Abstract
Background
Immune checkpoint inhibitors (ICIs) have become standard treatments for lung cancer patients. Immune checkpoint inhibitor-related pneumonitis (CIP) was the leading cause of death among ICIs-related adverse events (irAEs). Recurrent episodes of CIP without rechallenge of ICIs were reported in several cases and maybe a unique feature of CIP. Knowledge gaps remain regarding the rate and risk factors associated to CIP’s recurrence.
Methods
Data from 1,102 lung cancer patients receiving ICIs treatment between January 2016 and January 2021 were retrospectively collected and analyzed. CIP was diagnosed according to typical clinical features and/or new typical imaging changes. Recurrence of CIP (CIP-R) was defined as recurrent CIP after initial CIP improved after proper treatment. Logistic regression was used to assess risk factors associated with CIP recurrence.
Results
Eighty out of 1,102 (7.26%) patients were diagnosed with CIP. Twenty of those 78 (25.64%) patients suffered CIP-R, 2 patients died and were therefore excluded from the denominator. The median onset of initial pneumonitis for patients without and with recurrence was 3.49 months [interquartile range (IQR), 0.26–31.93 months] and 2.78 months (IQR, 1.22–20.93 months), respectively (P=0.48). The median interval duration between initial CIP and CIP-R was 1.54 months (IQR, 0.98–16.70 months). Recurrence of CIP was more common in males (P=0.03), squamous histology (P=0.016), and in patients who received chest radiotherapy (P=0.049). The duration of prednisolone equivalent dose ≥15 mg/day in CIP-R was significantly shorter, at 3.71 weeks (2.86–6.57 weeks) compared with 6.36 weeks in those without recurrence (IQR, 3.12–9.86 weeks) (P=0.001). Non-squamous histology [odds ratio (OR), 0.182; 95% confidence interval (CI): 0.038–0.860; P=0.031] and prolonged administration of prednisolone equivalent dose ≥15 mg/day for more than 4 weeks (OR, 0.082; 95% CI: 0.02–0.342; P=0.001) were independently associated with a decreased odds of CIP-R development.
Conclusions
CIP-R in a real-world lung cancer cohort is not uncommon, both in patients with and without rechallenge of ICIs. A duration of prednisolone equivalent dose ≥15 mg/day of at least 4 weeks during the tapering process of corticosteroids were recommend in patients with CIP.
Keywords: Lung cancer, immune checkpoint inhibitors (ICIs), ICIs-related adverse event (irAE), recurrent pneumonitis
Introduction
Immune checkpoint inhibitors (ICIs) have become standard treatments for lung cancer, including the first- and second-line therapy of advanced non-small cell lung cancer (NSCLC) without driver mutations, the first-line therapy of extensive-stage small cell lung cancer (SCLC) and the consolidative therapy of unresectable stage III NSCLC after concurrent chemoradiotherapy (1-5). However, despite clinical benefits characterized by a significant increase of long-term survival, ICIs use is associated with a wide spectrum of immune-related adverse events (irAEs) whose pathogenetic mechanisms are still largely unknown (6).
Although clinical trials suggest a rare incidence of immune checkpoint inhibitor-related pneumonitis (CIP) (approximately 4–6%), restrictive enrollment criteria may underestimate the true incidence in clinical practice (7,8). Indeed, the incidence of CIP reported in real-world lung cancer cohorts ranges from 5% to 19% (9,10). CIP is a serious adverse event in ICIs treated patients, and is the leading cause of death among the different irAEs, with an overall mortality rate of approximately 0.45% (11,12). However, the inflammatory characteristics of CIP that result in morbidity and mortality are also associated with enhanced programmed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) inhibitor efficacy in NSCLC (13).
Although irAE is generally reversible after appropriately corticosteroids treatment, several retrospective studies have reported recurrence of CIP (CIP-R) (14-18). Nishino et al. first reported that a patient with lung cancer receiving anti-PD-1 treatment experienced recurrent pneumonitis in absence of ICIs-rechallenge after a documented resolution, named a “pneumonitis flare” (14). Asher et al. reported three patients with melanoma who experienced unprovoked CIP-R after complete resolution without ICIs-rechallenge, named a “unprovoked recurrent pneumonitis” (16). Dolladille et al. reported a 28.8% recurrence rate of the same irAEs after a rechallenge with the same ICIs, and pneumonitis was associated with a higher recurrence rate compared with other irAEs (17). Recurrent CIP seem to be not uncommon, CIP-R occurred without rechallenge of ICIs maybe a unique feature of CIP, which was similar to the durable and persisted response after ICIs discontinuation (14). However, there is limited information on patients of CIP-R in recent research, especially regarding unprovoked recurrent pneumonitis.
