Abstract
Rationale & Objective
Chronic kidney disease (CKD) has a far-reaching impact on both patients and care partners, which can be further compounded by frequent complications such as anemia. This study assessed the burden experienced by patients with CKD and the care partners of patients with CKD, with and without anemia.
Study Design
Online survey.
Setting & Participants
Adult patients with CKD and the care partners of adult patients with CKD living in the United States were recruited through the American Association of Kidney Patients and a third-party online panel (January 9, 2020-March 12, 2020).
Outcomes
Patient and care partner characteristics, care received or provided; health-related quality of life, and work productivity.
Analytical Approach
Descriptive statistics were reported separately based on the presence or absence of anemia.
Results
In total, 410 patients (anemia: n=190, no anemia: n=220) and 258 care partners (anemia: n=110, no anemia: n=148) completed the survey. Most patients reported receiving paid or unpaid care because of their health condition (anemia: 58.9%, no anemia: 50.9%), with an overall average of 14.2 and 11.3 h/wk among the anemia and no anemia patients, respectively. The care partners also reported providing numerous hours of care (anemia: 33.6 h/wk, no anemia: 38.0 h/wk), especially care partners living with their care recipient (anemia: 52.6 h/wk, no anemia: 42.8 h/wk). Among the patients, those with anemia reported a numerically lower average health-related quality of life (Functional Assessment of Cancer Therapy-Anemia score, anemia: 110.1; no anemia: 121.6). Most care partners reported a severe or very severe burden (Burden Scale for Family Caregivers-Short Version score≥15, anemia: 69.1%; no anemia: 58.8%). The work productivity impairment was substantial among employed patients (anemia: 44.9%, no anemia: 35.4%) and employed care partners (anemia: 47.9%, no anemia: 40.7%).
Limitations
The survey results may have been subject to selection and recall biases; moreover, the observational nature of the study does not allow for causal inferences.
Conclusions
Patients with CKD and the care partners of patients with CKD experience a considerable burden, especially when anemia is present.
Index Words: Anemia, burden, care partners, chronic kidney studies, quality of life, survey, work productivity
Plain-Language Summary.
Patients with chronic kidney disease (CKD) experience the loss of kidney function, which can lead to complications such as anemia. Prior studies have shown that patients with CKD experience a substantial burden, but little is known about how this burden varies among patients with and without anemia and how burdensome it is to provide care for someone with CKD. An online survey was conducted to assess the burden among patients with CKD and the care partners of patients with CKD. The results showed that CKD affected multiple aspects of both patients’ and care partners’ lives, including health-related quality of life and reduced work productivity. This highlights the need for support strategies for patients with CKD and their care partners.
Chronic kidney disease (CKD) is an umbrella term that encompasses several heterogeneous disorders that affect the structure and function of the kidneys.1 The irreversible loss of kidney function that characterizes the disease is associated with numerous disabling symptoms and impairments, which can result in a substantial burden on both patients and care partners. Patients with CKD commonly experience fatigue, muscle weakness, leg cramps, itching, functional disability in work, and impaired sleep and libido.2, 3, 4, 5 In addition to the symptoms associated with CKD, the time-consuming nature of some CKD treatments that require in-clinic administration, such as dialysis for patients with kidney failure, may further contribute to the overall burden of CKD.
As the disease progresses, several complications of CKD may emerge and further compound the burden of CKD for patients.6 Anemia is a common complication whose prevalence increases from 8% in patients with stage 1 CKD to 53% in those with stage 5 CKD not receiving dialysis.7 Various disease- and treatment-related factors can cause or worsen anemia in patients with CKD, including impaired erythropoiesis and iron absorption in the gut, a decreased red blood cell lifespan, and blood loss during hemodialysis.6 In addition to the symptoms of CKD, anemia may cause or worsen fatigue, weakness, low exercise tolerance, difficulty concentrating, and dizziness.6,8 Together, these symptoms can significantly interfere with patients’ daily activities and health-related quality of life (HRQoL).
The care partners of patients with CKD may also experience a substantial burden in terms of HRQoL and work productivity impairment,9, 10, 11, 12, 13 which may similarly be exacerbated when anemia is present. However, existing studies that assessed the burden of CKD and anemia focused primarily on the burden experienced by patients rather than care partners.14 Therefore, the current study aimed to assess the burden experienced by patients with CKD and the care partners of patients with CKD, with and without anemia, in terms of HRQoL, work productivity, and care received or provided. Because of the increased burden that may be experienced by patients requiring dialysis and their care partners, the analyses were further stratified by kidney replacement therapy (KRT) status (ie, CKD with KRT or CKD without KRT) to better understand how experiences and perceptions may differ in these populations.
Methods
Survey Design and Data Source
The data for this study were collected from January 9, 2020 to March 12, 2020 through 2 online surveys: 1 was conducted among adult patients with CKD (Item S1) and the other among adult care partners of patients with CKD (Item S2). Patient and care partner respondents were independently recruited (ie, the inclusion of a given patient was not contingent on the participation of that patient’s care partner and vice versa). Email invitations were sent to the members of the American Association of Kidney Patients and to the members of an online panel maintained by a third-party recruiter (Dynata). Both the panels included individuals from all US regions. The sampling approach included quotas by anemia and KRT status, which were enforced to obtain approximately equal numbers of patients and care partners of patients with and without anemia as well as approximately equal numbers of patients with and without KRT to conduct both the main and sensitivity analyses described below. The sample size was determined based on the maximum number of patients and care partners that could be recruited based on a feasibility assessment. The final sample included 410 patients (272 [66.3%] from the American Association of Kidney Patients; 138 [33.6%] from Dynata’s panel) and 358 care partners (53 [14.8%] from the American Association of Kidney Patients; 305 [85.2%] from Dynata’s panel).
An initial group of 5 patients and 5 care partners completed semistructured interviews via telephone, conducted to review the survey content, ensure comprehension, and refine the questions as needed. Each survey took approximately 15 minutes to complete, including a short screening section to confirm respondent eligibility and agreement to participate in the study. The participating respondents were not aware of the identity of the study sponsor, and the survey did not collect any personal identifiable information. The respondents were compensated for completing the survey. This study was approved under the exempted category by the New England Institutional Review Board before the start of data collection.
Study Population
Patients were eligible to participate in this study if they met the following criteria: (1) they were ≥18 years of age; (2) they were, at minimum, somewhat comfortable reading and understanding English; (3) they were diagnosed with CKD; (4) they had no previous kidney transplant; (5) they had no history of cancer; and (6) they knew whether they were undergoing KRT regularly (ie, hemodialysis [in-center], hemodialysis [home], or peritoneal dialysis).
Similarly, care partners were eligible to participate in this study if they met the following criteria: (1) they were ≥18 years of age; (2) they were, at minimum, somewhat comfortable reading and understanding English; (3) they had provided care within 4 weeks before data collection to an adult patient diagnosed with CKD who had no previous kidney transplant or history of cancer (ie, care recipient); and (4) they knew whether the care recipient was undergoing KRT regularly (ie, hemodialysis [in-center], hemodialysis [home], or peritoneal dialysis).
