Summary
Telomeres form unique nuclear compartments that prevent degradation and fusion of chromosome ends by recruiting shelterin proteins and regulating access of DNA damage repair factors. To understand how these dynamic components protect chromosome ends, we combine in vivo biophysical interrogation and in vitro reconstitution of human shelterin. We show that shelterin components form multicomponent liquid condensates with selective biomolecular partitioning on telomeric DNA. Tethering and anomalous diffusion prevent multiple telomeres from coalescing into a single condensate in mammalian cells. However, telomeres coalesce when brought into contact via an optogenetic approach. TRF1 and TRF2 subunits of shelterin drive phase separation, and their N-terminal domains specify interactions with telomeric DNA in vitro. Telomeric condensates selectively recruit telomere-associated factors and regulate access of DNA damage repair factors. We propose that shelterin mediates phase separation of telomeric chromatin, which underlies the dynamic yet persistent nature of the end-protection mechanism.
Introduction
The nucleus contains the biological software of the cell – the genome – which is organized into individual chromosomes. Eukaryotic chromosomes end with telomeres, nucleoprotein structures containing repetitive DNA, which protect the genome over successive cell divisions (d'Adda di Fagagna et al., 2003; Maciejowski and de Lange, 2017). Unlike germline cells in which the average telomere length is set, the telomeres in somatic cells shorten over time (Baird et al., 2003; Blackburn, 1991; Harley et al., 1990). This mechanism has been viewed as a tumor-suppressing pathway, as the gradual shortening of telomeres leads to replicative senescence or cell death (Maciejowski and de Lange, 2017).
In humans, telomeres consist of 2-20 kilobases of double-stranded TTAGGG (dsTEL) repeats followed by 50-200 bases of single-stranded telomeric (ssTEL) overhang (Palm and de Lange, 2008). Telomeres associate with the six-protein complex shelterin (Nandakumar and Cech, 2013), which prevents degradation, chromosome end-to-end fusions, and unwanted DNA damage repair (DDR) (d'Adda di Fagagna et al., 2003; Maciejowski and de Lange, 2017). The homologous shelterin components TRF1 and TRF2 specifically bind to dsTEL tracts and recruit other subunits to telomeres. POT1/TPP1 binds to the ssTEL overhang, and TIN2 interconnects TRF1, TRF2, and TPP1. RAP1 binds to the hinge region of TRF2 (Janoušková et al., 2015; O'Connor et al., 2004). These proteins suppress a wide variety of DDR pathways at telomeres by masking the chromosome ends from being improperly recognized as DNA break sites (Galati et al., 2013; Ray et al., 2014). In particular, TRF2 inhibits the ataxia-telangiectasia mutated (ATM) pathway and non-homologous end-joining (NHEJ) of telomeres (Okamoto et al., 2013). Moreover, TRF1 prevents replication fork stalling (Bower and Griffith, 2014; Maestroni et al., 2017), POT1/TPP1 specifically suppresses the ataxia telangiectasia and Rad3-related (ATR) pathway, and TIN2 suppresses ATM, ATR, and NHEJ pathways (Palm and de Lange, 2008).
End-protection by telomeres is mechanistically attributed to the formation of t-loops, wherein TRF2 enables the ssTEL overhang to invade dsTEL tracts and form lasso-like structures (Doksani et al., 2013; Griffith et al., 1999). The t-loop model provides an explanation for how shelterin sequesters the chromosome ends from the ATM and NHEJ pathways (Doksani et al., 2013; Griffith et al., 1999). However, this model does not adequately explain how cells enter senescence while their telomeres still contain kilobases of telomeric repeats (Chiba et al., 2017; Herbig et al., 2004; Smogorzewska et al., 2000) since t-loops have been observed for telomeric DNA as short as one kilobase (Stansel et al., 2001; Kar et al., 2016). Shelterin is also hypothesized to protect telomere ends through the three-dimensional compaction of telomeric chromatin (Bandaria et al., 2016), but decompaction of telomeres upon shelterin knockdown has not been observed by others (Janissen et al., 2018; Timashev et al., 2017). The network of interactions between shelterin components and telomeric DNA could also function as a selectivity barrier to regulate the preferential binding of shelterin and prevention of DNA damage response signaling (Bandaria et al., 2016), but interactions between shelterin and telomeric DNA are too dynamic to serve as a steric barrier. Thus, it remains unclear what physical picture best describes telomere organization and function.
Liquid-liquid phase separation (LLPS) has emerged as a mechanism to create membraneless cellular compartments or “condensates,” such as nucleoli, Cajal bodies, and stress granules (Banani et al., 2017; Shin and Brangwynne, 2017). These structures contain high local concentrations of proteins and nucleic acids that condense into liquid-like assemblies through multivalency and noncovalent interactions, selectively excluding non-interacting molecules (Altmeyer et al., 2015; Riback et al., 2020). LLPS has recently been implicated in controlling chromatin structure (Shin et al., 2018) and in heterochromatin domain formation (Larson et al., 2017; Strom et al., 2017), raising the possibility that telomeric DNA may also form condensates with associated shelterin components. Consistent with this hypothesis, TRF1 and TRF2 display many of the characteristics common in phase separating systems, including intrinsically disordered regions (IDRs), a dimerization domain, and a DNA binding domain (Okamoto et al., 2013; Palm and de Lange, 2008). However, this liquid phase model has not been tested.
Here, we combine intracellular biophysical interrogation and in vitro reconstitution to reveal that shelterin components and telomeric DNA organize into liquid-like condensates. Using an optogenetic approach to bring two telomeres together, we find that telomeres are capable of undergoing coalescence, forming a single larger telomeric body. In living cells, we show that telomeres exhibit quantitative signatures of multicomponent LLPS, but their hindered diffusivity results in extremely few coalescence events. We reconstitute the human shelterin complex and find that the interactions between shelterin and telomeric DNA promote the formation of liquid condensates. TRF1 and TRF2 drive phase separation of the shelterin complex, and these liquid droplets selectively recruit telomere-associated factors in vitro. We propose that LLPS of shelterin components builds the telomere compartment and could protect chromosome ends by selectively recruiting telomere-associated factors while limiting access of DDR factors.
Results
Telomeres in living cells are liquid-like
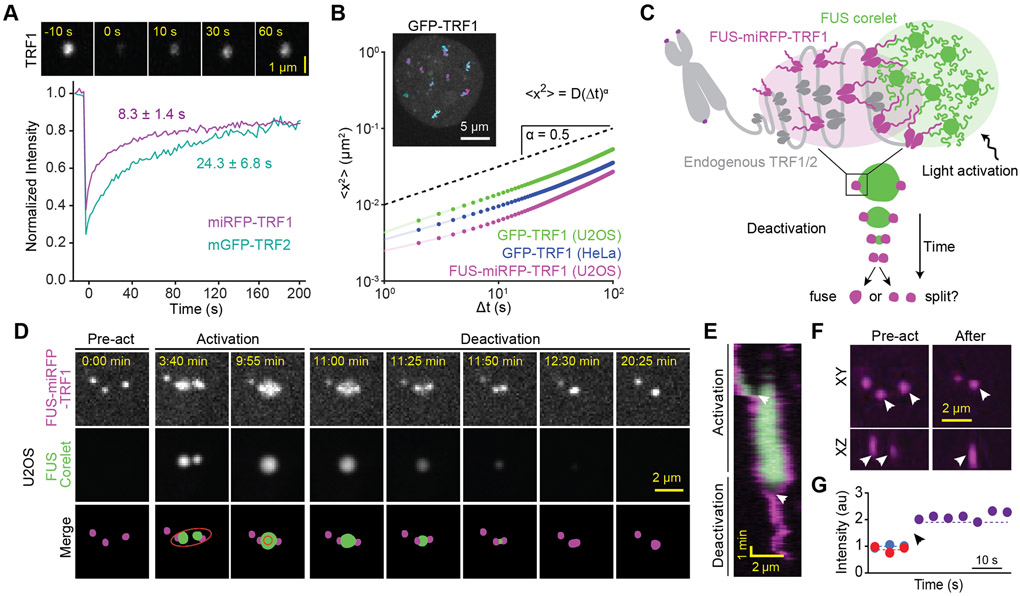
We first investigated whether telomeres exhibit liquid-like features in human cells. We expressed TRF1 (miRFP-TRF1) and TRF2 (mGFP-TRF2 and miRFP-TRF2) and confirmed that TRF1 and TRF2 formed distinct puncta in nuclei of U2OS cells (Figure S1A-B). As previously reported (Mattern et al., 2004) fluorescence recovery after photobleaching (FRAP) assays showed that TRF1 and TRF2 rapidly exchange between telomeres and the nucleoplasm (Figure 1A), which is typical for phase separating systems (Alshareedah et al., 2021; Taylor et al., 2019). If telomeres are liquid-like, we expect them to coalesce and round up due to surface tension. Consistent with previous studies (Bronshtein et al., 2015; Molenaar et al., 2003; Wang et al., 2008), telomeres exhibit subdiffusive motion and typically do not encounter one another (Figure 1B, Movie S1), likely because they are in a viscoelastic environment and tethered to chromosomes (Feric and Brangwynne, 2013; Lee et al., 2021). Based on mean-squared displacement (MSD) analysis, we estimate that it would take ~5 days for a telomere to reach its nearest neighbor (2.4 ± 1.2 μm, mean ± SD) and as long as ~200 days to reach the average pairwise distance between telomeres (6.8 ± 3.2 μm) via diffusion (Figure S1C-D). Consistently, we were able to detect only one potential coalescence event after imaging 60 cells for 1 h (Figure S1E), demonstrating that telomeres do not frequently merge with one another and remain distinct within living cells due to their suppressed diffusivity.
Figure 1. Telomeres in living cells exhibit liquid-like behavior.
A. (Top) FRAP of miRFP-TRF1 at a telomere in a U2OS cell. (Bottom) Recovery of mGFP-TRF2 or miRFP-TRF1 fluorescence at telomeres in U2OS cells (±SD, n = 9 and 11 telomeres respectively). B. (Inset) Trajectories of individual telomeres are colored separately by trajectory duration in a HeLa cell expressing GFP-TRF1. MSD analysis of these trajectories revealed subdiffusive motion with exponent α = 0.54 ± 0.01 and diffusion coefficient D = 2.8 ± 0.1 × 10−3 μm2 s−α (±SE). The slope of the dashed line serves as a reference for α = 0.5. C. Schematic of the optogenetically-induced telomere coalescence experiment: FUSN-miRFP-TRF1 serves as a seed at telomeres to recruit FUSN Corelet droplets upon local light activation. After two of these droplets merge, light is deactivated to pull telomeres together as the FUSN droplet shrinks. D. Pre-activation, activation, and deactivation of FUSN-miRFP-TRF1 and FUSN Corelets in U2OS cells. The ellipse in the schematic merged images shows the local activation pattern. E. Kymograph shows that the two telomeres coalesce and remain as a single spot after deactivation. White arrowheads indicate the merging of FUSN Corelet droplets and telomeres. F. XY and XZ views of the telomeres before and after activation. White arrowheads mark two telomeres that merge. G. The average intensities of the two telomeres add up (dashed lines) as they coalesce (black arrowhead) into a single spot. See also Figure S1 and Movie S1-3.
Due to the infrequency of telomere coalescence, the liquid phase model could not be tested through passive microscopic examination of telomeres in living cells. To controllably pull two or more telomeres into contact, we developed an optogenetic approach based on the Corelet system (Figure 1C) (Bracha et al., 2018). The synthetic Corelet droplets are made by triggering interaction of a phase-separation prone protein (in this case, FUSN) with a multivalent (24-mer Ferritin) core through light-triggered heterodimerization between sspB, attached to FUSN, and iLID, attached to the core. We tether the droplets to telomeres with FUSN-miRFP-TRF1, which binds the telomeric DNA and interacts with the droplet through homotypic FUSN interactions. With light activation, two closely-positioned telomeres can be induced to nucleate FUSN droplets, which fuse to create one FUSN droplet stably interacting with two telomeres (Figure 1D, Movie S2). Following removal of the blue light stimulus (‘deactivation’), the FUSN droplet shrinks, surface tension pulls telomeres inwards, and the telomeres ultimately coalesce into a single spot in three dimensions (Figure 1F). In 46% (11 out of 24) of our attempts, we observed these droplet-guided telomere coalescence events (Figures 1E-G and S1F), which remained a single spot for at least 8 minutes following the dissolution of the FUSN droplets. In several instances, the telomeres detached from the FUSN droplet before contacting each other and relaxed back to their original or more distal positions (Figure S1F-G, Movie S2), indicating that the local viscoelastic constraints on telomeres tend to maintain their relative separation (Shin et al., 2018).
To rule out the possibility that coalescence of telomeres is driven by linking FUSN to TRF1, we linked iLID to TRF1 and FUSN to mCherry-sspB (Figure S1H, Movie S3). In this case, iLID-miRFP-TRF1 only becomes a seed when FUSN-mCherry-sspB is bound upon light-activation, and FUSN-mCherry-sspB is released from the telomere after deactivation. We observed droplet-guided telomere coalescence events in 10 out of 13 attempts (77%) (Figure S1I-J), demonstrating that observed liquid-like telomere coalescence is driven by the endogenous telomere protein interactions. We observed these merger events in both U2OS (Figure 1D-F) and telomerase-positive hTERT-RPE1 cells (Figure S1F), indicating that telomere coalescence is not due to alternative lengthening of telomeres (ALT)-associated PML bodies (APBs) in U2OS cells (Grobelny et al., 2000; Min et al., 2019; Potts and Yu, 2007; Zhang et al., 2020). Taken together, these data suggest that inducing contact of two telomeres causes their coalescence, which is consistent with telomeres behaving as liquid-like condensates.
