- 35.Yang XY, Sun L, Xu P, Gong LL, Qiang GF, Zhang L, et al. Effects of salvianolic scid A on plantar microcirculation and peripheral nerve function in diabetic rats. Eur J Pharmacol. 2011;665:40–6. doi: 10.1016/j.ejphar.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y, Ge Y, Wu M, Xie Y, Wang M, Chen Y, et al. Long non coding RNAs regulate inflammation in diabetic peripheral neuropathy by acting as ceRNAs targeting miR-146a-5p. Diabetes Metab Syndr Obes. 2020;13:413–22. doi: 10.2147/DMSO.S242789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng Y, Chen L, Luo Q, Wu M, Chen Y, Shi X. Involvement of microRNA-146a in diabetic peripheral neuropathy through the regulation of inflammation. Drug Des Devel Ther. 2018;12:171–7. doi: 10.2147/DDDT.S157109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Li B, Chen B, Shao Y, Luo Q, Shi X, et al. Thymoquinone alleviates the experimental diabetic peripheral neuropathy by modulation of inflammation. Sci Rep. 2016;6:31656. doi: 10.1038/srep31656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He J, Yuan GH, Zhang JQ, Guo XH. Approach to creating early diabetic peripheral neuropathy rat model. Beijing Da Xue Xue Bao Yi Xue Ban. 2019;51:1150–4. doi: 10.19723/j.issn.1671-167X.2019.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coppey LJ, Shevalye H, Obrosov A, Davidson EP, Yorek MA. Determination of peripheral neuropathy in high-fat diet fed low-dose streptozotocin-treated female C57Bl/6J mice and Sprague-Dawley rats. J Diabetes Investig. 2018;9:1033–40. doi: 10.1111/jdi.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oza MJ, Kulkarni YA. Formononetin ameliorates diabetic neuropathy by increasing expression of SIRT1 and NGF. Chem Biodivers. 2020;17:e2000162. doi: 10.1002/cbdv.202000162. [DOI] [PubMed] [Google Scholar]
- 42.Davidson EP, Coppey LJ, Holmes A, Yorek MA. Effect of inhibition of angiotensin converting enzyme and/or neutral endopeptidase on vascular and neural complications in high fat fed/low dose streptozotocin-diabetic rats. Eur J Pharmacol. 2012;677:180–7. doi: 10.1016/j.ejphar.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson EP, Coppey LJ, Holmes A, Dake B, Yorek MA. Effect of treatment of high fat fed/low dose streptozotocin-diabetic rats with Ilepatril on vascular and neural complications. Eur J Pharmacol. 2011;668:497–506. doi: 10.1016/j.ejphar.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu YB, Jia GL, Wang JW, Ye XY, Lu JH, Chen JL, et al. Activation of CaMKII and GluR1 by the PSD-95-GluN2B couplingdependent phosphorylation of GluN2B in the spinal cord in a rat model of type-2 diabetic neuropathic pain. J Neuropathol Exp Neurol. 2020;79:800–8. doi: 10.1093/jnen/nlaa035. [DOI] [PubMed] [Google Scholar]
- 45.Zhou YF, Ying XM, He XF, Shou SY, Wei JJ, Tai ZX, et al. Suppressing PKC-dependent membrane P2X3 receptor upregulation in dorsal root ganglia mediated electroacupuncture analgesia in rat painful diabetic neuropathy. Purinergic Signal. 2018;14:359–69. doi: 10.1007/s11302-018-9617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou CH, Zhang MX, Zhou SS, Li H, Gao J, Du L, et al. SIRT1 attenuates neuropathic pain by epigenetic regulation of mGluR1/5 expressions in type 2 diabetic rats. Pain. 2017;158:130–9. doi: 10.1097/j.pain.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 47.He XF, Wei JJ, Shou SY, Fang JQ, Jiang YL. Effects of electroacupuncture at 2 and 100 Hz on rat type 2 diabetic neuropathic pain and hyperalgesia-related protein expression in the dorsal root ganglion. J Zhejiang Univ Sci B. 2017;18:239–48. doi: 10.1631/jzus.B1600247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahlawat A, Sharma S. A new promising simultaneous approach for attenuating type II diabetes mellitus induced neuropathic pain in rats: iNOS inhibition and neuroregeneration. Eur J Pharmacol. 2018;818:419–28. doi: 10.1016/j.ejphar.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Jain D, Bansal MK, Dalvi R, Upganlawar A, Somani R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J Integr Med. 2014;12:35–41. doi: 10.1016/S2095-4964(14)60001-7. [DOI] [PubMed] [Google Scholar]
