Abstract
Chronic obstructive pulmonary disease (COPD) is one of the major diseases threatening human life and health. According to the report released by the World Health Organization (WHO) in 2020, COPD has become the third leading cause of death in the world, featuring a sustainable growth of incidence rate as well as population age. The purpose of this review focuses on the advancement of bioactive natural compounds, such as baicalin, quercetin, resveratrol, and curcumin, which demonstrate promising therapeutic/interventional effects on CODP in vitro and in vivo. Information emphasizing on COPD was systematically collected from several authoritative internet databases including Web of Science, PubMed, Elsevier, Wiley Online Library, and Europe PMC, with a combination of keywords containing “COPD” and “natural small molecular compounds”. The new evidence indicated that these valuable molecules featured unique functions in the treatment of COPD through various biological processes such as anti-inflammatory, anti-oxidant, anti-apoptosis, and anti-airway fibrosis. Moreover, we found that the promising effects of these natural compounds on COPD were mainly achieved through JAK3/STAT3/NF-κB and MAPK inflammatory signaling pathways, Nrf2 oxidative stress signaling pathway, and TGF-β1/Smad 2/3 fibrosis signaling pathway, which referenced to multiple targets like TNF-α, IL-6, IL-8, TIMP-1, MMP, AKT, JAK3, IKK, PI3K, HO-1, MAPK, P38, ERK, etc. Current challenges and future directions in this promising field are also discussed at the end of this review. For the convenience of the readers, this review is divided into ten parts according to the structures of potential natural small molecular compounds. We hope that this review brings a quick look and provides some inspiration for the research of COPD.
Keywords: chronic obstructive pulmonary disease, natural compounds, flavonoids, polyphenol, alkaloid
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of mortality worldwide characterized by bronchitic and emphysematous components (Vogelmeier et al., 2020; Radicioni et al., 2021). The epidemiological survey of COPD shows that the prevalence of COPD in Spain has rapidly risen from 10.2 to 12.4%, and the proportion of men and women increases with age (Miravitlles et al., 2009). While there are about 175 million people around the world suffering from COPD, and the expenses for COPD-related treatment are as high as tens of billions of dollars every year. Only in the United States, the direct expenditure on COPD treatment in 2010 was $32 billion (Guarascio et al., 2013). It is generally believed that COPD is a series of pathophysiologic changes caused by inhaling pollutants (mainly cigarette smoke) or pathogens such as Haemophilus influenzae, Moraxella catalhalis, and Streptococcus pneumoniae (Leung et al., 2017; Szucs et al., 2019). The former can lead to airway inflammation by activating lung epithelium and inflammatory cells, while the latter can trigger pathogen-associated molecular patterns through pattern recognition receptors expressed on epithelial cells and innate immune cells, and activate nuclear factors κB (NF-κB), mitogen activated protein kinase (MAPK), phosphatidylinositol-3-kinase (PI3K), and IFN regulator signal, which lead to the production of pro-inflammatory mediators such as cytokines and chemokines, and cause a sustained harmful immune response (Sethi et al., 2006; Hallstrand et al., 2014). Subsequently, these persistent immune and inflammatory reactions will gradually introduce airway structural changes, and cause obstruction and respiratory symptoms (Rovina et al., 2013).
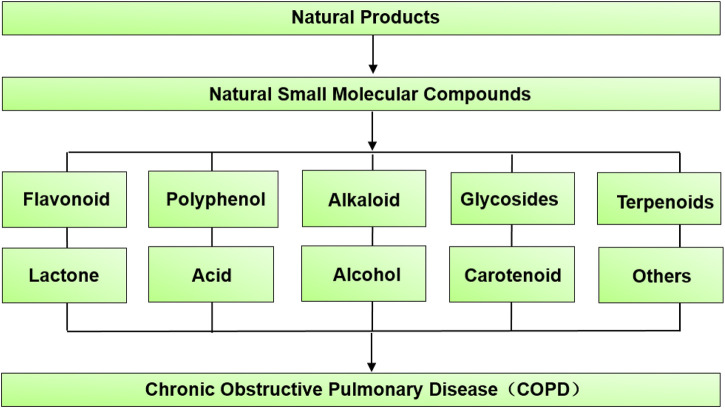
An increasing number of evidences indicate that inflammation serves as the turning point of vascular reconstitution in COPD, which is associated with the untimely activation of epithelial cells and innate immune cells (such as neutrophils, eosinophils, and macrophages) with inflammatory mediators (i.e. inflammatory peptides, lipid mediators, growth factors, reactive oxygen and nitrogen species, chemokines cytokines, and cellular proteases) (Barnes, 2016; Kuźnar-Kamińska et al., 2018; Capron et al., 2019). Recent studies have shown that numerous natural compounds possess obvious therapeutic effects on the symptoms of COPD model animals (Figure 1). For example, not only can quercetin, a
FIGURE 1.
The 10 kinds of natural small molecular compounds that have an effect on COPD.
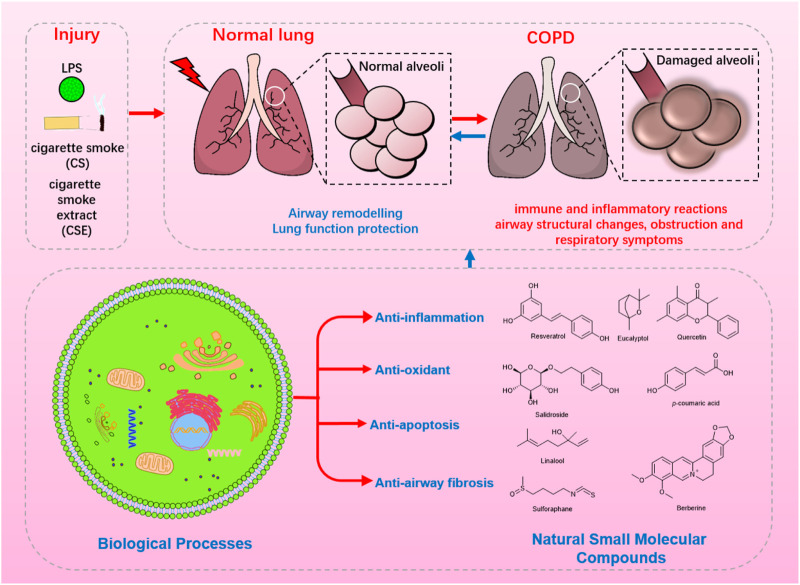
representation of flavonoid, significantly reduce pulmonary oxidative stress, inflammation, and mucus production in COPD model animals, but also improve corticosteroid resistance by promoting AMPK activation and Nrf2 expression (Ganesan et al., 2010; Braun et al., 2011; Mitani et al., 2017). The polyphenol compound curcumin enables the promotion of airway inflammation and airway remodeling in COPD model animals by regulating the NF-κB signaling pathway (Yuan et al., 2018), and improves skeletal muscle dysfunction by up-regulating the PGC-1α/SIRT3 pathway (Zhang et al., 2017). In addition, curcumin demonstrates an inhibition on the expression of pro-inflammatory genes and the level of chemokines, and regulations towards corticosteroid resistance (Gan et al., 2016). Given these multiple effects on improving COPD symptoms, a prospective review regarding the advancements of potential natural small molecular compounds is highly needed. However, to the best of our knowledge, less attention has been paid in this promising field despite scattered summaries in several reviews (Goncalves and Romeiro, 2019). The main purpose of this review concentrates on summarizing the latest and representative information on therapeutic/interventional effects of reported natural compounds on COPD in recent years (Figure 2), to excavate the potential of these bioactive molecules and furnish basic information for research in the future, as well as provide a useful supplement to reviews related to COPD (Figure 3).
FIGURE 2.
The biological processes and mechanisms of natural small molecular compounds in the treatment of COPD.
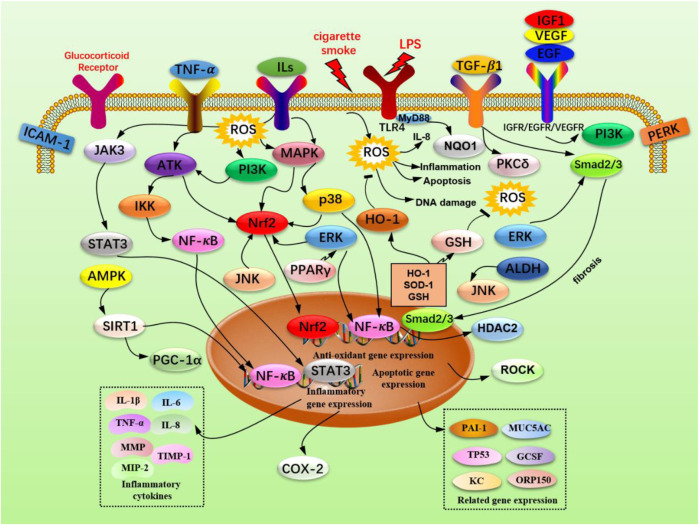
FIGURE 3.
The signaling pathways of natural small molecular compounds in the treatment of COPD.
Flavonoid
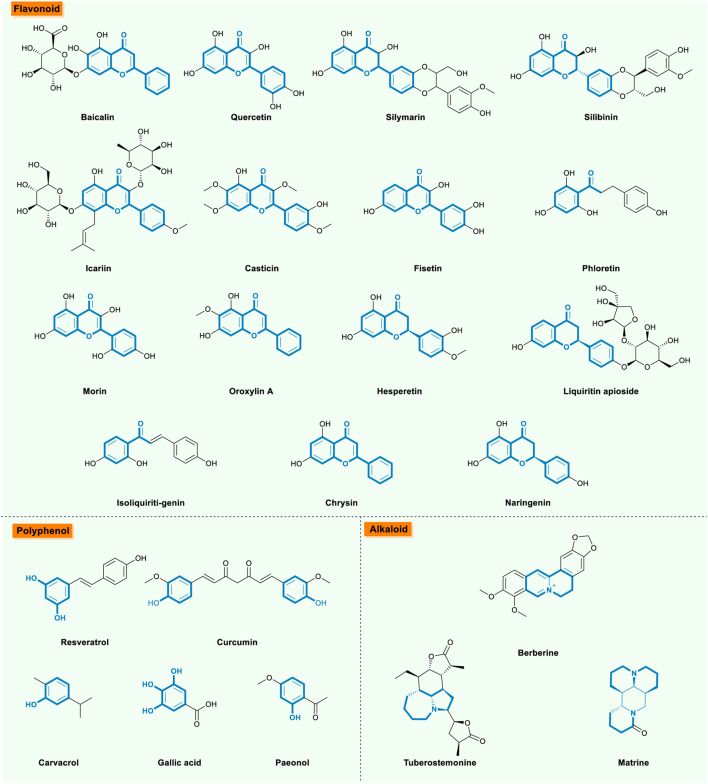
Flavonoids possess a variety of biological properties such as anti-inflammatory, anti-apoptosis, and anti-oxidant properties to improve COPD symptoms (Table 1; Figure 4). Baicalin is a flavonoid compound isolated from the root of Scutellaria baicalensis Georgi, possessing multiple biological activities, such as anti-inflammatory and anti-oxidant properties. To clarify the effects of baicalin on COPD, the mice and cell models were established by using cigarette smoke (CS) and cigarette smoke extract (CSE), respectively. Results showed that baicalin could regulate pro-infammatory and anti-infammatory balance and exert great lung function protection on COPD (Lixuan et al., 2010; Li et al., 2012; Wang et al., 2018a; Hao et al., 2021; Zhang et al., 2021). The anti-inflammatory effect was likely achieved via inhibiting the nuclear factor-kappa B(NF-κB) activation (Lixuan et al., 2010), up-regulating histone deacetylase 2(HDAC2) protein expression, along with inhibiting HDAC2 phosphorylation (enhancing HDAC2 activity) (Li et al., 2012), and modulating HDAC2/NF-κB/PAI-1 signaling pathways (Zhang et al., 2021).
TABLE 1.
The effects of flavonoid on COPD.
