Abstract
Introduction
Group A β-haemolytic Streptococcus (GAS), a Gram-positive bacterium, causes skin, mucosal and systemic infections. Repeated GAS infections can lead to autoimmune diseases acute rheumatic fever (ARF) and rheumatic heart disease (RHD). Aboriginal and Torres Strait Islander peoples in Australia have the highest rates of ARF and RHD in the world. Despite this, the contemporaneous prevalence and incidence of GAS pharyngitis and impetigo in remote Australia remains unknown. To address this, we have designed a prospective surveillance study of GAS pharyngitis and impetigo to collect coincident contemporary evidence to inform and enhance primary prevention strategies for ARF.
Methods and analysis
The Missing Piece Study aims to document the epidemiology of GAS pharyngitis and impetigo through collection of clinical, serological, microbiological and bacterial genomic data among remote-living Australian children. The study comprises two components: (1) screening of all children at school for GAS pharyngitis and impetigo up to three times a year and (2) weekly active surveillance visits to detect new cases of pharyngitis and impetigo. Environmental swabbing in remote schools will be included, to inform environmental health interventions. In addition, the application of new diagnostic technologies, microbiome analysis and bacterial genomic evaluations will enhance primary prevention strategies, having direct bearing on clinical care, vaccine development and surveillance for vaccine clinical trials.
Ethics and dissemination
Ethical approval has been obtained from the Western Australian Aboriginal Health Ethics Committee (Ref: 892) and Human Research Ethics Committee of the University of Western Australia (Ref: RA/4/20/5101). Study findings will be shared with community members, teachers and children at participating schools, together with academic and medical services. Sharing findings in an appropriate manner is important and will be done in a suitable way which includes plain language summaries and presentations. Finally, findings and updates will also be disseminated to collaborators, researchers and health planners through peer-reviewed journal publications.
Keywords: Epidemiology, Public health, INFECTIOUS DISEASES
Strengths and limitations of this study.
The prospective study design provides a platform to apply new medical diagnostic technologies, microbiome and genomic analysis using comprehensive and validated methods.
The incidence and prevalence observed in the school-based setting may be more representative of the burden of disease in children in the community.
Reliance on self-report of sore throat and once a week active surveillance visits has potential to miss some children if they are absent or not seen between surveillance visits.
Introduction
Group A Streptococcus (GAS) is a human-adapted bacterial pathogen responsible for a broad spectrum of diseases ranging from superficial infections (eg, pharyngitis and impetigo), to invasive diseases (eg, bacteraemia, necrotising fasciitis, toxic shock syndrome) and autoimmune diseases (eg, acute rheumatic fever (ARF), rheumatic heart disease (RHD) and acute poststreptococcal glomerulonephritis).1 RHD is estimated to affect 40 million people globally (95% CI 37.9 million to 40.8 million)2 with an estimated 319 000 deaths each year (95% CI 297 000 to 337 300).3 Notably, the highest reported incidence (374–508 per 100 000 children per year)4 5 and mortality rates (23.8 per 100 000 children per year) are among Aboriginal and Torres Strait Islander peoples in Australia.5 6
A causal association between GAS pharyngitis and ARF/RHD is well defined and supported by over 60 years of evidence gathered in temperate climates where GAS impetigo was uncommon.7 8 In Australia, where GAS impetigo is endemic and clinical pharyngitis is uncommon, impetigo is thought to be implicated in the development of ARF/RHD, however, this proposed association lacks systematic population-level surveillance to ‘quantify’ causal associations. There has been at least one reported case of ARF following a documented GAS skin infection,9 however, the published evidence is sparse. The high incidence of ARF/RHD among Aboriginal and Torres Strait Islander communities, where impetigo is pervasive10 11 and pharyngitis is seemingly uncommon, suggest an association between impetigo and ARF/RHD.8 In Aboriginal and Torres Strait Islander children, the median prevalence of impetigo is as high as 45% (IQR 34.0%–49.2%)12 with the majority of children presenting with GAS impetigo to a primary healthcare facility at least once before their first birthday.13–15 Within this setting of alarmingly high GAS impetigo, the fundamental ‘Missing Piece’ of evidence is whether GAS pharyngitis is truly uncommon16 or whether it is underdiagnosed and undertreated in remote Australian communities. Connected to these questions is the need to develop a more accurate understanding of how impetigo may contribute to ARF pathogenesis.
A well-designed prospective surveillance programme with predefined research questions, using feasible clinical assessment methodologies, provides a platform to concurrently study the epidemiology of GAS pharyngitis and impetigo, and explore additional pathogenesis and molecular epidemiology questions of interest.
The Missing Piece Surveillance study is a project designed to understand the concurrent burden of laboratory-confirmed GAS pharyngitis and impetigo infections in remote Australian settings. We will also collect information on and store group C Streptococcus (GCS) and group G Streptococcus (GGS) isolates that are identified during the latex testing of suspected group A streptococci (β-haemolytic morphology on blood agar). Since these species have similar virulence factors to GAS, their incidental identification may be informative.17 18 This multicentre study aims to establish a prospective registry and biorepository of laboratory-confirmed GAS pharyngeal, skin and environmental isolates in remote Australian school settings. This school-based, prospective surveillance programme is the only one of its kind collecting clinical, microbiological and bacterial genomic information on GAS impetigo and pharyngitis in children in Australia and in the world. We aim to enhance primary prevention strategies for ARF and RHD by revolutionising recognition and diagnostics, and with the application of novel microbiome analysis.