- 50.Davidson EP, Coppey LJ, Holmes A, Yorek MA. Changes in corneal innervation and sensitivity and acetylcholine-mediated vascular relaxation of the posterior ciliary artery in a type 2 diabetic rat. Invest Ophthalmol Vis Sci. 2012;53:1182–7. doi: 10.1167/iovs.11-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coppey L, Davidson E, Shevalye H, Obrosov A, Torres M, Yorek MA. Progressive loss of corneal nerve fibers and sensitivity in rats modeling obesity and type 2 diabetes is reversible with omega-3 fatty acid intervention: supporting cornea analyses as a marker for peripheral neuropathy and treatment. Diabetes Metab Syndr Obes. 2020;13:1367–84. doi: 10.2147/DMSO.S247571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes A, Coppey LJ, Davidson EP, Yorek MA. Rat models of diet-induced obesity and high fat/low dose streptozotocin type 2 diabetes: effect of reversal of high fat diet compared to treatment with enalapril or menhaden oil on glucose utilization and neuropathic endpoints. J Diabetes Res. 2015;2015:307285. doi: 10.1155/2015/307285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barriere DA, Noll C, Roussy G, Lizotte F, Kessai A, Kirby K, et al. Combination of high-fat/high-fructose diet and low-dose streptozotocin to model long-term type-2 diabetes complications. Sci Rep. 2018;8:424. doi: 10.1038/s41598-017-18896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coppey L, Davidson E, Shevalye H, Obrosov A, Yorek M. Effect of early and late interventions with dietary oils on vascular and neural complications in a type 2 diabetic rat model. J Diabetes Res. 2019;2019:5020465. doi: 10.1155/2019/5020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davidson EP, Coppey LJ, Shevalye H, Obrosov A, Kardon RH, Yorek MA. Impaired corneal sensation and nerve loss in a type 2 rat model of chronic diabetes is reversible with combination therapy of menhaden oil, α-lipoic acid, and enalapril. Cornea. 2017;36:725–31. doi: 10.1097/ICO.0000000000001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yorek MS, Davidson EP, Poolman P, Coppey LJ, Obrosov A, Holmes A, et al. Corneal sensitivity to hyperosmolar eye drops: a novel behavioral assay to assess diabetic peripheral neuropathy. Invest Ophthalmol Vis Sci. 2016;57:2412–9. doi: 10.1167/iovs.16-19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davidson EP, Holmes A, Coppey LJ, Yorek MA. Effect of combination therapy consisting of enalapril, α-lipoic acid, and menhaden oil on diabetic neuropathy in a high fat/low dose streptozotocin treated rat. Eur J Pharmacol. 2015;765:258–67. doi: 10.1016/j.ejphar.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 58.Davidson EP, Coppey LJ, Kardon RH, Yorek MA. Differences and similarities in development of corneal nerve damage and peripheral neuropathy and in diet-induced obesity and type 2 diabetic rats. Invest Ophthalmol Vis Sci. 2014;55:1222–30. doi: 10.1167/iovs.13-13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coppey LJ, Holmes A, Davidson EP, Yorek MA. Partial replacement with menhaden oil improves peripheral neuropathy in high-fat-fed low-dose streptozotocin type 2 diabetic rat. J Nutr Metab. 2012;2012:950517. doi: 10.1155/2012/950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferhatovic L, Banozic A, Kostic S, Sapunar D, Puljak L. Sex differences in pain-related behavior and expression of calcium/calmodulin-dependent protein kinase II in dorsal root ganglia of rats with diabetes type 1 and type 2. Acta Histochem. 2013;115:496–504. doi: 10.1016/j.acthis.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Davidson EP, Coppey LJ, Shevalye H, Obrosov A, Yorek MA. Effect of dietary content of menhaden oil with or without salsalate on neuropathic endpoints in high-fat-fed/low-dose streptozotocin-treated Sprague Dawley Rats. J Diabetes Res. 2018;2018:2967127. doi: 10.1155/2018/2967127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang R, Li L, Yuan H, Liu H, Gong Y, Zou L, et al. Quercetin relieved diabetic neuropathic pain by inhibiting upregulated P2X4 receptor in dorsal root ganglia. J Cell Physiol. 2019;234:2756–64. doi: 10.1002/jcp.27091. [DOI] [PubMed] [Google Scholar]