| Flavonoids | Sources | Models | Effects | Dose | Application | Ref |
|---|---|---|---|---|---|---|
| Baicalin | Scutellaria baicalensis Georgi | In vivo: COPD mice model was established by cigarette smoke (CS) exposure | Inhibition of the NF-kB pathway | 20–80 mg/kg | In vivo | Lixuan et al. (2010) |
| In vitro: cell model was established by using cigarette smoke extract (CSE) to stimulate type-II pneumocytes | 5–20 μM | In vitro | ||||
| CS-induced inflammatory models in mice; CSE-induced inflammatory models in A549 cells | Modulating HDAC2/anti-inflammatory | 25–100 mg/kg | In vivo | Li et al. (2012) | ||
| 10–100 μM | In vitro | |||||
| CS-induced rat model of COPD | Anti-infammatory/anti-airway remodeling/antioxidant | 40–160 mg/kg | In vivo | Wang et al. (2018a) | ||
| CS/CSE-induced airway inflammation in mice or human bronchial epithelial (HBE) cells | Anti-infammatory | 40–160 mg/kg | In vivo | Zhang et al. (2021) | ||
| 10–40 μM | In vitro | |||||
| CSE-induced MLE-12 cells; CS-induced COPD mice model | Regulation of HSP72-mediated JNK pathway | 25–100 mg/kg | In vivo | Hao et al. (2021) | ||
| 5–20 μmol/L | In vitro | |||||
| Quercetin | Polygoni avicularis herba | CSE-induced muman monocytic U937 cells and peripheral blood mononuclear cells (PBMC) collected from patients with COPD | Increased AMPK activation and Nrf2 expression, and restored corticosteroid resistance | 10 μM | In vitro | Mitani et al. (2017) |
| CSE-induced mice model/human airway epithelial NCI-H292 cells | Inhibiting the NF-κB pathway and EGFR phosphorylation | 25–50 mg/kg | In vivo | Yang et al. (2012) | ||
| 5–20 μM | In vitro | |||||
| Primary human osteoblasts exposed to cigarette smoke medium (CSM) | Activation of the anti-oxidative enzymes HO-1 and SOD-1 | 25–100 μM | In vitro | Braun et al. (2011) | ||
| Elastase/lipopolysaccharide (LPS)-exposed mice | Negatively regulating MMP expression | 10 mg/kg | In vivo | Ganesan et al., 2010 | ||
| Rhinovirus-infected mice with COPD phenotype | Preventing progression of lung disease in COPD | 0.1% quercetin containing diet | In vivo | Farazuddin et al. (2018) | ||
| Silymarin | Silybum marianum | CS-induced mice mode | Suppression of inflammation and oxidative stress by inhibiting the ERK/p38 MAPK pathway | 25–50 mg/kg | In vivo | Li et al. (2015) |
| CSE-induced human bronchial epithelial cell line (BEAS-2B) model | Inhibition of autophagy and the ERK/p38 MAPK pathway | 10–40 μM | In vitro | Li et al. (2016a) | ||
| Silibinin | Silybum marianum | CS and LPS exposure-induced mice model | Inhibited the pulmonary fibrosis induced by CS via suppression of TGF-β1/Smad 2/3 signaling | 10–20 mg/kg | In vivo | Ko et al. (2017) |
| CS-/LPS-induced COPD model mice; CS condensate-stimulated H292 cells | Inhibition in ERK phosphorylation | 20–40 mg/kg | In vivo | Park et al. (2016) | ||
| 6.25–50 μg/ml | In vitro | |||||
| Icariin | Epimedium | CSE-exposed BEAS-2B cells model | Reversing Glucocorticoids (GC)resistance | 20–80 µM | In vitro | Hu et al. (2020) |
| CS-induced lung inflammation using BALB/c mice; CSE-exposed A549 epithelial cells | Ameliorated inflammation by suppressing NF-kB activation and modulating glucocorticoid receptor (GR) protein expression | 25–100 mg/kg | In vivo | Li et al. (2014) | ||
| 10–100 µM | In vitro | |||||
| Casticin | Vitex rotundifolia and Vitex agnus-castus | CS-induced C57BL/6 mice model | Inhibition of inflammatory cytokines and chemokines | 1–10 mg/kg | In vivo | Lee et al. (2015) |
| CS-exposed mice | Attenuated oxidative Stress and inflammation via inhibition of NF-ĸB | 10–30 mg/kg | In vivo | Li et al. (2020) | ||
| Fisetin | Gleditsiae spina | Human airway epithelial cells | Inhibiting the TNF-α/NF-κB signaling pathway | 2.5–10 μM | In vitro | Lee et al. (2018a) |
| CS-exposed mice | Up-regulation of Nrf2 expression | 50 mg/kg | In vivo | Hussain et al. (2019) | ||
| Phloretin | Crotonis fructus; Rubi fructus | CS-induced mice model; CSE-induced NCI-H292 cells model | Inhibition of epidermal growth factor receptor (EGFR)/MAPK signaling pathways | 10–20 mg/kg | In vivo | Wang et al. (2018b) |
| 1–10 μM | In vitro | |||||
| Morin | Cudrania tricuspidata | CS-induced mice model | Anti-inflammation via inhibiting the P13K/ATK/NF-κB signaling pathway | 10–40 mg/kg | In vivo | Cai et al. (2018) |
| Oroxylin A | Scutellaria baicalensis Georgi | CS-stimulated BEAS-2B cells and RAW264.7 cells; CS-induced mice | Activating the Nrf2 signaling pathway | 15–60 mg/kg | In vivo | Li et al. (2016b) |
| 50–150 μM | In vitro | |||||
| Hesperetin | Citrus reticulata | CSE-induced mice model | Regulation of SIRT1/PGC-1α/NFκ-B signaling axis | 25–50 mg/kg | In vivo | Wang et al. (2020) |
| CS- and urethane-induced lung cancer with COPD in mice | Preventing COPD progression to lung cancer | 25–100 mg/kg | In vivo | Zhou et al. (2021) | ||
| Liquiritin apioside | Glycyrrhiza uralensis | CSE-induced cell injury in the A549 lung epithelial cell; CS-induced mice inflammation model | Inhibiting TGF-β and TNF-α expression and increasing levels of GSH | 3–30 mg/kg | In vivo | Guan et al. (2012) |
| 108–106 M | In vitro | |||||
| Isoliquiriti-genin | liquorice | CS-induced mice model | Regulating the Nrf2 and NF-κB signaling pathways | 10–30 mg/kg | In vivo | Yu et al. (2018a) |
| Chrysin | Flowers | CS-induced airway inflammation in mice | Inhibition of ERK and p38 phosphorylation | 10–20 mg/kg | In vivo | Shen et al. (2015) |
| Naringenin | Amacardi-um occidentale L | CS-induced mice model; CSE-exposed A549 cells | Suppression of NF-κB | 20–80 mg/kg | In vivo | Liu et al. (2018) |
| In vitro |
FIGURE 4.
The bioactive natural compounds from flavonoid, polyphenol, and alkaloid.
As a flavonoid abundant in fruits and vegetables, quercetin has attracted much attention for its beneficial health effects including anti-oxidant and anti-inflammation activity. It was found that quercetin successfully reduced oxidative stress, lung inflammation, and mucus production via negating MMP expression in elastase/LPS-exposed mice (Ganesan et al., 2010), or via inhibiting the NF-κB pathway and EGFR phosphorylation both in the CS/CSE-induced mice model and NCI-H292 cell model (Yang et al., 2012). Smokers frequently suffer from impaired fracture healing often due to poor bone quality and stability induced by increasing formation of reactive oxygen species (ROS). One research found that quercetin could protect primary human osteoblasts from the toxic effects of smoking through activation of the anti-oxidative enzymes HO-1 and SOD-1 (Braun et al., 2011). Besides, acute exacerbations are the major cause of morbidity and mortality in patients with COPD, Mohammad Farazuddin et al. disclosed that quercetin effectively mitigated rhinovirus-induced progression of lung disease on COPD mice models (Farazuddin et al., 2018). To remove a major barrier known as corticosteroid resistance for the effective treatment of COPD, quercetin also provided access to restore corticosteroid sensitivity in cells from patients with COPD via the mechanism of increasing AMPK activation and Nrf2 expression (Mitani et al., 2017).
Separated from the milk thistle (Silybum marianum), silymarin attenuated inflammation and oxidative stress induced by CS/CSE on mice and in the BEAS-2B cell (human bronchial epithelial cells). The anti-inflammatory and anti-oxidant effects of silymarin might be related to the inhibition of autophagy and ERK/p38 MAPK pathway (Li et al., 2015; Li D. et al., 2016). Silibinin, an active constitute of silymarin, could markedly reduce the production of fibrotic mediators in CS + LPS-exposed mice via suppression of TGF-β1/Smad 2/3 signaling (Ko et al., 2017), as well as clearly decrease the pro-inflammatory mediators and airway mucus production expression in CS condensate-stimulated H292 cells and COPD mice model via the inhibition in ERK phosphorylation (Park et al., 2016). Among dihydroflavones, Naringenin, hesperetin, and liquiritin apioside (LA) also exhibited positive effects on COPD, among which hesperetin could not only effectively alleviate inflammation and oxidative stress responses in CES-induced COPD mice by virtue of NAD-dependent protein deacetylase sirtuin-1(SIRT1)/PGC-1α/NF-κB signaling axis (Wang et al., 2020), but also suppress the protein expression of AKT1, IL6, VEGFA, and MMP9 and up-regulate TP53 to reduce the risk of COPD progressing to lung cancer (Zhou et al., 2021). Besides, LA offered protection to lung epithelial cell from CS-induced injuries by inhibiting the transforming growth factor-β (TGF-β) and tumor necrosis factor-α (TNF-α) expression and increasing anti-oxidative levels of glutathione (GSH) (Guan et al., 2012). Notably, naringenin smoothly attenuated inflammation in COPD on CS-induced mice models via suppressing NF-κB pathway (Liu et al., 2018).
As a major constituent of flavonoids isolated from the herb Epimedium, icariin exerted a therapeutic effect in numerous chronic inflammatory diseases. However, COPD tends to be glucocorticoid (GC) resistant, and Lingli Hu et al. noted that icariin was able to decrease CSE-induced inflammation, airway remodeling, and ROS production by mitigating GC resistance in CSE-induced BEAS-2B cells models (Hu et al., 2020). Besides, icariin owned anti-inflammatory effects on CS-induced inflammatory models, which was possibly achieved by suppressing NF-κB activation and modulating the glucocorticoid receptor (GR) protein expression (Li et al., 2014). Except for icariin suppressing NF-κB activation, casticin, which was a poly-methylflavone obtained from Vitex species such as Vitex rotundifolia and Vitex agnus-castus, was found possessing significant effects on attenuating oxidative stress and lung inflammation induced by CS (Lee et al., 2015), which was related to the inhibition of NF-ĸB pathway (Li et al., 2020). Apart from the extractions from herbs, the natural flavonoid fisetin (3,7,3′,4′-tetrahydroxyflavone) demonstrated its abilities on effectively alleviating lung oxidative stress and inflammation induced by the powerful pro-oxidant CS through the incremental expression of Nrf2 as well as its downstream target anti-oxidant gene (Hussain et al., 2019). Furthermore, Seoghyun Lee et al. found that fisetin acted as a good drug candidate for improving the lung function of patients with COPD by suppressing the TNF-α/NF-κB signaling cascade (Lee S. et al., 2018). Additionally, the valuable chalcone phloretin existing in Crotonis fructus and Rubi fructus featured diverse biologic properties. Hao Wang et al. reported that phloretin-based pre-treatment remarkably blocked mucins secretion, inflammatory cytokine release, and inflammatory cell infiltration on CS-induced mice models, as well as an interruption of CSE-induced expression of MUC5AC and IL-1β in NCI-H292 bronchial epithelial cells. Those previously mentioned protections were possibly achieved by attenuating the functions of P38, ERK and EGFR in vivo and in vitro (Wang et al., 2018b).
Despite the similarity of morin, oroxylin A and chrysin on structures, the three natural products provided positive effects on COPD through different mechanisms. Briefly, morin (3,5,7,2′,4′-pentahydroxyflavone), a major component of a traditional medicinal herb Cudrania tricuspidata, demonstrated protective effects on CS-induced lung inflammation probably by blocking P13K/ATK/NF-κB signaling pathway (Cai et al., 2018); Known as a natural flavonoid extracted from the traditional herb Scutellaria baicalensis Georgi, oroxylin A attenuated CS-induced lung histopathologic changes, expression of cytokines TNF-α, IL-1β in a mice model with a dose-dependent manner, as well as significantly up-regulated Nrf2 expression in CSE-stimulated cells (Li J. et al., 2016). Furthermore, as a naturally-occurring flavone commonly found in flowers, chrysin effectively inhibited CSE-induced airway inflammation in mice through inhibition of ERK and p38 phosphorylation (Shen et al., 2015). Beyond these molecules mentioned previously, as a variant of flavonoid, isoliquiriti-genin (ILG) derived from the root of liquorice was reported to antagonize COPD on CS-induced mice model by suppressing inflammatory and oxidative stress through up-regulating the expression of Nrf2 and down-regulating the expression of NF-κB signaling pathways (Yu D. et al., 2018).
Polyphenol
Polyphenol belongs to a group of chemical substances in plants featuring multiple phenol groups (Table 2; Figure 4). Resveratrol (3,4′,5-trihydroxystilbene; RESV), a natural polyphenol phytoalexin identified from a variety of plant species, exhibited a protective effect against CSE-induced apoptosis in cells (Zhang L. et al., 2015; Song et al., 2017; Zong et al., 2021). The anti-apoptotic effect may be exerted through the activation of a pathway involving SIRT1 and ORP150 in CSE-induced HBEpC cell (Zhang L. et al., 2015), and activation of Notch1 signaling mediated autophagy in CSE-induced HUVECs models (Zong et al., 2021), or via up-regulating mitofusin 2 (MFN2) in a CSE-induced HBEpC cell (Song et al., 2017). Recently, studies have found that resveratrol could protect against oxidative damage and pulmonary inflammation on the COPD mice model (Liu et al., 2014a; Wang et al., 2017), where the mechanism might be related with decreasing NF-κB activity and elevated HO-1 expression, and activating the SIRT1/PGC-1α signaling pathways (Wang et al., 2017). Alongside the functions mentioned previously, resveratrol could not only effectively attenuate the release of inflammatory cytokines from human bronchial smooth muscle cells (HASMCs) in COPD (Knobloch et al., 2010; Knobloch et al., 2014), but also inhibit the NF-κB, TNF-α, and MMP-9-associated pathways, simultaneously slowing the dysfunction of dendritic cells (DCs) in patients with COPD (Wang et al., 2015; Liu et al., 2016). These findings proved that resveratrol was able to ameliorate cardiac oxidation stress and apoptosis and increase the expression of SIRT1, as well as attenuate left ventricular remodeling, while these factors might assist the left ventricular impairment process in old mice with COPD induced by CS and LPS exposure. (Hu et al., 2013). Overall, resveratrol prophylaxis by inhalation is a potential approach for slowing down ageing-related deterioration of the lung function and structure in prematurely ageing telomerase null (terc−/−) mice, which could be developed as a potentially novel approach to maintaining lung health, prior to the irreversible onset of ageing-related structural and functional decline in the lungs (Navarro et al., 2017).
TABLE 2.