Telomeric DNA acts as an oligomerizing scaffold to promote TRF1 and TRF2-mediated condensation
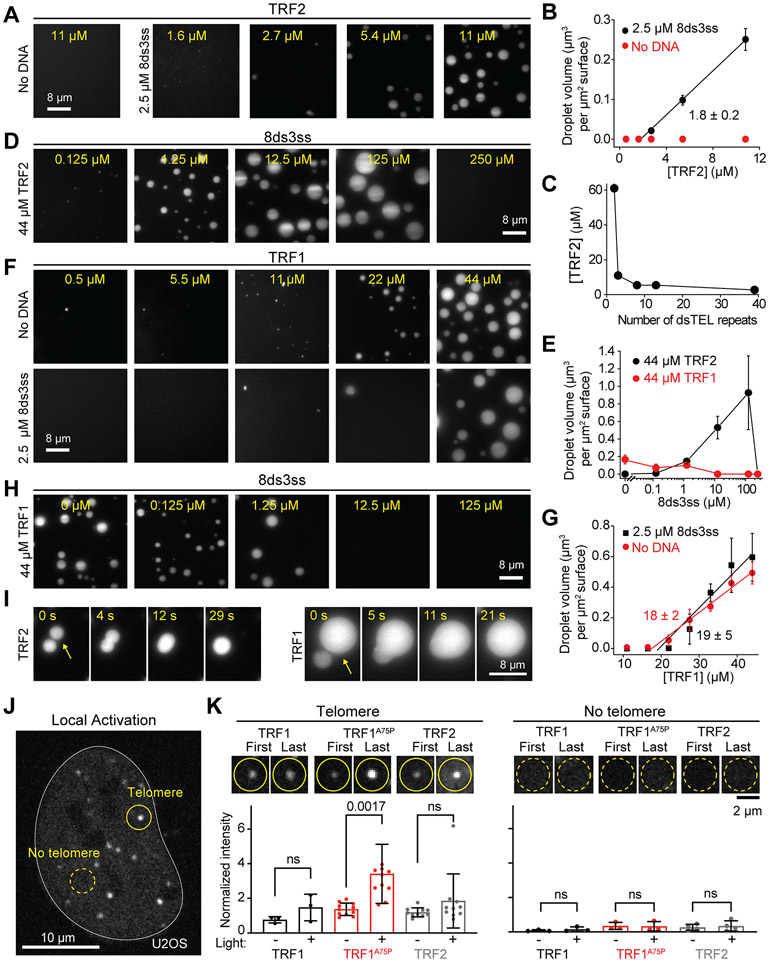
To examine whether components of the shelterin complex drive LLPS of telomeres, we purified human shelterin complex proteins and tested if they could create a biomolecular condensate with telomeric DNA in vitro. We first characterized whether TRF1 and TRF2 phase separate under physiological salt concentration (150 mM NaCl). While TRF2 did not form liquid droplets in the absence of DNA, the addition of short telomeric DNA with multiple TRF2 binding sites (8 dsTEL and 3 ssTEL repeats; 8ds3ss) initiated the formation of TRF2 droplets (Figures 2A and S2A-C, Table S1). We also observed that TRF2 did not form droplets with nontelomeric DNA at the same length as 8ds3ss, but still formed droplets with a 3-kb long nontelomeric DNA, suggesting that both telomeric DNA sequence and the length of the DNA backbone contribute to TRF2 phase separation.
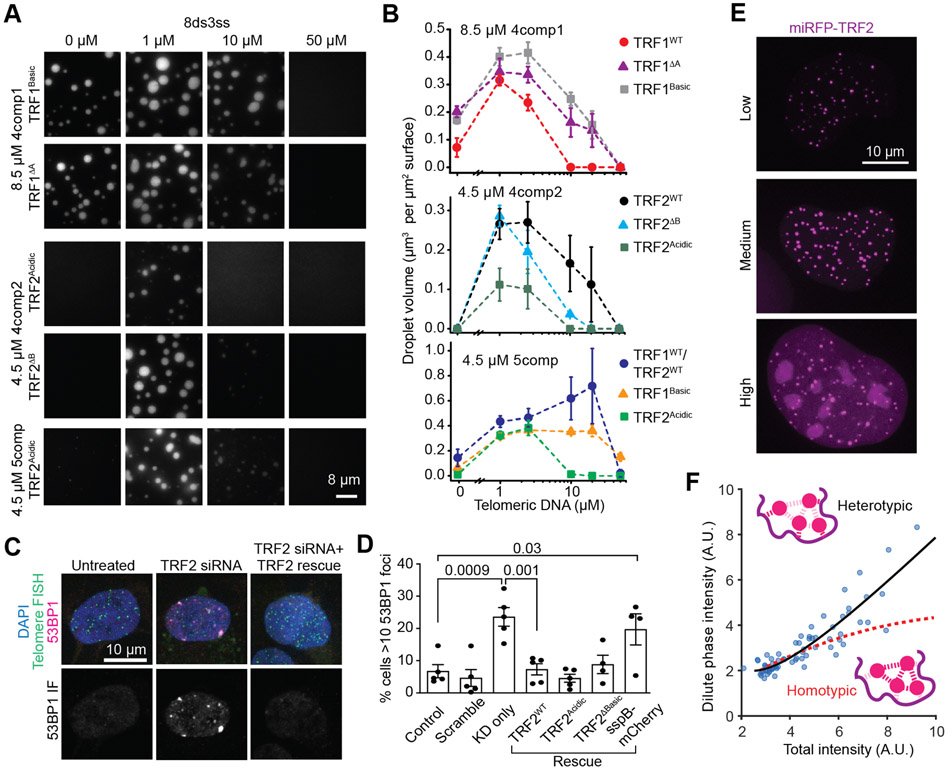
Figure 2: Telomeric DNA acts as an oligomerizing scaffold to promote TRF1 and TRF2-mediated condensation.
A. Example images of Cy3-TRF2 in the presence and absence of 8ds3ss telomeric DNA. B. The total volume of TRF2 condensates settled per micron squared area on the coverslip in the presence or absence of 2.5 μM 8ds3ss (mean ± SD, n = 30 with three technical replicates). Linear fit (solid line) reveals csat (± SE). C. The minimum TRF2 concentration that exhibits phase separation with a variable number of dsTEL repeats per DNA substrate. The total concentration of dsTEL tracts was fixed to 20 μM. D. TRF2 has a reentrant phase behavior as a function of 8ds3ss concentration. E. The total volume of TRF1 or TRF2 condensates settled per micron squared area on the coverslip under variable 8ds3ss concentration (mean ± SD, n = 20 with two technical replicates). F. Example images of Cy3-TRF1 in the presence and absence of 8ds3ss. G. The total volume of TRF1 condensates settled per micron squared area on the coverslip in the presence or absence of 2.5 μM 8ds3ss (mean ± SD, n = 30 with three technical replicates). Linear fits (solid lines) reveal csat (± SE). H. An increase in 8ds3ss concentration inhibits TRF1 phase separation. I. Fusion of TRF2 (22 μM, left) and TRF1 (44 μM, right) droplets formed in the presence of 2.5 μM 8ds3ss. J. U2OS expressing sspB-mCherry-TRF2. TRF corelets were locally activated at a single telomere (solid circle) or away from any telomere (dotted circle). K. (Top) Example images show first and last frames of locally activated TRF1, TRF1A75P, and TRF2 at telomeres (left; n = 3, 11, 10 cells analyzed, respectively) and away from telomeres (right; n = 3, 3, 4 cells analyzed, respectively) in U2OS cells. (Bottom) Quantification of change in intensity upon local activation, at and away from existing telomeres for WT TRF1, TRF1A75P, and WT TRF2. The intensity of each locally activated telomere or region was normalized to the average intensity of all other telomeres within the same activated cell. P-values were quantified by one-way ANOVA with multiple comparisons. See also Figures S2 and S3, Table S1, and Movie S4.
In the presence of telomeric DNA (2.5 μM 8ds3ss), the volume of the droplets increased linearly with TRF2 concentration (Figures 2B and S2D). The minimum TRF2 concentration that triggered phase separation (csat) was 1.8 ± 0.2 μM (Figure 2B, see Methods), which is comparable to the estimated nuclear concentration of TRF2 (~1 μM) and lower than the local concentration of TRF2 at telomeres (>100 μM) (Bandaria et al., 2016; Takai et al., 2010). A telomeric substrate that cannot recruit more than one TRF2 (2ds0ss) nucleated small droplets only at the highest TRF2 concentration tested (61 μM, Figure S2E), whereas increasing the length of the dsTEL tracts to 39 repeats substantially reduced the TRF2 concentration required for triggering phase separation (Figures 2C and S2E, Table S1). These results indicate that the multivalency of the DNA scaffold increases the local concentration of TRF2 dimers and drives phase separation. Interestingly, TRF2 exhibited first an increasing and then decreasing tendency to phase separate as a function of telomeric DNA concentration, and the addition of excess DNA abolished TRF2 condensation (Figure 2D-E). Such reentrant phase behavior has been reported for nucleoprotein assemblies (Banerjee et al., 2017; Sanders et al., 2020; Soranno et al., 2021) and indicates that the stoichiometry of TRF2 and dsTEL repeats is critical for their pairwise interactions to result in phase separation.
TRF1 also formed liquid droplets in vitro but under markedly different conditions than TRF2. We found that TRF1 forms liquid droplets in the absence of DNA (Figure 2F). The addition of DNA, either 8ds3ss or nontelomeric DNA, played an inhibitory role in TRF1 phase separation (Figures 2F and S2F), with TRF1 droplets no longer present at increased 8ds3ss concentrations (Figures 2E and 2H). The csat of TRF1 with or without DNA (19 ± 5 μM and 18 ± 2 μM, respectively, Figure 2G) was higher than that of TRF2 with DNA, indicating that TRF1 has a lower propensity to phase separate.
We also found that TRF1 and TRF2 droplets readily dissolve at high salt (0.5 M NaCl, Figure S2G) and coalesce when they encounter each other (Figure 2I), indicating that they exhibit liquid-like material properties. The average fusion time of TRF1 droplets (21 ± 2 s after contact, ±SE) was comparable to that of TRF2 droplets (27 ± 4 s) in the presence of DNA (Figure S2H, Movie S4). TRF1 droplets fused an order of magnitude faster in the absence of DNA (Figure S2H), suggesting that strong interactions between TRF1 with telomeric DNA increase the viscosity of these droplets.
Our in vitro findings highlight how telomeric DNA can strongly impact the phase behavior of shelterin components. Because TRF1 phase separates in the absence of DNA (Figure 2F), we used the Corelet system to test whether TRF1-TRF1 interactions could create liquid condensates independent of telomeres in living cells (Figure 2J). We marked telomeres in U2OS cells by expressing miRFP-TRF2, and fused sspB to either TRF1WT or the TRF1 dimerization mutant (TRF1A75P) (Bandaria et al., 2016; Fairall et al., 2001) to synthetically oligomerize up to 24 TRF1 molecules on the Ferritin core upon local light activation. When the Ferritin core was recruited to a single telomere, enrichment of TRF1WT or TRF1A75P at that telomere was slightly increased (Figure 2K). Interestingly, de novo TRF1 puncta were not observed to form when we locally activated a region away from a telomere (Figure 2K), except under very high expression conditions (Figure S2I-J). This is consistent with the concept that high concentration and valency are required for shelterin-mediated phase separation. We also obtained similar results with TRF2 in both U2OS and hTERT-RPE1 cell lines (Figures 2K and S2I-J), demonstrating that TRF1 and TRF2 condensation is dependent on multivalent interactions with the telomeric DNA in living cells.
Differences in TRF1 and TRF2 phase separation are driven by their N-terminal domains.
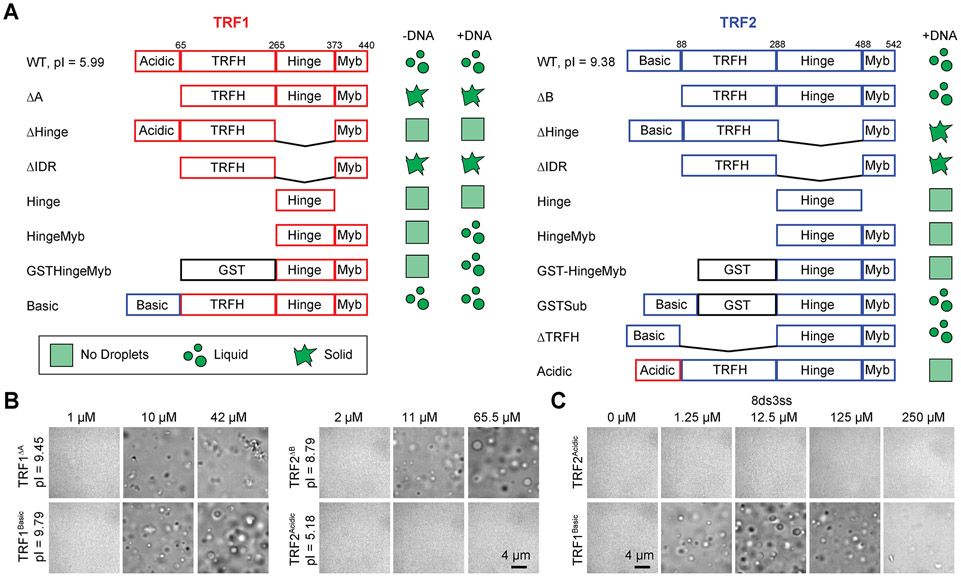
TRF1 and TRF2 are homologous proteins with flexible N-terminal charged domains (acidic in TRF1; basic in TRF2), structured TRFH dimerization domains (Court et al., 2005), flexible hinge domains, and C-terminal DNA-binding Myb domains (Figure 3A) (de Lange, 2018). To investigate which domains were primarily responsible for phase separation, we generated fragments and domain swapping mutants of TRF1 and TRF2 (Figures 3A and S3A-B). Deleting the N-terminal acidic domain of TRF1 (TRF1ΔA) triggered the formation of irregularly shaped, solid-like condensates across conditions, underscoring its role in the solubility of TRF1 (Figure 3B). Interestingly, replacing the acidic domain of TRF1 with the basic domain of TRF2 (TRF1Basic) resulted in a reentrant response of phase separation to DNA concentration, similar to TRF2WT (Figure 3C). In addition, swapping the acidic domain of TRF1 into TRF2 (TRF2Acidic) inhibited phase separation of TRF2 in the presence of DNA (Figures 3B-C and S3D), similar to TRF1WT. These results suggest that the TRF2 basic domain promotes phase separation through its interactions with telomeric DNA (Biffi et al., 2012; Poulet et al., 2009), whereas the TRF1 acidic domain weakens electrostatic interactions with the DNA backbone and reduces phase separation in the presence of DNA. We also observed that removing the basic domain from TRF2 (TRF2ΔB) did not strongly change its phase behavior (Figure 3B), presumably because this construct is still highly positively charged and the TRFH domain of TRF2 may be sufficient for favorable interactions with telomeric DNA (Amiard et al., 2007) in the absence of the basic domain. Differences in phase separation were not due to reduced DNA binding, as these mutants maintained a high affinity to bind telomeric DNA (Figure S3B).
Figure 3. Differential drivers of TRF1 and TRF2 phase separation.
A. Domain organization and condensate state of full-length, truncated, and engineered TRF1 and TRF2 constructs in the presence and absence of 2.5 μM 8ds3ss (pI: isoelectric point). B. Brightfield images taken in the presence of 2.5 μM 8ds3ss DNA show that TRF1ΔA, TRF1Basic, and TRF2ΔB form condensates, whereas TRF2Acidic does not form condensates. C. TRF1Basic phase separation exhibits reentrant response to 0 - 250 μM 8ds3ss, whereas TRF2Acidic does not phase separate in any conditions (protein concentration was set to 20.1 μM). See also Figure S3.
