Abstract
Background
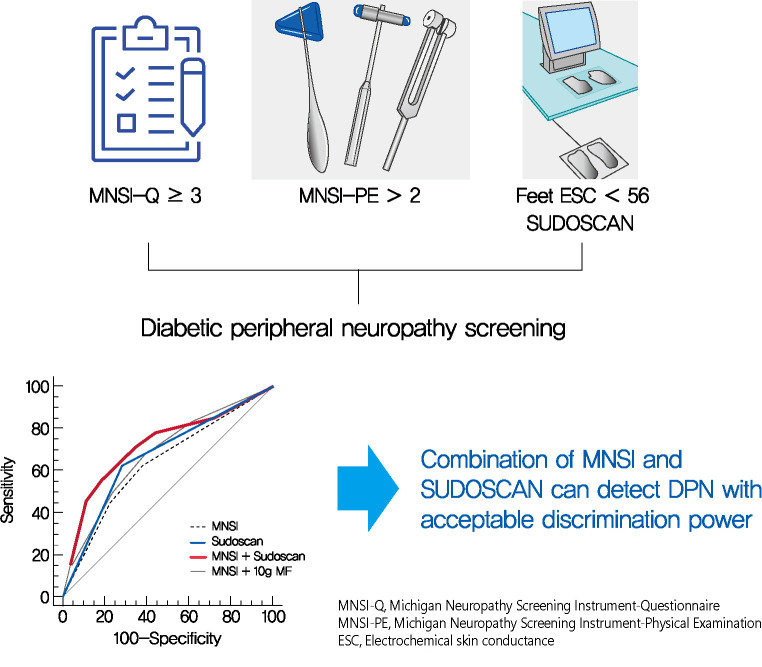
Screening for diabetic peripheral neuropathy (DPN) is important to prevent severe foot complication, but the detection rate of DPN is unsatisfactory. We investigated whether SUDOSCAN combined with Michigan Neuropathy Screening Instrument (MNSI) could be an effective tool for screening for DPN in people with type 2 diabetes mellitus (T2DM) in clinical practice.
Methods
We analysed the data for 144 people with T2DM without other cause of neuropathy. The presence of DPN was confirmed according to the Toronto Consensus criteria. Electrochemical skin conductance (ESC) of the feet was assessed using SUDOSCAN. We compared the discrimination power of following methods, MNSI only vs. SUDOSCAN only vs. MNSI plus SUDOSCAN vs. MNSI plus 10-g monofilament test.
Results
Confirmed DPN was detected in 27.8% of the participants. The optimal cut-off value of feet ESC to distinguish DPN was 56 μS. We made the DPN screening scores using the corresponding odds ratios for MNSI-Questionnaire, MNSI-Physical Examination, SUDOSCAN, and 10-g monofilament test. For distinguishing the presence of DPN, the MNSI plus SUDOSCAN model showed higher areas under the receiver operating characteristic curve (AUC) than MNSI only model (0.717 vs. 0.638, P=0.011), and SUDOSCAN only model or MNSI plus 10-g monofilament test showed comparable AUC with MNSI only model.
Conclusion
The screening model for DPN that includes both MNSI and SUDOSCAN can detect DPN with acceptable discrimination power and it may be useful in Korean patients with T2DM.
Keywords: Diabetes mellitus, type 2; Diabetic neuropathies; Diagnostic screening programs
Graphical abstract
INTRODUCTION
Diabetic peripheral neuropathy (DPN) is an important microvascular complication of diabetes and is prevalent in people with diabetes (28% to 50%) and even in those with prediabetes (11% to 49%) [1-3]. As in other microvascular complications such as retinopathy and nephropathy, DPN is likely to be asymptomatic at first and then progress to the development of severe consequences. There is at present no disease-modifying therapy [4,5], and early detection and prevention are essential. According to a recent position statement by the American Diabetes Association on diabetic neuropathy, many screening modalities are dependent on the physician’s history taking and physical examination, such as the 10-g monofilament test [6]. A neurophysiological study is a confirmative test and is highly reproducible [7], and we diagnose confirmed DPN if the patient had symptom or sign of DPN with abnormality of the study [8]. However, neurophysiological study is rarely necessary for DPN screening, and this type of study requires a specialist and it is time-consuming and difficult to standardise in the busy outpatient clinical setting. In real clinical practice, we asked symptom of DPN, and performed physical examination for small- and large- fiber nerve function. In fact, primary physicians did not evaluate the presence of neuropathy in more than one-third of patients from their clinic [9]. In addition, no single test can detect the diverse clinical manifestations of DPN, which can be easily overlooked. For example, nerve conduction study (NCS) detects only large fiber neuropathy and skin biopsy is used for evaluation of small fiber nerve [10].