The patient and care partner respondents were classified into mutually exclusive cohorts (ie, anemia cohort or no-anemia cohort) based on the patient’s or care recipient’s anemia status.
Study Measures and Outcomes
Because the patients and care partners were recruited independently from each other, patients under the care of care partner respondents—hereafter referred to as “care recipients”—were a group distinct from patient respondents. Thus, some study measures were reported among care recipients (ie, from the care partner’s perspective) in addition to the patient and care partner respondents.
Information on demographic and clinical characteristics, such as age, sex, race, and comorbidities, was reported separately among the patients, care recipients, and care partners. Among patients and care recipients with anemia, anemia-related characteristics, such as anemia severity and treatments, were reported. Patient respondents receiving anemia treatment reported their satisfaction with current anemia treatments, and both patients and the care partners of patients receiving anemia treatment reported their preference for the route and frequency of anemia treatment administration. Information on care received or provided, such as the number of hours of care per week, was also reported from the patient and care partner perspectives, respectively.
HRQoL was assessed among all the patient and care partner respondents. The patients’ HRQoL was assessed using the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire, an instrument that has been validated in the CKD population.15 The total FACT-An score is calculated by summing the Functional Assessment of Cancer Therapy-General (FACT-G) subscale, which is used to measure the general HRQoL, and the FACT-An subscale, which considers the impact of fatigue and anemia-related symptoms on patients’ HRQoL.16 The total FACT-An score ranges from 0 to 188, with lower scores indicating a lower HRQoL. The care partners’ HRQoL was assessed using the Burden Scale for Family Caregivers–Short Version. The Burden Scale for Family Caregivers–Short Version is a validated instrument that is used to measure the degree of the subjective burden of caregiving.17,18 The total Burden Scale for Family Caregivers–Short Version score ranges from 0 to 30, with higher scores reflecting a greater burden.17
Information on employment and work productivity was also reported among all the patient and care partner respondents, separately. The patients’ and care partners’ work productivity was measured using a validated instrument, Work Productivity and Activity Impairment-Specific Health Problem and Work Productivity and Activity Impairment-Caregiver, respectively.19 Job-related decisions, such as changing the number of work hours, taking a leave of absence, or declining a job advancement, were also assessed.
Statistical Analyses
Study measures and outcomes were descriptively summarized separately for the patients, care recipients, and care partners (as applicable) in the anemia and no-anemia cohorts. Means, medians, and standard deviations were reported for continuous variables; frequency counts and percentages were reported for categorical variables. No statistical comparisons between the cohorts were conducted; all differences reported in this study are numerical.
Sensitivity Analysis
In addition to the main stratification by anemia status, the cohorts were further stratified by KRT status (ie, CKD with KRT or CKD without KRT) to better understand how the burden may differ in these subgroups.
Results
The surveys were completed by 410 patients (anemia cohort: n=190, no-anemia cohort: n=220) and 258 care partners (anemia cohort: n=110, no-anemia cohort: n=148). There were 559 patients and 825 care partners who entered the survey but did not provide informed consent or did not meet the eligibility criteria.
Demographic and Clinical Characteristics: Patients and Care Recipients
The majority of the patients were aged ≥55 years (anemia cohort: 61.6%, no-anemia cohort: 67.7%), and most patients were women (anemia cohort: 67.4%, no-anemia cohort: 50.9%). The proportion of African American or Black patients was 21.1% in the anemia cohort and 13.6% in the no-anemia cohort (Table 1). The most frequently reported comorbidities among the patients included hypertension (anemia cohort: 65.8%, no-anemia cohort: 59.1%), high cholesterol (anemia cohort: 40.5%, no-anemia cohort: 32.3%), and diabetes (anemia cohort: 37.4%, no-anemia cohort: 31.8%; Table 2). The largest difference in the patients’ comorbid conditions between the cohorts was observed for depression (anemia cohort: 32.6%, no-anemia cohort: 18.6%). Similar trends in the demographic and clinical characteristics between the cohorts were observed among the care recipients (Tables 1 and 2).
Table 1.
Demographic Characteristics of Patients and Care Recipients
| Number of Patients or Care Recipients, n | Patient Characteristics |
Care Recipient Characteristics |
||||||
|---|---|---|---|---|---|---|---|---|
| Anemia Cohort |
No-Anemia Cohort |
Anemia Cohort |
No-Anemia Cohort |
|||||
| n=190 | n=220 | n=110 | n=148 | |||||
| Age (y), n (%) | ||||||||
| 18-34 | 14 | (7.4%) | 7 | (3.2%) | 8 | (7.3%) | 8 | (5.4%) |
| 35-44 | 29 | (15.3%) | 23 | (10.5%) | 16 | (14.5%) | 14 | (9.5%) |
| 45-54 | 30 | (15.8%) | 41 | (18.6%) | 17 | (15.5%) | 13 | (8.8%) |
| 55-64 | 48 | (25.3%) | 48 | (21.8%) | 24 | (21.8%) | 25 | (16.9%) |
| 65-74 | 48 | (25.3%) | 67 | (30.5%) | 24 | (21.8%) | 43 | (29.1%) |
| 75-84 | 16 | (8.4%) | 31 | (14.1%) | 10 | (9.1%) | 29 | (19.6%) |
| ≥85 | 5 | (2.6%) | 3 | (1.4%) | 11 | (10.0%) | 16 | (10.8%) |
| Sex, n (%) | ||||||||
| Male | 62 | (32.6%) | 108 | (49.1%) | 51 | (46.4%) | 87 | (58.8%) |
| Female | 128 | (67.4%) | 112 | (50.9%) | 59 | (53.6%) | 61 | (41.2%) |
| Race, n (%)a | ||||||||
| African American or Black | 40 | (21.1%) | 30 | (13.6%) | 20 | (18.2%) | 12 | (8.1%) |
| Asian or Pacific Islander | 3 | (1.6%) | 5 | (2.3%) | 3 | (2.7%) | 5 | (3.4%) |
| Native American or Alaskan Native | 8 | (4.2%) | 6 | (2.7%) | 4 | (3.6%) | 6 | (4.1%) |
| Hispanic or Latino | 13 | (6.8%) | 15 | (6.8%) | 15 | (13.6%) | 17 | (11.5%) |
| White | 137 | (72.1%) | 171 | (77.7%) | 71 | (64.5%) | 116 | (78.4%) |
| Other | 1 | (0.5%) | 0 | (0.0%) | 1 | (0.9%) | 0 | (0.0%) |
| Prefer not to answer | 2 | (1.1%) | 3 | (1.4%) | 0 | (0.0%) | 0 | (0.0%) |
| Region of residence, n (%) | ||||||||
| Northeast | 29 | (15.3%) | 30 | (13.6%) | 24 | (21.8%) | 28 | (18.9%) |
| West | 32 | (16.8%) | 60 | (27.3%) | 19 | (17.3%) | 24 | (16.2%) |
| Midwest | 45 | (23.7%) | 27 | (12.3%) | 23 | (20.9%) | 29 | (19.6%) |
| South | 84 | (44.2%) | 103 | (46.8%) | 44 | (40.0%) | 67 | (45.3%) |
Note: Patient characteristics were measured in the patient survey, and care recipient characteristics were measured in the care partner survey. Both patient and care recipient characteristics were measured at the time of data collection.
