Abstract
Objectives
There is inadequate information on the economic burden of hypertension treatment in Ethiopia. Therefore, this study was conducted to determine the societal economic burden of hypertension at selected hospitals in Southern Ethiopia.
Methods
Prevalence-based cost of illness study from a societal perspective was conducted. Disability-adjusted life years (DALYs) were determined by the current WHO’s recommended DALY valuation method. Adjustment for comorbidity and a 3% discount was done for DALYs. The data entry, processing and analysis were done by using SPSS V.21.0 and Microsoft Excel V.2013.
Results
We followed a cohort of 406 adult patients with hypertension retrospectively for 10 years from September 2010 to 2020. Two hundred and fifty (61.6%) of patients were women with a mean age of 55.87±11.03 years. Less than 1 in five 75 (18.5%) of patients achieved their blood pressure control target. A total of US$64 837.48 direct cost was incurred due to hypertension. A total of 11 585 years and 579.57 years were lost due to hypertension-related premature mortality and morbidity, respectively. Treated and uncontrolled hypertension accounted for 50.83% (6027) of total years lost due to premature mortality from treated hypertension cohort. Total productivity loss due to premature mortality and morbidity was US$449 394.69. The overall economic burden of hypertension was US$514 232.16 (US$105.55 per person per month).
Conclusion
Societal economic burden of hypertension in Southern Ethiopia was substantial. Indirect costs accounted for more than 8 out of 10 dollars. Treated and uncontrolled hypertension took the lion’s share of economic cost and productivity loss due to premature mortality and morbidity. Therefore, designing and implanting strategies for the prevention of hypertension, early screening and detection, and improving the rate of blood pressure control by involving all relevant stakeholders at all levels is critical to saving scarce health resources.
Keywords: health economics, health economics, cardiology, hypertension
Strengths and limitations of this study.
Using the cardiovascular disease policy model adapted to sub-Saharan African perspective.
Including productivity loss costs associated with hypertension (premature mortality and morbidity).
Obtaining all simulation variables and transition probability data from valid sources (systematic reviews, randomised controlled trials and prospective cohort studies) were the strengths of this study.
Uncertainty in age-specific and sex-specific prevalence of undiagnosed hypertension and variability in employment rate which require due consideration during applying the findings of this study were limitations.
Introduction
Hypertension doubles the risk of death from stroke, heart disease, vascular diseases, diabetes, atherosclerosis and kidney disease.1 According to the national STEPS survey, only 28.4% of patients with hypertension were taking antihypertensive medication prescribed by professionals in Ethiopia.2 According to the International Society of Hypertension Global Hypertension Practice Guideline 2020, hypertension remains the leading cause of death globally, accounting for 10.4 million deaths per year.3
Hypertension is associated with societal and economic consequences particularly in low-income and middle-income countries (LMICs). In addition to the direct costs associated with healthcare utilisation for the management of complications, hypertension causes significant productivity loss from disability and premature death.4 5 WHO report from South East Asian region also indicated huge impact of hypertension in national finances due to premature death, disability, personal and family disruption, loss of income and healthcare expenditure.6 According to a WHO report in 2017, stroke, coronary heart disease (CHD) and hypertension caused 39 571, 46 943 and 11 050 deaths, respectively, (ie, 30 patients per day die due hypertension) in Ethiopia.7
Cost of illness (COI) study is used to measure the economic burden of disease to individuals, communities and society as a whole. It can provide information to support the political process and healthcare decision-making if it is conducted from a societal perspective by using an appropriate approach and bottom-up costing strategy.8–12 Despite this huge impact on national economies, the economic burden of hypertension is not studied in Ethiopia particularly Southern Ethiopia. To fill this evidence gap, this study was conducted to determine the economic burden of hypertension at selected public hospitals in Southern Ethiopia by using the prevalence-based COI method from a societal perspective to estimate the direct and indirect costs of hypertension in a given year (2021) in Southern Ethiopia.
Methods and materials
Study design, area and period
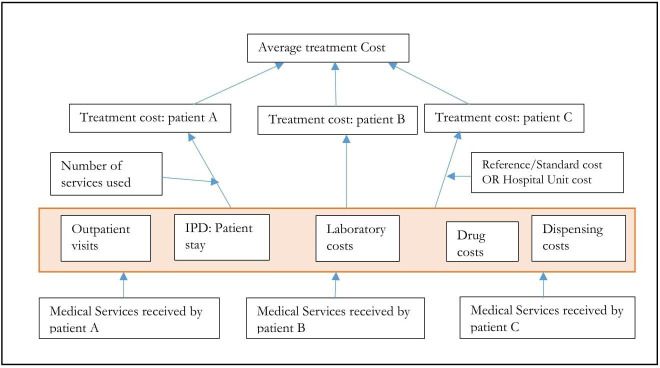
A prevalence-based retrospective COI study from societal perspective focusing on quantifying direct and indirect costs was conducted from September 2010 to September 2020 at three selected public hospitals in Southern Ethiopia. The bottom-up approach was used to estimate the economic burden of hypertension in Southern Ethiopia (figure 1). The human capital approach was used to calculate indirect costs separately in men and women and also among different age groups. A prevalence-based COI model was constructed in which patients with hypertension were simulated from diagnosis through active treatment, palliative care and death over 15–64 years. Age-specific and sex-specific mortality rates, measures of productivity, and workforce statistics were used to simulate the progression of these cohorts until death or age 64 years. First, the model estimated cumulative years of life and disability-adjusted life years lived for the working-age population who had hypertension. Then the model re-simulated with the hypothetical assumption that they did not have hypertension, with relevant changes to mortality rates and productivity. We estimated the probability of death separately for1 all-cause mortality in absence of hypertension and related complications and2 mortality attributable to the included disease states. The first component was estimated using WHO Life Tables, and the second component was calculated based on standardised mortality ratios extracted from the literature. The natural history study conducted in 1974 showed that the mortality rate was 1.85 (3.01 in men and 1.62 in women).13 Interventional trials suggested that it could be possible to achieve effective blood pressure (BP) targets in about 70% of patients by improving adherence and/or intensifying therapy.14
Figure 1.
Micro-costing bottom-up approach for healthcare costs. Adapted from Riewpaiboon et al. Cost analysis for efficient management: diabetes treatment at a public district hospital in Thailand. IPD, inpatient department.
Study populations
The study populations were selected adult patients with hypertension at three selected public hospitals. According to the world population prospect 2020 estimate,15 in the same year, the population of the Gamo Zone accounted for 1.5% of the total population and Gofa and South Omo Zone accounted for 1.5% of the total population. The target population is 3.0% of the total population of Ethiopia or 20% of the Southern Ethiopian population (6 208 034). Based on age distribution: 0–14 years are children, 15–24 years are early working age, 25–54 years are prime working age, 55–64 years are mature working age and ≥65 years are elderly.13
Inclusion and exclusion criteria
We included all adult patients with hypertension having at least 5 years of follow-up visits before data collection and receiving care during the study period from selected facilities. However, patients who are unwilling to participate in this study, patients who have less than 5 years of follow-up, and incomplete patient records (do not contain follow-up BP records and refill medications, laboratory requests and results) were excluded.
Study variables
Dependent variables
Economic burden of hypertension.
