Abstract
Dominantly inherited Alzheimer’s disease (DIAD) causes predictable biological changes decades before the onset of clinical symptoms, enabling testing of interventions in the asymptomatic and symptomatic stages to delay or slow disease progression. We conducted a randomized, placebo-controlled, multi-arm trial of gantenerumab or solanezumab in participants with DIAD across asymptomatic and symptomatic disease stages. Mutation carriers were assigned 3:1 to either drug or placebo and received treatment for 4–7 years. The primary outcome was a cognitive end point; secondary outcomes included clinical, cognitive, imaging and fluid biomarker measures. Fifty-two participants carrying a mutation were assigned to receive gantenerumab, 52 solanezumab and 40 placebo. Both drugs engaged their Aβ targets but neither demonstrated a beneficial effect on cognitive measures compared to controls. The solanezumab-treated group showed a greater cognitive decline on some measures and did not show benefits on downstream biomarkers. Gantenerumab significantly reduced amyloid plaques, cerebrospinal fluid total tau, and phospho-tau181 and attenuated increases of neurofilament light chain. Amyloid-related imaging abnormalities edema was observed in 19.2% (3 out of 11 were mildly symptomatic) of the gantenerumab group, 2.5% of the placebo group and 0% of the solanezumab group. Gantenerumab and solanezumab did not slow cognitive decline in symptomatic DIAD. The asymptomatic groups showed no cognitive decline; symptomatic participants had declined before reaching the target doses.
AD is a progressive neurodegenerative disorder that causes pathological changes in the brain decades before the onset of clinical symptoms. It is hypothesized that accumulation of amyloid beta (Aβ) plaques in the brain initiates a cascade of destructive mechanisms including inflammation and aggregation of tau protein in neurofibrillary tangles1,2. DIAD is a rare form of the disease, estimated at <1% of all cases where dementia develops at a relatively predictable age as determined by specific genetic mutations3,4. The Dominantly Inherited Alzheimer Network (DIAN) launched an observational study (DIAN–OBS) of DIAD in 2008 (ref.5). By tracking clinical, cognitive and biomarker measures, the study found that biomarker changes, such as amyloid plaque deposition and tau changes, begin at least two decades before the onset of clinical symptoms6–8.
Trials of drugs focused on removing or interrupting the accumulation of Aβ in sporadic late-onset AD have not included individuals with DIAD and have been mostly disappointing9–12. Potential reasons for past negative results include treating too late in the disease course, inadequate dosing or target engagement, incorrect target or non-AD contributions of dementia in trial populations13,14. Because individuals with DIAD develop AD dementia at a predictable age, manifest disease pathology many years before symptom onset and are unlikely to have comorbidities that contribute to cognitive decline, this population provides an opportunity for testing early-stage interventions to prevent or slow disease progression3,4.
The DIAN–Trials Unit (DIAN–TU) was established in 2012 as a public–private collaboration to test drug interventions across the stages of DIAD15,16. The DIAN–TU platform design17 enables simultaneous testing of multiple treatments with a single protocol and shared placebo group. We launched the first trial (DIAN–TU-001) in 2012 as a two-year biomarker study to test two anti-amyloid monoclonal antibodies in parallel, gantenerumab (an anti-fibrillar Aβ antibody) and solanezumab (an anti-soluble Aβ antibody) in asymptomatic and mild symptomatic stages of DIAD. In 2015, the study was transitioned to a four-year treatment trial with a cognitive primary end point to investigate the drugs’ potential to slow or prevent cognitive decline17.
Results
Participant characteristics.
Randomization and group assignments are presented in Fig. 1. Fifty-two individuals carrying a mutation (60% asymptomatic, defined as a Clinical Dementia Rating (CDR)18 score of 0, cognitively normal) were assigned to gantenerumab and 52 (60% asymptomatic) were assigned to solanezumab; 40 (55% asymptomatic) were assigned to shared placebo. Data from participants in a previous observational study, the DIAN–OBS study6,7, who met the DIAN–TU inclusion criteria were used as natural history controls for improved estimates of the placebo group in the primary analyses19. Of the 123 eligible participants from the DIAN–OBS study, 54 (74% asymptomatic) were run-in participants previously enrolled in DIAN–OBS who transitioned to DIAN–TU-001; 69 (51% asymptomatic) were in the DIAN–OBS study only (Supplementary Fig. 1). Baseline demographics in the three arms and the eligible DIAN–OBS group were balanced across characteristics (Table 1). The mean (s.d.) treatment duration was 4.02 (1.336) years, ranging from 0.90 to 6.49 years. For gantenerumab, 48 (92.3%) participants started the dose escalation and had an average duration on escalated doses of 2.41 (0.92) years; for solanezumab, 39 (75.0%) started the dose escalation and had an average duration on escalated doses of 1.44 (0.48) years (Supplementary Fig. 2).
Fig. 1 |. Randomization, group assignment and follow-up.
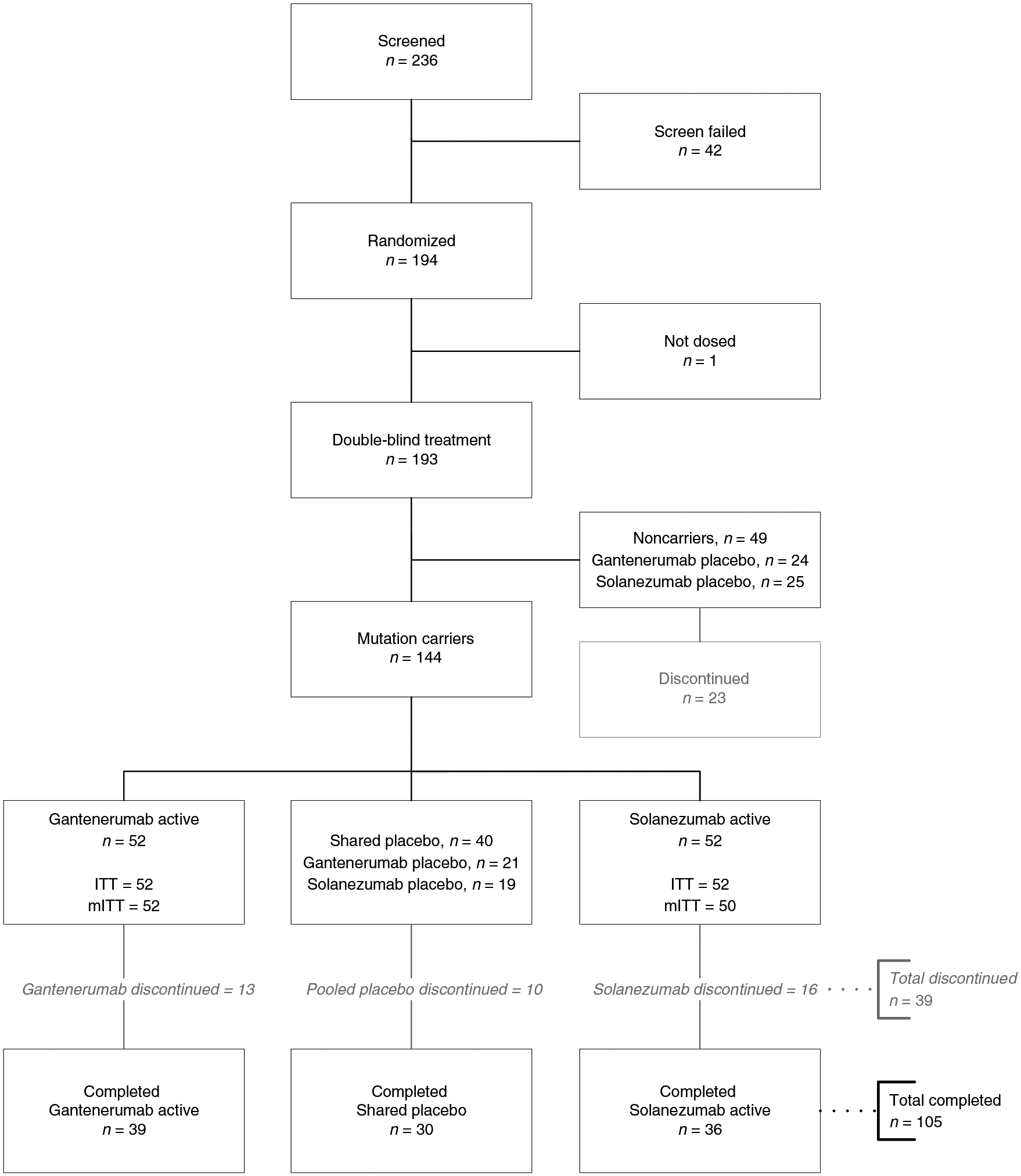
There were no significant between-group differences in the reasons for trial discontinuation; the most common reason was disease progression. Noncarriers were assigned to placebo and their data were not used in the reported study analyses. Those who chose to learn their mutation status were automatically discontinued from the trial. Thirty-nine participants discontinued: 61.6% due to withdrawal by participant or their proxy (13 due to disease progression, 5 to trial-related burden, 6 other); 33.3% due to physician decision (7 due to disease progression, 6 due to AEs/met protocol discontinuation criteria); 5.1% other. ITT, intention to treat; mITT, modified ITT.
