Abstract
Objective
Blood-based biomarkers for the early diagnosis of Alzheimer’s disease (AD) are a ‘Holy Grail’ of AD research. Growing evidence shows that levels of apolipoproteins and various inflammation-related factors are altered in the peripheral blood of patients with AD. The purpose of this study was to clear and definite whether these biomarkers are differentially expressed at the early stages of AD, and could be a biomarker as an early diagnosis of the disease.
Design
Observation study.
Setting
This study was a part of the Sino Longitudinal Study on Cognitive Decline, an ongoing prospective cohort study (ClinicalTrials.gov identifier: NCT03370744) that centres on Xuanwu Hospital (Beijing, China) in cooperation with an alliance of 94 hospitals from 50 cities across China.
Participants
In the present study, 416 right-handed Chinese Han subjects were recruited through standardised public advertisements from 2014 to 2019.
Outcome measures
Concentrations of plasma apolipoprotein A1, apolipoprotein CIII (ApoCIII), apolipoprotein E (ApoE), A-2-macroglobulin (A2M), complement C3 (C3) and complement factor H (FH) were determined using a commercial multiplex Luminex-based panel in normal controls (NC), subjective cognitive decline (SCD), mild cognitive impairment and AD groups.
Results
For individual analysis, pairwise comparisons showed that: (1) For SCD versus NC, no biomarker showed significant difference; (2) For amnestic mild cognitive impairment (aMCI) versus NC, levels of ApoCIII, ApoE, A2M, C3 and FH increased significantly; and (3) For AD versus NC, amounts of C3 increased. For models differentiating clinical groups, discriminant analysis was performed by including all protein markers, age, sex, genotype and education level in the model. This approach could distinguish between patients with aMCI (area under the curve (AUC): 0.743) and AD (AUC: 0.837) from NC.
Conclusion
Our results suggest that concentrations of certain apolipoproteins and inflammation-related factors are altered at the early stage of AD, and could be useful biomarkers for early diagnosis.
Trial registration number
Keywords: neurobiology, neurology, neuropathology, dementia
Strengths and limitations of this study.
We used a more sensitive method to simultaneously detect the concentration of apolipoproteins and inflammation-related factors in the plasma of 416 participants at various clinical stages.
Apolipoproteins and inflammation-related factors were altered at the early stage of Alzheimer’s disease (AD), and clinical information combined with these proteins had a high specificity to distinguish AD and mild cognitive impairment from normal controls.
The sample size is relatively small, especially for patients with AD, which may negatively influence statistical power.
The present study was a cross-sectional study. A longitudinal design investigating changes of these apolipoproteins and inflammation-related factors along with AD progression would definitely provide new insight into the roles of these protein markers.
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder in the elderly that leads to dementia.1 Subjective cognitive decline (SCD) is regarded as the first clinical manifestation in the AD continuum, and describes self-experienced persistent cognitive decline compared with a previously normal status.2 SCD is prevalent in adults older than 60 years,3 and approximately 14% of patients with SCD convert to AD within 4 years.4 Amnestic mild cognitive impairment (aMCI) is an intermediate state between SCD and dementia. The prevalence of aMCI in adults older than 60 years is approximately 14%.5 For patients with aMCI, the annual rate of progression to dementia is approximately 10% to 15%,6 and individuals with aMCI may have a higher risk of developing AD.7
Apolipoproteins are a group of proteins related to cholesterol and lipid metabolism.8 9 The levels of apolipoproteins in the peripheral blood of patients with AD have been extensively studied. Most studies show that apolipoprotein A1 (ApoA1) and apolipoprotein CIII (ApoCIII) levels are significantly lower in AD.10–13 Studies on apolipoprotein E (ApoE) levels in blood are less consistent, but a recent meta-analysis supported decreased levels of blood ApoE in patients with AD, supporting its potential value as an important risk factor for AD.14
Growing evidence indicates inflammation and complement dysregulation play essential roles in AD pathogenesis. A-2-macroglobulin (A2M) is a major component of the innate immune system and a chaperone protein that functions as a pan-protease inhibitor.15 Proteomic studies show an increase in plasma A2M concentration in patients with AD compared with controls.16–18 Complement C3 (C3) and complement factor H (FH) are key regulators of the complement system. Although a meta-analysis reported that differences in C3 and FH concentrations between AD and healthy controls are not significant in peripheral blood,19 a recent study showed differential levels of C3 and FH in patients with aMCI compared with healthy controls.20
Based on the described research, concentrations of ApoA1, ApoCIII, ApoE, A2M, C3 and FH in peripheral blood were inconsistent, yet all proteins had already changed at the dementia stage of AD. Therefore, we hypothesised that these proteins may also be differentially expressed at an early stage and could be used as biomarker(s) for early diagnosis. Further, establishment of blood-based biomarkers that can be used to screen for SCD and aMCI are critical and would be beneficial for trials testing potential disease-modifying treatments. To test this hypothesis, we used a more sensitive method (ie, a commercial multiplex Luminex-based panel) to simultaneously detect the concentration of those six proteins in the plasma of 416 participants at various clinical stages.