The aim of this study was to characterize the clinical and radiological features, cancer-related and CIP-related factors of lung cancer patients experiencing CIP-R, in order to identify potential risk factors to CIP-R either related or not to ICIs rechallenge. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-168/rc).
Methods
Patient selection
This retrospective study was conducted on patients who were diagnosed with lung cancer and received ICIs treatment in the First Medical Center of Chinese People’s Liberation Army (PLA) General Hospital between January 2016 and January 2021. Patients who were ultimately diagnosed with CIP were included in analysis.
The inclusion criteria included patients who were pathologically diagnosed with locally advanced/advanced lung cancer (including NSCLC and SCLC); patients who received at least 1 cycle of PD-1/PDL-1 inhibitors and/or CTLA-4 inhibitors, whether monotherapy or combined with other drugs; and patients who developed typical clinical features and/or new typical imaging changes after ICIs treatment and were ultimately diagnosed with CIP after evaluation by a multidisciplinary team. The exclusion criteria included patients with other lung diseases with a clear alternative etiology, such as carcinomatous lymphangitis or active lung infection.
For patients who received corticosteroids for CIP treatment, a cumulative prednisolone equivalent dose was calculated. Since equivalent prednisolone 15 mg/day is the maintenance dose commonly used in rheumatic diseases (19), this time point is marked as a key node of corticosteroid therapy. CIP-R was defined as recurrent CIP after initial CIP improved with regard to both chest CT and clinical symptoms after proper corticosteroid treatment according to NCCN guideline and the dose of corticosteroid tapered to less than equivalent prednisolone 15 mg/day (methylprednisolone 12 mg), regardless of complete discontinuation or rechallenge with immunotherapy lung.
Data collection
For all the patients, detailed clinical data were retrospectively collected from medical records, including demographic characteristics, histologic subtype, medical history, molecular pathology and PD-L1 expression status, previous and concurrent cancer treatments, duration of ICIs therapy, antitumor efficacy of ICIs, clinical manifestations and outcomes of CIP, rechallenge with ICIs and recurrence of CIP. A chest CT scan obtained 3 months before the initiation of ICIs treatment was recorded as baseline. Baseline chest CT scan findings, including the presence of emphysema and ILA, were also recorded.
ILA were defined by at least one of the following CT findings in no less than two lobes of the lung: ground glass appearance (GGA), reticular opacity, honeycombing or traction bronchiectasis, diffuse centrilobular nodularity, or nonemphysematous cysts on CT images (20,21). Patients with a history of drug-induced pneumonitis or those with radiation-related fibrotic lesions alone in the lung were excluded from the ILA group. Composite obstructive lung disease includes chronic obstructive pulmonary disease (COPD) by history, obstruction on spirometry, or emphysema on baseline CT images (22). CT images were evaluated by two pulmonologists who were unaware of patient outcomes by a sequential reading method. Pneumonitis grading was evaluated by the treating investigators according to the Common Toxicity Criteria for Adverse Events (CTCAE version 4.0).
Follow-up was up to August 15, 2021, allowing sufficient time to observe pneumonitis recurrence.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Chinese PLA General Hospital (No. S2021-562-01). Individual consent for this retrospective analysis was waived.
Statistical analysis
Time to the onset of CIP was defined as the time from the first dose of an ICIs to the first occurrence of CIP-related symptoms or imaging findings in asymptomatic patients. The time to CIP-R was defined as from the time of initial CIP to the time of CIP-R. Continuous variables are described as the median [interquartile range (IQR)] and were compared using t-tests or the Mann-Whitney U test. Categorical variables were compared using the chi-square test or Fisher’s exact test. Univariate and multivariate logistic regression analyses were performed on risk factors for the odds of CIP-R, including patient demographics (e.g., sex), medical histories (e.g., ILA), cancer-related factors (e.g., tumor histology), CIP-related factors (e.g., CTCAE grade). The multivariate analysis included predictors associated with CIP risk at the 0.15 significance level from the univariate analysis. Six patients who did not receive corticosteroids were excluded from the analysis of risk factors.