SUDOSCAN (Impeto Medical, Paris, France) is a non-invasive and convenient tool for assessing the function of unmyelinated c-fibers innervating sweat glands [11]. This device measures electrochemical skin conductance (ESC), which has been shown to be related to DPN [11] and autonomic neuropathy [12]. SUDOSCAN was recently further validated by comparison with quantification of nerve fiber density of skin and sweat gland biopsy samples [13] and sudomotor function evaluated using a quantitative sudomotor axon reflex test [14]. It also showed high reproducibility [15,16]. Given that SUDOSCAN has been validated against various evaluation tools for identification of diabetic neuropathies and the ability to test small-fiber neuropathy, which usually precedes large-fiber neuropathy [17,18], we reasoned that this modality may be useful for screening for diabetic neuropathy in a clinical setting. However, the clinical usefulness was not guaranteed when SUDOSCAN was used alone in screening DPN [19].
In this study, we tested the clinical effectiveness of screening modality for DPN using SUDOSCAN alone or in combination with the Michigan Neuropathy Screening Instrument (MNSI) compared to MNSI model in people with type 2 diabetes mellitus (T2DM).
METHODS
Participants
We aimed to develop a new examination tool which can effectively screen and predict DPN. The current study has exploratory nature to aim to discover the accurate score system for DPN including SUDOSCAN, and therefore we cannot calculate accurate sample size. Previous studies including T2DM (n=47) and healthy control (n=16) [14], or including type 1 diabetes mellitus (n=45) and healthy volunteers (n=25) [11] showed that feet ESC yielded the diagnostic accuracy of areas under the receiver operating characteristic curve (AUC) 0.705 and 0.64, respectively. We only enrolled subjects with T2DM and did not include healthy control. In this circumstance, the difference of feet ESC levels between subjects with DPN and without DPN might be less profound than previous studies. In this study, we enrolled 235 subjects with T2DM from a single tertiary hospital and analysed the data for 144 of these patients from 2017 to 2019 in whom we can assess the presence of confirmed DPN according to the definition of Toronto Diabetic Neuropathy criteria [8]. We included subjects aged 20 to 79 years and whose anti-hyperglycaemic medications had not changed in the past 3 months to exclude the possible effect of a change in medication on ESC. We also used the information contained in a comprehensive medical history taking and biochemical study to exclude other cause of neuropathy such as thyroid dysfunction and vitamin B12 deficiency. Other key exclusion criteria were renal dysfunction, estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 to exclude uremic neuropathy, pregnancy, or steroid use. When using SUDOSCAN, we also excluded patients who could not stand by themselves or who had active wounds on their hands or feet. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB Number: B-1611-372-305). All participants provided written informed consent before participation.
Clinical and laboratory evaluations
After an overnight fast, the participants visited the endocrinology outpatient clinic. Body weight and height were measured using electronic scales. Body mass index (BMI) was calculated by dividing body weight (kg) by height squared (m2) and is expressed as kg/m2. Smoking history was categorised as never, former, and current. Never smoking was defined as having smoked <100 cigarettes in a lifetime. Alcohol intake was evaluated according to average daily alcohol consumption as follows: high (>30 g/day for men or >20 g/day for women), moderate (<30 g/day for men or <20 g/day for women), and none. Positive physical activity was defined as exercising for >150 min/week. Fasting plasma glucose concentration, glycosylated hemoglobin (HbA1c) level, blood lipid concentrations, and renal function test were measured at the central laboratory.