- 63.Yuan H, Ouyang S, Yang R, Li S, Gong Y, Zou L, et al. Osthole alleviated diabetic neuropathic pain mediated by the P2X4 receptor in dorsal root ganglia. Brain Res Bull. 2018;142:289–96. doi: 10.1016/j.brainresbull.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Fink B, Coppey L, Davidson E, Shevalye H, Obrosov A, Chheda PR, et al. Effect of mitoquinone (Mito-Q) on neuropathic endpoints in an obese and type 2 diabetic rat model. Free Radic Res. 2020;54:311–8. doi: 10.1080/10715762.2020.1754409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rao S, Liu S, Zou L, Jia T, Zhao S, Wu B, et al. The effect of sinomenine in diabetic neuropathic pain mediated by the P2X3 receptor in dorsal root ganglia. Purinergic Signal. 2017;13:227–35. doi: 10.1007/s11302-016-9554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng H, Zou L, Xie J, Wu H, Wu B, Zhu G, et al. lncRNA NONRATT021972 siRNA decreases diabetic neuropathic pain mediated by the P2X3 receptor in dorsal root ganglia. Mol Neurobiol. 2017;54:511–23. doi: 10.1007/s12035-015-9632-1. [DOI] [PubMed] [Google Scholar]
- 67.Jia T, Rao J, Zou L, Zhao S, Yi Z, Wu B, et al. Nanoparticle-encapsulated curcumin inhibits diabetic neuropathic pain involving the P2Y12 receptor in the dorsal root ganglia. Front Neurosci. 2018;11:755. doi: 10.3389/fnins.2017.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niphakis MJ, Cognetta AB 3rd, Chang JW, Buczynski MW, Parsons LH, Byrne F, et al. Evaluation of NHS carbamates as a potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci. 2013;4:1322–32. doi: 10.1021/cn400116z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aghdam AM, Shahabi P, Karimi-Sales E, Ghiasi R, SadighEteghad S, Mahmoudi J, et al. Swimming exercise induced reversed expression of miR-96 and its target gene NaV1.3 in diabetic peripheral neuropathy in rats. Chin J Physiol. 2018;61:124–9. doi: 10.4077/CJP.2018.BAG531. [DOI] [PubMed] [Google Scholar]
- 70.Elkholy SE, Elaidy SM, El-Sherbeeny NA, Toraih EA, ElGawly HW. Neuroprotective effects of ranolazine versus pioglitazone in experimental diabetic neuropathy: targeting Nav1.7 channels and PPAR-γ. Life Sci. 2020;250:117557. doi: 10.1016/j.lfs.2020.117557. [DOI] [PubMed] [Google Scholar]
- 71.Boric M, Jelicic Kadic A, Puljak L. Cutaneous expression of calcium/calmodulin-dependent protein kinase II in rats with type 1 and type 2 diabetes. J Chem Neuroanat. 2014;61-62:140–6. doi: 10.1016/j.jchemneu.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Boric M, Skopljanac I, Ferhatovic L, Jelicic Kadic A, Banozic A, Puljak L. Reduced epidermal thickness, nerve degeneration and increased pain-related behavior in rats with diabetes type 1 and 2. J Chem Neuroanat. 2013;53:33–40. doi: 10.1016/j.jchemneu.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 73.Davidson EP, Coppey LJ, Shevalye H, Obrosov A, Yorek MA. Vascular and neural complications in type 2 diabetic rats: improvement by sacubitril/valsartan greater than valsartan alone. Diabetes. 2018;67:1616–26. doi: 10.2337/db18-0062. [DOI] [PubMed] [Google Scholar]
- 74.Wu YB, Li HQ, Ren MS, Li WT, Lv XY, Wang L. CHOP/ORP150 ratio in endoplasmic reticulum stress: a new mechanism for diabetic peripheral neuropathy. Cell Physiol Biochem. 2013;32:367–79. doi: 10.1159/000354444. [DOI] [PubMed] [Google Scholar]