Kaplan-Meier estimates for median progression-free survival (PFS) and overall survival (OS) after ICIs initiation were produced for patients; log-rank and Wilcoxon tests were conducted to assess differences. The hazard ratio (HR) was estimated by a Cox regression model. All reported p values were two sided, and a significant difference was considered at the 5% level. Statistical analyses were conducted using SPSS Statistics for Windows (Version 25.0; IBM), and the figures were made by GraphPad Prism 9 (GraphPad Software Inc.).
Results
Patient characteristics
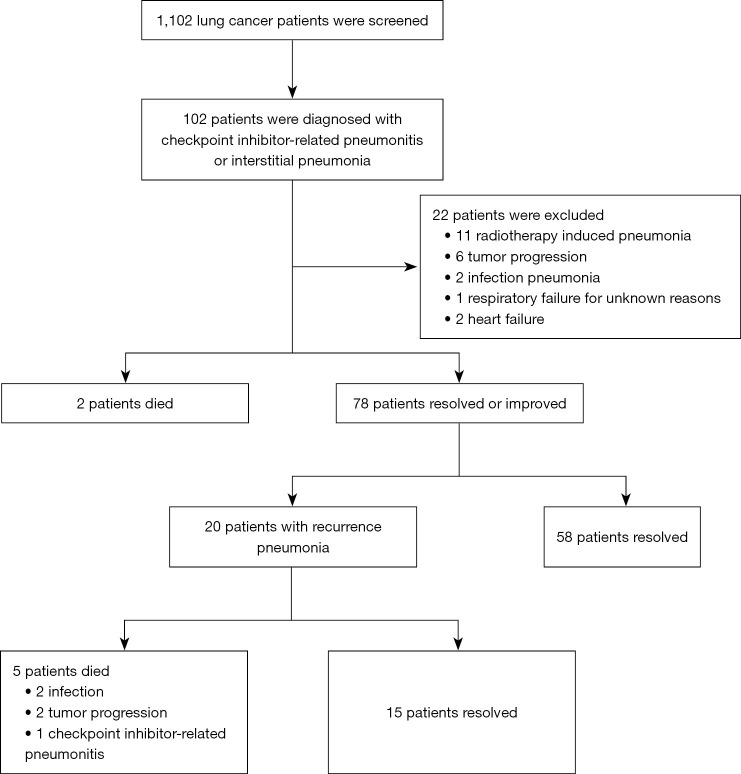
From January 2016 to January 2021, 1,102 patients with lung cancer were enrolled in this study. After reviewing the medical records and imaging data, 102 demonstrated features of pneumonitis. Of those, 80 patients (7.26%) were included in analysis, and 22 patients were excluded because of radiotherapy-induced pneumonitis, infection pneumonitis, and heart failure (Figure 1). Seventy-eight out of 80 patients who experienced CIP clinical improvement or resolution after initial treatment were definitively included in this study (2 patients died of pneumonitis although given high-dose steroids), Among these, 20 patients (25.64%) experienced CIP-R, and 5 patients (6.4%) died during treatment of CIP.
Figure 1.
Screening flow chart.
Detailed patients’ demographic, therapeutic, and clinical information are reported in Table 1. Demographic characteristics were similar between the CIP-R and no recurrence groups (CIP-NR) except for both sex and tumor type. Indeed all the patients with CIP-R were male, with squamous cell carcinoma as the predominant histological subtype, Conversely SCLC accounted for a larger proportion of the no recurrence group (P=0.016).