Materials and methods
Participants
This study was a part of the Sino Longitudinal Study on Cognitive Decline, an ongoing prospective cohort study (ClinicalTrials.gov identifier: NCT03370744) that centres on Xuanwu Hospital (Beijing, China) in cooperation with an alliance of 94 hospitals from 50 cities across China. All protocols were conducted in accordance with the Declaration of Helsinki.21 All subjects provided written informed consent. In the present study, 416 right-handed Chinese Han subjects were recruited through standardised public advertisements from 2014 to 2019. SCD was defined with the following criteria, according to the framework proposed in 2014:22 (1) Presence of a self-perceived continuous cognitive decline compared with a previously normal status, which was unrelated to an acute event; (2) Concerns or worries associated with cognitive complaints; and (3) Normal performance on standardised neuropsychological tests and failure to meet the criteria for aMCI or dementia. aMCI was defined based on an actuarial neuropsychological method proposed by Jak et al23 when the individual met any one of the following three criteria and failed to meet the criteria for dementia: (1) Having impaired scores (defined as >>1 SD below the age-corrected normative means) on both measures in at least one cognitive domain (memory, language or speed/executive function); (2) Having impaired scores in each of the three cognitive domains sampled (memory, language or speed/executive function); and (3) Functional Activities Questionnaire ≥9. The diagnosis of AD in the present study was based on the core clinical criteria for probably AD dementia delivered by National Institute on Aging and Alzheimer's Association (NIA-AA) in 2011 as follows: (1) Symptoms have a gradual onset over months to years, not sudden over hours or days; (2) Clear-cut history of worsening of cognition by report or observation; (3) The initial and most prominent cognitive deficit was an amnestic presentation based on history and examination; (4) Without substantial concomitant cerebrovascular disease, core features of dementia with Lewy bodies, prominent features of frontotemporal dementia or evidence for another concurrent active neurological disease. Besides clinical symptoms, all subjects completed routine MRI scanning of T1, T2 and fluid attenuated inversion recovery (FLAIR). All the subjects involved had hippocampal atrophy. According to MRI, cerebrovascular disease and intracranial occupancy were excluded. The diagnoses were confirmed by two neurologists from Xuanwu Hospital.24 Normal controls (NCs) were recruited from local communities or through online media advertising. The inclusion criteria for NC included Han Chinese nationality, right-handedness, no cognitive complaints or any concerns, normal scores in standardised neuropsychological tests, a negative result for nervous system physical examinations, without any relevant medical histories or family histories, and no diseases revealed on accessory examinations that could cause cognitive decline. All diagnoses were performed by two neurologists from the Neurology Department, Xuanwu Hospital, Capital Medical University (Beijing, China).
The exclusion criteria were: (1) A history of stroke; (2) Current major psychiatric diagnoses such as severe depression and anxiety; (3) Other neurological conditions causing cognitive decline (eg, brain tumour, Parkinson’s disease, encephalitis or epilepsy) rather than AD spectrum disorders; (4) Other systematic diseases causing cognitive decline (eg, thyroid dysfunction, severe anaemia, syphilis or HIV); (5) A history of psychosis or congenital mental developmental delay; and (6) Cognitive decline caused by traumatic brain injury.
Patient and public involvement
Patients and the public will not be involved in the development of the research question or the design of the study. Patients will not be involved in the recruitment of participants or the conduct of the study. The general results will be disseminated to participants through public education activities.
Neuropsychological tests
All subjects underwent standardised clinical and neuropsychological evaluation. The Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment-Basic (MoCA-B) were administered for global cognition.