Patients and care partners could select more than 1 option (not mutually exclusive).
Table 2.
Clinical Characteristics of Patients and Care Recipients
| Number of Patients and Care Recipients, n | Patient Characteristics |
Care Recipient Characteristics |
||||||
|---|---|---|---|---|---|---|---|---|
| Anemia Cohort |
No-Anemia Cohort |
Anemia Cohort |
No-Anemia Cohort |
|||||
| n=190 | n=220 | n=110 | n=148 | |||||
| Registration on a waitlist for kidney transplant, n (%) | 49 | (25.8%) | 41 | (18.6%) | 52 | (47.3%) | 38 | (25.7%) |
| KRT on a regular basis, n (%) | 80 | (42.1%) | 110 | (50.0%) | 68 | (61.8%) | 80 | (54.1%) |
| Time since CKD diagnosis (y), mean ± SD (median) | 8.8 ± 9.2 (6.0) | 9.2 ± 9.7 (6.0) | 5.3 ± 7.2 (3.0) | 4.9 ± 5.2 (4.0) | ||||
| Comorbidities, n (%)a | ||||||||
| Hypertension | 125 | (65.8%) | 130 | (59.1%) | 62 | (56.4%) | 81 | (54.7%) |
| High cholesterol | 77 | (40.5%) | 71 | (32.3%) | 43 | (39.1%) | 52 | (35.1%) |
| Diabetes | 71 | (37.4%) | 70 | (31.8%) | 56 | (50.9%) | 53 | (35.8%) |
| Depression | 62 | (32.6%) | 41 | (18.6%) | 48 | (43.6%) | 30 | (20.3%) |
| Thyroid problems | 60 | (31.6%) | 49 | (22.3%) | 30 | (27.3%) | 27 | (18.2%) |
| Heart disease | 47 | (24.7%) | 44 | (20.0%) | 43 | (39.1%) | 56 | (37.8%) |
| Chronic respiratory disease | 37 | (19.5%) | 20 | (9.1%) | 32 | (29.1%) | 23 | (15.5%) |
| Gout | 35 | (18.4%) | 34 | (15.5%) | 18 | (16.4%) | 23 | (15.5%) |
| Hyperkalemia | 22 | (11.6%) | 20 | (9.1%) | 13 | (11.8%) | 11 | (7.4%) |
| Cerebrovascular disease | 7 | (3.7%) | 5 | (2.3%) | 13 | (11.8%) | 8 | (5.4%) |
| Malnutrition | 2 | (1.1%) | 2 | (0.9%) | 9 | (8.2%) | 1 | (0.7%) |
| Other | 25 | (13.2%) | 21 | (9.5%) | 5 | (4.5%) | 12 | (8.1%) |
| Anemia-related characteristics | ||||||||
| Time since anemia diagnosis (y), mean ± SD (median) | 8.4 ± 11.1 (5.0) | – | 4.3 ± 7.0 (2.0) | – | ||||
| Anemia severity level, n (%) | ||||||||
| Mild | 49 | (25.8%) | – | 17 | (15.5%) | – | ||
| Moderate | 85 | (44.7%) | – | 69 | (62.7%) | – | ||
| Severe | 25 | (13.2%) | – | 15 | (13.6%) | – | ||
| Unknown | 31 | (16.3%) | – | 9 | (8.2%) | – | ||
| Current anemia treatment(s), n (%)ab | ||||||||
| Red blood cell transfusion | 18 | (9.5%) | – | 23 | (20.9%) | – | ||
| ESA | 54 | (28.4%) | – | 25 | (22.7%) | – | ||
| Intravenous iron supplement | 46 | (24.2%) | – | 37 | (33.6%) | – | ||
| Oral iron supplement | 87 | (45.8%) | – | 50 | (45.5%) | – | ||
| Vitamin B12 supplement | 67 | (35.3%) | – | 51 | (46.4%) | – | ||
| Folic acid supplement | 44 | (23.2%) | – | 33 | (30.0%) | – | ||
| Other vitamin supplement | 8 | (4.2%) | – | 6 | (5.5%) | – | ||
| Dietary modification | 29 | (15.3%) | – | 36 | (32.7%) | – | ||
| Other treatment | 2 | (1.1%) | – | 2 | (1.8%) | – | ||
| None | 26 | (13.7%) | – | 8 | (7.3%) | – | ||
| Had at least 1 current anemia treatment, n (%) | 164 | (86.3%) | – | 102 | (92.7%) | – | ||
| Patient-reported treatment efficacy in helping to alleviate symptoms, n (%)c | ||||||||
| Yes | 113 | (68.9%) | – | – | – | |||
| No | 21 | (12.8%) | – | – | – | |||
| Unknown | 30 | (18.3%) | – | – | – | |||
| Patient-reported satisfaction with current anemia treatment(s), n (%)b | ||||||||
| Extremely satisfied | 21 | (12.8%) | – | – | – | |||
| Satisfied | 90 | (54.9%) | – | – | – | |||
| Neither satisfied nor dissatisfied | 46 | (28.0%) | – | – | – | |||
| Dissatisfied | 7 | (4.3%) | – | – | – | |||
| Extremely dissatisfied | 0 | (0.0%) | – | – | – | |||
Note: Patient characteristics were measured in the patient survey, and care recipient characteristics were measured in the care partner survey. Both patient and care recipient characteristics were measured at the time of data collection.
Abbreviations: CKD, chronic kidney disease; ESA, erythropoiesis stimulating agents; SD, standard deviation.
Patients and care partners could select more than 1 option (not mutually exclusive).
Current treatments included those received within the month before completing the survey.
Evaluated among patients who indicated currently being treated with at least 1 treatment (n=164).
Anemia-Related Characteristics: Patients and Care Recipients
Among the patients in the anemia cohort, the majority reported having moderate or severe anemia (57.9%; Table 2). Furthermore, most patients (86.3%) reported receiving at least 1 anemia treatment in the month before data collection. The most common anemia treatments received were an oral iron supplement (45.8%), vitamin B12 supplement (35.3%), and erythropoiesis-stimulating agents (28.4%). Among patients who received erythropoiesis-stimulating agents, 79.6% reported receiving in-clinic administrations and 20.4% reported receiving at-home administrations. Additional erythropoiesis-stimulating agent characteristics, including the frequency of administration and the time needed for administration, can be found in Table S1.