Table 1. Patient and treatment characteristics.
| Characteristics | All patients* | CIP without recurrence | CIP-R | P value |
|---|---|---|---|---|
| Total | 80 | 58 | 20 | – |
| Age, years | 0.8 | |||
| ≤65 | 38 | 26 | 10 | |
| >65 | 42 | 32 | 10 | |
| Gender | 0.03 | |||
| Male | 68 | 46 | 20 | |
| Female | 12 | 12 | 0 | |
| Present/past smoker | 0.22 | |||
| Yes | 61 | 47 | 13 | |
| No | 19 | 11 | 7 | |
| ECOG PS | 0.33 | |||
| 0–1 | 63 | 45 | 18 | |
| ≥2 | 17 | 13 | 2 | |
| Tumor histology | 0.016 | |||
| Non-squamous NSCLC | 33 | 28 | 4 | |
| Squamous NSCLC | 30 | 17 | 13 | |
| SCLC | 17 | 13 | 3 | |
| Stage of tumor | 0.61 | |||
| I–IIIA | 2 | 2 | 0 | |
| IIIB–IIIC | 23 | 16 | 7 | |
| IV | 55 | 40 | 13 | |
| Baseline lung disease | 0.43 | |||
| ILA | 16 | 13 | 2 | |
| Composite obstructive lung disease** | 20 | 15 | 5 | |
| No | 44 | 30 | 13 | |
| Thoracic radiotherapy | 0.049 | |||
| Concurrent chest radiotherapy | 21 | 12 | 9 | |
| Previous chest radiotherapy | 12 | 8 | 4 | |
| No | 47 | 38 | 7 | |
| Line of ICIs treatment | 0.94 | |||
| First-line | 53 | 39 | 13 | |
| Second-line | 16 | 12 | 4 | |
| ≥ Third-line | 11 | 7 | 3 | |
| PD-L1 expression | 0.69 | |||
| 0 | 19 | 15 | 3 | |
| 1–49% | 31 | 22 | 9 | |
| ≥50% | 20 | 13 | 6 | |
| Unknown | 10 | 8 | 2 | |
| EGFR/ALK/Ros1/Ret mutation/fusion | 0.7 | |||
| Yes | 5 | 3 | 1 | |
| No | 46 | 35 | 10 | |
| Unknown | 29 | 20 | 9 | |
| Regimen of immune therapy | 0.69 | |||
| Monotherapy | 14 | 11 | 3 | |
| Combination therapy | 66 | 47 | 17 | |
| PD-1/PD-L1 + chemotherapy | 56 | 41 | 14 | |
| PD-1/PD-L1 + antiangiogenesis | 6 | 4 | 1 | |
| PD-1 + ipilimumab | 4 | 2 | 2 | |
| Best objective response of ICIs | 0.81 | |||
| Complete/partial response | 52 | 48 | 14 | |
| Stable disease | 20 | 14 | 5 | |
| Progression of disease | 7 | 6 | 1 | |
| Not evaluated | 1 | 1 | 0 | |
*, includes 2 patients with grade 5 CIP; **, includes COPD by history, obstruction on spirometry, or emphysema on baseline chest CT scan. ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICIs, immune checkpoint inhibitors; CIP, checkpoint inhibitor-related pneumonitis; CIP-R, recurrence of CIP; ILA, interstitial lung alterations; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; Ros1, ROS proto-oncogene 1, receptor tyrosine kinase; Ret, rearranged during transfection.
A history of composite obstructive lung disease or ILA was identified in 20 patients (25%) and 16 patients (20%), respectively. Fifty-five patients (68.8%) were stage IV, and ICIs were used predominantly administered as first-line therapy in 53 patients (66.3%). PD-L1 expression was evaluated in 70 cases (87.5%), and 51 (72.8%) showed positive expression (PD-L1 ≥1%). Three patients had EGFR mutations, 1 patient had ALK fusion and 1 patient had RET fusion. Sixty-six patients (82.5%) received PD-1/PD-L1 based combination therapy, including 56 combined with chemotherapy, 6 with antiangiogenics and 4 with CTLA-4 antibody. Thirty-three patients (41.3%) had a prior history of chest radiotherapy or concurrent chest radiotherapy (within 6 weeks of ICIs therapy). In detail the percentage of patients who received chest radiotherapy (previous or concurrent) was significantly higher among patients with CIP-R subgroup (P=0.049).
Clinical features of CIP
The clinical features of the CIP-R patients included in the study are shown in Table 2. The median onset of pneumonitis for patients without and with recurrence was 3.49 months (IQR, 0.26–31.93 months) and 2.78 months (IQR, 1.22–20.93 months), respectively, with no significant difference in onset between the two groups (P=0.48). The median interval duration between initial CIP and CIP-R was 1.54 months (IQR, 0.98–16.70 months).
