Abstract
Aims
The arrival of anti-vascular endothelial growth factor (anti-VEGF) therapies represented a treatment shift for several ophthalmological disorders and led to an increasing number of patients undergoing intravitreal injections. The aims of this observational study were to assess the expansion of anti-VEGF intravitreal injections in the Portuguese National Health System (NHS) and to identify factors correlated with geographical variations in episode rates.
Methods
Administrative database on discharge from Portuguese NHS hospitals was analysed for annual values and rates of intravitreal anti-VEGF injections at a national and regional level, between 2013 and 2018.
Results
The number of episodes of anti-VEGF treatment and patients treated increased 16% and 9% per year, respectively, between 2013 and 2018. During the study period around 72% of patients were treated in the Metropolitan areas of Lisbon and Porto and in the Central region. Intravitreal anti-VEGF treatment rates in 2018 were 560 per 100 000 population and presented high variability between municipalities. Higher anti-VEGF treatment rates at the municipality level were associated with shorter distances between their residence and the hospital. At the hospital level, higher ratio of ophthalmologists and higher organisational level were associated with higher anti-VEGF treatment rates.
Conclusion
The number of episodes and patients treated with anti-VEGF injections has been growing in recent years. Proximity to healthcare, more access to ophthalmologists and hospitals with higher organisational levels are associated with higher anti-VEGF treatment rates. Improving access is crucial to reduce regional discrepancies and ensure optimal treatment frequency, which may improve health outcomes.
Keywords: ophthalmology, public health, medical retina
Strengths and limitations of this study.
This is an administrative database study using the universe of inpatient and day cases stays of National Health System (NHS) hospitals in Portugal between 2013 and 2018.
For the characterisation of anti-vascular endothelial growth factor (anti-VEGF) intravitreal injections, a selection of surgical codes (International Classification of Diseases (ICD) ninth version-Clinical Modification and ICD 10th version) for intravitreal procedures was used as a proxy for intravitreal anti-VEGF injections.
Patient level data is available which, for example, makes it possible to analyse the real-world average number of injections per patient per year.
This administrative database gives us the universe of the Portuguese NHS but excludes the private setting.
Although clinical data are collected, this is not primarily a clinical database but an administrative database to inform financing of inpatient and day cases stays in NHS hospitals in Portugal.
Introduction
The availability of anti-vascular endothelial growth factor (anti-VEGF) therapies represented a treatment shift for a range of ophthalmological disorders, with a dramatic impact on serious conditions that were previously untreatable resulting in irreversible damages and loss of sight.1 2 Anti-VEGF intravitreal injections act by reducing neovascular progression and were initially approved for the treatment of neovascular age-related macular degeneration (nAMD).3 4 Currently, anti-VEGF therapies are indicated for the treatment of a vast number of other ocular diseases such as diabetic macular oedema (DME), choroidal neovascularisation (CNV) and retinal vein occlusion (RVO).2 Clinical trials have showed that anti-VEGF intravitreal injections prevented vision loss in the majority of patients and, in some cases, significantly improved vision.2 3 5 The positive impact of anti-VEGF injections in visual outcomes2 6–8 combined with the lack of previous efficient treatments, led to rapid diffusion of anti-VEGF treatments in many countries.4 6 9 10
The main barriers for treatment with anti-VEGF are the high costs of the drugs, the need for multiple treatments and the need for the treatments to be administered by specially trained personnel at hospitals.6 11 Access is hindered in countries such as the USA11 and in many Asian countries,6 where the drugs are not reimbursed by the health systems. Even in countries for which anti-VEGF treatments are reimbursed by the health system, such as England, Norway and Portugal, studies report considerable geographical variation in treatment rates.4 10 12 The study in Norway showed that the geographical variations in episode rates are challenges to the policy goals regarding equitable access and care, calling for further investigation.4 The study in Portugal indicated that the number of hospital episodes related with anti-VEGF injections increased from 1815 in 2001 to 25 106 in 2012, which is a mean annual increase of 32%.10
In Portugal, ranibizumab has been reimbursed by the National Health System (NHS) since 2008,10 and by 2018 bevacizumab and aflibercept were also reimbursed.13 Despite the equity-oriented nature of the Portuguese health system and the low copayment values, a study covering the 2002–2012 period found unequal geographical distribution in treatment rates across the country.10 Patients from regions without ophthalmology departments and lower population density received fewer treatments than other regions.10 More recent estimates on the diffusion of anti-VEGF intravitreal injections are needed to understand how this treatment has expanded with the existence of additional elective pharmaceuticals.
