Abstract
Background
Cross-reactivity between shrimp and house dust mite (HDM) proteins has been widely documented. However, a significant geographical variability in sensitization patterns and cross-reactive allergens has been reported which may impact the diagnosis and management of shrimp allergy among HDM-shrimp co-sensitized patients. This study aimed to investigate the prevalence of shrimp and tropomyosin sensitization among HDM-allergic patients in order to understand the local epidemiology to inform the development of targeted diagnostic and therapeutic tools.
Methods
Four hundred forty-six (446) HDM-allergic patients and 126 atopic controls were screened for shrimp-specific IgE using the IMMULITE 2000 XPI® System. HDM-shrimp sensitized subjected were also tested for IgE tropomyosin (nPen m 1) and thoroughly interviewed about their shellfish consumption habits. Tropomyosin sensitized patients were subjected to further analysis including measurement of IgE specific to squid and crab.
Results
The prevalence of shrimp sensitization in the HDM-allergic population was 20.4% vs 0% in the control group. Of them 63.7% were clinically allergic to shrimp, while 9 cases had no history of allergic reaction to this food and 24 patients reported not having consumed shrimp before. Besides, 72.5% of the HDM-shrimp sensitized subjects had tropomyosin-specific IgE with a positivity rate of 82.8% among shrimp-allergic patients. Among tropomyosin reactors, 95.5% were sensitized to crab and 89.5% to squid, none of them had previously ingested neither crab nor squid. Nevertheless, one-third of HDM-shrimp sensitized patients who never consumed shrimp before did not react to tropomyosin.
Conclusions
Shrimp allergy seems to be strictly dependent on HDM sensitization, at least in this geographical area. Therefore, HDM allergic patients should be systematically screened for shrimp sensitization and asked about the consumption of shellfish. Tropomyosin is a major and clinically relevant shrimp allergen that accounts for shellfish-HDM cross-reactivity. However, other components could be involved.
Keywords: Allergy, Cross-reactivity, House dust mites, Shrimp, Tropomyosin
Abbreviations: AK, arginine kinase; CRD, component resolved diagnostic; CLEIA, chemiluminescent enzyme immunoassay; DBPCFC, double-blind placebo-controlled food challenge; MLC, myosin light chain; OFC, oral food challenge; SCBP, sarcoplasmic calcium-binding protein; SPT, skin prick test; TM, tropomyosin
Introduction
Shellfish, including crustaceans and mollusks, is 1 of the “big 8” allergenic classes of food1 that can be associated with severe allergic reactions including anaphylaxis in both children and adults.2,3 Reported prevalence rates range from 0.2% to 7.7% depending on geographic locations, dietary habits, age, and the method of diagnosis.4 The majority of involved species are the crustaceans, with shrimp being by far the most prominent.
Tropomyosin (TM) was the first major allergen identified in shellfish.5 It is an alpha-helical coiled-coil dimeric protein associated with actine filaments that regulate muscle fibers contraction.6 Only invertebrate TMs are allergenic, and their primary structure is highly conserved which can explain the clinical cross-reactivity among various shellfish species that led to them being defined as a pan-allergen.7
More interestingly, TM is also responsible for cross-reactivity with arthropods such as house dust mites (HDM) and cockroach,8 where they were identified as minor aeroallergens. The molecular basis for this cross-reactivity was initially reviewed by Ayuso et al who demonstrated that mite (Der p 10) and shrimp tropomyosin (Pen a 1) share a high sequence homology of 81%.8,9 This homology can explain the link between HDM and shrimp allergy.10, 11, 12, 13
Despite the central role of tropomyosin, advances in molecular biology allowed the identification of other crustaceans allergens such as arginine kinase (AK),14 sarcoplasmic calcium-binding protein (SCBP),15 myosin light chain (MLC),16 hemocyanin, troponin,17 and triosephosphate isomerase,18 of which the clinical relevance still needs to be fully defined. Nevertheless, some of them potentially cross-react with homologous HDM allergens such as HDM-arginine kinase (Der p 20)19 and high molecular weight allergens in both shrimp and HDM, such as hemocyanin.20, 21, 22
These cross-reactivities raised several questions about the diagnosis and management of shrimp allergy among HDM-shrimp co-sensitized patients. In fact, a clinically irrelevant sensitization may always be considered due to the probability of finding positive IgE without any clinical implication. The beneficial or harmful effect of HDM immunotherapy in shrimp allergy is still controversial and might be related to differences in the total dose of tropomyosin present in the immunotherapy preparations23 as well as the route of administration. Indeed, HDM immunotherapy has been reported to induce either shrimp allergy in non-allergic patients,24 shrimp tolerance in shrimp-allergic patients,23 or no effect on shrimp sensitization.25
In order to tailor immunotherapy for individual patients and develop more reliable diagnostic tools, more investigations are needed to understand the mechanisms and temporal relationship between HDM and shellfish sensitization. However, the most difficult challenges in reaching this goal are the complex shellfish IgE reactivity profile and differences in mite-shellfish cross reactivity based on the climate and geographic locations.26
Considering the importance of these data, the authors aimed to investigate the prevalence of shrimp and tropomyosin sensitization among HDM-allergic Algerian patients, in order to understand the local epidemiology and determine the best approach to evaluate the risk of allergic reactions associated with crustaceans’ ingestion in mites-allergic patients.
Methods
Patient selection and study design
Clinical records of all HDM allergic patients who presented to the Department of Medical Immunology of Beni-Messous Teaching Hospital Center in Algiers, Algeria, between January 2018 and July 2021 were reviewed. The diagnosis of HDM allergy was done by a physician based on a history of perennial respiratory symptoms and a positive specific IgE result (≥0.35 kU/l, IMMULITE 2000 XPI®, chemiluminescent enzyme immunoassay (CLEIA)) to at least 1 of the HDM extracts: Dermatophagoides pteronyssinus or Dermatophagoides farinae. Specific IgE results to other airborne allergens (cat and dog dander, grass, weed, and tree pollen) were also retrieved from the electronic medical records of the patients.
Cases with 1 of the following criteria were excluded: those for whom serum was not available at our serum bank, those who had lost contact, and those who were previously treated by immunotherapy.