Table 2. Clinical features and management of CIP.
| Characteristics | CIP without recurrence | CIP-R | P value | |
|---|---|---|---|---|
| Time to CIP, median (IQR) | 3.49 (0.26–31.93) | 2.78 (1.22–20.93) | 0.48 | |
| Time to recurrence (month), median (IQR) | n/a | 1.54 (0.98–16.7) | ||
| Radiologic features | 0.96 | |||
| COP | 18 | 7 | ||
| NSIP | 9 | 3 | ||
| AIP | 9 | 3 | ||
| HP | 6 | 1 | ||
| NOS | 16 | 6 | ||
| CTCAE grade | 0.331 | |||
| 1 | 6 | 0 | ||
| 2 | 33 | 10 | ||
| 3 | 12 | 6 | ||
| 4 | 7 | 4 | ||
| Starting dose of equivalent MP | 0.053 | |||
| 0 | 6 | 0 | ||
| 0–1 mg/kg | 16 | 2 | ||
| 1–2 mg/kg | 23 | 14 | ||
| >2 mg/kg | 13 | 4 | ||
| Duration of prednisolone equivalent dose>15 mg/day | ||||
| Median (weeks) (IQR) | 6.36 (3.12–9.86) | 3.71 (2.86–6.57) | 0.001 | |
| 0 | 6 | 0 | 0 | |
| <4 weeks | 7 | 13 | ||
| 4–6 weeks | 18 | 6 | ||
| 6–8 weeks | 17 | 1 | ||
| >8 weeks | 10 | 0 | ||
| Other organs involved with irAEs | 0.61 | |||
| Yes | 22 | 9 | ||
| No | 36 | 11 | ||
| Rechallenges with ICIs | 1 | |||
| Yes | 16 | 5 | ||
| No | 42 | 15 | ||
IQR, interquartile range; ICIs, immune checkpoint inhibitors; CIP, checkpoint inhibitor-related pneumonitis; CIP-R, recurrence of CIP; COP, cryptogenic organizing pneumonia; NSIP, on-specific interstitial pneumonia; AIP, acute interstitial pneumonia; HP, hypersensitivity pneumonitis; NOS, non-specified; CTCAE, Common Toxicity Criteria for Adverse Events; MP, methylprednisolone.
According to the CTCAE v4.0 criteria, 49 (62.8%) patients experienced grade 1 or 2 CIP, while 32 (41.2%) patients experienced grade 3 or 4 CIP. After recurrence, 1 patient experienced grade 5 CIP, 4 patients experienced grade 4 CIP, 13 patients experienced grade 3 CIP, and 2 patients experienced grade 2 CIP. Although there was no grade 1 in the CIP-R group, severe CIP (grade 3–4) was similar between the 2 groups. The grade of CIP was upgraded in 8 patients experiencing recurrence: 5 patients from G2 to G3, 2 patients from G3 to G4, and 1 patient from G4 to G5. The CIP grade was downgraded in 1 patient (from G4 to G3).
Concurrent irAEs occurred in 31 patients (39.7%), including hypothyroidism (10 cases), dermatitis (9 cases), hepatitis (4 cases), nephritis (2 cases), hypophysis (2 cases), myocarditis (2 case) and one case each of pancreatitis, thrombocytopenia and tuberculosis.
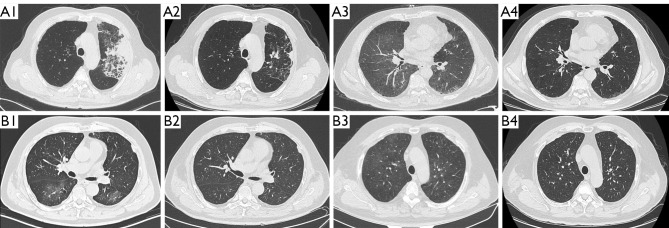
The baseline chest CT lesions for patients with untreated CIP included ground glass opacity (GGO) (32/78, 41.0%), consolidation (24/78, 30.8%), traction bronchiectasis (20/78, 25.6%) and reticular opacities (18/78, 23.1%). According to the classification of idiopathic interstitial pneumonia imaging patterns, 32.1% (25/78) met the pattern for cryptogenic organizing pneumonia (COP), 15.4% (12/78) met the pattern for non-specific interstitial pneumonia (NSIP), 15.4% (12/78) met the pattern for acute interstitial pneumonia (AIP), 9.0% (7/78) met the pattern for hypersensitivity pneumonitis (HP), and 28.2% (22/78) were non-specified (NOS). The radiological pattern may be changed (12/20, 60%) or not changed (8/20, 40%) in CIP-R cases (Figures 1,2).