Independent variables
Patient-related (sociodemographic characteristics, heart disease knowledge, healthy lifestyle and heart disease risk perception, presence of comorbidity, type of medications, treatment adherence, shared decision-making, health-related quality of life).
Cost-related variables
Medical costs (inpatient hospital stay/hospitalisation cost, outpatient clinic visit, drug acquisition costs, drug administration cost, laboratory test and imaging study costs).
Non-medical costs (transportation, meal, patient time cost due to treatment, cost due to informal care by family or friends).
Indirect costs (absenteeism, presenteeism, unemployment, early retirement, disability, premature death).
Sample size and sampling technique
Sample size determination
The sample size was determined by using the single population proportion formula by taking prevalence of patients who controlled their BP as 14% from WHO 2016 BP control rate report16–18 and Z value of 1.96 at 95% CI. We added 10% for non-response rate and two for design effect due to multistage sampling technique involvement. Finally, a formula giving a larger sample size was used. Total 407 adult patients with hypertension who are on follow-up care will be included.
Where: n=is the sample size,
Z2=standard normal deviation, set at 1.96, corresponding to the 95% CI,
d=is the desired level of precision/margin of error (0.05),
p=prevalence of patients taking anti-hypertensive medications (p=28.4%), and q is 1-p.
Sampling techniques
A multistage simple random sampling technique was used. We randomly selected 3 zones from a total of 12 zones found in the Southern region. Three general public hospitals with experience of providing cardiovascular disease (CVD) care for at least 5 years from selected four zones were included in this study. The total sample size was allocated to these hospitals based on an estimated number of adult patients with hypertension attending respective hospitals (ie, we included 212 patients from Arba Minch General Hospital, 107 patients from Jinka General Hospital and 88 patients from Sawula General Hospital). Finally, a consecutive sampling technique was applied in each facility until the desired sample size was achieved.
Data collection tools and procedures
Model input parameters
Key model input variables include; 2020 population of selected zones, hypertension prevalence by treatment and control status, transition probabilities to death and healthy state, cost of diagnosis and management. Among those with treated hypertension, treated and controlled hypertension was defined based on BP control target of International Society of Hypertension (ISH) 2020 guideline.3 We used national STEPS survey data to estimate the prevalence of cardiovascular risk factors (myocardial infarction (MI), angina, heart failure, stroke, transient ischaemic attack (TIA)). Incorporating the risk factor prevalence data in the relevant Framingham risk equation, the age-specific and sex-specific probability of CHD and cerebrovascular disease (ie, stroke and TIA) events were estimated. The probability of each health state was calculated using the age-specific and sex-specific CHD and cerebrovascular disease event distributions.2 19 To estimate the corresponding probabilities, separate relative risk estimates were used for CHD events (stable angina, unstable angina and MI) and cerebrovascular diseases (stroke and TIA), assuming that antihypertensive treatment affects the probability of every disease state similarly across all age and sex groups. Relative risk reductions attributable to antihypertensive treatment were extracted from the peer-reviewed literature.20–22
The 2020 world population prospect estimate was used for the baseline population and number of 33-year-olds projected to enter the model population from 2020 to 2070.15 The annual probability of CHD and stroke was based on national STEPS survey,2 and Framingham Heart Study23 and the Framingham Offspring Study,24 by contextualising to Ethiopian scenario. Incident CHD events were allocated to angina pectoris, MI or cardiac arrest. Prevalence, joint distributions and means of Ethiopia risk factor values were estimated from the national STEPS survey.2 Annual transition rates between risk factor levels were calculated to preserve age-range trends over time. Betas for risk function for non-BP risk factors were estimated separately for the risk of incident CHD events, incident strokes and non-CVD deaths, using examinations 1–8 of the Framingham Offspring cohort.24 Risk factors are assumed to affect the incidence of MI, arrest and angina in proportion to the overall incidence of CHD, except tobacco smokers are assumed to have a higher relative risk for infarction and arrest25; and a proportionately lower coefficient for angina. Environmental tobacco exposure is assumed to carry a relative risk of 1.26 for MI and cardiac arrest compared with non-exposed non-smokers26 but not to influence angina. The number of hospitalised MI were obtained from the national STEPS survey.2 Case-fatality rates and rates of MI in subgroups were estimated from national data and other complementary sources. Prehospital arrest deaths and out-of-hospital cardiac arrests surviving to hospital discharge were estimated from our effectiveness study (online supplemental table 1).
bmjopen-2021-056627supp001.pdf (644.5KB, pdf)
Survival after a CHD event was estimated and calibrated based on national or international data sources.27 28 Rates of coronary revascularisations was estimated from the National Hospital Discharge Survey, with mortalities estimated from aggregated historical data. Stroke incidence was assumed to be independent of the risk of new-onset CHD in the same year. The number of hospitalised strokes cases was obtained from national and regional studies. The annual probabilities of stroke after MI29 30 and the probability of CHD in patients who had a stroke were based on natural history studies and systematic reviews of BP control trials.31–36 A 30-day heart failure mortality and re-hospitalisation data were from the THESEUS-HF registry37 and Korean Acute Heart Failure Registry38 39 (online supplemental tables 2 and 3).
The background prevalence of CVD by age, sex and CVD state (stroke, CHD or both stroke and CHD) in 2020 was estimated from the National Health Survey data2 and GBD 2017.40 The background prevalence of prior coronary revascularisation was estimated from revascularisations before 2019 and estimated survival after revascularisation, while model projections were used to infer the distribution of revascularisation by CVD state. Age-specific and sex-specific healthcare costs were estimated using national data, and our effectiveness data. Hospitalised stroke and CHD costs and acute stroke rehabilitation costs were estimated using WHO-CHOICE41 inflated to 2021. Outpatient consultations, and inpatient stay and bed days were also estimated from WHO-CHOICE41 inflated to 2021. Chronic outpatient CVD costs additional to average background healthcare costs for the first year after the event and subsequent years were estimated for patients with a stroke or CHD diagnosis was pooled from the 2015 national STEPS survey. Average annual non-cardiovascular costs were estimated from the national STEPS survey,2 and Ethiopia Demographic and Health Survey (EDHS) 2016 survey.13
Cost estimation
The outcomes measures are total discounted societal costs, cost/year and cost/patient-year. This is the amount of health budget that could be saved by effective prevention and control of hypertension. The direct costs were divided into two subcategories: direct medical costs and direct non-medical costs. Direct medical costs include; inpatient stays, outpatient clinic visits, medical services, drug acquisition, dispensing, administration, monitoring, laboratory test and imaging study costs. The costs associated with outpatient/inpatient visits were estimated by multiplying the numbers of outpatient visits related to hypertension by the outpatient costs per year (ie, 12 times WHO cost per outpatient visit for secondary hospitals inflated to 2021).41
Data concerning medications prescribed for the management of hypertension, and associated comorbidities, and laboratory tests and imaging studies done were collected by patient chart abstraction in index year (2020). The cost of medications used for management of hypertension and associated comorbidities was taken from Ethiopian Pharmaceutical supply agency Arba Minch regional hub selling price and retail price of Arba Minch General Hospital in 2020. The retail price of Arba Minch General Hospital was used because of the minimum distance from the pharmaceutical supply agency hub, which could minimise markup added on retail price due to transportation cost. Costs of laboratory procedures were also taken from Arba Minch Hospital Laboratory’s service price list. The prices of relevant laboratory tests and imaging studies were based on the average price of included hospitals. The salary scale of the health workforce was based on the Federal Ministry of Health of Ethiopia (online supplemental table 4).
