Abstract
The gene encoding the Pif1 helicase was first discovered in a Saccharomyces cerevisiae genetic screen as a mutant that reduces recombination between mitochondrial respiratory mutants and was subsequently rediscovered in a screen for genes affecting the telomere length in the nucleus. It is now known that Pif1 is involved in numerous aspects of DNA metabolism. All known functions of Pif1 rely on binding to DNA substrates followed by ATP hydrolysis, coupling the energy released to translocation along DNA to unwind duplex DNA or alternative DNA secondary structures. The interaction of Pif1 with higher-order DNA structures, like G-quadruplex DNA, as well as the length of single-stranded (ss)DNA necessary for Pif1 loading have been widely studied. Here, to test the effects of ssDNA length, sequence, and structure on Pif1’s biochemical activities in vitro, we used a suite of oligonucleotide-based substrates to perform a basic characterization of Pif1 ssDNA binding, ATPase activity, and helicase activity. Using recombinant, untagged S. cerevisiae Pif1, we found that Pif1 preferentially binds to structured G-rich ssDNA, but the preferred binding substrates failed to maximally stimulate ATPase activity. In helicase assays, significant DNA unwinding activity was detected at Pif1 concentrations as low as 250 pM. Helicase assays also demonstrated that Pif1 most efficiently unwinds DNA fork substrates with unstructured ssDNA tails. As the chemical step size of Pif1 has been determined to be 1 ATP per translocation or unwinding event, this implies that the highly structured DNA inhibits conformational changes in Pif1 that couple ATP hydrolysis to DNA translocation and unwinding.
Graphical Abstract
INTRODUCTION
Pif1 proteins belong to the superfamily 1B (SF1B) helicases that translocate and unwind DNA in the 5'-3' direction.1,2 Members of the Pif1 helicase family have been discovered in all eukaryotes, as well as in many prokaryotes and some viruses.3 The Saccharomyces cerevisiae Pif1 is the best studied family member and plays important roles in many aspects of DNA metabolism, including DNA replication,4–6 Okazaki fragment maturation,7,8 DNA repair,9,10 DNA end resection,11–13 centromere segregation,14 mitochondrial DNA stability,15,16 removing obstructing proteins bound to DNA,17,18 and regulation of telomerase activity.19–21
In addition, Pif1 is also capable of unwinding DNA/RNA hybrids (when the DNA strand is the 5'-3' strand that Pif1 translocates along) more efficiently than DNA/DNA duplexes.22 Pif1 exhibits increased processivity on DNA/RNA hybrids compared to DNA/DNA duplexes, leading to increased rates of unwinding activity.23 This aspect of Pif1 biochemistry is hypothesized to be used by the helicase to disrupt the interaction between the telomeric single-stranded (ss)DNA and the telomerase RNA, a critical step in controlling the telomerase activity and the telomere length homeostasis.24 Acting as a DNA/RNA helicase, Pif1 also reorganizes nucleic acid structures that can interfere with DNA replication, such as R-loops.25 Indeed, a recent report using a mutated version of CRISPR/Cas9 demonstrates that Pif1 resolves a trapped DNA/RNA bubble formed by the CRISPR guide RNA.18
Like many SF1B helicases, S. cerevisiae Pif1 is monomeric in solution.26 However, Pif1 binding to and dimerization on a fork substrate and a 3'-tailed substrate have been demonstrated, though the 3'-tailed substrate is not unwound due to the 5'-3' directionality of Pif1.27 Single-molecule experiments using a variety of duplex DNA substrates with 3' ssDNA tails indicate that Pif1 specifically binds to the double-stranded (ds)DNA/ssDNA junction and, instead of translocation toward the end of the ssDNA, reels the ssDNA toward itself.28 This is a reiterative process in which Pif1 releases the ssDNA when it reels in the 3' end and then begins to reel it in again. This patrolling mechanism enables the unwinding of DNA-DNA and RNA-DNA duplexes, as well as the disruption of protein-DNA complexes that Pif1 encounters during the reeling cycle.28,29 Such a mechanism would be valuable for a helicase to remove proteins from 3' overhangs at DNA double-strand breaks (DSBs), at telomeres to avoid natural chromosome ends from being recognized as DNA breaks, or at other sites that could impede DNA replication—all functions attributed to Pif1 family helicases.30
The combination of bulk-phase assays and single-molecule experiments has yielded large amounts of data regarding S. cerevisiae Pif1 DNA binding, stimulation of its ATPase activity by DNA, and unwinding of an array of different substrates.31–35 To date, it is known that Pif1 requires 3–5 nt of 5'-ssDNA to load onto forked DNA, and blunt-ended dsDNA substrates are not unwound.27 Pif1 can also bind to short lengths of ssDNA, with a minimum binding requirement of 6–8 nt. Several modes of DNA binding have been reported, with Pif1 binding as a monomer, translocating to the ssDNA/dsDNA junction, and then forming a dimer that can actively unwind duplex DNA.26,29 Single-molecule analysis has shown that DNA unwinding can occur under conditions where only Pif1 monomers are likely to form, rather than as dimers observed at higher Pif1 concentrations.26,28,36
Telomere length homeostasis in S. cerevisiae is regulated in part by Pif1 activity, and this has been demonstrated both in vitro and in vivo.21,37–41 It has been reported that at lengths >34 bp, telomerase becomes insensitive to Pif1 activity on short artificial telomeres induced in vivo using the HO endonuclease.41 Another group using a similar system argues that Pif1 activity is the highest on the longest telomeres, thus allowing short telomeres to be lengthened preferentially.39 Again, with evidence from the same HO cleavage system, it has been reported that Pif1 is an active telomerase inhibitor at all telomere lengths.40 Using a Brownian motion detection system, another group reports that the ssDNA length increases the ability of Pif1 to evict telomerase from substrates up to 82 nt, but Pif1 unwinding activity does not increase with 3'-ends longer than 34 nt.38 Overall, it is clear that Pif1 functions at telomeres, but the effect of DNA length on Pif1 activity is an area that requires further investigation.
Another important variable controlling the activities of Pif1 is the DNA secondary structure, which affects Pif1 DNA binding, translocation, and unwinding.42–44 G-rich regions of chromosomes can form G-quadruplex (G4) DNA structures during replication.45 Many groups have reported in vitro unwinding of a range of different types of G4 DNA substrates by Pif1.33,42–44 Evidence for G4 DNA unwinding by Pif1 in vivo is more limited, and Pif1 is not essential for movement of replication forks past G4 DNA obstacles.33,46 Curiously, both stimulation and inhibition of Pif1 unwinding of G4 structure-containing fork substrates have been reported.42,43 Reviews of this subject suggest that the different types of G4 structures (e.g., intramolecular vs intermolecular) could be a cause of the apparent discrepancies.35,45 G4 DNA structures can be stabilized by replacing sodium ions with potassium ions in reaction buffers, and this may also account for the discrepancies in the outcome of unwinding assays.32,34 A somewhat overlooked variable that could additionally be significant for the regulation of Pif1 activity is the rate of folding/refolding by G4 structures. The refolding rates of G-rich structures at telomeres are reported to be critical for control of the binding of both Cdc13 (a telomeric ssDNA binding protein) and Pif1, providing a link between G4 DNA folding and the cell cycle.47
Considering the array of known activities involving Pif1 and the discrepancies found in the literature concerning Pif1 activity with regard to various DNA substrates, we here report the results of experiments to determine the basic biochemical parameters for the interaction of S. cerevisiae Pif1 with an array of ssDNA and dsDNA substrates of different lengths and degrees of the secondary structure. Using gel shift assays, we found that Pif1 exhibits high affinity binding to G-rich, highly structured DNA, as has been previously reported.33 ATPase assays with Pif1 demonstrate an inverse relationship between high affinity binding and ATP hydrolysis. Pif1 had the greatest a affinity for highly structured DNA that failed to maximally stimulate ATPase activity, suggesting that such structures may inhibit Pif1 translocation. Helicase activity was also significantly reduced by a higher-order structure in the 5' loading strand and, to a lesser extent, in the 3' nonloading strand of fork substrates. Human telomeric repeat DNA sequence in the loading strand of fork substrates reduced unwinding rates by 2 orders of magnitude compared to unstructured poly(dT) loading strand substrates. Increasing the poly(dT) ssDNA length from 5 to 30 nt significantly stimulated ATPase activity, but further increases in the primer length from 35 to 100 nt led to only small increases in the ATP hydrolysis rates. With Pif1 helicase assays, increasing the loading strand length from 25 to 75 nt had at most a 2-fold effect on the unwinding rate. Therefore, we conclude that the DNA sequence—and secondary structures formed by this sequence—is a much more significant feature of substrates than the length for controlling the rates of Pif1 activity.