Methods and analysis
Setting
The Kimberley is home to one of the most remote living populations in Australia and is located in the North Western region of the country, covering an area of 421 000 km2 with a tropical monsoon climate defined by a distinct wet and dry season. The estimated population size is approximately 35 000 people, of whom 47% are Aboriginal (Australian Bureau of Statistics: 2016 Census QuickStats). The Kimberley Aboriginal Medical Service and the Western Australian Country Health Services (Kimberley) manage health clinics, staffed by remote area nurses, general practitioners and Aboriginal health practitioners. The region has six hospitals in the towns of Broome, Derby, Kununurra, Halls Creek, Fitzroy Crossing and Wyndham. The Kimberley has the second highest incidence of ARF in Australia with a rate of 166/100 000 Aboriginal people under the age of 45 years.19 The two study sites, based in Broome and Derby, is a distance of 225 km from each other by road (figure 1). Weekly and screening surveillance activities will be conducted from June 2019 to December 2022.
Figure 1.

Map showing the two study sites in the Kimberley region of North Western Australia.
Patient and public involvement
During the development and design of this surveillance programme, we consulted community leaders, community members and stakeholders, and incorporated their input and feedback to ensure cultural appropriateness and to streamline the operational aspects of surveillance activities. We will continue to seek and respond to feedback received throughout the study and during the dissemination of the findings.
Study design
This multicentre prospective observational surveillance study involves schools in remote communities in the Kimberley, Western Australia and comprises two components.
Screening asymptomatic and symptomatic children up to three times a year: Every child consented and present in school will be clinically assessed for pharyngitis and impetigo at participating schools. The surveillance toolkit will be applied to healthy and symptomatic children including the collection of throat swabs. The data collected during these screening visits will be used to determine prevalence estimates for GAS throat carriage and to supplement prevalence data collected in the active weekly surveillance component of symptomatic pharyngitis and impetigo. Screening visits will take place up to three times per year, preferably in the beginning, mid-year and at the end of the year.
Active surveillance once a week: A weekly surveillance visit at participating schools will be conducted and include children who self-report symptomatic pharyngitis or impetigo. The data collected in this component will be used to determine incidence estimates of GAS pharyngitis and GAS impetigo over the study period.
A flow chart of screening and surveillance procedures for the Missing Piece Study is depicted in figure 2.
Figure 2.
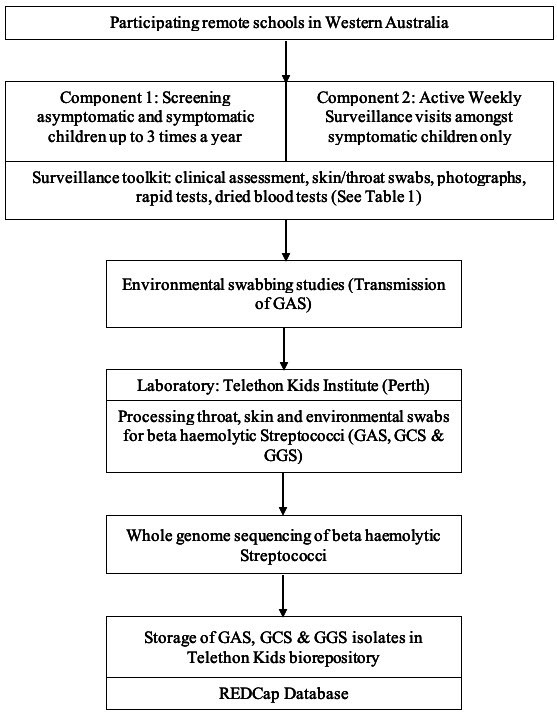
Flow chart of the surveillance procedures for the missing piece study. GAS, group A Streptococcus; GCS, group C Streptococcus; GGS, group G Streptococcus; REDCap, Research Electronic Data Capture.
The study objectives are to:
Determine the prevalence and incidence of laboratory-confirmed GAS pharyngitis and impetigo in remote-living school children aged 5–15 years.
Determine the prevalence of GAS pharyngeal carriage in asymptomatic remote-living school children.
Describe and compare the molecular epidemiology of GAS pharyngitis and impetigo in remote-living school children.
Determine the specificity, sensitivity, positive and negative predictive value of a rapid antigen detection test (RADT) (bioNexia Strep A plus, France) and a point-of-care molecular test (ID NOW Strep A 2, USA) for GAS pharyngitis in remote-living school children.
Evaluate the performance of validated clinical diagnostic rules for GAS pharyngitis and GAS impetigo in remote-living school children.
Assess the anti-streptolysin O titres (ASOT) in dried blood spots collected from remote-living school children.
Describe the uptake and treatment practices for GAS pharyngitis and impetigo in the Kimberley, Australia in remote-living school children referred to the clinics for symptomatic, superficial GAS diseases.