Ongoing programme costs for hypertension care was estimated from WHO tool outputs for CVD and diabetes care and National strategic action plan for prevention and control of non-communicable diseases in Ethiopia 2014–2016 and adjusted for 2021 inflation target population.42 Adjustment for the study population was done by multiplying the national cost by the proportion of the study population (ie, 3%). National and regional cost estimates were based on the proportion of patients studied (ie, 3% and 20%). We considered this strategy since the age and sex distribution of hypertension among different regions in the country did not vary significantly. The collected cost data added up and averaged by using a bottom-up approach (figure 1). Facility-based or reference costs were used during computing costs. The total medical cost of hypertension treatment was calculated as the sum of the product of medical costs with their respective unit prices. Costs were discounted at an annual rate of 3% and reported in 2021 US$.43 44
Direct non-medical costs include transportation costs and patient time costs due to care. The cost of patient time due to care was estimated by using the average daily wage of patients (97.00 Ethiopian birr (ETB)) which was calculated from 2912±2732.24 average monthly income. Transportation cost was determined by using the cost of average travelling distance and local transportation tariff (42.00 ETB) in January 2021. According to EDHS 2016 survey showed that 33% of women and 88% of men are currently employed.13 This proportion was used to determine the patient time cost due to care for employed groups. For the unemployed proportion, the average daily wage of daily labourers workers working 8 hours per day for 6 days per week was used (26.53 ETB) from the monthly wage of 796.00 ETB (420–1172 ETB).45
Indirect costs include cost of hospitalisation, productivity loss due to illness and cost of death. Cost of hypertension-related hospitalisation was taken from WHO-CHOICE,41 costs per inpatient stay and cost per inpatient bed day times duration of hospitalisation inflated for 2021, and professional time (physician, nurse laboratory professional and pharmacist time). If a patient had multiple admissions during the year, the costs for each admission were aggregated as the total costs.46
Mortality and morbidity estimations
Age-specific and sex-specific mortality rates among the adult general population in Ethiopia were taken from EDHS 2016 survey and extrapolated to selected populations.13 According to EDHS 2016, the probability of dying before age 50 years among adults ≥15 years were 10% and 12%, in women and men, respectively.13 Due to the absence of mortality data specific to hypertension treatment and control status in Ethiopia, mortality risk in the general population was attributed to those with and without hypertension using sex-specific estimates of the relative risk of all-cause mortality associated with hypertension by treatment and control status was derived from a study conducted in India was used.47 A cohort study conducted in India among adults aged 20 years and above to determine the rate and risk of all-cause mortality among people with hypertension showed that the incidence of deaths in the study was 4.28% during the follow-up period of 6 years. The relative risk of mortality was 3.13 (95% CI: 2.91 to 3.37) and 1.2 in the high BP group and at age of 60 years. The age-adjusted HR of all-cause mortality for the high BP group was 2.96 (2.56–3.42)47 (online supplemental tables 5 and 6).
In 2020 crude death rate of the Ethiopian population-based on global estimates was 6.29 deaths per 1000 population.48 The estimated prevalence of hypertension among adults was calculated from national STEPS survey 2016, systematic review and meta-analysis, and WHO report and local studies and the mean estimated prevalence of hypertension was 21.39%.2 13 47 49–52 Only 28.4% of patients with hypertension are taking antihypertensive medication.2 The mean relative risk of all-cause mortality among hypertensive population when compared with those without hypertension was 1.39 (95% CI: 0.95 to 1.95)53 (online supplemental table 3).
Years of life lost (YLL) due to hypertension morbidity was determined by first calculating disability weights for specific ages based on blood pressure control status (X). Then subtract this value (X) from the life expectancy of the Ethiopian population (ie, 66.7 years for men, and 70.4 years for women) (Y). The productivity loss cost due to hypertension morbidity was calculated by multiplying Y with sex-specific employment rate based on a monthly average income of 2059.078 ETB from the national STEPS survey 2015 adjusted for 2021 inflation (13.13/9.57=1.372) STEPS Survey, 2015.2 The EDHS 2016 survey showed that 33% of women and 88% of men are currently employed13 and for unemployed, 2019 minimum average monthly earnings (ETB) of daily labourers reported by the Ministry of Labor and Social Affairs 796 ETB (420–1172 ETB).45 Concerning, cost of productivity lost due to premature mortality: first we calculated potential YLL by subtracting life expectancy from sex-specific age of death at which the death is recorded (Z). Then Z is multiplied by the number of deaths in each age group (Xi). Finally, we multiplied Xi with sex-specific employment rates like productivity loss due to hypertension-related morbidity above.54 Excess mortality and morbidity due to hypertension were determined by subtracting age-specific and sex-specific morbidity and mortality among the general population from the hypertensive cohort. Both were determined by using age, sex and BP treatment status mortality rate per 1000 person-years (online supplemental table 6).
Morbidity adjustment
Patients with hypertension may have more than one disease, the addition of years of life lived with disabilities (YLDs) across causes may result in overestimation of the total loss of health.55 Therefore, it is recommended to estimate comorbidities using the assumption of independence within age–sex groups56:
Where P1+2 is the prevalence of the two comorbid diseases 1 and 2,
P1 is the prevalence of disease 1 and P2 is the prevalence of disease 2.
The combined disability weight for individuals with multiple conditions is estimated assuming a multiplicative model as follows:
Since prevalence YLDs are calculated for each cause as:
Two preceding equations can be combined into a single calculation resulting in:
Assumptions and transition probabilities
The counterfactual comparator (hypothetical cohort of normotensive individuals) with a probability of developing CVD events among the general population. Both in case and comparator cohorts, the probability of non-cardiovascular death does not depend on the health state and is similar for both hypertensive and normotensive populations57 and we chose not to model differential use of antihypertensive medication classes in order not to bias cost-of-treatment. Antihypertensive dose intensification and frequency of BP monitoring were based on ISH 2020 guidelines for BP control. We did not simulate the effects of any particular medication; instead, we simulated ‘standard dose’ effects and assumed average drug prices across classes.58 The amount of BP change was assumed to be a function of the baseline BP and the effect of a standard-dose antihypertensive agent at that pretreatment level.21 We also assumed the medication adherence rate as 75% based on clinical trials.21 Other important assumptions include COI due to hypertension or associated morbidities were calculated based on the monthly earnings during data collection; all costs incurred before 1 year were adjusted/accounted to today’s value (2021 US$ equivalent) and discounted at 3%; YLL and YLDs were not discounted as per the recent WHO recommendations.
Data quality control, processing and analysis
Questionnaires are prepared in English and the patient interview part of the questionnaire was translated into Amharic and translated back into English to check its consistency. The Amharic version of the patient interview questionnaire and English version of the health professional interview, data abstraction form and health system interview questionnaires were used for data collection. The questionnaire was pretested on 30 adult patients with hypertension in Arba Minch General Hospital to ensure that the respondents could understand the questions and to check for consistency and possible amendments were made based on findings. Six professional nurses (BSc) for data collection and one senior professional working in the respective health facilities for supervision were oriented before data collection about data collection approaches and contents of data collection format for one day by the principal investigator. Continuous follow-up and supervision were made by the principal investigator throughout the data collection period. The collected data were checked for completeness and consistency by the principal investigator on daily basis at the spot during the data collection time. Then data were transcribed back to English for the patient interview part and entry was made using EpiData V.3.1 software. After data processing, analysis was done by using SPSS V.21.0 and Microsoft Excel 2010. A summary of descriptive statistics was reported for sociodemographic factors; cost of hypertension and life years lost due to hypertension-related morbidity and premature mortality and presented in tables and figures.