APOE genotyping
Genomic DNA was extracted from peripheral blood leucocytes using TIANamp Genomic DNA Kit (Tiangen Biothch, Beijing, China). Genotyping of two APOE single nucleotide polymorphisms was undertaken to determine the APOE genotype, which is comprised of three alleles (ε2, ε3, ε4). In brief, APOE was amplified with the following primers: 5′-ACGCGGGCACGGCTGTCCAAGG-3′ (forward) and 5 ′-GGCGCTCGCGGATGGCGCTGA-3′ (reverse), using the following conditions: 1 cycle of 98°C for 10 s, 35 cycles of 72°C for 5 s and 1 cycle of 72°C for 5 min. PCR was performed in a final volume of 30 µl, containing 10 pmol of forward and reverse primers and 50 ng of genomic DNA template, using PrimeSTAR HS DNA Polymerase with GC Buffer (Takara Bio, Shiga, Japan), according to the manufacturer’s instructions. APOE was then genotyped using the standard Sanger sequencing method (Sangon, Shanghai, China).
Measurement of protein markers in plasma samples
Venous blood was obtained from all participants. A trained technician drew blood from fasting participants in the morning using 10 mL EDTA tubes, and then centrifuged the samples at 2500×g for 10 min at 4°C within 30 min of blood collection. The extracted plasma sample was aliquoted and stored at −80°C until analysis. The relevant proteins were measured using a commercial Luminex xMAP Human Neurodegenerative Disease Magnetic Bead Panel kit (EMD Millipore Corporation, Billerica, Massachusetts, USA) according to the manufacturer’s instructions. Plates were read on the Luminex 200 multiplexing instrument (Luminex Corp, Austin, Texas, USA). The assay sensitivity for ApoA1, ApoCIII, ApoE, A2M, C3 and FH was 0.3 ng/mL, 0.001 ng/mL, 0.003 ng/mL, 0.177 ng/mL, 0.012 ng/mL and 0.177 ng/mL, respectively. The intra-assay and interassay precisions were 10% and 20%, respectively.
Statistical analysis
All statistical analyses were performed using SPSS V.22.0 (IBM Corp, New York, New York, USA). Data distribution was assessed for normality with the Shapiro-Wilk test. Sex and APOE genotype distribution were analysed using the χ2 test. Age was analysed using Tukey’s multiple comparison. MMSE Score, MoCA-B Score and education level were analysed using Dunn’s test for multiple comparisons. Multiple linear regression covariate with age, sex, APOE genotype and education were used to compare the plasma of six protein markers in different groups. Discriminant analysis and receiver operating characteristics (ROC) curve analysis were used to reveal the diagnostic ability of six protein markers combined with age, education, sex and APOE genotype. A two-sided value of p<0.05 was considered statistically significant.
Results
Demographic data
Demographic and clinical information is given in table 1. In this study, 416 people were included. However, the protein values in 13 people appeared out of detectable concentration range, therefore these people were excluded, and the remaining 403 people were included in the study. In total 148 NC, 138 patients with SCD, 74 patients with aMCI and 43 patients with AD patients were included. Significant differences were observed between clinical groups in terms of age, sex, education level, MMSE Score and MoCA-B Score. There was a higher percentage of female subjects in the SCD group compared with the NC group. Patients with aMCI and AD were significantly older than NC subjects. Patients with aMCI and AD had a lower education level than NC subjects. Patients with aMCI and AD scored lower in MMSE and MoCA-B than NC subjects.
Table 1.
Subject demographics and clinical features
| NC | SCD | aMCI | AD | P value | |
| N | 148 | 138 | 74 | 43 | |
| Sex (M/F) | 65/83 | 38/100 | 31/43 | 17/26 | 0.001 |
| Age, years | 64.85±7.22 | 64.66±7.02 | 68.07±7.96 | 70.43±9.36 | <0.001 |
| Years of education | 12.67±3.36 | 12.84±3.05 | 11.19±4.12 | 10.24±4.28 | <0.001 |
| MMSE Score | 29.00 (28.00, 30.00) | 29.00 (28.00, 30.00) | 26.00 (24.00, 28.00) | 18.50 (13.75, 21.00) | <0.001 |
| MoCA-B Score | 26.00 (24.00, 28.00) | 26.00 (24.00, 27.00) | 21.00 (19.00, 23.00) | 14.00 (11.00, 16.00) | <0.001 |
Age and years of education are presented as mean±SD, MMSE and MoCA-B Scores are presented as median (IQR). χ2 test was used for categorical variables and Tukey’s or Dunn’s test for continuous variables.
AD, Alzheimer’s disease; aMCI, amnestic mild cognitive impairment; MMSE score, Mini-Mental State Examination score; MoCA-B score, Montreal Cognitive Assessment-Basic score; NC, normal controls; SCD, subjective cognitive decline.