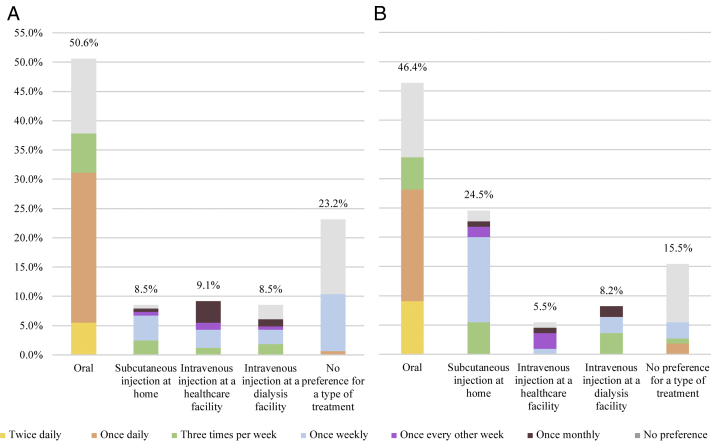
When patients receiving anemia treatment were asked about their level of satisfaction with their current anemia treatment(s), 67.7% reported being either satisfied or extremely satisfied (Table 2). The most commonly reported reasons for satisfaction included “improved symptoms” (55.4%), “convenience of administration” (49.7%), and “no or only mild adverse events or side effects” (46.5%). Conversely, the most commonly reported reasons for dissatisfaction with current anemia treatments included “no (or few) improvements in symptoms” (52.8%), “inconvenient monitoring requirements” (18.9%), and “diminished quality of life” (15.1%). If patients receiving at least 1 anemia treatment were to initiate a new anemia treatment, 44.2% indicated that they would prefer an oral treatment, of which 50.0% would prefer a once-daily formulation (Fig 1). The anemia-related characteristics were largely consistent among the care recipients (Table 2; Fig 1).
Figure 1.
(A) Patients’ and (B) care partners’ preferences for the route and frequency of administration of anemia treatment. The preferred frequency of administration was asked only to the subset of patients and care partners who indicated that a particular route of administration was preferred. The patients’ preference for the route and frequency of administration was assessed among patients who were currently receiving an anemia treatment. The care partners’ preference was assessed among all the care partners. An intravenous injection at a dialysis facility was administered only to patients who indicated receiving dialysis (n=80) and care partners who indicated providing care to a care recipient receiving dialysis (n=68).
Care Received: Patients
Most patients reported receiving paid or unpaid care because of their health condition (anemia cohort: 58.9%, no-anemia cohort: 50.9%; Table S2). Furthermore, the average number of hours of care received weekly was 14.2 hours in the anemia cohort and 11.3 hours in the no-anemia cohort.
Care Partner Characteristics
The majority of the care partners were <55 years of age (anemia cohort: 69.1%, no-anemia cohort: 51.4%) and were women (anemia cohort: 72.7%, no-anemia cohort: 78.4%; Table 3). The proportion of African American or Black care partners in the anemia and no-anemia cohorts was 16.4% and 8.8%, respectively. The comorbidities and symptoms most frequently reported by the care partners included anxiety (anemia cohort: 43.6%, no-anemia cohort: 32.4%), depression (anemia cohort: 36.4%, no-anemia cohort: 30.4%), headache (anemia cohort: 35.5%, no-anemia cohort: 31.8%), and sleep disturbances (anemia cohort: 30.9%, no-anemia cohort: 31.1%).
Table 3.
Care Partner Characteristics
| Number of Care Partners, n | Anemia Cohort |
No-Anemia Cohort |
||
|---|---|---|---|---|
| n=110 | n=148 | |||
| Demographic characteristics | ||||
| Age (y), n (%) | ||||
| 18-34 | 30 | (27.3%) | 15 | (10.1%) |
| 35-44 | 30 | (27.3%) | 27 | (18.2%) |
| 45-54 | 16 | (14.5%) | 34 | (23.0%) |
| 55-64 | 20 | (18.2%) | 34 | (23.0%) |
| 65-74 | 11 | (10.0%) | 35 | (23.6%) |
| 75-84 | 3 | (2.7%) | 3 | (2.0%) |
| ≥85 | 0 | (0.0%) | 0 | (0.0%) |
| Sex, n (%) | ||||
| Male | 29 | (26.4%) | 32 | (21.6%) |
| Female | 80 | (72.7%) | 116 | (78.4%) |
| Prefer not to answer | 1 | (0.9%) | 0 | (0.0%) |
| Race, n (%)a | ||||
| African American or Black | 18 | (16.4%) | 13 | (8.8%) |
| Asian or Pacific Islander | 6 | (5.5%) | 3 | (2.0%) |
| Native American or Alaskan Native | 3 | (2.7%) | 3 | (2.0%) |
| Hispanic or Latino | 15 | (13.6%) | 15 | (10.1%) |
| White | 79 | (71.8%) | 120 | (81.1%) |
| Other | 2 | (1.8%) | 0 | (0.0%) |
| Prefer not to answer | 0 | (0.0%) | 0 | (0.0%) |
| Clinical characteristics | ||||
| Comorbidities, n (%)a | ||||
| Anxiety | 48 | (43.6%) | 48 | (32.4%) |
| Depression | 40 | (36.4%) | 45 | (30.4%) |
| Headache(s) or migraine(s) | 39 | (35.5%) | 47 | (31.8%) |
| Sleep disturbance(s) or insomnia | 34 | (30.9%) | 46 | (31.1%) |
| Hypertension | 23 | (20.9%) | 48 | (32.4%) |
| Gastrointestinal symptoms | 21 | (19.1%) | 18 | (12.2%) |
| Diabetes | 19 | (17.3%) | 20 | (13.5%) |
| Substantial weight loss or gain | 12 | (10.9%) | 21 | (14.2%) |
| Chronic fatigue syndrome | 12 | (10.9%) | 6 | (4.1%) |
| Chronic respiratory disease | 11 | (10.0%) | 14 | (9.5%) |
| Heart disease | 6 | (5.5%) | 8 | (5.4%) |
| Kidney disease | 4 | (3.6%) | 3 | (2.0%) |
| Cancer | 4 | (3.6%) | 2 | (1.4%) |
| Alzheimer disease or dementia | 3 | (2.7%) | 2 | (1.4%) |
| Cerebrovascular disease | 1 | (0.9%) | 5 | (3.4%) |
| Other | 10 | (9.1%) | 20 | (13.5%) |
| None | 17 | (15.5%) | 26 | (17.6%) |
| Caregiving characteristics | ||||
| Primary care partner, n (%) | 94 | (85.5%) | 132 | (89.2%) |
| Relationship with care recipient, n (%) | ||||
| Spouse or partner | 36 | (32.7%) | 67 | (45.3%) |
| Parent or stepparent | 32 | (29.1%) | 36 | (24.3%) |
| Sibling or stepsibling | 3 | (2.7%) | 9 | (6.1%) |
| Child or stepchild | 5 | (4.5%) | 16 | (10.8%) |
| Aunt, uncle, or other relative | 13 | (11.8%) | 6 | (4.1%) |
| Friend or neighbor | 18 | (16.4%) | 12 | (8.1%) |
| Other | 0 | (0.0%) | 1 | (0.7%) |
| Unknown or prefer not to answer | 3 | (2.7%) | 1 | (0.7%) |
| Time since started caring for the care recipient (y), mean ± SD (median) | 3.5 ± 5.1 (2.0) | 3.7 ± 3.9 (3.0) | ||
| Care partner lives with the care recipient, n (%) | 56 | (50.9%) | 90 | (60.8%) |
| Number of h per wk spent caring for the care recipient, mean ± SD (median)b | 33.6 ± 35.8 (20.5) | 38.0 ± 40.9 (25.0) | ||
| Number of h per wk spent caring if living with the care recipient | 52.6 ± 42.5 (40.0) | 42.8 ± 41.6 (30.0) | ||
Note: Care partner characteristics were measured at the time of data collection.