- 75.Ferhatovic L, Banozic A, Kostic S, Kurir TT, Novak A, Vrdoljak L, et al. Expression of calcium/calmodulin-dependent protein kinase II and pain-related behavior in rat models of type 1 and type 2 diabetes. Anesth Analg. 2013;116:712–21. doi: 10.1213/ANE.0b013e318279b540. [DOI] [PubMed] [Google Scholar]
- 76.Chandramoorthy HC, Bin-Jaliah I, Karari H, Rajagopalan P, Ahmed Shariff ME, Al-Hakami A, et al. MSCs ameliorates DPN induced cellular pathology via [Ca2+ ]i homeostasis and scavenging the pro-inflammatory cytokines. J Cell Physiol. 2018;233:1330–41. doi: 10.1002/jcp.26009. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Z, Ding X, Zhou Z, Qiu Z, Shi N, Zhou S, et al. Sirtuin 1 alleviates diabetic neuropathic pain by regulating synaptic plasticity of spinal dorsal horn neurons. Pain. 2019;160:1082–92. doi: 10.1097/j.pain.0000000000001489. [DOI] [PubMed] [Google Scholar]
- 78.Lin JY, He YN, Zhu N, Peng B. Metformin attenuates increase of synaptic number in the rat spinal dorsal horn with painful diabetic neuropathy induced by type 2 diabetes: a stereological study. Neurochem Res. 2018;43:2232–9. doi: 10.1007/s11064-018-2642-4. [DOI] [PubMed] [Google Scholar]
- 79.Kelany ME, Hakami TM, Omar AH, Abdallah MA. Combination of sitagliptin and insulin against type 2 diabetes mellitus with neuropathy in rats: neuroprotection and role of oxidative and inflammation stress. Pharmacology. 2016;98:242–50. doi: 10.1159/000448043. [DOI] [PubMed] [Google Scholar]
- 80.Li K, Shi X, Luo M, Wu P, Zhang M, et al. Taurine protects against myelin damage of sciatic nerve in diabetic peripheral neuropathy rats by controlling apoptosis of schwann cells via NGF/Akt/GSK3β pathway. Exp Cell Res. 2019;383:111557. doi: 10.1016/j.yexcr.2019.111557. [DOI] [PubMed] [Google Scholar]
- 81.Hong QX, Xu SY, Dai SH, Zhao WX. Expression profiling of spinal genes in peripheral neuropathy model rats with type 2 diabetes mellitus. Int J Clin Exp Med. 2016;9:6376–84. [Google Scholar]
- 82.Xi P, Zhang C, Li Y. Association of TNF-alpha with type-2 diabetic mechanical hyperalgesia in rats. Biomed Res. 2017;28:305–8. [Google Scholar]
- 83.Kumar NP, Annamalai AR, Thakur RS. Antinociceptive property of Emblica officinalis Gaertn (Amla) in high fat diet-fed/low dose streptozotocin induced diabetic neuropathy in rats. Indian J Exp Biol. 2009;47:737–42. [PubMed] [Google Scholar]
- 84.Meng B, Shen LL, Shi XT, Gong YS, Fan XF, Li J, et al. Effects of curcumin on TTX-R sodium currents of dorsal root ganglion neurons in type 2 diabetic rats with diabetic neuropathic pain. Neurosci Lett. 2015;605:59–64. doi: 10.1016/j.neulet.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 85.Nanjundan PK, Arunachalam A, Thakur RS. Antinociceptive property of Trigonella foenum graecum (Fenugreek seeds) in high fat diet-fed/low dose streptozotocin induced diabetic neuropathy in rats. Pharmacologyonline. 2009;2:24–36. [Google Scholar]
- 86.Dawane JS, Pandit VA, Bhosale MS, Khatavkar PS. Evaluation of effect of nishamalaki on STZ and HFHF diet induced diabetic neuropathy in Wistar rats. J Clin Diagn Res. 2016;10:FF01–5. doi: 10.7860/JCDR/2016/21011.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang S, Wang Z, Li L, Zou L, Gong Y, Jia T, et al. P2Y12 shRNA treatment decreases SGC activation to relieve diabetic neuropathic pain in type 2 diabetes mellitus rats. J Cell Physiol. 2018;233:9620–8. doi: 10.1002/jcp.26867. [DOI] [PubMed] [Google Scholar]
- 88.Li L, Sheng X, Zhao S, Zou L, Han X, Gong Y, et al. Nanoparticle-encapsulated emodin decreases diabetic neuropathic pain probably via a mechanism involving P2X3 receptor in the dorsal root ganglia. Purinergic Signal. 2017;13:559–68. doi: 10.1007/s11302-017-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu W, Zhao GQ, Cao RJ, Zhu ZH, Li K. LncRNA NONRATT021972 was associated with neuropathic pain scoring in patients with type 2 diabetes. Behav Neurol. 2017;2017:2941297. doi: 10.1155/2017/2941297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Szkudelski T. Streptozotocin-nicotinamide-induced diabetes in the rat: characteristics of the experimental model. Exp Biol Med (Maywood) 2012;237:481–90. doi: 10.1258/ebm.2012.011372. [DOI] [PubMed] [Google Scholar]