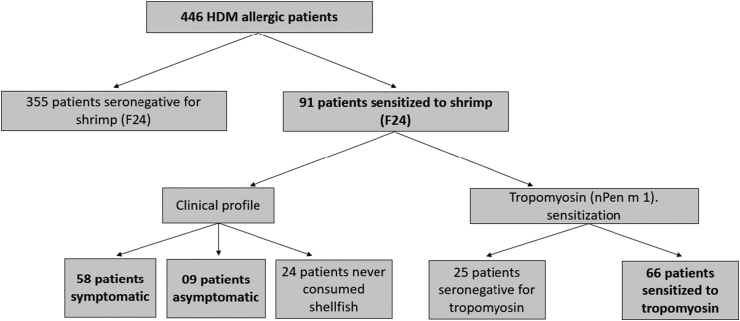
To achieve the aims of the study, we carried out the following steps (Fig. 1):
-
-
Patients who fulfilled the above criteria were initially selected.
-
-
Regardless of whether they reported allergic symptoms or not, all the selected patients were evaluated for shrimp sensitization by specific IgE to the whole shrimp extract.
-
-
Shrimp-sensitized patients were subjected to further detailed analysis including measurement of tropomyosin-specific IgE.
-
-
Among shrimp-sensitized patients, shrimp allergy was diagnosed according to history of immediate allergic symptoms after shrimp ingestion.
Fig. 1.
Schematic of the study design.
One hundred and twenty-six atopic patients, sensitized to different airborne allergens (cat epithelium (39.7%), tree pollen (38.1%), grass pollen (31%), weed pollen (12.7%), cockroach (4.8%) except HDM, were selected from our data base and included as a control group. They were screened for shrimp sensitization in the same way as the population of study.
Specific IgE measurement
Patients' and controls’ sera were obtained from the serum bank of our department. Specific IgE (sIgE) were measured using the IMMULITE 2000 XPI® system (Siemens Medical Solutions Diagnostics, Los Angeles, CA) following the manufacturer's guidelines.
First, all selected patients and controls were analyzed for specific IgE (sIgE) to shrimp (F24). Second, those with positive results were also tested for sIgE tropomyosin (nPen m 1). Finally, depending on the availability of blood serum, the tropomyosin sensitized patients were tested for sIgE to squid (F258) and crab (F23) as well as nDer p 1 and nDer p 2.
Results were expressed in concentrations (kU/l) and a value of sIgE ≥0.35 kU/L was regarded as positive according to the manufacturer's recommendation. Sera yielding sIgE levels above 100 kU/l were diluted (1:10) to determine the exact value of the measurement.
Diagnosis of shrimp allergy
All patients with shrimp sensitization, were contacted by phone and thoroughly interviewed about their seafood consumption habits and their tolerance to shellfish at the time of the initial attendance. Patients were asked about the offending food, symptoms, the lapse between the intake and the onset of symptoms, the required treatment, the duration of symptoms, and associated allergies. Those reporting a clinical history of food hypersensitivity (ie, immediate cutaneous reactions, oral allergy syndrome, digestive signs, or life-threatening symptoms) after ingestion of shrimp were defined as allergic to shrimp. The severity of reported symptoms was assessed into 03 categories based on the grading system proposed by L. Blazowski et al:27 mild (grade I), moderate (grade II or III), severe (grade IV).
-
-
mild: mucocutaneous symptoms (ie, pruritus, urticaria, flashing or angioedema—not laryngeal).
-
-
moderate: the presence of gastrointestinal (ie, abdominal pain, diarrhea, vomiting) or respiratory symptoms (ie, feeling of difficult breathing, upper airway angioedema).
-
-
Severe: life-threatening symptoms (ie, respiratory failure, loss of consciousness).
Ethics approval
The study was approved by the institutional ethics committee of Beni-Messous Teaching Hospital Center in Algiers (CHUBM-26-18), Algeria. Blood samples were initially collected for diagnosis purposes and all participants or their legal tutors gave a written informed consent for possible subsequent uses of their samples for research.
Statistical analysis
SPSS software (IBM Statistic 20.0) and RStudio (version February 1, 5033) were used for statistical analysis and data visualization. Categorical variables were expressed as frequency rates or percentages and significance was detected by chi-square testing or Fisher's exact test. Shapiro-Wilk normality test was conducted to estimate the distribution of the continuous variables, where p < 0.05 indicate that sIgE allergens levels were not normally distributed. Continuous variables were expressed as medians and interquartiles (Q1-Q3). Differences between 2 independent groups were evaluated using the Mann-Whitney U-test. The comparison of quantitative variables in multiples independent groups was completed using Kruskal Wallis test followed by a post-hoc analysis with Bonferroni adjustment, to compensate for multiple comparisons. Correlations between quantitative variables were determined using the Spearman rank correlation analysis. For all statistical analysis, p < 0.05 was considered statistically significant.
Results
Prevalence of shrimp sensitization in house dust mite allergic patients
Four hundred and forty-six HDM allergic patients were enrolled in the study. The basic data of patients and controls are summarized in Table 1. The prevalence of shrimp sensitization in the HDM allergic population was 91/446 (20.4%) vs 0/126 (0%) in the control group (p < 0.001). Among HDM allergic patients, no significant differences were found in gender and age characteristics between those with and without shrimp sensitization (p > 0.05). Interestingly, compared to the shrimp non-sensitized patients, the prevalence of asthma was higher in those who were sensitized (40.6% vs 56%; p = 0.008) with higher sIgE levels to D.pteronyssinus (22.3 (3.7–70.4) kU/l vs 62.2 (20.6–142) kU/l; p < 0.001) and D.farinae (12.9 (2.1–42.6) kU/l vs 49 (16.4–96.9) kU/l; p < 0.001).
Table 1.