Patient and public involvement
There was no identifiable patient involvement in this research. Patients’ demographic characteristics and disease-related variables were obtained by using questionnaire-based interview after obtaining verbal consent from the patient. No patient identifier information was collected. Finally, most of the variables were taken from published national and international literatures, and all relevant sources were acknowledged through citation.
Results
Description of study participants
In this study, we estimated the regional and national economic burden of hypertension (direct and indirect costs) by using the CVD policy model adapted to the sub-Saharan Africa perspective59 (online supplemental figure 1). Total costs of treated hypertension and hypertension-related excess mortality and YLL due to hypertension were determined. We followed a cohort of 406 patients with hypertension retrospectively for 10 years from September 2003 to 2013 Ethiopian calendar (September 2010–2020) for baseline assessment and simulated the cost of hypertension for lifelong from a societal perspective. About two-thirds, 250 (61.6%) of patients were women with a mean age of 55.87±11.03 years. Less than 1 in 5, 75 (18.5%) of patients achieved their BP control target based on international society of hypertension 2020 guidelines (table 1).
Table 1.
Patient characteristics and disease-related factors among adult patients with hypertension on regular follow-up at selected public hospitals in Southern Ethiopia, January 2021 (n=406)
| Sociodemographic factors | Frequency | |
| Sex | Male | 156 (38.4%) |
| Female | 250 (61.6%) | |
| Age in years | Below 40 years | 15 (3.7%) |
| 40–65 years | 286 (70.4%) | |
| 65 years and above | 105 (25.9%) | |
| Religion | Orthodox | 215 (53.0%) |
| Muslim | 37 (9.1%) | |
| Protestant | 144 (35.5%) | |
| Catholic | 10 (2.5%) | |
| Annual gross income before tax (n=406) | Less than 12 000 | 117 (28.8%) |
| 12 000–18 000 | 89 (21.9%) | |
| 18 000–23 000 | 200 (49.2%) | |
| Level of education | Illiterate | 259 (63.8%) |
| Grades 1–8 | 46 (11.3%) | |
| Grades 9–12 | 22 (5.4%) | |
| College and above | 73 (18.0%) | |
| Post-graduate degree | 6 (1.5%) | |
| Occupation | Employed | 65 (16.0%) |
| Merchant | 63 (15.5%) | |
| Farmer | 79 (19.5%) | |
| Housewife | 149 (36.7%) | |
| Disease-related factors | ||
| Duration of hypertension since diagnosis | 5–9 years | 262 (64.5%) |
| 10–14 years | 131 (32.3%) | |
| 15 and above years | 13 (3.2%) | |
| Family history of cardiovascular diseases | First-degree relative | 133 (32.7%) |
| Second-degree relative | 16 (3.9%) | |
| None | 257 (63.3%) | |
| Presence of comorbidities (n=406) | Yes | 310 (76.4%) |
| No | 96 (23.6%) | |
| History of hospitalisation | Yes | 250 (61.6%) |
| No | 156 (38.4%) | |
| Duration of hospitalisation (n=250) | Below 5 days | 56 (22.4%) |
| 5–10 days | 112 (44.8%) | |
| More than 10 days | 82 (32.8%) | |
| Target BP achieved based on ISH 2020 guideline | Yes | 75 (18.5%) |
| No | 331 (81.5%) | |
| Antihypertensive regimen | Monotherapy | 136 (33.5%) |
| Two drug combination | 234 (57.6%) | |
| Three and more drug combination | 36 (8.8%) | |
BP, blood pressure; ISH, International Society of Hypertension.
Cost of hypertension
Direct (medical and non-medical) costs
Direct medical costs include programme costs, cost of drugs for hypertension and comorbidities, laboratory costs, hospitalisation costs, annual outpatient visit costs and costs of medical supplies. A total of US$64 837.48 direct cost was incurred due to hypertension. Out of this, 80.0% (US$51 915.40) was direct medical cost. From direct medical costs, annual outpatient visit cost 33.55% (US$17 419.73), cost of comorbidity 26.21% (US$13 612.15), and laboratory test costs 8.17% (US$4263.29) took the largest share. While, total direct non-medical costs of hypertension was US$9866.58 (ie, transportation costs and patient time costs due to care). The regional and national annual estimated direct cost of hypertension were US$324 187.40 and US$2 161 249.33, respectively (table 2).
Table 2.
Direct annual costs of treating hypertension among adults in Southern Ethiopia, January 2021 (n=406)
| Cost category | Annual total in ETB Total (mean±SD) |
Annual cost in July 2021 US$ | Percentage from total direct cost |
| Direct medical total | 2 258 319.97 | 51 915.40 | 80.0% |
| Programme costs | 403 275.70 (993.0±0.00) | 9173.40 | |
| Cost of antihypertensives | 119 847.64 (295.19±107.78) | 2726.20 | |
| Cost of drugs for comorbidity | 598 409.00 (2266.7±1114.52) | 13 612.15 | |
| Cost for hospitalisation | 179 377.03 (3360.76±1594.69) | 4080.33 | |
| Laboratory tests | 187 420.00 (461.63±226.98) | 4263.29 | |
| Annual outpatient visit costs | 765 795.60 (1886.20±0.00) | 17 419.73 | |
| Cost of medical supplies | 4195.00 (85.60±0.00) | 95.42 | |
| Professional time total | 128 362.01 | 2950.85 | 4.6% |
| Physician time | 92 032.08 (226.68±0.00) | 2093.47 | |
| Nurse time | 2060.28 (43.84±17.81) | 46.87 | |
| Pharmacy time | 4453.01 (10.97+0.00) | 101.29 | |
| Laboratory time | 29 816.64 (73.44±0.00) | 678.25 | |
| Direct non-medical costs | 433 748.59 (1068.84±384.78) | 9866.58 | 15.37% |
| Total direct cost of treated hypertension | 2 820 430.57 | 64 837.48 | 100.00% |
US$1=43.9614 ETB on 13 July 2021
ETB, Ethiopian birr; US$, US dollar.
Life years lost due to premature mortality and morbidity
We determined the YLL due to premature mortality (excess mortality) and YLL due to hypertension morbidity for the productive age population (30–64 years) among a cohort of simulated adult patients with hypertension patients. Excess mortalities are all-cause deaths observed in those with hypertension compared with the same cohort assuming no hypertension. The excess mortality and YLL were different among the hypertensive cohort and simulated population with no hypertension. A total of 11 858 (6159, men and 5699 women) life years were lost due to hypertension-related premature mortality among 30–64 years old adults with hypertension. This equates US$428 969.78 (US$270 076.91, men and US$158 892.78, women). The estimated regional and national life years lost due to premature mortality was 59 290 and 395 267, respectively. This is equivalent to US$2 144 848.58 and US$14 298 990.51 respectively. From 15 232 years lost due to premature mortality in the hypertension cohort, treated and uncontrolled hypertension accounted for more than 6824 (44.8%) total yeas lost due to premature mortality followed by treated controlled hypertension 5832 (38.29%) and untreated hypertension 2575 (16.9%) (tables 3 and 4).