MATERIALS AND METHODS
Protein Production.
Escherichia coli strain NiCo21(DE3) pLysS was used for the overexpression of SUMO-tagged Pif1 (P07271) and SUMO protease (Q02724). The purification procedure has been reported previously.21 Briefly, cells were transformed with a SUMO-Pif1 fusion protein expression plasmid and maintained on LB medium supplemented with 50 μg/mL kanamycin and 34 μg/mL chloramphenicol. Liquid cultures for Pif1 protein overproduction were grown in 2x YT medium supplemented with 10 mM MgSO4, 1 mM ZnOAc, and the same antibiotics listed above. Pif1 overproduction was achieved by induction with 0.2 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG) followed by incubation at 18 °C for 16 h. Preparations of wildtype Pif1 were compared to recombinant Pif1-K264A (catalytically inactive) generated by the same procedure21 as a control to verify that catalytic activity was due to Pif1 and not an E. coli contaminant (not shown).
EMSAs.
Substrate oligonucleotides for all assays were purchased from Integrated DNA Technologies (IDT) and are listed in supplementary Table S1. All substrates for electrophoresis mobility shift assays (EMSAs) were labeled with 56-FAM for fluorescent detection. Binding reactions were performed in 1× binding buffer [25 mM HEPES (pH 8.0), 5% glycerol (w/v), 50 mM NaOAc, 150 mM NaCl, 7.5 mM MgCl2, and 0.01% Tween 20 (w/v)]. Fluorescently labeled substrates were added to binding reactions to a final concentration of 2 nM. Binding reactions were incubated at 30 °C for 30 min and mixed with 6× nonfluorescent loading buffer [50 mM Tris (pH 8.0), 25% glycerol (w/v), and 0.025% Orange G]. The reactions were separated on native 8% 19:1 acrylamide/bisacrylamide gels in 1× TG running buffer [25 mM Tris and 185 mM glycine (pH 8.8)]. Gels were run at 100 V for 30–45 min and were imaged and quantified using a Typhoon 9500 scanner with ImageQuant software. All data were plotted and, where appropriate, fit with curves using GraphPad software.
ATPase Assays.
All ATPase reaction components were prepared on ice in a 96-well plate format. ATPase reactions were performed in ATPase buffer [25 mM Na-HEPES (pH 8.0), 5% glycerol, 50 mM NaOAc (pH 7.5), 150 μM NaCl, and 0.01% NP-40], including the following reagents: 5 mM ATP (pH 7.0) (DOT Scientific), 5 mM MgCl2, 0.5 mM phosphor(enol)pyruvic acid (Sigma), 0.4 mM NADH (MP Biomedicals, LLC), 5 U/mL rabbit pyruvate kinase (Roche), and 8 U/mL lactate dehydrogenase from oyster (Sigma). For most reactions, the helicase concentration was 10 nM, and DNA was added to a final concentration of 100 nM. All reactions and controls were prepared in triplicate. Plates were scanned in a BioTek Synergy H1 microplate reader prewarmed to 30 or 37 °C. To check for possible confounding effects caused by secondary structures that oligonucleotides may form, the substrates were diluted to their working concentrations, boiled, and snap-cooled prior to addition to ATPase assays where indicated. To determine if Pif1 activity was affected by using equal concentrations of nucleotides (nt) rather than equimolar substrate concentrations (i.e., numbers of 5'-oligonucleotide ends), we also performed assays with 160 ng of primer in each reaction, giving a range of concentrations from 1 μM to 38.6 nM depending on the length of the ssDNA substrate. Reactions were monitored for absorbance at 340 nm, sampling at 1 min intervals from 0 to 60 min. Absorbance readings were converted to ATP turnover based on NADH concentration and corrected for spontaneous ATP hydrolysis using control reactions lacking Pif1. It was assumed that 1 μmole of NADH oxidized is proportional to 1 μmole ATP hydrolyzed. The linear portions of reaction curves for each sample were used to determine the hydrolysis rates (Figure S1). These rates could be converted into relative levels of ATPase stimulation normalized to reactions containing Pif1 but lacking added DNA.
Helicase Assays.
Fork substrates for helicase assays were constructed by incubating two partially complementary oligonucleotides (both at 1 μM) for annealing overnight at 37 °C in a buffer consisting of 20 mM Tris-HCl (pH 8.0), 4% glycerol, 0.1 mM ethylene diamine tetraacetic acid, 40 μg/mL bovine serum albumin, 10 mM dithiothreitol, and 10 mM MgOAc. All DNA fork substrates used for helicase assays are listed in Table S2. We used either 5'-IR, near infra-red, labeled probes (IDT) or 5'-32P-labeled probes to visualize the results. Oligonucleotides were 32P-labeled with T4 polynucleotide kinase and [γ-32P]-ATP under standard conditions. Radiolabeled [γ-32P]-ATP was purchased from PerkinElmer Life Sciences. Labeled oligonucleotides were separated from unincorporated label using G-50 microcolumns (GE Healthcare). Helicase reactions were performed at 30 °C for 30 min in 1× EMSA binding buffer supplemented with ATP to a final concentration of 5 mM. Labeled fork substrates were added to final concentrations of 0.2 nM for 32P-labeled probes or 2 nM for IR-labeled probes. Reactions were initiated by the addition of Pif1. Stop buffer was added to each reaction to a final concentration of 0.008% sodium dodecyl sulfate and 1.67 μg/mL proteinase K and incubated at 30 °C for 30 min, then mixed with 6× nonfluorescent loading, and placed on ice. Reactions were separated on 10% native acrylamide gels with the same conditions as described above for EMSAs. Gels with radioactive label were dried, imaged, and quantified using a Typhoon 9500 scanner with ImageQuant software. Gels with IR labels were visualized using a LI-COR scanner, and images were quantified using Image Studio Lite version 5.2.
RESULTS
Effects of ssDNA Sequence on Pif1 Binding.
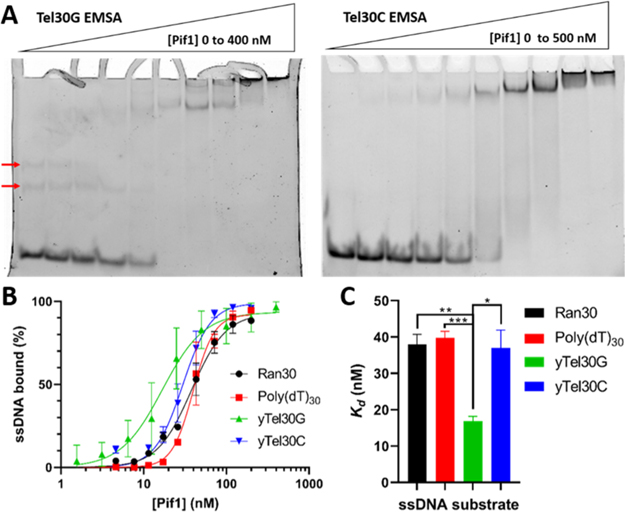
We have previously reported using a SUMO-tagged Pif1 construct obtained from Kevin Raney’s group to overproduce recombinant S. cerevisiae Pif1 protein.37,48 This construct allows for the clean removal of an N-terminal SUMO/His affinity tag, yielding a protein sequence identical to the wildtype nuclear isoform of Pif1. Because much of the previously reported biochemical characterization of Pif1 was performed with tagged versions of the helicase (e.g., ref 33), we decided to assess DNA binding, ATPase activity, and DNA unwinding by untagged Pif1. As a first step, we performed EMSAs with a set of 30 nt ssDNA substrates that varied in sequence, including a mimic of the yeast telomeric G-strand (yTel30G), the yeast telomeric C-strand (yTel30C), a random sequence ssDNA with an approximately 50% G + C ratio (Ran30), and a poly(dT) 30mer. Representative gel images and binding curves are shown in Figure 1A,B.