Evaluate the accuracy of photographs as a surveillance tool to assess pharyngitis and impetigo in remote-living school children.
Determine whether viable GAS can be detected on inanimate objects in the school environment, which may play a role in transmission.
Investigate the pharyngeal microbial profile as a predictor of acquiring GAS pharyngitis.
Case definitions
Pharyngitis will be defined as: (1) Self-reported sore throat, or >2 symptoms associated with a sore throat including finding it hard to swallow and one other symptom of not eating, not drinking, fever, tonsillectomy or croaky voice; or (2) ≥2 signs consistent with pharyngitis for example, fever, enlarged tonsils, tonsil erythema, tonsillar exudate, tender/enlarged nodes.
Carriage will be defined as a GAS positive culture obtained from a throat swab of an asymptomatic participant.
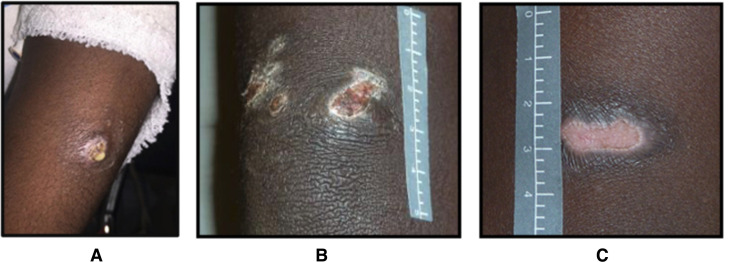
Impetigo, depicted in figure 3, can be classified as either: (1) Purulent sores: skin sores that start as round or oval honey-coloured pus-filled blisters or papules, or the evidence of pus seen in the context of a crusted sore; (2) Crusted sores: skin sores that form a crust on the skin that is typical for impetigo and (3) Flat or dry sores: The crust resolves and the skin beneath the preceding crust is healed. These flat, dry sores are evidence of recent infection.
Figure 3.
Photographs of the different types of sores referred to as (A) purulent, (B) crusted and (C) flat/dry. Note: the skin sore pictures depicted in the figure above are not patients in this study and have been taken with the participants knowledge (https://infectiousdiseases.telethonkids.org.au/our-research/skin-guidelines/).
Active impetigo as referred to in this study will be either purulent or crusted skin sores.
Study eligibility
All children aged between 5 and 15 years old attending the participating schools will be invited to participate in this study. Parents and guardians will be approached to provide informed consent prior to inclusion. Children will be told about the study at each visit, asked to assent and those who do not assent will be excluded.
Inclusion criteria
All children aged 5–15 years present at school and with consent will be assessed in the screening visits. Only consented children will be asked about symptoms of pharyngitis and impetigo at the weekly surveillance visits.
Exclusion criteria
Children will be excluded if they are below 5 or above 15 years or if no informed consent or assent was obtained.
Follow-up
Children with signs and symptoms of GAS pharyngitis or active impetigo will be referred to a medical service within the region for further assessment. Patient care will be determined by the attending healthcare provider following local guidelines (https://kahpf.org.au/clinical-protocols). Treatment practices will be assessed on an annual basis by a retrospective chart review at the clinical sites. There is no follow-up visit of individual episodes of GAS pharyngitis or impetigo included in the study design.
Data collection
Data will be collected with five key components that are consistent across pharyngitis and impetigo as shown in figure 4 at all screening and surveillance visits as described below.
Figure 4.
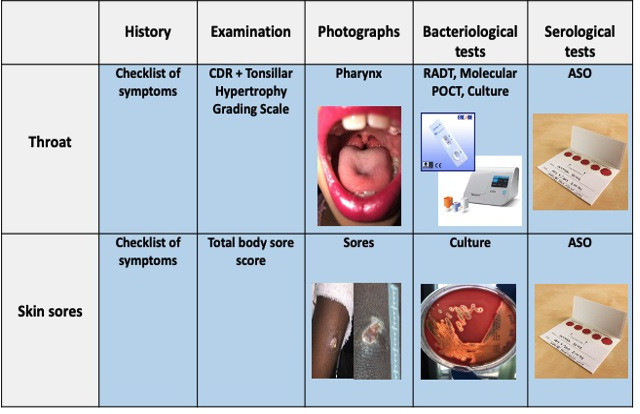
Components of the missing piece toolkit for the screening and surveillance visits.Note: the skin sore and sore throat pictures depicted in the figure above are not patients in this study and have been taken with the participants knowledge. (https://infectiousdiseases.telethonkids.org.au/our-research/skin-guidelines/). CDR, clinical decision rule; RADT, rapid antigen detection test; ID NOW Strep A 2 Point of Care test; ASO, anti-streptolysin O titres processed from a Dried Blood Spot (DBS) card shown in the photo.