Assessment of DPN
We diagnosed the confirmed DPN if NCS was abnormal with the presence of a symptom or a sign of neuropathy [8]. The NCS was performed at a Department of Rehabilitation Medicine. A trained examiner performed the tests for median nerve, ulnar nerve, peroneal nerve, sural nerve, and tibial nerve. Abnormalities of peroneal nerve velocity, amplitude, and distal latency, tibial compound muscle action potential, and sural amplitude were marked according to the reference values [20]. Abnormal NCS results were defined as more than one abnormal test in two separate nerves. The final diagnosis of DPN was confirmed by certified physicians who were not involved in this study. The MNSI-Questionnaire (MNSI-Q) was previously translated into Korean and Korean version was evaluated by the Neuropathy Study Group of the Korean Diabetes Association [21], and considered to be abnormal if MNSI-Q was 3 and higher [22]. A trained research nurse performed the MNSI-Physical Examination (MNSI-PE). MNSI-PE was consisted of an inspection of feet (appearance and ulceration), ankle reflexes, and vibration sensation assessed by using a 128 Hz tuning fork. The maximum value was 8 for both feet and, we considered abnormal value if MNSI-PE was >2.0 [22,23]. A 10-g monofilament test (Semmes–Weinstein 5.07 monofilaments) was performed at 10 sites on each foot and abnormal value was adopted from the previous Korean study (fewer than seven) [22]. All participants also underwent the SUDOSCAN test. They were instructed to place their palms and soles on the electrodes, and the ESC was measured automatically within 3 minutes. To reduce observer bias, the research nurse performed tests in the order of questionnaire, physical examination, and SUDOSCAN. The score was lastly calculated after SUDOSCAN.
Development of the DPN screening algorithm
We classified the participants as having or not having DPN and compared the neuropathy examination results. For the SUDOSCAN data, the appropriate cut-off value for feet ESC was determined first and used to categorise the participants. We created a score for each parameter according to the odds ratio (OR).
Statistical analysis
The data are presented as mean±standard deviation, median with interquartile range (IQR), or as number and percentage. Clinical and biochemical characteristics were compared between participants with and without DPN. Differences between two groups were identified using Student’s t-test for parametric variables, the Mann–Whitney U test for non-parametric variables, and the chi-square test for categorical variables. Spearman correlation analysis was conducted to assess the relationship between two continuous variables. The AUC was calculated to measure the predictive power. The cut-off values for feet ESC and a scoring model were determined using the maximum sum of sensitivity and specificity using the Youden index [24]. The association between parameters and the presence of DPN was assessed by logistic regression analysis. To test discrimination power, we calculated AUC of our new model in total and in each gender group. We compared AUCs of each model using the method of DeLong et al. [25]. Statistical analysis was conducted using IBM SPSS Statistics for Windows version 22.0 (IBM Co., Armonk, NY, USA) and R version 3.6.1 software (R Foundation for Statistical Computing, Vienna, Austria). Two-sided P values <0.05 were considered to be significant.
RESULTS
The clinical and biochemical characteristics according to the presence of DPN are shown in Table 1. The prevalence of DPN was 27.8% and study flow chart is shown in Supplementary Fig. 1. More male subjects were diagnosed with DPN, and age and BMI were lower in DPN group than their counterpart. High density lipoprotein-cholesterol and eGFR were lower in participants with DPN than in those without DPN. There was no difference in HbA1c levels between groups, but more participants with DPN were treated with insulin compared to those without DPN. The most frequently described medication for DPN was pregabalin, and there was no difference in frequency of medications for DPN between groups. Smoking, alcohol consumption, and physical activity status also did not differ significantly between groups. The MNSI-Q score but not MNSI-PE score was higher in participants with DPN than those without DPN. The median feet ESC was 51.5 μS (IQR, 29.8 to 67.8) and 62.0 μS (IQR, 53.5 to 71.0) in participants with and without DPN, respectively (P=0.002). The feet ESC was negatively associated with MNSI-Q (rho=–0.223, P=0.007) and MNSI-PE (rho=–0.177, P=0.033).
Table 1.