- 91.Deuis JR, Dvorakova LS, Vetter I. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 2017;10:284. doi: 10.3389/fnmol.2017.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fellmann L, Nascimento AR, Tibirica E, Bousquet P. Murine models for pharmacological studies of the metabolic syndrome. Pharmacol Ther. 2013;137:331–40. doi: 10.1016/j.pharmthera.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007;125:451–72. [PubMed] [Google Scholar]
- 94.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13:18–9. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 95.Peterson RG, Shaw WN, Neel MA, Little LA, Eichberg J. Zucker diabetic fatty rat as a model for non-insulin-dependent diabetes mellitus. Ilar J. 1990;32:16–9. doi: 10.1093/ilar.32.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, et al. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47:358–64. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- 97.Shibata T, Takeuchi S, Yokota S, Kakimoto K, Yonemori F, Wakitani K. Effects of peroxisome proliferator-activated receptor-alpha and -gamma agonist, JTT-501, on diabetic complications in Zucker diabetic fatty rats. Br J Pharmacol. 2000;130:495–504. doi: 10.1038/sj.bjp.0703328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shiota M, Printz RL. Diabetes in Zucker diabetic fatty rat. Methods Mol Biol. 2012;933:103–23. doi: 10.1007/978-1-62703-068-7_8. [DOI] [PubMed] [Google Scholar]
- 99.Hempe J, Elvert R, Schmidts HL, Kramer W, Herling AW. Appropriateness of the Zucker diabetic fatty rat as a model for diabetic microvascular late complications. Lab Anim. 2012;46:32–9. doi: 10.1258/la.2011.010165. [DOI] [PubMed] [Google Scholar]
- 100.Sugimoto K, Rashid IB, Kojima K, Shoji M, Tanabe J, Tamasawa N, et al. Time course of pain sensation in rat models of insulin resistance, type 2 diabetes, and exogenous hyperinsulinaemia. Diabetes Metab Res Rev. 2008;24:642–50. doi: 10.1002/dmrr.903. [DOI] [PubMed] [Google Scholar]
- 101.Oltman CL, Coppey LJ, Gellett JS, Davidson EP, Lund DD, Yorek MA. Progression of vascular and neural dysfunction in sciatic nerves of Zucker diabetic fatty and Zucker rats. Am J Physiol Endocrinol Metab. 2005;289:E113–22. doi: 10.1152/ajpendo.00594.2004. [DOI] [PubMed] [Google Scholar]
- 102.Lirk P, Verhamme C, Boeckh R, Stevens MF, ten Hoope W, Gerner P, et al. Effects of early and late diabetic neuropathy on sciatic nerve block duration and neurotoxicity in Zucker diabetic fatty rats. Br J Anaesth. 2015;114:319–26. doi: 10.1093/bja/aeu270. [DOI] [PubMed] [Google Scholar]
- 103.Davidson EP, Coppey LJ, Holmes A, Lupachyk S, Dake BL, Oltman CL, et al. Characterization of diabetic neuropathy in the Zucker diabetic Sprague-Dawley rat: a new animal model for type 2 diabetes. J Diabetes Res. 2014;2014:714273. doi: 10.1155/2014/714273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Singh J, Yousuf MS, Jones KE, Shelemey PT, Joy T, Macandili H, et al. Characterization of the Nile Grass Rat as a unique model for type 2 diabetic polyneuropathy. J Neuropathol Exp Neurol. 2018;77:469–78. doi: 10.1093/jnen/nly030. [DOI] [PubMed] [Google Scholar]