Understanding the trends in anti-VEGF treatments in terms of number of episodes and patients is of great importance for assessing health technologies. Assessing access to and impact of health technologies is paramount in investigating the number of episodes and patients treated. Periodic investigations about access to health technologies is vital to prevent health inequalities and to learn how to proceed if different technologies arise. The aim of this study was twofold: to analyse the expansion of anti-VEGF intravitreal injections in the Portuguese NHS between 2013 and 2018 and to identify factors associated with geographical variation in treatment rates.
Materials and methods
Data source and inclusion/exclusion criteria
This observational study used an administrative database on hospital discharges from public hospital institutions in mainland Portugal, which includes information about sex, age, municipality of residence, principal and secondary diagnosis and procedures, discharge hospital and a unique patients’ identifier from all inpatient and day case episodes. Use of this database was authorised for research purposes by the Portuguese Health System Central Administration (ACSS). The database is anonymised, guaranteeing the confidentiality of individuals, and it was therefore not necessary to obtain patients’ consent or approval by an ethics committee for this study.
Episodes related to intravitreal injections with anti-VEGF between 2013 and 2018 were selected according to procedures records coded with International Classification of Diseases (ICD) ninth version-Clinical Modification (ICD-9CM) and ICD 10th version (ICD-10) for episodes registered from 2017. As in previous studies, ICD-9CM procedures codes 1474, 1475, 1479 and 149 and ICD-10 procedures codes 3E0C30M and 3E0C3GC were used as proxy to anti-VEGF treatments.10 12 Note, however, that these codes might also capture intravitreal injections for other drugs such as injectable antibiotics or corticosteroids.10 12
Subsequently, the criteria for classification and exclusion of episodes were applied to assign a diagnosis for each episode. Episodes with missing data on sex, age, diagnosis and procedures and discharge hospitals were excluded. ICD-10 bilateral episodes were counted as two injections, while the number of patients was counted as one. The online supplemental appendix 1 contains details on the ICD codes used and the criteria to assign a diagnosis for each episode.
bmjopen-2021-055478supp001.pdf (147.3KB, pdf)
Data analysis
We examined the number of episodes and patients treated by year, by diagnosis and by region (according to patient’s municipality of residence). The number of patients treated per year was estimated using the unique patients’ identifier, regardless of whether they were already in treatment in the previous years or if they entered the database in that specific year. Then, using the patient as unit of observation, we computed the average number of injections per year for each diagnosis (nAMD, CNV, DME or RVO). Finally, we proceeded with the investigation of factors associated with geographical variations in anti-VEGF standardised treatment rates.
Statistical analysis was conducted to investigate factors associated with geographical variations in anti-VEGF standardised treatment rates. This ecological analysis was performed in two parts: the first had as unit of analysis the municipality of residence of the patient and in the second the unit of analysis was the hospital where the injection was performed. For analysis refinement, only patients aged 50 years or older were included in the analysis of associated factors, as the conditions for which anti-VEGF injections are indicated affects mostly people in this age category.2 12
For the ecological analysis at the municipality level the rate of episodes related to intravitreal injections with anti-VEGF treatments per 100 000 population was the dependent variable. The independent variables analysed were patients’ characteristics (mean age, proportion by sex, mean distance to hospital in kilometres—according to patient’s municipality of residence and municipality where the hospital is located), and municipalities’ characteristics (purchasing power, number of ophthalmologists per 20 000 persons and number of ophthalmology consultations per 1000 persons). The purchasing power variable is provided in relation to the national value, set equal to 100; and the purchasing power of the municipality can be a value above or below 100. The characteristics of the patients were retrieved from the hospital discharge database, and the characteristics of the municipality variables obtained from Statistics Portugal.14 The mean distance to the hospital was obtained through Google Maps, as these represent the distance to be travelled by patients. For the characteristics of patients, municipalities were separated into two categories for each year: ‘Higher rates’ category for the municipalities with episode rates higher than the median and ‘Lower rates’ category for the municipalities with episode rates lower than the median. The Mann-Whitney test was used to compare patients’ characteristics according to these two categories. For the characteristics of the municipalities, associations were analysed according to Spearman’s correlation analysis and multivariate linear regression models, with treatment rates as dependent variables and the independent variables (purchasing power, number of ophthalmologists per 20 000 persons and number of ophthalmology consultations per 1000 persons) added following the stepwise method.