Figure 1.
Effects of DNA sequence on Pif1 binding affinity. (A) EMSA gel images with the yTel30G or yTel30C oligonucleotide. Red arrows indicate putative secondary structures formed by this substrate that display slower mobility. (B) Compiled binding curves of triplicate EMSAs. (C) Bar graph display of Kd values for all EMSAs performed in triplicate. Statistical significance was determined via multiple t tests using the Holm-Sidak method, with α = 0.05. *p < 0.01, **p < 0.001, and ***p < 0.0001.
We found that Pif1 exhibits the highest binding affinity for the yTel30G substrate, with an apparent Kd (the concentration of Pif1 necessary to bind 50% of the substrate) of 16.87 ± 1.33 nM (Figure 1C). All of the other substrates were bound approximately 2-fold weaker (Kd ≈ 35 nM), representing a significant decrease in the binding affinity (p < 0.01; Figure 1C). yTel30C is comprised of the complementary sequence to yTel30G, which can be bound by Pif1 during Okazaki fragment processing at telomeres.4 Pif1 displayed a 2-fold reduced affinity for yTel30C compared to yTel30G, essentially equal to its affinity for Ran30 ssDNA.
Several studies demonstrate that Pif1 preferentially binds a variety of G4 structures with high affinity and that it plays an important role in the unfolding of G4 DNA that forms during replication.33,42,46,49 Even if G4 structures do not spontaneously fold from G-rich ssDNA, protein binding can chaperone the ssDNA to form stable structures. For instance, Est1, a S. cerevisiae telomerase subunit, has been shown to stimulate formation of Mg++-dependent inter- and intramolecular G4 structures that are stabilized by the addition of K+.44,50–52 While our work did not address this issue directly, we were curious about possible secondary structures that could occur within our DNA substrates. All ssDNAs that we used for EMSAs were assessed with the mFold program,53 and no stable secondary structures were predicted (data not shown). The yTel30G substrate is comprised of the G-rich S. cerevisiae telomeric G-strand repeat sequence (TG1–3) and was thus also analyzed with QGRS mapper for its potential to fold into a G4 structure,54 but no such structure was predicted. Despite the lack of a predicted higher-order structure for yTel30G, we observed two bands with slower mobility than the linear ssDNA substrate itself in native acrylamide gels following electrophoresis (Figure 1A). These bands were also bound by Pif1 along with the ssDNA form, but similar slower migrating species were not observed with the other ssDNA substrates used for EMSAs (Figure 1A). Our buffer solutions contain Mg2+ ions without added K+ for G4 stabilization, so these may represent metastable G4 structures or unknown secondary structures that can form in the absence of added potassium.28,29
Effects of ssDNA Sequence and Length on Pif1 ATPase Activity.
ATP hydrolysis fuels translocation along ssDNA and unwinding of nucleic acid duplexes by helicases, and the ATPase activity of most DNA helicases is stimulated by DNA. Thus, we next tested the effects of a variety of ssDNA substrates (Table S1) on Pif1 ATP hydrolysis. These included S. cerevisiae telomeric repeat sequences, random sequences, different lengths of poly(dT) from 5–100 nt, and homopolymers composed of one of each of the common nucleic acid bases.
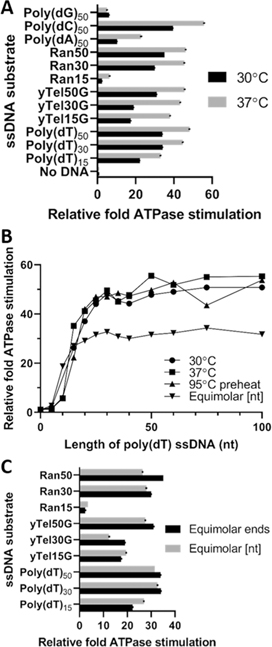
We first addressed how Pif1 ATPase activity would be affected by altering the standard assay conditions, that is, reducing the temperature from 37 to 30 °C (the optimal growth temperature for S. cerevisiae). In most cases, ssDNA-stimulated ATPase activity fell by ~30%, while Pif1 ATP hydrolysis in the presence of yTel15G, yTel30G, poly(dA)50, and Ran15 substrates decreased by more than 2-fold when the temperature was decreased (Figure 2A). In preliminary experiments, increasing the amounts of pyruvate kinase and lactate dehydrogenase by up to 20% in the coupled ATPase assay yielded approximately the same reductions in ATPase activity, suggesting that these results were due to lower Pif1 activity at lower temperature rather than decreased activity by the ATP regeneration system (data not shown). For the remaining ATPase assays, we compared the standard assay conditions (37 °C) with the assays performed at 30, 30 with a 95 °C pre-heating step for the DNA to melt potential secondary structures, and 30 °C with equimolar concentrations of substrate nucleotides versus equimolar ssDNA 5'-ends (Figure 2B,C).
Figure 2.
Effects of changing the length or concentration of ssDNA on Pif1 ATPase activity. All data are expressed as the relative ATPase activity stimulation normalized to Pif1-only controls. (A) Relative Pif1 ATPase activity at 30 and 37 °C. (B) Effects of increasing length of poly(dT) ssDNA on the relative stimulation of Pif1 ATPase activity. (C) Comparison of the relative stimulation of Pif1 ATPase activity using 160 ng or 100 nM of ssDNA.
It has been reported that Pif1 acts as a force-controlled helicase in single-molecule experiments using Förster resonance energy transfer analysis and magnetic tweezers.55 We hypothesized that increasing the length of a ssDNA substrate should allow increased loading of Pif1, thereby increasing the force and ATPase activity proportionately. To investigate this, ATPase assays were performed using a set of poly(dT) substrates of increasing length from 5 to 100 nt (Table S1). The poly(dT)5 ssDNA failed to significantly stimulate Pif1 ATPase activity under any of the conditions tested (Figure 2B). However, significant increases in ATPase activity occurred as the polymer length was increased from 10 to 30 nt, accounting for ~90% of ATPase stimulation observed (Figure 2B, Table S3). Further increases in the polymer length from 35 to 100 nt led to relatively small increases in ATPase activity (Figure 2B, Table S3). It has been estimated that Pif1 can form dimers on an 8 nt poly(dT) substrate.27 Our results support this finding, with a 10 nt poly(dT) substrate stimulating Pif1 ATPase but a 5 nt poly(dT) substrate failing to do so. This indicates a minimal size of >5 and <10 nt for Pif1 ssDNA binding and translocation events.
ATPase assays were also performed with 1.32 ng/μL (or 160 ng) of substrate for each reaction set compared against our standard assay containing 100 nM ssDNA. This resulted in the ssDNA concentration ranging from 1 μM for poly(dT)5 to 36.8 nM for poly(dT)100, so all reactions were still performed with a molar excess of substrate over Pif1. Our results indicate that all of the ssDNAs of 5, 10, and 15 nt in length resulted in increased ATPase activity relative to assays with equimolar substrate ends, reflecting their increased concentration (Figure 2C, Table S3). For instance, increasing the poly(dT)10 concentration 4-fold led to a 3-fold increase in ATPase activity, whereas increasing the poly(dT)15 concentration 3-fold yielded only a 1.2-fold increase in activity. Interestingly, despite the fact that the concentration of the poly(dT)20–40 ssDNAs was >100 nM, activity fell below that observed in the 30 °C control assay for each primer. In fact, ATPase activity failed to increase significantly with increasing primer length above 30 nt (Figure 2C, Table S3).