Figure 2.
Representative radiological features of recurrent CIP related to ICIs rechallenge (A) and unprovoked recurrent pneumonitis (B). (A1) A 70-year-old male with squamous lung cancer. CT shows new consolidations in the left upper lobe after 3 cycles of nivolumab and ipilimumab, classified as grade 2; (A2) consolidations are nearly completely absorbed 4 weeks after glucocorticoid treatment; (A3) 3 days after rechallenge with nivolumab, CT shows diffuse ground glass opacities, suspicious for CIP recurrence, grade 3; (A4) 8 weeks after glucocorticoid treatment, resolution of the pulmonary opacities is observed. (B1) A 66-year-old male with squamous lung cancer and new flake ground glass shadows in both lungs after 1 year of pembrolizumab treatment, classified as grade 2; (B2) 3 weeks after glucocorticoid treatment, CT shows resolution of the CIP; (B3) 4 weeks later, CT shows diffuse ground glass opacities without rechallenge of ICIs; (B4) 8 weeks after steroid treatment shows resolution of the pulmonary opacities. CIP, checkpoint inhibitor-related pneumonitis; ICIs, immune checkpoint inhibitors; CT, computed tomography.
In addition to treatment discontinuation, 72 (92.3%) patients received corticosteroids and 7 patients received additional immunosuppressive treatments (including 4 infliximab and 3 tocilizumab), with 2 of them still experiencing a recurrence of pneumonitis. After recurrence, 5 patients (25%) received further immunosuppressive treatments, including 1 infliximab and 4 tocilizumab.
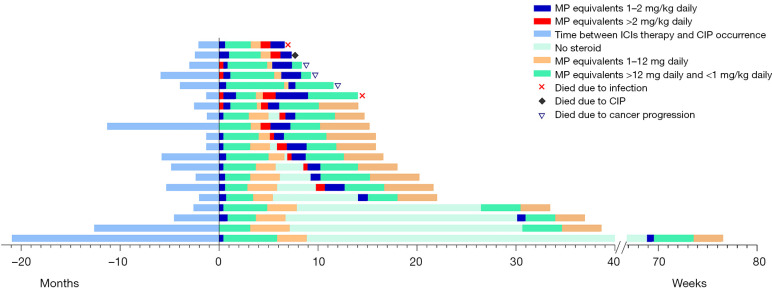
For the initial case of CIP, 17 patients received the dose of equivalent MP >2 mg/kg, 37 patients received a dose of 1–2 mg/kg and 18 patients received a dose of 0–1 mg/kg. The starting dose of corticosteroids was similar between the CIP-NR and the CIP-R groups, However, the duration of prednisolone equivalent dose ≥15 mg/day was significantly longer in the CIP group with 6.36 weeks (IQR, 3.12–9.86 weeks) as compared to the CIP-R group with 3.71 weeks (2.86–6.57 weeks) (Figure 3).
Figure 3.
Clinical course of recurrence of CIP. Individual clinical courses of patients with pneumonitis, from initiation of ICIs therapy, the first episode of CIP, the recurrence of CIP, to last follow-up or death. In most patients, there is sufficient follow-up time that minimizes the possibility of the recurrence of pneumonitis. The median onset of CIP for patients without recurrence and with recurrence was 3.49 months (IQR, 0.26–31.93 months) and 2.78 months (IQR, 1.22–20.93 months), respectively. The median interval duration between initial and recurrent CIP was 1.54 months (IQR, 0.98–20.93 months). CIP, checkpoint inhibitor-related pneumonitis; ICIs, immune checkpoint inhibitors; MP, methylprednisolone; IQR, interquartile range.
Twenty-one patients were rechallenged after recovery from initial CIP, 5 patients (23.8%) developed CIP-R, and 15 patients (26.3%) without rechallenges of ICIs experienced CIP-R. Sixteen patients accepted the same ICIs as rechallenge, including 15 patients treated with PD-1/PD-L1 inhibitors and 1 patient treated with PD-1 combined with CTLA-4 inhibitors. Four patients accepted another PD-1 inhibitor, while 1 patient who previously accepted a PD-1 inhibitor received double ICIs therapy (combined with a CTLA-4 inhibitor).