Table 1 |.
Demographic and baseline characteristics of the study participants
| DIAN-TU-001 mutation carriers | DIAN-OBS mutation carriers | |||
|---|---|---|---|---|
| Active gantenerumab n = 52 | Active solanezumaba n = 50 | Shared placebo n = 40 | DIAN-OBSb controls n = 69 | |
| Age (years) | 46.0 ± 10.8 | 42.5 ± 9.5 | 44.2 ± 9.6 | 42.3 ± 9.6 |
| Female (n (%)) | 21 (40) | 29 (58) | 22 (55) | 47 (68) |
| Gene (n (%)) | ||||
| APP | 6 (12) | 8 (16) | 5 (13) | 10 (14) |
| PS1 | 43 (83) | 40 (80) | 32 (80) | 55 (80) |
| PS2 | 3 (6) | 2 (4) | 3 (8) | 4 (6) |
| APOE4c (n (%)) | 16 (31) | 14 (28) | 13 (32) | 18 (26) |
| Enrollment EYO | −3.5 ± 7.1 | −2.4 ± 7.1 | −3.5 ± 7.6 | −3.6 ± 6.9 |
| CDRd 0 (n (%)) | 31 (60) | 30 (60) | 22 (55) | 35 (51) |
| CDR 0.5 (n (%)) | 15 (29) | 13 (26) | 15 (38) | 27 (39) |
| CDR 1 (n (%)) | 6 (12) | 7 (14) | 3 (8) | 7 (10) |
| Digit symbole | 46.96 ± 20.56 | 46.06 ± 19.94 | 46.63 ± 19.12 | 50.34 ± 18.57 |
| MMSEf | 27.10 ± 3.45 | 26.72 ± 4.11 | 26.68 ± 3.97 | 26.96 ± 3.22 |
| Logical memoryg | 9.90 ± 6.33 | 9.86 ± 6.86 | 9.40 ± 6.45 | 9.32 ± 6.42 |
| ISLTh | 5.96 ± 4.04 | 6.56 ± 3.95 | 5.80 ± 4.42 | NA |
| CDR-SBi | 1.33 ± 2.08 | 1.37 ± 2.01 | 1.43 ± 1.87 | 1.19 ± 1.70 |
| Amyloid burden (PiB-PET composite SUVR)j | 2.64 ± 1.23 | 2.75 ± 1.32 | 2.62 ± 1.20 | 2.39 ± 1.12 |
| Amyloid burden (Centiloid) | 64.75 ± 51.87 | 66.56 ± 54.11 | 63.97 ± 49.46 | 51.57 ± 46.78 |
Plus-minus values are the mean ± s.d.
Fifty-two participants were randomized to the solanezumab arm; 2 did not have post-baseline data and were excluded from the modified intent-to-treat population.
Excludes 54 run-in participants previously enrolled in DIAN–OBS who transitioned to the DIAN–Tu-001 trial; therefore, their demographic data are included in the DIAN–Tu active groups.
APOE refers to presence of at least one ε4 allele of apolipoprotein E.
CDR scores range from 0 to 3, with higher scores indicating worse cognition and daily function.
Digit Symbol scores range from 0 to 93, with lower scores indicating poorer cognitive performance.
MMSE scores range from 0 to 30, with lower scores indicating poorer cognitive performance.
Logical Memory scores range from 0 to 25, with lower scores indicating poorer cognitive performance.
ISLT scores range from 0 to 12, with lower scores indicating poorer cognitive performance.
CDR-SB scores range from 0 to 18, with higher scores indicating worse cognition and daily function.
PiB-PET composite SuVR refers to brain amyloid burden measured by the average SuVR of cortical regions of interest (superior frontal, rostral middle frontal, superior temporal, middle temporal, lateral orbitofrontal, medial orbitofrontal and precuneus), assessed by PiB-PET. NA, not applicable.
Clinical and cognitive outcomes.
The primary outcome was a measure of cognition referred to as the DIAN Multivariate Cognitive End Point (DIAN–MCE)20.
A Bayesian multivariate cognitive disease progression model (DPM) was developed that (1) utilized the predictability of estimated years to clinical symptom onset (EYO)4 in DIAD and (2) assumed a proportional treatment effect across both asymptomatic and symptomatic stages of the disease20. The multivariate cognitive DPM evaluated components of the DIAN–MCE across EYO and estimated a single cognitive progression ratio (CPR) for each treatment compared to the dynamically pooled21 control group. A CPR = 1 indicated no treatment effect and a CPR < 1 indicated a beneficial treatment effect. Results were considered significant if the probability that CPR < 1 exceeded 0.981. The CPR for gantenerumab, compared to the pooled control group, had a posterior mean of 1.063; the probability of the CPR ratio being <1 was 0.144, indicating no treatment benefit. The CPR for solanezumab had a posterior mean of 1.255 and the probability of the CPR ratio being <1 was P < 0.0001, also indicating no treatment benefit.
Some model assumptions were not met and the model itself did not converge. These model assumptions included proportionality, monotonic decline over EYO and the same variance for both asymptomatic and symptomatic participants. Trial data showed (1) a lack of decline in asymptomatic participants (Supplementary Fig. 3), (2) the presence of learning effect in the asymptomatic group for one of the components (Logical Memory Delayed Recall Test (Logical Memory)) and (3) improvement in some of the four components over time. These observations did not meet model expectations for monotonically decreasing outcomes of the component end points or placebo behavior similar to natural history.