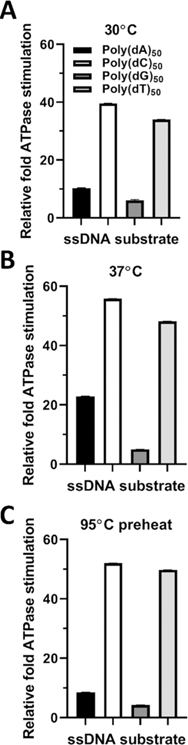
Previous reports demonstrate that G-rich telomeric ssDNA, including poly(dG) homopolymers, are bound by Pif1 with the highest affinity when compared to lower G + C ratio substrates or pyrimidine homopolymers.21,33,56 Our results in Figure 1 also support these conclusions. As Pif1 is known to play a significant role in the resolution of G4 DNA structures that form during telomere extension and as replication intermediates,30,33,45 we next tested the effects of each DNA polymer type on Pif1 ATP hydrolysis. We used a set of 50 nt homopolymers, one for each common nucleotide, as substrates for the ATPase assay (Table S1). Our results clearly demonstrate that pyrimidine homopolymers stimulated higher levels of ATP hydrolysis compared to purine homopolymers (Figure 3). The relative order of stimulation was poly(dC) ≥ poly(dT) » poly(dA) > poly(dG) for all conditions tested (Figure 3A–C).
Figure 3.
Stimulation of Pif1 ATPase activity is inhibited by highly structured ssDNA homopolymers. The stimulation of Pif1 ATPase activity at (a) 37, (b) 30, and (c) 30 °C with a 95 °C ssDNA preheat step.
Considering that G-rich ssDNAs were bound with the highest affinity by Pif1 (Figure 1), we next sought to determine if an inhibitory secondary structure was responsible for the low ATPase activity observed with polypurine polymers. Therefore, we diluted the substrates to their working concentration, heated them to 95 °C for 5 min, and then snap-cooled and held them on ice until the start of the assay at 30 °C. Curiously, following heat treatment, the poly(dC) and poly(dT) ssDNAs yielded significantly increased (p < 0.000001) Pif1 ATPase activity by 1.31- and 1.46-fold, respectively (Figure 3B,C). Heat treatment of the poly(dA) and poly(dG) polypurine substrates decreased the Pif1 ATPase activity by 0.83- and 0.71-fold, respectively (Figure 3B,C, Table S3). Therefore, if secondary structures that inhibit Pif1 ATPase activity are formed by the polypurine primers, then heat treatment did not disrupt them, or they reformed stable structures after heat treatment that inhibit Pif1 ATP hydrolysis (Figure 3C).
We also analyzed our ssDNA substrate sequences with the G4 structure prediction program QGRS.54 Surprisingly, substrates comprised of the G-rich yeast telomeric repeat sequence were not predicted to form G4 structures, even when 50 nt long. However, we observed evidence of two slower migrating bands of the yTel30G substrate during native gel electrophoresis, suggesting that stable higher-order structures do form (Figure 1A). The identities of the secondary structures formed by this substrate have not been investigated but could be intermolecular G4 structures based on their apparent higher molecular weight compared to the linear ssDNA itself. Four-stranded G4 structures have been shown to migrate slower in native acrylamide gels than their unfolded single-stranded forms.45
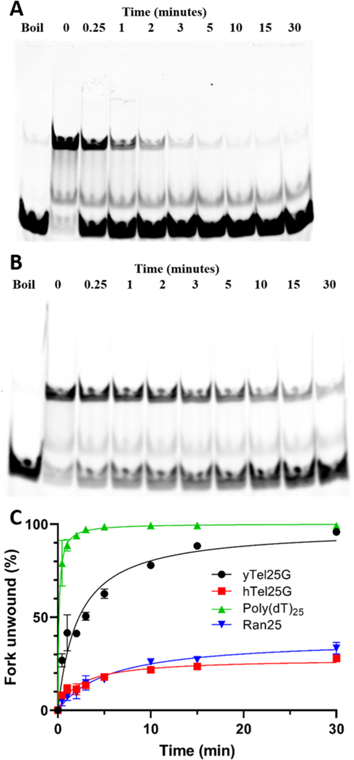
Effects of ssDNA Sequence and Length on Pif1 Helicase Activity.
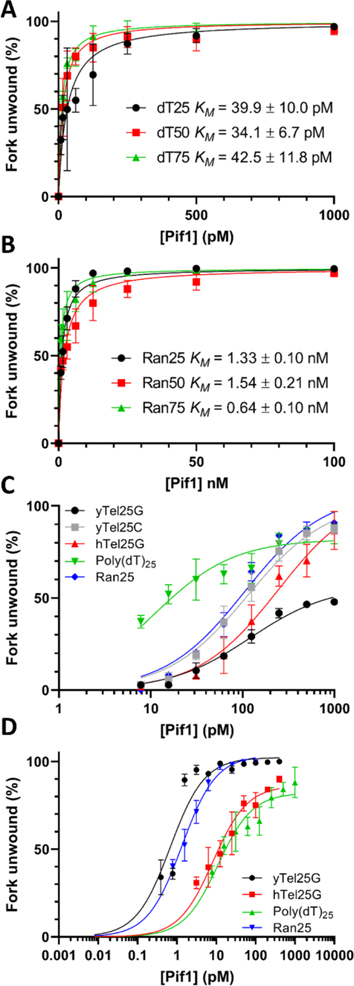
Having found that DNA sequence had opposite effects on Pif1 DNA binding and ATPase activity, we next wanted to determine the effects of altering the length and sequence of the 5' ssDNA tail (defined here as the Pif1 loading strand) of forked DNA substrates on Pif1 helicase activity. Helicase assays were performed using forks with poly(dT) and random-sequence loading strands of 25, 50, and 75 nt, keeping the 3' ssDNA tail (defined here as the nonloading strand) as a constant poly(dT) sequence of 25 nt in length (Table S2). Unwinding results are shown in Figure 4A,B, and the KM (concentration of Pif1 necessary for 50% unwinding) values for all experiments are listed in supplementary Table S3. We found that increasing the length of the poly(dT) loading strand from 25 to 50 to 75 nt had little effect on the KM (39.9 ± 10.0, 34.1 ± 6.7, and 42.5 ± 11.8 pM, respectively). Similarly, increasing the length of the random-sequence loading strand from 25 to 50 nt also had no significant effect, but a further increase to 75 nt did lead to a 2-fold decrease in the KM (1328 ± 96.7 vs 643 ± 102 pM, respectively). The KM of fork unwinding for all random sequence lengths tested was an order of magnitude greater compared to unwinding forks with poly(dT) in the loading strand (Figure 4A,B).
Figure 4.
A highly structured loading strand inhibits unwinding of fork substrates by Pif1. (A) Effects of increasing loading strand length on unwinding of poly(dT)x/poly(dT)25 forks. (B) Effects of increasing loading strand length on unwinding of RanX/poly(dT)25 forks. (C) Effects of varying the sequence of the loading strand on unwinding. (D) Effects of varying the sequence of the nonloading strand on unwinding.
To test the effects of DNA sequence on Pif1 unwinding, we designed two new sets of substrates. The first set included forks with varied 25 nt ssDNA loading strands [poly(dT), random sequence, yeast telomeric G-strand, or human telomeric G-strand (hTelG)] and constant 25 nt poly(dT) nonloading strands. The second set reversed this, varying the sequence of the nonloading strand and keeping the loading strand fixed as a 25 nt poly(dT) ssDNA tail. Each set of forks had an identical 20 bp dsDNA region. Results from the assays performed with forks containing loading strands of varied sequence display a clear pattern with relative KMs of unwinding being poly(dT) > Random > yTelG > hTelG (Figure 4C, Table S3). The KMs varied from 39.9 ± 10.0 pM for the fork with poly(dT) in both tails to 8295 ± 1187 pM with the human telomeric repeat G-strand sequence in the loading strand (Table S3). With both sets of fork substrates, the relative rate of unwinding increased when the poly(dT) sequence was in the loading strand (Figure 4C,D, Table S2). For example, comparing the hTelG/poly(dT) fork with the poly(dT)/hTelG fork (loading strand listed first), placing the poly(dT) sequence in the loading strand significantly lowered the KM value from 8295 ± 1187 to 244.4 ± 40.1 pM (Table S3). As expected, the fork substrate containing both poly(dT) tails had very similar KM values whether it was labeled with an IR700 dye at the 5'-end of the nonloading strand or with 32P on the 5'-end of the loading strand (39.9 ± 10 pM vs 11.09 ± 1.96, respectively; Table S3).