For the ecological analysis at the hospital level, the dependent variable was the episode rates, and the independent variables were the number of ophthalmologists per 20 000 persons in the hospital’s catchment area and the organisational level of the hospital’s ophthalmology departments (hospitals’ ophthalmology units were divided into three groups, classified according to the general requirements established by the national network of hospital specialties and referral for ophthalmology,15 as shown in the online supplemental appendix 2). As these independent variables were not available per year, the years 2013–2018 were collapsed into a single period of analysis. The association with ophthalmologist specialists was analysed using Spearman’s correlation analysis. The Kruskal-Wallis test was used to compare the episode rate between the three groups of hospitals. Hospitals in group III have a wider range of healthcare activities, longer opening hours and greater equipment availability than hospitals in group II, and the same for group II in relation to group I hospitals. Data on number of ophthalmologists and more details on organisational level of hospitals by groups can be found in the report of the national network of hospital specialty and referral for ophthalmology.15
A 5% significance level was adopted. Statistical analysis was performed using the IBM SPSS Statistics V.26.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Evolution, characteristics and distribution of anti-VEGF treatments
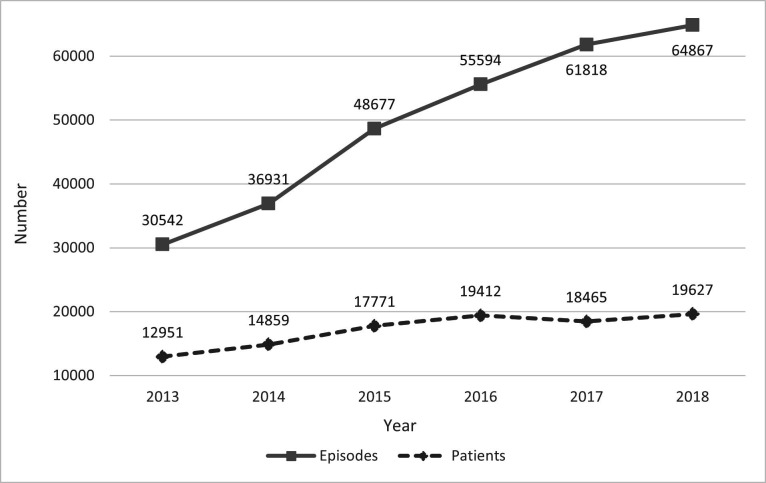
There were 298 429 episodes of anti-VEGF treatment between 2013 and 2018, and 65 534 patients treated. As illustrated in figure 1, the number of episodes increased from 30 542 in 2013 to 64 867 in 2018, which corresponds to a mean annual increase of 16%. The number of patients treated in 2013 was 12 951, growing to 19 627 in 2018 (mean annual increase of 9%). In 2018, the anti-VEGF standardised treatment rate was 560 per 100 000 persons.
Figure 1.
Number of hospital episodes of anti-vascular endothelial growth factor treatments and patients treated per year, from 2013 to 2018. Portugal.
The majority of patients (71%) were treated with intravitreal anti-VEGF in the Metropolitan area of Lisbon, Central region and Metropolitan area of Porto (table 1). The Algarve had the lowest proportion of patients treated between 2013 and 2018 (2.6%). If we assume a homogeneous prevalence of these diseases across the country, the proportion of the population can be used as a proxy as those who would qualify for anti-VEGF therapy treatments in each area. There are substantial differences in the proportion of resident population and the proportion of patients treated with anti-VEGF injections in the Metropolitan area of Porto and Algarve region. Online supplemental table S1 shows the proportion of patients treated with anti-VEGF injections, from 2013 and 2018, per region and per diagnosis (online supplemental appendix 3).
Table 1.
Proportion of patients treated with anti-vascular endothelial growth factor injections, between 2013 and 2018, per year, Portugal
| Region | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | Total | Proportion population 2018 |
| Alentejo | 6.32% | 6.46% | 6.74% | 7.51% | 6.88% | 7.54% | 7.53% | 7.21% |
| Algarve | 2.03% | 1.97% | 1.99% | 2.71% | 3.33% | 3.21% | 2.58% | 4.49% |
| Metropolitan area of Lisbon | 23.72% | 23.03% | 23.59% | 23.50% | 23.96% | 23.64% | 24.32% | 29.10% |
| Metropolitan area of Porto | 24.70% | 25.34% | 24.41% | 22.68% | 27.30% | 26.81% | 23.44% | 17.61% |
| Central region | 25.73% | 24.77% | 25.39% | 25.39% | 17.22% | 18.35% | 23.69% | 22.66% |
| Northern region | 17.50% | 18.44% | 17.88% | 18.20% | 21.31% | 20.46% | 18.43% | 18.92% |
As summarised in table 2, the most common diagnosis was nAMD, followed by DME and RVO. These three diagnoses accounted for 70% of episodes. nAMD was the most common condition in every year analysed, except 2016, when DME was the most common.
Table 2.