The effects of polyphenol on COPD.
| Polyphenol | Sources | Models | Effects | Dose | Application | Ref | |
|---|---|---|---|---|---|---|---|
| Resveratrol | Various plants, nuts and fruits | CSE-induced HBE cell model | Anti-apoptotic effect through the activation SIRT1 and ORP150 | 20 μmol/L | In vitro | Zhang et al. (2015b) | |
| CSE-induced Human umbilical vein endothelial cells (HUVECs) model | Anti-apoptosis | 40 μM | In vitro | Zong et al. (2021) | |||
| CSE-induced HBE cells model | Reduced apoptosis | 20 μM | In vitro | Song et al. (2017) | |||
| CS-induced mice model | Decreased NF-κB activity and the elevated HO-1 expression and activity | 1–3 mg/kg | In vivo | Liu et al. (2014a) | |||
| CS- and LPS-induced lung inflammation in a mouse model of COPD | Activating SIRT1/PGC-1α signaling pathways | 50 mg/kg | In vivo | Wang et al. (2017) | |||
| Human bronchial smooth muscle cells (HASMCs) exposed to lipoteichoic acid (LTA) | Anti-inflammation | 10-6-10-4M | In vitro | Knobloch et al. (2014) | |||
| Lymphocytes isolated from patients with COPD | Inhibited the translocation of NF κB, and decreased TNF α | 12.5 μmol/l | In vitro | Liu et al. (2016) | |||
| Human airway smooth muscle cells | Anti-inflammatory | 10-7-10–3 M | In vitro | Knobloch et al. (2010) | |||
| Dendritic cells (DCs) from COPD patients | Inhibited dysfunction of dendritic cells (DCs) | 10 μmol/ml | In vitro | Wang et al. (2015) | |||
| Old mice with COPD induced by CS exposure and LPS instillation | Attenuated left ventricular remodeling | 25 mg/kg | In vivo | Hu et al. (2013) | |||
| Prematurely ageing telomerase null (terc−/−) mice | Slowed ageing-related degenerative changes in mouse lungs | 1 mg/kg | In vivo | Navarro et al. (2017) | |||
| Curcumin | Curcuma longa | In mice model of COPD-like airway inflammation induced by non-typeable haemophilus influenzae exposure (NTHi) | Inhibition of inflammation and lung cancer progression | 0.2–2% | In vivo | Moghaddam et al. (2009) | |
| LPS- and CS-induced COPD murine models; LPS-stimulated BEAS-2B cells | Inhibiting NF-κB Signaling and COX-2 | 100–200 mg/kg | In vivo | Yuan et al. (2018) | |||
| 0.1–10 μmol/L | In vitro | ||||||
| CSE-treated BEAS-2B cells; CS-induced COPD mice models | Modulating the PPARγ-NF-κB signaling pathway | 100 mg/kg | In vivo | Li et al. (2019) | |||
| 2.5–7.5 mΜ | In vitro | ||||||
| Patients with mild COPD | Reduced serum atherosclerotic low-density lipoprotein levels in patients with mild COPD | 180 mg | In vivo | Funamoto et al. (2016) | |||
| Mice model of COPD established by CSE combined with lipopolysaccharide | Up-regulation of PGC-1α/SIRT3 signaling pathway | 100 mg/kg | In vivo | Zhang et al. (2017) | |||
| In vitro model of CSE-induced inflammation using human monocytic cell line (U937) | Restored corticosteroid function in monocytes exposed to oxidants by maintaining HDAC2 | 1–10,000 nM | In vitro | Meja et al. (2008) | |||
| CSE-induced mice model with COPD | Modulating HDAC2 expression and its effect on histone modification | 100 μM | In vitro | Gan et al. (2016) | |||
| Carvacrol | Zataria multiflora Boiss | Elastase-induced emphysema mice | Anti-inflammatory via suppression of NF-κB | 20 mg/kg | In vivo | Games et al. (2016) | |
| Guinea pigs model of COPD induced by CSE | Attenuated systemic inflammation | 60–240 μg/ml | In vitro | Mahtaj et al. (2015) | |||
| Guinea pigs model of COPD exposed to CS | Prevention of tracheal responsiveness and emphysema | 60–240 μg/ml | In vitro | Gholami Mahtaj et al. (2015) | |||
| Guinea pigs model of COPD exposed to CS | Against lung inflammation and oxidative stress | 60–240 μg/ml | In vivo | Boskabady and Gholami Mahtaj, (2015) | |||
| Gallic acid | Rheum palmatum L | Elastase (ET-) + LPS- induced COPD exacerbation like condition in mice model | Prevented the activation of NF κB and elevated the expression of Nrf2 | 200 mg/kg | In vivo | Singla et al. (2021) | |
| ET- and CS-induced mice model | Suppressed phosphorylation of p65NF-κB and IκBα along with down-regulation of IL-1β/TNF-α/KC/MIP-2/GCSF genes | 200 mg/kg | In vivo | Singla et al. (2020) | |||
| Paeonol | Paeonia suffruticosa | CS-induced mice model/CSE-induced HBE cell model | Inhibition of the MAPKs/NF-κB signaling | 10 mg/kg | In vivo | Liu et al. (2014b) | |
| 0.05–0.4 mM | In vitro | ||||||
Curcumin [(1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione] is a naturally occurring polyphenolic phytochemical isolated from the rhizome of the medicinal plant Curcuma longa. Dietary administration of curcumin effectively suppressed NTHi-induced COPD-like airway inflammation and lung cancer progression in mice (Moghaddam et al., 2009). Curcumin could also attenuate CS-induced inflammation both in vivo and in vitro by modulating the PPARγ-NF-κB signaling pathway (Li et al., 2019), along with attenuating airway inflammation and remodeling by blocking NF-κB and COX-2 signaling on CS-induced COPD mice (Yuan et al., 2018). Theracurmin®, a highly absorptive curcumin, with improved bioavailability using a drug delivery system, reduced levels of the atherosclerotic α1-antitrypsin-low-density lipoprotein (AT-LDL) complex. This result suggested that curcumin was beneficial to prevent the development of vascular events in patients with COPD (Funamoto et al., 2016). Among the multiple symptoms induced by COPD, skeletal muscle
dysfunction is one of the most extrapulmonary symptoms in COPD patients, where mitochondria manifestation plays an important role in the duration. Therefore, protecting mitochondria from injury is crucial to prophylaxis skeletal muscle dysfunction during the progression of COPD. Under this context, Ming Zhang et al. found that curcumin smoothly attenuated skeletal muscle mitochondrial impairment in COPD mice via up-regulating the PGC-1α/SIRT3 signaling pathway (Zhang et al., 2017). In addition, recent studies have suggested that histone modification showed a positive impact on various aspects associated with the progression of COPD where histone deacetylase 2 (HDAC2) could suppress proinflammatory gene expression through deacetylation of core histones. Thus, Lixing Gan et al. investigated the functions variation of histone modification via a combination with the expression of chemokines in type-II alveolar epithelial cells (AEC II) and HDAC2 caused by curcumin on a mice model with COPD induced by CS, and the results indicated that curcumin might inhibit chemokines and rebuild corticosteroid resistance in COPD through modulating HDAC2 expression, as well as show influence on histone modification (Gan et al., 2016). Similarly, another study found that curcumin could restore CS-impaired HDAC2 activity and corticosteroid efficacy in monocytes (Meja et al., 2008). All in all, curcumin showed potential to reverse corticosteroid resistance, which is commonly observed in patients with COPD.
Carvacrol, C6H3CH3(OH) (C3H7) as a constituent of Zataria multiflflora Boiss, was reported to own preventive therapeutic potential on lung infection and oxidative stress on CS-induced guinea pig models with COPD, which was comparable to or more potent than the effect of dexamethasone at used concentrations (Boskabady and Gholami Mahtaj, 2015; Gholami Mahtaj et al., 2015; Mahtaj et al., 2015). Subsequently, Ellen Games et al. found that carvacrol could protect mice against elastase-induced emphysema through a suppression of the NF-κB pathway (Games et al., 2016). Like other naturally occurring phenolic compounds, gallic acid is known to possess anti-oxidant/anti-inflammatory activities. Researchers revealed that the gallic acid protected against COPD exacerbation manifestations through inversing modulation of redox sensitive transcription factors-NF-κB and Nrf2 (Singla et al., 2021). Meanwhile, gallic acid ameliorated elastase (ET)-induced inflammation and emphysema by the restoration of redox imbalance and inhibition of NF-κB activation (Singla et al., 2020). As the representative of phenolic, paeonol existing in the Chinese herb Paeonia suffruticosa has been identified with the optimistic effects on alleviating oxidative stress and lung inflammation on CS-induced mice models. In addition, paeonol could also suppress CSE-induced IL-8 and ROS in human bronchial epithelial cells (HBECs) via inhibition of the MAPKs/NF- kB signaling (Liu et al., 2014b).
Alkaloid
Alkaloid, a class of naturally occurring organic nitrogen-containing bases, participates in diverse physiological functions of the human body (Table 3; Figure 4). As for COPD discussed in this review, berberine, as a protoberberine alkaloid, could effectively attenuate CS-induced lung inflammation in mice (Lin et al., 2013; Xu et al., 2015; Wang et al., 2019). Studies further confirmed that the anti-inflammation effect of berberine were associated with the suppression CS-induced NF-κB activation (Lin et al., 2013), inhibition of TGF-β1/Smads signaling (Wang et al., 2019), or inhibition of ERK and P38 pathway (Xu et al., 2015). Besides, as an alkaloid-type phytochemical from Stemona tuberosa, tuberostemonine (TS) attenuated CS-induced lung inflammation and decreased alveoli size in lung tissue through the inhibition of the infiltration of inflammatory cells by decreasing the chemokine expression related to lung inflammation (Jung et al., 2016a; Jung et al., 2016b). Apart from previously mentioned alkaloids, matrine, an alkaloid compound existed in Sophora flavescens Ait (Kushen) with a useful bioactivity of anti-inflammatory effect, Xuhua Yu et al. disclosed it could reduce CS-induced neutrophilic inflammation by inducing neutrophil apoptosis (Yu X. et al., 2019).
TABLE 3.
The effects of alkaloids on COPD.
| Alkaloid | Sources | Models | Effects | Dose | Application | Ref | |
|---|---|---|---|---|---|---|---|
| Berberine | Coptidis Rhizoma | CS-induced mice model | Suppressed CS-induced NF-κB activation | 50 mg/kg | In vivo | Lin et al. (2013) | |
| CSE-induced airway inflammation in mice | Inhibition of TGF-β1/Smads signaling | 25 mg/kg | In vivo | Wang et al. (2019) | |||
| Mice exposed to CS | Inhibition of ERK and P38 pathway | 5–10 mg/kg | In vivo | Xu et al. (2015) | |||
| Tuberostemonine | Stemona tuberosa | CS-induced lung inflammation in mice | Suppressed inflammation | 1–10 mg/kg | In vivo | Jung et al. (2016b) | |
| CS-induced mice model | Suppressed inflammation | 1–10 mg/kg | In vivo | Jung et al. (2016a) | |||
| Matrine | Sophora flavescens Ait | CS-induced mice model | Inducing neutrophil apoptosis | 100 mg/kg | In vivo | Yu et al. (2019b) | |
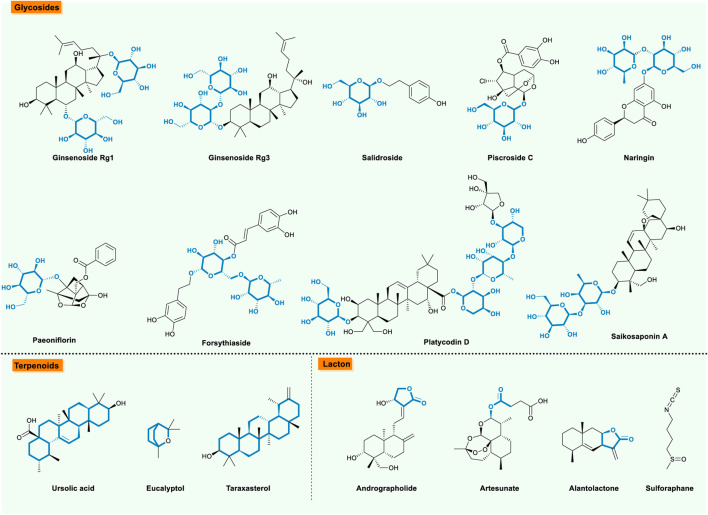
Glycosides
Glycosides are formed in nature by the interaction of the nucleotide glycosides with the alcoholic or phenolic group, which is categorized as O-glycosides, S-glycosides, N-glycosides, and C-glycosides. Among them, this review focuses on O-glycosides, the most numerous ones found in nature (Table 4; Figure 5). Ginsenoside Rg1 attenuated CS-induced pulmonary epithelial-mesenchymal transition airway fibrosis by suppressing the TGF-β1/Smad Pathway in both COPD rats and HBE cells (Guan et al., 2017a; Guan et al., 2017b). Subsequently, ginsenoside Rg3 was confirmed that it could suppress neutrophil migration through down-regulating the PI3K pathway, by which ameliorated acute exacerbation of COPD in chronic CS-induced COPD and NTHi-induced acute exacerbation in mice, as well as in BEAS-2B cell models (Guan et al., 2020), which might alleviate acute exacerbation of chronic obstructive pulmonary disease (AECOPD) induced by exacerbation-mediated neutrophilia. Salidroside, one of the extracted compounds of Rhodiola rosea L., was reported to effectively ameliorate an inflammatory response and oxidative stress in COPD model mice induced by CS, which negated the MAPK/NF-kB pathway (Luo et al., 2017). Alongside it, salidroside also mitigated the long-term CS-induced emphysema and skeletal muscle atrophy in rats by inhibiting oxidative stress and inflammatory responses and regulating muscle-specific transcription factor expression (Zhang et al., 2019). Piscroside C, a novel iridoid glycoside isolated from Pseudolysimachion rotundum var. Subinegrum, was capable of effectively inhibiting inflammatory responses induced by CS, intervening a vital part of COPD development by the way of IKK/NF-κB inhibition (Song et al., 2015). Related research further found that piscroside C inhibited the TNF-α/NF-κB pathway by obstructing the interaction of protein kinase C (PKCδ) towards a TNF receptor 1 signaling complex (TNF-RSC) formation with a model of TNF-α-stimulated human airway epithelial cells (NCI-H292 cells) (Lee SU. et al., 2018). As for naringin, a well-known compound equipped with an effective anti-inflammatory activity, attenuated chronic pulmonary neutrophilic inflammation in CS-exposed rats (Nie et al., 2012). Apart from inflammatory protection, glycosides exhibit diverse biological effects on attenuating COPD progression, for instance, paeoniflorin, a monoterpene glycoside, was reported to re-balance the relationship between oxidant and anti-oxidant in CS-induced mice lung tissues with COPD via a Nrf2-dependent mechanism (Lin et al., 2016). Then, forsythiaside, an active constituent isolated from the Chinese medicinal herb Forsythia suspensa, offered protection against CS-induced mice lung injury via activating the Nrf2 and inhibiting the NF-κB signaling pathway (Cheng et al., 2015). Moreover, platycodin D, a major saponin derived from the roots of Platycodon grandiflflorum, had been shown to have protection towards CS-induced lung inflammation via suppressing an inflammatory and oxidative response by activating the Nrf2 signaling pathway. This phenomenon indicated that platycodin D might be a promising therapeutic agent for lung inflammation induced by CS (Gao et al., 2017). As for saikosaponin a, a triterpenoid saponin existed in Radix bupleuri, was found to ameliorate CS-induced oxidant stress and inflammatory via inhibiting CS-induced NF-κB activation and up-regulating the expression of Nrf2 and HO-1, proving its therapeutic potential towards CS-induced lung inflammation (Chen et al., 2018).