Clinical and biochemical characteristics according to the presence of DPN
| Characteristic | Total (n=144) | DPN (–) (n=104) | Confirmed DPN (+) (n=40) | P value |
|---|---|---|---|---|
| Age, yr | 59.4±8.9 | 60.4±8.8 | 57.1±8.9 | 0.046 |
| Male sex | 85 (59.0) | 53 (51.0) | 32 (80.0) | 0.002 |
| Height, cm | 162.7±8.4 | 161.0±8.4 | 167.2±7.0 | <0.001 |
| Body weight, kg | 67.5±11.5 | 67.1±10.7 | 68.7±13.4 | 0.458 |
| BMI, kg/m2 | 25.4±3.5 | 25.8±3.3 | 24.4±3.7 | 0.027 |
| SBP, mm Hg | 131.1±13.2 | 129.9±13.1 | 134.0±13.2 | 0.099 |
| DBP, mm Hg | 75.0±9.5 | 74.5±9.4 | 76.3±9.8 | 0.293 |
| Diabetes duration, yr | 9.5 (6.0–17.0) | 9.5 (6.0–17.0) | 9.5 (4.5–18.3) | 0.732 |
| FPG, mg/dL | 137 (116–156) | 137 (116–157) | 138 (109–154) | 0.940 |
| HbA1c, % | 7.1 (6.6–7.7) | 7.1 (6.6–7.5) | 7.1 (6.6–8.1) | 0.723 |
| Cholesterol, mg/dL | 150 (133–171) | 152 (134–172) | 145 (128–168) | 0.168 |
| Triglyceride, mg/dL | 111 (86–160) | 107 (86–155) | 130 (79–183) | 0.306 |
| HDL-C, mg/dL | 46.5 (40.0–55.0) | 48 (41–57) | 44 (36–51) | 0.007 |
| LDL-C, mg/dL | 87.8±23.6 | 88.2±23.2 | 86.9±24.9 | 0.774 |
| eGFR, mL/min/1.73 m2 | 96.6±22.2 | 99.0±21.9 | 90.4±21.9 | 0.037 |
| Hypertension | 81 (56.3) | 37 (48.7) | 17 (53.1) | 0.833 |
| Lipid-lowering drugs | 109 (75.7) | 56 (73.7) | 23 (71.9) | 0.999 |
| Insulin | 40 (27.8) | 14 (18.4) | 12 (37.5) | 0.048 |
| Metformin | 134 (93.1) | 68 (89.5) | 31 (96.9) | 0.276 |
| Sulfonylurea | 44 (30.6) | 24 (31.6) | 10 (31.3) | 0.999 |
| DPP-4 inhibitor | 77 (53.5) | 40 (52.6) | 18 (56.3) | 0.833 |
| SGLT-2 inhibitor | 22 (15.3) | 8 (10.5) | 2 (6.3) | 0.720 |
| Thiazolidinedione | 19 (13.2) | 8 (10.5) | 3 (9.4) | 0.999 |
| Medications for DPN | ||||
| Pregabalin | 29 (20.1) | 18 (17.3) | 11 (27.5) | 0.245 |
| Tricyclic antidepressant | 3 (2.1) | 3 (2.9) | 0 | 0.560 |
| Duloxetine | 4 (2.8) | 3 (2.9) | 1 (2.5) | 0.999 |
| Alpha-lipoic acid | 14 (9.7) | 8 (7.7) | 6 (15.0) | 0.213 |
| Smoking | 0.856 | |||
| Never | 67 (46.5) | 35 (46.1) | 13 (40.6) | |
| Former | 49 (34.0) | 27 (35.5) | 12 (37.5) | |
| Current | 28 (19.4) | 14 (18.4) | 7 (21.9) | |
| Alcohol intake | 0.495 | |||
| None | 67 (46.5) | 34 (44.7) | 16 (50.0) | |
| Moderate | 72 (50.0) | 39 (51.3) | 16 (50.0) | |
| High | 5 (3.5) | 3 (3.9) | 0 | |
| Physically active | 95 (66.0) | 52 (68.4) | 19 (59.4) | 0.383 |
| MNSI-PE score | 2.3 (1.0–4.0) | 2.0 (1.0–3.0) | 3.0 (1.6–3.5) | 0.117 |
| MNSI-Q score | 2 (1–4) | 2 (1–4) | 3 (2–5) | 0.003 |
| 10-g monofilament (score) | 9 (9–10) | 10 (9–10) | 9 (7–10) | <0.001 |
| Feet ESC, μS | 61.0 (48.0–70.0) | 62.0 (53.5–71.0) | 51.5 (29.8–67.8) | 0.002 |
Values are presented as mean±standard deviation, number (%), or median (interquartile range). The P values were derived from the Student t-test, Mann-Whitney U test, or chi-square test.
DPN, diabetic peripheral neuropathy; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; eGFR, estimated glomerular filtration rate; DPP-4, dipeptidyl peptidase-4; SGLT-2, sodium glucose cotransporter‐2; MNSI-PE, Michigan Neuropathy Screening Instrument Physical Examination; MNSI-Q, MNSI Questionnaire; ESC, electrochemical skin conductance.