Overall, altering the sequence of the nonloading strand resulted in a similar trend as above, with the fork containing a poly(dT) nonloading tail being unwound the fastest and that containing the hTelG sequence being unwound most poorly among this substrate set (Table S3). In all cases, having poly(dT) as the loading strand stimulated Pif1 unwinding. In addition to the equilibrium unwinding assays reported above, time course assays using fork substrates with poly(dT)25 in the loading strand and varying sequence in the nonloading strand were performed. These results confirmed the pattern observed during equilibrium assays, with relative t1/2 values (the time necessary to achieve 50% unwinding) being poly(dT) > Random > yTelG > hTelG (Figure 5, Table S3).
Figure 5.
Forks with unstructured loading strands are unwound at the fastest rate by Pif1. All time course experiments shown here were performed using 1 nM Pif1. (A) Gel image of a representative time course helicase assay with the poly(dT)25/poly(dT)25 fork. (B) Gel image of a representative time course helicase assay with the Ran25/poly(dT)25 fork. (C) Effects of varying the sequence of the loading strand on the unwinding rate.
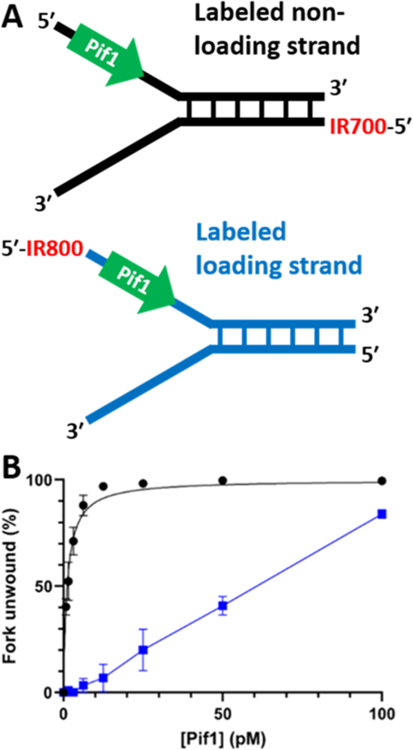
IR Label Placement also Affects Pif1 DNA Unwinding.
A third set of fork substrate experiments was also planned with 25 nt of random-sequence ssDNA as the fixed loading strand, labeled at the 5' end with an IR800 dye (Figure 6, Table S1). However, preliminary experiments revealed that all fork substrates constructed with this loading strand had significantly lower levels of unwinding than the identical DNA constructs labeled with an IR700 dye on the nonloading strand (data not shown). One example assay, performed in triplicate, is included here and demonstrates that the IR800 label on the 5' end of the loading strand drastically reduced Pif1 helicase activity (Figure 6A). The unwinding of forks with the IR800 label on the 5' end of the loading strand as a function of Pif1 concentration displayed a linear rather than hyperbolic relationship (Figure 6B). Because this unwinding curve had not saturated, we were unable to calculate a KM to compare to that of the fork containing the IR700-labeld nonloading strand with the otherwise identical DNA sequence (113.2 ± 12.76 pM; Table S3). These data indicate that some aspect of the IR800 label is interfering with Pif1 loading or unwinding, despite the presence of 25 nt of potential ssDNA binding sites on the labeled strand. We could find no other reports of 5'-labels interfering with Pifl unwinding activity. Based on these results, we did not proceed with the remaining planned experiments with the third set of forks but will discuss the implications of these observations below.
Figure 6.
IR800 labeling of the 5' end of the loading strand significantly reduces unwinding by Pif1. (A) Labeling scheme for the IR700- and IR800-based forks. (B) Unwinding curve comparing identical DNA forks with either a 5' IR800 label on the loading strand (blue) or a 5' IR700 label on the nonloading strand (black).
DISCUSSION
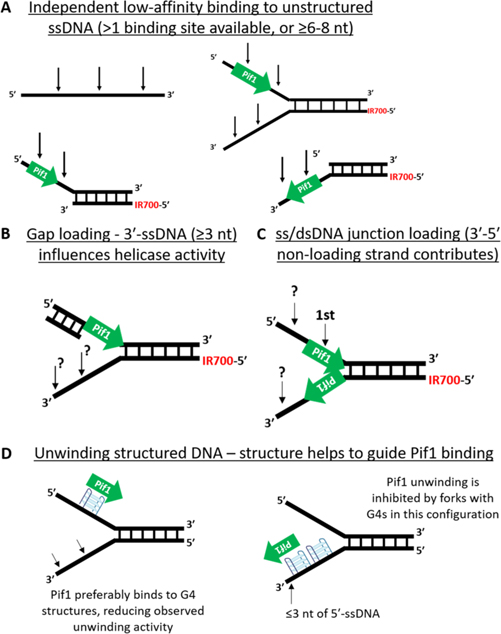
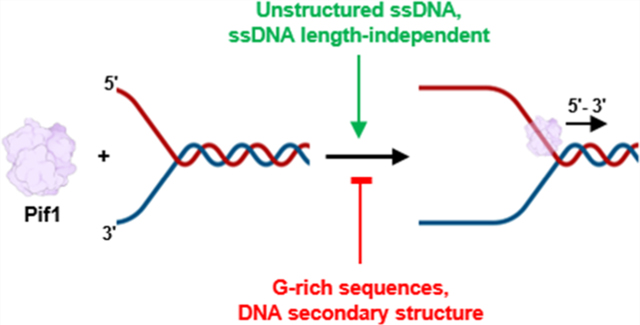
Here, we report the results of DNA binding assays, ATPase assays, and multiturnover helicase assays with DNA traps to avoid reannealing of dsDNA. Together with previous biochemical analyses, the presented data support the view that Pif1 has high affinity for G-rich substrates including G4 DNA.33,42 There is a clear hierarchy of higher affinity Pif1 binding to G-rich substrates followed by stacked DNA > random-sequence DNA > unstructured DNA. ATPase data reveals another pattern where the maximum amount of hydrolysis (and presumably Pif1 translocation) is the fastest on the least structured DNA, with slower movement on ssDNA forming secondary structures.42 Fork unwinding assays reflect the ATPase results, where highly structured loading strand sequences inhibit Pif1 unwinding.42,57 Human telomeric repeat sequences in either the loading strand or the nonloading strand of the fork are both predicted to form G4 structures, and both types of these forks significantly inhibited unwinding compared with forks containing two poly(dT) ssDNA tails. These results support a model in which Pif1 surveilles ssDNA exposed during DNA replication, DNA repair, and telomere maintenance, with higher affinity binding to regions where highly structured DNA forms (Figure 7).
Figure 7.
Model of Pif1 binding to a variety of DNA substrates. (A) Pif1 binds with moderate affinity to ssDNA, requiring 6–8 nt for efficient binding. The black arrows indicate potential Pif1 binding sites on the ssDNA portions of various substrates. The green arrows represent Pif1 monomers oriented in the 5'-3' direction on the substrates. (B) Pif1 binding to short gapped sequences is influenced by dsDNA 5' to the bound Pif1, allowing stable binding to shorter regions of ssDNA. (C) Nonloading strand (i.e., 3' strand) contributes to binding and unwinding of fork DNA substrates. (D) Highly structured DNA, such as G4 DNA, binds Pif1 with high affinity. On the loading strand, this would reduce unwinding due to high affinity trapping. On the nonloading strand, Pif1 would be sequestered in an orientation directed away from the fork junction.
