Abstract
Intercellular adhesion (IcaADBC) operon is necessary for PNAG (Polyβ-1,6-N-acetyl-D-glucosamine) biosynthesis of biofilm formation in Staphylococcus epidermidis. IcaC protein has a wide range of functions in terms of growth phase variation, migration, transposon insertion, PNAG modification, biofilm formation. Unusual TTTA signature motifs were identified from nucleotide sequence. Asparagine-linked glycosylation consensus motifs were identified at position 169 and 240. S. epidermidis was a close evolutionary association with S. haemolyticus and other Staphylococcus spp. Due to the non-availability of crystal structure, protein threading procedure was selected for constructing a full length IcaC three-dimensional structure. QMEANBrane structure quality assessment with model scores −100000 range within predicted integral membrane structure. IcaC motif constitutes 18 transmembrane helix, 37 helix-helix interaction, 8 beta turn, 2 gamma turn. Binding free energy was calculated with their succinate ligand docking form hydrogen bond with critical amino acids showed ΔG score −2.574 kJ/mol using Schrödinger. Serine (Ser96), Glutamic acid (Glu99), Tryptophan (Trp191) were active site amino acids form the catalytic core required for O-succinyltransferase function. Molecular dynamics simulation (MDS) was performed to evaluate the stability of IcaC protein and IcaC-Succinate binding complexes with the active site amino acids throughout trajectories captured with time scale 100 ns simulation period using GROMACS 4.5.
Keywords: Staphylococcus epidermidis, Biofilm, Intercellular adhesion, Protein threading, Molecular docking, Molecular simulation
Graphical abstract
Highlights
-
•
Structural characterization of Intercellular adhesion (IcaC) protein from Staphylococcus epidermidis.
-
•
Understanding of nucleotide, protein signature sequence, secondary structure motifs and phylogenetic association among Staphylococcus sp. homologs.
-
•
IcaC-Succinate docking and molecular dynamics simulation for determined structural stability of protein.
1. Introduction
Quorum sensing (QS) is the type of behavior coordinating bacterial cell-cell interaction involved in EPS production, cell motility, toxin secretion, biofilm formation (CDC, 2004). S. epidermidis is gram-positive bacteria dwelling on medical devices causes contamination during operation and become life-threatening diseases (Uckay et al., 2009). RP62A is clinical biofilm-positive isolate and genome sequence is available in the public database (Rogers et al., 2009; Costerton et al., 1999). Polysaccharide intercellular adhesion (PIA) is known as exopolysaccharide, which is long mature β-1,6-linked-poly-N-acetylglucosamine (dPNAG) have pathogenicity role in the animal model to prevent phagocytosis (Mack et al., 1996). IcaC is membrane bounded O-succinyltransferase involved in PNAG-O-Succinate addition were separated from Q-Sepharose column, gel filtration chromatography. It’s belongs to type-II antigen polysaccharide (PNAG), which constitutes <20% i.e. non N-acetylated and anionic in nature (Mark et al., 1996). This O-succinylation modification constitutes 6% succinate molecule arranged in a random passion provides anionic charges to dPNAG (Mack et al., 1996; Vu et al., 2009; Branda et al., 2005). Complete 93 CoNS strains out of 76 were S. epidermidis (81.7%) strains detected from neonate’s blood culture for investigated virulence-associated genes associated with SCCmec (Staphylococcal Cassette Chromosome mec) and antibiotics susceptibility for Neonatal sepsis reported for high mortality and morbidity among neonates in maternity hospital Kuwait (Udoc et al., 2020). Higher accumulation of IcaC protein than other IcaADB protein has been elucidated in transcription factor σB mutants of S. aureus strain which is directly proportional to more PNAG biosynthesis (Lasa et al., 2019). Screening of IcaADBC genes among S.epidermidis clinical isolates for stronger biofilm producers attached to the host cells was putatively identified as IcaC gene was found to predominant 82.8% by multiple colony PCR (Mousav et al., 2020). IcaA and IcaD positive isolates was lacking with PNAG production and biofilm formation, due to absence of IcaC expression in Staphylococcus epidermidis (Ziebuhr et al., 1999).
In S. aureus, high resolution nuclear magnetic resonance spectroscopic analysis performed on TTTA repeats of IcaC gene encodes in the coding region forming mini-loop and dumbbell structure resulting in strand slippage during DNA replication, therefore premature stop codon and truncated protein synthesis (Guo et al., 2015). This slipped-stranded mispairing resulting in bacteria growth phase variation. This process is RecA-independent, reversible mechanism by expansion and contraction of simple tetranucleotide tandem repeat within IcaC. Loss of overproducing PIA gained fitness cost advantage of IcaC negative genotype. However, IcaABD produces PIA by the unknown mechanism for survival advantage (Guo et al., 2015). Well understood mechanism of dPNAG biosynthesis inactivation promotes growth phase variation was reported through the transposition of IS256 insertion sequence within IcaC (Ziebuhr et al., 1999).