We also tested the possible roles of IDRs and dimerization domains in TRF1 and TRF2 phase separation. Deletion of the hinge (ΔHinge) or both the hinge and N-terminal domains (ΔIDR) reduced the solubility and inhibited LLPS of TRF1 and TRF2 (Figures S3C and E). While Hinge-Myb of TRF2 was unable to drive LLPS in the presence or absence of DNA, Hinge-Myb of TRF1 formed droplets only at very high concentrations (>100 μM) (Figure S3C). Artificial dimerization of Hinge-Myb triggered phase separation in TRF1 with telomeric DNA, but additionally required the N-terminal basic domain in TRF2 (GSTSub) for phase separation (Figures 3A, S3C and F). Taken together, dimerization and IDRs of TRF1 and TRF2 are essential for phase separation, and the N-terminal domain regulates interactions with telomeric DNA in liquid droplets.
The shelterin complex phase separates in vitro
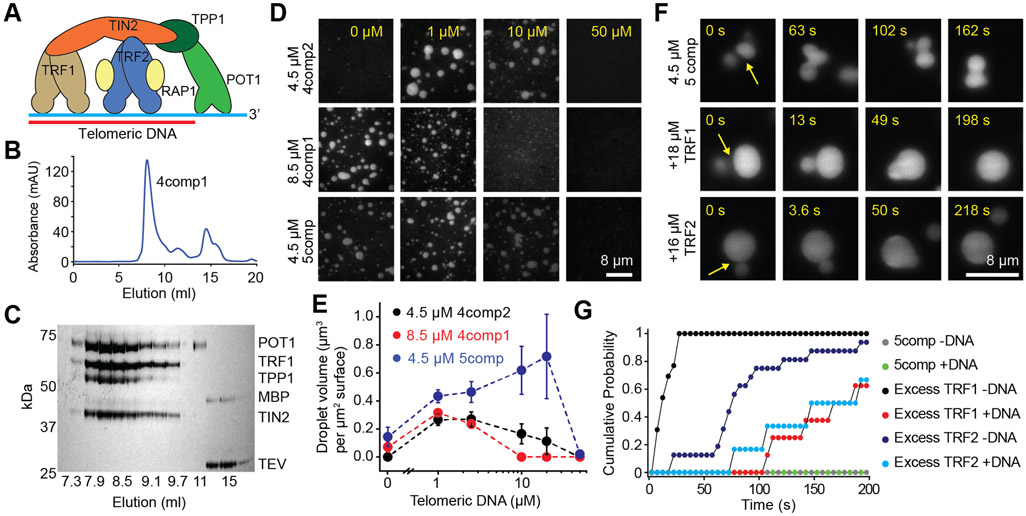
To examine the role of interactions among TRF1, TRF2, and the rest of the shelterin components in driving telomeric phase separation, we co-expressed human shelterin components in insect cells (Figure 4A). Four component complexes containing TRF1, TIN2, TPP1, and POT1 (4comp1); TRF2, TIN2, TPP1, and POT1 (4comp2); and the five-component complex that contains both TRF1 and TRF2 (5comp) eluted as a stable complex from gel filtration (Figures 4B-C and S4A). Unlike TRF1 and TRF2, neither POT1 nor co-purified TPP1 and TIN2 formed liquid condensates (Figure S4C), showing that these proteins do not phase separate on their own. Although POT1 does not specifically bind to TRF1 or TRF2 (Lim et al., 2017; Liu et al., 2004), it partitioned into TRF1 and TRF2 droplets (Figure S4D-E), indicating that interactions within the condensate can recruit POT1 in the absence of TPP1-TIN2 and ssTEL tracts.
Figure 4. The shelterin complex phase separates in vitro.
A. A schematic of the human shelterin complex. TRF1 and TRF2 are homodimers that bind to dsTEL, and POT1/TPP1 binds to ssTEL. TIN2 interconnects TRF1, TRF2, and TPP1, and RAP1 binds to TRF2. B-C. UV absorbance (B) and denaturing gel (C) show that 4comp1 elutes as a single complex from a gel filtration column. D. 4comp2 and 5comp exhibit reentrant responses to increasing DNA concentration similar to TRF2 droplets, while 4comp1 is inhibited by increasing DNA concentration similar to TRF1 droplets. E. The total volume of shelterin droplets settled per micron squared area on the coverslip under variable 8ds3ss concentration (mean ± SD, n = 20 with two technical replicates). F. In the presence of 2.5 μM 8ds3ss, 5comp droplets do not fuse on relevant time scales, whereas the addition of excess TRF1 or TRF2 reduces the fusion time. G. Cumulative probability of 5comp droplet fusion in the presence and absence of excess TRF1 or TRF2 after forming a contact at t = 0 s (n = 7, 4, 13, 16, 15 and 7 events from top to bottom). See also Figure S4 and Movie S4.
We then characterized the phase behavior of the shelterin complexes with and without telomeric DNA (Figures 4D and S4F). Similar to TRF1 condensates, 4comp1 efficiently formed liquid droplets in the absence of telomeric DNA, and its phase separation was inhibited by the addition of telomeric DNA. Similar to TRF2 condensates, 4comp2 only phase-separated in the presence of DNA and formed droplets across a range of telomeric DNA concentrations (Figures 4D-E and S4F). 5comp, containing equimolar TRF1 and TRF2, phase-separated across a broader range of telomeric DNA (8ds3ss) concentrations (Figure 4D) and in the absence of DNA (Figure S4F). Therefore, TRF1 and TRF2 are synergistic in driving phase separation of shelterin, possibly due to complex coacervation between their charged domains.
Next, we investigated the material state of the shelterin condensates in vitro. 5comp droplets could adhere to one another and change shape, but they could not complete fusion into a spherical droplet within 200 s (Figure 4F, Movie S4). Therefore, shelterin droplets appear to be more viscoelastic than TRF1 or TRF2 droplets and exhibit gel-like properties in the presence of telomeric DNA. Because shelterin components form subcomplexes with fewer subunits (de Lange, 2018), and TRF1 and TRF2 are more abundant than POT1-TPP1 at telomeres (Takai et al., 2010), we asked whether changing the stoichiometry of shelterin subunits affect these condensates. The equimolar mixtures of separately purified TRF1, TRF2, TPP1-TIN2, and POT1 also formed liquid droplets with all components present and responded to changes in DNA concentration similar to co-purified 5comp (Figure S4G-H). The addition of ~3-fold excess TRF1 or TRF2 substantially reduced fusion times of shelterin droplets (Figure 4F-G and S4I-K). These results suggest that the stoichiometry of shelterin components could serve to regulate the viscoelasticity of telomeres in vivo.
Telomeres exhibit quantitative signatures of multicomponent liquids
To determine how altered phase behavior of TRF1 and TRF2 mutants might affect phase separation of shelterin complexes in vitro, we assembled shelterin complexes using N-terminal swap or deletion mutants of TRF1 and TRF2 (Figure S5A). Replacing TRF1WT with TRF1ΔA or TRF1Basic resulted in phase separation of 4comp1 over a wider range of DNA concentrations (Figure 5A-B). Replacing TRF2WT with TRF2ΔB in 4comp2 and 5comp did not inhibit phase separation (Figure 5A-B and S5B), but adding TRF2Acidic reduced the size and number of droplets of the complex (Figures 5A-B).
Figure 5. Telomeric condensates exhibit quantitative signatures consistent with multicomponent phase-separated liquids both in vitro and in living cells.
A. Example images show phase separation of 4comp1, 4comp2, and 5comp assembled using N-terminal swap or truncation mutants of TRF1 and TRF2. B. The total volume of shelterin droplets assembled with native or mutant TRF1 and TRF2 settled per micron squared area on the coverslip under different 8ds3ss concentrations (mean ± SD, n = 20 with two technical replicates). C. 53BP1 staining (magenta) of hTERT-RPE1 cells that are treated or untreated with TRF2 siRNA. Telomeres are stained with a telomeric DNA FISH probe (green). Nuclei are labeled with DAPI (blue). D. The percentage of hTERT-RPE1 cells with more than 10 53BP1 foci per nucleus under knockdown and rescue conditions. Error bars represent SEM of five biological replicates for all conditions except for TRF2ΔB and sspB-mCherry (four replicates). n > 1000 cells analyzed for all conditions. P-values were calculated by one-way ANOVA with multiple comparisons. E. Overexpression of miRFP-TRF2 leads to an increased dilute phase (nucleoplasmic) partitioning in U2OS cells. F. The dilute phase intensity increases nonlinearly as a function of the total intensity of the miRFP-TRF2 signal in U2OS cells. The data fit to a nonlinear heterotypically stabilized model (black solid curve) but not to homotypic interactions (red dashed curve). The ‘homotypic’ curve is not a flat line due to the presence of endogenous protein (see Riback et al., 2020). See also Figure S5.
We next tested whether altered phase separation of TRF2 affects end-protection of telomeres in living cells, by expressing TRF2WT, TRF2ΔB, or TRF2Acidic upon knockdown of endogenous TRF2 in hTERT-RPE1 cells (Figure 5C-D, Figure S5C-F). We quantified the number of DNA damage foci formed by the localization of p53 binding protein 1 (53BP1), a downstream signaling protein recruited to these DDR foci (Figure 5C) (Schultz et al., 2000). As previously reported (Takai et al., 2003), knockdown of endogenous TRF2 led to a significant increase in the percent of nuclei with greater than ten 53BP1 foci (23.6% compared to 6.7% of untreated cells, Figures 5D and S5C-F). Consistent with previous studies (Okamoto et al., 2013; Rai et al., 2016), both TRF2WT and TRF2ΔB rescued telomere end-protection, with few cells exhibiting greater than ten 53BP1 foci (7.3% and 8.8%, respectively; Figure 5D). We found that TRF2Acidic also rescued end-protection (4.5%, Figure 5D), consistent with phase separation of this mutant with the rest of the shelterin complex in vitro.
To further probe whether telomere compartmentalization requires interactions between multiple components in living cells, we quantified the relative importance of homotypic vs heterotypic interactions in telomere formation in U2OS cells. We measured the nucleoplasmic, or dilute phase concentration (cdil) of miRFP-TRF2 at increasing expression levels (Figure 5E-F). If homotypic interactions of miRFP-TRF2 dominate its phase separation, cdil would remain constant as miRFP-TRF2 concentration is increased (Riback et al., 2020). However, we observed that cdil continues to increase with miRFP-TRF2 overexpression (Figure 5E-F). Our results suggest that telomeres are thermodynamically stabilized by heterotypic interactions, which is consistent with the necessity of telomeric DNA for shelterin condensation in live cells.
Phase separation of shelterin is modulated by telomere-associated factors
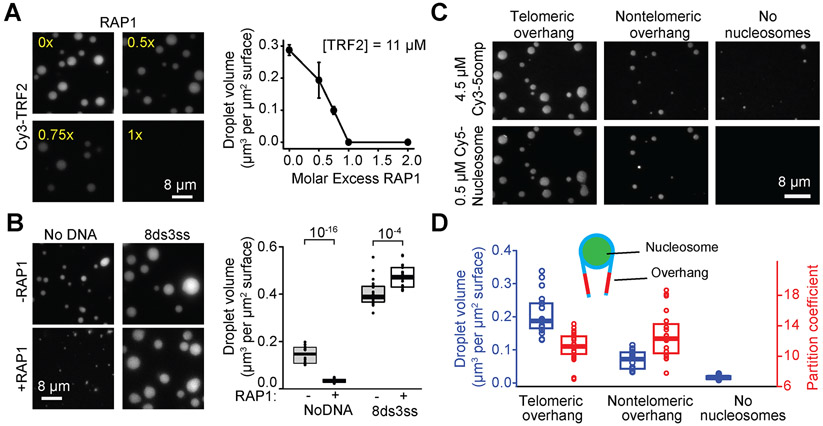
In mammalian cells, telomeres also associate with the sixth component of shelterin, RAP1, and nucleosomes, and we sought to examine their impact on telomeric phase separation in vitro. We found that RAP1 fully inhibits phase separation of TRF2 when mixed at an equimolar concentration (Figure 6A), presumably because RAP1 binding to the TRF2 hinge domain prevents this region to contribute to phase separation (Soranno et al., 2021). RAP1 also reduced the total volume of shelterin droplets without DNA when mixed at equal concentrations, and it moderately reduced the volume of 4comp2 droplets in the presence of DNA (Figures 6B and S6A-B). However, the addition of RAP1 only had a minor effect in the presence of telomeric DNA when all six shelterin subunits were present (Figure 6B), suggesting that the telomeric DNA scaffold and the interactions of the other shelterin subunits counteract RAP1’s inhibitory effect on phase separation.
Figure 6. Telomere-associated proteins modulate phase separation of shelterin droplets.
A. (Left) Increasing the molar ratio of RAP1 inhibits phase separation of TRF2 droplets. Droplets were formed in the presence of 2.5 μM 8ds3ss DNA. (Right) The total volume of TRF2 condensates settled per micron squared area on the coverslip as a function of RAP1 concentration (mean ± SD, n = 20 with two technical replicates). B. (Left) 5comp droplets formed with or without equimolar RAP1 and in the presence or absence of 2.5 μM 8ds3ss DNA. Complex concentration was set at 4.5 μM. (Right) The total volume of shelterin condensates settled per micron squared area on the coverslip in the absence or presence of RAP1. The center and edges of the box represent the median with the first and third quartile (n = 20 with two technical replicates). The p-values were calculated from a two-tailed t-test. C. Example images show phase separation of 4.5 μM 5comp in the presence and absence of nucleosomes wrapped with telomeric or nontelomeric DNA. D. Volume of droplets settled per micron squared area and partition coefficient of nucleosomes into 5comp droplets. The center and edges of the box represent the median with the first and third quartiles (n = 20 droplets with two technical replicates). See also Figure S6.
We also purified mono-nucleosomes wrapped with Widom positioning DNA that contains either a telomeric or a nontelomeric overhang. We observed that mono-nucleosomes do not form liquid droplets on their own (Figure S6C) but are sequestered strongly into 5comp droplets in the absence of additional DNA (Figure 6C). The telomeric nucleosomes stimulated phase separation of 5comp, while less droplet formation occurred in the nontelomeric nucleosomes or buffer-only conditions (Figure 6D and S6D). These results indicate that heterotypic interactions between shelterin and telomeric DNA drive phase separation, even in the presence of other abundant factors like nucleosomes that localize to telomeres.
Phase-separated shelterin selectively recruits associated factors.