Baseline characteristics of the study population and comparative analysis between patients with co-sensitization to mite and shrimp and those sensitized only to mites.
| Control group |
All HDM allergic patient | Shrimp sensitization |
|||
|---|---|---|---|---|---|
| HDM allergy and Shrimp - | HDM allergy and Shrimp + | p | |||
| Number of patients | 126 | 446 | 335/466 (79,6%) | 91/466 (20,4%) | / |
| Age, years, mean ± SD [range] | 23,3 ± 10,8 [4-73] | 21,7 ± 14,5 [0,6–74] | 21,4 ± 14,6 | 23,1 ± 14,3 | 0,190 |
| Gender | 0,579 | ||||
| Males, % (n/N) | 46,8% (59/126) | 46,9% (209/446) | 46,2% (164/355) | 49,5% (45/91) | |
| Females, % (n/N) | 53,2% (67/126) | 53,1% (237/446) | 53,8% (191/355) | 50,5% (46/91) | |
| HDM related symptoms | |||||
| Asthma, % (n/N) | 47,6% (60/126) | 43,7% (195/446) | 40,6% (144/355) | 56% (51/91) | 0,008 |
| Rhinitis, % (n/N) | 63,5% (80/126) | 61% (272/446) | 59,4% (211/355) | 67% (61/91) | 0,185 |
| Co-sensitization aeroallergens, % (n/N) | na | 38,3% (171/446) | 38,3% (136/355) | 38,5% (35/91) | 0,979 |
| Co-sensitization food allergensa, % (n/N) | na | 07% (31/446) | 6,2% (22/355) | 9,9% (9/91) | 0,217 |
| Der p sensitization | |||||
| % (n/N) | 0% (0/126) | 98,7% (440/446) | 98,3% (349/355) | 100% (91/91) | 0,607 |
| sIgE level (kU/l) | na | 27,8 (4,7–84,4) | 22,3 (3,7–70,4) | 62,2 (20,6–142) | <0,001 |
| Der f sensitization | |||||
| % (n/N) | 0% (0/126) | 94,2% (420/446) | 92,7% (329/355) | 100% (91/91) | 0,004 |
| sIgE level (kU/l) | na | 17,3 (2,9–56,3) | 12,9 (2,1–42,6) | 49 (16,4–96,9) | <0,001 |
Data are presented as medians and inter-quartiles (Q1-Q3) or % (n/N) (%), where N is the total number of patients. p values comparing patients with and without shrimp sensitization are from Mann-Whitney U test, χ2 test or Ficher's exact test as appropriate. na: not available.
Others than shellfish
Clinical relevance of shrimp sensitization
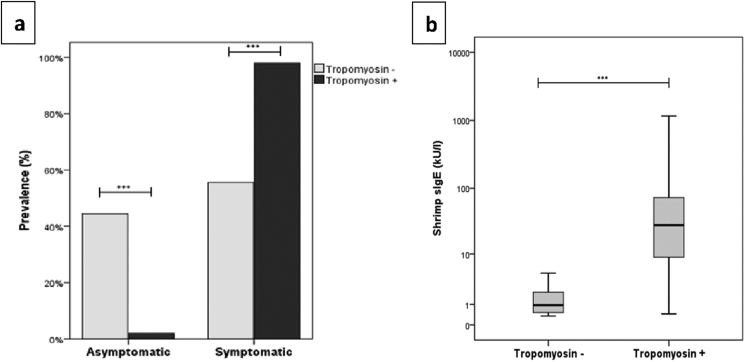
Fifty-eight of 91 patients sensitized to shrimp (63.7%) were clinically allergic to this food while 9 (9.9%) cases had no history of allergic reaction to shrimp and 24 (26.4%) patients reported not having consumed shrimp before (Fig. 1). It is worth noting that, those with shrimp allergy showed a significantly higher median level of sIgE to shrimp than patients reporting good tolerance (56.6 (19.75–177.3) kU/l vs 0.45 (0.4–1.2) kU/l) (Fig. 2a).
Fig. 2.
Clinical symptoms associated to shrimp ingestion. (a) comparison of sIgE levels to shrimp between symptomatic and asymptomatic patients. (b) prevalence of symptoms and (c) sIgE to shrimp according to the grade of severity. Shrimp allergic patients vs asymptomatic: (d) sIgE levels to Der p (e) sIgE level to Der f (f) Asthma (g) Sensitization status. ns: not significant, ∗p<0.05, ∗∗∗p<0.001.
As shown in Fig. 2b, among shrimp allergic patients, mild symptoms (mucocutaneous reactions) were the most frequent (37/58, 63.8%) while anaphylaxis was reported in only 17.2% (10/58) of the cases. Furthermore, there was a significant difference among sIgE levels to shrimp in the different groups of sensitized patients, grouped according to severity of symptoms (p < 0.001) (Fig. 2c). Indeed, shrimp-specific IgE level was significantly lower in patients with mild reactions (5.6 (1.7–20.9) kU/l) compared to those with moderate or severe reactions (85.2 (31.2–175) kU/l and 33.5 (27.3–109) kU/l respectively). Whereas, no statistically significant difference in the level of sIgE to shrimp has been observed between patients with moderate and severe reaction (p > 0.05).
Median sIgE levels to D.pteronyssinus, D.farinae and frequency of asthma were higher in shrimp allergic patients as compared to non-allergic but not statistically significant (Fig. 2d, e and 2f). Alternatively, patients monosensitized to HDM had higher prevalence of shrimp allergy than those who reacted to different airborne allergens (95.1% vs 73.1%, p = 0.022, Fig. 2g).
Tropomyosin as a major shrimp allergen and cross-reactive allergen
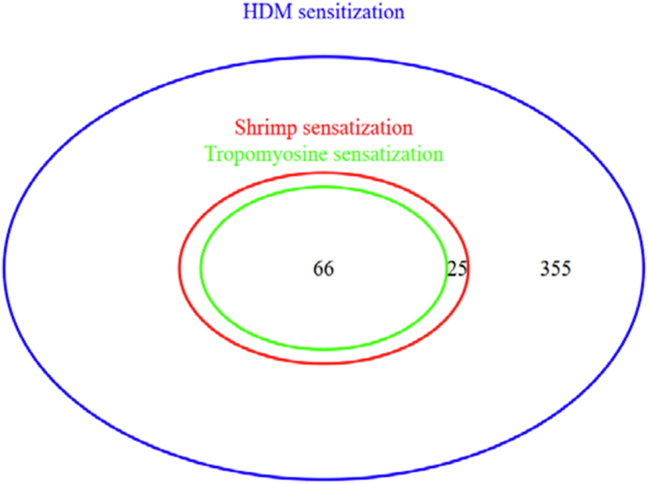
In the study population, among the 91 patients co-sensitized to HDM and shrimp, tropomyosin sIgE was positive in 72.5% (66/91) of the cases (Fig. 3) with a positivity rate of 82.8% (48/58) among shrimp allergic patients. Besides, tropomyosin sensitized subjects reported more frequently allergic reactions associated with shrimp's ingestion than the non-sensitized (98% vs 55.6%; p < 0.001) (Fig. 4a) and had higher shrimp-specific IgE levels (28.1 (8.8–73.8) kU/l vs 0.9 (0.5–2) kU/l; p < 0.001) (Fig. 4b). Nonetheless, 10/58 (17.2%) of patients clinically allergic to shrimp, did not react against tropomyosin (Table 2).
