We read with great interest recent articles published in the International Multidisciplinary Team Discussion (iMTD) section of the Journal of Thoracic Disease (1-7). The authors and international experts provided valuable information and opinions on controversial clinical issues in the diagnosis and treatment of thoracic diseases including non-small cell lung cancer (NSCLC). Here, we focus on unmet medical needs in treating patients with NSCLC harboring mutations in the epidermal growth factor receptor (EGFR) gene, as is discussed in the majority of the articles (1-3,6,7).
Platinum-doublet chemotherapy had been recommended as first-line treatment for advanced NSCLC. However, the development of tyrosine kinase inhibitors (TKIs) for NSCLC harboring oncogenic driver alterations such as EGFR-mutations and re-arrangements of anaplastic lymphoma kinase (ALK) gene has become a “game-changer”. Several randomized clinical trials (RCTs) using first-generation and second-generation EGFR-TKIs for EGFR-mutated NSCLC showed superior tumor response and survival benefit as compared with platinum-doublet chemotherapy. Accordingly, systemic treatment with EGFR-TKIs has established as a standard treatment of care for patients with advanced NSCLC with common EGFR mutations such as deletions in the exon 19 (Del19) and a point mutation in the exon 21 causing the substitution of arginine for leucine at position 858 (L858R) (8,9). Du and coworkers presented a case of double primary lung adenocarcinomas showing different efficacy, in which one tumor harboring L858R mutation in the left lower lobe responded well to systemic treatment using a first-generation EGFR-TKI (gefitinib) but another tumor without EGFR-mutation did not (1). Molecular characteristics and responses to targeting treatment may provide useful information in discrimination between multiple primary lung cancers and pulmonary metastases.
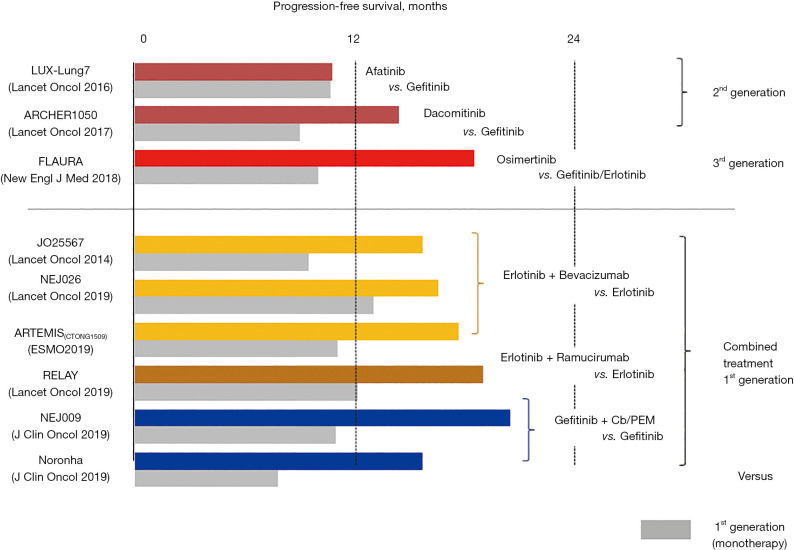
Several EGFR-TKI-based treatment options including combined therapy with a first-generation EGFR-TKI plus an antiangiogenic agent are currently available as first-line treatment for advanced EGFR-mutated NSCLC (Figure 1). Among them, monotherapy with a third-generation EGFR-TKI (osimertinib), which is a mutant-specific and irreversible inhibitor of EGFR kinase activity, is preferably prescribed due to its favorable toxicity profile and superior survival benefit over first-generation EGFR-TKIs (9-11). However, in daily clinical practice, we may encounter a variety of issues as follows.
Figure 1.
First-line treatment options for advanced EGFR-mutated non-small cell lung cancer. Comparison of progression-free survival in randomized controlled trials (RCTs).