The Succinyl-CoA binds with IcaC protein (O-Succinyltransferase) necessitates a nucleophilic attack with putative active site amino acid, demonstrated a ping-pong enzymatic mechanism for the transfer of succinate group across cytoplasmic membrane to the synthesized dPNAG (Zubieta et al., 2007). dPNAG structure is identical in S. aureus and O-succinlylation modification was present at minimum 10% level of the GlcNAc moiety. The modification is frequently observed at the O3 position of PNAG and least level was observed at the O4 position, which is predicted from the instantaneous accumulation of the succinyl group over the ring structure after addition at O3 position (Atkin et al., 2014). Protein threading has been implemented to propose the three-dimensional IcaC structure function. Procedure to evaluate their binding pattern of ligand molecule docking score with minimum binding energy were manually examined using molecular visualization tools. Following MD simulation done with individual protein and protein-ligand complexes in GROMACS 4.5. During the trajectory period of the molecular simulation were determined for complete structural stability of the protein. To cross-check, the binding affinity of protein-ligand complexes was investigated with binding free energy. Results uncovered succinate binding sites were predicted as a trans-succinylation regulation site. These studies appear to be highly effective in blocking drug-resistant S. epidermidis using FDA approved drugs in the future.
2. Materials and methods
2.1. IcaC protein characterization, sequence alignment and phylogenetic tree construction
RP62A was selected from literature and IcaC gene sequence was retrieved from GenBank (http://www.ncbi.nlm.nih.gov/BLAST). Physiochemical characterization was analyzed using ProtParam tools (https://web.expasy.org/protparam/). Scratch protein predictor used for In-silico protein overexpression in bacterial host, solubility nature, antigenicity (http://scratch.proteomics.ics.uci.edu/). The protein domain was searched using a CDD-conserved domain database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). The basic local alignment search tool was analyzed on the BLASTp tool (http://www.ncbi.nlm.nih.gov/BLAST). Protein-protein interaction network was generated using the STRING online database (https://string-db.org/). Multiple sequence alignment was performed using ClustalW (https://www.ebi.ac.uk/Tools/msa/clustalw2/). The evolutionary tree was constructed using MEGA 7.0 and the UPGMA method was implemented for tree construction (Kumar et al., 2016).
2.2. Protein 3D structure prediction
Three-dimensional protein structure prediction was done by using threading method on I-tasser online server (https://zhanggroup.org/I-TASSER/). The generated structure was subjected for loop refinement using ModBase online tool (http://modbase.compbio.ucsf.edu/modloop/). The predicted model was validated using SAVES server [http://nihserver.mbi.ucla.edu/SAVES/]. ProFunc database was used for prediction of secondary structure and biochemical function of protein (http://www.ebi.ac.uk/thornton-srv/databases/ProFunc/). The transmembrane model quality score was checked from QMEANBrane server (Schwede et al., 2014). PyMOL software was used for 3D structure visualization (DeLano WL, 2002).
2.3. Protein-ligand docking
Active site of protein was predicted using CASTp tools (http://sts.bioe.uic.edu/castp/calculation). Highest volume and area were chosen for active site. Protein-ligand docking study was executed using schrodinger software (Banks et al., 2005). The succinate ligand structure was retrieved from PubChem database [CID_160419] (http://pubchem.ncbi.nlm.nih.gov/). Protein and ligand were prepared with protein preparation and LigPrep module of Schrodinger suite (Banks et al., 2005). Grid box was defined to cover the active site of protein structure. UCFC chimera molecular visualization tool were used to get insights into the docking pattern, and Schrodinger was used to obtain 2D interaction plot (Levy et al., 2006; Pettersen et al., 2004).
2.4. Molecular dynamics simulations
To evaluate the stability of IcaC protein and IcaC-Succinate ligand complex using molecular dynamics simulation was performed with Gromacs 5.1.4 (Pronk et al., 2013; Malde et al., 2011). Automated topology builder (ATB) was used to create topology files for succinate. IcaC and IcaC-Succinate complex were solvated with SPC water molecule in cubic shape box placed at the center (Oostenbrink et al., 2004). 1 nm distance was given between simulation box edge and protein complex, to move freely in the immersed state completely. Electrostatic energy calculation was done with Particle Mesh Ewald (PME) algorithms permits Ewald summation and covalent bond constraints used to solve with linear constraint solver (LINCS) algorithms (Wang et al., 2010). Four chloride ions (Cl-) was added to neutralize the system. Energy minimization of IcaC and IcaC-succinate complex was done with the steepest descent approach at 100 ps. Constant pressure, volume, temperature (300 K) was maintained to system equilibration at 100 ps with IcaC-Succinate complex. 100 ns time scale was set for the final MD simulation run and trajectories were saved for further analysis. Root mean square deviation (RMSD), Root mean square fluctuation (RMSF), Radium of gyration, Hydrogen bond graph and Solvent accessible were generated using Xmgrace tools (Pronk et al., 2013).