Distribution of APOE genotypes in different groups is shown in table 2. Isoforms of ε2/ε2 genotypes were absent in SCD and AD groups, and accounted for 1% in NC and aMCI groups. The proportion of ε2/ε3 genotype, ε2/ε4 genotype and ε3/ε4 genotype in each group was about 11%, 3% and 30%, respectively; ε3/ ε3 genotype was the highest expression level was found in all genotypes, of which 32.6% in group AD and 50% in other groups; ε4/ε4 genotype accounted for about 10% in groups of aMCI and AD.
Table 2.
Distribution of APOE genotypes
| NC (n=148) | SCD (n=138) | aMCI (n=74) | AD (n=43) | |||||
| N % | N % | N % | N % | |||||
| ε2/ε2 | 2 | 1.4 | 0 | 0 | 1 | 1.4 | 0 | 0 |
| ε2/ε3 | 11 | 7.4 | 16 | 11.6 | 12 | 16.2 | 5 | 11.6 |
| ε2/ε4 | 8 | 5.4 | 5 | 3.6 | 2 | 2.7 | 1 | 2.3 |
| ε3/ε3 | 81 | 54.7 | 73 | 52.9 | 37 | 50 | 14 | 32.6 |
| ε3/ε4 | 45 | 30.4 | 42 | 30.4 | 15 | 20.3 | 19 | 44.2 |
| ε4/ε4 | 1 | 0.7 | 2 | 1.4 | 7 | 9.5 | 4 | 9.3 |
AD, Alzheimer’s disease; aMCI, amnestic mild cognitive impairment; NC, normal controls; SCD, subjective cognitive decline.
Quantification of six protein markers
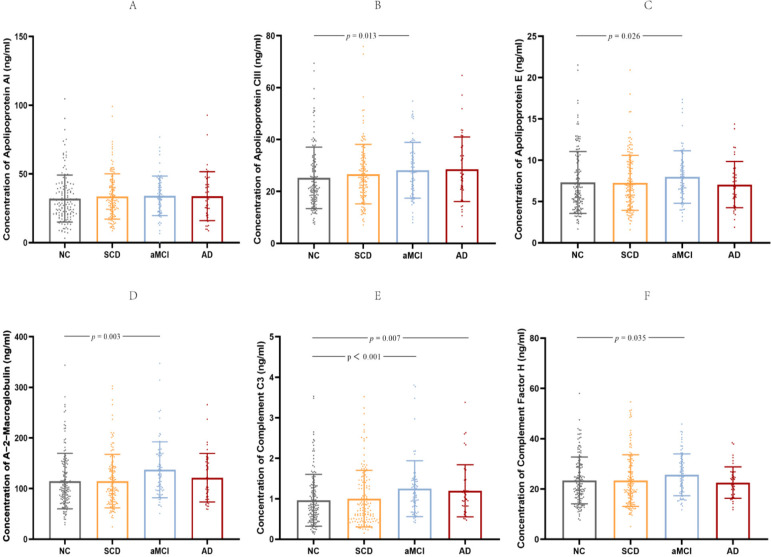
Participants with abnormal liver function were excluded based on history and there was no significant difference in the level of low-density lipoprotein in blood among the groups (F=0.609, p=0.545). The human neurodegenerative disease magnetic bead panel was chosen to simultaneously examine the amounts of ApoA1, ApoCIII, ApoE, A2M, C3 and FH in plasma. The kit contains quality control materials. The experimental results showed that the concentrations of these two quality control values were within the range shown in the manual, indicating that the data of each plate are reliable. Accessory holes were made for each plasma sample. Our results showed that ApoE, A2M, C3 and FH were detectable in all samples, while 3 samples (1 from aMCI and 2 from NC) from ApoA1 were lower than minimum detectable concentration and 13 samples (2 from SCD, 2 from aMCI and 9 from NC) from ApoCIII were higher than maximum detectable concentration, respectively. Multiple linear regression was performed using the covariates of age, sex, APOE genotype and education level to examine comparisons between groups. (1) For SCD versus NC, there were no significant differences; (2) For aMCI versus NC, increased ApoCIII, ApoE, A2M, C3 and FH were observed; and (3) For AD versus NC, increased C3 was observed (table 3; figure 1A–F).
Table 3.