Table 3.
Excess deaths among adult patients with hypertension by treatment and control status over the working lifetime simulated from life table modelling in Southern Ethiopia, January 2021
| Age group | Deaths in treated hypertension cohort | Deaths in ‘hypertension cohort’ assuming no hypertension | Excess deaths in those with treated hypertension | Deaths in those with hypertension by treatment and control status* | ||
| Treated and controlled | Treated and uncontrolled | Untreated | ||||
| Men | ||||||
| 30–34 | 1436 | 448 | 988 | 487 | 501 | 295 |
| 35–39 | 1180 | 381 | 799 | 401 | 398 | 242 |
| 40–44 | 1027 | 428 | 599 | 357 | 242 | 191 |
| 45–49 | 1735 | 224 | 1511 | 1167 | 344 | 163 |
| 50–54 | 989 | 166 | 823 | 370 | 453 | 123 |
| 55–59 | 731 | 123 | 608 | 273 | 335 | 91 |
| 60–64 | 932 | 101 | 831 | 362 | 469 | 127 |
| Total | 8030 | 1871 | 6159 | 3417 | 2742 | 1232 |
| Women | ||||||
| 30–34 | 1401 | 415 | 986 | 434 | 552 | 310 |
| 35–39 | 1187 | 212 | 975 | 368 | 607 | 263 |
| 40–44 | 1019 | 287 | 732 | 324 | 408 | 205 |
| 45–49 | 832 | 279 | 553 | 265 | 288 | 167 |
| 50–54 | 887 | 91 | 796 | 350 | 446 | 137 |
| 55–59 | 805 | 72 | 733 | 277 | 456 | 109 |
| 60–64 | 1071 | 147 | 924 | 396 | 528 | 154 |
| Total | 7202 | 1503 | 5699 | 2414 | 3285 | 1345 |
| Box sex total | 15 232 | 3374 | 11 858 | 5831 | 6027 | 2577 |
*Excess deaths are all-cause deaths observed in those with hypertension compared with the same cohort assuming no hypertension.
Table 4.
Years of life lost (YLL) by adults with hypertension by treatment and control status over the lifetime simulated from life table modelling in Southern Ethiopia, January 2021
| Age group | Years of life lived in treated hypertension cohort | Years of life lived in ‘hypertension cohort’ assuming no hypertension | YLL lost to treated hypertension (excess) | YLL lost due to hypertension by treatment and control status* | Years of life lived in untreated hypertension cohort | YLL lost due to untreated hypertension | |
| Treated and controlled | Treated and uncontrolled | ||||||
| Men | |||||||
| 33–39 | 199.87 | 181.2 | 18.67 | 18.67 | NA | 122.67 | 58.53 |
| 40–44 | 357.48 | 324.1 | 33.38 | 16.67 | 17.71 | 219.42 | 104.68 |
| 45–49 | 587.08 | 522.5 | 64.58 | NA | 64.58 | 353.73 | 168.77 |
| 50–54 | 341.9 | 295.3 | 46.6 | NA | 46.6 | 199.92 | 95.38 |
| 55–59 | 161.63 | 140.1 | 21.53 | NA | 21.53 | 94.85 | 45.25 |
| 60–64 | 129.88 | 109.4 | 20.48 | NA | 20.48 | 74.06 | 35.34 |
| Total | 1777.84 | 1572.6 | 205.24 | 35.34 | 169.9 | 1064.65 | 507.95 |
| Women | |||||||
| 33–39 | 318.33 | 288.6 | 29.73 | 29.73 | NA | 195.38 | 93.22 |
| 40–44 | 791.95 | 718 | 73.95 | 73.95 | NA | 486.09 | 231.91 |
| 45–49 | 1147.34 | 1040.2 | 107.14 | NA | 107.14 | 704.22 | 335.98 |
| 50–54 | 953.59 | 863.8 | 89.79 | NA | 89.79 | 279.01 | |
| 55–59 | 491.71 | 445.8 | 45.91 | NA | 45.91 | 309.52 | 143.99 |
| 60–64 | 297.81 | 270 | 27.81 | NA | 27.81 | 182.79 | 87.21 |
| Total | 4000.73 | 3626.4 | 374.33 | 103.68 | 270.65 | 1878.00 | 1171.33 |
| Grand total | 5778.57 | 5199 | 579.57 | 139.02 | 440.55 | 2942.65 | 1679.28 |
*YLL by those with hypertension compared with the same cohort assuming no hypertension.
NA, no patient is reported in this age group.
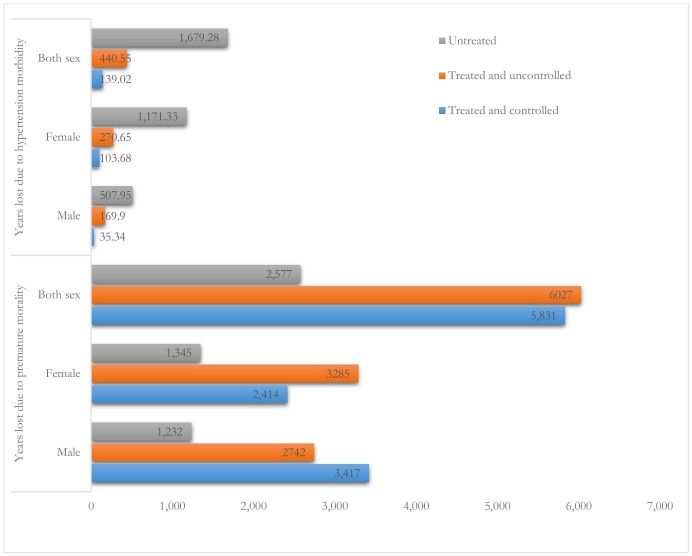
A total of 579.57 (205.24 men and 374.33 women) years of life were lost due to hypertension morbidity. This equates to US$19 436.56. A total of 11 858 (6159 men and 5699 women) years of life were lost due to hypertension-related premature mortality. This equates to US$$429 958.12. Total productivity loss due to premature mortality and morbidity was US$449 394.68 (table 5). Treated and uncontrolled hypertension accounted for 2937.72 (50.84%) of productive life years lost, followed by untreated hypertension 1679.28 (29.06%). Treated uncontrolled hypertension contributed to more YLL due to premature mortality in both sexes 6824 (44.8%), and life years lost due to hypertension morbidity 9378 (50.84%) (figure 2).
Table 5.
Mean annual productivity loss associated premature mortality and hypertension morbidity, Southern Ethiopia, January, 2021
| Variable | Sex | Excess years lost | Lost productivity ETB | Lost productivity in 2021 US$ |
| Years lost due to premature morality | Male | 6159 | 11 748 345.71 | 270 699.21 |
| Female | 5699 | 6 911 836.90 | 159 258.91 | |
| Both | 11 858 | 18 660 182.62 | 429 958.12 | |
| Years lost due to hypertension morbidity | Male | 205.24 | 391 497.07 | 8999.93 |
| Female | 374.33 | 453 993.32 | 10 436.63 | |
| Both | 579.57 | 845 490.39 | 19 436.56 | |
| Total productivity loss | 19 505 673.01 | 449 394.69 | ||
Note: productivity loss is calculated by taking 88% employment rate for men and 33% employment rate for women. Monthly wage of employed was 2059.078 from EDHS 2016 and national STEPS survey 2015 which is adjusted for current inflation (1.3689). Unemployment/unpaid monthly wage was 796 ETB.