The ability of the AUC based on feet ESC to distinguish DPN was 0.663 (95% confidence interval [CI], 0.556 to 0.771; P=0.002). The optimal cut-off value of feet ESC was 56 μS. Therefore, we used this cut-off value to categorise subjects according to normal and abnormal feet ESC. The associations between various parameters and DPN in these groups are shown in Table 2. The ORs for MNSI-Q, MNSI-PE, 10-g monofilament test and feet ESC were 2.67 (95% CI, 1.26 to 5.66), 1.75 (95% CI, 0.83 to 3.67), 4.95 (95% CI, 1.51 to 16.21), and 4.11 (95% CI, 1.91 to 8.86), respectively. We applied a score of 3 for MNSI-Q, 2 for MNSI-PE, 5 for 10-g monofilament test, and 4 of feet ESC according to the ORs.
Table 2.
ORs and 95% CIs for the association between various parameters and diabetic peripheral neuropathy
| Parameter | OR (95% CI) | P value | Score |
|---|---|---|---|
| MNSI-Q score | |||
| <3 | Reference | ||
| ≥3 | 2.67 (1.26–5.66) | 0.011 | 3 |
| MNSI-PE score | |||
| ≤2 | Reference | ||
| >2 | 1.75 (0.83–3.67) | 0.139 | 2 |
| 10-g Monofilament test score | |||
| ≥7 | Reference | ||
| <7 | 4.95 (1.51–16.21) | 0.008 | 5 |
| Feet ESC, μS | |||
| ≥56 | Reference | ||
| <56 | 4.11 (1.91–8.86) | <0.001 | 4 |
OR, odds ratio; CI, confidence interval; MNSI-Q, Michigan Neuropathy Screening Instrument Questionnaire; MNSI-PE, MNSI Physical Examination; ESC, electrochemical skin conductance.
When we compared the predictive power of the screening strategies, the MNSI plus SUDOSCAN score, which included the scores for MNSI-Q, MNSI-PE, and feet ESC, had better discrimination power than the MNSI-only model, which included MNSI-Q and MNSI-PE (Table 3). However, MNSI plus 10-g monofilament test did not improve the discrimination power compared to MNSI score. The diagnostic power of screening scores of MNSI plus SUDOSCAN model showed AUC of 0.717 with a sensitivity of 70.0% and 66.3% specificity at the cut-off value of 5 (Fig. 1). When we calculated AUC for our screening tool in men or women separately, the AUC value was 0.719 (95% CI, 0.601 to 0.838; P=0.001) in men and 0.836 (95% CI, 0.730 to 0.941; P=0.002) in women.
Table 3.
Area under the ROC curves for each model
| ROC area | 95% CI | P value | P compared to MNSI model | |
|---|---|---|---|---|
| MNSI model | 0.638 | 0.553–0.744 | 0.011 | NA |
| SUDOSCAN model | 0.668 | 0.585–0.744 | 0.002 | 0.609 |
| MNSI+SUDOSCAN model | 0.717 | 0.636–0.789 | <0.001 | 0.020 |
| MNSI+10-g MF model | 0.674 | 0.591–0.750 | 0.001 | 0.278 |
ROC, receiver operating characteristic curve; CI, confidential interval; MNSI, Michigan Neuropathy Screening Instrument; NA, not available; MF, monofilament.
Fig. 1.
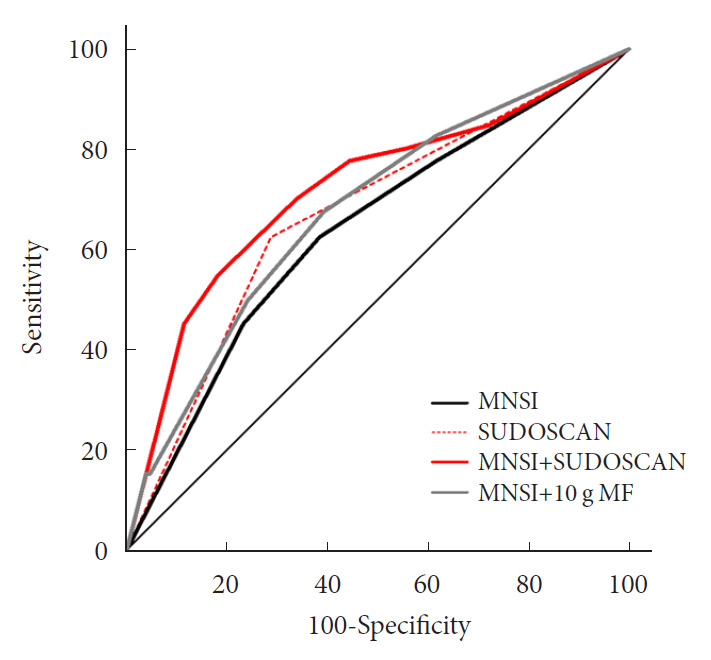
ROC curves showing the ability of Michigan Neuropathy Screening Instrument (MNSI) (black solid line), SUDOSCAN (red dotted line), MNSI plus SUDOSCAN (red solid line), and MNSI plus 10-g monofilament (MF) test (grey solid line) to detect diabetic peripheral neuropathy.