Six protein markers across different clinical states
| Analyte | NC (n=148) (Mean±SD) |
SCD (n=138) (Mean±SD) |
aMCI (n=74) (Mean±SD) |
AD (n=43) (Mean±SD) |
P value SCD vs NC |
P value aMCI vs NC | P value AD vs NC |
| ApoA1 (ng/ml) | 32.07±17.13 | 33.63±16.47 | 34.02±14.39 | 33.81±17.83 | NS | NS | NS |
| ApoCIII (ng/ml) | 25.22±11.87 | 26.68±11.43 | 28.19±10.77 | 28.55±12.46 | NS | 0.013 | NS |
| ApoE (ng/ml) | 7.30±3.75 | 7.26±3.33 | 7.97±3.18 | 7.05±2.80 | NS | 0.026 | NS |
| A2M (ng/ml) | 114.69±54.82 | 114.83±52.88 | 137.10±55.18 | 121.51±47.93 | NS | 0.003 | NS |
| C3 (ng/ml) | 0.96±0.64 | 1.00±0.70 | 1.25±0.69 | 1.20±0.64 | NS | <0.001 | 0.007 |
| FH (ng/ml) | 23.42±9.29 | 23.37±10.28 | 25.67±8.28 | 22.55±6.24 | NS | 0.035 | NS |
All data are expressed as mean±SD. Multiple linear regression with age, sex, APOE genotype and education level used as covariates.
AD, Alzheimer’s disease; A2M, A-2-macroglobulin; aMCI, amnestic mild cognitive impairment; ApoA1, apolipoprotein A1; ApoCIII, apolipoprotein CIII; ApoE, apolipoprotein E; C3, complement C3; FH, complement factor H; NC, normal controls; SCD, subjective cognitive decline.
Figure 1.
Six protein markers in the NC, SCD, aMCI and AD groups. The concentrations of plasma apolipoprotein A1 (A), apolipoprotein CIII (B), apolipoprotein E (C), A-2-macroglobulin (D), complement C3 (E) and complement factor H (F) in different clinical groups are each shown in scatter plots with SD. The values of p shown were obtained following multiple linear regression using age, sex, APOE genotype and education level as covariates. Statistically significant p values are shown. aMCI, amnestic mild cognitive impairment; AD, Alzheimer’s disease; NC, normal controls; SCD, subjective cognitive decline.
Developing models to differentiate clinical groups
Discriminant analysis was performed by including all protein markers in the model and it was able to distinguish patients with aMCI (Wilk’s λ, 0.923; p=0.007) and AD (Wilk’s λ, 0.895; p=0.001) from NC.
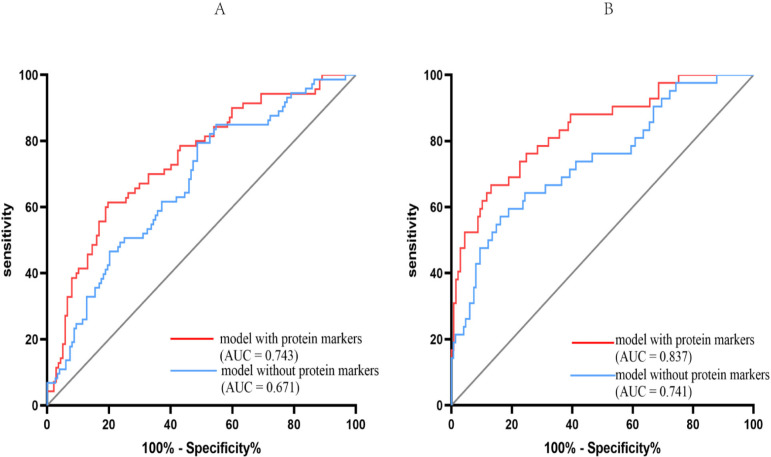
To differentiate aMCI from NC, a D-score was calculated as follows: D-score=1.098 × A2M – 0.833×ApoA1 – 0.019×ApoCIII + 0.077×ApoE + 0.965×complement C3 – 0.661×FH. ROC analysis was also performed (sensitivity 0.614 and specificity 0.803) to distinguish between patients with aMCI from NC but including protein biomarkers with age, sex, APOE genotype and education level in the model. It showed that including protein markers into the model strengthened the discriminatory power (model with protein markers: area under the curve (AUC), 0.743; 95% CI 0.671 to 0.814; p<0.001; model without protein markers: AUC, 0.671; 95% CI 0.597 to 0.746; p<0.001; figure 2A).
Figure 2.
Receiver operating characteristics (ROC) curve analysis for models distinguishing clinical states. ROC curves were generated representing models that differentiated between amnestic mild cognitive impairment (aMCI) (A) or Alzheimer’s disease (AD) (B) from controls. in each case, the area under the curve (AUC) for selected models was calculated and is shown. The red line represents the model including protein markers as covariates. The blue line represents the model without protein markers as covariates.