Total episodes of anti-vascular endothelial growth factor between 2013 and 2018, by diagnosis and year, Portugal
| Diagnosis | Total | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||||||||
| N | % | % cumulative | N | % | N | % | N | % | N | % | N | % | N | % | |
| Neovascular age-related macular degeneration | 100 168 | 33.57 | 33.57 | 11 575 | 37.90 | 13 415 | 36.32 | 16 357 | 33.60 | 16 094 | 28.95 | 20 857 | 33.74 | 2187 | 33.72 |
| Diabetic macular oedema | 85 997 | 28.82 | 62.38 | 6578 | 21.54 | 8044 | 21.78 | 13 371 | 27.47 | 18 181 | 32.70 | 19 769 | 31.98 | 20 054 | 30.92 |
| Retinal vein occlusion | 18 716 | 6.27 | 68.65 | 1451 | 4.75 | 2104 | 5.70 | 2841 | 5.84 | 3500 | 6.30 | 3956 | 6.40 | 4864 | 7.50 |
| Unspecified macular degeneration | 16 042 | 5.38 | 74.03 | 1750 | 5.73 | 1862 | 5.04 | 2712 | 5.57 | 3979 | 7.16 | 2724 | 4.41 | 3015 | 4.65 |
| Proliferative diabetic retinopathy | 15 737 | 5.27 | 79.30 | 1846 | 6.04 | 2297 | 6.22 | 2726 | 5.60 | 2144 | 3.86 | 3250 | 5.26 | 3474 | 5.36 |
| Choroidal neovascularisation | 13 783 | 4.62 | 83.92 | 1698 | 5.56 | 2190 | 5.93 | 2619 | 5.38 | 3040 | 5.47 | 2154 | 3.48 | 2082 | 3.21 |
| Retinal oedema | 12 581 | 4.22 | 88.14 | 1256 | 4.11 | 1890 | 5.12 | 1690 | 3.47 | 1677 | 3.02 | 2575 | 4.17 | 3493 | 5.38 |
| Other diagnosis | 35 405 | 11.86 | 100 | 4388 | 14.37 | 5129 | 13.89 | 6361 | 13.07 | 6979 | 12.55 | 6533 | 10.57 | 6015 | 9.27 |
| Total | 298 429 | 100 | 30 542 | 100 | 36 931 | 100 | 48 677 | 100 | 55 594 | 100 | 61 818 | 100 | 64 867 | 100 | |
Table 3 summarises the average increase in the number of injections per year per patient, by diagnosis. The highest number of injections per year per patient was for nAMD, which increased from 2.72 in 2013 to 3.37 in 2018. In contrast, CNV had the lowest values, reaching 2.01 injections per year per patient in 2018.
Table 3.
Average number of injections per year per patient, by diagnosis, 2013–2018, Portugal
| Diagnosis | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
| Neovascular age-related macular degeneration | 2.72 | 2.77 | 2.96 | 2.72 | 3.4 | 3.37 |
| Diabetic macular oedema | 2.33 | 2.32 | 2.64 | 2.88 | 2.77 | 2.80 |
| Choroidal neovascularisation | 1.35 | 1.43 | 1.41 | 1.51 | 2.06 | 2.01 |
| Retinal vein occlusion | 1.88 | 2.08 | 2.25 | 2.38 | 2.42 | 2.48 |
Factors associated with geographical distribution of anti-VEGF injections
Table 4 shows the comparison of characteristics of patients at the municipality level. In 2016, patients treated with anti-VEGF intravitreal injections who lived in municipalities with episode rates higher than the median (‘Higher rates’ category) were older. In 2013, municipalities in the ‘Higher’ category had a significantly higher proportion of women. For the distance between municipality of residence and hospital, significant differences were found for all years, with the average distance being shorter for municipalities in the ‘Higher’ category.
Table 4.