TABLE 4.
The effects of glycosides on COPD.
| Glycosides | Sources | Models | Effects | Dose | Application | Ref | |
|---|---|---|---|---|---|---|---|
| Ginsenoside Rg1 | Panax ginseng | CSE-induced COPD mice; Human embryonic lung fibroblasts exposed to CSE | Suppressed airway fibrosis | 20 mg/kg | In vivo | Guan et al. (2017a) | |
| 40 μM | In vitro | ||||||
| CSE-induced COPD mice and HBE cells model | Attenuated Pulmonary Epithelial-Mesenchymal Transition (EMT) | 5–20 mg/kg | In vivo | Guan et al. (2017b) | |||
| 5–160 μM | In vitro | ||||||
| Ginsenoside Rg3 | Panax ginseng | AECOPD murine model established by CS exposure and NTHi infection; CS- and NTHi stimulation on BEAS-2B | Inhibition of PI3K | 10–40 mg/kg | In vivo | Guan et al. (2020) | |
| 10–160 μM | In vitro | ||||||
| Salidroside | Rhodiola rosea L | CS-induced COPD in mice | Mitigated skeletal muscle atrophy | 50–200 mg/kg | In vivo | Zhang et al. (2019) | |
| CS-induced COPD in mice | Inhibition the MAPK/NF-kB pathway | 20–40 mg/kg | In vivo | Luo et al. (2017) | |||
| Piscroside C | Pseudolysimachion rotundum var. subintegrum | TNF-α-stimulated human airway epithelial cells (NCI-H292 cells) | Inhibited TNF-α/NF-κB pathway by suppression of PKCδ activity for TNF-RSC formation | 2.5–20 μM | In vitro | Lee et al. (2018b) | |
| CS- and LPS-induced COPD mice model; TNF-stimulated human airway epithelial NCIH292 cells | Suppression of IKK/NF-κB activation | 15–30 mg/kg | In vivo | Song et al. (2015) | |||
| 2.5–20 μM | In vitro | ||||||
| Naringin | Grape fruit and citrus fruits | CS-induced COPD mice model | Anti-inflammatory | 20–80 mg/kg | In vivo | Nie et al. (2012) | |
| Paeoniflorin | Paeonia lactiflora | CS-exposed COPD mice model | Attenuated oxidative stress via an Nrf2-dependent mechanism | 40 mg/kg | In vivo | Lin et al. (2016) | |
| Forsythiaside | Forsythia suspensa | CS-induced mice model | Activating Nrf2 and inhibiting NF-κB signaling pathways | 15–60 mg/kg | In vivo | Cheng et al. (2015) | |
| Platycodin D | Platycodon grandiflflorum | CS-induced mice model | Activating the Nrf2 signaling pathway | 20–80 mg/kg | In vivo | Gao et al. (2017) | |
| Saikosaponin a | Radix bupleuri | CS-induced mice model | Inhibited oxidant stress and inflammatory by activating the Nrf2 and inhibiting the NF-κB signaling pathway | 5–20 mg/kg | In vivo | Chen et al. (2018) | |
FIGURE 5.
The bioactive natural compounds from glycosides, terpenoids, and lacton.
Terpenoids
Terpenoids represent a highly diverse group of natural products with wide applications. Among these, several molecules exhibited positive effects towards COPD (Table 5; Figure 5). Taking ursolic acid as an example, a pentacyclic triterpenoid compound exists in many plants, and has anti-oxidant/anti-inflammatory activities. Studies pointed out that ursolic acid could effectively attenuate CS-induced mice emphysema (Lin et al., 2017; Lin et al., 2019a; Lin et al., 2019b), which might be fullfiled by the down-regulation of the PERK pathway to attenuate apoptosis, with a combination of up-regulation of Nrf2
TABLE 5.
The effects of terpenoids on COPD.
| Terpenoids | Sources | Models | Effects | Dose | Application | Ref | |
|---|---|---|---|---|---|---|---|
| Ursolic acid | Loquat leaves, glossy privet leaves, forsythia, Prunella vulgaris | CSE treated normal human bronchial epithelial (NHBE) cell model; mice model established by A549 cells in nude mice in vivo | Prevented development of lung cancer | 10 mg/kg | In vivo | Liu et al. (2012) | |
| 3.2–25 μmol/L | In vitro | ||||||
| CS-induced mice emphysema model | Down-regulating PERK pathway and up-regulating Nrf2 pathway | 10–40 mg/kg | In vivo | Lin et al. (2017) | |||
| CS-induced emphysema mice | Alleviated airway-vessel remodeling and muscle consumption partly through IGF1 and TGF-β1/Smad2.3 signaling pathways | 10–40 mg/kg | In vivo | Lin et al. (2019b) | |||
| CES-exposed mice model | Alleviated CSE-induced emphysema and airway remodeling | 10–40 mg/kg | In vivo | Lin et al. (2017) | |||
| Eucalyptol | Eucalyptus globulus | CS-induced COPD mice model | Promoted lung repair | 1–10 mg/kg | In vivo | Kennedy-Feitosa et al. (2019) | |
| CS-induced COPD mice model | Anti-inflammatory and antioxidant effects via attenuating NF-κB p65 subunit activation | 1–10 mg/ml | In vivo | Kennedy-Feitosa et al. (2016) | |||
| CS-induced COPD mice model | Against bacterial invasion through attenuating ciliated cell Damage and suppressing MUC5AC expression | 260 mg/kg | In vivo | Yu et al. (2019a) | |||
| CS-induced COPD mice model | Mitigated lung injury by suppressing ICAM-1 gene expression | 260 mg/kg | In vivo | Yu et al. (2018b) | |||
| Taraxasterol | Taraxacum officinale | CS-induced mice model; CSE- induced HBE cells model | Inhibiting oxidative stress and inflammatory responses | 2.5–10 mg/kg | In vivo | Xueshibojie et al. (2016) | |
| 3–12 μg/ml | In vitro | ||||||
A pathway to modify oxidant stress in CS-induced mice lungs (Lin et al., 2017), following reports from Lin et al. further proved that ursolic acid could regulate IGF1 and TGF-β1/Smad2.3 signaling pathways (Lin et al., 2019b) and three unfolded protein response (UPR) pathways. Notably, ursolic acid could also attenuate downstream apoptotic pathways, as well as the activation of Smad2 and Smad3 (Lin et al., 2019a) regulating. Meanwhile, Wenbo Liu et al. uncovered that ursolic acid was able to inhibit CSE-induced NHBE cell injuries and prevent the development of lung cancer, which indicated that ursolic acid was a promising chemopreventive agent of lung cancer (Liu et al., 2012). As a saturated monoterpene, eucalyptol was reported as an anti-oxidant and anti-inflammatory candidate for the treatment of CS-induced COPD in mice (Kennedy-Feitosa et al., 2016; Yu N. et al., 2018; Kennedy-Feitosa et al., 2019) through the promotion of lung repair. As for the mechanisms of eucalyptol on anti-oxidant and anti-inflammation, preliminary work found that the desirable effects were related to the attenuation of NF-κB p65 subunit activation (Kennedy-Feitosa et al., 2016). Futhermore, Yu et al. indicated that these biological functions conducted by eucalyptol was not only highly associated with the suppression of intercellular adhesion molecule (ICAM)-1 gene expression in diseased lungs (Yu N. et al., 2018), but also with ciliated cell damage attenuation and MUC5AC expression inhibition, thus protecting the lungs from bacterial invasion through a joint mechanism (Yu N. et al., 2019). Finally, taraxasterol, a pentacyclic-triterpene isolated from Taraxacum officinale, could effectively work against CS-induced lung inflammation in mice and in HBE cells via inhibiting reactive oxygen species (ROS)-induced TLR4 trafficking to lipid rafts (Xueshibojie et al., 2016).
Lactone
Lactone, a class of cyclic organic esters, is known as the outstanding exponents of secondary metabolites because of their remarkable biological activities and chemical architectures (Table 6; Figure 5). Regarding the biological functions of lactone upon COPD, four representative nature products are listed below. Firstly, Andrographolide, a labdane diterpene lactone isolated from the Andrographis paniculata plant, was reported to be a great candidate for therapy on the CS-induced COPD model in vivo and in vitro due to its anti-lung inflammation and anti-oxidative stress injury (Guan et al., 2013; Li et al., 2013; Yang et al., 2013; Tan et al., 2018; Zhang et al., 2020) via the complex mechanisms including activation of HO-1 (Yang et al., 2013), inhibition of SIRT1/ERK signaling (Zhang et al., 2020), induction of microRNA-218 (Li et al., 2013), and the augmentation of Nrf2 activity (Guan et al., 2013; Tan et al., 2018). Secondly, artesunate, a semi-synthetic derivative of artemisinin, possessed characteristics of anti-inflammatory and anti-oxidative effects on CS-induced lung impairments by suppressing the PI3K and p42/22 MAPK signaling pathways, enhancing Nrf2 and catalase activities, and reducing the NOX2 level (Ng et al., 2014). Furthermore, Kunming Pan et al. revealed that the artesunate treatment significantly protected against CS-induced airway inflammation, as well as airway remodeling via PPAR-γ/TGF-β1/Smad2/3 signaling pathway in vivo and in vitro (Pan et al., 2021). Thirdly, the natural sesquiterpene lactone alantolactone (ALT), which was isolated from Inula helenium L, possessed the abilities of suppressing CSE-induced inflammation, apoptosis, and oxidative stress in BEAS-2B and NHBE cells via modulating the NF-κB and Nrf2/HO-1 axis (Dang et al., 2020). Lastly, sulforaphane, an isothiocyanate derived from cruciferous vegetables, was famous for its anti-inflammatory activities. Xiaoli Zeng et al. indicated that sulforaphane exerted anti-inflammatory activities in monocyte-derived macrophages (MDMs) from patients with COPD by modulating the toll-like receptors’ (TLRs) pathway, which suggested that sulforaphane may be a potential therapeutic agent for the treatment of COPD (Zeng et al., 2021).
TABLE 6.
The effects of lactone on COPD.
| Lactone | Sources | Models | Effects | Dose | Application | Ref | ||
|---|---|---|---|---|---|---|---|---|
| Andrographolide | Andrographis paniculata | CSE-exposed RAW 264.7 cells | Inhibition of SIRT1/ERK signaling pathway | 1–40 µM | In vitro | Zhang et al. (2020) | ||
| BEAS-2B cells exposed to CSE | Augmented Nrf2 antioxidant defense and facilitated autophagic flux blockade | 10–30 μM | In vitro | Tan et al. (2018) | ||||
| Human alveolar epithelial A549 cells exposed to CSE | Induction of microRNA-218 | 5 μM | In vitro | Li et al. (2013) | ||||
| CSE-exposed bronchial epithelial cells (BEAS-2); CS-exposed mice as COPD model | Augmentation of Nrf2 activity | 0.1–1 mg/kg | In vivo | Guan et al. (2013) | ||||
| 30 μM | In vitro | |||||||
| CS-exposed mice model | Activation of HO-1–mediated signaling | 1 mg/kg | In vivo | Yang et al. (2013) | ||||
| Artesunate | Artemisia annua L | CS-exposed COPD mice model; human bronchial smooth muscle cells exposure in CSE | Against airway inflammation and airway remodeling via PPAR-γ/TGF-β1/Smad2/3 signaling | 25–100 mg/kg | In vivo | Pan et al. (2021) | ||
| 1–100 μM | In vitro | |||||||
| CSE-exposed BEAS-2; CS-exposed mice as COPD model | Anti-inflammatory and anti-oxidative | 10–100 mg/kg | In vivo | Ng et al. (2014) | ||||
| 30 μM | In vitro | |||||||
| Alantolactone | Inula helenium L | CSE-exposed BEAS-2B and NHBE cells | Activation of Nrf2/HO-1 and inhibition of the NF-κB pathways | 1–10 μM | In vitro | Dang et al. (2020) | ||
| Sulforaphane | Cruciferous vegetables | Monocyte-derived macrophages (MDMs) from patients with COPD | Modulating the TLR pathway | 2.5–20 μmol/L | In vitro | Zeng et al. (2021) | ||
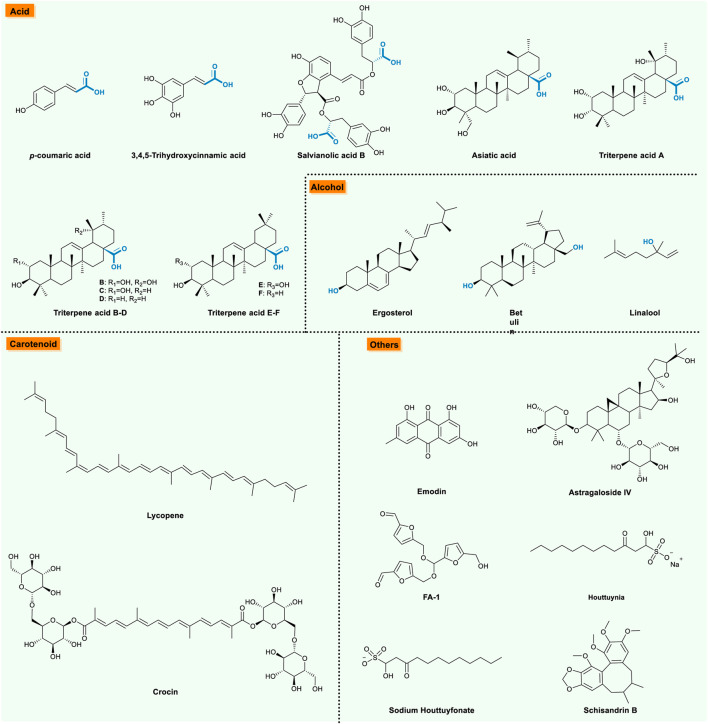
Acid
Organic acids are classified as compounds bearing carboxylic acid groups from the view of chemistry, which are widely distributed in nature. With regard to COPD, organic acids contribute anti-inflammatory and anti-oxidant effects (Table 7; Figure 6). For instance, p-Coumaric acid, a phenolic acid, effectively decreased the production of IL-8 in CSE-stimulated A549 cells as efficiently as dexamethasone, the standard drug for research of the inflammatory process (da Silva et al., 2019). Besides, Woogyeong Kim et al. described that p-coumaric acid displayed an anti-inflammatory effect in the CS-induced pulmonary inflammation mice model by inhibiting pro-inflammatory mediators such as cytokines and chemokine, via blocking NF-κB translocation to the nucleus (Kim et al., 2018). (query)3,4,5-trihydroxycinnamic acid, a derivative of hydroxycinnamic acid, ameliorated pulmonary inflammation in mice due to CS exposure and LPS administration by suppressing inflammatory molecules and inflammatory cell recruitment accompanied by suppressing MAPK (partial p38 and JNK) and NF-κB signaling. Notably, 3,4,5-trihydroxycinnamic acid pre-treatment reduced PMA-triggered IL-6 secretion in A549 or H292 cells by up-regulating NAD(P)H dehydrogenase (quinone 1) 1 (NQO1) expression (Min et al., 2020). Moreover, salvianolic acid B, a useful compound isolated from the Chinese herb Radix salviae Miltiorrhizae, exhibited both anti-oxidant and anti-inflammatory effects against CS-induced lung inflammation via activating Nrf-2 and inhibiting NF-κB activation, which suggested that salvianolic acid B treatment may be a potential therapy option while treating COPD (Zhang DF. et al., 2015). In addition, asiatic acid is one of the major components of the titrated extract of Centella asiatica (TECA), could effectively protect against pulmonary inflammation and mucus overproduction by inhibition of inflammatory molecules via suppressing the activation of MAPKs and NF-κB pathway, up-regulating HO-1 in the lung tissue of CS exposure mice at the meantime (Lee et al., 2016). As a series of bioactive acids extracted from loquat leaves, triterpene acids suppressed the production of inflammatory mediators on CS-induced COPD mice in a dose-dependent manner via modulating CS-induced AMPK/Nrf2 and NF-κB/iNOS signaling pathways (Jian et al., 2020).