DISCUSSION
We have tested the clinical effectiveness of SUDOSCAN for detecting DPN in adults with T2DM who were followed up in a tertiary academic hospital. Our screening model that includes the MNSI and SUDOSCAN showed acceptable discrimination power and it showed superior screening power than MNSI only model. In contrast, 10-g monofilament test did not improve the discrimination power compared to MNSI alone. Therefore, SUDOSCAN plus MNSI might be acceptable to screen DPN in Korean patients with T2DM.
Small-fiber nerve dysfunction is believed to be the earliest sign of DPN [17], and tests for assessing the small-fiber neuropathy can detect DPN with high sensitivity [26,27]. By contrast, even though a NCS can be used for screening for early stage DPN [28,29] it is controversial for the role of NCS in early stage detection [10]. SUDOSCAN is another tool for assessing small-fiber nerve function and its usefulness for the early detection of DPN has been previously tested in combination with a point-of-care NCS [30]. However, the normal reference values for nerve conduction measures is strongly influenced by age [31], and this issue should not be ignored. In contrast to the point-of-care NCS, a standard NCS requires a trained examiner and long examination time [10]. Therefore, the need to use a neurophysiological study might limit the clinical feasibility.
A position statement of the American Diabetes Association recommends an annual 10-g monofilament test [6]. The 10-g monofilament test is good for predicting foot ulceration and amputation, but it is not sensitive in the early detection of DPN [32]. Our analysis found that the MNSI plus SUDOSCAN was better for detecting DPN than the MNSI, but the MNSI plus 10-g monofilament test did not improve the diagnostic power compared with the MNSI alone. SUDOSCAN can measure feet ESC within 3 minutes and needs no special preparation. Our findings suggest that the MNSI plus SUDOSCAN may be practical and accurate for detecting DPN in real clinical settings, especially for running busy outpatient clinics without sufficient human resources. Prospective studies are needed to evaluate whether this newly developed scoring system using SUDOSCAN can predict foot ulceration or amputation.
In this study, we used own cut-off value of feet ESC from our study population and the accuracy for diagnosis of DPN was 65.7%. Others suggested different cut-off values; Selvarajah et al. [11] suggested cut-off point of 77.0 µS in subjects with type 1 diabetes mellitus, and Krieger et al. [14] suggested 80 µS in subjects with T2DM. These values were different from cut-off value of previously published data; >60 µS, no dysfunction; 60 to 40 µS, moderate sudomotor dysfunction; and <40 µS, severe sudomotor dysfunction [12,15]. When we used the cut-off value of 60 µS, the AUC of our model was 0.638 (95% CI, 0.536 to 0.739), which value was numerically lower than the model using cut-off value of 56 μS. In fact, ESC might be influenced by ethnicity [33]; therefore, it would be reasonable to use cut-off value of feet ESC according to ethnicity of population.
The main limitation of our study is that NCS was performed in the subgroup of subjects. Some patients did not have enough time to check NCS, because we enrolled participants from outpatient clinical setting. In addition, some of patients without neuropathic symptom were not willing to undergo NCS. In fact, the patients underwent NCS had higher scores of MNSI than those without the data of NCS (data not shown). Therefore, there might be the possibility to include relatively high risk patients for DPN. In addition, we did not define DPN if the patient had normal nerve conduction regardless of the presence of neuropathic symptom. This is why there was no difference in the prescription rate of neuropathy medications between groups. For medications of DPN, tricyclic antidepressants have anti-cholinergic effect, and we cannot exclude the effect of the usage of this medication on feet ESC level. In this study, we enrolled people with T2DM whose renal function was normal to exclude uremic neuropathy and who were recruited only from a tertiary academic hospital. Furthermore, we tried to rule out the possible interference of newly treated anti-hyperglycaemic medications, even though there was a lack of data in the influence of anti-hyperglycaemic medications on ESC values. Therefore, we cannot generalise our findings to all people with T2DM. Further study is needed to validate our model and to evaluate the effect of medications on ESC values in a larger population.