Fig. 3.
Venn diagram showing shrimp and tropomyosin sensitization among HDM allergic patients.
Fig. 4.
Tropomyosin sensitized patients vs non-sensitized (a) prevalence of symptoms (b) sIgE to shrimp. ∗∗∗p<0.001.
Table 2.
Comparative analysis between patients with and without tropomyosin sensitization in HDM-shrimp co-sensitized patients.
| Shrimp sensitization (N = 91) |
|||
|---|---|---|---|
| HDM + Shrimp + Tropomyosine − |
HDM + Shrimp + Tropomyosine + |
p | |
| % (n) | 27,5% (25/91) | 72,5% (66/91) | / |
| Age (median±SD) | 26,7 ± 13,6 | 21,8 ± 14,4 | 0,077 |
| Gender | 0,088 | ||
| Males, % (n) | 64% (16/25) | 43,9% (29/66) | |
| Females, % (n) | 36% (09/25) | 56,1% (37/66) | |
| HDM related symptoms | |||
| Asthma, % (n) | 56% (14/25) | 56,1% (37/66) | 1 |
| Rhinitis, % (n) | 68% (17/25) | 66,7% (44/66) | 1 |
| Shellfish consumption | |||
| Never consumed, % (n) | 28% (07/25) | 25,8% (17/66) | / |
| Shrimp consumption, % (n) | 72% (18/25) | 74,2% (49/66) | / |
| Squid consumption, % (n) | 28% (07/25) | 0% (0/66) | / |
| Crab consumption, % (n) | 0% (0/25) | 0% (0/66) | / |
| Shrimp related symptoms | |||
| Asymptomatic, % (n) | 44,4% (08/18) | 2% (01/49) | <0,001 |
Symptomatic, % (n)
|
55,6% (10/18)90% (09/10)0% (0/10)10% (01/10) | 98% (48/49)58,3% (28/48)22,9% (11/48)18,8% (09/48) | /// |
| Co-sensitization aeroallergens, % (n) | 60% (15/25) | 30,3% (20/66) | 0,015 |
| Co-sensitization food allergensa, % (n) | 4% (01/25) | 12,1% (08/66) | 0,435 |
| Der p sensitization, sIgE level (kU/l) | 40,3 (7,2–106,8) | 72,2 (29,9–160,8) | 0,029 |
| Der f sensitization, sIgE level (kU/l) | 33,5 (5,5–59,2) | 58,7 (20,6–105,3) | 0,007 |
| nDer p 1 sensitizationb | |||
| % (n/N) | na | 69% (40/58) | / |
| sIgE level (kU/l) | na | 2,7 (0,1–41,8) | / |
| nDer p 2 sensitizationb | |||
| % (n/N) | na | 70,7% (41/58) | / |
| sIgE level (kU/l) | na | 3,1 (0,1–291,8) | / |
| Shrimp sensitization, sIgE level (kU/l) | 0,9 (0,5–2) | 28,1 (8,8–73,8) | <0,001 |
| Crab sensitizationc | |||
| % (n/N) | na | 95,5% (42/44) | / |
| sIgE level (kU/l) | na | 35,4 (9,5–141,3) | / |
| Squid sensitizationd | |||
| % (n/N) | na | 89,5% (51/57) | / |
| sIgE level (kU/l) | na | 8,4 (1,8–31,7) | / |
Data are presented as medians and inter-quartiles (Q1-Q3) or % (n/N) (%), where N is the total number of patients. p values comparing patients with and without shrimp sensitization are from Mann-Whitney U test, χ2 test or Ficher's exact test as appropriate. na: not available.
Others than shellfish.
Tested for 58 patients.
Tested for 44 patients.
Tested for 57 patients
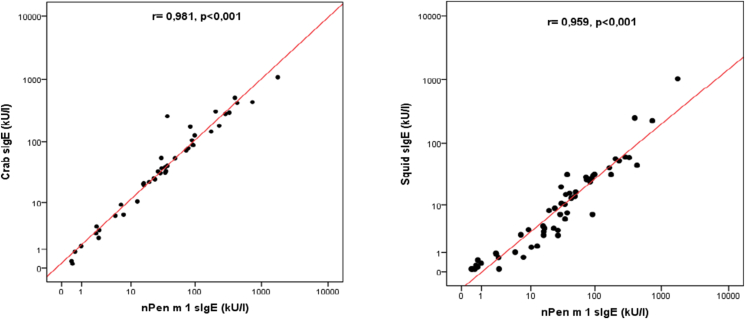
Since they share tropomyosin as a pan-allergen, sensitization profile to crab and squid were also examined. Among tropomyosin reactors, 95.5% were sensitized to crab and 89.5% to squid (Table 2). However, it is important to note that none of the sensitized patients had ingested neither crab nor squid before. Moreover, correlations between the level of sIgE to nPen m 1 and sIgE to crab and squid were analyzed. It turned out a significant positive and strong correlation between sIgE nPen m 1 and other sIgE (p < 0.001) (Fig. 5).
Fig. 5.
Correlations between the level of sIgE to nPen m 1 and sIgE to crab and squid.
Notably, a third of HDM-shrimp sensitized patients who never consumed shrimp before didn't react to tropomyosin (Table 2).
Sensitization to major HDM allergens among the shrimp/tropomyosin sensitized patients
All shrimp/tropomyosin sensitized patients had increased sIgE levels to both species of HDM. sIgE levels to D.pteronyssinus and D.farinae were significantly higher in tropomyosin sensitized patients than in non-sensitized (72.2 (29.9–160.8) kU/l vs 40.3 (7.2–106.8) kU/l; p = 0.029 and 58.7 (20.6–105.3) kU/l vs 33.5 (5.5–59.2) kU/l; p = 0.007 respectively). Besides, a moderate positive correlation was found between sIgE to nPen m 1 and sIgE to D.farinae (r = 0.603; p < 0.001); whereas the strength of correlation turned out to be lower between nPan m 1 and D.pteronyssinus (r = 0.485; p < 0.001) (Fig. 6).