Mann-Whitney test for individual variables by municipality category
| Year | Age | Sex (proportion of men) | Distance in kilometres | |||||||||
| Mean (SD) | Mean (SD) | Mean (SD) | ||||||||||
| Lower rates | Higher rates | U | Signif.* | Lower rates | Higher rates | U | Signif. | Lower rates | Higher rates | U | Signif.* | |
| 2013 | 70.70 (4.64) | 71.43 (2.65) | 8737 | 0.168 | 0.511 (0.214) | 0.465 (0.130) | 8256* | 0.036 | 88.50 (50.25) | 46.13 (30.58) | 4187* | <0.001 |
| 2014 | 70.90 (4.50) | 71.02 (2.64) | 9466 | 0.772 | 0.499 (0.198) | 0.486 (0.121) | 9025 | 0.343 | 84.11 (52.25) | 46.08 (32.22) | 4835* | <0.001 |
| 2015 | 70.62 (4.07) | 71.35 (2.92) | 8553 | 0.098 | 0.519 (0.179) | 0.486 (0.110) | 8484 | 0.079 | 81.04 (51.11) | 42.62 (25.65) | 4701* | <0.001 |
| 2016 | 70.58 (3.71) | 71.61 (2.62) | 7656* | 0.004 | 0.500 (0.169) | 0.503 (0.099) | 9218 | 0.576 | 73.52 (49.44) | 40.99 (28.36) | 5098* | <0.001 |
| 2017 | 72.30 (5.37) | 71.66 (2.71) | 7826 | 0.135 | 0.480 (0.244) | 0.511 (0.127) | 7989 | 0.218 | 69.69 (53.74) | 41.89 (32.51) | 6238* | <0.001 |
| 2018 | 72.26 (4.70) | 72.02 (2.56) | 8553 | 0.449 | 0.523 (0.233) | 0.484 (0.107) | 8246 | 0.216 | 82.88 (72.94) | 66.42 (65.37) | 7586* | 0.002 |
*Significant difference by Mann-Whitney U-test between categories of municipalities (p<0.05)
In the bivariate correlation analysis of the rate of anti-VEGF treatments with the independent ecological variables, a positive correlation was found for: purchasing power in the years 2016 (p value<0.001) and 2018 (p value<0.001); rate of ophthalmologists in 2015 (p value=0.042) and 2016 (p value=0.016); ophthalmology consultations in all hospitals in 2013 (p value=0.047) and 2016 (p value=0.018), and consultations in public hospitals in 2013 (p value=0.040) and in 2016 (p value=0.030) (online supplemental table S2).
Stepwise linear regression models were generated for each year. Between 2013 and 2015 the variable ophthalmology consultations was included with a positive coefficient. For 2016–2018, the variable that remained in the model was purchasing power, with a positive coefficient. The models had low adjusted R2 (the highest was 0.043 in 2018) and the analysis of residues was inconclusive regarding the quality of the models. (online supplemental table S3).
In the ecological analysis at the hospital level, the bivariate Spearman’s correlation between the rate of anti-VEGF treatments between 2013 and 2018 and the ratio of ophthalmologists had a positive correlation (ρ=0359; n=40; p value=0.023). The Kruskal-Wallis test showed a statistically significant difference in episode rates with anti-VEGF according to the hospital’s organisational level (H(2) = 7.054; p value=0.029). More specifically, the results indicate that hospitals in group III had a higher episode rate than hospitals in group II. These, in turn, had higher episode rates than group I hospitals.
Discussion
The aim of this study was to analyse the expansion of anti-VEGF intravitreal treatments in the Portuguese NHS and to identify factors associated with geographical variations. Results indicate that access to treatment with anti-VEGF injection has been increasing in Portugal, and that they were first used to treat nAMD, followed by DME, CNV and RVO. An increase in the number of injections per patient per year was observed for all diagnoses. More than half of the episodes with anti-VEGF were recorded in the metropolitan areas of Lisbon and Porto.
Given the positive impact of anti-VEGF injections on health outcomes for many ocular neovascular diseases, the expansion in injections performed and patients treated seems justified. The evolution of anti-VEGF treatments found from 2013 to 2018 was consistent with values reported by Marques et al10 from 2002 to 2012. The total number of injections per year in Portugal varied from less than 2000 to over 60 000 in 16 years. As anti-VEGF injections are covered by the Portuguese NHS10 13 16 and are safe and highly effective,17 there are reasons to expect that this upward tendency will continue to be observed in the coming years.
Neovascular AMD and DME diagnosis corresponded to 63% of episodes associated with anti-VEGF treatment between 2013 and 2018. An analysis of the literature revealed that AMD was the eye pathology most often addressed in scientific publications between 2013 and 2018,18 and it was the most common condition for which anti-VEGF intravitreal injections were used in countries like England,12 Norway4 and the USA.19
The number of injections per year per patient for nAMD increased within the period analysed, reaching 3.37 injections per year in 2018. The on-label treatment guidelines for treatment of nAMD for both ranibizumab and aflibercept supported monthly injections in the first 3 months followed by treat and extend regimen (flexible, according to the needs of the patient).20 21 Therefore, in a first year of treatment, it would correspond to between 6 and 12 injections (due to loading dose), while in the second year and thereafter it would correspond to between 4 and 12 injections. Although there was no information on which drug was used to treat the patients analysed, the values of the on-label standards are greater than what was observed in this study. This low frequency of injections per year was also found in Portugal before 2013,10 England (2.7 in 2008)12 and Norway (4.1 in 2015).4 On the one hand, these results may indicate difficulties to access the treatment, leaving patients undertreated.22–25 On the other hand, some clinical studies indicate that variable frequency of anti-VEGF injections is also effective in the treatment of nAMD, and therefore this flexible regimen may have been increasingly adopted.1 26
The geographical variations in episode rates in Portugal observed between 2002 and 2012 were associated with the availability of anti-VEGF therapies and ophthalmology services, as well as population density.10 These results indicate that patients from distant cities or rural areas may have delayed access to treatments and were more likely to miss follow-up appointments.10 The findings for the period from 2013 to 2018 corroborate this possibility, as the distance between municipality of residence and hospital was significantly different between municipalities with higher and lower episode rates. A systematic review of factors associated with non-adherence to anti-VEGF treatment has also identified greater distance to hospital as a potential contributing factor.27 Lower numbers of ophthalmologist and consultations were also associated with lower episode rates.