With this model, Pif1 residence time at structured DNA would be longer, even as unwinding events are occurring, due to high affinity binding. This would favor dissociation of a nonprocessive helicase like Pif1 from unstructured DNA while favoring reassociation with DNA structures that could impede replication and repair. One issue with this model is that structured DNA can occur on either strand, leading to Pif1 binding on the 3'-strand such that it translocates away from a dsDNA junction. Biologically, this could have significant importance, particularly during lagging strand synthesis at telomeres where Pif1 plays important roles in replication, repair, telomere length control, and Okazaki fragment maturation.4,10,24,36 In the case of telomeres, telomere lengthening of the G-strand and completion of C-strand replication are highly coordinated, and Pif1 helicase activity is required on both strands to avoid telomere loss.7,58 It also has implications for the view that Pif1 binding is favored at ssDNA/dsDNA junctions, as mimicked with fork substrates.59,60 If binding at dsDNA junctions is favored, then Pif1 will most likely bind to ssDNA at a junction adjacent to structured DNA rather than at a standard ssDNA/dsDNA junction.
G4 DNA presents many complications to the interpretation of Pif1 unwinding data due to the wide range of possible structures the G4 motifs can adopt.35,45 Inhibition of Pif1 unwinding activity with G4-containing substrates has been reported, while others have reported stimulation of Pif1 unwinding in the presence of G4 structures.34,42–44 While this debate is beyond the scope of this work, the loading strand of our hTelG/poly(dT) fork is predicted to fold into a G4 structure with a 3 nt 5'-leader sequence, which would be expected to be unwound because there is no requirement for a leader sequence for G4 DNA unwinding in the presence of Na+ ions.27,28 Unlike some previous reports, there is no gap between the predicted G4 structure and the dsDNA duplex portion of this substrate. Despite this, Pif1 was still able to unwind this fork, though less efficiently than the yTelG/poly(dT) fork substrate. S. cerevisiae telomeres may adopt an alternate higher order structure that Pif1 is better adapted to and that is different from the G4 structures formed by human telomeric ssDNA. Regardless of the higher-order structure, these data support a model where Pif1 will preferentially bind to telomeres, rDNA, and G-rich DSBs due to its high affinity for G-rich ssDNA.61
A critical focus of this work was the role of the length of ssDNA loading strands of potential Pif1 substrates. Efficient unwinding of immobilized gapped forks by Pif1 requires a ssDNA gap size of >5 nt in a unique set of single-molecule experiments using Brownian motion for detection of unwinding.38 Even gaps of 3 nt supported 40% unwinding in these assays, while nicked (0 nt gap) substrates were not unwound. Here, we showed that a 50 nt poly(dG) ssDNA that is predicted to form a G4 structure with no 5'-ssDNA leader fails to stimulate Pif1 ATPase activity. Therefore, some G4 structures such as this that fail to stimulate Pif1 ATP hydrolysis are unlikely to be unwound by Pif1 helicase activity alone.62 Slower unwinding of human telomeric sequence forks compared to fork duplexes has also been reported but with a 3 nt gap between the G4 DNA and the DNA duplex region to stabilize the G4 structure.34 Here, we found that S. cerevisiae Pif1 was able to unwind a fork substrate with a hTelG loading strand, which is predicted to fold into a G4 structure with a 3 nt 5'-leader and with no gap between the G4 DNA and the DNA duplex, though with lower efficiency than the other fork substrates tested (Figure S3).
We also tested the effects of changing the length or sequence of both ssDNA and the ssDNA portions of fork substrates on Pif1 ATPase and helicase activities. In both types of assays, we found that sequence effects outweigh changes in the substrate length. In the literature, there is a debate concerning the role of telomere length in modifying the effects of Pif1 at S. cerevisiae telomeres.38–41,63,64 Telomere extension has been shown to increase at telomeres <125 nt in length.63 It has been proposed that this transition point is due to decreased processivity of Pif1 at telomeres <125 nt.64 Several groups report that Pif1 more efficiently binds to and unwinds longer telomeric sequences, both in vitro and in vivo with HO cleavage assays.38,39 Another group reports a transition at 34 bp of telomeric sequence where DNA ends become insensitive to the activity of Pif1, proposing a model involving Cdc13 for how cells recognize DSBs from telomeres.41 However, results from iSTEX assays indicate that Pif1 inhibits telomerase at all telomere lengths.40 While we did not address this directly, our data together suggest that Pif1 activity is stimulated by increasing the length of ssDNA loading strands from 10 to 30 nt, with only minor further increases with ssDNA lengths of 35–100 nt (Figure 2B).
How can we explain the inverse relationship between ssDNA with high Pif1 binding affinity and poorer unwinding of substrates containing such sequences? Careful biochemical analysis of Pif1 indicates that it hydrolyzes one ATP per nucleotide translocated or per basepair unwound.57 Given that this is the chemical step size for Pif1, then each basepair unwound would utilize the same amount of energy, despite the energetics of the particular structure being unwound. This implies that the energy from ATP hydrolysis is coupled to changes in Pif1 conformation, powering unwinding of DNA in excess of the amount of energy required to unwind any given substrate DNA. Therefore, differences in Pif1 unwinding of different substrates should be reflected by the increased time rather than the increased energy demand for ATP.
Recent studies of bacterial and truncated eukaryotic Pif1 helicases can lend support to this interpretation of Pif1 unwinding.35 Crystal structures of human PIF1 reveal positional and conformational changes in highly conserved protein motifs upon ATP binding, increasing the affinity for ssDNA and flexibility in the ssDNA binding domain.65 The 2B domain of Thermus oshimai Pif1 undergoes repetitive transitions from a closed to open confirmation upon DNA unwinding.66 A 1 bp step mechanism has been proposed for Bacteroides sp. Pif1, where dimers interact with ssDNA/dsDNA junctions, creating a sharp bend at the 5'-end of bound DNA.60 The authors propose a model where each monomer is active, with the loading strand Pif1 monomer breaking the first basepair and the Pif1 monomer on the 3'-strand stabilizing the unwound basepair and preparing to engage the next basepair. This mechanism would require ATP hydrolysis by the loading strand Pif1 monomer, leading to an active conformational change causing a change that is transmitted to the second monomer and leading to a disruption of the subsequent basepair.
These data are consistent with a model in which the rate of protein conformational changes within Pif1 following substrate binding controls the DNA unwinding rate. Energetically stable DNA structures would lead to slower rates of protein transition and, therefore, slower rates of observed DNA unwinding. Coupling between the ATPase status of the E. coli replicative helicase DnaB to recognition of DnaC and ssDNA substrates has been reported to affect intermolecular contacts between proteins (Puri et al., 2021). We propose a working model in which Pif1 independently binds to any ssDNA of sufficient length, but in the context of a fork, high-affinity G-rich (and likely structured) DNA in the loading or nonloading strand can interfere with DNA unwinding (Figure 7). For example, Pif1 could bind tightly to G4 DNA and would require additional time to move through this DNA compared to an unstructured linear ssDNA of equal length. This model also suggests that the high affinity of Pif1 for ssDNA/dsDNA junctions is due to stabilization of an initially bound Pif1 monomer on the loading strand by the formation of a Pif1 dimer upon binding of the second monomer to the second strand of ssDNA if present, as in a fork. The patrolling mode of Pif1 bound at the junction on the nonloading strand is consistent with this model of Pif1 DNA binding. Initial Pif1 binding could take place on either ssDNA strand at the fork junction, with subsequent dimerization occurring by Pif1 binding to the opposite strand, leading to an allosteric binding event.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Bochman lab for thoughtful comments on this manuscript. We also acknowledge the Physical Biochemistry Instrumentation Facility at Indiana University Biochemistry for use of equipment.
Funding
This work was funded by grants from the National Health Institutes (R35GM133437) and the American Cancer Society (RSG-16-180-01-DMC) to M.L.B.