Fig. 6.
Correlations between the level of sIgE for nPen m 1 and those specific for Der p and Der f.
Regarding the HDM allergen components, among the 66 tropomyosin reactors, the frozen blood serum of 58 patients was still available for testing. 69% (40/58) of them were sensitized to nDer p 1, 70.7% (41/58) to nDer p 2 and 63.8% (37/58) were co-sensitized to both of them (Table 2). Nonetheless, 24.1% (14/58) did not show sIgE to any of the 2 major HDM allergens; even though they all presented perennial respiratory symptoms with history of clinical expression upon household exposure to dust mites. Interestingly, within this last group, there was a very strong positive correlation between sIgE to nPen m1 and sIgE to D.pteronyssinus (r = 0.938; p < 0.001) and D.farinae (r = 0.960; p < 0.001) (data not shown).
Discussion
This is the first study that has ever evaluated the prevalence of shrimp sensitization among a large cohort of Algerian patients with clinically defined HDM allergy, which also allowed to observe the role of tropomyosin as major allergen in shrimp sensitized patients. The strength of the current work is that we analyzed not only serological parameters but also clinical symptoms, providing a number of interesting results that are worth discussing.
First, our results highlighted the link between HDM and shrimp sensitization. In fact, none of the atopic control vs 20.4% of the HDM-allergic subjects react in vitro to shrimp extract. A similar finding has been described by Celi et al in an Italian study13 suggesting that shrimp sensitization is closely related to HDM-allergy. Furthermore, of the 91 patients reacting to shrimp in our study, 26.4% of the cases denied having consumed shrimp before. These results indicated that IgE sensitization to shrimp in this last group occurred as a result of indirect sensitization to cross-reacting allergens. This is in line with what have been previously reported by many authors where shrimp sensitization was observed in HDM-sensitized subjects who have never been exposed to shrimps due to their religious eating habits28 or a vegetarian diet.29 Nonetheless, it is important to point out that this close causal relationship between HDM and shellfish sensitization has been questioned in some populations. Indeed, Hemmer et al30 reported, in a study from Central Europe, that only 34.3% of shellfish allergic patients were positive for the dust mite allergens Der p/f 1 or 2.
Another relevant observation from the results of this study and others from the literature, is that the positivity rates of shrimp sIgE among HDM-allergic patients was higher than those in the general population. Indeed, a systematic review of fish and shellfish allergy prevalence,31 including 61 studies, concluded that shellfish sensitization ranged from 0% to 10.3%, a lower percentage compared to those reported in HDM-allergic patients in which 19.2%–32.9% had sIgE to shrimp.12,32,33 These epidemiological data provide further proof on the potential role of inhalant mite allergens in shrimp sensitization and such prevalence suggests that the cross-reactivity involves minor mite allergens. Tropomyosin has been the main panallergen implicated so far. IgE initially produced against mites' tropomyosin (Der p 10), also react to tropomyosin of shrimp (Pen a 1).8 Moreover, indirect sensitization by cross-reacting tropomyosins is not limited to shrimp but also occur with other shellfish. In fact, in our study among HDM-allergic patients with tropomyosin reactivity, 95.5% were sensitized to crab and 89.5% to squid, whereas none of them had ingested crab nor squid before. This serological cross-reactivity is related to a high molecular homology between different tropomyosin molecules.9 Indeed, like other allergenic components, tropomyosin from phylogenetically related crustacean demonstrates a significant amino acid similarity attains up to 98%,9,34 whereas homology between crustacean and molluskan is lower (57%–64%).9,35 However, our results indicate that tropomyosin is not the only cross-reacting allergen. One-third of HDM-shrimp sensitized patients who never consumed shrimp before did not react to tropomyosin.
Alternatively, tropomyosin is the major allergen in shrimp and in all edible crustacean species,5 as confirmed by our results where 82.8% (48/58) of shrimp allergic patients reacted to tropomyosin. This is in line with the results of Ayaso et al,36 Gamez et al,37 and Yang et al38 who reported positivity rates of 57% in the United States, 89% in Spain, and 71.4% in Brazil, respectively. However, in other studies involving different populations (Italy, Japan, Singapore),13,21,39,40 less than 50% of shrimp allergic patients were found to be reactive to tropomyosin. As noted by Tsedendorj et al,39 sensitization to tropomyosin may be dependent on the geographic area.
As with other IgE mediated food allergies, a convincing clinical history and evaluation of sensitization with SPT and/or detection of serum sIgE are essential for the diagnosis of shrimp allergy.41 Interestingly, compared to non-sensitized patients; those with tropomyosin sensitization reported more frequently allergic reactions associated with ingestion of shrimp (55.6% vs 98%, p < 0.001). This suggested that in vitro determination of IgE antibodies to tropomyosin is more effective than IgE to the whole extract. In fact, Yang et al38 had demonstrated that despite a similar sensitivity and negative predictive value, determination of the sIgE to tropomyosin was more specific and had a higher positive predictive value of a clinical allergy (Sp:92.8%, VPP:71.4%) than SPT (Sp:64.2%, VPP:33.3%) or IgE to whole shrimp extract (Sp:75%, VPP:41.6%). This IgE reactivity to whole extract without clinical correlation could be attributed to some allergens, which seem more likely associated to a mite-shellfish cross reactivity than real shrimp allergy such as hemocyanin.42 Indeed, hemocyanin was more frequently recognized by tolerant patients (negative double-blind placebo-controlled food challenge, DBPCFC) than shrimp allergic ones,42,43 being also recognized by subjects allergic to HDM and/or cockroach with no sensitization to shrimp, whereas tropomyosin and SCBP were the principal allergens associated with clinical reactivity to shrimp.42 Nevertheless, a 2014 case study reported anaphylaxis to shrimp caused by hemocyanin.44
In spite of tropomyosin clinical relevance, oral food challenge (OFC) is often necessary in HDM allergic subjects with tropomyosin sensitization who had never before consumed shrimp or other shellfish to avoid false positive diagnosis. Actually, even with a positive IgE-tropomyosin result, some patients can be tolerant (Yang et al:38 7.1% [2/28], Gamez et al:37 33% [6/18], Pascal et al:42 28.6% [8/28]). On the other hand, in the present study 27.5% of the shrimp sensitized subjects did not react to tropomyosin and 55.6% of them showed clinical symptoms after eating shrimp with one patient reporting severe reaction, suggesting that allergens other than tropomyosin may be sufficiently relevant to induce life-threatening reactions upon ingestion.