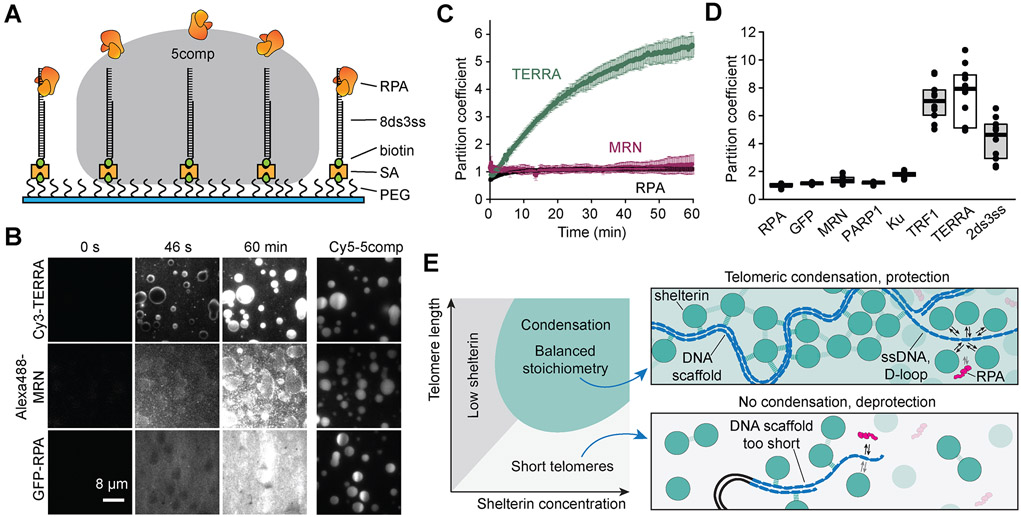
To investigate selective permeability of shelterin droplets in an in vitro system that could mimic protection of the ssTEL overhang, we settled 5comp droplets onto surfaces coated with 8ds3ss (Figure 7A) and flowed fluorescently labeled queries into the chamber. When TRF1, telomeric noncoding RNA (TERRA) (Chu et al., 2017), or telomeric DNA were introduced into the chamber, they strongly partitioned into the droplets within a few minutes (Figures 7B-C and S7A), indicating that these settled droplets can accumulate favorable biomolecules. Consistently, TERRA partitioned less strongly into shelterin droplets containing TRF2ΔB or TRF2Acidic (Figure S7B), likely due to the loss of the interactions between TERRA and TRF2’s basic domain (Deng et al., 2009).
Figure 7. Shelterin droplets selectively recruit telomere-associated factors.
A. 5comp droplets are settled onto PEG surfaces decorated with 8ds3ss. (PEG: polyethylene glycol, SA: streptavidin; not to scale). B. 100 nM Cy3-TERRA, 15 nM Alexa488-MRN complex, or 100 nM GFP-RPA are introduced to 7.6 μM Cy5-5comp droplets. TERRA partitions strongly into the droplets, while MRN and GFP-RPA are initially excluded from the droplets and uniformly distributed after 60 min incubation. C. Partitioning of 100 nM Cy3-TERRA, 15 nM Alexa488-MRN, or 100 nM GFP-RPA into 7.6 μM 5comp droplets over time (mean ± SD, n = 3 droplets per condition). D. Partition coefficients of DDR proteins and telomere-associated factors in 7.6 μM 5comp droplets after 60 min incubation. The center and edges of the box represent the median with the first and third quartile (n = 10 droplets per condition). E. (Left) Multicomponent phase diagram of telomere condensation with balanced stoichiometry. No condensation results at low shelterin concentrations and/or short telomeres. (Top) Telomere condensation, formed by both heterotypic (dark dashes) and homotypic (light dashes) interactions, selectively recruit telomere-associated factors while acting as a diffusion barrier against other components that target telomeric DNA, like RPA. The enrichment of shelterin, and thus POT1, outcompetes RPA binding to ssTEL. (Bottom) Shortened telomere scaffold cannot recruit enough shelterin to form a condensate, which could fail to protect the ssTEL overhang against RPA binding. See also Figure S7 and Movie S5.
We then tested access of replication protein A (RPA), which activates the ATR pathway by binding to the ssTEL overhang (Denchi and de Lange, 2007; Gong and de Lange, 2010; Takai et al., 2011; Wold, 1997), and the Mre11-Rad50-Nbs1 (MRN) complex, which activates the ATM pathway at DNA double-strand breaks (DSBs) (Lamarche et al., 2010; Myler et al., 2017). Both GFP-RPA and Alexa488-MRN were distributed uniformly inside and outside the droplet after 10 min, rather than being enriched inside the droplet (Figure 7B-D, Movie S5). Downstream DDR factors PARP1, which is involved in the homologous recombination (HR) pathway (Rai et al., 2016), and the Ku70-Ku80 complex (Ku), which binds DSBs and is part of the non-homologous end joining (NHEJ) pathway (de Lange, 2018), also exhibited near-uniform partitioning inside and outside shelterin droplets (Figures 7D and S7C-E). Furthermore, GFP-RPA diffused into the droplets more slowly than telomere-associated factors and GFP (Figure S7A), suggesting that telomeric condensates could act as a diffusion barrier to biomolecules with a large Stokes radius (Frey and Gorlich, 2007; Wei et al., 2017). The addition of excess TRF1 or TRF2 did not speed up the diffusion of RPA (Figure S7F), suggesting that slow diffusion of RPA is not due to the high viscosity of 5comp droplets. However, RPA partitioned more strongly into 5comp shelterin containing TRF2Acidic (Figure S7G), likely due to weakening of phase separation under these conditions. These results suggest that LLPS of shelterin selectively recruits and enriches telomere-associated factors independent of their size (Figure 7E).
Discussion
In this study, we use in vivo and in vitro biophysical interrogation to demonstrate that telomeres represent a phase-separated liquid-like compartment. This compartment is formed through protein-protein and protein-DNA interactions, which give rise to the unique physicochemical properties of telomeres. We propose that the repetitive nature of telomeric DNA serves as a “super-scaffold” (Söding et al., 2020), effectively oligomerizing phase separation-prone proteins to drive the formation of a liquid compartment that protects the chromosome terminus (Fig 7E). In addition, our findings elucidate important organizing principles that likely underlie the formation of other genomic compartments.
Due to their constrained diffusion in mammalian cells, telomeres do not coalesce into a single condensate as would be expected in an equilibrium system. We speculate that random merger events would promote telomeric DNA end-to-end fusions and genome instability, and therefore the cell maintains telomeres as multiple distinct condensates. However, telomere clustering has been reported in TERT positive human cells (Adam et al., 2019) and ALT cells show fewer telomere puncta than the number of chromosome ends (Draskovic et al., 2009). It remains to be determined whether end-to-end fusion of telomeres is due to higher mobility or interactions with other phase separating condensates (such as APBs) in these cells. We repurposed the Corelet system to bring telomeres together on-demand, and showed that telomeres coalesce upon contact. This optogenetic method can be used to bring other chromatin loci together and could thus be a powerful approach to study the role of genomic compartmentalization in gene regulation and cellular function.
Previous examples of intracellular phase separation have primarily focused on the role of homotypic IDR-IDR interactions that behave as single component systems and exhibit a fixed csat (Nott et al., 2015; Wang et al., 2018). However, many phase separating systems utilize both homotypic and heterotypic interactions to form a complex network of multicomponent interactions (Alberti et al., 2019; Banani et al., 2016). Here, we show that the telomere is a multicomponent compartment whose formation relies on heterotypic interactions of the shelterin components and the scaffolding telomeric DNA (Riback et al., 2020). Consistently, synthetic oligomerization of shelterin components cannot form de novo condensates away from telomeres in living cells, except at exceedingly high concentration and valence.
Using an in vitro reconstitution approach, we found that the TRF1 and TRF2 subunits of human shelterin form liquid droplets, in agreement with an emerging study on in vitro phase separation of TRF2 (Soranno et al., 2021). Both IDRs and dimerization domains are required for TRF1 and TRF2 phase separation, and the differentially charged N-terminal domains are responsible for their distinct properties of condensation in the presence and absence of telomeric DNA. Consistent with our in vivo results, TRF1 and TRF2 together drive phase separation when in complex with other shelterin components and telomeric DNA.
Collectively, our results are consistent with a model of telomeres as condensed liquid compartments, in which shelterin components drive local condensation around the valence-amplifying super-scaffold of telomeric DNA. A balance between the length of telomeric DNA and the stoichiometry of the component factors affect the formation and composition of telomere condensation (Fig 7E). In accordance with this model, telomere function in vivo is controlled by protein levels of TRF1 and TRF2 (Celli and de Lange, 2005; Sfeir and de Lange, 2012; Ye et al., 2004) and the length of telomeric repeats (Galati et al., 2013; Shay and Wright, 2005). An imbalance of these factors may destabilize the structure and deprotect the telomere ends. Partial knockdown of TRF1 and TRF2 triggers several DDR pathways even though TRF1 and TRF2 are still abundant at these telomeres (Cesare et al., 2013; Orun et al., 2016). Additionally, as telomeres shorten in aging tissues, they fail to recruit sufficient shelterin to suppress DDR signals (Lackner et al., 2011). Consistent with these observations, we have shown that increasing the length of the telomeric DNA triggers phase separation at a lower concentration of TRF2 in vitro. Phase separation could also explain the mechanism of action of a dominant-negative allele of TRF2 within the context of the multicomponent network. This mutation is capable of dimerization with endogenous TRF2 but lacks DNA binding and the N-terminal domain, which may alter the interaction valence of the shelterin complex, leading to loss of compartmentalization and end-protection (van Steensel et al., 1998).
We found that in vitro shelterin droplets are more enriched with telomere-associated factors than the DDR proteins. These results suggest that through selective permeability the telomeric condensate could potentially recruit specific complexes like the DNA replication machinery and telomerase to the telomeric DNA, while limiting access of DDR factors during large-scale rearrangements of telomeric DNA. Selective partitioning of POT1, but not RPA, into shelterin droplets may also explain how POT1 can outcompete RPA binding to the displacement loop (D-loop) and the ssTEL overhang even though RPA is more abundant and has similar affinity for the ssTEL (Lazzerini-Denchi and Sfeir, 2016; Takai et al., 2011).
Limitations of the Study
We present evidence that telomeres form liquid condensates using optogenetic manipulation in live cells and in vitro reconstitution of the shelterin complex. The phase separation model we propose is not mutually exclusive with the t-loop model, but provides an explanation for how shelterin compartmentalizes telomeric chromatin and regulates telomere function through selective recruitment of telomere-associated factors. The physiological relevance of LLPS and selective permeability for telomere end protection remain to be defined in vivo.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ahmet Yildiz (yildiz@berkeley.edu).
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact upon request.
Data and Code Availability
This study did not generate any datasets/code amenable for depositing into public repositories. All data reported in this paper will be shared by the lead contact upon request. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell Culture
Recombinant proteins were purified from Sf9-ESF S. frugiperda insect cells (RRID:CVCL_0549; female) grown at 27 °C in ESF 921 Insect Cell Culture Medium (Expression Systems, NC903611) supplemented with 1% fetal bovine serum (Corning, 35-010-CV) and 1% antibiotic-antimycotic (Thermo Fisher Scientific, 15240062). All human cell lines were incubated in and grown at 37°C with 5% CO2. U2OS cells were obtained from the ATCC and hTERT-RPE1 cells (p53 −/−, Rb −/−) were cultured in DMEM (GIBCO, 11995065) with 10% FBS (Atlanta Biological, S11150H) and 1% streptomycin and penicillin (GIBCO, 15140122), grown at 37°C with 5% CO2. The HeLa RMCE GFP-TRF1 cell line was cultured in DMEM with 10% FBS (Atlanta Biological, S11150H), 1% streptomycin and penicillin, and 1 μg/ml puromycin (Sigma, P7255). The U2OS cells were authenticated via ATCC's STR profiling. The other cell lines were not authenticated.