History and examination
A checklist will be used to elicit symptoms of sore throat (fever, difficulty swallowing, throat pain) and impetigo (itch, pain/discomfort, purulent or crusted) (see online supplemental material S1). Examination of the throat will use criteria based on previously developed clinical prediction rules used in resource-limited settings, which include scores developed by McIsaac,20 Steinhoff,21 Engel22 and the WHO23 (online supplemental material S1). Each of these clinical rules generate a score, based on the presence or absence of selected signs and symptoms summarised in online supplemental material S2. In addition to age, sex, ethnic background and place of residence, we will record the presence or absence of the following signs of pharyngitis: cough, rhinorrhoea, hoarseness, fever (>38°C), tonsillar erythema or swelling, pharyngeal or tonsillar exudate, tenderness of an anterior cervical lymph node on palpation, an anterior cervical lymph node >1.5 cm in diameter (ie, swelling) and rash. Swelling of the tonsils will be examined in more detail by the tonsillar hypertrophy grading scale (Brodsky Grading Scale),24 a standardised and reproducible measurement often used in paediatrics (online supplemental material S1).25 The visible skin will be examined, regions with sores documented using a body map, and the appearance of the sores (flat/dry/crusted/or purulent) noted. Clinical diagnosis of skin infection will use the Recognition and Treatment of Skin Infections protocol.26
bmjopen-2021-057296supp001.pdf (2.9MB, pdf)
bmjopen-2021-057296supp002.pdf (51.2KB, pdf)
Photographs
Photography of the throat and skin will use established protocols27 which specifies standardised equipment, camera settings, participant positioning and photography technique. Sore throat and skin sores will be documented using digital images collected by an amateur photographer by means of an iPhone adapted version of the protocol specific to research within a remote setting.28 To streamline the process, only the most severe skin sore (purulent or crusted) on each child will be photographed. Photographs of tonsils and skin sores will not contain any identifying information.
Bacteriological tests
A total of three throat swabs will be obtained during both screening and surveillance visits; one stored in skim milk, trypticase glucose glycogen broth (SGGB) for microbiological culture (Pathwest, Perth Australia), one stored in Molecular Transport Medium (PrimeStore, USA) for microbiome analysis, and one for the processing of either a GAS RADT (bioNexia Strep A plus, France)29 or a GAS molecular point of care test (ID NOW Strep A 2, USA).30 Swabs will slowly be passed across one tonsil or tonsillar fossa, then across the posterior pharynx, and finally across the opposite tonsil or tonsillar fossa. The collector will avoid touching the tongue or the mouth with the swab. Participants will be reassured throughout the swabbing procedure. A child with purulent or crusted skin sores will have a single sore swabbed (pus or crust swabbed). Specimens will be couriered to the Telethon Kids Institute in Perth and testing will be processed in batches.
We will evaluate the performance of a molecular point of care diagnostic test (ID NOW) for GAS pharyngitis since these tests have not been approved for impetigo.30 The ID NOW test will be performed immediately following throat swab collection, and children with positive test results would be referred to the clinic for treatment with intramuscular benzathine penicillin or oral penicillin.31 We will calculate the sensitivity, specificity, positive and negative predictive values of the test against gold-standard laboratory culture results to determine the performance of this test in children living in remote Western Australia. The performance of the RADT will be evaluated in the same way as the ID NOW and will also be used to guide treatment.
Serological tests
Dried blood samples will be collected according to predefined standard operating procedures to measure ASOT. In brief, blood will be collected from participants using a sterile lancet to prick the finger and blood will be absorbed into the Whatman 903 filter paper. At least three full circles of blood will be collected, and the cards will be air dried at room temperature for 24 hours before storage in a zip lock bag with desiccant in a refrigerator (4°C–8°C). Samples will then be transported to the Pathwest biochemistry laboratory for processing on a nephelometer. Briefly, a 6 mm ‘chat’ is eluted and processed to detect antistreptolysin O (ASO) antibodies. This method has been developed and validated.32
Microbiome analysis
Pharyngeal swabs will be collected from healthy, asymptomatic children during the screening component of the surveillance programme (baseline). During the weekly active surveillance component, we will collect pharyngeal swabs from symptomatic GAS pharyngitis children. We will then compare the bacteriome in baseline swabs of those who subsequently acquire GAS infection with those who remain asymptomatic throughout the study period. In the active surveillance component of the study, sequential pharyngeal swabs will be obtained from children with new or repeated episodes of GAS pharyngitis. For these children, pharyngeal swabs will undergo microbiome analysis to explore the impact of antibiotic treatment on the pharyngeal bacteriome. Total DNA will be extracted from the samples and full-length 16S rRNA sequencing will be performed for bacterial profiling, allowing discrimination to bacterial species and strain level. 16S microbiome library preparation and full-length 16S rRNA sequencing will be done on a PacBio Sequel II sequencer using circular consensus sequencing and established protocols. The DADA233 bioinformatics pipeline will be used to resolve sequence variants and obtain species and strain-level relative abundance of the oral bacteriome. Machine learning methods will be used to establish key bacterial taxa and identify those that may be protective or predictive for development of GAS pharyngitis.
Environmental sampling
It is not known whether environmental reservoirs of GAS may contribute to the heavy GAS burden seen in remote Aboriginal communities of Australia. During one of the screening visits that will be conducted over the study period, we will conduct a once-off environmental swabbing study which will involve sampling of up to 100 high touch surfaces at participating schools to identify whether GAS is present in the environment. Environmental sampling will be conducted according to established protocols using environmental surface swabs (Copan FLOQSwabs, Italy) and the handling, storage and processing of the swabs will follow the same procedures described below for storage, transport, culture and DNA extraction for whole genome sequencing (WGS). WGS will be used to determine whether the GAS strains found on environmental surfaces are directly related to clinical samples of children with GAS pharyngitis and impetigo.