Based on current evidences, in absence of clinical reaction, HDM-allergic patients should not be advised to avoid shrimp or related shellfish spices only according to a positive sIgE result to tropomyosin. Also, it should not be the only component used for the diagnosis of shrimp allergy. Therefore, allergen panels combining major and minor allergens has been proposed to improve diagnostic accuracy.18,42 Pascal et al42 recommended in a multi-center study in the United States, Brazil, and Spain, an interesting diagram for shrimp allergy diagnosis based on 3 allergenic components (TM, SCBP, MLC) avoiding a systemic need for a food challenge. However, even with the complete panel of 10 allergens (TM, SCBP isoform alpha and beta, MLC, AK, hemocyanin, fatty-acid-binding protein, and 3 troponin C protein), 03 patients (5.2%) did not show IgE binding to any recombinant allergen suggesting the existence of other shrimp-allergens that remain unidentified. Taken together, these observations showed that despite the promise of component resolved diagnostic (CRD), shellfish allergy remains particularly challenging to diagnose due to the complex shellfish IgE reactivity profile.
Beside tropomyosin sensitization, other risk factors for shrimp allergy have been previously identified including monosensitization to HDM with high sIgE levels, the presence of HDM-induced asthma, and anti-shrimp IgE levels.13,33,37,38,42,43 These factors are potential markers that could allow to select cases who require (or not) a shrimp oral challenge. In the current study, a significant higher prevalence of shrimp allergy was found among patients monosensitized to HDM as compared to those who reacted to different airborne allergens (95.1% vs 73.1%, p = 0.022). Celi et al13 suggested that extensive sIgE reactivity toward a large number of allergens is protective against the development of food allergy or severe allergic reactions like in subjects with food allergy to lipid transfer protein.45 Otherwise, in our case, sIgE levels to HDM and the frequency of asthma were significantly associated with shrimp sensitization but not with shrimp allergy.
It is worth nothing that our study has some limitations. First, we did not perform inhibition analyses to confirm HDM-shrimp cross-reactivity. Moreover, it was a single center study limited to patients from the north-center regions of Algeria. The primary sensitizer and cross-reactive allergens may be different in locations with different climate and supposed different levels of mites. In fact, IgE reciprocal inhibition assays in a Spanish cohort identified mite as the primary sensitizer in patients living in a humid climate while shrimp was observed to be the primary sensitizer in dry climate population.26 Therefore, a multi-center study, including patients from different area of the country, would significantly enhance the strength of our results and explore possible differences in the sensitization profiles according to the studied population. Finally, although patients were thoroughly interviewed about their shellfish consumption habits, the discrepancy between self-reported allergy and real allergy cannot be underrated.31,46 Allergic reactions could be amplified by other cofactors, such as physical exercise, concomitant infections, alcohol and anti-inflammatory drugs intake.47 Thus, DBPCFC remains the gold standard, despite its potential risk, for a definitive diagnosis.
In conclusion, shrimp allergy seems to be closely related to HDM sensitization, at least in this geographical area. Therefore, HDM allergic patients should be systematically screened for shrimp sensitization and asked about the consumption of shellfish. Tropomyosin is a major and clinically relevant shrimp allergen that account for shellfish-HDM cross-reactivity. However, our results indicated that other components seem to be involved. Further studies, with inhibition assays and OFC, are needed to assess the prevalence and clinical relevance of sensitization to shrimp allergens other than tropomyosin and their implication in mite-shellfish cross-reactions among Algerian patients.
Authors’ contributions
LLM designed the study, collected the data, performed the statistical analysis, interpreted the data, and wrote the manuscript. BB, LMB, FM critically reviewed the manuscript. SYR, AAK, IB, WS contributed to data collection. IA critically reviewed the manuscript. RD supervised the research group and critically reviewed the manuscript. All authors read and approved the final manuscript.
Ethics approval
The study was approved by the institutional ethics committee of Beni-Messous Teaching Hospital center (CHUBM-26-18), Algiers, Algeria. Blood samples were initially collected for diagnosis purposes and all participants or their legal tutors gave a written informed consent for possible subsequent uses of their samples for research.
Declaration of competing interest
The authors have no conflict of interest to declare.
Submission declaration
We confirm that this manuscript is original, has not been published before, is not currently being considered for publication elsewhere, and has not been posted to a preprint server.
Consent for publication
All authors have reviewed this manuscript and consent to its publication.
Funding source
This study was funded by Beni-Messous Teaching Hospital center, Algiers, Algeria.