3. Results
3.1. Characterization of intercellular adhesion (IcaC) protein
IcaC gene accession no. is U43366.1 and protein_Id is AAC06119.1 retrieved from NCBI. The gene sequence encodes 1068 nucleotides with unique signature motif 24 TTTA tandem repeats (Supplementary data). The polypeptide encodes 355 amino acids with deduced 42.0 kDa molecular weight and theoretical isoelectric points (pI) 8.49. The non-polar amino acids are predominant as leucine (14.6%), isoleucine (13.2%), valine (4.8%), alanine (2.5%), phenylalanine (10.7%). 20 positively charged amino acids are arginine and lysine and 17 negatively charged amino acids are aspartate and glutamate. The aliphatic index and grand average of hydropathicity (GRAVY) are computed to be 125.18 and 0.774. The atomic composition carbon 2036, hydrogen 3040, nitrogen 436, oxygen 499, and sulphur 15. The instability index is computed as 32.05, considered as stable. The total number of cysteine amino acid is 3, and predicted number of disulphide bond is one at the position of 263 and 321. Deduced N-glycosylation modification was predicted at Asn169 and Asn240 amino acid position as NDTF and NVTS (Supplementary data). Putative domain encodes 318 amino acids between Tyr10 to Leu328 belongs to acyltransferase family with e-value 8.54e−76 (Supplementary data). Predicted solubility upon bacteria host overexpression with range is 0.8523 and probability of antigenicity is 0.0384.
3.2. Evolutionary clade analysis
BLASTp analysis revealed that IcaC hit with a wide range of staphylococcus homologous species. Multiple sequence alignment of IcaC protein sequence of S. epidermidis and relative genus homologs at asparagine residue 169 aa position showing sequence inversion at serine amino acid in S. saprophyticus and S. capitis, whereas asparagine 240 aa position showing inversion at tryptophan amino acid in S. delphini (Fig. 1). Active site amino acid position at Serine (Ser96), Glutamic acid (Glu99), Tryptophan (Trp191), in which glutamic acid and tryptophan is highly conserved among species, whereas Serine (Ser96) showing inversion at that position as cysteine in S. saprophyticus, S. saccharolyticus, S. cohnii, S. condimenti, S. piscifermentans and alanine in S. pettonkoferi, S. argensis. One gap was present at 128 aa position in S. saccharolyticus, which 2nd closet genome is sharing with S. epidermidis and isoleucine present in that position. Cysteine at 263 aa and 321aa position is highly conserved among staphylococcus homologs showing inversion in sequence as threonine and alanine in S. pettonkoferi, S. argensis and S. cohnii. Overall, IcaC protein among staphylococcus homologous sp. showing more than 90% identity (Fig. 1). Phylogenetic tree analysis of IcaC protein homologs retrieved from distinct Staphylococcus spp. selected for evolutionary clade analysis. In this phylogenetic analysis, IcaC homologs identified from staphylococcus species are evolutionary association and indicated with bootstrap values (Fig. 2). We demonstrated that IcaC was well associated with gram positive bacteria. However, the low bootstrap value represents far from staphylococcus sp. genome evolution. Results predicted that S. epidermidis are showing 100% identical to S. haemolyticus and least divergence to S. stepanovicii (Fig. 2).
Fig. 1.
Multiple sequence alignment with IcaC protein from different Staphylococcus sp. Conserved amino acids indicated in the star symbol (Glu99, Trp191, Cys321), gap (127), inversion showing in box for active site amino (Ser96), post-translational predicted site (Asn169, Asn240) and disulfide bridge (Cys263).
Fig. 2.
Phylogenetic tree constructed of IcaC protein from 19 distinct staphylococcus species using UPGMA method.