Handling, processing and storage of biological samples
The collection, handling and processing of all biological specimens will be in accordance with the relevant standard operating procedures contained within the study specific Laboratory Instructions Manual. The throat and skin swabs stored in SGGB and throat swabs in Primestore media will be stored at refrigeration temperature (4°C–8°C) for up to 5 days before being transported by air to Perth at 4°C–8°C and then frozen at −80°C until processed at the Telethon Kids Institute laboratory. Swabs stored in SGGB will be defrosted, vortexed and a 20 µL aliquot of SGGB fluid inoculated onto horse blood agar (HBA) and HBA supplemented with colistin and nalidixic acid plates. These plates will be cultured at 37°C in CO2 and cultures will be examined at 24–48 hours following initial plating as above for GAS and Staphylococcus aureus. Pure colonies of beta-haemolytic streptococci identified as GAS, GCS or GGS using bacitracin sensitivity and latex agglutination tests (Oxoid, United Kingdom) will be removed from the plates in a sterile fashion and placed in SGGB for storage. Duplicate vials will be made for each isolate and checked for sterility. The cryovials will be labelled with identifier numbers and placed in the −80°C freezer.
Transport of specimens
Shipment procedures will be handled by laboratory couriers in accordance with protocols for transporting biological samples and maintaining the cold chain and special packaging requirements. Detailed specimen tracking logs will be maintained. Results will be stored in the electronic database.
Whole genome sequencing
GAS is a genetically diverse pathogen, where strains that share the same epidemiological marker (such as emm type) may represent genetically distinct populations.34 To fully characterise the relatedness of GAS isolates retrieved, WGS will be undertaken. GAS isolates will be cultured from frozen stocks overnight, and genomic DNA will be extracted. Samples will be submitted for short-read WGS using an Illumina-based platform (Doherty Applied Microbial Genomics, University of Melbourne, Australia). Illumina sequencing reads will be processed based on our streptococcal population genomics pipeline35 where quality control, draft genome assemblies, in silico epidemiological markers (such as emm-type and multi-locus sequence type) as well as antimicrobial resistance gene profile will be defined. This approach will also include phylogenetic approaches and single nucleotide polymorphism genome-based clustering to define highly related strains.
Sample size calculations
Using existing prevalence estimates of 10% (95% CI 5.1% to 15.6%) for GAS pharyngitis,16 in order to achieve a precision of 5% around this point estimate, a minimum sample size of 138 participants (range 74–202) presenting with sore throat will need to be enrolled in our study.36 37 Considering prevalence estimates of 40% (95% CI 34.8% to 45.0%) for impetigo and a precision of 5%, a minimum sample size of 369 participants (range 349–380) with impetigo will need to be enrolled.37 During weekly surveillance, only symptomatic children with pharyngitis and impetigo will participate in surveillance activities on the day. Among the two sites, there are approximately 450 children between the ages of 5–15 years eligible to participate.
Data analysis plan
Data entry and management will be conducted using a secure web-based password protected database program Research Electronic Data Capture, USA. From screening and surveillance assessments and laboratory investigations, and using the case definitions provided above, the prevalence and incidence rates of GAS pharyngitis and GAS impetigo will be calculated with 95% confidence intervals. GAS pharyngeal carriage rates will also be calculated with 95% CIs.
Analysis will involve descriptive statistics to assess the prevalence of GAS pharyngitis and impetigo, clinical symptoms and demographic factors of participants. The Shapiro-Wilk test will be used to assess data normality of the continuous variables. We will summarise the participants demographic characteristics (eg, sex, age, ethnicity and community) using means and SD and or medians and IQRs depending on the distribution pattern of the continuous and interval data. For categorical data, we will estimate the proportions and 95% CIs. Descriptive statistics, for example, χ2 test will be used to compare the clinical syndromes associated with pharyngitis and impetigo, ethnicity, sex and community. We will assess the ASO titre results by sex, ethnicity and infection vs carriage status. The performance of rapid tests will be evaluated by calculating the specificity, sensitivity, positive and negative predictive values and will be compared with gold standard laboratory culture methods for identifying GAS. Statistical analysis of the dataset will be conducted using STATA V.16 (StataCorp. 2019. Stata Statistical Software: Release 16., StataCorp).
Discussion
This study is a key informant for the primary prevention aspects of the roadmap towards the elimination of ARF and RHD in Australia.38 The END RHD roadmap highlights research priority questions and policy recommendations to address evidence gaps. Our surveillance study will directly address these critical gaps in scientific knowledge to improve primary prevention strategies for ARF by collecting comprehensive information to investigate (1) the concurrent burden of GAS pharyngitis and impetigo, (2) genomic and molecular data associated with GAS pharyngitis and impetigo, (3) the performance of GAS rapid tests in remote populations, (4) potential for environmental transmission of GAS and (5) treatment practices in remote populations. To the best of our knowledge, this is the first prospective school-based surveillance study of epidemiological, clinical, serological, microbiological and molecular characteristics of GAS pharyngitis and impetigo in remote settings within Australia.