US$1=43.5 ETB.
ETB, Ethiopian birr; US$, US dollar.
Figure 2.
Number of premature deaths and years of life lost due to morbidity among adults with hypertension by sex, treatment and control status over productive life years simulated from life table modelling in Southern Ethiopia.
The overall estimated hypertension-related economic burden (direct and indirect cost) was US$514 232.16 in the study area (tables 2 and 5). Since the study population is estimated to be 20% of the Southern region, the estimated economic burden of hypertension in the region is US$2 571 160.8 in the region. More than eight out of ten 87.37% dollars were due productivity loss. Productivity loss is calculated by taking 88% employment rate for men and 33% employment rate for women. Monthly wage of employed was 2059.078 from EDHS 2016 and national STEPS survey 2015 which is adjusted for current inflation (1.3689). Unemployment/unpaid monthly wage was 796 ETB (table 5).
Discussion
In this prevalence-based retrospective COI study, we estimated the economic burden of hypertension among productive age population from societal perspective. A total direct (medical and non-medical) annual cost incurred due to hypertension in the study population was US$64 837.48 (US$13.308 per person per month). Out of direct costs, 80.0% (US$51 915.40) was direct medical cost. While, the total indirect annual cost incurred due to hypertension was US$449 394.69 (US$92.24 per person per month). The total annual economic burden of hypertension was US$514 232.16 (US$1266.58 per person per year). This is higher than findings from another institution-based cross-sectional study conducted to evaluate cost of hypertension illness among patients attending hospitals in Southwest Shewa Zone that showed the mean monthly total cost of hypertension illness was US$22.3 (95% CI: 21.3 to 23.3).60 Findings from an institution-based cross-sectional study conducted to estimate the direct and indirect costs of hypertension at Gondar Specialized Hospital showed that total cost of hypertension was US$91.72±78.65 per patient per year.61 The COI study conducted among 202 patients with hypertension in Ghana that showed the total annual treatment cost of hypertension was US$76 275.60 (US$31.47 per person per month).62 This variation could be explained by some uncertainties in our estimation (ie, uncertainty in age-specific and sex-specific prevalence of undiagnosed hypertension and variability in employment rate). Consideration of fixed employment rate according to EDHS 2016 survey (ie, 33% of women and 88% of men) could contribute to the relatively higher annual economic burden of hypertension in our study area.13
However, this is less than findings from and a study conducted in Canada also showed that annual individual healthcare cost of hypertension was US$2341,63 and study conducted in the USA showed that individuals with hypertension had US$1920 higher annual incremental expenditure.64 This variation could be explained by variation in socioeconomic and population health status, and asymptomatic nature of hypertension,65 a significant number of undiagnosed hypertension among adults and difference in healthcare system and level of care.
In this study, indirect cost accounted for more than three-fourth of hypertension-related costs 85.6% (US$449 394.69). This is against evidence generated by a cross-sectional study conducted to determine the burden of out-of-pocket payments among patients with CVD in public and private hospitals in Ibadan, South West, Nigeria, showed that across all the hospital facilities, the annual direct and indirect outpatient costs were US$1164.2±US$2363.8 and US$52.87±US$148.05, respectively.66 An institution-based cross-sectional study conducted to estimate the direct and indirect costs of hypertension at Gondar Specialized Hospital showed that the direct medical and non-medical cost constituted 60.81% and 12.17% of the total cost of hypertension, respectively.61 An institution-based cross-sectional study conducted to evaluate cost of hypertension illness among patients attending hospitals in Southwest Shewa Zone showed that the mean monthly total cost of hypertension illness was US$22.3 (direct cost of US$11.39 and indirect cost of US$10.89).60 This is also higher than evidence that suggested about a half of the costs associated with CVD burden are caused by direct healthcare costs.67 The findings from a study conducted in Ghana direct cost accounting for almost 70% of the total cost of managing hypertension.62 Similarly, a study conducted in rural Yunnan Province of China showed that direct costs represented the largest component of the economic cost of hypertension.68 The variation could be explained by significant number of productive age populations affected hypertension in the study area and poor BP control. Therefore, it is important to promote existing strategies and develop country/region-specific strategies for hypertension prevention and control (ie, annual screening of the high-risk population and promoting healthy lifestyles) by all stakeholders could reduce the economic burden of hypertension in Ethiopia.69 70
Concerning premature mortality, a total of 11 858 (6159 men and 5699 women) years were lost due to hypertension-related premature mortality. This equates US$429 958.12. Concerning health-related life loss, about 26 678 deaths per study population were due to hypertension. This is higher than the number of hypertension-related death occurred in 2017, which is 11 050.7 This could be explained by the increasing trend of hypertension in the country.
From 11 585 years lost due to premature death in the treated hypertension cohort more than one-half of related deaths 6027 (50.83%) were due to treated uncontrolled hypertension. This is supported by evidence from other studies that revealed uncontrolled BP cost of US$370 billion globally in 2001.71 This is because the relative risk of all-cause mortality is higher among treated and uncontrolled (1.62) than untreated (1.40) and treated controlled (1.12) patients.53
Untreated hypertension accounted for 1679.28 (507.95 men and 1171.33 women) YLL. Treated and uncontrolled hypertension accounted for 440.55 (76.01%) of productive life years lost from treated hypertension cohort. This is higher than findings from a study conducted to estimate the economic burden of hypertension in a given year in rural Yunnan Province of China showed that the overall prevalence of and YLL/1000 population because of hypertension was 24.8% and 1.5 years for the survey population, respectively.68 A total of 579.57 (205.24 men; 374.33 women) years of life were lost due to treated hypertension. The estimated national life years lost due to hypertension is 19 319 (ie, US$846 413.56). This is supported by evidence from a study conducted in Australia that revealed hypertension caused 609 801 productivity-adjusted life years loss (equating to $A137.2 billion) over the working lifetime.72 Therefore, prevention of hypertension and improving the rate of BP control is important to reduce hypertension-related complications and productive life-year loss in the region as well as in the country.73
Conclusion
The societal economic burden of hypertension in Southern Ethiopia was substantial. Indirect costs accounted for more than 8 out of 10 dollars economic burden. Prevention of hypertension could result in US$2 571 160.8 annual economic savings in the Southern Region. Therefore, designing and implanting strategies for prevention of hypertension, early screening and detection, and improving the rate of BP control by involving all relevant stakeholders at all levels (national, regional, zonal, community and patient-level) is critical to saving scarce health resources.
Supplementary Material
Footnotes
Contributors: All authors read and approved the manuscript. MM conceived the research, framed the format design and developed the manuscript for publication. MM is responsible for the overall content as guarantor. MD participated in data analysis and reviewed the manuscript and AK reviewed the manuscript and write-up process; NS participated in literature review and polished the language of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by Tehran University of Medical Sciences, Faculty of Pharmacy, Department of Pharmacoeconomics and Pharmaceutical Administration Ethical Review Board with Approval ID: IR.TUMS.MEDICINE.REC.1399.674 and Arba Minch University College of Medicine and Health Sciences Institutional Review Board with Reference number: IRB/T10/2012. After clarifying the study objective and confidentiality of the information; verbal informed consent was obtained from each respective hospital before data collection.