ABBREVIATIONS
- SF1B
superfamily 1B
- ssDNA
single-stranded DNA
- dsDNA
double-stranded DNA
- DSB
double-strand break
- G4
G-quadruplex
- EMSA
electrophoretic mobility shift assay
- yTel30G
yeast telomeric G-strand 30mer
- yTel30C
yeast telomeric C-strand 30mer
- Ran30
random-sequence 30mer
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.biochem.1c00614.
Oligonucleotides used in this study; oligonucleotide pairs annealed to form substrates; KM and t1/2 values for DNA unwinding reactions; and ATP hydrolysis plots (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.biochem.1c00614
The authors declare no competing financial interest.
UniProt accession ID for Saccharomyces cerevisiae Pifl:P07271.
REFERENCES
- (1).Lahaye A; Stahl H; Thines-Sempoux D; Foury F PIF1: a DNA helicase in yeast mitochondria. EMBO J. 1991, 10, 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Berger JM SnapShot: nucleic acid helicases and translocases. Cell 2008, 134, 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Bochman ML; Judge CP; Zakian VA The Pif1 family in prokaryotes: what are our helicases doing in your bacteria? Mol. Biol. Cell 2011, 22, 1955–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Budd ME; Reis CC; Smith S; Myung K; Campbell JL Evidence Suggesting that Pif1 Helicase Functions in DNA Replication with the Dna2 Helicase/Nuclease and DNA Polymerase δ. Mol. Cell. Biol. 2006, 26, 2490–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Buzovetsky O; Kwon Y; Pham NT; Kim C; Ira G; Sung P; Xiong Y Role of the Pif1-PCNA Complex in Pol δ-Dependent Strand Displacement DNA Synthesis and Break-Induced Replication. Cell Rep. 2017, 21, 1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Dahan D; Tsirkas I; Dovrat D; Sparks MA; Singh SP; Galletto R; Aharoni A Pif1 is essential for efficient replisome progression through lagging strand G-quadruplex DNA secondary structures. Nucleic Acids Res. 2018, 46, 11847–11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Rossi ML; Pike JE; Wang W; Burgers PMJ; Campbell JL; Bambara RA Pif1 helicase directs eukaryotic Okazaki fragments toward the two-nuclease cleavage pathway for primer removal. J. Biol. Chem. 2008, 283, 27483–27493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Pike JE; Burgers PMJ; Campbell JL; Bambara RA Pif1 helicase lengthens some Okazaki fragment flaps necessitating Dna2 nuclease/helicase action in the two-nuclease processing pathway. J. Biol. Chem. 2009, 284, 25170–25180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Wilson MA; Kwon Y; Xu Y; Chung W-H; Chi P; Niu H; Mayle R; Chen X; Malkova A; Sung P; Ira G Pif1 helicase and Polδ promote recombination-coupled DNA synthesis via bubble migration. Nature 2013, 502, 393–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Vasianovich Y; Harrington LA; Makovets S Break-induced replication requires DNA damage-induced phosphorylation of Pif1 and leads to telomere lengthening. PLoS Genet. 2014, 10, No. e1004679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Dewar JM; Lydall D Pif1- and Exo1-dependent nucleases coordinate checkpoint activation following telomere uncapping. EMBO J. 2010, 29, 4020–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Budd ME; Campbell JL Dna2 is involved in CA strand resection and nascent lagging strand completion at native yeast telomeres. J. Biol. Chem. 2013, 288, 29414–29429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Jimeno S; Camarillo R; Mejías-Navarro F; Fernández-Ávila MJ; Soria-Bretones I; Prados-Carvajal R; Huertas P The Helicase PIF1 Facilitates Resection over Sequences Prone to Forming G4 Structures. Cell Rep. 2018, 25, 3543. [DOI] [PubMed] [Google Scholar]
- (14).Chen C-F; Pohl TJ; Pott S; Zakian VA Two Pif1 Family DNA Helicases Cooperate in Centromere Replication and Segregation in Saccharomyces cerevisiae. Genetics 2019, 211, 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Foury F; Lahaye A Cloning and sequencing of the PIF gene involved in repair and recombination of yeast mitochondrial DNA. EMBO J. 1987, 6, 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Bochman ML Roles of DNA helicases in the maintenance of genome integrity. Mol. Cell. Oncol. 2014, 1, No. e963429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Koc KN; Singh SP; Stodola JL; Burgers PM; Galletto R Pif1 removes a Rap1-dependent barrier to the strand displacement activity of DNA polymerase δ. Nucleic Acids Res. 2016, 44, 3811–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Schauer GD; Spenkelink LM; Lewis JS; Yurieva O; Mueller SH; van Oijen AM; O’Donnell ME Replisome bypass of a protein-based R-loop block by Pif1. Mol. Cell. Oncol. 2020, 117, 30354–30361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Schulz VP; Zakian VA The Saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell 1994, 76, 145–155. [DOI] [PubMed] [Google Scholar]
- (20).Zhou J-Q; Monson EK; Teng S-C; Schulz VP; Zakian VA Pif1p Helicase, a Catalytic Inhibitor of Telomerase in Yeast. Science 2000, 289, 771–774. [DOI] [PubMed] [Google Scholar]
- (21).Nickens DG; Rogers CM; Bochman ML The Saccharomyces cerevisiae Hrq1 and Pif1 DNA helicases synergistically modulate telomerase activity in vitro. J. Biol. Chem. 2018, 293, 14481–14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Boule J-B; Zakian VA The yeast Pif1p DNA helicase preferentially unwinds RNA DNA substrates. Nucleic Acids Res. 2007, 35, 5809–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Chib S; Byrd AK; Raney KD Yeast Helicase Pif1 Unwinds RNA:DNA Hybrids with Higher Processivity than DNA:DNA Duplexes. J. Biol. Chem. 2016, 291, 5889–5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Boulé J-B; Vega LR; Zakian VA The yeast Pif1p helicase removes telomerase from telomeric DNA. Nature 2005, 438,57–61. [DOI] [PubMed] [Google Scholar]
- (25).Zheng K.-w.; Xiao S; Liu J.-q.; Zhang J.-y.; Hao Y.-h.; Tan Z Co-transcriptional formation of DNA:RNA hybrid G-quadruplex and potential function as constitutional cis element for transcription control. Nucleic Acids Res. 2013, 41, 5533–5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Galletto R; Tomko EJ Translocation of Saccharomyces cerevisiae Pif1 helicase monomers on single-stranded DNA. Nucleic Acids Res. 2013, 41, 4613–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Barranco-Medina S; Galletto R DNA binding induces dimerization of Saccharomyces cerevisiae Pif1. Biochemistry 2010, 49, 8445–8454. [DOI] [PubMed] [Google Scholar]
- (28).Zhou R; Zhang J; Bochman ML; Zakian VA; Ha T Periodic DNA patrolling underlies diverse functions of Pif1 on R-loops and G-rich DNA. Elife 2014, 3, No. e02190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Lu C; Le S; Chen J; Byrd AK; Rhodes D; Raney KD; Yan J Direct quantification of the translocation activities of Saccharomyces cerevisiae Pif1 helicase. Nucleic Acids Res. 2019, 47, 7494–7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Bochman ML; Sabouri N; Zakian VA Unwinding the functions of the Pif1 family helicases. DNA Repair 2010, 9, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Lahaye A; Leterme S; Foury F PIF1 DNA helicase from Saccharomyces cerevisiae. Biochemical characterization of the enzyme. J. Biol. Chem. 1993, 268, 26155–26161. [PubMed] [Google Scholar]