Similar results were found in Norway4 and England.12 National rates of intravitreal injections in England had a 50-fold variation in age-standardised rates between regions.12 In Norway, the age adjusted number of episodes across counties varied from 19 to 55 per 1000 persons aged 50 years or older.4 These studies demonstrated challenges associated with the arrival of this treatment that include frequent and long-term administration and high allocation of resources. Despite the effort to guarantee geographical equity of access afforded by the health systems in England, Norway and Portugal, the variations in anti-VEGF rates indicate that challenges remain.
Because anti-VEGF drugs are injected directly into the vitreous body, there are requirements for use of this treatment that can include specialised training and the setting up of a location dedicated to injection.28 These requirements might be difficult to achieve in small hospitals due to financial or technical limitations.10 The results showed significant differences in anti-VEGF treatment rates between hospitals, according to the number of specialists and their organisational level.
The present study has found that despite the considerable expansion of anti-VEGF treatments between 2013 and 2018 in Portugal, geographical variations still remain. Substantial treatment coverage discrepancies may be observed among regions, if we assume that prevalence does not change across the Portuguese territory and if we compare the percentages of residents, at the same age group, and the percentages of patients treated with an anti-VEGF in each region. In a previous study,10 it was shown that people in the rural areas were receiving less treatments. It is possible to speculate that the needs for treatments are likely to be similar in urban and rural areas. Although the methodology chosen did not produce robust evidence to accurately identify the reasons behind these variations, there are strong indications that barriers previously discussed by Marques et al10 and also observed in England12 and Norway4 are possibly a root cause, and in any event remain a challenge.
Strengths of this study reside in the use of nationwide information and long period of analysis. The geographical and temporal analysis performed produced important results to monitor the diffusion of anti-VEGF treatments in Portugal, while raising awareness of persisting inequalities. The statistical methods employed allowed the identification of factors that should be addressed to ensure the treatment of patients with ophthalmological needs. However, there are also limitations associated with its use that are important to mention. The procedures and ICD codes were used as a proxy to identify episodes with anti-VEGF and the associated diagnosis, since there are no further details about the intravitreal injection such as the drugs used in each episode. Thus, it is possible that in some cases anti-VEGF have not been administered, overestimating the findings reported herein. Additionally, the administrative database used is not primarily a clinical database. Clinical data are collected to inform financing of inpatient and day cases stays in NHS hospitals in Portugal, thus procedures carried out in the autonomous regions of Azores and Madeira are excluded. The database does not comprise episodes of intravitreal anti-VEGF injected at the private setting. There is also no available information for other relevant clinical data (eg, smoking behaviour, cardiovascular diseases and previous cardiovascular events, blood pressure, cholesterol and medication use). Future studies may collect more accurate information on episodes to ensure correspondence to anti-VEGF intravitreal injections and clinical characteristics of patients. At the time of analysis, data for 2017 and 2018 were provisional, as two hospitals had under-reported information.
Conclusion
The development of anti-VEGF drugs has brought effective treatment for retinal diseases that can lead to severe visual impairment. This study shows that the number of episodes related to anti-VEGF treatment as well as the number of treated patients increased between 2013 and 2018. However, the distribution of treatment with anti-VEGF showed regional asymmetries. Factors such as proximity to healthcare, greater access to ophthalmologists and hospitals having ophthalmological departments with more human resources, more equipment and higher differentiation level were associated with higher rates of anti-VEGF treatment. Improving access to treatment is crucial to address the regional discrepancies found and to ensure that treatment follows patients’ clinical needs and enhances better health outcomes. The increasing number of treatment episodes related to anti-VEGF, the low number of injections per patient per year and the regional discrepancies detected impose challenges to the NHS in terms of budget and access. Given the ageing of the population and the fact that more anti-VEGF drugs have been developed and approved, both demand and supply of these treatments are likely to increase.