Our findings suggest that SUDOSCAN combined with the MNSI can be used for screening of DPN. To combine these tools, the discrimination power for detecting DPN will be increased compared to using MNSI solely. This strategy may be applicable in a real clinical setting and does not require a trained examiner. Further study is necessary to confirm whether our suggesting tool actually improves examination rate for DPN and could prevent severe foot complication.
Acknowledgments
The authors acknowledge the assistance of the research nurses and study participants. We especially thank Ms Ji-hyong Kook for support with the statistical analyses.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: T.J.O., H.C.J., S.H.C.
Acquisition, analysis, or interpretation of data: T.J.O., Y.S.
Drafting the work or revising: T.J.O., S.H.C.
Final approval of the manuscript: T.J.O., Y.S., H.C.J., S.H.C.
FUNDING
This work was supported by a grant (Tae Jung Oh, 2016F-5) from the Korean Diabetes Association. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Materials
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0014.
Study flow chart. DPN, diabetic peripheral neuropathy.
REFERENCES
- 1.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:150–4. doi: 10.1007/BF00400697. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, KORA Study Group Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31:464–9. doi: 10.2337/dc07-1796. [DOI] [PubMed] [Google Scholar]
- 3.Lee CC, Perkins BA, Kayaniyil S, Harris SB, Retnakaran R, Gerstein HC, et al. Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: the PROMISE Cohort. Diabetes Care. 2015;38:793–800. doi: 10.2337/dc14-2585. [DOI] [PubMed] [Google Scholar]
- 4.Albers JW, Herman WH, Pop-Busui R, Martin CL, Cleary P, Waberski B, et al. Subclinical neuropathy among Diabetes Control and Complications Trial participants without diagnosable neuropathy at trial completion: possible predictors of incident neuropathy? Diabetes Care. 2007;30:2613–8. doi: 10.2337/dc07-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JH, Kim DS. The necessity of the simple tests for diabetic peripheral neuropathy in type 2 diabetes mellitus patients without neuropathic symptoms in clinical practice. Diabetes Metab J. 2018;42:442–6. doi: 10.4093/dmj.2017.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:136–54. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohara N, Kimura J, Kaji R, Goto Y, Ishii J, Takiguchi M, et al. F-wave latency serves as the most reproducible measure in nerve conduction studies of diabetic polyneuropathy: multicentre analysis in healthy subjects and patients with diabetic polyneuropathy. Diabetologia. 2000;43:915–21. doi: 10.1007/s001250051469. [DOI] [PubMed] [Google Scholar]
- 8.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–93. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh TJ, Lee JE, Kim S, Yoo S, Jang HC. Mobile healthcare system provided by primary care physicians improves quality of diabetes care. Cardiometab Syndr J. 2021;1:88–97. [Google Scholar]
- 10.Petropoulos IN, Ponirakis G, Khan A, Almuhannadi H, Gad H, Malik RA. Diagnosing diabetic neuropathy: something old, something new. Diabetes Metab J. 2018;42:255–69. doi: 10.4093/dmj.2018.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvarajah D, Cash T, Davies J, Sankar A, Rao G, Grieg M, et al. SUDOSCAN: a simple, rapid, and objective method with potential for screening for diabetic peripheral neuropathy. PLoS One. 2015;10:e0138224. doi: 10.1371/journal.pone.0138224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther. 2013;15:948–53. doi: 10.1089/dia.2013.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duchesne M, Richard L, Vallat JM, Magy L. Assessing sudomotor impairment in patients with peripheral neuropathy: comparison between electrochemical skin conductance and skin biopsy. Clin Neurophysiol. 2018;129:1341–8. doi: 10.1016/j.clinph.2018.04.608. [DOI] [PubMed] [Google Scholar]