To differentiate AD from NC, a D-score was calculated as follows: D-score=1.016 × A2M – 0.442×ApoA1+0.231 × ApoCIII – 0.211×ApoE + 1.101×complement C3 – 0.661×FH. ROC analysis was also performed (sensitivity 0.667 and specificity 0.869) to discriminate between patients with AD from NC but including protein biomarkers with age, sex, APOE genotype and education level in the model. It showed that including protein markers into the model again strengthened the discriminatory power (model with protein markers: AUC, 0.837; 95% CI 0.765 to 0.909; p<0.001; model without protein markers: AUC, 0.741; 95% CI 0.652 to 0.830; p<0.001; figure 2B).
Discussion
A blood-based biomarker or biomarker set that aids early diagnosis of AD is of great importance. Many putative biomarkers have been reported,25 however their robust replication and clinical usefulness are still an enormous challenge. In the present study, concentrations of a set of apolipoproteins and inflammation-related factors were examined at various clinical stages of AD. It showed that some analyses were differentially expressed at the early stage of AD. A discriminating model with all protein markers, age, sex, genotype and education level included as covariates could distinguish between patients with aMCI (AUC: 0.743) and AD (AUC: 0.837) from NC.
Six protein markers, including ApoA1, ApoCIII, ApoE, A2M, C3 and FH were examined in the present study. Recent studies show that apolipoproteins play an important role in neurodegenerative progress. ApoE and ApoA1 have the potential ability to clear beta amyloid (Aβ) or prevent Aβ aggregation and thereby prevent induced neurotoxicity.26 27 ApoCIII can also bind to Aβ and indirectly regulate Aβ deposition in the brain.28 Inflammation and complement dysregulation are important components of AD pathogenesis.29 30 A2M is a major serum antiprotease that has the capacity to bind and regulate a variety of cytokines. In addition, A2M can also form a complex with Aβ and prevent Aβ fibril formation. Complement proteins such as C3 and FH can promote phagocytosis activity and induce Aβ clearance, while excessive complement activation can result in extreme cell damage and systemic inflammatory responses.
The levels of these six protein markers have been examined in AD plasma. Most studies have shown that concentrations of ApoA1 and ApoCIII levels are significantly lower in AD.10–12 31 Studies on ApoE levels in blood are less consistent, but a recent meta-analysis supported decreased levels of blood ApoE in patients with AD. Proteomic studies show an increase in plasma A2M concentration in patients with AD compared with controls.16–18 Although a recent meta-analysis showed that the differences in C3 and FH concentration between AD and healthy controls were not significant in peripheral blood. Nevertheless, the study included in this meta-analysis with a larger study population showed increased peripheral blood C3 concentration in AD.19 Levels of these markers at the early stage of AD are largely unexplored. A few studies have shown increased concentrations of ApoE, A2M, C3 and FH in patients with aMCI.16 20 31 In our study, amounts of these six proteins were examined in patients with SCD, aMCI and dementia. No marker showed significant differences at the SCD stage. Increased concentrations were identified for A2M, ApoCIII, ApoE, C3 and FH at the aMCI stage, while only C3 remained upregulated at the AD stage. These results may reflect a dynamic change in these proteins in the process of disease development, and A2M, ApoCIII, ApoE, C3 and FH may play a reverse role in the AD stage. Our findings together with previous reports suggest that levels of these protein markers vary with clinical condition.
Another interesting finding is that we managed to establish a model to distinguish between patients with aMCI and AD from NC by combining basic clinical information and levels of these protein markers. Diagnostic accuracy in distinguishing NC from aMCI was moderate (AUC: 0.743), and was better in distinguishing NC from AD (AUC: 0.837). Recently, Jia et al, reported that examination of blood exosomal Aβ42, T-tau, and P-T181-tau could be useful for AD and aMCI diagnosis.32 Compared with their neuronal-derived exosome-based strategy, our method is straightforward. Target proteins could be examined directly in plasma. Of note, all six protein markers were included as covariates in our model, although only some were differentially expressed significantly. In support of our approach to establish a model, Jia et al established a highly accurate diagnosis model by combining four different synaptic protein markers, changes in whose concentrations were minimal.33
There are some limitations to our studies. First, the sample size is relatively small, especially for patients with AD, which may negatively influence statistical power. Second, all the participants were recruited from Beijing, which may lead to a geographical bias. Third, there were some mismatches in terms of age and sex between clinical groups. Fourth, the diagnosis of SCD, aMCI and AD relied mainly on neuropsychological tests. Therefore, clinical misdiagnosis may not be fully avoided. Fifth, the distribution of isoforms of APOE genotype in different groups were analysed, but their distribution in each group was uneven and the sample size was relatively small, so it is not clear if different isoforms of APOE genotype were detected equally by this assay. Therefore, increasing the number of different isoforms of gene carriers is helpful to systematically analyse the effects of different APOE gene isoforms on the levels of ApoE. Finally, the present study was a cross-sectional study. A longitudinal design investigating changes of these apolipoproteins and inflammation-related factors along with AD progression would definitely provide new insight into the roles of these protein markers. Considering these limitations, longitudinal multicentre cooperation should be promoted in future studies.