3.3. Structural and functional insights of O-succinyltransferase
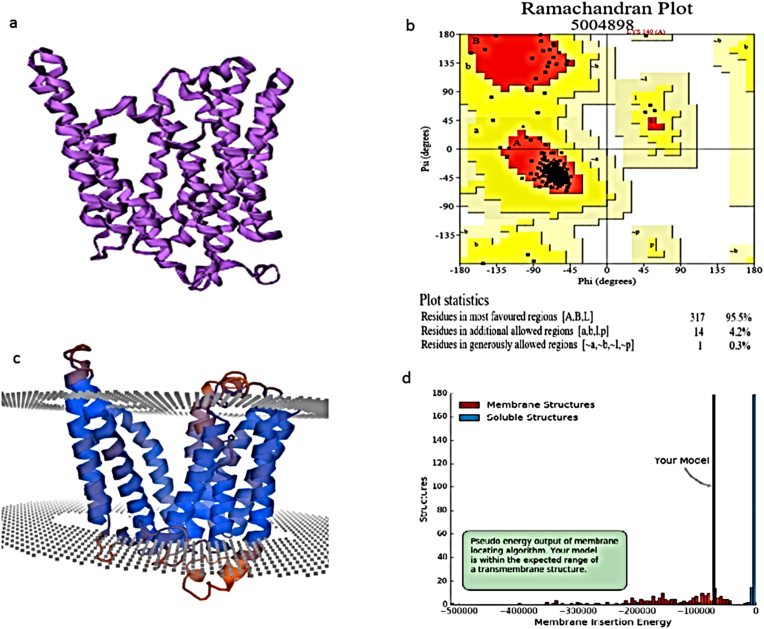
Suitable PDB structure was not available from PDB-PSI BLAST and three-dimensional structure was predicted from threading method using I-TASSER sever. Out of five model, fourth model was selected from c-score −2.43 and ramachandran plot score taken for loop refinement. Ramachandran plot shows that 95.5% amino acid residues were found in the favored region and 4.2% amino acids in allowed regions and no amino acid in the outlier region (Fig. 3). PDB secondary structure were predicted as 18 helices, 37 helix-helix interaction, 8 beta turns, 2 gamma turns (Fig. 4). IcaC protein structure resembles like V-Shaped funnel like alpha-helix that was visualized with PyMOL (Fig. 6a). The cleft, tunnel and pore analysis reveals that interior surface of funnel was found to be 40, 6 and 7 amino acid residues of aliphatic amino acids (Fig. 5). Due to transmembrane protein, the overall quality of 3D model assessment is predicted using QMEANBane. The scoring function reveals −100000 was found that predicted model is within the expected range and reliable (Fig. 3d).
Fig. 3.
a. Predicted three-dimensional structure of IcaC protein, b. Ramachandran plot, c.Transmembrane model quality assessment with cytoplasmic membrane and d.QMEANBane model scoring.
Fig. 4.
Secondary structure prediction from PDB co-ordinates of IcaC protein.
Fig. 6.
Screenshot from STRING protein-protein interaction of IcaC protein, results showing a set of 11 proteins involved in biofilm formation. The protein nodes corresponding to this pathway are highlighted in color. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
Both a. and b. Cleft structure, c. Tunnel, d. Pore analysis of IcaC protein reveals amino acid.
3.4. Active site prediction and protein-ligand docking
Predicted protein-protein interaction envisages that neighborhood partners are IcaB, IcaD, IcaA, IcaR and significant score is 0.985, 0.980, 0.951 and 0.901, while occurrence pathway proteins as aap, bhp, tnpC-3, tnpC-2, tnpC-1 and score is 0.668, 0.527, 0.520, 0.453, 0.453, 0.453 and coexpression profile as IcaB, IcaD (Fig. 6). CASTp tool predicted 79 active sites amino acids from IcaC PDB model. The predicted active site amino acids were Ser96, Glu99, Trp191. IcaC-succinate docking with minimum binding energy was found as −2.5 kcal/mol. Docking pose analysis revealed four hydrogen bonds (H_bonds) interactions between succinate and active site amino acids residues of protein. Here, we observed single H_bond with Ser96, Trp191 residues of bond length 1.877 Å, 2.801 Å, while two H_bond formations were observed with Glu99 of bond length 1.908 Å and1.633 Å. Eventually, it revealed that succinate with three side-chain H_bond interaction with Ser96, Glu99 and one backbone H_bond interaction with Trp191 (Fig. 7).
Fig. 7.
a. IcaC-Succinate docking, b. RMSD, c. RMS fluctuation, d. Radius of gyration, e. Hydrogen bonds, f. Solvent accessible surface.