However, the overall conclusions were unchanged after evaluating sensitivity and exploratory analyses using mixed models for repeated measures (MMRM). The mean change from baseline for each component in the DIAN–MCE and for the secondary outcomes CDR-Sum of Boxes22 (CDR-SB) and Functional Assessment Scale (FAS)23 was estimated using the MMRM24 and no cognitive or clinical benefit was observed for either drug versus the shared placebo (Fig. 2 and Supplementary Table 1). Overall, the analyses indicate that there was no difference in cognitive decline between the gantenerumab and control groups and suggest a faster cognitive decline in the solanezumab group versus the control groups.
Fig. 2 |. Cognitive and clinical results.

Estimated mean change from baseline with 95% confidence intervals for treatment and pooled placebo groups using MMRM analyses. a, Digit Symbol scores range from 0 to 93, with lower scores indicating poorer cognitive performance. b, MMSE scores range from 0 to 30, with lower scores indicating poorer cognitive performance. c, Logical Memory scores range from 0 to 25, with lower scores indicating poorer cognitive performance. d, ISLT Delayed Recall scores range from 0 to 12, with lower scores indicating poorer cognitive performance. e, CDR-SB scores range from 0 to 18, with higher scores indicating worse cognition and daily function. f, FAS scores range from 0 to 30, with higher scores indicating worse instrumental activities of daily living. The sample sizes at yearly assessments are listed below the x axes. Mean values and statistics are shown in Supplementary Table 1. Note that the primary analysis used the DIAN–MCE. *P < 0.05 for solanezumab versus shared placebo.
Participants who were asymptomatic did not demonstrate cognitive decline over the trial duration, whereas participants who were symptomatic did (Supplementary Fig. 3). Cognitive performance on the Logical Memory test showed improvement, possibly due to a practice effect in the asymptomatic group. CDR-SB scores indicate that participants who were symptomatic across treatment groups had declined substantially by the time the drug doses were increased (CDR-SB mean score (s.d.) = 3.2 (1.9) at baseline versus 5.9 (4.4) at the start of dose escalation)).
Safety.
The safety profiles of gantenerumab and solanezumab were consistent with trials in sporadic AD. No new safety issues were identified for either drug. The adverse events (AEs) reported more frequently with gantenerumab or solanezumab than with the shared placebo are listed in Table 2.
Table 2 |.
AEs by treatment group
| Shared placebo n = 40 | Gantenerumab n = 52 | p a | |
|---|---|---|---|
| Gantenerumab and shared placebo | |||
| AEs (n (%)) | |||
| Injection site reactions | 18 (45) | 47 (90) | <0.0001 |
| Nasopharyngitis | 11 (28) | 20 (38) | 0.3738 |
| Back pain | 11 (28) | 16 (31) | 0.8192 |
| Contact dermatitis | 5 (13) | 10 (19) | 0.5703 |
| Nasal congestion | 4 (10) | 9 (17) | 0.3782 |
| Bronchitis | 5 (13) | 8 (15) | 0.7700 |
| Muscle spasms | <4 (<10) | 8 (15) | - |
| Sinusitis | 4 (10) | 8 (15) | 0.542 |
| Fall | <4 (<10) | 6 (12) | - |
| Oropharyngeal pain | <4 (<10) | 6 (12) | - |
| ARIA post-baseline (n (%)) | |||
| ARIA-E | 1 (3) | 10 (19) | 0.0205 |
| ARIA-Hb associated with ARIA-E (n (%)) | |||
| Microhemorrhage | 1 (3) | 5 (10) | 0.2277 |
| Superficial siderosis | 0 | 2 (4) | 0.5031 |
| ARIA-H not associated with ARIA-E (n (%)) | |||
| Microhemorrhage | 4 (10) | 13 (25) | 0.1028 |
| Superficial siderosis | 0 | 2 (4) | 0.5031 |
| Solanezumab and shared placebo | |||
| AEs (n (%)) | |||
| Headache | 20 (50) | 28 (54) | 0.8337 |
| Nasopharyngitis | 11 (28) | 18 (35) | 0.5049 |
| Post-lumbar puncture syndrome | 11 (28) | 17 (33) | 0.6522 |
| Back pain | 11 (28) | 15 (29) | >0.9999 |
| Sinusitis | 4 (10) | 13 (25) | 0.1028 |
| Influenza | 6 (15) | 12 (23) | 0.4298 |
| Insomnia | 4 (10) | 9 (17) | 0.3782 |
| Rhinorrhea | <4 (<10) | 9 (17) | - |
| Urinary tract infection | 6 (15) | 9 (17) | >0.9999 |
| Depression | 4 (10) | 6 (12) | >0.9999 |
| Pain in extremity | 4 (10) | 6 (12) | >0.9999 |
| Toothache | <4 (<10) | 6 (12) | - |
| ARIA post-baseline (n (%)) | |||
| ARIA-E | 1 (3) | 0 | 0.4348 |
| ARIA-H associated with ARIA-E (n (%)) | |||
| Microhemorrhage | 1 (3) | 0 | 0.4348 |
| Superficial siderosis | 0 | 0 | NA |
| ARIA-H not associated with ARIA-E (n (%)) | |||
| Microhemorrhage | 4 (10) | 6 (12) | >0.9999 |
| Superficial siderosis | 0 | 0 | NA |
Non-ARIA AEs that had an incidence >10% and were more frequent in the active treatment group are presented. AEs that occurred in fewer than 4 participants are listed as <4 to maintain blinding. The same AEs were also collected for mutation-negative participants but are not presented in this table. ARIA-E cases were identified on scheduled safety MRIs. ARIA refers to amyloid-related imaging abnormalities, ARIA-E to amyloid-related imaging abnormalities of cerebral edema and ARIA-H to amyloid-related imaging abnormalities of incident microhemorrhage, superficial siderosis or microhemorrhage. AE and ARIA categories are not mutually exclusive.
Two-sided P values using Fisher’s exact test; P values for AEs that listed placebo as <4 were not provided to maintain blinding.
ARIA due to haemosiderin deposition.
Amyloid-related imaging abnormalities-cerebral edema (ARIA-E) were observed in 19.2% of the gantenerumab group, 2.5% of the shared placebo group and 0% in the solanezumab group. The ARIA-E findings were mostly asymptomatic (8 out of 11); if symptoms occurred (3 out of 11), they were mild in nature (headache (1 out of 3), dizziness (1 out of 3) and balance disorder with ear pain (1 out of 3)) and resolved. The mean time for ARIA-E resolution was 85.5 d (s.d. = 54.3). ARIA-E events were managed by holding the dose and resuming at similar or lower doses, with most participants reaching the target dose.
Biological measures.
The primary outcomes of the original two-year biomarker study were changes in amyloid deposition measured by Pittsburgh compound-B positron emission tomography (PiB-PET) for gantenerumab and cerebrospinal fluid (CSF) total Aβ42 concentrations for solanezumab. Gantenerumab significantly reduced brain amyloid deposition assessed by PiB-PET compared to placebo at years 2 (P < 0.001) and 4 (P < 0.001) (Fig. 3 and Supplementary Table 2). The relative change from baseline resulted in a 9% between-group difference at year 2 (4% reduction with gantenerumab versus 5% increase with placebo). This effect was more pronounced at year 4, when a larger number of participants had experienced the higher 1,200 mg dose for a longer duration and the between-group difference was 24.3% (12.7% reduction with gantenerumab versus 11.6% increase with placebo). Although not powered to measure subgroup differences, noteworthy numerical differences were seen in biomarker changes in the symptomatic and asymptomatic groups. As defined by amyloid PET signal, but not neuropathology, the amount of amyloid plaque lowering after treatment with gantenerumab was similar in participants who were asymptomatic and symptomatic (Supplementary Fig. 4), but the relative change from baseline compared to shared placebo was larger in the asymptomatic group (33.3%: 15.9% reduction with gantenerumab versus 17.4% increase with placebo) compared to the symptomatic group (19.1%: 10.7% reduction with gantenerumab versus 8.4% increase with placebo).