TABLE 7.
The effects of acid on COPD.
| Acid | Sources | Models | Effects | Dose | Application | Ref |
|---|---|---|---|---|---|---|
| p-coumaric acid | Bambusae Caulis | A549 cells exposed to CSE to induce inflammatory process | Anti-inflammatory | 10–100 µM | In vitro | da Silva et al. (2019) |
| CS-induced inflammatory mice model | Suppressed CS-induced pulmonary inflammation | 5–10 mg/kg | In vivo | Kim et al. (2018) | ||
| 3,4,5-Trihydroxycinnamic acid | ||||||
| Cinnamomum cassia Presl | COPD model elicited by CS and LPS; phorbol myristate acetate (PMA)-stimulated A549 or H292 airway epithelial cells | Down-regulation of MAPK (partial p38 and JNK)/NF-κB signaling and upregulation of NQO1 and SIRT1 expression | 20–40 mg/kg | In vivo | Min et al. (2020) | |
| 5–50 µM | In vitro | |||||
| Salvianolic acid B | Radix Salviae Miltiorrhizae | CS-induced mice model | Attenuated inflammation via activating Nrf-2 and inhibiting NF-κB activation | 6–25 mg/kg | In vivo | Zhang et al. (2015a) |
| Asiatic acid | Centella asiatica | CS-exposed mice model | Up-regulation of HO-1 and inhibition of the activation of MAPKs and NF-kB pathway | 15–30 mg/kg | In vivo | Lee et al. (2016) |
| Triterpene acids | Eriobotrya japonica | CS-induced mice model | Regulating the AMPK/Nrf2 and NFκB Pathways | 50–100 mg/kg | In vivo | Jian et al. (2020) |
FIGURE 6.
The bioactive natural compounds from acid, alcohol, carotenoid –and others.
Alcohol
Alcohol, a class of organic compounds characterized by one or more hydroxyl (―OH) groups attached to a carbon atom of an alkyl group. Among numerous alcohols exist in nature, several compounds exhibit therapeutic effects on the COPD model (Table 8; Figure 6). Citing ergosterol for instance, the main bioactive ingredient in Cordyceps sinensis (C. sinensis), suppressed COPD inflammatory, oxidative stress, and apoptosis in both CSE-induced 16HBE cells and Balb/c mice via inhibiting the activation of NF-κB/p65, suggesting that ergosterol may be partially responsible for the therapeutic effects on COPD patients (Sun et al., 2019). More evidences, like Wang Huan et al., demonstrated the protective effects of ergosterol on CS-induced COPD mice manifesting as an anti-inflammatory response possibly by inhibiting the JAK3/STAT3/NF-κB pathway (Huan et al., 2017). As for betulin, a pentacyclic triterpene alcohol, which is extracted from the bark of the birch tree, was reported to show protective effects on CS-induced COPD mice by inhibiting inflammatory response and oxidative stress via inhibiting the ROCK/NF-kB pathway (Chunhua et al., 2017). Apart from the two mentioned previously, Linalool, a natural compound existing in the volatile oil of several aromatic plant species, dramatically alleviated CS-induced lung inflammation due to the inhibition the inflammatory cell infiltration and TNF-α, IL-6, IL-1β, and IL-8 production by inhibiting CS-induced NF-κB activation in a dose-dependent manner (Ma et al., 2015).
TABLE 8.
The effects of alcohol on COPD.
| Alcohol | Sources | Models | Effects | Dose | Application | Ref | |
|---|---|---|---|---|---|---|---|
| Ergosterol | Cordyceps sinensis (C. sinensis) | CSE-induced COPD model both in 16HBE cells and Balb/c mice | Suppressed COPD inflammatory and oxidative stress and apoptosis through the suppression of NF-κB/p65 activation | 20–40 mg/kg | In vivo | Sun et al. (2019) | |
| 5–20 μM | In vitro | ||||||
| CS-induced COPD mice model | Inhibiting the JAK3/STAT3/NF-κB pathway | 25–50 mg/kg | In vivo | Huan et al. (2017) | |||
| Betulin | Birch tree bark | CS-induced COPD mice model | Inhibiting the inflammatory response and oxidative stress possibly through the ROCK/NF-κB pathway | 20–40 mg/kg | In vivo | Chunhua et al. (2017) | |
| Linalool | Aromatic plants species | CS-induced COPD mice model | Against inflammation by inhibiting CS-induced NF-κB activation | 10–40 mg/kg | In vivo | Ma et al. (2015) | |
Carotenoid
Carotenoids are lipid-soluble pigments and naturally exist in flora and fauna, which offer multiple beneficial functions (Table 9; Figure 6). With regard to the theme this review focuses on, lycopene, a carotenoid found in plant foods, was found to demonstrate anti-oxidant and anti-inflammatory properties in mice exposed to long/short-term CS exposure (Campos et al., 2017; Campos et al., 2019). Overall, the consumption of lycopene in the diet might contribute to the prevention of and therapy for treatment of patients with COPD. Besides, crocin, a valuable constituent of Crocus sativus L, effectively against CS-induced COPD complicated with comorbid depression, due to its inhibition of the inflammatory response via PI3K/Akt-mediated NF-κB signaling (Xie et al., 2019). For another, Mahin Dianat et al. found that crocin could protect the lungs against injuries and related cardiac dysfunction caused by COPD via modulation of the Nrf2 pathway among CS exposure mice models. (Dianat et al., 2018).
TABLE 9.
The effects of carotenoids on COPD.
| Carotenoid | Sources | Models | Effects | Dose | Application | Ref | |
|---|---|---|---|---|---|---|---|
| Lycopene | Tomatoes | CS-exposed mice model | Anti-oxidant and anti-inflammatory | 25–50 mg/kg | In vivo | Campos et al. (2019) | |
| J774A.1 (Macrophages) cells exposed to CSE; CS-exposed mice model | Anti-oxidant and anti-inflammatory | 25–50 mg/kg | In vivo | Campos et al. (2017) | |||
| 0.5–25 µM | In vitro | ||||||
| Crocin | Crocus sativus L | CS-induced mice model | Activation of Nrf2 pathway | 50 mg/kg | In vivo | Dianat et al. (2018) | |
| CS-exposed C57BL/6 mice model | Preventing the activation of PI3K/Akt mediated NF-κB inflammatory pathways | 50 mg/kg | In vivo | Xie et al. (2019) | |||
Others
Apart from the valuable natural compounds previously summarized, there are other numerous natural products with different scaffolds that contribute therapeutic functions towards COPD (Table 10; Figure 6). For instance, emodin, an active compound of Rheum palmatum L., demonstrated protective effects against lung inflammation and oxidative injury induced by CS in mice model via enhancing the expression and activities of HO-1 and Nrf-2 (Xue et al., 2015). Alongside it, astragaloside IV, the best biological activity among Astragalus polysaccharide, could provide protection both on CS-induced COPD in mice and in human bronchial epithelial cell models via blocking the JAK3/STAT3/NF-κB pathway (Meiqian et al., 2018). Meanwhile, polysaccharides from Dendrobium huoshanense stems alleviated CS-induced lung inflammation in mice via inhibiting the NF-κB and MAPK signaling pathways (Ge et al., 2018), while 5,5'-((((5-(hydroxymethyl)furan-2-yl)methylene)bis (oxy))bis (methylene))bis (furan-2-carbaldehyde) (FA-1) isolated from a concentrated Japanese apricot extract (JAE), enabled protection against cytotoxicity, DNA damage, and oxidative stress in CSE-exposed HBE cells and normal human epidermal keratinocyte (NHEK) cells via augmenting aldehyde dehydrogenase (ALDH) and DNA repair (Jang et al., 2018). Furthermore, houttuynia, one of the main components of the cordate houttuynia, could alleviate lung injury in the rats’ lung tissues of COPD induced by smoking combined with intratracheal instillation of LPS via inhibiting the TLR4/MyD88/NF-κB activation sequence (Wang et al., 2021). As a bioactive compound extracted from houttuynia, sodium houttuyfonate (SH) significantly alleviated the pulmonary inflammation via suppressing the TLR4/NF-κB pathway, thus protecting the lung tissue on the CS-/LPS-induced mice model with COPD (Wu et al., 2017), and schisandrin B, a dibenzocyclooctadiene derivative identified from Schisandra chinensis, was reported to fight against CS-induced lung inflammation in mice by activating the Nrf2 and inhibiting NF-κB signaling pathway (Jia et al., 2017).
TABLE 10.
The effects of other compounds on COPD.
| Compound | Sources | Models | Effects | Dose | Application | Ref |
|---|---|---|---|---|---|---|
| Emodin | Rheum palmatum L | CS-induced lung injury in a mouse model | Enhancing the expression and activities of HO-1 and Nrf-2 | 20–40 mg/kg | In vivo | Xue et al. (2015) |
| Astragaloside IV | Astragalus mongholicus | CS-induced mice model; CSE-stimulated NHBE cells model | Inhibition of the JAK3/STAT3/NF-κB pathway | 10–40 mg/kg | In vivo | Meiqian et al. (2018) |
| 10–40 μM | In vitro | |||||
| Polysaccharides from Dendrobium huoshanense | Dendrobium huoshanense | CS-induced mice model | Inhibition of the NF-κB and MAPK signaling pathways | 100–400 mg/kg | In vivo | Ge et al. (2018) |
| FA-1 | Prunus mume | CSE-induced immortalized HBE cells and normal human epidermal keratinocytes (NHEK) | Augmenting ALDH and DNA repair | 150 nM | In vitro | Jang et al. (2018) |
| Houttuynia | Houttuynia cordata Thunb | Mice model of COPD established by smoking combined with intratracheal instillation of LPS | Inhibiting the activation of the TLR4/MyD88/NF-κB (p65) signaling pathway | 5–25 mg/kg | In vivo | Wang et al. (2021) |
| Sodium Houttuyfonate | Houttuynia cordata Thunb | CS- and LPS-induced mice model | Suppressing the TLR4/NF-κB pathway | 24.3 mg/kg | In vivo | Wu et al. (2017) |
| Schisandrin B | Schisandra chinensis | CS-induced mice model | Activating Nrf2 and inhibiting the NF-κB signaling pathway | 20–80 mg/kg | In vivo | Jia et al. (2017) |
Summary
This review discloses that LPS, cigarette smoke, and cigarette smoke extract contribute to the development of COPD, and the cellular biological processes concerning COPD mainly involve immune inflammatory response, apoptosis, fibrosis, and oxidative stress, which gradually lead to airway structural changes, obstruction, and destruction of the alveolar structure and respiratory symptoms. Moreover, these reported natural small molecular compounds demonstrated unique functions in the treatment of COPD through numerous biological processes such as anti-inflammatory, anti-oxidant, anti-apoptosis, and anti-airway fibrosis, as shown in Figure 2. The main signaling pathways involved in the regulation of physiological functions of lung cell or tissue refer to the JAK3/STAT3/NF-κB and MAPK inflammatory signaling pathways, the Nrf2 oxidative stress signaling pathway, TGF-β1/Smad 2/3 fibrosis signaling, and so on; related targets are mainly about TNF-α, IL-6, IL-8, TIMP-1, MMP, AKT, JAK3, IKK, PI3K, HO-1, MAPK, P38, ERK, etc. as shown in Figure 3. It is worth noting that a few compounds (like baicalin, quercetin, resveratrol, curcumin, and ursolic acid) have shown impressive effects on improving COPD symptoms, considering the great potential of these valuable molecules, continuous efforts should be paid in this field, especially from a simple molecular level to a mechanism level. Besides, the efficacy of the single-drug curative strategy is far from the clinical needs in the current CODP treatment, and this inspires researchers that a combination strategy utilizing two or more bioactive natural compounds seems to be a potential direction of COPD research (Terry and Dhand, 2020). Not only could this therapeutic combination increase the degree of bronchiectasis, but also reduce the toxic and side effects by reducing the dosage and enhancing complementary therapeutic effects of the bioactive molecule used. In brief, natural small molecular compounds demonstrate great potential in the area of COPD treatment, and we hope that this review can bring a quick look and provide some inspiration for the research in relevant fields.