Abbreviation: SD, standard deviation.
More than 1 option could be selected (not mutually exclusive).
Measured at the time of data collection, based on recollection from the past 4 weeks.
The majority of the care partners identified themselves as the primary care partner (anemia cohort: 85.5%, no-anemia cohort: 89.2%) and indicated that they lived with the care recipient (anemia cohort: 57.7%, no-anemia cohort: 67.7%). Overall, the care partners reported providing a substantial number of hours of care per week (anemia cohort: 33.6 hours, no-anemia cohort: 38.0 hours). Among those living with the care recipient, the time spent on care per week was 52.6 hours in the anemia cohort and 42.8 hours in the no-anemia cohort.
HRQoL: Patients and Care Partners
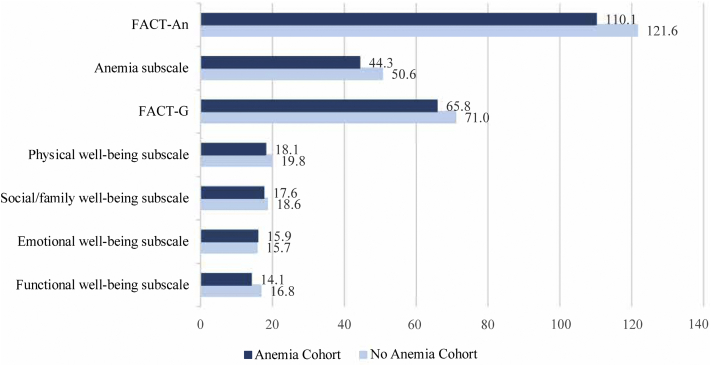
Regarding the patients’ HRQoL, the total FACT-An score was 110.1 in the anemia cohort and 121.6 in the no-anemia cohort (the commonly accepted minimal clinically important difference was 7.0; Fig 2).20 This trend was consistent in both the FACT-G subscale (anemia cohort: 65.8, no-anemia cohort: 71.0) and the anemia subscale (anemia cohort: 44.3, no-anemia cohort: 50.6).
Figure 2.
Total Functional Assessment of Cancer Therapy-Anemia and subscale scores. The Functional Assessment of Cancer Therapy-Anemia scores were derived through the addition of the anemia subscale score and the Functional Assessment of Cancer Therapy-General score. The total Functional Assessment of Cancer Therapy-General scores were derived through the addition of scores for the physical well-being, social or family well-being, emotional well-being, and functional well-being domains. For physical well-being, social or family well-being, and functional well-being, the scores ranged from 0 to 28. For emotional well-being, the scores ranged from 0 to 24. For the anemia subscale, the scores ranged from 0 to 80. For the Functional Assessment of Cancer Therapy-General subscale, the scores ranged from 0 to 108. The total Functional Assessment of Cancer Therapy-Anemia scores ranged from 0 to 188. For all the scales, a higher score indicated better health-related quality of life. Abbreviations: FACT-An, Functional Assessment of Cancer Therapy-Anemia; FACT-G, Functional Assessment of Cancer Therapy-General.
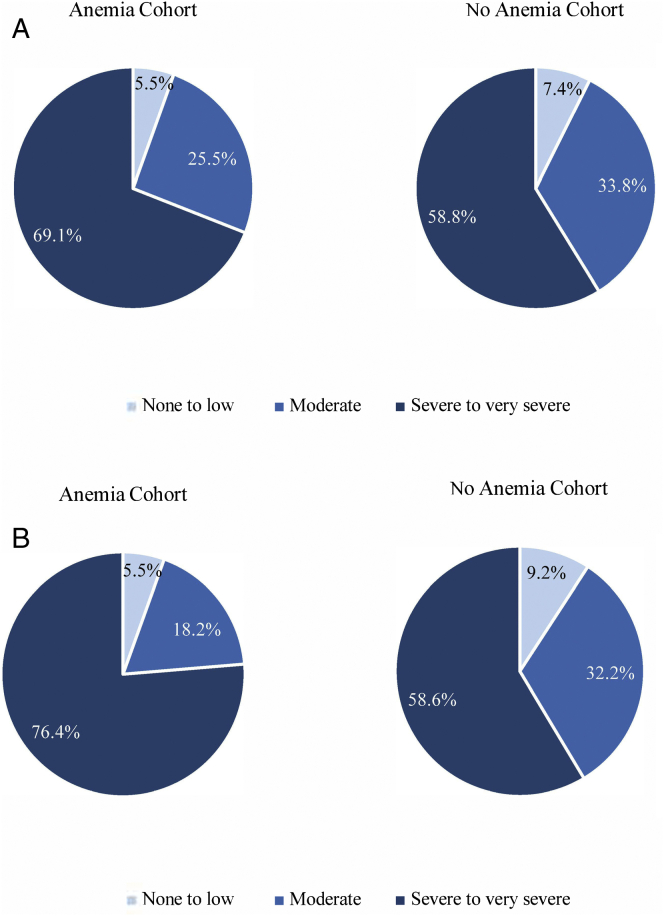
Similarly, most care partners reported a severe-to-very-severe burden due to caregiving (ie, Burden Scale for Family Caregivers–Short Version score≥15).18 The proportion of care partners who reported having a severe-to-very-severe burden was 69.1% for those who cared for patients with anemia and 58.8% for those who cared for patients without anemia (Fig 3A). Among care partners living with the care recipient, this trend appeared even more pronounced (anemia cohort: 76.4%, no-anemia cohort: 58.6%; Fig 3B).
Figure 3.
Burden of caregiving, measured using the Burden Scale for Family Caregivers–Short Version, among (A) all care partners and (B) care partners living with the care recipient. The Burden Scale for Family Caregivers-Short Version score ranged from 0 to 30, with higher scores indicating a greater care partner burden. Scores ranging from 0 to 4 indicate a degree of subjective burden ranging from none to low, with no increased risk of physical psychosomatic complaints. Scores ranging from 5 to 14 indicate a moderate degree of subjective burden, with an increased risk of physical psychosomatic complaints. Scores ranging from 15 to 30 indicate a severe-to-very-severe degree of subjective burden and a very much increased risk of physical psychosomatic complaints.