METHOD DETAILS
Shelterin protein purification
Constructs for expressing individual components of the human shelterin complex were tagged with an N terminal ZZ affinity tag, TEV cleavage site, and YBBR labeling site and cloned into a Baculovirus vector. A construct expressing both TPP1 and TIN2 (TIN2 did not express on its own or without a solubility tag) and constructs expressing four- or five-component shelterin were cloned using a BigBac vector as described (Ferro et al., 2019). For the four-component shelterin BigBac construct, POT1 was given an N terminal YBBR tag, POT1 and TRF2 were each given an N terminal ZZ affinity tag and a TEV cleavage site, and TIN2 and TPP1 were each given an N-terminal His-MBP affinity tag and a TEV cleavage site. For a full list of constructed plasmids, see the Key Resources Table. Protein was purified from insect cells as previously described (Ferro et al., 2019). Briefly, plasmids containing genes of interest were transformed into DH10Bac competent cells (Berkeley MacroLab), and Bacmid DNA was purified using ZymoPURE miniprep buffers (Zymo Research, D4210) and ethanol precipitation.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-TRF2 | Novus Biologicals | Cat#NB110-57130 |
| Mouse anti-Histone H3 | Abcam | Cat#Ab10799 |
| Anti-Mouse IgG, Peroxidase Conjugated, Goat | Jackson ImmunoResearch | Cat#115-035-062 |
| Anti-Rabbit IgG, Peroxidase Conjugated, Goat | Jackson ImmunoResearch | Cat#111-035-144 |
| Rabbit anti-53BP1 | Novus Biologicals | Cat#NB100-305 |
| Goat anti-Rabbit IgG, AlexaFluor 647 conjugated | Thermo Fisher Scientific | Cat#A-21245 |
| Anti-FLAG M2, mouse | Sigma-Aldrich | Cat#F1804 |
| Bacterial and Virus Strains | ||
| XL1Blue | MacroLab, University of California Berkeley | N/A |
| Rosetta | MacroLab, University of California Berkeley | N/A |
| DH10Bac | MacroLab, University of California Berkeley | N/A |
| BL21(DE3)pLysS | Sigma-Aldrich | Cat#69451 |
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Fugene HD Transfection Reagent | Promega | Cat#E2311 |
| TEV protease | MacroLab, University of California Berkeley | Addgene Cat#8827 |
| Streptavidin | ThermoFisher Scientific | Cat#434301 |
| LD655-CoA | Lumidyne Technologies | Custom synthesis |
| LD555-CoA | Lumidyne Technologies | Custom synthesis |
| Bradford Reagent | Bio-Rad | Cat#500-0006 |
| Benzonase nuclease | Sigma Aldrich | Cat#E1014-25KU |
| Blocking reagent | Millipore Sigma | Cat#11096176001 |
| Fetal Bovine Serum, Premium, Heat-Inactivated | Atlanta Biologicals | Cat#S11150H |
| Fibronectin Bovine Plasma | Millipore Sigma | Cat#F1141 |
| FITC-TelC, C-rich telomere probe, FITC labeled | PNA bio | Cat#F1009 |
| Formamide (Deionized) | Thermo Fisher | Cat#AM9342 |
| GIBCO DMEM, High Glucose, Pyruvate | Thermo Fisher Scientific | Cat#11995065 |
| GIBCO DPBS, no calcium, no magnesium | Thermo Fisher Scientific | Cat#14190144 |
| GIBCO Opti-MEM Reduced Serum Medium | Thermo Fisher Scientific | Cat#31985062 |
| GIBCO Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher Scientific | Cat#15140122 |
| Hoechst 33342, 10mg/mL | Thermo Fisher Scientific | Cat#H3570 |
| In-Fusion HD Cloning Plus | Takara Bio | Cat#638910 |
| Lenti-X Concentrator | Takara Bio | Cat#631231 |
| Normal Goat Serum Blocking Solution | Vector Laboratories | Cat#S-1000-20 |
| NuPAGE™ LDS Sample Buffer (4X) | Thermo Fisher | Cat#NP0007 |
| Oligofectamine | Thermo Fisher | Cat#12252011 |
| Paraformaldehyde (16%) | Electron Microscopy Science | Cat#15710 |
| Phusion® High-Fidelity DNA Polymerase | New England Biolabs | Cat#M0530L |
| Pierce RIPA buffer | BCA | Cat#89901 |
| Protease Inhibitor tablets (EDTA-free) | Sigma | Cat#4693132001 |
| Puromycin dihydrochloride from Streptomyces alboniger | Sigma | Cat#P7255 |
| SuperSignal West Pico PLUS Chemiluminescent Substrate | Thermo Fisher | Cat#34577 |
| Transit293 Transfection Reagent | Mirus | Cat#MIR 2700 |
| TRIS-buffered saline (TBS, 10X) pH 7.4 | Fisher Scientific | Cat#AAJ62938K2 |
| Triton X-100 | Promega | Cat#H5142 |
| Tween-20 | Thermo Fisher | Cat#BP337-100 |
| Vectashield Plus Antifade Mounting Medium with DAPI | Vectashield | Cat#H-2000-10 |
| ESF 921 Insect Cell Culture Medium | Expression Systems | Cat#NC903611 |
| Antibiotic-antimycotic | Thermo Fisher Scientific | Cat#15240062 |
| Fetal Bovine Serum | Corning | Cat#35-010-CV |
| ZymoPURE miniprep kit | Zymo Research | Cat#D4210 |
| Alexa Fluor 488 antibody labeling kit | Thermo Fisher Scientific | Cat#A20181 |
| Atto488 maleimide dye | Sigma-Aldrich | Cat#28562 |
| SYBR-Safe | Thermo Fisher Scientific | Cat#S33102 |
| Genejet PCR purification kit | Thermo Fisher Scientific | Cat#K0701 |
| Cy3 Label IT kit | Mirus Bio | Cat#MIR 3600 |
| Biotin-BSA | Sigma-Aldrich | Cat#9048-46-8 |
| Streptavidin | Thermo Fisher Scientific | Cat#434301 |
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| SF9-ESF S Frugiperda | Berkeley Cell Culture Facility | RRID:CVCL_0549 |
| Human: U-2 OS | ATCC | ATCC® HTB-96™ |
| Human: Lenti-X™ 293T | Takara Bio | Cat#632180 |
| Human: hTERT-RPE1 (p53−/−, Rb−/−) | Titia de Lange, Rockefeller Univ. | N/A |
| Human: HeLa RMCE GFP-TRF1 | Huaiying Zhang, Carnegie Mellon Univ. | N/A |
| Experimental Models: Organisms/Strains | ||
| Oligonucleotides | ||
| See Table S1 | ||
| siRNA targeting human TRF2 (#2 sequence from Takai et al. 2003): 5′-UGU GCU GGA GAU GAU UAA AAC-3′ | IDT | N/A |
| siRNA targeting human TRF2 (#4 sequence from Takai et al. 2003): 5′-AUC GCU GGC GGA CCA UGA A-3′ | IDT | N/A |
| siRNA targeting human TRF2 (sequence from Yang et al. 2015): 5′-CCA GAA GGA UCU GGU UCU UTT-3′ | IDT | N/A |
| Scrambled RNAi (sequence from Yang et al. 2015): 5′-UUC UCC GAA CGU GUC ACG UTT-3′ | IDT | N/A |
| Silencer™ Cy™3-labeled Negative Control No.1 siRNA | Thermo Fisher Scientific | Cat#AM4621 |
| Recombinant DNA | ||
| CDS: iLID | Bracha et al. 2018 | N/A |
| CDS: TRF1 (NCBI Reference sequence: NM_003218.3) | IDT gBlock with codon optimization | N/A |
| CDS: TRF2 (NCBI Reference Sequence: NM_005652.5) | IDT gBlock with codon optimization | N/A |
| Plasmid: FM5-iLId-miRFP-TRF1 | This paper | N/A |
| Plasmid: FM5-GFP-TRF2 | This paper | N/A |
| Plasmid: FM5-miRFP-TRF1 | This paper | N/A |
| Plasmid: FM5-miRFP-TRF2 | This paper | N/A |
| Plasmid: FM5-sspB-mCherry | Sanders et al. 2020 | N/A |
| Plasmid: FM5-sspB-mCherry-TRF1A75P | This paper | N/A |
| Plasmid: FM5-sspB-mCherry-TRF2 | This paper | N/A |
| Plasmid: FM5-sspB-mCherry-TRF2Acidic | This paper | N/A |
| Plasmid: FM5-sspB-mCherry-TRF2ΔB | This paper | N/A |
| Plasmid: FM5-TRF1-mCherry-sspB | This paper | N/A |
| Plasmid: pCMV-dR8.91 | Toettcher Lab, Princeton University | N/A |
| Plasmid: pMD2.G | Toettcher Lab, Princeton University | N/A |
| Plasmid: pHR-FUSN-mCherry-sspB | Bracha et al. 2018 | N/A |
| Plasmid: pHR-FUSN-miRFP-TRF1 | Shunsuke Shimobayashi (Brangwynne Lab, Princeton University) | N/A |
| Plasmid: pHR-NLS-iLID-EGFP-FTH1 | Bracha et al. 2018 | N/A |
| Plasmid: PSP | Sanders et al. 2020 | N/A |
| Plasmid: VSVG | Sanders et al. 2020 | N/A |
| Plasmid: EGFP-pBAD | Davidson Lab, Florida State University | Addgene Cat#54762 |
| Plasmid: pet29b-SFP-His | Worthington & Burkart, 2006 | Addgene Cat#75015 |
| Plasmid: pET-H2A | Luger et al., 1999 | N/A |
| Plasmid: pET-H2B | Luger et al., 1999 | N/A |
| Plasmid: pET-H3 | Luger et al., 1999 | N/A |
| Plasmid: pET-H4 | Luger et al., 1999 | N/A |
| Plasmid: pGEM-3z/601 | Lowary and Widom, 1998 | Addgene Cat#26656 |
| Plasmid: pOmnibac zz TEV YBBR TRF1 | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF1ΔA | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF1ΔHinge | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF1ΔIDR | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF1Hinge | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF1HingeMyb | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF1GSTHingeMyb | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF1Basic | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF2 | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF2ΔB | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF2ΔHinge | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF2ΔIDR | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF2Hinge | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF2HingeMyb | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF2GSTHingeMyb | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF2GSTSub | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF2 ΔTRFH | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF2Acidic | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF1A74D | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR TRF2Y102F | This paper | N/A |
| Plasmid: pOmnibac zz TEV YBBR POT1 | This paper | N/A |
| Plasmid: pBig1a zz TEV YBBR TPP1 MBP TEV TIN2 | This paper | N/A |
| Plasmid: pBig2ab zz TEV YBBR POT1 ZZ TEV TPP1 MBP TEV TIN2 ZZ TEV TRF1 (4comp1) | This paper | N/A |
| Plasmid: pBig1a zz TEV YBBR POT1 MBP TEV TPP1 MBP TEV TIN2 ZZ TEV TRF2 (4comp2) | This paper | N/A |
| Plasmid: pBig1a zz TEV YBBR RAP1 ZZ TEV TRF2 | This paper | N/A |
| Plasmid: pLIB MBP TEV YBBR RAP1 | This paper | N/A |
| Plasmid: pRST5-Spinach-39xTelG | This paper | N/A |
| Software and Algorithms | ||
| Fiji (ImageJ 1.52p) | NIH | https://imagej.nih.gov/ij/ |
| 3D objects Counter (Fiji) | Bolte and Cordelières, 2006 | |
| 3D Multicoloc in 3D ImageJ suite (Fiji) | Ollion et al. 2013 | |
| GraphPad PRISM 9.1.0 | GraphPad | https://graphpad.com |
| MATLAB 2019b | MathWorks | https://www.mathworks.com/products/MATLAB.html |
| Python 3.7.10 | Python Software Foundation | https://python.org |
| Origin 8.5.0 SR1 | OriginLab Corporation | https://www.originlab.com/ |
| Other | ||
| IgG Sepharose beads | GE Healthcare | Cat#17096902 |
| HisPur Ni-NTA beads | Thermo Fisher Scientific | Cat#88221 |
| Amylose beads | New England BioLabs | Cat# E8021S |
| Superdex 200 Increase 10/300 GL | Cytiva | Cat#28-9909-44 |
| Superdex 200 10/300 GL | Cytiva | Cat#17517501 |
| NuPAGE 4-12% Bis-Tris gel | Thermo Fisher Scientific | Cat#NP0322BOX |
| PEG-Biotin cover slips | MicroSurfaces, Inc | Cat# Bio_02 |
| Trans-Blot Turbo Mini 0.2 um PVDF transfer pack | Bio-Rad | Cat#1704156 |
| 40kDa Zeba spin desalting column | Thermo Fisher Scientific | Cat#87766 |
| HiTrap SP HP | GE Life Sciences | Cat#95056-076 |
| HiTrap DEAE-FF | Cytiva | Cat#17515401 |
| Amersham Typhoon | GE Life Sciences | Cat#29238583 |
Insect cells were transfected using Fugene HD transfection reagent (Promega, E2311). The virus was amplified in progressively larger cultures. 1 mL of the P1 virus was used to infect 50 mL of Sf9 cells at 1 million cells/mL for 72 h. 10 mL of the P2 virus was used to infect 1 L of Sf9 cells at 1 million cells/mL and expression proceeded for 72 h. Cells expressing the protein of interest were harvested at 4,000 g for 10 min and resuspended in 50 mL lysis buffer (50 mM HEPES pH 7.4, 1 M NaCl, 1 mM PMSF, 1 mM DTT, and 1 tablet of protease inhibitor (Sigma, 4693132001)). Lysis was performed using 15 loose and 15 tight plunges of a Wheaton glass dounce. The lysate was clarified using a 45 min, 360,000 g spin in a Ti70 rotor. The supernatant was incubated with 1 mL IgG beads (IgG Sepharose 6 Fast Flow, GE Healthcare, 17096902) for ZZ-tagged TRF1, TRF2, and POT1 constructs or 1 mL amylose beads (New England BioLabs, E8021S) for co-expressed TPP1 and TIN2 and shelterin constructs for 1 h. Beads were washed with 40 mL of labelling buffer (50 mM HEPES pH 7.4, 300 mM NaCl, 10 mM MgCl2, 1 mM EGTA, 10% glycerol, 1 mM DTT). Beads were then collected and incubated with purified SFP protein (Addgene #75015)and a fluorescent dye functionalized with CoA (Lumidyne, custom synthesis) at room temperature for 30 min. Beads were washed in 40 mL labeling buffer, collected, and incubated with TEV protease (Berkeley Macrolab, Addgene #8827) for 1 h at room temperature to elute the protein. For shelterin protein preps, the protein was additionally incubated with 0.3 mL Ni-NTA beads (HisPur, Thermo Fisher Scientific, 88221) in 20 mM imidazole to remove the His-MBP and TEV in solution. After 30 min of incubation at °C, the beads were pelleted and the unbound protein was collected from the supernatant.
For the TRF1 and TRF2 mutant proteins, all purification steps were carried out in 1M NaCl to prevent aggregation. After TEV cleavage, the mutant proteins were concentrated and resuspended to reduce NaCl concentration to 300 mM. Finally, the protein was concentrated using Amicon Ultra 30K concentrators, concentration was measured using Bradford reagent (Bio-Rad, 500-0006), and aliquots were snap-frozen in liquid nitrogen. Isoelectric points were calculated with ExPASy ProtParam.
4comp2 was purified using the BigBac system, where subunits were coexpressed from the same vector. 4comp1 was created by mixing known concentrations of purified TRF1, coexpressed TPP1 and TIN2, and POT1 on ice. 5comp consisted of purified 4comp2 mixed with purified TRF1. 4comp1 and 4comp2 were run through a Superdex 200 Increase 10/300 GL size exclusion column (Cytiva, 28-9909-44) to separate assembled complexes from individual proteins and subcomplexes. Mixing purified proteins on ice produced results comparable to coexpressing the components (Figure S4G-H).
DDR protein purification
GFP-RPA was purified as previously described (Schaub et al., 2018). GFP was expressed in Rosetta cells (Berkeley MacroLab) using the GFP plasmid (Addgene 54762). This culture was added to 1 L of LB media and grown for 3 h until OD600 reaches 0.7. Cells were induced with 0.2% L-arabinose and incubated for 4.5 h at 37 °C in a shaker. After harvesting cells at 4,785 g for 15 min in a JLA 8.1 rotor, 500 mL cell pellets were incubated with 40 mL lysis buffer (50 mM HEPES pH 7.4, 300 mM NaCl, 20 mM imidazole, 1 mM PMSF, 1 mM DTT, and 1 tablet of protease inhibitor (Sigma, 11836170001)). Cells were lysed with a sonicator and spun in a Ti70 rotor at 117,734 g for 30 min. The supernatant was incubated with 2 mL of washed Ni-NTA beads (HisPur, Thermo Scientific, 88221) for 1 h at 4 °C. Beads were collected in a Bio-Rad column and washed in lysis buffer. Protein was cleaved off the beads with TEV protease at room temperature for 1 h and concentrated in an Amicon Ultra 10K concentrator. Protein concentration was measured using Bradford reagent (Bio-Rad, 500-0006). Protein was aliquoted and snap-frozen in 10% glycerol.
Mre11-Rad50-Nbs1 (MRN) complex and Ku were purified from Sf21 insect cells as previously described (Myler et al., 2017), with the addition of a 3x FLAG tag on the C-terminus of Mre11 and a 3x HA tag on the C-terminus of Ku80. PARP-1 was purified from Rosetta Escherichia coli cells as previously described (Caron et al., 2019; Langelier et al., 2011), with the addition of an N-terminal His-SUMO-HA tag. To label MRN, anti-FLAG (Sigma-Aldrich, F1804) was labeled using an Alexa Fluor 488 antibody labeling kit (Thermo Fisher Scientific, A20181). Alexa488 anti-FLAG at 3x molar excess was then incubated with the MRN complex on ice for 10 minutes. Ku and PARP-1 were labeled by incubating 2 hr at room temperature with 5-fold molar excess maleimide-coupled Atto488 dye (Sigma-Aldrich, 28562). Free dye was removed using a 40kDa Zeba spin desalting column (Thermo Fisher Scientific, 87766).