In Aboriginal and Torres Strait Islander communities where ARF is endemic, pharyngitis is seemingly uncommon,8 16 while the burden of impetigo is among the highest reported in the world.8 It has been more than 15 years since the last attempt to understand the epidemiology of GAS pharyngitis in remote Australia, with only two published studies reporting conflicting results. The first study conducted in the Top End of the Northern Territory16 reported an incidence rate of 4 (95% CI 1.4 to 15.5) per 100 person-years and the second study conducted in Central Australia, NT39 reported an incidence of 32 (95% CI 8 to 77) per 100 person-years for GAS pharyngitis. Impetigo incidence was not reported in these studies, but prevalence in children<15 years with one or more episodes per year was found to be 5.5% (95% CI 1.8 to 12.3) and 26.5% (95% CI 23.1 to 30.1), respectively. The burden of impetigo however is well established with a reported median prevalence of 45% (IQR 34.0%–49.2%) in Australian Aboriginal and Torres Strait Islander communities.12 Eighty-four per cent of Australian Aboriginal and Torres Strait Islander children present to a healthcare clinic at least once before their first birthday with impetigo40; the median age of first infection being 7 months (95% CI 6.9 to 9.5).
To date, primary prevention strategies for ARF and efforts in GAS vaccine development have been based on the concept that ARF is caused solely by GAS pharyngitis. In light of these observed low rates of pharyngitis and high rates of impetigo in Aboriginal and Torres Strait Islander populations, primary prevention efforts have been redirected at reducing GAS impetigo.28 41 However, in the absence of clear evidence for GAS impetigo causing ARF or recent, accurate estimates of GAS pharyngitis in this population at high risk of ARF, it is difficult to accurately inform with evidence the guidelines for primary prevention. In a recent report on the cost of inaction on RHD in Australia, it was estimated that more than 10 000 Australian children would develop ARF or RHD by 2031 if no action is taken.42 In addition, it would cost the health system at least US$317 million to provide care for these patients which includes >1000 cardiac surgery procedures and >500 deaths (mostly in young people).42 Without this prospective surveillance study, we remain at an impasse in our ability to inform the prioritisation of treatment for sore throats, skin sores or both, in remote-living Aboriginal and Torres Strait Islander children. This surveillance study will serve to enhance our prevention strategies for ARF and RHD by investigating gaps in research to inform evidence-based policy recommendations in remote Australia.
Limitations include that it is a small, school-based study where children with symptomatic infections may not be present at school. School attendance rates are on average 70% for the region and hence may miss inclusion of children for other non-attendance reasons. Our research over the last decade has confirmed the school setting as an effective location for assessment of children.28
Findings from this study could improve clinical management of GAS pharyngitis and impetigo through the introduction of new diagnostic technologies to fast-track accurate diagnosis, treatment and improve antibiotic prescription practices. This is the first field-research to incorporate the molecular point of care testing in Australia and will inform national guidelines on its future use. Our study will also provide clinicians with a better understanding of the proportion of GAS positive sore throats and skin sore infections that can be expected in this population. Findings will provide an understanding of the interactions between pharyngitis and impetigo through genomic evaluations and comparison of GAS emm-types. In addition, an evaluation of immune responses to these infections will supplement the burden of GAS infection data in our study cohort. The application of novel microbiome analysis will have implications for the development of probiotic therapeutics43 as an alternative to antibiotic therapy, and may assist in identifying children at high risk of GAS pharyngitis. Lastly, data on GAS transmission in our surveillance study will inform evidence-based environmental health practices to reduce transmission among children attending schools in remote regions.
Ethics and dissemination
Ethics approval for the Missing Piece Surveillance Study has been obtained from the Western Australian Aboriginal Health Ethics Committee (HREC/REF: 892), The University of Western Australia (REF: RA/4/20/5101) and Catholic Education Western Australia (REF: RP2018/55). Study findings will be shared with community members, teachers and children at participating schools, together with academic and medical services. Sharing findings in an appropriate manner is of utmost importance and will be done in a suitable way which includes plain language summaries and presentations. Finally, findings and updates will also be disseminated to collaborators, researchers and health planners through peer-reviewed journal publications.
Supplementary Material
Acknowledgments
We acknowledge the students, families, school staff and elders on Yawuru and Nyikina country in the Kimberley region who have participated in the design of the study and who have guided aspects of this programme to ensure it will be conducted in a culturally appropriate manner. We acknowledge the support of the Kulunga team at Telethon Kids Institute for guidance on study design and implementation.
Footnotes
Twitter: @crocodylbert, @ashabowen
Contributors: AB, CS and JC conceived and conducted the pilot study to inform this protocol, and developed the methods for photography of the throat, dried blood spot assessment and history taking. DDB and AB further developed the full protocol to strengthen the surveillance design. MN, JP and MRD advised on the design and sampling of the microbiome and genomic work. DDB and CC wrote the first draft of the revised protocol. DDB, MJM, CS, CC, JP, MN, MRD, JC and AB read and critically revised the final design of this study prior to submission. DDB, AB, MJM, JC and MN will advise on the analysis and interpretation of the data. DDB is responsible for the study management and coordination.