Treatment for NSCLC with uncommon EGFR mutations
EGFR-TKIs generally provide significant clinical benefit for patients with NSCLC harboring common activating mutations (Del19 and L858R) that comprise 80-90% of EGFR mutations. The other uncommon mutations consist of insertions in the exon 20, activating point mutations in the exon 18-21 (G719X, S768I and L861Q), and point mutations associated with acquired resistance (T790M, C797X and L718Q), for which first-generation EGFR-TKIs may not be effective (2,3,7,9,12-14). A combined analysis of 3 RCTs showed that a second-generation EGFR-TKI (afatinib) was active for uncommon activating point mutations (G719X, S768I and L861Q) but less active for exon 20 insertions (13). For uncommon activating point mutations, osimertinib also showed a favorable clinical activity (14). Based on these results, afatinib or osimertinib monotherapy may be offered for patients with advanced NSCLC harboring uncommon activating point mutations, and platinum-doublet chemotherapy is generally prescribed for patients with NSCLC harboring exon 20 insertions (9). Recently, a novel EGFR-TKI (mobocertinib) targeting exon 20 insertions as well as a bispecific antibody (amivantamab) targeting EGFR and mesenchymal-epithelial transition (MET) have been recently approved in the United States, which may provide a new insight in the treatment of EGFR-mutated advanced NSCLC (15).
Overcoming resistance to EGFR-TKIs
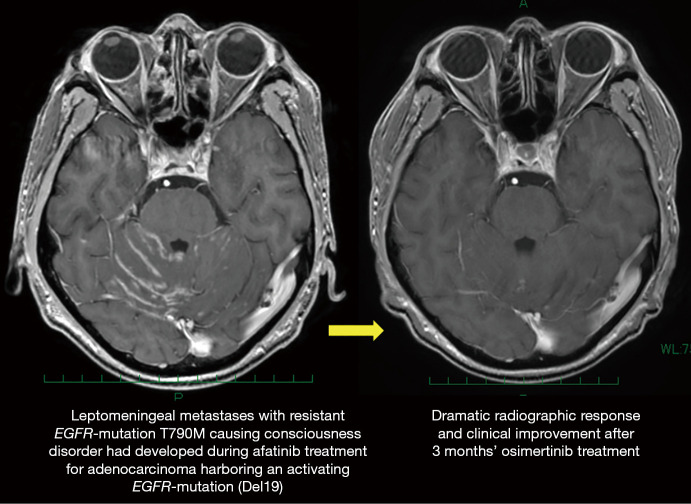
The second mutation in the exon 20, T790M, is responsible for acquired resistance in 50–60% of patients treated with a first-generation EGFR-TKI, as shown in a case presented by Zang and coworkers (7). Osimertinib may overcome T790M-mediated acquired resistance, and provide significant survival benefit for patients who have disease progression caused by T790M resistant-mutation during prior EGFR-TKI treatment (16). Osimertinib also may be effective for tumor progression in the central nervous system (CNS), as is characterized by high CNS penetration (17,18). In fact, Zheng and coworkers presented a case of successful treatment with osimertinib for T790M-mediated acquired CNS resistance (3). We also experienced a case of leptomeningeal metastasis with Del19 plus T790M that had developed during prior afatinib treatment and was successfully treated with osimertinib (Figure 2).
Figure 2.
A case of leptomeningeal metastases successfully treated with osimertinib. A 51-year-old Japanese man presented with consciousness disorder caused by leptomeningeal metastases that had developed during systemic treatment using afatinib following multiple treatment including erlotinib plus bevacizumab, platinum-doublet chemotherapy plus bevacizumab, and docetaxel plus ramucirumab for adenocarcinoma harboring an activating EGFR-mutation (Del 19). A second EGFR-mutation (T790M) causing acquired resistance was detected. Clinical symptoms were dramatically improved after the initiation of osimertinib, and magnetic resonance imaging (MRI) also revealed good radiographic response.
Acquired resistance to osimertinib is the most critical issues in the treatment of advanced EGFR-mutated NSCLC. A wide variety of mechanisms such as resistant EGFR-mutations (C797X and L718Q), mutations in genes other than EGFR (PIK3CA, ALK, BRAF, KRAS, and TP53), amplification of MET gene and human epidermal growth factor 2 (HER2) gene, and histological transformation are associated with acquired resistance to osimertinib (19). Platinum-doublet chemotherapy is generally prescribed at the time of tumor progression after osimertinib treatment (9), which may provide an only modest survival benefit.