Supplementary Material
Acknowledgments
We acknowledge the Central Administration of the Health System for providing the hospital morbidity database.
Footnotes
Twitter: @V-9571-2017
Contributors: APM, MA-S, PL and RS conceived and designed the study. JVR and APM had full access to the data and conducted initial analysis. JVR, APM, MA-S, ASA and JF conducted the analysis and interpreted the results. AFM and PL advised on interpretation of the results. JVR and MP drafted the manuscript. AFM, ASA and JF participated in the discussions and provided the clinical feedback. MA-S and RS provided critical feedback to the manuscript. JVR acts as the guarantor. All the authors revised the manuscript for important intellectual content, contributed to the data interpretation and writing and critically reviewed the manuscript at all stages and approved the final copy.
Funding: This analysis was funded by Novartis Farma, Produtos Farmacêuticos SA (no grant number).
Competing interests: MA-S, PL, ASA, JF and MP are employees of Novartis Farma, Produtos Farmacêuticos SA, Porto Salvo, Portugal, the funder the study. Novartis is the manufacturer of brolucizumab and ranibizumab.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data of hospitalisations are the property of Central Administration of the Health System (Administração Central do Sistema de Saúde (ACSS), I.P.). However the data are available from the authors upon request and with permission of the ACSS. The data of hospitalisations are not publicly available, however the authors confirm that interested researchers can ask for access to these data by contacting ACSS directly at the following: Parque da Saúde da Lisboa, Edifício 16, Avenida do Brasil, 53 1700-063 Lisboa, Portugal (e-mail: geral@acss.min-saude.pt).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Khanna S, Komati R, Eichenbaum DA, et al. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: a comparative review. BMJ Open Ophthalmol 2019;4:e000398. 10.1136/bmjophth-2019-000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tah V, Orlans HO, Hyer J, et al. Anti-Vegf therapy and the retina: an update. J Ophthalmol 2015;2015:1–13. 10.1155/2015/627674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim LS, Mitchell P, Seddon JM, et al. Age-Related macular degeneration. Lancet 2012;379:1728–38. 10.1016/S0140-6736(12)60282-7 [DOI] [PubMed] [Google Scholar]
- 4.Kristiansen IS, Haugli Bråten R, Jørstad Øystein Kalsnes, et al. Intravitreal therapy for retinal diseases in Norway 2011-2015. Acta Ophthalmol 2020;98:279–85. 10.1111/aos.14262 [DOI] [PubMed] [Google Scholar]
- 5.Gemenetzi M, Patel PJ. A systematic review of the treat and extend treatment regimen with anti-VEGF agents for neovascular age-related macular degeneration. Ophthalmol Ther 2017;6:79–92. 10.1007/s40123-017-0087-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai TYY, Cheung CMG, Mieler WF. Ophthalmic application of anti-VEGF therapy. Asia Pac J Ophthalmol 2017;6:479-480. 10.22608/APO.2017500 [DOI] [PubMed] [Google Scholar]
- 7.Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-Year outcomes in ranibizumab-treated patients in anchor, marina, and horizon: a multicenter cohort study (seven-up). Ophthalmology 2013;120:2292–9. 10.1016/j.ophtha.2013.03.046 [DOI] [PubMed] [Google Scholar]
- 8.Bressler NM, Chang TS, Suñer IJ, et al. Vision-related function after ranibizumab treatment by better- or worse-seeing eye: clinical trial results from marina and anchor. Ophthalmology 2010;117:747–56. 10.1016/j.ophtha.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 9.Stein JD, Hanrahan BW, Comer GM, et al. Diffusion of technologies for the care of older adults with exudative age-related macular degeneration. Am J Ophthalmol 2013;155:688–96. 10.1016/j.ajo.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marques AP, Macedo AF, Perelman J, et al. Diffusion of anti-VEGF injections in the Portuguese National health system. BMJ Open 2015;5:e009006. 10.1136/bmjopen-2015-009006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erie JC, Barkmeier AJ, Hodge DO, et al. High variation of intravitreal injection rates and Medicare anti-vascular endothelial growth factor payments per injection in the United States. Ophthalmology 2016;123:1257–62. 10.1016/j.ophtha.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 12.Keenan TDL, Wotton CJ, Goldacre MJ. Trends over time and geographical variation in rates of intravitreal injections in England. Br J Ophthalmol 2012;96:413–8. 10.1136/bjophthalmol-2011-300338 [DOI] [PubMed] [Google Scholar]
- 13.Administração Central do Sistema de Saúde, INFARMED, Serviços Partilhados do Ministério da Saúde . Circular informatiova conjunta No 8/2016/ACSS/INFARMED/SPMS [Internet], 2016. Available: http://www2.acss.min-saude.pt/Portals/0/Circularconjunta08__SPMS_ACSS_INFARMED(2).pdf [Accessed 03 Dec 2020].