Nucleosome preparation
Histones were expressed from pET-H2A, pET-H2B, pET-H3, and pET-H4 constructs (Luger et al., 1999) in BL21(DE3)pLysS cells (Sigma-Aldrich, 69451) and purified according to previously described procedures (Dyer et al., 2004). Inclusion bodies were solubilized in DMSO and unfolding buffer (7M Guanidine HCl, 20 mM Tris-HCl, pH 7.5, 10 mM DTT) and purified using anion exchange chromatography (HiTrap SP HP, GE Life Sciences, 95056-076) in SAU buffer (7M deionized urea, 20 mM sodium acetate pH 5.2, 5 mM beta-mercaptoethanol (BME), 1 mM EDTA), eluting with a salt gradient from 0.2 to 0.6 M NaCl. Following dialysis of the peak fractions overnight in water + 2 mM BME, histones were buffer exchanged into unfolding buffer and concentrated. Octamers were assembled by mixing equimolar amounts of each histone and dialyzing overnight in refolding buffer (2M NaCl, 10 mM Tris pH 7.5, 1 mM EDTA, 5 mM BME). Next, octamers were purified in refolding buffer with a Superdex 10/300 GL column (GE Life Sciences, 17517501) using an Akta Pure chromatography system. Octamer formation in peak fractions was verified by Coomassie staining of SDS-PAGE gels. Nucleosome DNA sequences were amplified with Phusion polymerase (New England BioLabs, M0530L) from pGEM-3z/601 (Addgene plasmid #26656) (Lowary and Widom, 1998) using the following primer pairs for the standard nucleosome: 5’-Cy5-CTGGAGAATCCCGGTGCCG-3’ and 5’-ACAGGATGTATATATCTGACACG-3’, and for the telomere-tagged nucleosome the primer pair 5’-Cy5-TCGAATTCTTAGGGTTAGGGTTACCCTGGAGAATCCCGGT-3’ and 5’- CTGGATCCTAACCCTAACCCTAAGCACAGGATGTATATATCTGA-3’ were used. Oligonucleotides were synthesized by IDT. PCR products were verified on SYBR-Safe (Thermo Fisher Scientific, S33102) stained gels and purified using the Genejet PCR purification kit (Thermo Scientific, K0701). Nucleosomal core particles (NCPs) were then reconstituted by mixing the DNA and octamer at a molar ratio of 1.1:1 and slowly dialyzing into low salt, according to the procedure of Chua et al. (Chua et al., 2016) except that the dialysis was stopped at 0.1 M KCl. NCPs were purified on a HiTrap DEAE-FF (Cytiva, 17515401) column, first binding to the column in TCS buffer (20 mM Tris, pH 7.5, 1 mM EDTA, 1 mM DTT) and then eluting with an increasing gradient of KCl in TES buffer (10 mM Tris, pH 7.5, 0.5 mM EDTA). Peak fractions were examined on 0.5% agarose gels for Cy5 fluorescence (Amersham Typhoon; GE Life Sciences, 29238583). Fractions containing NCPs were pooled and dialyzed overnight in TCS buffer, then concentrated using Millipore centrifugal filters.
Formation and labeling of DNA substrates
For all DNA substrates except 39ds0ss, ssDNA sequences were ordered from IDT. Solutions of equimolar complementary sequences suspended in annealing buffer (10 mM Tris pH 7.5, 50 mM LiCl) were mixed and incubated in a hot plate at 95 °C for 5 min. The sample was then removed from the hot plate and allowed to cool to room temperature over 2 h. Comparing the molecular weights of the ssDNA oligos with that of the annealed dsDNA on an agarose gel confirms that annealing efficiency is high (Figure S2C). The 39ds0ss substrate was created by PCR amplifying a region of telomeric repeats from a plasmid, and the purity and length of the resulting DNA was verified on a 0.8% agarose gel. DNA was Cy3 labeled using a Label IT kit (Mirus Bio, MIR 3600).
Imaging of condensates
Slides were incubated with wash buffer (50 mM HEPES pH 7.4, 150 mM NaCl, 10 mM MgCl2, 1 mM EGTA, 1 mM DTT, 1% pluronic) for 5 min. Samples were settled onto the coverslip for 25 min before imaging. Imaging was performed using a Nikon Ti-E Eclipse microscope equipped with a 100X 1.49 N.A. Plan Apo oil immersion objective (Nikon). The samples were excited in near-TIRF using 488, 561, and 633 nm laser beams (Coherent). The emission signal was passed through a filter wheel and detected by Andor Ixon EMCCD Camera (512x512 pixels). The effective pixel size was 106 nm after 1X magnification and 160 nm after 1.5X magnification. Image processing is described in the “Quantification and Statistical Analysis” section.
For experiments involving DNA bound to the surface of the slide, chambers were incubated with 1 mg/mL Biotin-BSA (Sigma-Aldrich, 9048-46-8) for 2 min, incubated with 1 mg/mL streptavidin (Thermo Fisher Scientific, 434301) for 2 min, washed twice with 20 μL wash buffer, incubated with 1 μM biotinylated 8ds3ss DNA (IDT) for 2 min and washed twice with 20 μL wash buffer. Shelterin droplets were formed in a test tube, flowed into the chamber, and settled on the coverslip for 10 min. 5 μL solution containing the protein or DNA being tested was introduced to the chamber, and the sample was imaged using time-lapsing for 1 s every 10 s for 1 hr.
Cell culture
All DNA fragments of interest were PCR-amplified using Phusion High-Fidelity DNA Polymerase (New England BioLabs, M0530L). The hTRF1 and hTRF2 gene fragments were synthesized by IDT as gBlocks, with synonymous codon optimization to reduce repetitive DNA tracts. These fragments and point mutants were cloned into a linearized FM5 lentiviral vector. FM5 lentiviral vectors carried standardized linkers to insert the PCR fragments using the In-Fusion HD cloning kit (Takara Bio, 638910) (Sanders et al., 2020). Corelet constructs, unless otherwise noted, were cloned into the pHR lentiviral vector and confirmed by GENEWIZ Sanger sequencing.
Lentiviruses were generated by plating Lenti-X 293T cells (Takara Bio, 632180) into 6-well plates to reach ~70% confluence at the time of transfection. 24-36 hours after plating the Lenti-X cells, the transfer plasmid (1.50 μg), pCMVdR8.91 (1.33 μg), pMD2.G (0.17 μg) were transfected into the cells using FuGENE HD incubated in OptiMEM (modified from (Shin et al., 2018)). Transfer plasmids for the 53BP1 counting assay were transfected into Lenti-X cells with the helper plasmids VSVG and PSP with the Transit293 transfection reagent (Mirus, MIR 2700), following the protocol listed in Sanders et al. 2014 (Sanders et al., 2020). The supernatant-containing viruses were harvested 48 hours after transfection and filtered with a 0.45 μm filter (Pall Life Sciences), then used immediately or stored at −80°C. U2OS and hTERT-RPE1 cells plated at low (10-20%) confluency in 96-well glass-bottom plates (Cellvis) were transduced for 2-3 days before the washout of the virus, replacement with fresh media, and subsequent live-cell imaging experiments. Virus used for the formation of TRF1-mCh-sspB droplets away from telomeres at exceedingly high TRF1 concentrations was concentrated 10x using the Lenti-X Concentrator (Takara Bio, 631231), following the manufacturer’s protocol.
Live cell imaging
Cells plated on 96-well glass-bottom plates were incubated at 37°C and 5% CO2 by an Okolab microscope stage incubator with 96-well insert during all imaging experiments. Confocal microscopy was performed on a spinning disk (Yokogawa CSU-X1) confocal microscope with an Andor DU-897 EMCCD camera on a Nikon Eclipse Ti body using a 100x oil immersion Apo TIRF objective (NA 1.49). The following wavelength lasers were used to image the respective constructs: constructs with mGFP (488 nm), mCherry (561 nm), miRFP (640 nm). Fixed samples in the 53BP1 counting assay also used the 405 nm laser to detect nuclei stained with Hoechst (Thermo Fisher Scientific, H3570) or DAPI (Vectashield, H-2000-10).
Estimation of telomere component concentration in vivo
Both estimate that there are on the order of thousands of TRF2 dimers in a cell. Telomeric puncta are estimated to be 60-300 nm in diameter, and because virtually all TRF2 localize at telomeres (Palm and de Lange, 2008), the local concentration of dimers within TRF2 puncta would be hundred-micromolar.
FRAP assays
FRAP experiments were performed on a Nikon A1 laser scanning confocal microscope using a 60x oil immersion objective. A single telomere marked by shelterin proteins of interest was photobleached with the 488 nm and 640 nm laser each at bleaching power of ~ 400 kW cm-2. The cell was imaged every 2 s for 10 s of pre-bleach, and every 2 s post-bleach for 200 s. FRAP data were normalized by using the normalization method (Day et al., 2012). The first post-bleach point was set to zero.
Mean Squared Displacement measurements
Time-lapse movies were taken of U2OS cells with GFP-TRF1 or FUSN-miRFP-TRF1 overexpression and HeLa RMCE GFP-TRF1. Cells were plated 24 h before imaging on 96-well glass-bottom plates coated with fibronectin to reach high confluency. Each movie was 1 h long, imaging 1 s per frame.
Optogenetic telomere coalescence
Local activation was performed by using a Mightex Polygon digital micromirror device (DMD) to pattern blue light (488nm) stimulation from a Lumencor SpectraX light engine using Nikon Elements software. U2OS cells expressing the optogenetic telomere coalescence constructs FUSN-miRFP-TRF1, NLS-GFP-iLId-Fe and FUSN-mCherry-sspB were imaged using a specific local activation protocol, as follows. Pre-activation, imaging the mCherry (541 nm beam) and miRFP (640 nm beam) channels every 5 s for 15 s. Activation, wherein an elliptical region of interest (ROI) was used to locally activate two telomere foci to nucleate and grow FUSN Corelet droplets using the 485 nm DMD laser every 5 s for 6 min. A second activation sequence used a smaller, circular ROI aimed at the junction between two FUSN Corelet droplets every 5 s for 4 min to encourage them to fuse. Finally, the FUSN droplet was deactivated for 10 min by only imaging the mCherry and miRFP channels every 5 s, which allows the droplets to dissolve and pull together any attached telomeres. The second set of telomere coalescence constructs (iLId-miRFP-TRF1) uses a similar local activation protocol but only a single circular activation ROI for 3 min and a longer deactivation sequence (15-30 min).
Corelet experiments
TRF1WT, TRF2WT, and TRF1 mutant Corelet experiments were imaged every 5 s and followed this protocol: 15 s pre-activation (561 and 640 nm lasers) and 10 min of activation for local activation (488, 561, and 640 nm lasers). Each locally activated telomere and region away from telomere was normalized by subtracting the background from the ROI and divided by the average intensity of all other telomeres in the same cell minus the background. The first and last frames of activation were quantified.
siRNA TRF2 knockdown
Endogenous TRF2 levels were knocked down using siRNAs made by IDT with sequences from Takai et al. 2003 (Takai et al., 2003) and Yang et al. 2015 (Yang et al., 2015). siRNA transfection efficiency was estimated by transfecting a Cy3 labeled control siRNA (ThermoFisher Scientific, AM4621). To quantify transfection efficiency, three biological trials were transfected, fixed, stained with Hoechst or DAPI, and imaged. Nuclei were segmented with DAPI/Hoechst channel in FIJI and the intensity of Cy3 within each nucleus was recorded. Background intensity was subtracted from each, and the percent of cells with Cy3 signal at least 200 A.U. above background was plotted. The 1x condition was used for the 53BP1 counting assay. TRF2 knockdown efficiency was then validated by western blots (Figure S5B).
Western blot analysis
hTERT-RPE1 cells were plated on 6-well plates 24 hrs before siRNA treatment, and cells with or without siRNA treatment (siRNA #2 from Takai et al., 2003 (Takai et al., 2003) and a control scramble siRNA from Yang et al., 2015 (Yang et al., 2015) were grown for 48 hr before harvesting. Cell pellets were resuspended in 300ul RIPA buffer (BCA, 89901) with protease inhibitor (Sigma Aldrich, 4693132001). 1ul of benzonase (Sigma Aldrich, E1014-25KU) was added to each sample and left on ice for 30 min, each sample was spun down for an additional 30 min at 4°C, 30ul of lysate was resuspended in sample buffer (Thermo Fisher, NP0007), boiled at 100°C for 5 min with 15ul of the mix loaded for SDS-PAGE. Samples were run on a NuPAGE 4-12% Bis-Tris protein gel (Thermo Fisher, NP0322BOX) and transferred onto Trans-Blot Turbo Mini 0.2 um PVDF transfer pack (Bio-Rad, 1704156) for 30 min. Membranes were blocked for 2 hr with 5% NFDM in 1X TBST (Fisher Scientific, AAJ62938K2), and incubated in block with the anti-TRF2 antibody 1:2000 (Novus Biologicals, NB110-57130) and anti-Histone H3 antibody 1:2000 (Abcam, ab10799) for the loading control overnight at 4°C. Membranes were washed three times 5 min each with 1X TBST, incubated with either the Peroxidase AffiniPure Goat anti-mouse IgG 1:10,000 (Jackson ImmunoResearch, 115-035-062) or the Peroxidase AffiniPure Goat anti-rabbit IgG secondary antibodies 1:10,000 (JacksonImmunoResearch, 111-035-144) for 30 min at room temperature. Membranes were washed three times 5 min each with 1X TBST and developed using the SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher, 34577), following the manufacturer’s protocol. To determine the knockdown efficiency, the background intensity was subtracted from each band intensity, then normalized relative to loading control and plotted as a ratio relative to scrambled RNAi (set at 100% for each trial).
53BP1 foci counting assay
The siRNA #2 from Takai et al., 2003 (Takai et al., 2003) and a control scramble siRNA from Yang et al., 2015 (Yang et al., 2015) were used for the 53BP1 counting assay that used an IF-FISH protocol adapted from both the de Lange lab’s IF-FISH protocol and PNA bio’s FISH protocol. hTERT-RPE1 cells plated on glass-bottom 96 well plate 24 hr before transfection and were transfected twice with Oligofectamine (Thermo Fisher, 12252011) according to the manufacturer’s protocol (the second transfection was 24 hr after the first). Cells were transduced with 50-70ul of the rescue construct lentiviruses simultaneously with the siRNA treatment. Cells were then fixed with 4% paraformaldehyde for 5 min 48 hours after the first siRNA transfection, washed three times 5 min each with 1X TBST, and permeabilized for 15 min in 0.5% Triton X-100 buffer, incubated with block (10% goat serum and 0.1% Triton X-100 in 1X TBST) at room temperature for 1 hr, and incubated in block with anti-53BP1 antibody 1:50 (Novus Biologicals, NB100-305) overnight at 4°C. Cells were then washed four times 5 min each with 1X TBST, incubated with Goat anti-Rabbit IgG secondary antibody conjugated to Alexa fluor 647 (Thermo Fisher, A-21245) for 2 hours, washed four times, and fixed again with 4% paraformaldehyde. After three washes of 5 min each with 1X TBST, cells were dehydrated in 70%, 85%, 100% cold ethanol for 5 min each, air-dried for 15 min, denatured for 10 min with the hybridization buffer at 80°C. The hybridization buffer contained 70% formamide, 0.5% blocking reagent (Millipore Sigma, 11096176001), 20mM Tris-HCl pH 7.5, and a FITC labeled C-rich telomere probe (PNA bio, F1009). The samples were then incubated in the dark at room temperature for 2 hr, washed twice with 70% formamide, 10mM Tris-HCl pH 7.5 for 15 min each, washed three times for 5 min each with 1X TBST, left to air dry before mounting in Vectashield Plus Antifade Mounting Medium with DAPI (H-2000-10). 2x2 tiled images were taken from 31 z-stacks of 0.2um spacing on a spinning disk (Yokogawa CSU-X1) confocal microscope. 3D Objects Counter (Bolte and Cordelières, 2006) and 3D Multicoloc included in the 3D ImageJ suite (Ollion et al., 2013) were used to detect the nuclei, 53BP1, telomeres, and the number of colocalizations per stack. 3D segmented data was parsed in Python 3.7.10 with a custom Python script that counts the number of 53BP1 foci per nucleus.
miRFP-TRF2 dilute phase vs. total concentration
U2OS cells were transduced with 50 μl of miRFP-TRF2 lentivirus to result in differential overexpression levels. Images were taken from 11 z-planes of 0.5 μm spacing. Total concentration was calculated by taking the average intensity of the miRFP-TRF2 signal in an entire segmented nucleus, and dilute phase calculated by taking the average intensity of miRFP-TRF2 in the nucleoplasm, with bright telomeres masked out. cdil is measured as the ‘background’ concentration when a condensed phase is present. In a single-component phase separating system, cdil will saturate at a single ‘saturation concentration’ (csat), while in a multicomponent phase separating system, cdil may vary as a function of total system concentration.