Work Productivity and Work-Related Decisions: Patients and Care Partners
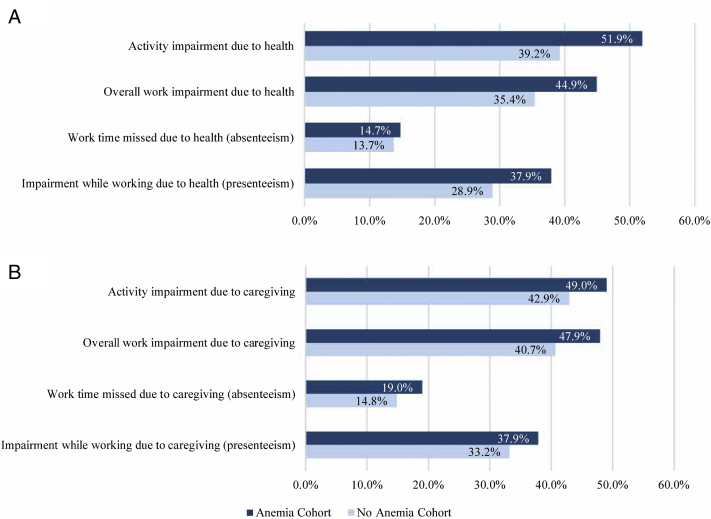
The reported average impairment in nonwork-related activities was substantial among employed and unemployed patients (anemia cohort: 51.9%, no-anemia cohort: 39.2%; Fig 4A) and care partners (anemia cohort: 49.0%, no-anemia cohort: 42.9%; Fig 4B). In the subset of employed respondents, a substantial average reduction in work productivity was reported by the patients (anemia cohort: 44.9%, no-anemia cohort: 35.4%; Fig 4A) and care partners (anemia cohort: 47.9%, no-anemia cohort: 40.7%; Fig 4B). The degree of absenteeism and presenteeism was also noteworthy in both the patients and the care partners (Fig 4).
Figure 4.
(A) Patient work productivity, assessed using the Work Productivity and Activity Impairment (WPAI)-Specific Health Problem, was evaluated based on the last 7 days at the time of data collection. The WPAI-Specific Health Problem outcomes are expressed as impairment percentages, with higher numbers indicating greater impairment and less productivity. Activity impairment refers to the impact of health problems on the ability to complete daily activities and was measured among all the patients (n=410). Overall work impairment, presenteeism, and absenteeism refer to the impact of health problems on the ability to work and were measured among employed patients (n=133, anemia cohort: n=55, no-anemia cohort: n=78). (B) Care partner work productivity, assessed using the WPAI-Caregiver, was evaluated based on the last 7 days at the time of data collection. The WPAI-Caregiver outcomes are expressed as impairment percentages, with higher numbers indicating greater impairment and less productivity. Activity impairment refers to the impact of caregiving on the ability to complete daily activities and was measured among all the care partners (n=258). Overall work impairment, presenteeism, and absenteeism refer to the impact of caregiving on the ability to work and were measured among employed care partners (n=141, anemia cohort: n=67, no-anemia cohort: n=74).
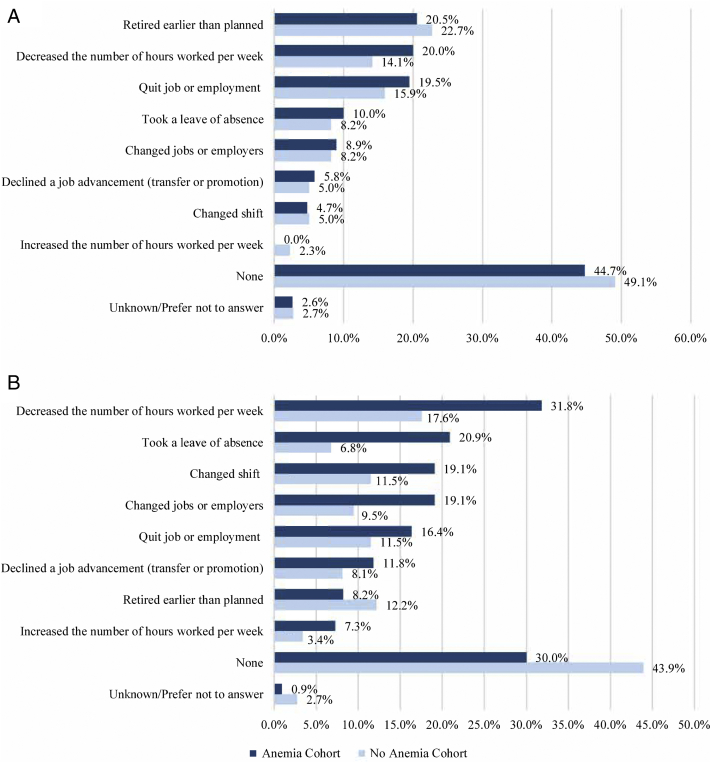
Overall, approximately half of the patients (anemia cohort: 52.6%, no-anemia cohort: 48.2%; Fig 5A) and the majority of the care partners (anemia cohort: 69.1%, no-anemia cohort: 53.4%; Fig 5B) made at least 1 job-related decision because of the patient’s health. Among the patients, the most commonly reported decisions included retiring early (anemia cohort: 20.5%, no-anemia cohort: 22.7%), decreasing the number of hours worked (anemia cohort: 20.0%, no-anemia cohort: 14.1%), and quitting their job or employment (anemia cohort: 19.5%, no-anemia cohort: 15.9%; Fig 5A). Among the care partners, the most commonly reported decisions included decreasing the number of hours worked per week (anemia cohort: 31.8%, no-anemia cohort: 17.6%) and taking a leave of absence (anemia cohort: 20.9%, no-anemia cohort: 6.8%; Fig 5B).
Figure 5.
Job-related decisions among (A) patients and (B) care partners. More than 1 option could be selected (not mutually exclusive).
Sensitivity Analysis
Overall, the results of the analyses stratified by KRT status were generally consistent with the results presented above and suggested that the patient and care partner respondents in both the CKD-with-KRT and CKD-without-KRT subgroups experienced an important burden, which was particularly high in the CKD-with-KRT subgroup. In addition, within each subgroup, the patients and care partners in the anemia cohort tended to report a greater burden than those in the no-anemia cohort. This is perhaps best illustrated by the trends observed in HRQoL. Among the patient respondents, the FACT-An score in the CKD-with-KRT subgroup (anemia cohort: 108.4, no-anemia cohort: 114.4) appeared lower than that in the CKD-without-KRT subgroup (anemia cohort: 111.3, no-anemia cohort: 128.7), indicating a potentially lower HRQoL among CKD patients with KRT. Similarly, the proportion of care partners who reported a severe-to-very-severe burden due to caregiving appeared greater in the CKD-with-KRT subgroup (anemia cohort: 73.5%, no-anemia cohort: 67.5%) than in the CKD-without-KRT subgroup (anemia cohort: 61.9%, no-anemia cohort: 48.5%). Additional results stratified by KRT status can be found in Tables S3 and S4.
Discussion
The present study suggests that CKD imposes a substantial burden on both patients and care partners, as evidenced by the poor HRQoL and reduced work productivity, and that the burden appears to be exacerbated by anemia. Prior real-world studies have exclusively focused on the burden of CKD experienced by patients,21,22 such that the extent of the burden experienced by care partners remained largely unknown. The findings of this study build on previous work by describing the burden experienced by both patients with CKD and the care partners of patients with CKD in terms of HRQoL, work productivity, and care received or provided, in the presence or absence of anemia.