Fig. 3 |. Key biomarker results.

Estimated mean change from baseline with 95% confidence intervals for the treatment and shared placebo groups using MMRM analyses. a, Estimated mean change from baseline in brain amyloid burden for gantenerumab measured by the average SuVR of cortical regions of interest (superior frontal, rostral middle frontal, superior temporal, middle temporal, lateral orbitofrontal, medial orbitofrontal and precuneus), assessed by PiB-PET. The transformation of SuVR to Centiloid is shown in Supplementary Fig. 4. b, Estimated mean change from baseline in CSF total Aβ42 (free + bound) for solanezumab. c,d, Estimated mean change from baseline in CSF phospho-tau181 for gantenerumab (c) and solanezumab (d), respectively. e,f, Estimated mean change from baseline in CSF total tau for gantenerumab (e) and solanezumab (f), respectively. g,h, Estimated mean change from baseline in CSF NfL (pg ml−1, log-transformed) for gantenerumab (g) and solanezumab (h), respectively. Sample sizes at yearly assessments are listed below the x axes. Mean values and statistics are shown in Supplementary Tables 2 and 3. Each drug group was compared to the shared placebo group independently using the MMRM model. *P < 0.05, **P < 0.01, ***P < 0.001.
Gantenerumab significantly increased CSF Aβ42 compared to placebo at year 4 (Supplementary Fig. 5, P < 0.001), with a between-group difference of 42.6% (19.3% increase with gantenerumab versus 23.3% reduction with placebo). Gantenerumab significantly reduced CSF total tau (P < 0.001) and phospho-tau181 (P < 0.001) at year 4, with a between-group difference of 20.6% (15.3% reduction with gantenerumab versus 5.3% increase with placebo) and 32.8% (23.4% reduction with gantenerumab versus 9.4% increase with placebo), respectively and significantly slowed increases in CSF neurofilament light chain (NfL) at year 4 (P < 0.05) with a between-group log difference of 2.2% (1.7% increase with gantenerumab versus 3.9% increase with placebo; the non-log-transformed difference was 5.8%, not significant; Fig. 3 and Supplementary Table 2).
Differences in downstream markers of tau pathology and neuronal injury between gantenerumab and placebo appeared to be larger in the asymptomatic group versus the symptomatic group (Supplementary Fig. 6) but were not statistically significant: total tau (35.8 versus 11.0% in the asymptomatic and symptomatic groups, respectively); phospho-tau181 (50.2 versus 23.2%); and log-transformed CSF NfL (2.8 versus 1.2%).
Solanezumab significantly increased total CSF Aβ42 at years 2 (P < 0.001) and 4 (P < 0.001) compared to shared placebo, with between-group differences of 86.6% (93.3% increase with solanezumab versus 6.7% increase with placebo) and 200.5% (200.5% increase with solanezumab versus 0% increase with placebo), respectively (Fig. 3 and Supplementary Table 3). Log-transformed CSF NfL increased significantly more in the solanezumab group than in the shared placebo group at year 4 (P = 0.005); solanezumab did not have significant effects on amyloid PET, total tau or phospho-tau181. There were no notable differences between asymptomatic and symptomatic groups (Supplementary Fig. 7).
There were no significant between-group differences in biological measures of brain cortical metabolism 18F-FDG-PET or atrophy (precuneus thickness and hippocampal volume by volumetric magnetic resonance imaging (MRI) for either drug group compared to placebo (Supplementary Fig. 8)).
Discussion
The DIAN–TU trial developed an innovative trial platform and successfully recruited participants with a rare form of AD to test two anti-amyloid antibodies using a shared placebo group that was augmented by historical control data. This AD prevention trial was designed to adapt to new findings and incorporate new biomarkers16,17. Midway through the trial, the study transitioned from a two-year biomarker trial to a common close 4 year plus trial to determine the potential for cognitive benefits, increase the exposure and duration of drug treatment and assess prolonged drug effects on biomarkers. The dose of both drugs was also increased during the study based on external results from trials in sporadic AD. However, the first dose escalation for both drugs occurred relatively late, which limited exposure to higher doses.
The results indicate that under these trial conditions, neither drug demonstrated a beneficial effect on cognitive measures compared to the control group, but both drugs engaged their Aβ targets (fibrillar Aβ for gantenerumab and soluble Aβ for solanezumab), as evidenced by reductions in cortical amyloid by PET for gantenerumab and elevations in CSF total Aβ42 for solanezumab. While the trial was not powered to detect statistically significant subgroup differences, exploratory analyses investigating the effects of drug dose and stage of disease suggest potentially larger impacts of higher doses at earlier stages of disease.
The faster cognitive decline observed with solanezumab relative to the pooled control groups conflicts with the safety data and trend toward the clinical and cognitive benefits seen in three large phase 3 solanezumab trials in sporadic AD25,26. We believe the decline in cognitive scores is most likely related to the small sample size in the DIAN–TU-001 study, causing a chance selection of more rapid progressors in the solanezumab arm or slower progressors in the placebo group. Alternatively, solanezumab may not be effective in DIAD compared to previous sporadic AD studies.
Given the small sample sizes and the late increase in dose of both drugs during the trial, we were unable to determine whether these higher doses would have resulted in cognitive benefit in participants who were symptomatic. In addition, because cognitive decline was not observed in the asymptomatic subgroup, the results were not conclusive regarding treatment effects in this population (Supplementary Fig. 3). This also caused a substantial reduction in the power to detect an overall treatment effect. Because of the observed, limited cognitive decline of the placebo group, the actual power of this trial was only 8% to detect a 30% slowing of decline by MMRM and only 5% and 18% for the asymptomatic and symptomatic subgroups respectively.
These findings suggest several considerations for future DIAD trials, such as larger sample sizes, longer treatment duration for participants who are asymptomatic, a narrower range of baseline disease severity and cognitive measures less susceptible to practice effects and more sensitive to change in the earlier stages of disease progression27–29. Therefore, the DIAN–TU platform is implementing several changes to upcoming trials in the DIAN–TU cohort. These include reducing the range of disease stages for participants to be enrolled in future trials (primary prevention EYO −25 to −10; secondary prevention EYO −10 to +10; Supplementary Fig. 9), separate assignment of participants to treatment arms based on disease stage (for example, asymptomatic CDR = 0 versus symptomatic CDR > 0), and for the tau NexGen arms, Aβ+/tau PET+ and Aβ+/tau PET− will be analyzed independently.
No unexpected safety signals were observed and 76% of randomized participants completed the four-year placebo-controlled period. Participants in the solanezumab active arm, which targets soluble Aβ, had no incidence of ARIA-E, which is consistent with results in sporadic AD26. As expected with plaque-binding anti-amyloid antibodies, gantenerumab was associated with ARIA-E, which was usually radiographically mild, asymptomatic and reversible.