To elucidate precise molecular mechanisms of resistance during EGFR-TKI treatment, re-biopsy upon tumor progression is mandatory. However, in clinical practice, it is sometimes difficult to obtain adequate tumor tissues that are suitable for molecular characterization. In addition, tissue biopsy may represent only a snapshot of the biopsied part of tumor at the time of biopsy (20). Accordingly, liquid biopsy may be an alternative to monitor longitudinal gene-alteration status of circulating tumor DNA (ctDNA) during treatment, and several single-gene assays to detect EGFR-mutations in the plasma have been already approved (21). In addition, muti-gene assays using next generation sequencing (NGS) has been recently introduced into clinical practice (21), which may provide useful information to achieve long-term survival with precise medicine using the optimal drug at the optimal timing (20). In fact, Song and coworkers present a patient with metastatic lung adenocarcinoma with L858R who survived for 30 months despite development of multiple EGFR-TKI resistances including T790M, C797S and L718Q. The patient was treated with sequential use of multiple drugs (EGFR-TKIs and chemotherapy), which was decided based on results of tissue and liquid biopsies (2).
Surgical treatment for EGFR-mutated NSCLC
Systemic tumor progression is commonly observed at the time of acquired resistance to EGFR-TKI. However, isolated tumor progression may occur on some occasions, which can be well controlled with local treatment such as surgery. Zang and coworkers presented a case of salvage surgery for solitary lung metastasis that progressed after 27 months’ gefitinib treatment (7). Ohtaki and coworkers reported a nation-wide Japanese data of salvage surgery following systemic treatment using EGFR-TKI (n=33) or ALK-TKI (n=3), which showed that the 3-year overall survival (OS) rate after surgery was 75.1% and that no death was documented within 90days after surgery (22). These results may indicate that salvage surgery after EGFR-TKI treatment may be indicated in selected patients, which should be discussed by a multidisciplinary team (7). In addition, the safety of surgery after EGFR-TKI treatment, which was also reported by Du and coworkers (1), may support the use of an EGFR-TKI in neoadjuvant treatment of resectable EGFR-mutated NSCLC. An international RCT (NeoADAURA) will reveal the clinical efficacy of neoadjuvant osimertinib treatment (23).
The adjuvant use of EGFR-TKIs for resected early-stage EGFR-mutated NSCLC patients was evaluated in several RCTs (24). The ADAURA study is the landmark study to reveal the efficacy of adjuvant osimertinib treatment (25). The disease-free survival (DFS) among patients with pathologic stage II–IIIA disease was significantly prolonged (overall hazard ratio for disease recurrence or death, 0.17; P<0.001). Based on the results, the adjuvant use of osimertinib after standard adjuvant chemotherapy has been approved worldwide (25). However, whether adjuvant use of osimertinib may improve the rate of “cure” remains unknown due to inmaturity of overall survival data (25). The adjuvant use of osimertinib for should be considered according to the balance between toxicity and efficacy in each patient (6), as osimertinib may be also active at the time of recurrence.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-177/coif). FT reports research grant from Boehringer Ingelheim Japan, Ono Pharmaceutical, Taiho Pharmaceutical, Chugai Pharmaceutical. FT also reports payment for lectures from MSD, Bristol-Meyers Squibb, Boehringer Ingelheim Japan, Ono Pharmaceutical, Johnson & Johnson, Covidien Japan, Taiho Pharmaceutical, Astra Zeneca, Chugai Phamaceutical. The other authors have no conflicts of interest to declare.