The results of the patient survey are consistent with those of 2 previous international, real-world studies that evaluated the HRQoL and work productivity among patients with CKD.21,22 The numerically smaller difference in HRQoL between the anemia and no-anemia cohorts among CKD patients with KRT may have been because the additional burden ensuing from anemia was relatively modest compared with that ensuing from KRT and other symptoms in patients with a more advanced disease stage. In addition to this HRQoL burden, most patients reported receiving regular care for their anemia, which may also have contributed to increasing the burden associated with anemia in patients with CKD. Furthermore, employed patients reported an approximately 40% reduction in work productivity, which appeared more pronounced among patients with anemia. Taken together, these results suggest that patients with CKD experience a considerable burden, with anemia and KRT potentially further adding to this burden.
The current survey found that approximately two-thirds of the care partners reported a severe or very severe degree of subjective burden, and that proportion appeared to be even higher among the care partners of patients with anemia. Additionally, the care partners reported providing a substantial number of hours of care per week (anemia cohort: 33.6 hours, no-anemia cohort: 38.0 hours) and experienced a high level of work productivity impairment, which may have increased financial stress for the care partners and led to considerable indirect costs to society. Consistent with our findings, the National Alliance for Caregiving found that one-third of the care partners of patients across different diseases (eg, cancer, dementia, and diabetes) in the United States who provided more than 21 hours of care per week felt that their role had worsened their overall health. Additionally, 64% of all care partners reported moderate-to-high emotional stress.23 Given that CKD is a chronic condition and care partners play a crucial role in the patient journey, including for the administration of certain treatments, the impact of this burden on care partners can be far-reaching. Recently, the International Federation of Kidney Foundations emphasized that strategies to improve life participation (ie, the ability to do meaningful activities in life) should be equally applied to both patients and care partners.24 Our findings contextualize the burden of being a care partner of patients with CKD by capturing the care partners’ perspective, which is important for improving our understanding of the burden associated with CKD and anemia in patients with CKD among care partners. Conceivably, improvement in patients’ life participation (eg, through more convenient therapeutic options) may also help ameliorate the well-being of both patients and care partners.
A number of reasons may underlie the higher burden reported by the patients and care partners in the presence of anemia. Symptoms associated with anemia in patients with CKD are known to impair the patients’ HRQoL independently of disease stage. These symptoms likely contributed to the patients’ HRQoL and work productivity burden observed in this study and may also explain a part of the observed burden among the care partners. Moreover, frequent visits associated with anemia treatment and anemia monitoring may impose an additional burden on both patients and care partners, especially in the CKD-without-KRT population. Over three-quarters of the patients receiving erythropoiesis-stimulating agents in the current study reported receiving in-clinic administrations, which typically require substantial time and can disrupt daily activities for both patients and care partners. Taken together, these factors may explain why a substantial proportion of the patients and care partners expressed a preference for a once-daily, oral medication rather than subcutaneous or intravenous needle-based treatments to manage anemia in patients with CKD. Novel, oral hypoxia-inducible factor prolyl hydroxylase inhibitor treatments are currently undergoing clinical evaluation for the treatment of anemia in patients with CKD and could help alleviate this burden.25
The present study is subject to some limitations. First, the study sample may not be representative of the general population of patients with CKD and the care partners of patients with CKD in the United States. In particular, our sample included patients younger than the average CKD population in the United States. Recruiting was based on a sample of patient and care partner members of the American Association of Kidney Patients and Dynata’s panel who agreed to participate in the study, which may have led to a selection bias. For example, survey participants generally tend to be women, have a higher socioeconomic status, have a healthier lifestyle, and present with less morbidity.26 The proportion of African American or Black patients in the present survey was comparable with that in the general CKD population,27 likely because the sampling approach included quotas by anemia and KRT statuses. This approach may have favored the inclusion of African American or Black patients because this group tends to present with more severe forms of CKD (ie, with anemia and/or kidney failure), which partially reflects disparities in access to care and other social determinants of health.28 Second, although the respondents were asked to recall events that occurred in the recent past when possible, this study was subject to a recall bias or errors in the accuracy or completeness of the respondents’ recalled experiences. It was notable that approximately 50% of the patients in the no-anemia cohort reported receiving KRT, which may highlight the lack of awareness of anemia diagnoses among some patients. Because anemia treatments are often administered during KRT, these patients might not have been aware of their anemia diagnosis or whether they were receiving anemia treatments. Third, because of the observational nature of this study, no causal inferences can be made regarding the impact of anemia on the burden of CKD. In particular, the differences observed in the outcomes across the cohorts may have been influenced by differences in the characteristics (eg, age and comorbidities).
The findings of this survey study demonstrate that there is a considerable burden experienced by patients with CKD and the care partners of patients with CKD, especially when anemia is present. The crucial roles of care partners in the CKD patient journey should be recognized, and strategies are needed to improve their quality of life, which could lead to better care for these patients. Further studies are needed to better understand the extent of the burden associated with CKD and the impact of the burden among subgroups (eg, the elderly) as well as explore support strategies for patients and care partners.
Article Information
Authors’ Full Names and Academic Degrees
Steven N. Michalopoulos, MPH, Marjolaine Gauthier-Loiselle, PhD, Myrlene Sanon Aigbogun, MPH, Elizabeth Serra, MPH, Rebecca Bungay, MPH, Diana Clynes, BA, Martin Cloutier, MSc, Erin Kahle, MPA, CNP, Annie Guérin, MSc, Youssef M. K. Farag, MD, PhD, MPH, and Jay B. Wish, MD.
Authors’ Contributions
Research idea and study design: SNM, MG-L, MSA, ES, RB, DC, MC, EK, AG, YMKF, JBW; data acquisition: MG-L, ES, RB, MC, AG; and data analysis and interpretation: SNM, MG-L, MSA, ES, RB, DC, MC, EK, AG, YMKF, JBW. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was sponsored by Otsuka Pharmaceutical Development & Commercialization, Inc, and Akebia Therapeutics, Inc. The study sponsor was involved in all aspects of the research, including the design of the study; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Financial Disclosure
Dr Gauthier-Loiselle, Ms Serra, Ms Bungay, Mr Cloutier, and Ms Guérin are employees of Analysis Group, Inc., which provided paid consulting services to Otsuka Pharmaceutical Development & Commercialization Inc. for the conduct of the present study. Mr Michalopoulos and Ms Aigbogun were employees of Otsuka Pharmaceutical Development & Commercialization Inc. at the time the study was conducted but do not own stock or stock options. Ms Clynes and Ms Kahle are employees of the American Association of Kidney Patients, which has provided consulting services to Otsuka Pharmaceutical Development & Commercialization Inc. Dr Farag was an employee of Akebia Therapeutics, Inc. at the time the study was conducted. Dr Wish reports receiving consulting and advisory board honoraria from Akebia Therapeutics, Inc., Otsuka Pharmaceutical Development & Commercialization Inc., AstraZeneca, Vifor Pharma Group, and Rockwell Medical Inc., and also served on speakers’ bureaus for Akebia Therapeutics, Inc., and AstraZeneca.