References
- 1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Hypertension 2018;71:e13–115. 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 2.Institute EPH . Ethiopia steps report on risk factors for chronic non-communicable diseases and prevalence of selected NCDs 2016.
- 3.Unger T, Borghi C, Charchar F, et al. 2020 International Society of hypertension global hypertension practice guidelines. Hypertension 2020;75:1334–57. 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010;376:112–23. 10.1016/S0140-6736(10)60834-3 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . A heavy burden: the productivity cost of illness in Africa, 2019. [Google Scholar]
- 6.Region WSEA . Special issue on blood pressure-take control. India, World Health Day 2013.
- 7.WHO . Health profile: Ethiopia. World Health Rankings [onine], 2017. Available: https://www.worldlifeexpectancy.com/country-health-profile/ethiopia
- 8.Tarricone R. Cost-of-illness analysis: what room in health economics? Health Policy 2006;77:51–63. 10.1016/j.healthpol.2005.07.016 [DOI] [PubMed] [Google Scholar]
- 9.Lesyuk W, Kriza C, Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004-2016. BMC Cardiovasc Disord 2018;18:74. 10.1186/s12872-018-0815-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menzin J, Marton JP, Menzin JA, et al. Lost productivity due to premature mortality in developed and emerging countries: an application to smoking cessation. BMC Med Res Methodol 2012;12:87. 10.1186/1471-2288-12-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu JLY, Maniadakis N, Gray A, et al. The economic burden of coronary heart disease in the UK. Heart 2002;88:597–603. 10.1136/heart.88.6.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . WHO guide to identifying the economic consequences of disease and injury 2009.
- 13.CSA and ICF . Ethiopia demographic and health survey. Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF, 2016. [Google Scholar]
- 14.Massimo Volpe CS. Natural history of treated and untreated hypertension. In: Berbari A, Mancia G, eds. Disorders of blood pressure regulation. updates in hypertension and cardiovascular protection. Cham: Springer, 2018. [Google Scholar]
- 15.Desa U. World population prospects 2019: highlights. New York: United Nations Department for Economic and Social Affairs, 2019. [Google Scholar]
- 16.Norheim OF, Baltussen R, Johri M, et al. Guidance on priority setting in health care (GPS-Health): the inclusion of equity criteria not captured by cost-effectiveness analysis. Cost Eff Resour Alloc 2014;12:18. 10.1186/1478-7547-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . It’s time to walk the talk: WHO independent high-level commission on noncommunicable diseases final report. Geneva: World Health Organization, 2019. [Google Scholar]
- 18.Ruhil R. The changing wealth of nations 2018. building a sustainable future, by Glenn-Marie Lange, Quentin Wodon and Kevin Carey; Washington DC: World Bank Group. IASSI-Quarterly 2018;37:135–7. [Google Scholar]
- 19.Turin TC, Okamura T, Afzal AR, et al. Hypertension and lifetime risk of stroke. J Hypertens 2016;34:116–22. 10.1097/HJH.0000000000000753 [DOI] [PubMed] [Google Scholar]
- 20.Beyhaghi H, Viera AJ. Comparative cost-effectiveness of clinic, home, or ambulatory blood pressure measurement for hypertension diagnosis in US adults. Hypertension 2019;73:121–31. 10.1161/HYPERTENSIONAHA.118.11715 [DOI] [PubMed] [Google Scholar]
- 21.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665. 10.1136/bmj.b1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaptoge S, Pennells L, De Bacquer D, et al. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health 2019;7:e1332–45. 10.1016/S2214-109X(19)30318-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawber TR. The Framingham study: the epidemiology of atherosclerotic disease. Cambridge, MA: Harvard University Press, 1980. [Google Scholar]
- 24.Feinleib M, Kannel WB, Garrison RJ, et al. The Framingham offspring study. design and preliminary data. Prev Med 1975;4:518–25. 10.1016/0091-7435(75)90037-7 [DOI] [PubMed] [Google Scholar]
- 25.Parish S, Collins R, Peto R, et al. Cigarette smoking, TAR yields, and non-fatal myocardial infarction: 14,000 cases and 32,000 controls in the United Kingdom. The International studies of infarct survival (Isis) collaborators. BMJ 1995;311:471–7. 10.1136/bmj.311.7003.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Law MR, Morris JK, Wald NJ. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. BMJ 1997;315:973–80. 10.1136/bmj.315.7114.973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medical Expenditure Panel Survey . Medical expenditure panel survey public use files 1996-2001. Available: http://www.meps.ahrq.gov/Puf/PufSearch.asp?SearchOption=Keyword
- 28.Huffman MD, Mohanan PP, Devarajan R, et al. Effect of a quality improvement intervention on clinical outcomes in patients in India with acute myocardial infarction: the ACS Quik randomized clinical trial. JAMA 2018;319:567–78. 10.1001/jama.2017.21906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witt BJ, Brown RD, Jacobsen SJ, et al. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med 2005;143:785–92. 10.7326/0003-4819-143-11-200512060-00006 [DOI] [PubMed] [Google Scholar]
- 30.Yasui D, Asayama K, Ohkubo T, et al. Stroke risk in treated hypertension based on home blood pressure: the Ohasama study. Am J Hypertens 2010;23:508–14. 10.1038/ajh.2010.15 [DOI] [PubMed] [Google Scholar]
- 31.Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:1374–59. 10.1016/j.jvs.2006.10.008 [DOI] [PubMed] [Google Scholar]
- 32.Appelros P, Gunnarsson KE, Terent A. Ten-year risk for myocardial infarction in patients with first-ever stroke: a community-based study. Acta Neurol Scand 2011;124:383–9. [DOI] [PubMed] [Google Scholar]
- 33.Behar S, Tanne D, Abinader E, et al. Cerebrovascular accident complicating acute myocardial infarction: incidence, clinical significance and short- and long-term mortality rates. The sprint Study Group. Am J Med 1991;91:45–50. [DOI] [PubMed] [Google Scholar]
- 34.Lakshminarayan K, Schissel C, Anderson DC, et al. Five-year rehospitalization outcomes in a cohort of patients with acute ischemic stroke. Stroke 2011;42:1556–62. 10.1161/STROKEAHA.110.605600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prosser J, MacGregor L, Lees KR, et al. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke 2007;38:2295–302. 10.1161/STROKEAHA.106.471813 [DOI] [PubMed] [Google Scholar]
- 36.Touzé E, Varenne O, Chatellier G, et al. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: a systematic review and meta-analysis. Stroke 2005;36:2748–55. 10.1161/01.STR.0000190118.02275.33 [DOI] [PubMed] [Google Scholar]
- 37.Health MSf . International medical products price guide. 2015 edn, 2015. [Google Scholar]
- 38.Lee SE, Lee H-Y, Cho H-J, et al. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean acute heart failure registry (KorAHF). Korean Circ J 2017;47:341–53. 10.4070/kcj.2016.0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi D-J, Han S, Jeon E-S, et al. Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: a report from the Korean heart failure registry. Korean Circ J 2011;41:363–71. 10.4070/kcj.2011.41.7.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stenberg K, Lauer JA, Gkountouras G, et al. Econometric estimation of WHO-CHOICE country-specific costs for inpatient and outpatient health service delivery. Cost Eff Resour Alloc 2018;16:11. 10.1186/s12962-018-0095-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Health FMo . National strategic action plan (NSAP) for prevention & control of non-communicable diseases in Ethiopia, 2014-2016 2014:43–7.