- 105.Zhai X, Sun C, Rong P, Li S, McCabe MF, Wang X, et al. A correlative relationship between chronic pain and insulin resistance in Zucker fatty rats: role of downregulation of insulin receptors. J Pain. 2016;17:404–13. doi: 10.1016/j.jpain.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 106.Vera G, Lopez-Miranda V, Herradon E, Martin MI, Abalo R. Characterization of cannabinoid-induced relief of neuropathic pain in rat models of type 1 and type 2 diabetes. Pharmacol Biochem Behav. 2012;102:335–43. doi: 10.1016/j.pbb.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 107.Romanovsky D, Walker JC, Dobretsov M. Pressure pain precedes development of type 2 disease in Zucker rat model of diabetes. Neurosci Lett. 2008;445:220–3. doi: 10.1016/j.neulet.2008.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li F, Abatan OI, Kim H, Burnett D, Larkin D, Obrosova IG, et al. Taurine reverses neurological and neurovascular deficits in Zucker diabetic fatty rats. Neurobiol Dis. 2006;22:669–76. doi: 10.1016/j.nbd.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 109.Otto KJ, Wyse BD, Cabot PJ, Smith MT. Longitudinal study of painful diabetic neuropathy in the Zucker diabetic fatty rat model of type 2 diabetes: impaired basal G-protein activity appears to underpin marked morphine hyposensitivity at 6 months. Pain Med. 2011;12:437–50. doi: 10.1111/j.1526-4637.2011.01067.x. [DOI] [PubMed] [Google Scholar]
- 110.De Visser A, Hemming A, Yang C, Zaver S, Dhaliwal R, Jawed Z, et al. The adjuvant effect of hypertension upon diabetic peripheral neuropathy in experimental type 2 diabetes. Neurobiol Dis. 2014;62:18–30. doi: 10.1016/j.nbd.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 111.Li S, Sun C, Rong P, Zhai X, Zhang J, Baker M, et al. Auricular vagus nerve stimulation enhances central serotonergic function and inhibits diabetic neuropathy development in Zucker fatty rats. Mol Pain. 2018;14:1744806918787368. doi: 10.1177/1744806918787368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shi J, Jiang K, Li Z. Involvement of spinal glutamate transporter-1 in the development of mechanical allodynia and hyperalgesia associated with type 2 diabetes. J Pain Res. 2016;9:1121–9. doi: 10.2147/JPR.S118412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Yorek MA. Changes in endoneurial blood flow, motor nerve conduction velocity and vascular relaxation of epineurial arterioles of the sciatic nerve in ZDF-obese diabetic rats. Diabetes Metab Res Rev. 2002;18:49–56. doi: 10.1002/dmrr.257. [DOI] [PubMed] [Google Scholar]
- 114.Liu S, Zou L, Xie J, Xie W, Wen S, Xie Q, et al. LncRNA NONRATT021972 siRNA regulates neuropathic pain behaviors in type 2 diabetic rats through the P2X7 receptor in dorsal root ganglia. Mol Brain. 2016;9:44. doi: 10.1186/s13041-016-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feng H, Lu G, Li Q, Liu Z. Inhibition of adenylyl cyclase in the spinal cord alleviates painful diabetic neuropathy in Zucker diabetic fatty rats. Can J Diabetes. 2017;41:177–83. doi: 10.1016/j.jcjd.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 116.Paniagua N, Giron R, Goicoechea C, Lopez-Miranda V, Vela JM, Merlos M, et al. Blockade of sigma 1 receptors alleviates sensory signs of diabetic neuropathy in rats. Eur J Pain. 2017;21:61–72. doi: 10.1002/ejp.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang Y, Zhang Z, Guan J, Liu J, Ma P, Gu K, et al. Administrations of thalidomide into the rostral ventromedial medulla alleviates painful diabetic neuropathy in Zucker diabetic fatty rats. Brain Res Bull. 2016;125:144–51. doi: 10.1016/j.brainresbull.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 118.Ni GL, Cui R, Shao AM, Wu ZM. Salidroside ameliorates diabetic neuropathic pain in rats by inhibiting neuroinflammation. J Mol Neurosci. 2017;63:9–16. doi: 10.1007/s12031-017-0951-8. [DOI] [PubMed] [Google Scholar]
- 119.Zhuang HX, Wuarin L, Fei ZJ, Ishii DN. Insulin-like growth factor (IGF) gene expression is reduced in neural tissues and liver from rats with non-insulin-dependent diabetes mellitus, and IGF treatment ameliorates diabetic neuropathy. J Pharmacol Exp Ther. 1997;283:366–74. [PubMed] [Google Scholar]