Risk factors for CIP-R
Non-squamous NSCLC subtype (OR, 0.187; 95% CI: 0.052–0.667) and duration of prednisolone equivalent dose ≥15 mg/day (OR, 0.084; 95% CI: 0.025–0.283) were significantly associated with a decreased risk of CIP-R development, while concurrent chest radiotherapy (OR, 4.071; 95% CI: 1.249–13.275) was associated to an increased risk of CIP-R (Table 3).
Table 3. Logistic regression analysis of potential risk factors for CIP-R development.
| Characteristics | Univariate analyses | Multivariate analyses | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Gender (male vs. female) | 0 | 0–∞ | 0.999 | – | – | – | |
| Smoker (yes vs. no) | 2.301 | 0.774–7.114 | 0.148 | – | – | – | |
| ECOG PS (≥2 vs. 0–1) | 0.385 | 0.079–1.878 | 0.238 | – | – | – | |
| Histologic type (non-Sq vs. Sq) | 0.187 | 0.052–0.667 | 0.01 | 0.182 | 0.038–0.860 | 0.031 | |
| Histologic type (SCLC vs. Sq) | 0.302 | 0.071–1.284 | 0.105 | 0.576 | 0.1–3.318 | 0.537 | |
| Stage IV vs. others | 1.197 | 0.409–3.503 | 0.743 | – | – | – | |
| Driven gene mutation (yes vs. no) | 1.036 | 0.102–10.572 | 0.976 | – | – | – | |
| ILA vs. no | 0.769 | 0.231–2.562 | 0.669 | – | – | – | |
| Composite obstructive lung disease vs. no | 0.355 | 0.070–1.803 | 0.212 | – | – | – | |
| Second-line treatment vs. first-line treatment | 1.119 | 0.304–4.123 | 0.866 | – | – | – | |
| Third-line or later treatment vs. first-line treatment | 1.319 | 0.297–5.852 | 0.716 | – | – | – | |
| Concurrent chest radiotherapy vs. no | 4.071 | 1.249–13.275 | 0.02 | 3.245 | 0.748–14.081 | 0.116 | |
| Previous chest radiotherapy vs. no | 2.714 | 0.639–11.523 | 0.176 | 2.833 | 0.482–16.654 | 2.833 | |
| PD-1/PD-L1 antibody (monotherapy vs. combination therapy) | 1.349 | 0.330–5.514 | 0.677 | – | – | – | |
| Grade 3–4 vs. 1–2 | 2.053 | 0.730–5.772 | 0.173 | – | – | – | |
| Initial corticosteroids dose (equal methylprednisolone ≥2 vs. <2 mg/kg) | 0.865 | 0.246–3.043 | 0.822 | – | – | – | |
| Duration of prednisolone equivalent dose ≥15 mg/day (>4 vs. <4 weeks) | 0.084 | 0.025–0.283 | 0 | 0.082 | 0.02–0.342 | 0.001 | |
| Rechallenge with ICIs (yes vs. no) | 0.875 | 0.273–2.804 | 0.822 | – | – | – | |
CIP-R, recurrence of checkpoint inhibitor-related pneumonitis; ECOG PS, Eastern Cooperative Oncology Group Performance Status; SCLC, small cell lung cancer; Sq, squamous non-small cell lung cancer; non-Sq, non-squamous cell non-small cell lung cancer ILA, interstitial lung alteration; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; ICIs, immune checkpoint inhibitors; ILA, interstitial lung alterations; OS, overall survival; CI, confidence interval.
Multivariate logistic regression analysis was performed on 72 (92%) patients as 6 patients who did not receive corticosteroids were excluded. The results showed that both non-squamous NSCLC histology (OR, 0.182; 95% CI: 0.038–0.860) and duration of prednisolone equivalent dose ≥15 mg/day (OR, 0.082; 95% CI: 0.02–0.342) for more than 4 weeks were independently associated with a decreased odds for the CIP-R development.