Author Contributions
L-YL, C-HZ, and HZ contributed to the conception and design of the study. F-YZ, GZ, Y-FL, and KL organized the database and performed the statistical analysis. L-YL and C-TZ wrote the first draft of the manuscript. C-HZ and HZ contributed to the manuscript revision. All authors read and approved the submitted version.
Funding
Zigong Science and Technology Bureau, Grant/ Award Number: 2018SHFZ17, 2019YLSF43; Sichuan Provincial Medical Association, Grant/Award Number: 2018SHD2-3.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Barnes P. J. (2016). Inflammatory Mechanisms in Patients with Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 138, 16–27. 10.1016/j.jaci.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Boskabady M. H., Gholami Mahtaj L. (2015). Lung Inflammation Changes and Oxidative Stress Induced by Cigarette Smoke Exposure in guinea Pigs Affected by Zataria Multiflora and its Constituent, Carvacrol. BMC Complement. Altern. Med. 15, 39. 10.1186/s12906-015-0574-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K. F., Ehnert S., Freude T., Egaña J. T., Schenck T. L., Buchholz A., et al. (2011). Quercetin Protects Primary Human Osteoblasts Exposed to Cigarette Smoke through Activation of the Antioxidative Enzymes HO-1 and SOD-1. ScientificWorldJournal 11, 2348–2357. 10.1100/2011/471426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B., Gan X., He J., He W., Qiao Z., Ma B., et al. (2018). Morin Attenuates Cigarette Smoke-Induced Lung Inflammation through Inhibition of PI3K/AKT/NF-κB Signaling Pathway. Int. Immunopharmacol 63, 198–203. 10.1016/j.intimp.2018.07.035 [DOI] [PubMed] [Google Scholar]
- Campos K. K. D., Araújo G. R., Martins T. L., Bandeira A. C. B., Costa G. P., Talvani A., et al. (2017). The Antioxidant and Anti-inflammatory Properties of Lycopene in Mice Lungs Exposed to Cigarette Smoke. J. Nutr. Biochem. 48, 9–20. 10.1016/j.jnutbio.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Campos K. K. D., de Oliveira Ramos C., Martins T. L., Costa G. P., Talvani A., Garcia C. C. M., et al. (2019). Lycopene Mitigates Pulmonary Emphysema Induced by Cigarette Smoke in a Murine Model. J. Nutr. Biochem. 65, 93–100. 10.1016/j.jnutbio.2018.12.008 [DOI] [PubMed] [Google Scholar]
- Capron T., Bourdin A., Perez T., Chanez P. (2019). COPD beyond Proximal Bronchial Obstruction: Phenotyping and Related Tools at the Bedside. Eur. Respir. Rev. 28. 10.1183/16000617.0010-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. J., Guo X. Y., Cheng B. H., Gong Y. Q., Ying B. Y., Lin M. X. (2018). Saikosaponin a Inhibits Cigarette Smoke-Induced Oxidant Stress and Inflammatory Responses by Activation of Nrf2. Inflammation 41, 1297–1303. 10.1007/s10753-018-0778-7 [DOI] [PubMed] [Google Scholar]
- Cheng L., Li F., Ma R., Hu X. (2015). Forsythiaside Inhibits Cigarette Smoke-Induced Lung Inflammation by Activation of Nrf2 and Inhibition of NF-Κb. Int. Immunopharmacol 28, 494–499. 10.1016/j.intimp.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Chunhua M., Long H., Zhu W., Liu Z., Jie R., Zhang Y., et al. (2017). Betulin Inhibited Cigarette Smoke-Induced COPD in Mice. Biomed. Pharmacother. 85, 679–686. 10.1016/j.biopha.2016.11.079 [DOI] [PubMed] [Google Scholar]
- da Silva E. C. O., Dos Santos F. M., Ribeiro A. R. B., de Souza S. T., Barreto E., Fonseca E. J. D. S. (2019). Drug-induced Anti-inflammatory Response in A549 Cells, as Detected by Raman Spectroscopy: a Comparative Analysis of the Actions of Dexamethasone and P-Coumaric Acid. Analyst 144, 1622–1631. 10.1039/c8an01887a [DOI] [PubMed] [Google Scholar]
- Dang X., He B., Ning Q., Liu Y., Guo J., Niu G., et al. (2020). Alantolactone Suppresses Inflammation, Apoptosis and Oxidative Stress in Cigarette Smoke-Induced Human Bronchial Epithelial Cells through Activation of Nrf2/HO-1 and Inhibition of the NF-Κb Pathways. Respir. Res. 21, 95. 10.1186/s12931-020-01358-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianat M., Radan M., Badavi M., Mard S. A., Bayati V., Ahmadizadeh M. (2018). Crocin Attenuates Cigarette Smoke-Induced Lung Injury and Cardiac Dysfunction by Anti-oxidative Effects: the Role of Nrf2 Antioxidant System in Preventing Oxidative Stress. Respir. Res. 19, 58. 10.1186/s12931-018-0766-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farazuddin M., Mishra R., Jing Y., Srivastava V., Comstock A. T., Sajjan U. S. (2018). Quercetin Prevents Rhinovirus-Induced Progression of Lung Disease in Mice with COPD Phenotype. PLoS One 13, e0199612. 10.1371/journal.pone.0199612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto M., Sunagawa Y., Katanasaka Y., Miyazaki Y., Imaizumi A., Kakeya H., et al. (2016). Highly Absorptive Curcumin Reduces Serum Atherosclerotic Low-Density Lipoprotein Levels in Patients with Mild COPD. Int. J. Chron. Obstruct Pulmon Dis. 11, 2029–2034. 10.2147/copd.S104490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games E., Guerreiro M., Santana F. R., Pinheiro N. M., de Oliveira E. A., Lopes F. D., et al. (2016). Structurally Related Monoterpenes P-Cymene, Carvacrol and Thymol Isolated from Essential Oil from Leaves of Lippia Sidoides Cham. (Verbenaceae) Protect Mice against Elastase-Induced Emphysema. Molecules 21. 10.3390/molecules21101390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L., Li C., Wang J., Guo X. (2016). Curcumin Modulates the Effect of Histone Modification on the Expression of Chemokines by Type II Alveolar Epithelial Cells in a Rat COPD Model. Int. J. Chron. Obstruct Pulmon Dis. 11, 2765–2773. 10.2147/copd.S113978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S., Faris A. N., Comstock A. T., Chattoraj S. S., Chattoraj A., Burgess J. R., et al. (2010). Quercetin Prevents Progression of Disease in elastase/LPS-Exposed Mice by Negatively Regulating MMP Expression. Respir. Res. 11, 131. 10.1186/1465-9921-11-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Guo Y., Yang H. (2017). Platycodin D Protects against Cigarette Smoke-Induced Lung Inflammation in Mice. Int. Immunopharmacol 47, 53–58. 10.1016/j.intimp.2017.03.009 [DOI] [PubMed] [Google Scholar]
- Ge J. C., Zha X. Q., Nie C. Y., Yu N. J., Li Q. M., Peng D. Y., et al. (2018). Polysaccharides from Dendrobium Huoshanense Stems Alleviates Lung Inflammation in Cigarette Smoke-Induced Mice. Carbohydr. Polym. 189, 289–295. 10.1016/j.carbpol.2018.02.054 [DOI] [PubMed] [Google Scholar]
- Gholami Mahtaj L., Boskabady M. H., Mohamadian Roshan N. (2015). The Effect of Zataria Multiflora and its Constituent, Carvacrol, on Tracheal Responsiveness and Lung Pathology in Guinea Pig Model of COPD. Phytother Res. 29, 730–736. 10.1002/ptr.5309 [DOI] [PubMed] [Google Scholar]
- Gonçalves P. B., Romeiro N. C. (2019). Multi-target Natural Products as Alternatives against Oxidative Stress in Chronic Obstructive Pulmonary Disease (COPD). Eur. J. Med. Chem. 163, 911–931. 10.1016/j.ejmech.2018.12.020 [DOI] [PubMed] [Google Scholar]
- Guan S., Liu Q., Han F., Gu W., Song L., Zhang Y., et al. (2017a). Ginsenoside Rg1 Ameliorates Cigarette Smoke-Induced Airway Fibrosis by Suppressing the TGF-β1/Smad Pathway In Vivo and In Vitro . Biomed. Res. Int. 2017, 6510198. 10.1155/2017/6510198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S., Xu W., Han F., Gu W., Song L., Ye W., et al. (2017b). Ginsenoside Rg1 Attenuates Cigarette Smoke-Induced Pulmonary Epithelial-Mesenchymal Transition via Inhibition of the TGF-β1/Smad Pathway. Biomed. Res. Int. 2017, 7171404. 10.1155/2017/7171404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S. P., Tee W., Ng D. S., Chan T. K., Peh H. Y., Ho W. E., et al. (2013). Andrographolide Protects against Cigarette Smoke-Induced Oxidative Lung Injury via Augmentation of Nrf2 Activity. Br. J. Pharmacol. 168, 1707–1718. 10.1111/bph.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X., Yuan Y., Wang G., Zheng R., Zhang J., Dong B., et al. (2020). Ginsenoside Rg3 Ameliorates Acute Exacerbation of COPD by Suppressing Neutrophil Migration. Int. Immunopharmacol 83, 106449. 10.1016/j.intimp.2020.106449 [DOI] [PubMed] [Google Scholar]
- Guan Y., Li F. F., Hong L., Yan X. F., Tan G. L., He J. S., et al. (2012). Protective Effects of Liquiritin Apioside on Cigarette Smoke-Induced Lung Epithelial Cell Injury. Fundam. Clin. Pharmacol. 26, 473–483. 10.1111/j.1472-8206.2011.00956.x [DOI] [PubMed] [Google Scholar]
- Guarascio A. J., Ray S. M., Finch C. K., Self T. H. (2013). The Clinical and Economic burden of Chronic Obstructive Pulmonary Disease in the USA. Clinicoecon Outcomes Res. 5, 235–245. 10.2147/ceor.S34321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallstrand T. S., Hackett T. L., Altemeier W. A., Matute-Bello G., Hansbro P. M., Knight D. A. (2014). Airway Epithelial Regulation of Pulmonary Immune Homeostasis and Inflammation. Clin. Immunol. 151, 1–15. 10.1016/j.clim.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Hao D., Li Y., Shi J., Jiang J. (2021). Baicalin Alleviates Chronic Obstructive Pulmonary Disease through Regulation of HSP72-Mediated JNK Pathway. Mol. Med. 27, 53. 10.1186/s10020-021-00309-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Liu F., Li L., Zhang L., Yan C., Li Q., et al. (2020). Effects of Icariin on Cell Injury and Glucocorticoid Resistance in BEAS-2B Cells Exposed to Cigarette Smoke Extract. Exp. Ther. Med. 20, 283–292. 10.3892/etm.2020.8702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. X., Cui H., Fan L., Pan X. J., Wu J. H., Shi S. Z., et al. (2013). Resveratrol Attenuates Left Ventricular Remodeling in Old Rats with COPD Induced by Cigarette Smoke Exposure and LPS Instillation. Can. J. Physiol. Pharmacol. 91, 1044–1054. 10.1139/cjpp-2012-0464 [DOI] [PubMed] [Google Scholar]
- Huan W., Tianzhu Z., Yu L., Shumin W. (2017). Effects of Ergosterol on COPD in Mice via JAK3/STAT3/NF-Κb Pathway. Inflammation 40, 884–893. 10.1007/s10753-017-0533-5 [DOI] [PubMed] [Google Scholar]
- Hussain T., Al-Attas O. S., Alamery S., Ahmed M., Odeibat H. A. M., Alrokayan S. (2019). The Plant Flavonoid, Fisetin Alleviates Cigarette Smoke-Induced Oxidative Stress, and Inflammation in Wistar Rat Lungs. J. Food Biochem. 43, e12962. 10.1111/jfbc.12962 [DOI] [PubMed] [Google Scholar]
- Jang A. J., Lee J. H., Yotsu-Yamashita M., Park J., Kye S., Benza R. L., et al. (2018). A Novel Compound, "FA-1" Isolated from Prunus Mume, Protects Human Bronchial Epithelial Cells and Keratinocytes from Cigarette Smoke Extract-Induced Damage. Sci. Rep. 8, 11504. 10.1038/s41598-018-29701-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia R., Zhang H., Yang Z., Zhao H., Liu F., Wang H., et al. (2017). Protective Effects of Schisandrin B on Cigarette Smoke-Induced Airway Injury in Mice through Nrf2 Pathway. Int. Immunopharmacol 53, 11–16. 10.1016/j.intimp.2017.09.030 [DOI] [PubMed] [Google Scholar]
- Jian T., Ding X., Li J., Wu Y., Ren B., Li J., et al. (2020). Triterpene Acids of Loquat Leaf Improve Inflammation in Cigarette Smoking Induced COPD by Regulating AMPK/Nrf2 and NFκB Pathways. Nutrients 12. 10.3390/nu12030657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K. H., Beak H., Park S., Shin D., Jung J., Park S., et al. (2016a). The Therapeutic Effects of Tuberostemonine against Cigarette Smoke-Induced Acute Lung Inflammation in Mice. Eur. J. Pharmacol. 774, 80–86. 10.1016/j.ejphar.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Jung K. H., Kil Y. S., Jung J., Park S., Shin D., Lee K., et al. (2016b). Tuberostemonine N, an Active Compound Isolated from Stemona Tuberosa, Suppresses Cigarette Smoke-Induced Sub-acute Lung Inflammation in Mice. Phytomedicine 23, 79–86. 10.1016/j.phymed.2015.11.015 [DOI] [PubMed] [Google Scholar]