Solanezumab increased CSF total Aβ42 levels but had no effect on amyloid PET, CSF total tau or phospho-tau 181. The solanezumab group had significantly higher NfL levels compared to placebo at 4 years, which was directionally consistent with increased cognitive decline. These unexpected results may be the result of undetected imbalance in arms due to small sample sizes, the drug worsening progression in DIAD or another unknown factor.
Gantenerumab reduced amyloid plaques in a dose-dependent fashion. However, the amount of amyloid plaque lowering after treatment with gantenerumab at 4 years (PiB-PET standardized uptake value ratio (SUVR) 2.25 versus 2.62 at baseline; Centiloid 43.9 versus 64.7 at baseline) was above what would be considered a typical threshold of amyloid positivity (PiB-PET SUVR < 1.42, Centiloid < 33.8)30,31. This magnitude of effect differs from studies of gantenerumab in sporadic AD32, where treatment over 36 months after starting up-titration substantially lowered PiB-PET to below the level of amyloid positivity. Given that previous imaging and neuropathological work suggested that pathology accumulates faster and to a greater extent in DIAD than sporadic AD, higher doses and longer duration of treatment may be needed to reduce amyloid to an amyloid-negative level in the DIAD population33–35. However, participants in the DIAN–TU-001 initially received one-fifth of the final dose and did not start dose titration until midway through the study.
The effect of gantenerumab on plaque reduction and downstream biomarkers (that is, decreased CSF measures of tau and neurodegeneration), especially in participants who were asymptomatic, suggests the possibility that removal of amyloid plaques may be a viable strategy in preventing or slowing the biological progression of AD. While intriguing, this hypothesis should be tested in participants who are asymptomatic for a longer duration at the target dose. The less pronounced effect in participants who were symptomatic could be due to the duration of existing pathology in the brain, the amount of pathology present when treatment began or the presence of other pathologies, such as tauopathy. However, recent findings25,26,36–38 suggested clinical benefit in early symptomatic patients. Nevertheless, it is still controversial whether removing amyloid will provide a meaningful clinical benefit.
These results raise key questions for future study design. Would early aggressive reduction of amyloid plaques produce an even stronger effect on downstream AD processes? Would continued removal of amyloid plaques lead to clinical benefits, especially in cognitively normal mutation carriers close to their expected age of onset? Is reversing amyloid pathology, soluble tau and neurodegeneration markers a valid strategy to slow or delay clinical onset and progression? Some of these questions may be addressed by the ongoing exploratory open-label extension of the DIAN–TU-001 study with gantenerumab, while additional trials may address others.
DIAN–TU-001 is the first global treatment trial in DIAD and the first AD prevention trial to test amyloid-targeting drugs. The trial had excellent recruitment and retention due to the dedication of the DIAD participants and families, researchers and medical providers. This adaptive multi-drug multi-arm platform trial was supported by a strong public-private partnership of the National Institutes of Health (NIH), industry, advocacy groups and phil-anthropic organizations. The success of the DIAN–TU platform in completing the first drug arms and continuing ongoing arms demonstrates the potential for informative global platform trials, even with rare diseases (Supplementary Fig. 9)39. Finally, since several prevention trials in sporadic AD are now underway or planned, the results from the DIAN–TU-001 study have provided critical insight regarding the need for better and more sensitive cognitive measures in asymptomatic populations and the use of higher doses for longer periods to maximize target engagement. Although no cognitive or clinical benefit was observed, improvements in downstream biomarkers in participants treated with gantenerumab support the possibility of preventing or slowing the biological progression of AD via amyloid lowering, especially at the earlier stages of the disease.
Methods
Study participants.
Participants were referred from the DIAN Expanded Registry (DIAN–EXR), DIAN–OBS, DIAN–TU and partner sites. Eligibility criteria included participants known to have or at-risk for a DIAD mutation, between 15 years before to 10 years after the expected age of symptom onset4 and a CDR of 0 (cognitively normal) or 0.5–1 (early dementia)18. Participants could choose to remain blinded to their mutation status; mutation noncarriers were assigned to placebo and not included in prespecified analyses. DIAD mutation carriers were randomized 3:1 to active or placebo with a minimization procedure (Supplementary Table 4)40. All study personnel, sponsors and participants were blinded to active or placebo assignment but not to study drug arm. Data from participants in the DIAN–OBS study6,7 who met the DIAN–TU inclusion criteria were used as natural history controls for improved estimates of the placebo group19. The DIAN–OBS and DIAN–TU studies have similar protocols, including cognitive, clinical, imaging and biomarker measures. The trial registration number is NCT01760005.
Study design.
DIAN–TU-001 was conducted at 25 sites in 7 countries from December 2012 through to November 2019 (Supplementary Fig. 10). Investigators are listed in the Supplementary Information. Cognitive outcomes were assessed every 6 months, clinical outcomes annually and biomarkers at baseline and years 1, 2 and 4. A common close design ensured that double-blind treatment continued for all participants until the last participant reached 4 years. Based on the results of concurrent phase 2 and 3 trials in sporadic AD26,41, target drug doses were increased approximately midway or later through the study. Gantenerumab was increased from 225 mg (subcutaneously, every 4 weeks) to 1,200 mg in 2016. Solanezumab was increased from 400 mg (intravenously, every 4 weeks) to 1,600 mg in 2017 (Supplementary Fig. 2). To reduce participant burden, home health personnel administered most infusions16.
Primary and secondary outcomes.
The primary outcomes of the original two-year biomarker study were changes in amyloid deposition measured by PiB-PET for gantenerumab and CSF total Aβ42 concentrations for solanezumab. The primary outcome after transition to a four-year common close cognitive study was a multivariate measure of cognition referred to as the DIAN–MCE20. Rather than a composite score, which comprises the average of the component z-scores, the DIAN–MCE allows the use of a multivariate approach to model each component simultaneously. By modeling the four measures separately, the multivariate model provides substantially more precise measures of the effect of the treatment. This improved precision is based on two important factors: (1) averaging the four measures to a single composite measure loses precision compared to modeling them separately; and (2) the rate of decline of each of the four measures is different over the wide range of disease stages, so by modeling the four measures separately in the multivariate approach, each measure can contribute more effectively to the different stages of the disease.
The DIAN–MCE includes the Wechsler Memory Scale-Revised Logical Memory42, the Wechsler Adult Intelligence Scale Digit Symbol Substitution Test (Digit Symbol)43, the International Shopping List Test (ISLT) Delayed Recall score44,45 and the Mini-Mental State Examination (MMSE)46.
Secondary outcomes included the CDR-SB22 and FAS23. Biomarker outcomes were PiB-PET, 18F-fluorodeoxyglucose (FDG)-PET, volumetric MRI, CSF total Aβ, CSF total tau, CSF phospho-tau181 and CSF NfL.
Imaging methods.
MRI was performed using the Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocol. T1-weighted images (1.1 × 1.1 × 1.2 mm voxels) were acquired for all participants on a 3T MRI scanner. The ADNI Imaging Core screened images for protocol compliance, artifacts and ARIA47. Volumetric segmentation and cortical surface reconstruction were done using FreeSurfer v.5.3 (refs.48,49) to define cortical and subcortical regions of interest (ROIs). Segmentations were visually inspected by members of the DIAN–TU Imaging Core and edited as needed. Subcortical volumes were corrected for intracranial volume using a regression approach50. Cortical thickness was averaged and volume measures were summed across the hemispheres.