Funding: This work is supported by The END RHD Centre for Research Excellence (National Health and Medical Research Council ((NHMRC)) of Australia (GNT1080401)) and the Government of Western Australia (Western Australian Child Research Fund). AB is supported by an NHMRC fellowship (GNT1175509).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. The Lancet 2005;366:155–68. 10.1016/S0140-6736(05)66874-2 [DOI] [PubMed] [Google Scholar]
- 2.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1789–858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med Overseas Ed 2017;377:713–22. 10.1056/NEJMoa1603693 [DOI] [PubMed] [Google Scholar]
- 4.Carapetis JR, Currie BJ, Mathews JD. Cumulative incidence of rheumatic fever in an endemic region: a guide to the susceptibility of the population? Epidemiol Infect 2000;124:239–44. 10.1017/S0950268800003514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferretti JJ, Stevens DL, a FV. Streptococcus pyogenes: basic biology to clinical manifestations. In: Streptococcus pyogenes basic Biol to clin manifestations, 2016. https://pubmed.ncbi.nlm.nih.gov/26866208/ [Google Scholar]
- 6.Reglinski M, Sriskandan S. Streptococcus pyogenes. In: Molecular medical microbiology. 2nd edn, 2014: 675–716. [Google Scholar]
- 7.Wannamaker LW. The chain that links the heart to the throat. Circulation 1973;48:9–18. 10.1161/01.CIR.48.1.9 [DOI] [PubMed] [Google Scholar]
- 8.McDonald M, Currie BJ, Carapetis JR. Acute rheumatic fever: a chink in the chain that links the heart to the throat? Lancet Infect Dis 2004;4:240–5. 10.1016/S1473-3099(04)00975-2 [DOI] [PubMed] [Google Scholar]
- 9.Bisno AL, Shulman ST, Dajani AS. The rise and fall (and rise?) of rheumatic fever. JAMA 1988;259:728–9. 10.1001/jama.1988.03720050064027 [DOI] [PubMed] [Google Scholar]
- 10.Davidson L, Knight J, Bowen AC. Skin infections in Australian Aboriginal children: a narrative review. Med J Aust 2020;212:231–7. 10.5694/mja2.50361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies - disease burden and modern treatment strategies. J Infect 2016;72 Suppl:S61–7. 10.1016/j.jinf.2016.04.024 [DOI] [PubMed] [Google Scholar]
- 12.Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One 2015;10:e0136789. 10.1371/journal.pone.0136789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearns T, Clucas D, Connors C, et al. Clinic attendances during the first 12 months of life for Aboriginal children in five remote communities of northern Australia. PLoS One 2013;8:e58231. 10.1371/journal.pone.0058231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carapetis JR, Steer AC, Mulholland EK, et al. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005;5:685–94. 10.1016/S1473-3099(05)70267-X [DOI] [PubMed] [Google Scholar]
- 15.World Health Organisation . Epidemiology and management of common skin diseases in children in developing countries, 2005. Available: /entity/maternal_child_adolescent/documents/fch_cah_05_12/en/index.html
- 16.McDonald MI, Towers RJ, Andrews RM, et al. Low rates of streptococcal pharyngitis and high rates of pyoderma in Australian Aboriginal communities where acute rheumatic fever is hyperendemic. Clin Infect Dis 2006;43:683–9. 10.1086/506938 [DOI] [PubMed] [Google Scholar]
- 17.Bisno AL, Collins CM, Turner JC. M proteins of group C streptococci isolated from patients with acute pharyngitis. J Clin Microbiol 1996;34:2511–5. 10.1128/jcm.34.10.2511-2515.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bisno AL, Craven DE, McCabe WR. M proteins of group G streptococci isolated from bacteremic human infections. Infect Immun 1987;55:753–7. 10.1128/iai.55.3.753-757.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katzenellenbogen JM, Bond-Smith D, Seth RJ, et al. Contemporary incidence and prevalence of rheumatic fever and rheumatic heart disease in Australia using linked data: the case for policy change. J Am Heart Assoc 2020;9:e016851. 10.1161/JAHA.120.016851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mclsaac WJ, White D, Tannenbaum D. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ 1998;158. [PMC free article] [PubMed] [Google Scholar]
- 21.Steinhoff MC, Abd el Khalek MK, Khallaf N, et al. Effectiveness of clinical guidelines for the presumptive treatment of streptococcal pharyngitis in Egyptian children. Lancet 1997;350:918–21. 10.1016/S0140-6736(97)03317-5 [DOI] [PubMed] [Google Scholar]
- 22.Engel ME, Cohen K, Gounden R, et al. The Cape town clinical decision rule for streptococcal pharyngitis in children. Pediatr Infect Dis J 2017;36:250–5. 10.1097/INF.0000000000001413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health organization . The management of acute respiratory infections in children: practical guidelines for outpatient care, 1995. Available: https://apps.who.int/iris/handle/10665/41803
- 24.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am 1989;36:1551–69. 10.1016/S0031-3955(16)36806-7 [DOI] [PubMed] [Google Scholar]