In this study, six proteins in plasma were detected simultaneously, which reduced the heterogeneity of independent detection and increased prediction efficiency. The results of the previous studies were verified using a more sensitive method, which provided a more reliable basis for changes in these proteins at different stages of AD.
Conclusion
Our results showed that a set of apolipoproteins and inflammation-related factors were differentially expressed at the early stage of AD, which could be used to create diagnostic models for AD and aMCI.
Supplementary Material
Acknowledgments
The authors thank Rachel James, Ph.D., from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
TW, XW and YY contributed equally.
Contributors: YC designed the study. XW and YH recruited participants and performed the clinical investigation. TW, YY, CZ and CY performed the Luminex assay and genotyped APOE. TW and YY performed the data analysis and wrote the manuscript. All coauthors contributed to revising the manuscript for intellectual content and approved the final version for publication. The guarantor in this study is YC.
Funding: This work was supported by the National Key Research and Development Programme of China (nos. 2016YFC1306300, 2017YFC0909100, 2017YFC0909103, 2017YFC1310200), National Key R&D Program of China 2021YFC2501205, the National Natural Science Foundation of China (nos. 61633018, 81671246), and Science Innovation 2030 - Brain Science and Brain-Like Intelligence Technology Major Project #2021ZD0201100 Task 1 #2021ZD0201101
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The studies involving human participants were reviewed and approved by the Medical Research Ethics Committee and Institutional Review Board of Xuanwu Hospital (2017) 046.
References
- 1.Hsu J-L, Lee W-J, Liao Y-C, et al. The clinical significance of plasma clusterin and Aβ in the longitudinal follow-up of patients with Alzheimer's disease. Alzheimers Res Ther 2017;9:91. 10.1186/s13195-017-0319-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabin LA, Smart CM, Amariglio RE. Subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol 2017;13:369–96. 10.1146/annurev-clinpsy-032816-045136 [DOI] [PubMed] [Google Scholar]
- 3.Cedres N, Machado A, Molina Y, et al. Subjective cognitive decline below and above the age of 60: a multivariate study on neuroimaging, cognitive, clinical, and demographic measures. J Alzheimers Dis 2019;68:295–309. 10.3233/JAD-180720 [DOI] [PubMed] [Google Scholar]
- 4.Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol 2020;19:271–8. 10.1016/S1474-4422(19)30368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue J, Li J, Liang J, et al. The prevalence of mild cognitive impairment in China: a systematic review. Aging Dis 2018;9:706–15. 10.14336/AD.2017.0928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eshkoor SA, Hamid TA, Mun CY, et al. Mild cognitive impairment and its management in older people. Clin Interv Aging 2015;10:687–93. 10.2147/CIA.S73922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giau VV, Bagyinszky E, An SSA. Potential fluid biomarkers for the diagnosis of mild cognitive impairment. Int J Mol Sci 2019;20:4149. 10.3390/ijms20174149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millán J, Pintó X, Muñoz A, et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 2009;5:757–65. [PMC free article] [PubMed] [Google Scholar]
- 9.Eichner JE, Dunn ST, Perveen G, et al. Apolipoprotein E polymorphism and cardiovascular disease: a huge review. Am J Epidemiol 2002;155:487–95. 10.1093/aje/155.6.487 [DOI] [PubMed] [Google Scholar]
- 10.Lin Q, Cao Y, Gao J. Decreased expression of the APOA1-APOC3-APOA4 gene cluster is associated with risk of Alzheimer's disease. Drug Des Devel Ther 2015;9:5421–31. 10.2147/DDDT.S89279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H-C, Hu C-J, Chang J-G, et al. Proteomic identification of lower apolipoprotein A-I in Alzheimer's disease. Dement Geriatr Cogn Disord 2006;21:155–61. 10.1159/000090676 [DOI] [PubMed] [Google Scholar]
- 12.Kitamura Y, Usami R, Ichihara S, et al. Plasma protein profiling for potential biomarkers in the early diagnosis of Alzheimer's disease. Neurol Res 2017;39:231–8. 10.1080/01616412.2017.1281195 [DOI] [PubMed] [Google Scholar]