ROIs defined by FreeSurfer on the MRI scans were used for the regional processing of all PET data. Aβ imaging was performed using the 11C-Pittsburgh compound-B. Data from the 40–70-min postinjection window were converted to regional SUVRs relative to the cerebellar gray matter51 (https://github.com/ysu001/PUP). A composite to represent a global measure of Aβ was calculated using the averaged SUVR values in the lateral orbitofrontal, medial orbitofrontal, precuneus, rostral middle frontal, superior frontal, superior temporal and middle temporal regions. Metabolic imaging was performed with 18F-FDG with data from the 40–60-min postinjection window converted to SUVRs relative to cerebellar gray matter. Both types of PET data were partial volume-corrected using a regional spread function technique51,52.
Fluid biomarker methods.
CSF collection.
CSF for biomarker analysis was collected via lumbar puncture at DIAN–TU host sites under fasting conditions using a 22-G atraumatic Sprotte spinal needle. CSF was collected into a single 50-ml polypropylene tube via gravity drip methods, except when fluoroscopy was required. Baseline lumbar punctures were performed as close to 8:00 local time as possible. Subsequent lumbar punctures were performed as close to the baseline lumbar puncture time as possible. Collection was performed at baseline visit (V2) and at the week 52 (V15), 104 (V28) and 208 (V54) visits; samples were shipped overnight on dry ice to the central laboratory on the day of collection.
CSF processing.
Frozen bulk CSF samples were shipped to the DIAN–TU Biomarker Core laboratory at Washington University monthly, whereupon they were stored at −80 °C for a minimum of 48 h before thawing on wet ice and aliquoting into 2.0-ml polypropylene tubes in 500 μl volumes. Aliquots were flash-frozen upright on dry ice and stored at −80 °C until biomarker analysis.
Fluid biomarker measurements.
Samples underwent two freeze–thaw cycles before analysis for all analytes. For both participants taking solanezumab and gantenerumab, CSF total tau and phospho-tau181 concentrations were measured using validated, automated LUMIPULSE G1200 methods. Validated LUMIPULSE G1200 chemiluminescence enzyme immunoassay methods were used to determine Aβ1–40 and Aβ1–42 concentrations in participants taking gantenerumab. CSF total Aβ1–40 and Aβ1–42 concentrations in participants taking solanezumab were determined using validated proprietary immunoassay methods as described previously53. Concentrations of NfL for all participants were determined using a validated Quanterix single molecule array method.
Safety measures.
Safety assessments included AEs, routine laboratory assessments, physical examinations including electrocardiogram and MRIs for ARIA.
Statistical analysis.
The primary analysis population included DIAN–TU-001 participants who had at least one baseline and post-baseline assessment for the same cognitive test and used a pooled control group (gantenerumab and solanezumab shared placebo with dynamic borrowing21 of DIAN–OBS data). The use of a pooled placebo approach was planned a priori and aimed at determining each drug’s treatment effect while maximizing the number of participants on active drug (3:1) and incorporating natural history data. The eligibility of DIAN–OBS participant data was determined using the DIAN–TU-001 inclusion/exclusion criteria.
A Bayesian multivariate cognitive DPM was developed that (1) utilized the predictability of EYO4 in DIAD and (2) assumed a proportional treatment effect across both asymptomatic and symptomatic stages of disease20. The multivariate cognitive DPM evaluated components of the DIAN–MCE across the EYO and estimated a single CPR for each treatment compared to the dynamically pooled21 control group. A CPR = 1 indicated no treatment effect and a CPR < 1 indicated a beneficial treatment effect. Results were considered significant if the probability that CPR < 1 exceeded 0.981, which was found via simulation to ensure that each drug had a 2.5% one-sided type I error rate. Per protocol, the drugs were compared to controls but not to each other. Missing values in the DIAN–MCE were treated as unknown variables in the modeling54. All early discontinuations were treated as missing at random. These analyses used computational packages built using the Fortran programming language. Secondary outcomes were analyzed by MMRM24 using the SAS software (v.9.2 or higher).
Under a set of model assumptions and through simulation, we estimated that a sample size of 52 participants on active drug and 103 participants in the pooled control group (34 shared placebo plus 69 DIAN–OBS) would provide >95% power to detect a 30% attenuation of cognitive decline over 4 years.
Study oversight.
The study was conducted in accordance with the Declaration of Helsinki (version 7) and the International Conference on Harmonization and Good Clinical Practice guidelines and had ethics committee approval at each participating site. All participants provided written informed consent.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary Material
Acknowledgements
We gratefully acknowledge the outstanding commitment of the participants, family members and caregivers whose participation was critical to the success of the DIAN–TU trial. We thank the DIAN–TU Funding and Study Team (https://dian.wustl.edu/our-research/clinical-trial/funding/) for their exceptional dedication and accomplishments, which ensured the success of the trial. We thank the DIAN–EXR and DIAN–OBS study teams for their support on recruitment and commitment to family members. We acknowledge the robust intellectual collaboration between the DIAN–TU investigators, participants and family members, F. Hoffmann-La Roche, Ltd/Genentech and Eli Lilly and Company, the DIAN–TU Pharma Consortium (https://dian.wustl.edu/our-research/the-pharma-consortium/), the NIH, and regulatory representatives who were critical in making this study possible. We thank the Alzheimer’s Association, GHR Foundation, an anonymous organization, other industry partners (Avid Radiopharmaceuticals, a wholly-owned subsidiary of Eli Lilly and Company, Signant Health and Cogstate) and regulatory representatives for their support. We also thank L. Ryan from the National Institute on Aging (NIA) for her key contributions in leadership and scientific guidance on this project. The research reported in this publication was supported by the NIA of the NIH under award nos. U01AG042791, U01AG042791-S1 (FNIH and Accelerating Medicines Partnership), R01AG046179 and R01AG053267-S1. This research was also supported by the Alzheimer’s Association, Eli Lilly and Company, F. Hoffman-LaRoche Ltd, Avid Radiopharmaceuticals GHR Foundation and an anonymous organization. Cogstate and Signant Health offered in-kind support. The DIAN–OBS was supported by the NIA of the NIH (DIAN, U19AG032438), the German Center for Neurodegenerative Diseases, Raúl Carrea Institute for Neurological Research, partial support by the Research and Development Grants for Dementia from the Japan Agency for Medical Research and Development (AMED) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute.