- 25.Ng SK, Lee DLY, Li AM, et al. Reproducibility of clinical grading of tonsillar size. Arch Otolaryngol Head Neck Surg 2010;136:159–62. 10.1001/archoto.2009.170 [DOI] [PubMed] [Google Scholar]
- 26.Project EARHS, of Health Research MS . Recognising & treating skin conditions : how to recognise and treat scabies, skin sores, tinea and other skin conditions in Aboriginal and Torres Strait Islander people, 2009.. Menzies school of health research. Available: http://menzies.edu.au/sites/menzies.edu.au/files/images/file/child health/childhealthResources/RecognisingandTreatingSkinconditions2007.pdf
- 27.Bowen AC, Burns K, Tong SYC, et al. Standardising and assessing digital images for use in clinical trials: a practical, reproducible method that blinds the assessor to treatment allocation. PLoS One 2014;9:e110395–10. 10.1371/journal.pone.0110395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowen AC, Tong SYC, Andrews RM, et al. Short-Course oral co-trimoxazole versus intramuscular benzathine benzylpenicillin for impetigo in a highly endemic region: an open-label, randomised, controlled, non-inferiority trial. Lancet 2014;384:2132–40. 10.1016/S0140-6736(14)60841-2 [DOI] [PubMed] [Google Scholar]
- 29.Stewart EH, Davis B, Clemans-Taylor BL, et al. Rapid antigen group A Streptococcus test to diagnose pharyngitis: a systematic review and meta-analysis. PLoS One 2014;9:e111727. 10.1371/journal.pone.0111727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickering JL, Barth DD, Bowen AC. Performance and practicality of a rapid molecular test for the diagnosis of Strep a pharyngitis in a remote Australian setting. Am J Trop Med Hyg 2020;103:2530–2. 10.4269/ajtmh.20-0341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shulman ST, Bisno AL, Clegg HW, et al. Executive summary: clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the infectious diseases Society of America. Clin Infect Dis 2012;55:1279–82. 10.1093/cid/cis847 [DOI] [PubMed] [Google Scholar]
- 32.Joseph J, Kent N, Bowen A, et al. Immuno-nephelometric determination of group streptococcal anti-streptolysin O titres (ASOT) from dried blood spots: method for validating a new assay. J Immunol Methods 2017;448:59–65. 10.1016/j.jim.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 33.Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods 2016;13:581–3. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies MR, McIntyre L, Mutreja A, et al. Atlas of group A streptococcal vaccine candidates compiled using large-scale comparative genomics. Nat Genet 2019;51:1035–43. 10.1038/s41588-019-0417-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacey JA, James TB, Tong SYC, et al. Whole genome sequence analysis and population genomics of group A streptococci. Methods Mol Biol 2020;2136:81–111. 10.1007/978-1-0716-0467-0_7 [DOI] [PubMed] [Google Scholar]
- 36.Oliver J, Malliya Wadu E, Pierse N, et al. Group A Streptococcus pharyngitis and pharyngeal carriage: a meta-analysis. PLoS Negl Trop Dis 2018;12:e0006335. 10.1371/journal.pntd.0006335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altman DG. Statistics and ethics in medical research: III how large a sample? Br Med J 1980;281:1336–8. 10.1136/bmj.281.6251.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyber R, Noonan K, Halkon C, et al. Ending rheumatic heart disease in Australia: the evidence for a new approach. Med J Aust 2020;213 Suppl 10:S3–31. 10.5694/mja2.50853 [DOI] [PubMed] [Google Scholar]
- 39.McDonald M, Brown A, Edwards T, et al. Apparent contrasting rates of pharyngitis and pyoderma in regions where rheumatic heart disease is highly prevalent. Heart Lung Circ 2007;16:254–9. 10.1016/j.hlc.2007.02.087 [DOI] [PubMed] [Google Scholar]
- 40.Lydeamore MJ, Campbell PT, Cuningham W, et al. Calculation of the age of the first infection for skin sores and scabies in five remote communities in northern Australia. Epidemiol Infect 2018;146:1194–201. 10.1017/S0950268818001061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CARPA . CARPA standard treatment manual. 6th edn. Alice Springs: Centre for Remote Health, 2014. [Google Scholar]
- 42.Wyber R, Cannon J, Katzenellenbogen J. The cost of inaction on rheumatic heart disease: the predicted human and financial costs of rheumatic heart disease for Aboriginal and Torres Strait Islander people 2016 -2031. The end RhD CRE. Perth: Telethon Kids Institute, 2018. [Google Scholar]
- 43.Maya-Barrios A, Lira-Hernandez K, Jiménez-Escobar I, et al. Limosilactobacillus reuteri ATCC PTA 5289 and DSM 17938 as adjuvants to improve evolution of pharyngitis/tonsillitis in children: randomised controlled trial. Benef Microbes 2021;12:137–45. 10.3920/BM2020.0171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-057296supp001.pdf (2.9MB, pdf)
bmjopen-2021-057296supp002.pdf (51.2KB, pdf)