3.5. Molecular dynamics simulations
Root mean square deviations (RMSD) was calculated for IcaC, IcaC-succinate complex. The graphs were generated for protein backbone flexibility. Throughout simulation period, protein backbone stability was not disturbed and strong interaction with IcaC-succinate complex at 35 ns and IcaC at 50 ns, with no significant fluctuations was seen. Root Mean Square Fluctuation (RMSF) was evaluated the residual mobility of IcaC and IcaC-succinate molecule against residue number and graph was generated. High fluctuation was observed from 20 to 50 ns during trajectory period of MD simulation (Fig. 7). The radius of gyration (Rg) was calculated high secondary structure compactness and shown decreasing from 60,000 ps to 100,000 ps throughout 100 ns trajectory period of MD simulation. Rg graph starts at y-axis 2.28 nm of IcaC and IcaC-succinate complex, eventually reaching to the lowest range to 2.18 nm (Fig. 7). To determine hydrogen bond stability of IcaC-succinate binding sites were monitored throughout trajectory time scale 100 ns The analysis revealed that H_bonds graph starts at y-axis with the number of hydrogen bond from 0 to 3 and Y-axis with 0–100 ns period. Three hydrogen bond profiles were shown with 0–1, 1–2, 2–3. H_bond profile 0–1 range was calculated with no significant fluctuation and breaking was observed, eventually maintained stability and throughout 100 ns. Profile 1–2 range was calculated with no significant fluctuation, however least H_bond breakage was observed and maintained stability and throughout 100 ns. Profile 2–3 range has a significant range of fluctuation and higher H_bond breakage was observed (Fig. 7). Solvent accessibility surface area results were observed that IcaC and IcaC-Succinate resides at 205 nm2 and 220 nm2 in the simulation graph throughout trajectory time scale 100 ns IcaC and IcaC-Succinate complex showed a gradual decrease and reached to limit of 183 nm2 and 190 nm2 at 100 ns (Fig. 7). Binding affinity was deduced using prodigy server predicted as −5.3 kcal/mol for icaC and succinate complex.
4. Discussion
S.epidermidis causes prolonged bloodstream infection contaminated through medical device (Otto M., 2009). Molecular cloning and characterization of N-acetylglucosaminyl transferase activity involved in PNAG biosynthesis with perceptive to PIA-mediated cell aggregation function and pathogenesis of Staphylococcus epidermidis (Gerke et al., 1998). Due to the limitation of appropriate intercellular adhesion protein structure has been major factor for understanding bacterial biofilm formation and quorum sensing. Gene sequence comprises TTTA tandem repeats results in protein truncation leads to growth phase arrest and hamper PNAG overproduction gained fitness cost survival advantage. This characterization of gene sequence analysis of tetra nucleotide tandem repeats TTTA and might involve in non-B DNA structure and TT mini loop dumbbell structure resulting in DNA replication slippage. This replication slippage reveals that bacterial phase variation a reversible mechanism is switch on/off for IcaC protein expression. Eventually addition of one more tandem repeats results in mRNA expression changes and stop translation by codon frame shift (Guo et al., 2015). Subsequently, the process is RecA-independent; loss of overproducing strain of PNAG gained fitness cost advantage of IcaC negative genotype, however IcaADB producing PIA by unknown mechanism for survival advantage (Guo et al., 2015). IcaC metabolic pathway recruit intercellular adhesion operon IcaABD and IcaR undergo temporal expression under extreme environmental niches for bacterial biofilm formation, whereas extensive lineage of occurrence partners is aap, bhp, tnpC-3, tnpC-2, tnpC-1 predicted as similar cellular in function and expresses in other peculiar phenotype (Mack et al., 1996; Vu et al., 2009; Branda et al., 2005). Hence in-silico expression analysis reveals that IcaC, principle determinant of IcaADBC operon amenable for genetic alterations for biofilm formation (Mack et al., 1996; Vu et al., 2009; Branda et al., 2005). IcaC is most commonly expressing genes constitutes 69.7% among the other potent genes revealed from S. epidermidis clinical isolates (Udoc et al., 2020).
IcaC are evolutionarily conserved protein and underlying molecular function in both gram positive and gram-negative bacteria. Phylogenetic tree analysis of IcaC protein is showing closest evolutionary association to S. haemolyticus and other Staphylococcus spp. Sequence alignment of cysteine amino acid position in S. pettonkoferi, S. argensis, S. cohnii reveals disulphide linkage is irrespective to protein structure, query coverage sequence, E value homology may predict to be similar in other function. Ser96 amino acid in S. saprophyticus, S. cohnii, S. condiment, S. capitis, S. pettonkoferi, S. argensis is found to be indirectly proportional to succinate binding. Evolutionary clade analysis showed IcaC protein was evolved from common ancestor and changed at the time of divergence. Predicted Asn-X-Thr consensus sequence at position 169 and 240, whereas asparagine amino acid necessitates hydrophobic interaction relative for transmembrane protein fold to packing within phospholipid bilayer completely (Varki et al., 2009; Gavel et al., 1990). Asparagine residues is highly putative for interaction between hydrophobic and oppositely charged amino acids within α-helical topological sector (Wolynes et al., 2014). Two cysteine amino acid at N-terminal position of IcaC structure to form cystine disulfide bridge to provide protein folding and intra-stability to transmembrane structure were highly conserved among staphylococcus homologous (Cox et al., 2007; Galaktionov et al., 2001; Gandhi et al., 2008). Our perceptive that over evolutionary change cause mutation load with gaps and inversion were found in serine active site amino acid, two N-glycosylation consensus sequence and cysteine position predicting that is substantial for unique structure and function of IcaC protein in S.epidermidis (Galaktionov et al., 2001; Gandhi et al., 2008).