Competing interests
S.S. was the Project Arm Leader for the gantenerumab arm. He also receives research support and is a consultant to Eisai, Novartis, Genentech, F. Hoffmann-La Roche Ltd, GemVax, Avid Radiopharmaceuticals and Eli Lilly and Company. M. Farlow was the Project Arm Leader for the solanezumab arm. He receives research support and is consultant to Eli Lilly and Company, Eisai, Novartis, Green Valley, AbbVie, Biogen, Eisai, F. Hoffmann-La Roche Ltd, Suvan, vTv Therapeutics, Vaccinex, QR Pharma, Avanir Pharmaceuticals, AZTherapies, Cerecin, Cognition Therapeutics, Cortexyme, Longeveron, Otsuka Pharmaceutical, Samumed and Takeda. A.M.F. is the Biomarker Core Leader of DIAN–TU. She is a member of the scientific advisory boards (SABs) for Roche Diagnostics, Genentech and AbbVie and also consults for Araclon/Grifols, DiademRes, DiamiR and Otsuka Pharmaceuticals. T.L.S.B. has investigator-initiated research funding from the NIH, the Alzheimer’s Association, the Barnes-Jewish Hospital Foundation and Avid Radiopharmaceuticals. She participates as a site investigator in clinical trials sponsored by Avid Radiopharmaceuticals, Eli Lilly and Company, Biogen, Eisai, Janssen and F. Hoffmann-La Roche Ltd. She serves as an unpaid consultant to Eisai and Siemens. She is on the Speaker’s Bureau for Biogen. J.C.M. is the Friedman Distinguished Professor of Neurology, Director of the Charles F. and Joanne Knight Alzheimer’s Disease Research Center, Associate Director of DIAN and Founding Principal Investigator of DIAN. He is funded by NIH grant nos. P30 AG066444, P01AG003991, P01AG026276, U19 AG032438 and U19 AG024904. Neither J.C.M. nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. J.H. is an employee of Hitchcock Regulatory Consulting and has consulted for Acumen Pharmaceuticals, Axon Advisors, Critical Path Institute, Gerson Lehrman Group, H. Lundbeck, High Lantern Group, Regenera Pharma Ltd, UCB Biopharma SPRL, United Neuroscience, Vaccinex and Washington University. She is retired from Eli Lilly and Company and is an Eli Lilly and Company shareholder. E.M. is the Associate Director of the DIAN–TU. He reports serving on a Data Safety Committee for Eli Lilly and Company and Alector. He is scientific consultant for Eisai and Eli Lilly and Company and has received institutional grant support from Eli Lilly and Company, F. Hoffmann-La Roche Ltd. and Janssen. D.B.C. is Medical Director of DIAN–TU and serves as scientific consultant to Biogen, Takeda, Millennium, Genzyme, Amgen, F. Hoffmann-La Roche Ltd/Genentech, GlaxoSmithKline, Serono, Inhibikase Therapeutics, Dr Reddy’s Laboratories, Bristol Myers Squibb, Atara, Mitsubishi Tanabe Pharma, Excision BioTherapeutics, UpToDate and Wolters Kluwer. He serves on the Data and Safety Monitoring Board (DSMB)/Data Monitoring Committees for F. Hoffmann-La Roche Ltd/Genentech, Wave, EMD Serono, Shire, Pfizer and Sanofi. He has carried out legal consulting for Cook County, State Farm, Wilke & Wilke PC, Shevlin Smith and Sal Indomenico & Associates PC. He receives research support from the NIH National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, National Institute of Allergy and Infectious Diseases, National Center for Advancing Translational Sciences and NIA. A.J.A. has served as a consultant for Biogen and H. Lundbeck. J.H. is a paid consultant for F. Hoffmann-La Roche Ltd, Takeda and H. Lundbeck and is on the Data Safety and Monitoring Board for Eisai. C.R.J. Jr serves on an independent data monitoring board for F. Hoffmann-La Roche Ltd, has consulted for and served as a speaker for Eisai and consulted for Biogen, but he receives no personal compensation from any commercial entity. He receives research support from the NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. C.C. receives research support from Biogen, Eisai, Alector and Parabon. C.C. is a member of the advisory board of Vivid Genetics, Halia Therapeutics and ADx Healthcare. C.M. is a consultant for Biogen. L.S.H. is a consultant for Cortexyme, Eisai, Eli Lilly and Company, Medscape and Prevail. He receives research grant support from Abbvie, Alector, Biogen, Genentech, Eli Lilly and Company and F. Hoffmann-La Roche Ltd. J.P. is on the Speakers’ Bureau for Biogen. E.D.R. is a consultant for Biogen, Applied Genetic Technologies Corporation and AVROBIO, receives funding from Alector and is the owner of intellectual property related to tau. S.G. is a member of the SAB for Alzheon, Biogen, Eli Lilly and Company and TauRx and a member of the Data Safety Monitoring Board for Alzheimer’s Disease Cooperative Study, ATRI and Banner Health. C.H.V.D. has served as a consultant for F. Hoffmann-La Roche Ltd and Eisai and received grant support for clinical trials from F. Hoffmann-La Roche Ltd, Eli Lilly and Company, Biogen, Genetech, Merck, Janssen, Eisai, Novartis and Biohaven Pharmaceuticals. R.S.V. served on a SAB for Ionis Pharmaceuticals. B.D. receives research support from F. Hoffmann-La Roche Ltd. M.M. is a consultant to Arkuda Therapeutics, Ionis and Alector and receives research funding from F. Hoffmann-La Roche Ltd, Novartis and Alector. D.R.G. is a consultant to Biogen, Esai, Fujirebio and Amprion and is on the DSMB for Cognition Therapeutics. G.R.H. has received research support as a clinical trial site investigator for Anavax, Biogen and F. Hoffmann-La Roche Ltd. He has received research funding from the Canadian Institutes of Health Research, Alzheimer Society of Canada and the NIH. P.S.A. collaborates with Eli Lilly and Company and F. Hoffmann-La Roche Ltd on drug development in AD and has a research grant from Eli Lilly and Company. M.B., P.D., R.S.D., P.F., C.G. and G.A.K. are employees and shareholders of F. Hoffmann-La Roche Ltd. S.W.A., K.C.H., M.A.M., J.R.S. and R.Y. are employees and shareholders of Eli Lilly and Company. R.J.B. is Director of DIAN–TU and Principal Investigator of DIAN–TU-001. He receives research support from the NIA of the NIH, DIAN–TU trial pharmaceutical partners (Eli Lilly and Company, F. Hoffman-La Roche Ltd and Avid Radiopharmaceuticals), Alzheimer’s Association, GHR Foundation, Anonymous Organization, DIAN–TU Pharma Consortium (active: Biogen, Eisai, Eli Lilly and Company, Janssen, F. Hoffmann-La Roche Ltd/Genentech; previous: AbbVie, Amgen, AstraZeneca, Forum, Mithridion, Novartis, Pfizer, Sanofi, United Neuroscience). He has been an invited speaker and consultant for AC Immune, F. Hoffman-La Roche Ltd and Janssen and a consultant for Amgen and Eisai. D.H., the Department Head of Neurology where the research is being conducted, is an inventor on patents for solanezumab, currently being tested in the DIAN–TU clinical trials. If solanezumab is approved as a treatment for AD or dominantly inherited AD, Washington University and D.H. will receive part of the net sales of solanezumab from Eli Lilly and Company, which has licensed patents related to solanezumab from Washington University. The funders of the study had no role in the collection, analysis or interpretation of the data, the writing of the report or in the decision to submit the paper for publication. S.B.B., S.M.B., W.S.B., K.A.C., D.H., B.A.G., J.J.L., J.J.L.G., M. Formaglio, S.J., R.C., Y.L., R.K., I.Z.J.V., S.L.M., G.M.S., C.X., D.W. and J.R.B. do not declare any competing interests.
Footnotes
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41591-021-01369-8.
Code availability
All code for data cleaning and analysis associated with the current submission is available upon request to the corresponding author and is provided as part of the replication package.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41591-021-01369-8.
Data availability
Data access to the DIAN–TU trial data will follow the policies of the DIAN–TU data access policy55, which complies with the guidelines established by the Collaboration for Alzheimer’s Prevention. Patient-related data not included in the paper were generated as part of a clinical trial and may be subject to patient confidentiality. Any data and materials that can be shared will be released via a data/material sharing agreement. Requests to access the DIAN–TU-001 trial data can be made at https://dian.wustl.edu/our-research/for-investigators/diantu-investigator-resources/. All code for data cleaning and analysis associated with the current submission is available upon request to the corresponding author and is provided as part of the replication package.