- 120.Gilloteaux J, Subramanian K, Solomon N, Nicaise C. The leptin receptor mutation of the obese Zucker rat causes sciatic nerve demyelination with a centripetal pattern defect. Ultrastruct Pathol. 2018;42:377–408. doi: 10.1080/01913123.2018.1522405. [DOI] [PubMed] [Google Scholar]
- 121.Sugimoto K, Kojima K, Baba M, Yasujima M. Olmesartan ameliorates peripheral nerve dysfunction in Zucker diabetic fatty rats. J Hypertens. 2011;29:1337–46. doi: 10.1097/HJH.0b013e328347da0f. [DOI] [PubMed] [Google Scholar]
- 122.Rutten K, Gould SA, Bryden L, Doods H, Christoph T, Pekcec A. Standard analgesics reverse burrowing deficits in a rat CCI model of neuropathic pain, but not in models of type 1 and type 2 diabetes-induced neuropathic pain. Behav Brain Res. 2018;350:129–38. doi: 10.1016/j.bbr.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 123.Griggs RB, Donahue RR, Adkins BG, Anderson KL, Thibault O, Taylor BK. Pioglitazone inhibits the development of hyperalgesia and sensitization of spinal nociresponsive neurons in type 2 diabetes. J Pain. 2016;17:359–73. doi: 10.1016/j.jpain.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shevalye H, Watcho P, Stavniichuk R, Dyukova E, Lupachyk S, Obrosova IG. Metanx alleviates multiple manifestations of peripheral neuropathy and increases intraepidermal nerve fiber density in Zucker diabetic fatty rats. Diabetes. 2012;61:2126–33. doi: 10.2337/db11-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.La Fontaine J, Chen C, Hunt N, Jude E, Lavery L. Type 2 diabetes and metformin influence on fracture healing in an experimental rat model. J Foot Ankle Surg. 2016;55:955–60. doi: 10.1053/j.jfas.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 126.Brussee V, Guo G, Dong Y, Cheng C, Martinez JA, Smith D, et al. Distal degenerative sensory neuropathy in a long-term type 2 diabetes rat model. Diabetes. 2008;57:1664–73. doi: 10.2337/db07-1737. [DOI] [PubMed] [Google Scholar]
- 127.Garcia-Perez E, Schonberger T, Sumalla M, Stierstorfer B, Sola R, Doods H, et al. Behavioural, morphological and electrophysiological assessment of the effects of type 2 diabetes mellitus on large and small nerve fibres in Zucker diabetic fatty, Zucker lean and Wistar rats. Eur J Pain. 2018;22:1457–72. doi: 10.1002/ejp.1235. [DOI] [PubMed] [Google Scholar]
- 128.Shimoshige Y, Ikuma K, Yamamoto T, Takakura S, Kawamura I, Seki J, et al. The effects of zenarestat, an aldose reductase inhibitor, on peripheral neuropathy in Zucker diabetic fatty rats. Metabolism. 2000;49:1395–9. doi: 10.1053/meta.2000.17723. [DOI] [PubMed] [Google Scholar]
- 129.Russell JW, Berent-Spillson A, Vincent AM, Freimann CL, Sullivan KA, Feldman EL. Oxidative injury and neuropathy in diabetes and impaired glucose tolerance. Neurobiol Dis. 2008;30:420–9. doi: 10.1016/j.nbd.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thakur V, Sadanandan J, Chattopadhyay M. High-mobility group box 1 protein signaling in painful diabetic neuropathy. Int J Mol Sci. 2020;21:881. doi: 10.3390/ijms21030881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ten Hoope W, Hollmann MW, de Bruin K, Verberne HJ, Verkerk AO, Tan HL, et al. Pharmacodynamics and pharmacokinetics of lidocaine in a Rodent model of diabetic neuropathy. Anesthesiology. 2018;128:609–19. doi: 10.1097/ALN.0000000000002035. [DOI] [PubMed] [Google Scholar]
- 132.Marshall AG, Lee-Kubli C, Azmi S, Zhang M, Ferdousi M, Mixcoatl-Zecuatl T, et al. Spinal disinhibition in experimental and clinical painful diabetic neuropathy. Diabetes. 2017;66:1380–90. doi: 10.2337/db16-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Peterson RG. alpha-Glucosidase inhibitors in diabetes: lessons from animal studies. Eur J Clin Invest. 1994;24 Suppl 3:11–8. doi: 10.1111/j.1365-2362.1994.tb02250.x. [DOI] [PubMed] [Google Scholar]