Quantitative analysis of bacterial membrane proteins is still challenging due to dynamical entities of protein structure modification (Cox et al., 2007). Predicted protein solubility was found to be overexpressed in the E. coli system and soluble in nature. IcaC is stably folded throughout the point of time with the relative lowest Rg value considered with tight packing of IcaC protein in terms of Helix-helix interaction (Galzitskaya et al., 2008). From QMEANBrane structure elucidated IcaC form transmembrane helix covered by four loops on the outer membrane surface and predominately 9 negatively charged amino acid were present and structural interaction with phospholipid bilayer showing features of α-helical hydrophobicity and transmembrane transferase properties (Atkin et al., 2014; Marothy et al., 2015; Tusnady et al., 2016). The data suggest that transmembrane loop is essential for membrane integration as well and likely for anchor IcaC protein to cell membrane during dPNAG modification (Galaktionov et al., 2001). IcaC structure close to bacterial transition-mental transporter and rich in hydrophobic amino acids effective for protein stabilization within phospholipid bilayer (Nureki et al., 2015; Marothy et al., 2015). Arginine side chains attracts higher water and phosphate molecule in the cell membrane and may form predominate H-bonding to Arg-phosphate clusters (Allen et al., 2013). The hydrophilic amino acids side chain of lysine, arginine, histidine plays vital function in favours 6% anionic charge form perturbation and disrupt phospholipids on transmembrane helix model and contribute to protein stability at greater net charge were reported in other Staphylococcus spp. (Mack et al., 1996; Galaktionov et al., 2001; Tusnady et al., 2016; Allen et al., 2013).
Characterization of O-succinyltransferase from homoserine O-succinyltransferase (HTS) as serine active site amino acid bound with succinate group form enzymes-substrate complex, as highly conserved among HTS enzymes of various bacteria (Zubieta et al., 2007). Enzyme kinetics of bacterial HTS enzymes, which has structural characterized O-succinyltransferase undergo Ping-Pong mechanism forms intermediate as enzyme-succinate interaction with Ser96, Glu99, Trp191 found to be key catalytic residues and act as trans-succinylation core site for PNAG modification (Zubieta et al., 2007). Succinate is a metabolite in elimination and formation of reactive oxygen species as crucial signaling mediator for oxidative stress and hypoxia state regulation during pathological stimulus (Tretter et al., 2016). Succinate molecule triggers surface-associated biofilm dispersion to 80% effective biomass removal. This mechanism of dispersal phase of biofilm accumulation is much debated strategy, which regulates bacterial migration to new infectious sites in QS biology (Tretter et al., 2016). Negative regulation of biofilm formation favouring dispersal phase that disseminates bacteria to new infection sites. Interfering with QS system is much debated strategy to combat biofilm-related new infection (Arciola et al., 2015). IcaC protein constitutes 1.7% proline amino acids form a kink in transmembrane structure (Heijne et al., 1991). Mostly hydrophobic and hydrophilic amino acids are found to be exposed and buried in IcaC protein and possibility of predicting protein structure and folding characteristics from estimation of solvent accessibility surface area (Ahmad et al., 2014). Stability of IcaC and IcaC-succinate complex were compactly folded and conformation in the native state derived from solvent accessible surface area, hence hydrophobic exposed, hydrophobic imposed, hydrophilic exposed, hydrophilic imposed not disturbed among non-polar residues of hydrophobic interaction (Ahmad et al., 2014).The insilico method would support the design of IcaC inhibitors to prevent biofilm formation by S. epidermidis.
5. Conclusion
In this study, we first documented the three-dimensional structure of the IcaC protein of S.epidermidis from threading method. This prediction seems to have a wide range of functions in terms of gene regulation, phase variation, IcaC-succinate binding site and PNAG trans-succinylation mechanism. TTTA repeats in nucleotide sequence are first reported for bacterial phase variation with respective to PNAG production. The secondary domain architecture was predicted from PDB coordinates represented transmembrane helix, helix-helix interaction, beta turns, gamma turns. Virtually, no significant curable assets presently exist for S. epidermidis, it’s vital to understand the complete structural mechanism of IcaC function will be major interface between biology of QS and explore the role of potential drug targets for antibiotics.