- Kennedy-Feitosa E., Cattani-Cavalieri I., Barroso M. V., Romana-Souza B., Brito-Gitirana L., Valenca S. S. (2019). Eucalyptol Promotes Lung Repair in Mice Following Cigarette Smoke-Induced Emphysema. Phytomedicine 55, 70–79. 10.1016/j.phymed.2018.08.012 [DOI] [PubMed] [Google Scholar]
- Kennedy-Feitosa E., Okuro R. T., Pinho Ribeiro V., Lanzetti M., Barroso M. V., Zin W. A., et al. (2016). Eucalyptol Attenuates Cigarette Smoke-Induced Acute Lung Inflammation and Oxidative Stress in the Mouse. Pulm. Pharmacol. Ther. 41, 11–18. 10.1016/j.pupt.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Kim W., Lim D., Kim J. (2018). p-Coumaric Acid, a Major Active Compound of Bambusae Caulis in Taeniam, Suppresses Cigarette Smoke-Induced Pulmonary Inflammation. Am. J. Chin. Med. 46, 407–421. 10.1142/s0192415x18500209 [DOI] [PubMed] [Google Scholar]
- Knobloch J., Sibbing B., Jungck D., Lin Y., Urban K., Stoelben E., et al. (2010). Resveratrol Impairs the Release of Steroid-Resistant Inflammatory Cytokines from Human Airway Smooth Muscle Cells in Chronic Obstructive Pulmonary Disease. J. Pharmacol. Exp. Ther. 335, 788–798. 10.1124/jpet.110.166843 [DOI] [PubMed] [Google Scholar]
- Knobloch J., Wahl C., Feldmann M., Jungck D., Strauch J., Stoelben E., et al. (2014). Resveratrol Attenuates the Release of Inflammatory Cytokines from Human Bronchial Smooth Muscle Cells Exposed to Lipoteichoic Acid in Chronic Obstructive Pulmonary Disease. Basic Clin. Pharmacol. Toxicol. 114, 202–209. 10.1111/bcpt.12129 [DOI] [PubMed] [Google Scholar]
- Ko J. W., Shin N. R., Park S. H., Lee I. C., Ryu J. M., Kim H. J., et al. (2017). Silibinin Inhibits the Fibrotic Responses Induced by Cigarette Smoke via Suppression of TGF-β1/Smad 2/3 Signaling. Food Chem. Toxicol. 106, 424–429. 10.1016/j.fct.2017.06.016 [DOI] [PubMed] [Google Scholar]
- Kuźnar-Kamińska B., Mikuła-Pietrasik J., Mały E., Makowska N., Malec M., Tykarski A., et al. (2018). Serum from Patients with Chronic Obstructive Pulmonary Disease Promotes Proangiogenic Behavior of the Vascular Endothelium. Eur. Rev. Med. Pharmacol. Sci. 22, 7470–7481. 10.26355/eurrev_201811_16288 [DOI] [PubMed] [Google Scholar]
- Lee H., Jung K. H., Lee H., Park S., Choi W., Bae H. (2015). Casticin, an Active Compound Isolated from Vitex Fructus, Ameliorates the Cigarette Smoke-Induced Acute Lung Inflammatory Response in a Murine Model. Int. Immunopharmacol 28, 1097–1101. 10.1016/j.intimp.2015.07.041 [DOI] [PubMed] [Google Scholar]
- Lee J. W., Park H. A., Kwon O. K., Jang Y. G., Kim J. Y., Choi B. K., et al. (2016). Asiatic Acid Inhibits Pulmonary Inflammation Induced by Cigarette Smoke. Int. Immunopharmacol 39, 208–217. 10.1016/j.intimp.2016.07.010 [DOI] [PubMed] [Google Scholar]
- Lee S., Ro H., In H. J., In J. H., Kim M. O., Lee J., et al. (2018a). Fisetin Inhibits TNF-Α/nf-Κb-Induced IL-8 Expression by Targeting PKCδ in Human Airway Epithelial Cells. Cytokine 108, 247–254. 10.1016/j.cyto.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Lee S. U., Lee S., Ro H., Choi J. H., Ryu H. W., Kim M. O., et al. (2018b). Piscroside C Inhibits TNF-Α/nf-Κb Pathway by the Suppression of PKCδ Activity for TNF-RSC Formation in Human Airway Epithelial Cells. Phytomedicine 40, 148–157. 10.1016/j.phymed.2018.01.012 [DOI] [PubMed] [Google Scholar]
- Leung J. M., Tiew P. Y., Mac Aogáin M., Budden K. F., Yong V. F., Thomas S. S., et al. (2017). The Role of Acute and Chronic Respiratory Colonization and Infections in the Pathogenesis of COPD. Respirology 22, 634–650. 10.1111/resp.13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Hu J., Wang T., Zhang X., Liu L., Wang H., et al. (2016a). Silymarin Attenuates Cigarette Smoke Extract-Induced Inflammation via Simultaneous Inhibition of Autophagy and ERK/p38 MAPK Pathway in Human Bronchial Epithelial Cells. Sci. Rep. 6, 37751. 10.1038/srep37751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Xu D., Wang T., Shen Y., Guo S., Zhang X., et al. (2015). Silymarin Attenuates Airway Inflammation Induced by Cigarette Smoke in Mice. Inflammation 38, 871–878. 10.1007/s10753-014-9996-9 [DOI] [PubMed] [Google Scholar]
- Li J., Qiu C., Xu P., Lu Y., Chen R. (2020). Casticin Improves Respiratory Dysfunction and Attenuates Oxidative Stress and Inflammation via Inhibition of NF-ĸb in a Chronic Obstructive Pulmonary Disease Model of Chronic Cigarette Smoke-Exposed Rats. Drug Des. Devel Ther. 14, 5019–5027. 10.2147/dddt.S277126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Tong D., Liu J., Chen F., Shen Y. (2016b). Oroxylin A Attenuates Cigarette Smoke-Induced Lung Inflammation by Activating Nrf2. Int. Immunopharmacol 40, 524–529. 10.1016/j.intimp.2016.10.011 [DOI] [PubMed] [Google Scholar]
- Li L., Bao H., Wu J., Duan X., Liu B., Sun J., et al. (2012). Baicalin Is Anti-inflammatory in Cigarette Smoke-Induced Inflammatory Models In Vivo and In Vitro: A Possible Role for HDAC2 Activity. Int. Immunopharmacol 13, 15–22. 10.1016/j.intimp.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Li L., Sun J., Xu C., Zhang H., Wu J., Liu B., et al. (2014). Icariin Ameliorates Cigarette Smoke Induced Inflammatory Responses via Suppression of NF-Κb and Modulation of GR In Vivo and In Vitro . PLoS One 9, e102345. 10.1371/journal.pone.0102345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Sun J., Mohammadtursun N., Wu J., Dong J., Li L. (2019). Curcumin Inhibits Cigarette Smoke-Induced Inflammation via Modulating the PPARγ-NF-Κb Signaling Pathway. Food Funct. 10, 7983–7994. 10.1039/c9fo02159k [DOI] [PubMed] [Google Scholar]
- Li Y. J., Yu C. H., Li J. B., Wu X. Y. (2013). Andrographolide Antagonizes Cigarette Smoke Extract-Induced Inflammatory Response and Oxidative Stress in Human Alveolar Epithelial A549 Cells through Induction of microRNA-218. Exp. Lung Res. 39, 463–471. 10.3109/01902148.2013.857443 [DOI] [PubMed] [Google Scholar]
- Lin J., Xu F., Wang G., Kong L., Luo Q., Lv Y., et al. (2016). Paeoniflorin Attenuated Oxidative Stress in Rat COPD Model Induced by Cigarette Smoke. Evid. Based Complement. Alternat Med. 2016, 1698379. 10.1155/2016/1698379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K., Liu S., Shen Y., Li Q. (2013). Berberine Attenuates Cigarette Smoke-Induced Acute Lung Inflammation. Inflammation 36, 1079–1086. 10.1007/s10753-013-9640-0 [DOI] [PubMed] [Google Scholar]
- Lin L., Hou G., Han D., Kang J., Wang Q. (2019a). Ursolic Acid Protected Lung of Rats from Damage Induced by Cigarette Smoke Extract. Front. Pharmacol. 10, 700. 10.3389/fphar.2019.00700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Hou G., Han D., Yin Y., Kang J., Wang Q. (2019b). Ursolic Acid Alleviates Airway-Vessel Remodeling and Muscle Consumption in Cigarette Smoke-Induced Emphysema Rats. BMC Pulm. Med. 19, 103. 10.1186/s12890-019-0826-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Yin Y., Hou G., Han D., Kang J., Wang Q. (2017). Ursolic Acid Attenuates Cigarette Smoke-Induced Emphysema in Rats by Regulating PERK and Nrf2 Pathways. Pulm. Pharmacol. Ther. 44, 111–121. 10.1016/j.pupt.2017.03.014 [DOI] [PubMed] [Google Scholar]
- Liu H., Ren J., Chen H., Huang Y., Li H., Zhang Z., et al. (2014a). Resveratrol Protects against Cigarette Smoke-Induced Oxidative Damage and Pulmonary Inflammation. J. Biochem. Mol. Toxicol. 28, 465–471. 10.1002/jbt.21586 [DOI] [PubMed] [Google Scholar]
- Liu J., Yao J., Zhang J. (2018). Naringenin Attenuates Inflammation in Chronic Obstructive Pulmonary Disease in Cigarette Smoke Induced Mouse Model and Involves Suppression of NF-Κb. J. Microbiol. Biotechnol. 10.4014/jmb.1810.10061 [DOI] [PubMed] [Google Scholar]
- Liu M. H., Lin A. H., Lee H. F., Ko H. K., Lee T. S., Kou Y. R. (2014b2014). Paeonol Attenuates Cigarette Smoke-Induced Lung Inflammation by Inhibiting ROS-Sensitive Inflammatory Signaling. Mediators Inflamm. 2014, 651890. 10.1155/2014/651890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Tan X., Shu L., Sun H., Song J., Jin P., et al. (2012). Ursolic Acid Inhibits Cigarette Smoke Extract-Induced Human Bronchial Epithelial Cell Injury and Prevents Development of Lung Cancer. Molecules 17, 9104–9115. 10.3390/molecules17089104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. J., Bao H. R., Zeng X. L., Wei J. M. (2016). Effects of Resveratrol and Genistein on Nuclear factor-κB, T-umor N-ecrosis F-actor-α and M-atrix M-etalloproteinase-9 in P-atients with C-hronic O-bstructive P-ulmonary D-isease. Mol. Med. Rep. 13, 4266–4272. 10.3892/mmr.2016.5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lixuan Z., Jingcheng D., Wenqin Y., Jianhua H., Baojun L., Xiaotao F. (2010). Baicalin Attenuates Inflammation by Inhibiting NF-kappaB Activation in Cigarette Smoke Induced Inflammatory Models. Pulm. Pharmacol. Ther. 23, 411–419. 10.1016/j.pupt.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Luo F., Liu J., Yan T., Miao M. (2017). Salidroside Alleviates Cigarette Smoke-Induced COPD in Mice. Biomed. Pharmacother. 86, 155–161. 10.1016/j.biopha.2016.12.032 [DOI] [PubMed] [Google Scholar]
- Ma J., Xu H., Wu J., Qu C., Sun F., Xu S. (2015). Linalool Inhibits Cigarette Smoke-Induced Lung Inflammation by Inhibiting NF-Κb Activation. Int. Immunopharmacol 29, 708–713. 10.1016/j.intimp.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Mahtaj L. G., Feizpour A., Kianmehr M., Soukhtanloo M., Boskabady M. H. (2015). The Effect of Carvacrol on Systemic Inflammation in guinea Pigs Model of COPD Induced by Cigarette Smoke Exposure. Pharmacol. Rep. 67, 140–145. 10.1016/j.pharep.2014.08.017 [DOI] [PubMed] [Google Scholar]
- Meiqian Z., Leying Z., Chang C. (2018). Astragaloside IV Inhibits Cigarette Smoke-Induced Pulmonary Inflammation in Mice. Inflammation 41, 1671–1680. 10.1007/s10753-018-0811-x [DOI] [PubMed] [Google Scholar]
- Meja K. K., Rajendrasozhan S., Adenuga D., Biswas S. K., Sundar I. K., Spooner G., et al. (2008). Curcumin Restores Corticosteroid Function in Monocytes Exposed to Oxidants by Maintaining HDAC2. Am. J. Respir. Cel Mol Biol 39, 312–323. 10.1165/rcmb.2008-0012OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J. H., Kim M. G., Kim S. M., Park J. W., Chun W., Lee H. J., et al. (2020). 3,4,5-Trihydroxycinnamic Acid Exerts a Protective Effect on Pulmonary Inflammation in an Experimental Animal Model of COPD. Int. Immunopharmacol 85, 106656. 10.1016/j.intimp.2020.106656 [DOI] [PubMed] [Google Scholar]
- Miravitlles M., Soriano J. B., García-Río F., Muñoz L., Duran-Tauleria E., Sanchez G., et al. (2009). Prevalence of COPD in Spain: Impact of Undiagnosed COPD on Quality of Life and Daily Life Activities. Thorax 64, 863–868. 10.1136/thx.2009.115725 [DOI] [PubMed] [Google Scholar]
- Mitani A., Azam A., Vuppusetty C., Ito K., Mercado N., Barnes P. J. (2017). Quercetin Restores Corticosteroid Sensitivity in Cells from Patients with Chronic Obstructive Pulmonary Disease. Exp. Lung Res. 43, 417–425. 10.1080/01902148.2017.1393707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam S. J., Barta P., Mirabolfathinejad S. G., Ammar-Aouchiche Z., Garza N. T., Vo T. T., et al. (2009). Curcumin Inhibits COPD-like Airway Inflammation and Lung Cancer Progression in Mice. Carcinogenesis 30, 1949–1956. 10.1093/carcin/bgp229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro S., Reddy R., Lee J., Warburton D., Driscoll B. (2017). Inhaled Resveratrol Treatments Slow Ageing-Related Degenerative Changes in Mouse Lung. Thorax 72, 451–459. 10.1136/thoraxjnl-2016-208964 [DOI] [PubMed] [Google Scholar]
- Ng D. S., Liao W., Tan W. S., Chan T. K., Loh X. Y., Wong W. S. (2014). Anti-malarial Drug Artesunate Protects against Cigarette Smoke-Induced Lung Injury in Mice. Phytomedicine 21, 1638–1644. 10.1016/j.phymed.2014.07.018 [DOI] [PubMed] [Google Scholar]
- Nie Y. C., Wu H., Li P. B., Luo Y. L., Long K., Xie L. M., et al. (2012). Anti-inflammatory Effects of Naringin in Chronic Pulmonary Neutrophilic Inflammation in Cigarette Smoke-Exposed Rats. J. Med. Food 15, 894–900. 10.1089/jmf.2012.2251 [DOI] [PubMed] [Google Scholar]