Availability of data statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank Pr. Luis Caraballo for his critical reading of the manuscript and insightful suggestions.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Teuber S., Beyer K., Comstock S., Wallowitz M. In: Food Allergy. Maleki S.J., Wesley Burks A., Helm R.M., editors. 2006. The big eight foods: clinical and epidemiological overview. [Google Scholar]
- 2.Webb L.M., Lieberman P. Anaphylaxis: a review of 601 cases. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. juill. 2006;97(1):39–43. doi: 10.1016/S1081-1206(10)61367-1. [DOI] [PubMed] [Google Scholar]
- 3.Asero R., Pravettoni V., Scala E., Villalta D. Shrimp-induced anaphylaxis. Curr Treat Options Allergy. 1 sept 2020;7(3):381–389. [Google Scholar]
- 4.Wong L., Tham E.H., Lee B.W. An update on shellfish allergy. Curr Opin Allergy Clin Immunol. juin. 2019;19(3):236–242. doi: 10.1097/ACI.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 5.Shanti K.N., Martin B.M., Nagpal S., Metcalfe D.D., Rao P.V. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. J Immunol Baltim Md. 15 nov 1993;151(10):5354–5363. 1950. [PubMed] [Google Scholar]
- 6.Hitchcock-DeGregori S.E. Advances in Experimental Medicine and Biology. 2008. Tropomyosin: function follows structure; pp. 60–72. [DOI] [PubMed] [Google Scholar]
- 7.Reese G., Ayuso R., Lehrer S.B. Tropomyosin: an invertebrate pan-allergen. Int Arch Allergy Immunol. août. 1999;119(4):247–258. doi: 10.1159/000024201. [DOI] [PubMed] [Google Scholar]
- 8.Ayuso R., Reese G., Leong-Kee S., Plante M., Lehrer S.B. Molecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int Arch Allergy Immunol. sept. 2002;129(1):38–48. doi: 10.1159/000065172. [DOI] [PubMed] [Google Scholar]
- 9.Ayuso R., Lehrer S.B., Reese G. Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin) Int Arch Allergy Immunol. 1 janv. 2002;127(1):27–37. doi: 10.1159/000048166. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfield L., Tsoulis M.W., Milio K., Schnittke M., Kim H. High rate of house dust mite sensitization in a shrimp allergic southern Ontario population. Allergy Asthma Clin Immunol Off J Can Soc Allergy Clin Immunol. 2017;13:5. doi: 10.1186/s13223-017-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuano K., Davis C. Oral allergy syndrome in shrimp and house dust mite allergies. J Allergy Clin Immunol Pract. 2018;6:2163–2164. doi: 10.1016/j.jaip.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 12.Ukleja-Sokołowska N., Gawrońska-Ukleja E., Lis K., et al. Shrimp sensitization in house dust mite allergic patients. Int J Immunopathol Pharmacol. mars. 2020;34:1–8. doi: 10.1177/2058738420907188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celi G., Brusca I., Scala E., et al. House dust mite allergy and shrimp allergy: a complex interaction. Eur Ann Allergy Clin Immunol. sept. 2020;52(5):205. doi: 10.23822/EurAnnACI.1764-1489.108. [DOI] [PubMed] [Google Scholar]
- 14.Yu C.-J., Lin Y.-F., Chiang B., Chow L. Proteomics and immunological analysis of a novel shrimp allergen, pen m 21. J Immunol. 2003;170(1):445–453. doi: 10.4049/jimmunol.170.1.445. [DOI] [PubMed] [Google Scholar]
- 15.K S., Y S., S H., H M., K S. Sarcoplasmic calcium-binding protein: identification as a new allergen of the black tiger shrimp Penaeus monodon. Int Arch Allergy Immunol. 2008;146(2):91–98. doi: 10.1159/000113512. [DOI] [PubMed] [Google Scholar]
- 16.Ayuso R., Grishina G., Bardina L., et al. Myosin light chain is a novel shrimp allergen, Lit v 3. J Allergy Clin Immunol. 1 oct 2008;122(4):795–802. doi: 10.1016/j.jaci.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Piboonpocanun S., Jirapongsananuruk O., Tipayanon T., Boonchoo S., Goodman R.E. Identification of hemocyanin as a novel non-cross-reactive allergen from the giant freshwater shrimp Macrobrachium rosenbergii. Mol Nutr Food Res. oct 2011;55(10):1492–1498. doi: 10.1002/mnfr.201000602. [DOI] [PubMed] [Google Scholar]
- 18.Bauermeister K., Wangorsch A., Garoffo L.P., et al. Generation of a comprehensive panel of crustacean allergens from the North Sea Shrimp Crangon crangon. Mol Immunol. 1 sept 2011;48(15):1983–1992. doi: 10.1016/j.molimm.2011.06.216. [DOI] [PubMed] [Google Scholar]
- 19.Binder M., Mahler V., Hayek B., et al. Molecular and immunological characterization of arginine kinase from the indianmeal moth, plodia interpunctella, a novel cross-reactive invertebrate pan-allergen. J Immunol. 1 nov. 2001;167(9):5470–5477. doi: 10.4049/jimmunol.167.9.5470. [DOI] [PubMed] [Google Scholar]
- 20.Ayuso R., Grishina G., Pascal M., et al. Hemocyanin, troponin C and fatty acid-binding protein (FABP) may be cross-reactive allergens between Crustaceans, cockroach and dust mites. J Allergy Clin Immunol. 1 févr. 2011;127(2):AB235. [Google Scholar]
- 21.Asero R., Mistrello G., Amato S., et al. Shrimp allergy in Italian adults: a multicenter study showing a high prevalence of sensitivity to novel high molecular weight allergens. Int Arch Allergy Immunol. 2012;157(1):3–10. doi: 10.1159/000324470. [DOI] [PubMed] [Google Scholar]
- 22.Giuffrida M., Villalta D., Mistrello G., Amato S., Asero R. Shrimp allergy beyond Tropomyosin in Italy: clinical relevance of Arginine Kinase, Sarcoplasmic calcium binding protein and Hemocyanin. Eur Ann Allergy Clin Immunol. 2014;46(5):172–177. [PubMed] [Google Scholar]
- 23.Cortellini G., Spadolini I., Santucci A., et al. Improvement of shrimp allergy after sublingual immunotherapy for house dust mites: a case report. Eur Ann Allergy Clin Immunol. oct. 2011;43(5):162–164. [PubMed] [Google Scholar]
- 24.van Ree R., Antonicelli L., Akkerdaas J.H., Garritani M.S., Aalberse R.C., Bonifazi F. Possible induction of food allergy during mite immunotherapy. Allergy. 1996;51(2):108–113. [PubMed] [Google Scholar]
- 25.Asero R. Lack of de novo sensitization to tropomyosin in a group of mite-allergic patients treated by house dust mite-specific immunotherapy. Int Arch Allergy Immunol. mai. 2005;137(1):62–65. doi: 10.1159/000085105. [DOI] [PubMed] [Google Scholar]