References
- 1.Jack CR Jr. et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braak H, Thal DR, Ghebremedhin E & Del Tredici K Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol 70, 960–969 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Bateman RJ et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res. Ther 3, 1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryman DC et al. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology 83, 253–260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris JC et al. Developing an international network for Alzheimer’s research: the Dominantly Inherited Alzheimer Network. Clin. Investig. (Lond.) 2, 975–984 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman RJ et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med 367, 795–804 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDade E et al. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology 91, e1295–e1306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon BA et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: a longitudinal study. Lancet Neurol 17, 241–250 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sevigny J et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Selkoe D β-secretase inhibitors for Alzheimer’s disease: heading in the wrong direction? Lancet Neurol 18, 624–626 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Henley D et al. Preliminary results of a trial of atabecestat in preclinical Alzheimer’s disease. N. Engl. J. Med 380, 1483–1485 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Egan MF et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N. Engl. J. Med 380, 1408–1420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karran E & De Strooper B The amyloid cascade hypothesis: are we poised for success or failure? J. Neurochem 139, 237–252 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Cummings JL, Morstorf T & Zhong K Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res. Ther 6, 37 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moulder KL et al. Dominantly Inherited Alzheimer Network: facilitating research and clinical trials. Alzheimers Res. Ther 5, 48 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills SM et al. Preclinical trials in autosomal dominant AD: Implementation of the DIAN-TU trial. Rev. Neurol. (Paris) 169, 737–743 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bateman RJ et al. The DIAN-TU Next Generation Alzheimer’s prevention trial: adaptive design and disease progression model. Alzheimers Dement 13, 8–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JC The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414 (1993). [DOI] [PubMed] [Google Scholar]
- 19.Lim J et al. Reducing patient burden in clinical trials through the use of historical controls: appropriate selection of historical data to minimize risk of bias. Ther. Innov. Regul. Sci 54, 850–860 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Wang G et al. A novel cognitive disease progression model for clinical trials in autosomal-dominant Alzheimer’s disease. Stat. Med 37, 3047–3055 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viele K et al. Use of historical control data for assessing treatment effects in clinical trials. Pharm. Stat 13, 41–54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg L et al. Mild senile dementia of the Alzheimer type: 2. longitudinal assessment. Ann. Neurol 23, 477–484 (1988). [DOI] [PubMed] [Google Scholar]
- 23.Breines E The functional assessment scale as an instrument for measuring changes in levels of function of nursing home residents following occupational therapy. Can. J. Occup. Ther 55, 135–140 (1988). [Google Scholar]
- 24.Fitzmaurice GM, Laird NM & Ware JH Applied Longitudinal Analysis (John Wiley & Sons, 2012). [Google Scholar]
- 25.Siemers ER et al. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer’s disease patients. Alzheimers Dement 12, 110–120 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Honig LS et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N. Engl. J. Med 378, 321–330 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Cummings J et al. Drug development in Alzheimer’s disease: the path to 2025. Alzheimers Res. Ther 8, 39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cummings J Lessons learned from Alzheimer disease: clinical trials with negative outcomes. Clin. Transl. Sci 11, 147–152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDade E & Bateman RJ Stop Alzheimer’s before it starts. Nature 547, 153–155 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Su Y et al. Utilizing the Centiloid scale in cross-sectional and longitudinal PiB PET studies. Neuroimage Clin 19, 406–416 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlassenko AG et al. Imaging and cerebrospinal fluid biomarkers in early preclinical Alzheimer disease. Ann. Neurol 80, 379–387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein G et al. Thirty-six-month amyloid positron emission tomography results show continued reduction in amyloid burden with subcutaneous gantenerumab. J. Prev. Alzheimers Dis 8, 3–6 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Shepherd C, McCann H & Halliday GM Variations in the neuropathology of familial Alzheimer’s disease. Acta Neuropathol 118, 37–52 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Ringman JM et al. Neuropathology of autosomal dominant Alzheimer disease in the national Alzheimer coordinating center database. J. Neuropathol. Exp. Neurol 75, 284–290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon BA et al. Tau PET in autosomal dominant Alzheimer’s disease: relationship with cognition, dementia and other biomarkers. Brain 142, 1063–1076 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mintun MA et al. Donanemab in early Alzheimer’s disease. N. Engl. J. Med 384, 1691–1704 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Haeberlein SB et al. Emerge and Engage topline results: phase 3 studies of aducanumab in early Alzheimer’s disease. Alzheimers Dement 16, e047259 (2020). [Google Scholar]
- 38.Schuck EL et al. Population pharmacokinetic/pharmacodynamic analyses of BAN2401 in patients with early Alzheimer’s disease: correlation of BAN2401 exposure, pet standard uptake value ratio, and cognitive outcomes. Alzheimers Dement 15, P1582–P1583 (2019). [Google Scholar]
- 39.Woodcock J & LaVange LM Master protocols to study multiple therapies, multiple diseases, or both. N. Engl. J. Med 377, 62–70 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Taves DR Minimization: a new method of assigning patients to treatment and control groups. Clin. Pharmacol. Ther 15, 443–453 (1974). [DOI] [PubMed] [Google Scholar]
- 41.Ostrowitzki S et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res. Ther 9, 95 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wechsler D WMS-R: Wechsler Memory Scale–Revised: manual (Psychological Corporation, 1987). [Google Scholar]
- 43.Wechsler D Wechsler Adult Intelligence Scale–Revised (Psychological Corporation, 1981). [Google Scholar]
- 44.Lim YY et al. A method for cross-cultural adaptation of a verbal memory assessment. Behav. Res. Methods 41, 1190–1200 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Thompson TAC et al. Sensitivity and test–retest reliability of the International Shopping List Test in assessing verbal learning and memory in mild Alzheimer’s disease. Arch. Clin. Neuropsychol 26, 412–424 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Folstein MF, Folstein SE & McHugh PR “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12, 189–198 (1975). [DOI] [PubMed] [Google Scholar]
- 47.Sperling R et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol 11, 241–249 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischl B FreeSurfer. Neuroimage 62, 774–781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischl B et al. Automatically parcellating the human cerebral cortex. Cereb. Cortex 14, 11–22 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Buckner RL et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage 23, 724–738 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Su Y et al. Partial volume correction in quantitative amyloid imaging. Neuroimage 107, 55–64 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rousset OG, Ma Y & Correction AC for partial volume effects in PET: principle and validation. J. Nucl. Med 39, 904–911 (1998). [PubMed] [Google Scholar]
- 53.Farlow M et al. Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement 8, 261–271 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Robert CP & Casella G Monte Carlo Statistical Methods (Springer, 2004). [Google Scholar]
- 55.Weninger S et al. Collaboration for Alzheimer’s prevention: principles to guide data and sample sharing in preclinical Alzheimer’s disease trials. Alzheimers Dement 12, 631–632 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data access to the DIAN–TU trial data will follow the policies of the DIAN–TU data access policy55, which complies with the guidelines established by the Collaboration for Alzheimer’s Prevention. Patient-related data not included in the paper were generated as part of a clinical trial and may be subject to patient confidentiality. Any data and materials that can be shared will be released via a data/material sharing agreement. Requests to access the DIAN–TU-001 trial data can be made at https://dian.wustl.edu/our-research/for-investigators/diantu-investigator-resources/. All code for data cleaning and analysis associated with the current submission is available upon request to the corresponding author and is provided as part of the replication package.