QUANTIFICATION AND STATISTICAL ANALYSIS
In vitro droplet image processing
To calculate the saturation concentration (csat) of the proteins, droplets were identified using the Phansalkar function in Fiji (ImageJ 1.52p) with a 30-pixel radius and a minimum condensate size of 10 pixels. The volume of the condensates was estimated from 2D projections by taking the semi-principal axis in the z-plane as the geometric average of semi-principal axes in the XY plane. The total volume of the condensates settled per micron squared on the coverslip was quantified. Conditions that resulted in measurable condensate volumes were fit to linear regression in Origin. The x-intercept of the linear regression represents csat, the minimum protein concentration that results in condensate formation. csat for TRF2 in the presence of different DNA constructs (Figure 3D) was determined as the lowest protein concentration for which condensates are visible (Figure S2D). The aspect ratio was calculated using the Phansalkar function with a 30-pixel radius to detect particles and the Fit Ellipse function to determine the major axis length by the minor axis length in Fiji. Fusion times were calculated as the time between the last frame where two droplets appear separated (i.e. no overlap) and the first frame where the fused droplet appears spherical (aspect ratio ~ 1).
Image segmentation for time-lapse imaging of telomeres
All images were analyzed in Fiji (ImageJ 1.52p) and MATLAB 2019b (Mathworks). The first frame of each movie was used to calculate inter-telomere spacing. Briefly, nuclei were segmented using Otsu’s method; telomeres were then segmented by filtering using an LoG (Laplacian of Gaussians) kernel and applying a two standard deviation threshold. Average pairwise distance and nearest neighbors were then calculated (pdist2) based on the weighted centroids of all telomeres within each nucleus (extracted from the punctate mask regionprops). The local concentration of TRF2 at telomeres was estimated to be 400 μM from the measured radius of telomeres (~100 nm, (Bandaria et al., 2016; Jeynes et al., 2017)) and estimated number of TRF2 at each telomere in cells (~1,000 on average) from immunoblotting (Takai et al., 2010) and superresolution imaging assays (Bandaria et al., 2016).
Mean Squared Displacement analysis
To analyze telomere movement, images were registered to correct for whole-cell movement using StackReg plugin in Fiji. Then, Trackmate was used to track telomere movement with subpixel resolution using a Laplacian of Gaussian detector and object diameter of 500 nm. Trajectories of telomeres were then created using LAP tracking with maximum linking and gap-closing distances of 500 nm and zero-gap frames. Trajectories were only used if they spanned at least half the number of frames of the movie, then coordinates exported to MATLAB to calculate mean squared displacement.
Integrated intensity predictions and measurements
Telomeres were segmented using an LoG filter threshold method. Their respective total integrated intensity was calculated by summing over the intensity per pixel in the identified region. Since the integrated intensity should be directly proportional to the volume (Berry et al., 2015), the average integrated intensity of each telomere was calculated pre-coalescence (defined as all frames wherein two puncta were identified) and summed; the error was estimated and propagated by taking the standard error of the mean over pre-coalescence frames. The resulting summed integrated intensity and error bar were used as the independent prediction of the integrated intensity post-coalescence (defined as all frames wherein only one object was detected).
Statistical analysis
Statistics for the Corelet experiments and 53BP1 counting assay were performed using GraphPad PRISM version 9.1.0 software (GraphPad). Statistical significance (when reported) was calculated by one-way ANOVA with multiple comparisons or two-tailed t-test as noted in the figure legends. Number of replicates, size of n and precision measures (mean, median, ± SE and ± SD) are noted in the figure legends and captions.
Supplementary Material
Movie S1. Telomeres rarely coalesce due to their suppressed diffusivity in living cells; related to Figure 1. Time-lapse movie showing that telomeres marked by GFP-TRF1 in HeLa cells are mostly stationary due to their subdiffusive motion.
Movie S2. Specific local activation protocol using the Corelet system allows observation of liquid-like telomere coalescence in living cells; related to Figure 2. (Left) The shrinking of FUSN Corelet droplets pull in FUSN-miRFP-TRF1 marked telomeres to coalesce into a single telomere condensate. (Right) Telomeres relax back to more distal positions after detaching from FUSN droplets.
Movie S3. Telomere coalescence is not due to FUSN-FUSN interactions as iLID fused to TRF1 demonstrates liquid-like telomere merging events; related to Figure 2. iLID-miRFP-TRF1 acts as a seed only when it is bound to FUSN-mCherry-sspB upon light activation. The FUSN droplet shrinks and pulls in the telomeres to merge upon light deactivation once FUSN-mCherry-sspB is completely dissolved.
Movie S4. The fusion of TRF2, TRF1, and 5comp droplets in the presence of telomeric DNA; related to Figures 3 and 5. TRF2 and TRF1 droplets containing DNA fuse within 30 seconds, on average, while 5-component shelterin droplets containing DNA adhere and relax but do not become spherical over several hundred seconds. Droplets were imaged with a TIRF microscope at 4.3 frames/s. Droplets were formed with 2.5 μM 8ds3ss telomeric DNA using either 22 μM TRF2, 44 μM TRF1, or 4.5 μM 5comp.
Movie S5. RPA and TERRA diffuse into 5-comp droplets settled onto a DNA-coated slide; related to Figure 7. Droplets formed from 5.3 μM 5comp in physiological salt were settled onto a slide coated with 8ds3ss telomeric DNA for 10 min before 100 μM GFP-RPA and 100 μM 60nt-TERRA were flowed onto the slide. RPA is distributed uniformly across the surface over several minutes, while TERRA partitions strongly into the shelterin droplets. TIRF imaging began 55 s after the introduction of RPA and TERRA to the sample. Time-lapse images were acquired for 200 ms every 10 s.
Acknowledgments
A. J., Y.K., and D.S.W.L. are supported by the NSF GRFP fellowship (DGE-1752814, A.J.; DGE-2039656, Y.K.; DCE-1656466, D.S.W.L.). Y.K. was previously supported by the NIGMS (T32GM007388) while conducting experiments. A.R.S. is a Life Science Research Fellow through the Mark Foundation for Cancer Research (AWD1006303). L.F. was supported by the NIH F32 Fellowship (GM123655). This work was supported by NSF (MCB-1617028, A.Y.), NIGMS (GM 118773, A.Y.; GM120554, I.J.F.; and GM124463, E.H.K.), the NIH 4D Nucleome Program (U01 DA040601, C.P.B.), CPRIT (RP190301 to I.J.F.), the Welch Foundation (F-1808 to I.J.F.), and the Howard Hughes Medical Institute (C.P.B.). The content is solely the responsibility of the authors and does not represent the official views of these funding institutions.
We thank Joshua Riback, Jorine Eeftens, Yi-Che Chang, David Sanders, Evangelos Gatzogiannis, Yavuz S. Dagdas, John T. Canty, and other members of the Yildiz and Brangwynne laboratories for helpful discussions, Shunsuke Shimobayashi for the FUSN-miRFP-TRF1 construct, David Sanders for the FM5 vectors, Yi-Che Chang for helpful discussions on data analysis, Sofi Quinodoz for help with the control siRNA transfection efficiency experiment, Lindsay Becker for help with the immunofluorescence protocol, Titia de Lange (Rockefeller Univ.) for the hTERT-RPE1 cell line, Huaiying Zhang (Carnegie Mellon Univ.) for the HeLa RMCE GFP-TRF1 cell line, UC Berkeley MacroLab for the TEV protease and competent cells, and UC Berkeley Cell Culture Facility for the insect cell cultures.
Footnotes
Declaration of Interests
C.P.B. is a founder of and consultant for Nereid Therapeutics. All other authors declare no competing interests.
References
- Adam N, Degelman E, Briggs S, Wazen RM, Colarusso P, Riabowol K, and Beattie T (2019). Telomere analysis using 3D fluorescence microscopy suggests mammalian telomere clustering in hTERT-immortalized Hs68 fibroblasts. Commun Biol 2, 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S, Gladfelter A, and Mittag T (2019). Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 176, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshareedah I, Kaur T, and Banerjee PR (2021). Methods for characterizing the material properties of biomolecular condensates. Methods Enzymol 646, 143–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmeyer M, Neelsen KJ, Teloni F, Pozdnyakova I, Pellegrino S, Grofte M, Rask MD, Streicher W, Jungmichel S, Nielsen ML, and Lukas J (2015). Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). Nat Commun 6, 8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiard S, Doudeau M, Pinte S, Poulet A, Lenain C, Faivre-Moskalenko C, Angelov D, Hug N, Vindigni A, Bouvet P, et al. (2007). A topological mechanism for TRF2-enhanced strand invasion. Nature structural & molecular biology 14, 147–154. [DOI] [PubMed] [Google Scholar]
- Baird DM, Rowson J, Wynford-Thomas D, and Kipling D (2003). Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat Genet 33, 203–207. [DOI] [PubMed] [Google Scholar]
- Banani SF, Lee HO, Hyman AA, and Rosen MK (2017). Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani SF, Rice AM, Peeples WB, Lin Y, Jain S, Parker R, and Rosen MK (2016). Compositional Control of Phase-Separated Cellular Bodies. Cell 166, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaria JN, Qin P, Berk V, Chu S, and Yildiz A (2016). Shelterin Protects Chromosome Ends by Compacting Telomeric Chromatin. Cell 164, 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee PR, Milin AN, Moosa MM, Onuchic PL, and Deniz AA (2017). Reentrant Phase Transition Drives Dynamic Substructure Formation in Ribonucleoprotein Droplets. Angew Chem Int Ed Engl 56, 11354–11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J, Weber SC, Vaidya N, Haataja M, and Brangwynne CP (2015). RNA transcription modulates phase transition-driven nuclear body assembly. Proc Natl Acad Sci U S A 112, E5237–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi G, Tannahill D, and Balasubramanian S (2012). An intramolecular G-quadruplex structure is required for binding of telomeric repeat-containing RNA to the telomeric protein TRF2. J Am Chem Soc 134, 11974–11976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH (1991). Structure and function of telomeres. Nature 350, 569–573. [DOI] [PubMed] [Google Scholar]
- Bolte S, and Cordelières FP (2006). A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224, 213–232. [DOI] [PubMed] [Google Scholar]
- Bower BD, and Griffith JD (2014). TRF1 and TRF2 differentially modulate Rad51-mediated telomeric and nontelomeric displacement loop formation in vitro. Biochemistry 53, 5485–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha D, Walls MT, Wei MT, Zhu L, Kurian M, Avalos JL, Toettcher JE, and Brangwynne CP (2018). Mapping Local and Global Liquid Phase Behavior in Living Cells Using Photo-Oligomerizable Seeds. Cell 175, 1467–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronshtein I, Kepten E, Kanter I, Berezin S, Lindner M, Redwood AB, Mai S, Gonzalo S, Foisner R, Shav-Tal Y, and Garini Y (2015). Loss of lamin A function increases chromatin dynamics in the nuclear interior. Nat Commun 6, 8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron MC, Sharma AK, O'Sullivan J, Myler LR, Ferreira MT, Rodrigue A, Coulombe Y, Ethier C, Gagné JP, Langelier MF, et al. (2019). Poly(ADP-ribose) polymerase-1 antagonizes DNA resection at double-strand breaks. Nat Commun 10, 2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli GB, and de Lange T (2005). DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol 7, 712–718. [DOI] [PubMed] [Google Scholar]
- Cesare AJ, Hayashi MT, Crabbe L, and Karlseder J (2013). The telomere deprotection response is functionally distinct from the genomic DNA damage response. Mol Cell 51, 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba K, Lorbeer FK, Shain AH, McSwiggen DT, Schruf E, Oh A, Ryu J, Darzacq X, Bastian BC, and Hockemeyer D (2017). Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science 357, 1416–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HP, Cifuentes-Rojas C, Kesner B, Aeby E, Lee HG, Wei C, Oh HJ, Boukhali M, Haas W, and Lee JT (2017). TERRA RNA Antagonizes ATRX and Protects Telomeres. Cell 170, 86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EYD, Vogirala VK, Inian O, Wong ASW, Nordenskiöld L, Plitzko JM, Danev R, and Sandin S (2016). 3.9 Å structure of the nucleosome core particle determined by phase-plate cryo-EM. Nucleic Acids Research 44, 8013–8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court R, Chapman L, Fairall L, and Rhodes D (2005). How the human telomeric proteins TRF1 and TRF2 recognize telomeric DNA: a view from high-resolution crystal structures. Embo Rep 6, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, and Jackson SP (2003). A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198. [DOI] [PubMed] [Google Scholar]
- Day CA, Kraft LJ, Kang M, and Kenworthy AK (2012). Analysis of protein and lipid dynamics using confocal fluorescence recovery after photobleaching (FRAP). Curr Protoc Cytom Chapter 2, Unit2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T (2018). Shelterin-Mediated Telomere Protection. Annu Rev Genet 52, 223–247. [DOI] [PubMed] [Google Scholar]
- Denchi EL, and de Lange T (2007). Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448, 1068–1071. [DOI] [PubMed] [Google Scholar]
- Deng Z, Norseen J, Wiedmer A, Riethman H, and Lieberman PM (2009). TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell 35, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doksani Y, Wu JY, de Lange T, and Zhuang X (2013). Super-Resolution Fluorescence Imaging of Telomeres Reveals TRF2-Dependent T-loop Formation. Cell 155, 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draskovic I, Arnoult N, Steiner V, Bacchetti S, Lomonte P, and Londoño-Vallejo A (2009). Probing PML body function in ALT cells reveals spatiotemporal requirements for telomere recombination. Proc Natl Acad Sci U S A 106, 15726–15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer PN, Edayathumangalam RS, White CL, Bao Y, Chakravarthy S, Muthurajan UM, and Luger K (2004). Reconstitution of nucleosome core particles from recombinant histones and DNA. Methods Enzymol 375, 23–44. [DOI] [PubMed] [Google Scholar]
- Fairall L, Chapman L, Moss H, de Lange T, and Rhodes D (2001). Structure of the TRFH dimerization domain of the human telomeric proteins TRF1 and TRF2. Mol Cell 8, 351–361. [DOI] [PubMed] [Google Scholar]
- Feric M, and Brangwynne CP (2013). A nuclear F-actin scaffold stabilizes ribonucleoprotein droplets against gravity in large cells. Nat Cell Biol 15, 1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro LS, Can S, Turner MA, ElShenawy MM, and Yildiz A (2019). Kinesin and dynein use distinct mechanisms to bypass obstacles. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, and Gorlich D (2007). A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 130, 512–523. [DOI] [PubMed] [Google Scholar]