- 13.Ijsselstijn L, Papma JM, Dekker LJM, et al. Serum proteomics in amnestic mild cognitive impairment. Proteomics 2013;13:2526–33. 10.1002/pmic.201200190 [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Yu J-T, Wang H-F, et al. Meta-Analysis of peripheral blood apolipoprotein E levels in Alzheimer's disease. PLoS One 2014;9:e89041. 10.1371/journal.pone.0089041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scacchi R, Ruggeri M, Gambina G, et al. Alpha2-Macroglobulin deletion polymorphism and plasma levels in late onset Alzheimer's disease. Clin Chem Lab Med 2002;40:333–6. 10.1515/CCLM.2002.052 [DOI] [PubMed] [Google Scholar]
- 16.Varma VR, Varma S, An Y, et al. Alpha-2 macroglobulin in Alzheimer's disease: a marker of neuronal injury through the RCAN1 pathway. Mol Psychiatry 2017;22:13–23. 10.1038/mp.2016.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Lyutvinskiy Y, Herukka S-K, et al. Prognostic polypeptide blood plasma biomarkers of Alzheimer's disease progression. J Alzheimers Dis 2014;40:659–66. 10.3233/JAD-132102 [DOI] [PubMed] [Google Scholar]
- 18.Thambisetty M, Simmons A, Hye A, et al. Plasma biomarkers of brain atrophy in Alzheimer's disease. PLoS One 2011;6:e28527. 10.1371/journal.pone.0028527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krance SH, Wu C-Y, Zou Y, et al. The complement cascade in Alzheimer's disease: a systematic review and meta-analysis. Mol Psychiatry 2021;26:5532–41. 10.1038/s41380-019-0536-8 [DOI] [PubMed] [Google Scholar]
- 20.Morgan AR, Touchard S, Leckey C, et al. Inflammatory biomarkers in Alzheimer's disease plasma. Alzheimers Dement 2019;15:776–87. 10.1016/j.jalz.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Medical Association . World Medical association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 2014;10:844–52. 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 2009;17:368–75. 10.1097/JGP.0b013e31819431d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–9. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampel H, O'Bryant SE, Molinuevo JL, et al. Blood-Based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol 2018;14:639–52. 10.1038/s41582-018-0079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paula-Lima AC, Tricerri MA, Brito-Moreira J, et al. Human apolipoprotein A-I binds amyloid-beta and prevents Abeta-induced neurotoxicity. Int J Biochem Cell Biol 2009;41:1361–70. 10.1016/j.biocel.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki Y, Zhao N, Caulfield TR, et al. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol 2019;15:501–18. 10.1038/s41582-019-0228-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih Y-H, Tsai K-J, Lee C-W, et al. Apolipoprotein C-III is an amyloid-β-binding protein and an early marker for Alzheimer's disease. J Alzheimers Dis 2014;41:855–65. 10.3233/JAD-140111 [DOI] [PubMed] [Google Scholar]
- 29.International Genomics of Alzheimer's Disease Consortium (IGAP) . Convergent genetic and expression data implicate immunity in Alzheimer's disease. Alzheimers Dement 2015;11:658–71. 10.1016/j.jalz.2014.05.1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpanini SM, Torvell M, Morgan BP. Therapeutic inhibition of the complement system in diseases of the central nervous system. Front Immunol 2019;10:362. 10.3389/fimmu.2019.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song F, Poljak A, Crawford J, et al. Plasma apolipoprotein levels are associated with cognitive status and decline in a community cohort of older individuals. PLoS One 2012;7:e34078. 10.1371/journal.pone.0034078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia L, Qiu Q, Zhang H, et al. Concordance between the assessment of Aβ42, t-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement 2019;15:1071–80. 10.1016/j.jalz.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 33.Jia L, Zhu M, Kong C, et al. Blood neuro-exosomal synaptic proteins predict Alzheimer's disease at the asymptomatic stage. Alzheimers Dement 2021;17:49–60. 10.1002/alz.12166 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.