CRediT authorship contribution statement
Ramachandira Prabu: Contributed to the overall experiment, manuscript writing and executed protein characterization to 3D structure prediction. Executed Protein-ligand docking and Molecular dynamics simulation. Interpretation of results, discussion part and complete manuscript revision.
Amaresh Mohanty: Protein- ligand docking and Molecular dynamics simulation.
Susmida Seni Balakrishnan: Bioinformatics Support.
G. Jayalakshmi: Contributed in discussion part and manuscript revision.
Kothandapani Sundar: Corresponding author designed the work and finalizing the manuscript Journal Pre-proof.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors are thankful to the Pondicherry University for providing bioinformatics facilities to carry out the work.
handling editor: Natalie Strynadka
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.crstbi.2022.03.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Ahmad F., Ali S.A., Hassan M.I., Islam A. A review of methods available to estimate solvent-accessible surface areas of soluble proteins in the folded and unfolded states. Curr. Protein Pept. Sci. 2014;15(5):456–476. doi: 10.2174/1389203715666140327114232. [DOI] [PubMed] [Google Scholar]
- Allen T.W., Li L., Vorobyov I. The different interactions of lysine and arginine side chains with lipid membranes. J. Phys. Chem. B. 2013;117(40):11906–11920. doi: 10.1021/jp405418y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciola C.R., Campoccia D., Ravaioli S., Montanaro L. Polysaccharide intercellular adhesion in biofilm: structural and regulatory aspect. Front Cell Infect. Microbiol. 2015;10:5–7. doi: 10.3389/fcimb.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin K.E., MacDonald S.J., Brentnall A.S., Potts J.R., Thomas G.H. A different path:Revealing the function of staphylococcal proteins in biofilm formation. FEBS Lett. 2014;588(10):1869–1872. doi: 10.1016/j.febslet.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Banks J.L., Beard H.S., Cao Y., Cho A.E., Damm W., Farid R., Felts A.K., Halgren T.A., Mainz D.T., Maple J.R. Integrated modelling program, applied chemical theory (IMPACT) J. Comput. Chem. 2005;26:1752–1780. doi: 10.1002/jcc.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda S.S., Vik S., Friedman L., Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13(1):20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- CDC National nosocomial infections surveillance (NNIS) system report. Am. J. Infect. Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Cox J., Mann M. Is proteomics the new genomics? Cell. 2007;130(3):395–398. doi: 10.1016/j.cell.2007.07.032. [DOI] [PubMed] [Google Scholar]
- DeLano W.L. DeLano Scientific; San Carlos, CA, USA: 2002. The PyMOL User's Manual. [Google Scholar]
- Galaktionov S., Nikiforovich G.V., Marshall G.R. Ab initio modeling of small, medium, and large loops in proteins. Biopolymers. 2001;60(2):153–168. doi: 10.1002/1097-0282(2001)60:2<153::AID-BIP1010>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Galzitskaya O.V., Lobanov M.Y., Bogatyreva N.S. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. 2008;42:623–628. [PubMed] [Google Scholar]
- Gandhi N.S., Mancera R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008;72(6):455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- Gavel Y., Von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990;3(5):433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke C., Kraft A., Süssmuth R., Schweitzer O., Götz F. Characterization of the N-acetylglucosaminyl transferase activity involved in the biosynthesis of the staphylococcus epidermidis polysaccharide intercellular adhesin. J. Biol. Chem. 1998;273(29):18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- Guo P., Lam S.L. Unusual structures of TTTA repeats in Staphylococcus aureus. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2015;589(12):1296–1300. doi: 10.1016/j.febslet.2015.04.031. [DOI] [PubMed] [Google Scholar]
- Heijne G.V. Proline kinks in transmembrane alpha-helices. J. Mol. Biol. 1991;218(3):499–503. doi: 10.1016/0022-2836(91)90695-3. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33(7):1596–1599. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasa I., Valle J., Echeverz M. σB inhibits poly-N-acetylglucosamine Exopolysaccharide synthesis and biofilm formation in Staphylococcus aureus. J. Bacteriol. 2019;201(11) doi: 10.1128/JB.00098-19. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R.N., Friesner R.A., Murphy R.B., Repasky M.P., Frye L.L., Greenwood J.R. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Mack D., Fischer W., Krokotsch A., Leopold K., Hartmann R., Egge H., Laufs R. The intercellular adhesion involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta -1,6-linked glucosaminoglycan : purification and structural analysis. J. Bacteriol. 1996;178(1):175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malde A.K., Zuo L., Breeze M., Stroet M., Poger D., Pramod C., Nair P.C. An automated force field topology builder (ATB) and repository: version 1.0. J. Chem. Theor. Comput. 2011;7:4026–4037. doi: 10.1021/ct200196m. [DOI] [PubMed] [Google Scholar]