- 14.Instituto Nacional de Estatística . Estatísticas- População e Sociedade- Saúde [Internet]. Available: https://www.ine.pt/xportal/xmain?xpgid=ine_tema&xpid=INE&tema_cod=1117 [Accessed 03 Jun 2020].
- 15.Serviço Nacional de Saúde . Rede nacional de especialidade hospitalar e de referenciação de oftalmologia [Internet], 2016. Available: https://www.sns.gov.pt/wp-content/uploads/2016/05/Proposta-RNEHR-Oftalmologia-2016-ACSS-1_VFinal.pdf
- 16.INFARMED . Relatório público de avaliação (BEOVU- Brolucizumab) [Internet], 2021. Available: https://www.infarmed.pt/documents/15786/1424140/Relatório+de+avaliação+de+financiamento+público+de+Beovu+%28DCI%3A+brolucizumab%29+2021/02da132e-8bf4-fb93-e744-4f64ed596470
- 17.Moisseiev E, Loewenstein A. Abicipar pegol-a novel anti-VEGF therapy with a long duration of action. Eye 2020;34:605–6. 10.1038/s41433-019-0584-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung AWK, Abdel-Daim MM, Abushouk AI, et al. A literature analysis on anti-vascular endothelial growth factor therapy (anti-VEGF) using a bibliometric approach. Naunyn Schmiedebergs Arch Pharmacol 2019;392:393–403. 10.1007/s00210-019-01629-y [DOI] [PubMed] [Google Scholar]
- 19.Parikh R, Ross JS, Sangaralingham LR, et al. Trends of anti-vascular endothelial growth factor use in ophthalmology among privately insured and Medicare advantage patients. Ophthalmology 2017;124:352–8. 10.1016/j.ophtha.2016.10.036 [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency . Eylea [Internet], 2020. Available: https://www.ema.europa.eu/en/documents/overview/eylea-epar-medicine-overview_en.pdf
- 21.European Medicines Agency . Lucentis [Internet], 2018. Available: https://www.ema.europa.eu/en/documents/overview/lucentis-epar-medicine-overview_en.pdf
- 22.Holekamp NM, Liu Y, Yeh W-S, et al. Clinical utilization of anti-VEGF agents and disease monitoring in neovascular age-related macular degeneration. Am J Ophthalmol 2014;157:825–33. 10.1016/j.ajo.2013.12.018 [DOI] [PubMed] [Google Scholar]
- 23.Monés J, Singh RP, Bandello F, et al. Undertreatment of neovascular age-related macular degeneration after 10 years of anti-vascular endothelial growth factor therapy in the real world: the need for a change of Mindset. Ophthalmologica 2020;243:1–8. 10.1159/000502747 [DOI] [PubMed] [Google Scholar]
- 24.Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol 2015;99:220–6. 10.1136/bjophthalmol-2014-305327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciulla TA, Hussain RM, Pollack JS, et al. Visual Acuity Outcomes and Anti-Vascular Endothelial Growth Factor Therapy Intensity in Neovascular Age-Related Macular Degeneration Patients: A Real-World Analysis of 49 485 Eyes. Ophthalmol Retina 2020;4:19–30. 10.1016/j.oret.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 26.Holz FG, Amoaku W, Donate J, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the sustain study. Ophthalmology 2011;118:663–71. 10.1016/j.ophtha.2010.12.019 [DOI] [PubMed] [Google Scholar]
- 27.Ehlken C, Ziemssen F, Eter N, et al. Systematic review: non-adherence and non-persistence in intravitreal treatment. Graefes Arch Clin Exp Ophthalmol 2020;258:2077–90. 10.1007/s00417-020-04798-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michels S, Becker M, Wachtlin J, et al. The intravitreal injection: variations in regulations, cost and reimbursement in Europe. Spektrum Augenheilkd 2012;26:2–6. 10.1007/s00717-012-0072-2 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055478supp001.pdf (147.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data of hospitalisations are the property of Central Administration of the Health System (Administração Central do Sistema de Saúde (ACSS), I.P.). However the data are available from the authors upon request and with permission of the ACSS. The data of hospitalisations are not publicly available, however the authors confirm that interested researchers can ask for access to these data by contacting ACSS directly at the following: Parque da Saúde da Lisboa, Edifício 16, Avenida do Brasil, 53 1700-063 Lisboa, Portugal (e-mail: geral@acss.min-saude.pt).