- Galati A, Micheli E, and Cacchione S (2013). Chromatin structure in telomere dynamics. Front Oncol 3, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, and de Lange T (2010). A Shld1-controlled POT1a provides support for repression of ATR signaling at telomeres through RPA exclusion. Mol Cell 40, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, and de Lange T (1999). Mammalian telomeres end in a large duplex loop. Cell 97, 503–514. [DOI] [PubMed] [Google Scholar]
- Grobelny JV, Godwin AK, and Broccoli D (2000). ALT-associated PML bodies are present in viable cells and are enriched in cells in the G(2)/M phase of the cell cycle. J Cell Sci 113 Pt 24, 4577–4585. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, and Greider CW (1990). Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460. [DOI] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, and Sedivy JM (2004). Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 14, 501–513. [DOI] [PubMed] [Google Scholar]
- Janissen R, Arens MMA, Vtyurina NN, Rivai Z, Sunday ND, Eslami-Mossallam B, Gritsenko AA, Laan L, de Ridder D, Artsimovitch I, et al. (2018). Global DNA Compaction in Stationary-Phase Bacteria Does Not Affect Transcription. Cell 174, 1188–1199.e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoušková E, Nečasová I, Pavloušková J, Zimmermann M, Hluchý M, Marini V, Nováková M, and Hofr C (2015). Human Rap1 modulates TRF2 attraction to telomeric DNA. Nucleic Acids Res 43, 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeynes JCG, Geraki K, Jeynes C, Zhaohong M, Bettiol AA, Latorre E, Harries LW, and Soeller C (2017). Nanoscale Properties of Human Telomeres Measured with a Dual Purpose X-ray Fluorescence and Super Resolution Microscopy Gold Nanoparticle Probe. ACS Nano 11, 12632–12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar A, Willcox S, and Griffith JD (2016). Transcription of telomeric DNA leads to high levels of homologous recombination and t-loops. Nucleic Acids Res 44, 9369–9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner DH, Durocher D, and Karlseder J (2011). A siRNA-based screen for genes involved in chromosome end protection. PLoS One 6, e21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche BJ, Orazio NI, and Weitzman MD (2010). The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett 584, 3682–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelier MF, Planck JL, Servent KM, and Pascal JM (2011). Purification of human PARP-1 and PARP-1 domains from Escherichia coli for structural and biochemical analysis. Methods Mol Biol 780, 209–226. [DOI] [PubMed] [Google Scholar]
- Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, Agard DA, Redding S, and Narlikar GJ (2017). Liquid droplet formation by HP1alpha suggests a role for phase separation in heterochromatin. Nature 547, 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzerini-Denchi E, and Sfeir A (2016). Stop pulling my strings - what telomeres taught us about the DNA damage response. Nat Rev Mol Cell Biol 17, 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DSW, Wingreen NS, and Brangwynne CP (2021). Chromatin mechanics dictates subdiffusion and coarsening dynamics of embedded condensates. Nat. Phys 17, 531–538. [Google Scholar]
- Lim CJ, Zaug AJ, Kim HJ, and Cech TR (2017). Reconstitution of human shelterin complexes reveals unexpected stoichiometry and dual pathways to enhance telomerase processivity. Nat Commun 8, 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Safari A, O'Connor MS, Chan DW, Laegeler A, Qin J, and Songyang Z (2004). PTOP interacts with POT1 and regulates its localization to telomeres. Nat Cell Biol 6, 673–680. [DOI] [PubMed] [Google Scholar]
- Lowary PT, and Widom J (1998). New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J Mol Biol 276, 19–42. [DOI] [PubMed] [Google Scholar]
- Luger K, Rechsteiner TJ, and Richmond TJ (1999). Preparation of nucleosome core particle from recombinant histones. Methods Enzymol 304, 3–19. [DOI] [PubMed] [Google Scholar]
- Maciejowski J, and de Lange T (2017). Telomeres in cancer: tumour suppression and genome instability. Nat Rev Mol Cell Biol 18, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni L, Matmati S, and Coulon S (2017). Solving the Telomere Replication Problem. Genes (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern KA, Swiggers SJ, Nigg AL, Lowenberg B, Houtsmuller AB, and Zijlmans JM (2004). Dynamics of protein binding to telomeres in living cells: implications for telomere structure and function. Mol Cell Biol 24, 5587–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Wright WE, and Shay JW (2019). Clustered telomeres in phase-separated nuclear condensates engage mitotic DNA synthesis through BLM and RAD52. Genes Dev 33, 814–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar C, Wiesmeijer K, Verwoerd NP, Khazen S, Eils R, Tanke HJ, and Dirks RW (2003). Visualizing telomere dynamics in living mammalian cells using PNA probes. Embo j 22, 6631–6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myler LR, Gallardo IF, Soniat MM, Deshpande RA, Gonzalez XB, Kim Y, Paull TT, and Finkelstein IJ (2017). Single-Molecule Imaging Reveals How Mre11-Rad50-Nbs1 Initiates DNA Break Repair. Mol Cell 67, 891–898.e894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar J, and Cech TR (2013). Finding the end: recruitment of telomerase to telomeres. Nature reviews. Molecular cell biology 14, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones DP, Pawson T, Forman-Kay JD, and Baldwin AJ (2015). Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell 57, 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MS, Safari A, Liu D, Qin J, and Songyang Z (2004). The human Rap1 protein complex and modulation of telomere length. J Biol Chem 279, 28585–28591. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Bartocci C, Ouzounov I, Diedrich JK, Yates JR 3rd, and Denchi EL (2013). A two-step mechanism for TRF2-mediated chromosome-end protection. Nature 494, 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollion J, Cochennec J, Loll F, Escudé C, and Boudier T (2013). TANGO: a generic tool for high-throughput 3D image analysis for studying nuclear organization. Bioinformatics 29, 1840–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orun O, Tiber PM, and Serakinci N (2016). Partial knockdown of TRF2 increase radiosensitivity of human mesenchymal stem cells. Int J Biol Macromol 90, 53–58. [DOI] [PubMed] [Google Scholar]
- Palm W, and de Lange T (2008). How shelterin protects mammalian telomeres. Annu Rev Genet 42, 301–334. [DOI] [PubMed] [Google Scholar]
- Potts PR, and Yu H (2007). The SMC5/6 complex maintains telomere length in ALT cancer cells through SUMOylation of telomere-binding proteins. Nat Struct Mol Biol 14, 581–590. [DOI] [PubMed] [Google Scholar]
- Poulet A, Buisson R, Faivre-Moskalenko C, Koelblen M, Amiard S, Montel F, Cuesta-Lopez S, Bornet O, Guerlesquin F, Godet T, et al. (2009). TRF2 promotes, remodels and protects telomeric Holliday junctions. Embo j 28, 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R, Chen Y, Lei M, and Chang S (2016). TRF2-RAP1 is required to protect telomeres from engaging in homologous recombination-mediated deletions and fusions. Nat Commun 7, 10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Bandaria JN, Qureshi MH, Yildiz A, and Balci H (2014). G-quadruplex formation in telomeres enhances POT1/TPP1 protection against RPA binding. Proceedings of the National Academy of Sciences of the United States of America 111, 2990–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riback JA, Zhu L, Ferrolino MC, Tolbert M, Mitrea DM, Sanders DW, Wei MT, Kriwacki RW, and Brangwynne CP (2020). Composition-dependent thermodynamics of intracellular phase separation. Nature 581, 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DW, Kedersha N, Lee DSW, Strom AR, Drake V, Riback JA, Bracha D, Eeftens JM, Iwanicki A, Wang A, et al. (2020). Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization. Cell 181, 306–324.e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub JM, Zhang H, Soniat MM, and Finkelstein IJ (2018). Assessing Protein Dynamics on Low-Complexity Single-Stranded DNA Curtains. Langmuir 34, 14882–14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz LB, Chehab NH, Malikzay A, and Halazonetis TD (2000). p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol 151, 1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfeir A, and de Lange T (2012). Removal of Shelterin Reveals the Telomere End-Protection Problem. Science 336, 593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, and Wright WE (2005). Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis 26, 867–874. [DOI] [PubMed] [Google Scholar]
- Shin Y, and Brangwynne CP (2017). Liquid phase condensation in cell physiology and disease. Science 357. [DOI] [PubMed] [Google Scholar]
- Shin Y, Chang YC, Lee DSW, Berry J, Sanders DW, Ronceray P, Wingreen NS, Haataja M, and Brangwynne CP (2018). Liquid Nuclear Condensates Mechanically Sense and Restructure the Genome. Cell 175, 1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, and de Lange T (2000). Control of human telomere length by TRF1 and TRF2. Mol Cell Biol 20, 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soranno A, Incicco JJ, De Bona P, Tomko EJ, Galburt EA, Holehouse AS, and Galletto R (2021). Shelterin components modulate nucleic acids condensation and phase separation. bioRxiv, doi: 10.1101/2021.04.30.442189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansel RM, de Lange T, and Griffith JD (2001). T-loop assembly in vitro involves binding of TRF2 near the 3' telomeric overhang. Embo j 20, 5532–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom AR, Emelyanov AV, Mir M, Fyodorov DV, Darzacq X, and Karpen GH (2017). Phase separation drives heterochromatin domain formation. Nature 547, 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söding J, Zwicker D, Sohrabi-Jahromi S, Boehning M, and Kirschbaum J (2020). Mechanisms for Active Regulation of Biomolecular Condensates. Trends Cell Biol 30, 4–14. [DOI] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, and de Lange T (2003). DNA damage foci at dysfunctional telomeres. Curr Biol 13, 1549–1556. [DOI] [PubMed] [Google Scholar]
- Takai KK, Hooper S, Blackwood S, Gandhi R, and de Lange T (2010). In vivo stoichiometry of shelterin components. J Biol Chem 285, 1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai KK, Kibe T, Donigian JR, Frescas D, and de Lange T (2011). Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol Cell 44, 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NO, Wei MT, Stone HA, and Brangwynne CP (2019). Quantifying Dynamics in Phase-Separated Condensates Using Fluorescence Recovery after Photobleaching. Biophys J 117, 1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timashev LA, Babcock H, Zhuang X, and de Lange T (2017). The DDR at telomeres lacking intact shelterin does not require substantial chromatin decompaction. Genes Dev 31, 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel B, Smogorzewska A, and de Lange T (1998). TRF2 protects human telomeres from end-to-end fusions. Cell 92, 401–413. [DOI] [PubMed] [Google Scholar]
- Wang J, Choi JM, Holehouse AS, Lee HO, Zhang X, Jahnel M, Maharana S, Lemaitre R, Pozniakovsky A, Drechsel D, et al. (2018). A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 174, 688–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kam Z, Carlton PM, Xu L, Sedat JW, and Blackburn EH (2008). Rapid telomere motions in live human cells analyzed by highly time-resolved microscopy. Epigenetics Chromatin 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MT, Elbaum-Garfinkle S, Holehouse AS, Chen CC, Feric M, Arnold CB, Priestley RD, Pappu RV, and Brangwynne CP (2017). Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat Chem 9, 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold MS (1997). Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annual review of biochemistry 66, 61–92. [DOI] [PubMed] [Google Scholar]
- Yang X, Li Z, Yang L, Lei H, Yu H, Liao Z, Zhou F, Xie C, and Zhou Y (2015). Knockdown of telomeric repeat binding factor 2 enhances tumor radiosensitivity regardless of telomerase status. J Cancer Res Clin Oncol 141, 1545–1552. [DOI] [PubMed] [Google Scholar]
- Ye JZ, Donigian JR, van Overbeek M, Loayza D, Luo Y, Krutchinsky AN, Chait BT, and de Lange T (2004). TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. The Journal of biological chemistry 279, 47264–47271. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhao R, Tones J, Liu M, Dilley R, Chenoweth DM, Greenberg RA, and Lampson MA (2020). Nuclear body phase separation drives telomere clustering in ALT cancer cells. Mol Biol Cell, mbcE19100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Telomeres rarely coalesce due to their suppressed diffusivity in living cells; related to Figure 1. Time-lapse movie showing that telomeres marked by GFP-TRF1 in HeLa cells are mostly stationary due to their subdiffusive motion.
Movie S2. Specific local activation protocol using the Corelet system allows observation of liquid-like telomere coalescence in living cells; related to Figure 2. (Left) The shrinking of FUSN Corelet droplets pull in FUSN-miRFP-TRF1 marked telomeres to coalesce into a single telomere condensate. (Right) Telomeres relax back to more distal positions after detaching from FUSN droplets.
Movie S3. Telomere coalescence is not due to FUSN-FUSN interactions as iLID fused to TRF1 demonstrates liquid-like telomere merging events; related to Figure 2. iLID-miRFP-TRF1 acts as a seed only when it is bound to FUSN-mCherry-sspB upon light activation. The FUSN droplet shrinks and pulls in the telomeres to merge upon light deactivation once FUSN-mCherry-sspB is completely dissolved.
Movie S4. The fusion of TRF2, TRF1, and 5comp droplets in the presence of telomeric DNA; related to Figures 3 and 5. TRF2 and TRF1 droplets containing DNA fuse within 30 seconds, on average, while 5-component shelterin droplets containing DNA adhere and relax but do not become spherical over several hundred seconds. Droplets were imaged with a TIRF microscope at 4.3 frames/s. Droplets were formed with 2.5 μM 8ds3ss telomeric DNA using either 22 μM TRF2, 44 μM TRF1, or 4.5 μM 5comp.
Movie S5. RPA and TERRA diffuse into 5-comp droplets settled onto a DNA-coated slide; related to Figure 7. Droplets formed from 5.3 μM 5comp in physiological salt were settled onto a slide coated with 8ds3ss telomeric DNA for 10 min before 100 μM GFP-RPA and 100 μM 60nt-TERRA were flowed onto the slide. RPA is distributed uniformly across the surface over several minutes, while TERRA partitions strongly into the shelterin droplets. TIRF imaging began 55 s after the introduction of RPA and TERRA to the sample. Time-lapse images were acquired for 200 ms every 10 s.
Data Availability Statement
This study did not generate any datasets/code amenable for depositing into public repositories. All data reported in this paper will be shared by the lead contact upon request. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.