References
- 1.Du W, Zhao Y, Xuan Y, et al. Different efficacy in the non-small cell lung cancer patient with bilateral synchronous lesions treated with neoadjuvant gefitinib therapy: a case report. J Thorac Dis 2020;12:1582-7. 10.21037/jtd.2020.02.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song Y, Jia Z, Wang Y, et al. Potential treatment strategy for the rare osimertinib resistant mutation EGFR L718Q. J Thorac Dis 2020;12:2771-80. 10.21037/jtd.2020.03.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y, Zhou M, Arulananda S, et al. Management of non-small cell lung cancer with resistance to epidermal growth factor receptor tyrosine kinase inhibitor: case discussion. J Thorac Dis 2020;12:159-64. 10.21037/jtd.2020.01.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai J, Greiffenstein P, Petrella F, et al. Treatment of a lung lobectomy patient with severe post-surgical infection in the anterior thoracic wall by multiple debridement and drainage procedures: a case report. J Thorac Dis 2020;12:7481-7. 10.21037/jtd-20-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G, Lin Y, Zheng L, et al. A new method for accurately localizing and resecting pulmonary nodules. J Thorac Dis 2020;12:4973-84. 10.21037/jtd-20-2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia Z, Wang Y, Cao L, et al. First-line treatment selection with organoids of an EGFRm + TP53m stage IA1 patient with early metastatic recurrence after radical surgery and follow-up. J Thorac Dis 2020;12:3764-73. 10.21037/jtd-20-1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zang J, Horinouchi H, Hanaoka J, et al. The role of salvage surgery in the treatment of a gefitinib-resistant non-small cell lung cancer patient: a case report. J Thorac Dis 2021;13:4554-9. 10.21037/jtd-21-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoneda K, Imanishi N, Ichiki Y, et al. Treatment of Non-small Cell Lung Cancer with EGFR-mutations. J UOEH 2019;41:153-63. 10.7888/juoeh.41.153 [DOI] [PubMed] [Google Scholar]
- 9.Hanna NH, Robinson AG, Temin S, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol 2021;39:1040-91. 10.1200/JCO.20.03570 [DOI] [PubMed] [Google Scholar]
- 10.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 11.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. 10.1056/NEJMoa1913662 [DOI] [PubMed] [Google Scholar]
- 12.Beau-Faller M, Prim N, Ruppert AM, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol 2014;25:126-31. 10.1093/annonc/mdt418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. 10.1016/S1470-2045(15)00026-1 [DOI] [PubMed] [Google Scholar]
- 14.Cho JH, Lim SH, An HJ, et al. Osimertinib for Patients With Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J Clin Oncol 2020;38:488-95. 10.1200/JCO.19.00931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullard A. FDA approves first EGFR exon 20 targeted kinase inhibitor. Nat Rev Drug Discov 2021;20:806. 10.1038/d41573-021-00168-7 [DOI] [PubMed] [Google Scholar]
- 16.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629-40. 10.1056/NEJMoa1612674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu YL, Ahn MJ, Garassino MC, et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J Clin Oncol 2018;36:2702-9. 10.1200/JCO.2018.77.9363 [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi H, Wakuda K, Fukuda M, et al. A Phase II Study of Osimertinib for Radiotherapy-Naive Central Nervous System Metastasis From NSCLC: Results for the T790M Cohort of the OCEAN Study (LOGIK1603/WJOG9116L). J Thorac Oncol 2021;16:2121-32. 10.1016/j.jtho.2021.07.026 [DOI] [PubMed] [Google Scholar]
- 19.Hayashi H, Nadal E, Gray JE, et al. Overall Treatment Strategy for Patients With Metastatic NSCLC With Activating EGFR Mutations. Clin Lung Cancer 2022;23:e69-82. 10.1016/j.cllc.2021.10.009 [DOI] [PubMed] [Google Scholar]
- 20.Yoneda K, Imanishi N, Ichiki Y, et al. A liquid biopsy in primary lung cancer. Surg Today 2019;49:1-14. 10.1007/s00595-018-1659-2 [DOI] [PubMed] [Google Scholar]
- 21.Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol 2021;18:297-312. 10.1038/s41571-020-00457-x [DOI] [PubMed] [Google Scholar]
- 22.Ohtaki Y, Shimizu K, Suzuki H, et al. Salvage surgery for non-small cell lung cancer after tyrosine kinase inhibitor treatment. Lung Cancer 2021;153:108-16. 10.1016/j.lungcan.2020.12.037 [DOI] [PubMed] [Google Scholar]
- 23.Tsuboi M, Weder W, Escriu C, et al. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA. Future Oncol 2021;17:4045-55. 10.2217/fon-2021-0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaft JE, Shyr Y, Sepesi B, et al. Preoperative and Postoperative Systemic Therapy for Operable Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:546-55. 10.1200/JCO.21.01589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. 10.1056/NEJMoa2027071 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as