- Marothy M.T.D., Elofsson A. Marginally hydrophobic transmembrane α-helices shaping membrane protein folding. Protein Sci. 2015;24(7):1057–1074. doi: 10.1002/pro.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousav S.F., Mirzaei B., Faridifar P., Shahmoradi M., Shapouri R., Iranpour F., Haghi F. Genotypic and phenotypic analysis of biofilm formation Staphylococcus epidermidis isolates from clinical specimens. BMC Res. Notes. 2020;13:114. doi: 10.1186/s13104-020-04965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nureki O., Taniguchi R., Kato H.E., Font J., Deshpande C.N., Wada M., Ito K., Ishitani R., Jormakka M. Outward and inward-facing structures of a putative bacterial transition-metal transporter with homology to ferroportin. Nat. Commun. 2015;6:8545. doi: 10.1038/ncomms9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenbrink C., Villa A., Mark A.E., Van Gunsteren W.F.A. Biomolecular force field based on the free enthalpy of hydration and solvation: the GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 2004;25:1656–1676. doi: 10.1002/jcc.20090. [DOI] [PubMed] [Google Scholar]
- Otto M. Staphylococcus epidermidis–the “accidental” pathogen. Nat. Rev. Microbiol. 2009;7(8):555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pronk S., Pall S., Schulz R., Larsson P., Bjelkmar P., Apostolov R., Shirts M.R., Smith J.C. Gromacs 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics. 2013;29:845–854. doi: 10.1093/bioinformatics/btt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers K.L., Fey P.D., Rupp M.E. Coagulase-negative staphylococcal infections. Infect. Dis. Clin. 2009;23(1):73–98. doi: 10.1016/j.idc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Schwede T., Studer G., Biasini M. Assessing the local structural quality of transmembrane protein models using statistical potentials (QMEANBrane) Bioinformatics. 2014;30(17):i505–i511. doi: 10.1093/bioinformatics/btu457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L., Patocs A., Chinopoulos C. Succinate, an intermediate in metabolism, signal transduction, ROS, Hypoxia and tumorigenesis. Biochim. Biophys. Acta. 2016;1857(8):1086–1101. doi: 10.1016/j.bbabio.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Tusnady G.E., Molnar J., Szakacs G. Characterization of disease-associated mutations in human transmembrane proteins. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0151760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckay I., Pittet D., Vaudaux P., Sax H., Lew D., Waldvogel F. Foreign body infections due to Staphylococcus epidermidis. Ann. Med. 2009;41(2):109–119. doi: 10.1080/07853890802337045. [DOI] [PubMed] [Google Scholar]
- Udoc E.E., Al-Haqan A., Boswihi S.S., Pathan S. Antimicrobial resistance and virulence determinants in coagulase-negative staphylococci isolated mainly from preterm neonates. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0236713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A., Sharon N. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor; NY, USA: 2009. Historical background and overview. [Google Scholar]
- Vu B., Chen M., Crawford R.J., Ivanova E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules. 2009;14(7):2535–2554. doi: 10.3390/molecules14072535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Dommert F., Holm C. Optimizing working parameters of the smooth particle mesh Ewald algorithm in terms of accuracy and efficiency. J. Chem. Phys. 2010;133 doi: 10.1063/1.3446812. [DOI] [PubMed] [Google Scholar]
- Wolynes P.G., Kim B.L., Schafer N.P. Predictive energy landscapes for folding α-helical transmembrane proteins. Proc. Natl. Acad. Sci. U.S.A. 2014;111(30):11031–11036. doi: 10.1073/pnas.1410529111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr W., Krimmer V., Rachid S., Lossner I., Gotz F., Hacker J. A novel mechanism of phase variation of virulence in staphylococcus epidermidis evidence for control of the polysaccharide intercellular adhesion synthesis by altering insertion and excision of insertion sequence element IS256. Mol. Microbiol. 1999;32(2):345–356. doi: 10.1046/j.1365-2958.1999.01353.x. [DOI] [PubMed] [Google Scholar]
- Zubieta C., Krishna S.S., McMullan D., Miller M.D., Abdubek P., Agarwalla S. Crystal structure of homoserine O-succinyltransferase from Bacillus cereus at 2.4 Å resolution. Proteins. 2007;68(4):999–1005. doi: 10.1002/prot.21208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.