Prior Presentation
A synopsis of this study was presented on October 22, 2020, during the American Society of Nephrology Kidney Week 2020 Reimagined.
Peer Review
Received June 8, 2021. Evaluated by 2 external peer reviewers, with direct editorial input by the Statistical Editor and the Editor-in-Chief. Accepted in revised form December 29, 2021.
Footnotes
Complete author and article information provided before references.
Item S1: Patient survey.
Item S2: Care partner survey.
Table S1: Erythropoiesis-stimulating agent characteristics.
Table S2: Care received by patients.
Table S3: Care received, anemia-related characteristics, health-related quality of life, employment, and work productivity among patients, stratified by dialysis status.
Table S4: Care provided, anemia-related characteristics, health-related quality of life, employment, and work productivity among care partners, stratified by dialysis status.
Supplementary Materials
Item S1 and S2; Tables S1-S4.
References
- 1.Levey A.S., Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 2.Edey M.M. Male sexual dysfunction and chronic kidney disease. Front Med (Lausanne) 2017;4:32. doi: 10.3389/fmed.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heiwe S. Experienced physical functioning and effects of resistance training in patients with chronic kidney disease. Institutionen för Medicin/Department of Medicine. 2004. https://openarchive.ki.se/xmlui/handle/10616/43197
- 4.Klang B., Clyne N. Well-being and functional ability in uraemic patients before and after having started dialysis treatment. Scand J Caring Sci. 1997;11(3):159–166. doi: 10.1111/j.1471-6712.1997.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 5.Pai M.F., Hsu S.P., Yang S.Y., Ho T.I., Lai C.F., Peng Y.S. Sleep disturbance in chronic hemodialysis patients: the impact of depression and anemia. Ren Fail. 2007;29(6):673–677. doi: 10.1080/08860220701459642. [DOI] [PubMed] [Google Scholar]
- 6.Romagnani P., Remuzzi G., Glassock R., et al. Chronic kidney disease. Nat Rev Dis Primers. 2017;3(1):1–24. doi: 10.1038/nrdp.2017.88. [DOI] [PubMed] [Google Scholar]
- 7.Stauffer M.E., Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute of Diabetes and Digestive and Kidney Diseases Anemia in chronic kidney disease. 2020. https://www.niddk.nih.gov/health-information/kidney-disease/anemia
- 9.Hopps M., Iadeluca L., McDonald M., Makinson G.T. The burden of family caregiving in the United States: work productivity, health care resource utilization, and mental health among employed adults. J Multidiscip Healthc. 2017;10:437–444. doi: 10.2147/JMDH.S135372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gayomali C., Sutherland S., Finkelstein F.O. The challenge for the caregiver of the patient with chronic kidney disease. Nephrol Dial Transplant. 2008;23(12):3749–3751. doi: 10.1093/ndt/gfn577. [DOI] [PubMed] [Google Scholar]
- 11.Feinberg L., Choula R. Understanding the impact of family caregiving on work. AARP Public Policy Institute. 2012. https://www.aarp.org/caregiving/life-balance/info-2019/working-caregiver-tips.html
- 12.Gaugler J.E., Pestka D.L., Davila H., et al. The complexities of family caregiving at work: a mixed-methods study. Int J Aging Hum Dev. 2018;87(4):347–376. doi: 10.1177/0091415017752936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giovannetti E.R., Wolff J.L., Frick K.D., Boult C. Construct validity of the Work Productivity and Activity Impairment questionnaire across informal caregivers of chronically ill older patients. Value Health. 2009;12(6):1011–1017. doi: 10.1111/j.1524-4733.2009.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anatchkova M., Arregui M., Brooks A., et al. National Kidney Foundation Spring Clinical Meeting; 2019. Targeted literature review of patient-reported burden of anemia in chronic kidney disease. Paper presented on May 8-12, 2019. [Google Scholar]
- 15.Finkelstein F.O., van Nooten F., Wiklund I., Trundell D., Cella D. Measurement properties of the Short Form-36 (SF-36) and the Functional Assessment of Cancer Therapy-Anemia (FACT-An) in patients with anemia associated with chronic kidney disease. Health Qual Life Outcomes. 2018;16(1):111. doi: 10.1186/s12955-018-0933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yellen S.B., Cella D.F., Webster K., Blendowski C., Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 17.Graessel E., Berth H., Lichte T., Grau H. Subjective caregiver burden: validity of the 10-item short version of the Burden Scale for Family Caregivers BSFC-s. BMC Geriatr. 2014;14(1):1–9. doi: 10.1186/1471-2318-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pendergrass A., Malnis C., Graf U., Engel S., Graessel E. Screening for caregivers at risk: extended validation of the short version of the burden scale for family caregivers (BSFC-s) with a valid classification system for caregivers caring for an older person at home. BMC Health Serv Res. 2018;18(1):1–9. doi: 10.1186/s12913-018-3047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reilly Associates WPAI references. 2020. http://www.reillyassociates.net/WPAI_References2.html
- 20.Cella D., Eton D.T., Lai J.S., Peterman A.H., Merkel D.E. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson D., Teitsson S., Goldsmith D., Jackson J., van Nooten F. Cross-sectional descriptive study of the impact of anaemia in patients with chronic kidney disease on healthcare resource utilisation and work productivity across Europe. Value Health. 2015;18(7):A514. [Google Scholar]
- 22.van Haalen H., Jackson J., Spinowitz B., Milligan G., Moon R. Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: analysis of multinational real-world data. BMC Nephrol. 2020;21(1):88. doi: 10.1186/s12882-020-01746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.AARP & National Alliance for Caregiving Caregiving in the United States 2020. 2020. https://www.aarp.org/ppi/info-2020/caregiving-in-the-united-states.html
- 24.Kalantar-Zadeh K., Li P.K., Tantisattamo E., et al. Living well with kidney disease by patient and care-partner empowerment: kidney health for everyone everywhere. Clin Nephrol. 2021;95(3):115–122. doi: 10.5414/CN110436. [DOI] [PubMed] [Google Scholar]
- 25.Sanghani N.S., Haase V.H. Hypoxia-inducible factor activators in renal anemia: current clinical experience. Adv Chronic Kidney Dis. 2019;26(4):253–266. doi: 10.1053/j.ackd.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enzenbach C., Wicklein B., Wirkner K., Loeffler M. Evaluating selection bias in a population-based cohort study with low baseline participation: the LIFE-Adult-Study. BMC Med Res Methodol. 2019;19(1):135. doi: 10.1186/s12874-019-0779-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Chronic kidney disease surveillance system. https://nccd.cdc.gov/CKD/detail.aspx?Qnum=Q55
- 28.Norton J.M., Moxey-Mims M.M., Eggers P.W., et al. Social determinants of racial disparities in CKD. J Am Soc Nephrol. 2016;27(9):2576–2595. doi: 10.1681/ASN.2016010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1 and S2; Tables S1-S4.