- 14.Krieger SM, Reimann M, Haase R, Henkel E, Hanefeld M, Ziemssen T. Sudomotor testing of diabetes polyneuropathy. Front Neurol. 2018;9:803. doi: 10.3389/fneur.2018.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayaudon H, Miloche PO, Bauduceau B. A new simple method for assessing sudomotor function: relevance in type 2 diabetes. Diabetes Metab. 2010;36(6 Pt 1):450–4. doi: 10.1016/j.diabet.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Gin H, Baudoin R, Raffaitin CH, Rigalleau V, Gonzalez C. Non-invasive and quantitative assessment of sudomotor function for peripheral diabetic neuropathy evaluation. Diabetes Metab. 2011;37:527–32. doi: 10.1016/j.diabet.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Malik RA, Veves A, Tesfaye S, Smith G, Cameron N, Zochodne D, et al. Small fibre neuropathy: role in the diagnosis of diabetic sensorimotor polyneuropathy. Diabetes Metab Res Rev. 2011;27:678–84. doi: 10.1002/dmrr.1222. [DOI] [PubMed] [Google Scholar]
- 18.Breiner A, Lovblom LE, Perkins BA, Bril V. Does the prevailing hypothesis that small-fiber dysfunction precedes large-fiber dysfunction apply to type 1 diabetic patients? Diabetes Care. 2014;37:1418–24. doi: 10.2337/dc13-2005. [DOI] [PubMed] [Google Scholar]
- 19.Novak P. Electrochemical skin conductance: a systematic review. Clin Auton Res. 2019;29:17–29. doi: 10.1007/s10286-017-0467-x. [DOI] [PubMed] [Google Scholar]
- 20.Oh SJ. Clinical electromyography: nerve conduction studies. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2003. Chapter 7, Normal values for common nerve conduction tests; pp. 86–106. [Google Scholar]
- 21.Won JC, Kwon HS, Kim CH, Lee JH, Park TS, Ko KS, et al. Prevalence and clinical characteristics of diabetic peripheral neuropathy in hospital patients with type 2 diabetes in Korea. Diabet Med. 2012;29:e290–6. doi: 10.1111/j.1464-5491.2012.03697.x. [DOI] [PubMed] [Google Scholar]
- 22.Won JC, Park TS. Recent advances in diagnostic strategies for diabetic peripheral neuropathy. Endocrinol Metab (Seoul) 2016;31:230–8. doi: 10.3803/EnM.2016.31.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–9. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 24.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 26.Umapathi T, Tan WL, Loke SC, Soon PC, Tavintharan S, Chan YH. Intraepidermal nerve fiber density as a marker of early diabetic neuropathy. Muscle Nerve. 2007;35:591–8. doi: 10.1002/mus.20732. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed A, Bril V, Orszag A, Paulson J, Yeung E, Ngo M, et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care. 2012;35:821–8. doi: 10.2337/dc11-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisman A, Bril V, Ngo M, Lovblom LE, Halpern EM, Orszag A, et al. Identification and prediction of diabetic sensorimotor polyneuropathy using individual and simple combinations of nerve conduction study parameters. PLoS One. 2013;8:e58783. doi: 10.1371/journal.pone.0058783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63:2454–63. doi: 10.2337/db13-1819. [DOI] [PubMed] [Google Scholar]
- 30.Binns-Hall O, Selvarajah D, Sanger D, Walker J, Scott A, Tesfaye S. One-stop microvascular screening service: an effective model for the early detection of diabetic peripheral neuropathy and the high-risk foot. Diabet Med. 2018;35:887–94. doi: 10.1111/dme.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stetson DS, Albers JW, Silverstein BA, Wolfe RA. Effects of age, sex, and anthropometric factors on nerve conduction measures. Muscle Nerve. 1992;15:1095–104. doi: 10.1002/mus.880151007. [DOI] [PubMed] [Google Scholar]
- 32.Tan LS. The clinical use of the 10g monofilament and its limitations: a review. Diabetes Res Clin Pract. 2010;90:1–7. doi: 10.1016/j.diabres.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Vinik AI, Smith AG, Singleton JR, Callaghan B, Freedman BI, Tuomilehto J, et al. Normative values for electrochemical skin conductances and impact of ethnicity on quantitative assessment of sudomotor function. Diabetes Technol Ther. 2016;18:391–8. doi: 10.1089/dia.2015.0396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study flow chart. DPN, diabetic peripheral neuropathy.