- 26.Gámez C., Zafra M.P., Boquete M., et al. New shrimp IgE-binding proteins involved in mite-seafood cross-reactivity. Mol Nutr Food Res. sept. 2014;58(9):1915–1925. doi: 10.1002/mnfr.201400122. [DOI] [PubMed] [Google Scholar]
- 27.Blazowski L., Majak P., Kurzawa R., Kuna P., Jerzynska J. A severity grading system of food-induced acute allergic reactions to avoid the delay of epinephrine administration. Ann Allergy Asthma Immunol. 1 oct 2021;127(4):462–470.e2. doi: 10.1016/j.anai.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes J., Reshef A., Patton L., Ayuso R., Reese G., Lehrer S.B. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy. 2003;33(7):956–961. doi: 10.1046/j.1365-2222.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- 29.Shen C.-Y., Tsai J.-J., Liao E.-C. Cross-reactivity of sIgE to mite and shrimp induced allergies in different age groups and clinical profiles of shrimp sIgE in vegetarians. Sci Rep. déc. 2019;9(1):12548. doi: 10.1038/s41598-019-49068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemmer W., Wöhrl S., Sesztak-Greinecker G., Jarisch R., Wantke F. Evaluation of individual sensitisation patterns to shrimp allergens in patients with seafood allergy using Immunocap ISAC microarray. Clin Transl Allergy. 17 mars. 2014;4(2):P41. [Google Scholar]
- 31.Moonesinghe H., Mackenzie H., Venter C., et al. Prevalence of fish and shellfish allergy: a systematic review. Ann Allergy Asthma Immunol. 1 sept 2016;117(3):264–272. doi: 10.1016/j.anai.2016.07.015. e4. [DOI] [PubMed] [Google Scholar]
- 32.Hernández-Moreno K.E., Muñoz M., Calvo V., Diez-Zuluaga L.S., Sánchez J. Relationship between the sensitization to shrimp and mites. Exploration of cross-reactivity due tropomyosin. Rev Alerg Mex. 2019;66(2):205–216. doi: 10.29262/ram.v66i2.402. [DOI] [PubMed] [Google Scholar]
- 33.Diez S., Puerta L., Martínez D., Muñoz M., Hernández K., Sánchez J. Clinical relevance of shrimp sensitization in patients with allergic rhinitis: anti-der p 10 IgE as predictor. Int Arch Allergy Immunol. 2021;182(10):971–979. doi: 10.1159/000516005. [DOI] [PubMed] [Google Scholar]
- 34.Leung P.S.C., Chen Y., Gershwin M.E., Wong S.H., Kwan H.S., Chu K.H. Identification and molecular characterization of Charybdis feriatus tropomyosin, the major crab allergen. J Allergy Clin Immunol. 1 nov. 1998;102(5):847–852. doi: 10.1016/s0091-6749(98)70027-2. [DOI] [PubMed] [Google Scholar]
- 35.Motoyama K., Ishizaki S., Nagashima Y., Shiomi K. Cephalopod tropomyosins: identification as major allergens and molecular cloning. Food Chem Toxicol. 1 déc. 2006;44(12) doi: 10.1016/j.fct.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Ayuso R., Sánchez-Garcia S., Pascal M., et al. Is epitope recognition of shrimp allergens useful to predict clinical reactivity? Clin Exp Allergy J Br Soc Allergy Clin Immunol. févr. 2012;42(2):293–304. doi: 10.1111/j.1365-2222.2011.03920.x. [DOI] [PubMed] [Google Scholar]
- 37.Gámez C., Sánchez-García S., Ibáñez M.D., et al. Tropomyosin IgE-positive results are a good predictor of shrimp allergy. Allergy. oct 2011;66(10):1375–1383. doi: 10.1111/j.1398-9995.2011.02663.x. [DOI] [PubMed] [Google Scholar]
- 38.Yang A.C., Arruda L.K., Santos A.B.R., et al. Measurement of IgE antibodies to shrimp tropomyosin is superior to skin prick testing with commercial extract and measurement of IgE to shrimp for predicting clinically relevant allergic reactions after shrimp ingestion. J Allergy Clin Immunol. 1 avr. 2010;125(4):872–878. doi: 10.1016/j.jaci.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 39.Tsedendorj O., Chinuki Y., Ueda K., Kohno K., Adachi A., Morita E. Tropomyosin is a minor but distinct allergen in patients with shrimp allergies in Japan. J Cutan Immunol Allergy. 2018;1(3):100–108. [Google Scholar]
- 40.Thalayasingam M., Gerez I.F.A., Yap G.C., et al. Clinical and immunochemical profiles of food challenge proven or anaphylactic shrimp allergy in tropical Singapore. Clin Exp Allergy J Br Soc Allergy Clin Immunol. mars. 2015;45(3):687–697. doi: 10.1111/cea.12416. [DOI] [PubMed] [Google Scholar]
- 41.Muraro A., Werfel T., Hoffmann-Sommergruber K., et al. EAACI Food Allergy and Anaphylaxis Guidelines: diagnosis and management of food allergy. Allergy. 2014;69(8):1008–1025. doi: 10.1111/all.12429. [DOI] [PubMed] [Google Scholar]
- 42.Pascal M., Grishina G., Yang A.C., et al. Molecular diagnosis of shrimp allergy: efficiency of several allergens to predict clinical reactivity. J Allergy Clin Immunol Pract. août. 2015;3(4):521–529. doi: 10.1016/j.jaip.2015.02.001. e10. [DOI] [PubMed] [Google Scholar]
- 43.Farioli L., Losappio L.M., Giuffrida M.G., et al. Mite-induced asthma and IgE levels to shrimp, mite, tropomyosin, arginine kinase, and der p 10 are the most relevant risk factors for challenge-proven shrimp allergy. Int Arch Allergy Immunol. 2017;174(3-4):133–143. doi: 10.1159/000481985. [DOI] [PubMed] [Google Scholar]
- 44.Guillen D., Fiandor A., del Pozo V., et al. Anaphylaxis caused by hemocyanin contained in shrimp cephalothorax. Ann Allergy Asthma Immunol. 1 déc. 2014;113(6):674–675.e2. doi: 10.1016/j.anai.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Pastorello E.A., Farioli L., Pravettoni V., et al. Pru p 3-sensitised Italian peach-allergic patients are less likely to develop severe symptoms when also presenting IgE antibodies to Pru p 1 and Pru p 4. Int Arch Allergy Immunol. 1 janv. 2011;156(4):362–372. doi: 10.1159/000324440. [DOI] [PubMed] [Google Scholar]
- 46.Nwaru B.I., Hickstein L., Panesar S.S., et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69(8):992–1007. doi: 10.1111/all.12423. [DOI] [PubMed] [Google Scholar]
- 47.shin M. Food allergies and food-induced anaphylaxis: role of cofactors. Clin Exp Pediatr. 12 nov 2020;64(8):393–399. doi: 10.3345/cep.2020.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]