- 43.Tolla MT, Norheim OF, Memirie ST, et al. Prevention and treatment of cardiovascular disease in Ethiopia: a cost-effectiveness analysis. Cost Eff Resour Alloc 2016;14:10. 10.1186/s12962-016-0059-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan-Torres Edejer T, Acharya A, Ta A, et al. Making choices in health: WHO guide to cost-effectiveness analysis 2003.
- 45.Iftikhar A. Ethiopia Decent work check. Amsterdam: WageIndicator Foundation, 2019. [Google Scholar]
- 46.Wang G, Zhang Z, Ayala C. Hospitalization costs associated with hypertension as a secondary diagnosis among insured patients aged 18-64 years. Am J Hypertens 2010;23:275–81. 10.1038/ajh.2009.241 [DOI] [PubMed] [Google Scholar]
- 47.Kuriakose A, Nair Anish TS, Soman B, et al. Rate and risk of all cause mortality among people with known hypertension in a rural community of southern Kerala, India: the results from the prolife cohort. Int J Prev Med 2014;5:596–603. [PMC free article] [PubMed] [Google Scholar]
- 48.Atlas WD . Ethiopia - Crude death rate 2020.
- 49.Kibret KT, Mesfin YM. Prevalence of hypertension in Ethiopia: a systematic meta-analysis. Public Health Rev 2015;36:14. 10.1186/s40985-015-0014-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WHO . Non-communicable diseases country profiles 2018. Geneva: World Health Organization, 2018. [Google Scholar]
- 51.Helelo TP, Gelaw YA, Adane AA. Prevalence and associated factors of hypertension among adults in Durame town, southern Ethiopia. PLoS One 2014;9:e112790. 10.1371/journal.pone.0112790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shukuri A, Tewelde T, Shaweno T. Prevalence of old age hypertension and associated factors among older adults in rural Ethiopia. Integr Blood Press Control 2019;12:23–31. 10.2147/IBPC.S212821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou D, Xi B, Zhao M, et al. Uncontrolled hypertension increases risk of all-cause and cardiovascular disease mortality in US adults: the NHANES III linked mortality study. Sci Rep 2018;8:9418. 10.1038/s41598-018-27377-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Najafi F, Karami-Matin B, Rezaei S, et al. Productivity costs and years of potential life lost associated with five leading causes of death: evidence from Iran (2006-2010). Med J Islam Repub Iran 2016;30:412. [PMC free article] [PubMed] [Google Scholar]
- 55.Noh J, Kim HC, Shin A, et al. Prevalence of comorbidity among people with hypertension: the Korea National health and nutrition examination survey 2007-2013. Korean Circ J 2016;46:672–80. 10.4070/kcj.2016.46.5.672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organization . WHO methods and data sources for global burden of disease estimates 2000-2016. Global health estimates technical paper WHO/HIS/IER/GHE/20184. Geneva: WHO, 2018. [Google Scholar]
- 57.Suchard MA, Schuemie MJ, Krumholz HM, et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet 2019;394:1816–26. 10.1016/S0140-6736(19)32317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Law MR, Wald NJ, Morris JK, et al. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 2003;326:1427. 10.1136/bmj.326.7404.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sorato MM, Davari M, Kebriaeezadeh A, et al. Risk of fatal and nonfatal coronary heart disease and stroke events among adult patients with hypertension: basic Markov model inputs for evaluating cost-effectiveness of hypertension treatment: systematic review of cohort studies. J Pharm Health Ser Res 2021;12:283–302. 10.1093/jphsr/rmaa031 [DOI] [Google Scholar]
- 60.Zawudie AB, Lemma TD, Daka DW. Cost of hypertension illness and associated factors among patients attending hospitals in Southwest Shewa Zone, Oromia Regional State, Ethiopia. Clinicoecon Outcomes Res 2020;12:201–11. 10.2147/CEOR.S241591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adane E, Atnafu A, Aschalew AY. The cost of illness of hypertension and associated factors at the University of Gondar comprehensive specialized Hospital Northwest Ethiopia, 2018. Clinicoecon Outcomes Res 2020;12:133–40. 10.2147/CEOR.S234674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Offei S. Economic burden of hypertension among patients attending Nsawam-Government hospital in the Nsawam-Adoagyiri Municipality, Eastern Region, Ghana: University of Ghana, 2018. [Google Scholar]
- 63.Weaver CG, Clement FM, Campbell NRC, et al. Healthcare costs attributable to hypertension. Hypertension 2015;66:502–8. 10.1161/HYPERTENSIONAHA.115.05702 [DOI] [PubMed] [Google Scholar]
- 64.Kirkland EB, Heincelman M, Bishu KG, et al. Trends in healthcare expenditures among US adults with hypertension: national estimates, 2003-2014. J Am Heart Assoc 2018;7:e008731. 10.1161/JAHA.118.008731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen JD. Hypertension epidemiology and economic burden: refining risk assessment to lower costs. Manag Care 2009;18:51–8. [PubMed] [Google Scholar]
- 66.Adeniji F. Burden of out-of-pocket payments among patients with cardiovascular disease in public and private hospitals in Ibadan, South West, Nigeria: a cross-sectional study. BMJ Open 2021;11:e044044-e. 10.1136/bmjopen-2020-044044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pogosova N. Costs associated with cardiovascular disease create a significant burden for society and they seem to be globally underestimated. Eur J Prev Cardiol 2019;26:1147–9. 10.1177/2047487319842578 [DOI] [PubMed] [Google Scholar]
- 68.Le C, Zhankun S, Jun D, et al. The economic burden of hypertension in rural south-west China. Trop Med Int Health 2012;17:1544–51. 10.1111/j.1365-3156.2012.03087.x [DOI] [PubMed] [Google Scholar]
- 69.Sorato MM, Davari M, Kebriaeezadeh A, et al. Reasons for poor blood pressure control in eastern sub-Saharan Africa: looking into 4P’s (primary care, professional, patient, and public health policy) for improving blood pressure control: a scoping review. BMC Cardiovasc Disord 2021;21:123. 10.1186/s12872-021-01934-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoruk A, Boulos PK, Bisognano JD. The state of hypertension in sub-Saharan Africa: review and commentary. Am J Hypertens 2018;31:387–8. 10.1093/ajh/hpx196 [DOI] [PubMed] [Google Scholar]
- 71.Gaziano TA, Bitton A, Anand S, et al. The global cost of nonoptimal blood pressure. J Hypertens 2009;27:1472–7. 10.1097/HJH.0b013e32832a9ba3 [DOI] [PubMed] [Google Scholar]
- 72.Hird TR, Zomer E, Owen AJ. Productivity burden of hypertension in Australia: a life table modeling study. Hypertension 2019;73:777–84. [DOI] [PubMed] [Google Scholar]
- 73.Flack JM, Casciano R, Casciano J, et al. Cardiovascular disease costs associated with uncontrolled hypertension. Manag Care Interface 2002;15:28–36. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-056627supp001.pdf (644.5KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.