- (32).Bochman ML; Paeschke K; Zakian VA DNA secondary structures: stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012, 13, 770–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Paeschke K; Bochman ML; Garcia PD; Cejka P; Friedman KL; Kowalczykowski SC; Zakian VA Pif1 family helicases suppress genome instability at G-quadruplex motifs. Nature 2013, 497, 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Byrd AK; Bell MR; Raney KD Pif1 helicase unfolding of G-quadruplex DNA is highly dependent on sequence and reaction conditions. J. Biol. Chem. 2018, 293, 17792–17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Byrd AK; Raney KD Structure and function of Pif1 helicase. Biochem. Soc. Trans. 2017, 45, 1159–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Singh SP; Koc KN; Stodola JL; Galletto R A Monomer of Pif1 Unwinds Double-Stranded DNA and It Is Regulated by the Nature of the Non-Translocating Strand at the 3'-End. J. Mol. Biol. 2016, 428, 1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Nickens DG; Sausen CW; Bochman ML The Biochemical Activities of the Saccharomyces cerevisiae Pif1 Helicase Are Regulated by Its N-Terminal Domain. Genes 2019, 10, 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Li J-R; Yu T-Y; Chien I-C; Lu C-Y; Lin J-J; Li H-W Pif1 regulates telomere length by preferentially removing telomerase from long telomere ends. Nucleic Acids Res. 2014, 42, 8527–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Phillips JA; Chan A; Paeschke K; Zakian VA The pif1 helicase, a negative regulator of telomerase, acts preferentially at long telomeres. PLoS Genet. 2015, 11, No. e1005186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Stinus S; Paeschke K; Chang M Telomerase regulation by the Pif1 helicase: a length-dependent effect? Curr. Genet. 2018, 64, 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Strecker J; Stinus S; Caballero MP; Szilard RK; Chang M; Durocher D A sharp Pif1-dependent threshold separates DNA double-strand breaks from critically short telomeres. Elife 2017, 6, No. e23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Byrd AK; Raney KD A parallel quadruplex DNA is bound tightly but unfolded slowly by pif1 helicase. J. Biol. Chem. 2015, 290, 6482–6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Duan X-L; Liu N-N; Yang Y-T; Li H-H; Li M; Dou SX; Xi X-G G-quadruplexes significantly stimulate Pif1 helicase-catalyzed duplex DNA unwinding. J. Biol. Chem. 2015, 290, 7722–7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Zhang B; Wu W-Q; Liu N-N; Duan X-L; Li M; Dou SX; Hou X-M; Xi X-G G-quadruplex and G-rich sequence stimulate Pif1p-catalyzed downstream duplex DNA unwinding through reducing waiting time at ss/dsDNA junction. Nucleic Acids Res. 2016, 44, 8385–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Mendoza O; Bourdoncle A; Boulé J-B; Brosh RM Jr.; Mergny J-L G-quadruplexes and helicases. Nucleic Acids Res. 2016, 44, 1989–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Ribeyre C; Lopes J; Boulé J-B; Piazza A; Guédin A; Zakian VA; Mergny J-L; Nicolas A The yeast Pif1 helicase prevents genomic instability caused by G-quadruplex-forming CEB1 sequences in vivo. PLoS Genet. 2009, 5, No. e1000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Jurikova K; Gajarsky M; Hajikazemi M; Nosek J; Prochazkova K; Paeschke K; Trantirek L; Tomaska L Role of folding kinetics of secondary structures in telomeric G-overhangs in the regulation of telomere maintenance in Saccharomyces cerevisiae. J. Biol. Chem. 2020, 295, 8958–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Ramanagoudr-Bhojappa R; Blair LP; Tackett AJ; Raney KD Physical and functional interaction between yeast Pif1 helicase and Rim1 single-stranded DNA binding protein. Nucleic Acids Res. 2013, 41, 1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Ray S; Tillo D; Boer RE; Assad N; Barshai M; Wu G; Orenstein Y; Yang D; Schneekloth JS Jr.; Vinson C Custom DNA Microarrays Reveal Diverse Binding Preferences of Proteins and Small Molecules to Thousands of G-Quadruplexes. ACS Chem. Biol. 2020, 15, 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Zhang M-L; Tong X-J; Fu X-H; Zhou BO; Wang J; Liao X-H; Li Q-J; Shen N; Ding J; Zhou J-Q Yeast telomerase subunit Est1p has guanine quadruplex-promoting activity that is required for telomere elongation. Nat. Struct. Mol. Biol. 2010, 17, 202–209. [DOI] [PubMed] [Google Scholar]
- (51).Liu S-W; Chu J-F; Tsai C-T; Fang H-C; Chang T-C; Li H-W Assaying the binding strength of G-quadruplex ligands using single-molecule TPM experiments. Anal. Biochem. 2013, 436, 101–108. [DOI] [PubMed] [Google Scholar]
- (52).Li Q-J; Tong X-J; Duan Y-M; Zhou J-Q Characterization of the intramolecular G-quadruplex promoting activity of Est1. FEBS Lett. 2013, 587, 659–665. [DOI] [PubMed] [Google Scholar]
- (53).Zuker M Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Kikin O; D’Antonio L; Bagga PS QGRS Mapper: a web-based server for predicting G-quadruplexes in nucleotide sequences. Nucleic Acids Res. 2006, 34, W676–W682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Li J-H; Lin W-X; Zhang B; Nong D-G; Ju H-P; Ma JB; Xu C-H; Ye F-F; Xi XG; Li M; Lu Y; Dou S-X Pif1 is a force-regulated helicase. Nucleic Acids Res. 2016, 44, 4330–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Lu K-Y; Chen W-F; Rety S; Liu N-N; Wu W-Q; Dai Y-X; Li D; Ma H-Y; Dou S-X; Xi X-G Insights into the structural and mechanistic basis of multifunctional S. cerevisiae Pif1p helicase. Nucleic Acids Res. 2018, 46, 1486–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Ramanagoudr-Bhojappa R; Chib S; By rd, Aarattuthodiyil S; Pandey M; Patel SS; Raney KD Yeast Pif1 helicase exhibits a one-base-pair stepping mechanism for unwinding duplex DNA. J. Biol. Chem. 2013, 288, 16185–16195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Vega L; Phillips J; BR T; DP T; Onigbanjo M; Zakian V Sensitivity of yeast strains with long G-tails to levels of telomerebound telomerase. PLoS Genet. 2007, 3, No. e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Zhou X; Ren W; Bharath SR; Tang X; He Y; Chen C; Liu Z; Li D; Song H Structural and Functional Insights into the Unwinding Mechanism of Bacteroides sp Pif1. Cell Rep. 2016, 14, 2030–2039. [DOI] [PubMed] [Google Scholar]
- (60).Su N; Byrd AK; Bharath SR; Yang O; Jia Y; Tang X; Ha T; Raney KD; Song H Structural basis for DNA unwinding at forked dsDNA by two coordinating Pif1 helicases. Nat. Commun 2019, 10, 5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Hoerr RE; Ngo K; Friedman KL When the Ends Justify the Means: Regulation of Telomere Addition at Double-Strand Breaks in Yeast. Front. Cell Dev. Biol. 2021, 9, 655377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Maestroni L; Audry J; Luciano P; Coulon S; Géli V; Corda Y RPA and Pif1 cooperate to remove G-rich structures at both leading and lagging strand. Cell Stress 2020, 4,48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Teixeira MT; Arneric M; Sperisen P; Lingner J Telomere length homeostasis is achieved via a switch between telomerase-extendible and -nonextendible states. Cell 2004, 117, 323–335. [DOI] [PubMed] [Google Scholar]
- (64).Chang M; Arneric M; Lingner J Telomerase repeat addition processivity is increased at critically short telomeres in a Tel1-dependent manner in Saccharomyces cerevisiae. Genes Dev. 2007, 21, 2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Dehghani-Tafti S; Levdikov V; Antson AA; Bax B; Sanders CM Structural and functional analysis of the nucleotide and DNA binding activities of the human PIF1 helicase. Nucleic Acids Res. 2019, 47, 3208–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Dai Y-X; Chen W-F; Liu N-N; Teng F-Y; Guo H-L; Hou X-M; Dou S-X; Rety S; Xi X-G Structural and functional studies of SF1B Pif1 from Thermus oshimai reveal dimerization-induced helicase inhibition. Nucleic Acids Res. 2021, 49, 4129–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.