- 134.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Yorek MA. Treatment of Zucker diabetic fatty rats with AVE7688 improves vascular and neural dysfunction. Diabetes Obes Metab. 2009;11:223–33. doi: 10.1111/j.1463-1326.2008.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Adebara ET, et al. Vascular and neural dysfunction in Zucker diabetic fatty rats: a difficult condition to reverse. Diabetes Obes Metab. 2008;10:64–74. doi: 10.1111/j.1463-1326.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 136.Davis JE, Cain J, Banz WJ, Peterson RG. Age-related differences in response to high-fat feeding on adipose tissue and metabolic profile in ZDSD rats. ISRN Obes. 2013;2013:584547. doi: 10.1155/2013/584547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reinwald S, Peterson RG, Allen MR, Burr DB. Skeletal changes associated with the onset of type 2 diabetes in the ZDF and ZDSD rodent models. Am J Physiol Endocrinol Metab. 2009;296:E765–74. doi: 10.1152/ajpendo.90937.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peterson RG, Jackson CV, Zimmerman K, de Winter W, Huebert N, Hansen MK. Characterization of the ZDSD rat: a translational model for the study of metabolic syndrome and type 2 diabetes. J Diabetes Res. 2015;2015:487816. doi: 10.1155/2015/487816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang K, Gotzmann J, Kuny S, Huang H, Sauve Y, Chan CB. Five stages of progressive β-cell dysfunction in the laboratory Nile rat model of type 2 diabetes. J Endocrinol. 2016;229:343–56. doi: 10.1530/JOE-15-0517. [DOI] [PubMed] [Google Scholar]
- 140.Chaabo F, Pronczuk A, Maslova E, Hayes K. Nutritional correlates and dynamics of diabetes in the Nile rat (Arvicanthis niloticus): a novel model for diet-induced type 2 diabetes and the metabolic syndrome. Nutr Metab (Lond) 2010;7:29. doi: 10.1186/1743-7075-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Santos-Nogueira E, Redondo Castro E, Mancuso R, Navarro X. Randall-Selitto test: a new approach for the detection of neuropathic pain after spinal cord injury. J Neurotrauma. 2012;29:898–904. doi: 10.1089/neu.2010.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jensen TS, Yaksh TL. Comparison of the antinociceptive action of mu and delta opioid receptor ligands in the periaqueductal gray matter, medial and paramedial ventral medulla in the rat as studied by the microinjection technique. Brain Res. 1986;372:301–12. doi: 10.1016/0006-8993(86)91138-8. [DOI] [PubMed] [Google Scholar]
- 143.Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 144.Irwin S, Houde RW, Bennett DR, Hendershot LC, Seevers MH. The effects of morphine methadone and meperidine on some reflex responses of spinal animals to nociceptive stimulation. J Pharmacol Exp Ther. 1951;101:132–43. [PubMed] [Google Scholar]
- 145.Elzinga SE, Savelieff MG, O’Brien PD, Mendelson FE, Hayes JM, Feldman EL. Sex differences in insulin resistance, but not peripheral neuropathy, in a diet-induced prediabetes mouse model. Dis Model Mech. 2021;14:dmm048909. doi: 10.1242/dmm.048909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Eid SA, Feldman EL. Advances in diet-induced rodent models of metabolically acquired peripheral neuropathy. Dis Model Mech. 2021;14:dmm049337. doi: 10.1242/dmm.049337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu Y, Sebastian B, Liu B, Zhang Y, Fissel JA, Pan B, et al. Sensory and autonomic function and structure in footpads of a diabetic mouse model. Sci Rep. 2017;7:41401. doi: 10.1038/srep41401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schmidt RE. Autonomic neuropathy in experimental models of diabetes mellitus. Handb Clin Neurol. 2014;126:579–602. doi: 10.1016/B978-0-444-53480-4.00038-2. [DOI] [PubMed] [Google Scholar]
- 149.Morgenstern J, Groener JB, Jende JM, Kurz FT, Strom A, Gopfert J, et al. Neuron-specific biomarkers predict hypo- and hyperalgesia in individuals with diabetic peripheral neuropathy. Diabetologia. 2021;64:2843–55. doi: 10.1007/s00125-021-05557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