- Pan K., Lu J., Song Y. (2021). Artesunate Ameliorates Cigarette Smoke-Induced Airway Remodelling via PPAR-Γ/tgf-β1/Smad2/3 Signalling Pathway. Respir. Res. 22, 91. 10.1186/s12931-021-01687-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. W., Shin N. R., Shin I. S., Kwon O. K., Kim J. S., Oh S. R., et al. (2016). Silibinin Inhibits Neutrophilic Inflammation and Mucus Secretion Induced by Cigarette Smoke via Suppression of ERK-SP1 Pathway. Phytother Res. 30, 1926–1936. 10.1002/ptr.5686 [DOI] [PubMed] [Google Scholar]
- Radicioni G., Ceppe A., Ford A. A., Alexis N. E., Barr R. G., Bleecker E. R., et al. (2021). Airway Mucin MUC5AC and MUC5B Concentrations and the Initiation and Progression of Chronic Obstructive Pulmonary Disease: an Analysis of the SPIROMICS Cohort. Lancet Respir. Med. 10.1016/s2213-2600(21)00079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovina N., Koutsoukou A., Koulouris N. G. (2013). Inflammation and Immune Response in COPD: where Do We Stand? Mediators Inflamm. 2013, 413735. 10.1155/2013/413735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S., Maloney J., Grove L., Wrona C., Berenson C. S. (2006). Airway Inflammation and Bronchial Bacterial Colonization in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 173, 991–998. 10.1164/rccm.200509-1525OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Tian P., Li D., Wu Y., Wan C., Yang T., et al. (2015). Chrysin Suppresses Cigarette Smoke-Induced Airway Inflammation in Mice. Int. J. Clin. Exp. Med. 8, 2001–2008. [PMC free article] [PubMed] [Google Scholar]
- Singla E., Dharwal V., Naura A. S. (2020). Gallic Acid Protects against the COPD-Linked Lung Inflammation and Emphysema in Mice. Inflamm. Res. 69, 423–434. 10.1007/s00011-020-01333-1 [DOI] [PubMed] [Google Scholar]
- Singla E., Puri G., Dharwal V., Naura A. S. (2021). Gallic Acid Ameliorates COPD-Associated Exacerbation in Mice. Mol. Cel Biochem 476, 293–302. 10.1007/s11010-020-03905-5 [DOI] [PubMed] [Google Scholar]
- Song C., Luo B., Gong L. (2017). Resveratrol Reduces the Apoptosis Induced by Cigarette Smoke Extract by Upregulating MFN2. PLoS One 12, e0175009. 10.1371/journal.pone.0175009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H. H., Shin I. S., Woo S. Y., Lee S. U., Sung M. H., Ryu H. W., et al. (2015). Piscroside C, a Novel Iridoid Glycoside Isolated from Pseudolysimachion Rotundum Var. Subinegrum Suppresses Airway Inflammation Induced by Cigarette Smoke. J. Ethnopharmacol 170, 20–27. 10.1016/j.jep.2015.04.043 [DOI] [PubMed] [Google Scholar]
- Sun X., Feng X., Zheng D., Li A., Li C., Li S., et al. (2019). Ergosterol Attenuates Cigarette Smoke Extract-Induced COPD by Modulating Inflammation, Oxidative Stress and Apoptosis In Vitro and In Vivo . Clin. Sci. (Lond) 133, 1523–1536. 10.1042/cs20190331 [DOI] [PubMed] [Google Scholar]
- Szucs B., Szucs C., Petrekanits M., Varga J. T. (2019). Molecular Characteristics and Treatment of Endothelial Dysfunction in Patients with COPD: A Review Article. Int. J. Mol. Sci. 20. 10.3390/ijms20184329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W. S. D., Liao W., Peh H. Y., Vila M., Dong J., Shen H. M., et al. (2018). Andrographolide Simultaneously Augments Nrf2 Antioxidant Defense and Facilitates Autophagic Flux Blockade in Cigarette Smoke-Exposed Human Bronchial Epithelial Cells. Toxicol. Appl. Pharmacol. 360, 120–130. 10.1016/j.taap.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Terry P. D., Dhand R. (2020). Inhalation Therapy for Stable COPD: 20 Years of GOLD Reports. Adv. Ther. 37, 1812–1828. 10.1007/s12325-020-01289-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmeier C. F., Román-Rodríguez M., Singh D., Han M. K., Rodríguez-Roisin R., Ferguson G. T. (2020). Goals of COPD Treatment: Focus on Symptoms and Exacerbations. Respir. Med. 166, 105938. 10.1016/j.rmed.2020.105938 [DOI] [PubMed] [Google Scholar]
- Wang G., Mohammadtursun N., Lv Y., Zhang H., Sun J., Dong J. (2018a2018). Baicalin Exerts Anti-airway Inflammation and Anti-remodelling Effects in Severe Stage Rat Model of Chronic Obstructive Pulmonary Disease. Evid. Based Complement. Alternat Med. 2018, 7591348. 10.1155/2018/7591348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yang T., Wang T., Hao N., Shen Y., Wu Y., et al. (2018b). Phloretin Attenuates Mucus Hypersecretion and Airway Inflammation Induced by Cigarette Smoke. Int. Immunopharmacol 55, 112–119. 10.1016/j.intimp.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Wang S., He N., Xing H., Sun Y., Ding J., Liu L. (2020). Function of Hesperidin Alleviating Inflammation and Oxidative Stress Responses in COPD Mice Might Be Related to SIRT1/PGC-1α/nf-Κb Signaling axis. J. Recept Signal. Transduct Res. 40, 388–394. 10.1080/10799893.2020.1738483 [DOI] [PubMed] [Google Scholar]
- Wang W., Wu W., Wang B., Gao F. (2021). Effect of Houttuynia on Improving Lung Injury in Chronic Obstructive Pulmonary Disease by Regulating the TLR4 Signaling Pathway. Food Sci. Nutr. 9, 3389–3396. 10.1002/fsn3.1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zha G., Zou J. J., Wang X., Li C. N., Wu X. J. (2019). Berberine Attenuates Cigarette Smoke Extract-Induced Airway Inflammation in Mice: Involvement of TGF-β1/Smads Signaling Pathway. Curr. Med. Sci. 39, 748–753. 10.1007/s11596-019-2101-8 [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang C., Huang G., Han D., Guo Y., Meng X., et al. (2015). Resveratrol Inhibits Dysfunction of Dendritic Cells from Chronic Obstructive Pulmonary Disease Patients through Promoting miR-34. Int. J. Clin. Exp. Pathol. 8, 5145–5152. [PMC free article] [PubMed] [Google Scholar]
- Wang X. L., Li T., Li J. H., Miao S. Y., Xiao X. Z. (2017). The Effects of Resveratrol on Inflammation and Oxidative Stress in a Rat Model of Chronic Obstructive Pulmonary Disease. Molecules 22. 10.3390/molecules22091529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Tan B., Zhang H., Guo Y., Tu Y., Qiu F., et al. (2017). Effects of Sodium Houttuyfonate on Pulmonary Inflammation in COPD Model Rats. Inflammation 40, 2109–2117. 10.1007/s10753-017-0650-1 [DOI] [PubMed] [Google Scholar]
- Xie Y., He Q., Chen H., Lin Z., Xu Y., Yang C. (2019). Crocin Ameliorates Chronic Obstructive Pulmonary Disease-Induced Depression via PI3K/Akt Mediated Suppression of Inflammation. Eur. J. Pharmacol. 862, 172640. 10.1016/j.ejphar.2019.172640 [DOI] [PubMed] [Google Scholar]
- Xu D., Wan C., Wang T., Tian P., Li D., Wu Y., et al. (2015). Berberine Attenuates Cigarette Smoke-Induced Airway Inflammation and Mucus Hypersecretion in Mice. Int. J. Clin. Exp. Med. 8, 8641–8647. [PMC free article] [PubMed] [Google Scholar]
- Xue W. H., Shi X. Q., Liang S. H., Zhou L., Liu K. F., Zhao J. (2015). Emodin Attenuates Cigarette Smoke Induced Lung Injury in a Mouse Model via Suppression of Reactive Oxygen Species Production. J. Biochem. Mol. Toxicol. 29, 526–532. 10.1002/jbt.21723 [DOI] [PubMed] [Google Scholar]
- Xueshibojie L., Duo Y., Tiejun W. (2016). Taraxasterol Inhibits Cigarette Smoke-Induced Lung Inflammation by Inhibiting Reactive Oxygen Species-Induced TLR4 Trafficking to Lipid Rafts. Eur. J. Pharmacol. 789, 301–307. 10.1016/j.ejphar.2016.07.047 [DOI] [PubMed] [Google Scholar]
- Yang D., Zhang W., Song L., Guo F. (2013). Andrographolide Protects against Cigarette Smoke-Induced Lung Inflammation through Activation of Heme Oxygenase-1. J. Biochem. Mol. Toxicol. 27, 259–265. 10.1002/jbt.21483 [DOI] [PubMed] [Google Scholar]
- Yang T., Luo F., Shen Y., An J., Li X., Liu X., et al. (2012). Quercetin Attenuates Airway Inflammation and Mucus Production Induced by Cigarette Smoke in Rats. Int. Immunopharmacol 13, 73–81. 10.1016/j.intimp.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Yu D., Liu X., Zhang G., Ming Z., Wang T. (2018a). Isoliquiritigenin Inhibits Cigarette Smoke-Induced COPD by Attenuating Inflammation and Oxidative Stress via the Regulation of the Nrf2 and NF-Κb Signaling Pathways. Front. Pharmacol. 9, 1001. 10.3389/fphar.2018.01001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N., Sun Y. T., Su X. M., He M., Dai B., Kang J. (2019a). Eucalyptol Protects Lungs against Bacterial Invasion through Attenuating Ciliated Cell Damage and Suppressing MUC5AC Expression. J. Cel Physiol 234, 5842–5850. 10.1002/jcp.26359 [DOI] [PubMed] [Google Scholar]
- Yu N., Sun Y. T., Su X. M., He M., Dai B., Kang J. (2018b). Treatment with Eucalyptol Mitigates Cigarette Smoke-Induced Lung Injury through Suppressing ICAM-1 Gene Expression. Biosci. Rep. 38. 10.1042/bsr20171636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Seow H. J., Wang H., Anthony D., Bozinovski S., Lin L., et al. (2019b). Matrine Reduces Cigarette Smoke-Induced Airway Neutrophilic Inflammation by Enhancing Neutrophil Apoptosis. Clin. Sci. (Lond) 133, 551–564. 10.1042/cs20180912 [DOI] [PubMed] [Google Scholar]
- Yuan J., Liu R., Ma Y., Zhang Z., Xie Z. (2018). Curcumin Attenuates Airway Inflammation and Airway Remolding by Inhibiting NF-Κb Signaling and COX-2 in Cigarette Smoke-Induced COPD Mice. Inflammation 41, 1804–1814. 10.1007/s10753-018-0823-6 [DOI] [PubMed] [Google Scholar]
- Zeng X., Liu X., Bao H. (2021). Sulforaphane Suppresses Lipopolysaccharide- and Pam3CysSerLys4-Mediated Inflammation in Chronic Obstructive Pulmonary Disease via Toll-like Receptors. FEBS Open Bio 11, 1313–1321. 10.1002/2211-5463.13118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Cao L., Wang Z., Feng H., Cai X., Xu M., et al. (2019). Salidroside Mitigates Skeletal Muscle Atrophy in Rats with Cigarette Smoke-Induced COPD by Up-Regulating Myogenin and Down-Regulating Myostatin Expression. Biosci. Rep. 39. 10.1042/bsr20190440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. F., Zhang J., Li R. (2015a). Salvianolic Acid B Attenuates Lung Inflammation Induced by Cigarette Smoke in Mice. Eur. J. Pharmacol. 761, 174–179. 10.1016/j.ejphar.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Zhang H., Liu B., Jiang S., Wu J. F., Qi C. H., Mohammadtursun N., et al. (2021). Baicalin Ameliorates Cigarette Smoke-Induced Airway Inflammation in Rats by Modulating HDAC2/NF-Κb/pai-1 Signalling. Pulm. Pharmacol. Ther. 70, 102061. 10.1016/j.pupt.2021.102061 [DOI] [PubMed] [Google Scholar]
- Zhang L., Guo X., Xie W., Li Y., Ma M., Yuan T., et al. (2015b). Resveratrol Exerts an Anti-apoptotic Effect on Human Bronchial Epithelial Cells Undergoing Cigarette Smoke Exposure. Mol. Med. Rep. 11, 1752–1758. 10.3892/mmr.2014.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Tang J., Li Y., Xie Y., Shan H., Chen M., et al. (2017). Curcumin Attenuates Skeletal Muscle Mitochondrial Impairment in COPD Rats: PGC-1α/SIRT3 Pathway Involved. Chem. Biol. Interact 277, 168–175. 10.1016/j.cbi.2017.09.018 [DOI] [PubMed] [Google Scholar]
- Zhang X. F., Ding M. J., Cheng C., Zhang Y., Xiang S. Y., Lu J., et al. (2020). Andrographolide Attenuates Oxidative Stress Injury in Cigarette Smoke Extract Exposed Macrophages through Inhibiting SIRT1/ERK Signaling. Int. Immunopharmacol 81, 106230. 10.1016/j.intimp.2020.106230 [DOI] [PubMed] [Google Scholar]
- Zhou L., Gu W., Kui F., Gao F., Niu Y., Li W., et al. (2021). The Mechanism and Candidate Compounds of Aged Citrus Peel (Chenpi) Preventing Chronic Obstructive Pulmonary Disease and its Progression to Lung Cancer. Food Nutr. Res. 65. 10.29219/fnr.v65.7526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong D. D., Liu X. M., Li J. H., Ouyang R. Y., Long Y. J., Chen P., et al. (2021). Resveratrol Attenuates Cigarette Smoke Induced Endothelial Apoptosis by Activating Notch1 Signaling Mediated Autophagy. Respir. Res. 22, 22. 10.1186/s12931-021-01620-3 [DOI] [PMC free article] [PubMed] [Google Scholar]