ICIs response and survival in patients with CIP
Regardless of the tumor histology, stage, and ICIs regimen the objective response rate (ORR) was 47.2% (51/77), and the disease control rate (DCR) was 91.7% (70/77), with 4 cases of complete remission (CR), 47 cases of partial response (PR), 19 cases of stable disease (SD), 7 cases of progressive disease (PD) and 1 unevaluable patient. Up to August 15, 2021, 23 patients had not progressed, and 38 patients were still alive. The median follow-up time for the entire cohort is 17.40 months (range 2.92-64.85 months). The median PFS was 11.07 months (95% CI: 3.59–18.56 months) and 11.27 months (95% CI: 4.34–18.20 months) for the CIP-NR and CIP-R groups, respectively. The median OS after ICIs initiation was 25.92 months (95% CI: 13.52–38.33 months) and 19.29 months (95% CI: 11.58–26.98 months) for the CIP-NR and CIP-R groups, respectively. There was no difference in PFS or OS between the two groups (Figure S1).
Discussion
In this study, we present CIP recurrence incidence, clinical features and survival outcomes in a real-world lung cancer cohort receiving ICIs-therapies and report important risk factors for the development of CIP-R. In this cohort, the incidence of CIP was 7.26%, which is consistent with prior real-world clinical reports (9,22). The recurrence rate of CIP was 25.6%, including 26.3% of patients without and 23.8% of patients with ICIs-rechallenge, highlighting the importance of risk assessment for CIP recurrence in lung cancer patients.
The results of our study suggest that, in lung cancer patients, tumor histology, concurrent chest radiotherapy, and a duration of prednisolone equivalent dose ≥15 mg/day may be risk factors associated with CIP recurrence. Prior clinical trials have shown that non-squamous NSCLC may be associated with a lower risk of CIP, reported to be around 2.4%, compared with 4.5% in squamous NSCLC (23,24). Similar results were reported also in retrospective real-world studies (9). However, tumor histology may be associated with other patient clinical or biological characteristics, such as smoking status and baseline lung disease.
Whether radiotherapy may increase the risk of CIP is still under debate. A low rate of pneumonitis (1.1%) was reported by Avrillon et al. in a French study of 591 participants receiving durvalumab after definitive chemoradiotherapy, but a higher rate of pneumonitis (61%) was reported by Miura et al. in a similar patient group (25,26). In clinical trials, the risk of pneumonitis was increased in patients who received prior thoracic radiation (27). The observed increased recurrence rate of pneumonitis might be attributed to the poor lung function induced by prior thoracic radiotherapy or NSCLC itself, rather than anti-PD-1 therapy, since CIP induced by these agents is preferentially located within lung areas involved in radiation fields (28).
Although the exact pathogenetic mechanisms underlying the irAEs occurrence are still unknown, CIP may be considered as a special immune-mediated interstitial lung disease based on its immunologic mechanisms of action, since checkpoint protein play a crucial role in self-tolerance, inhibition of checkpoint may lead to a degree of autoimmunity (6). Corticosteroids represent the first line treatment for CIP, with success in approximately 70–80% of cases (9). However, the starting doses, duration and timing of combination with other immunosuppressive agents have not been fully studied and are unclear according to several available guidelines about the treatment of irAEs (29-31). Corticosteroid doses of prednisolone equivalent dose <15 mg/day are used as maintenance doses for different rheumatic diseases. High-dose corticosteroids have significant immunosuppressive effects and have been associated with a higher risk of infection (19). For example, an equal duration of high dose prednisolone (≥30 mg/day) was associated with a higher risk of acquired infections in cancer patients with CIP (12). The initial dose of corticosteroids was recommended according to the CIP grading, but the optimal timing of a prednisolone equivalent dose ≥15 mg/day has not been clarified yet. Our data showed that the duration of prednisolone equivalent dose ≥15 mg/day was significantly shorter in the recurrence versus non recurrence groups (median, 3.73 vs. 6.36 weeks). A duration less than 4 weeks emerged as an independent risk factor for CIP recurrence, and therefore a duration of at least 4 weeks of prednisolone equivalent dose ≥15 mg/day is recommended during the tapering process of corticosteroids.
The presence of baseline lung disease has been shown in several studies to increase the risk of CIP (32-34), however not related to recurrence of CIP. The presence of baseline lung disease with poor lung function may lead to worse prognosis due to worsening tolerance of CIP relative to patients with normal lung function (35). This raises the need for pretreatment pulmonary function screening when risk factors for baseline lung disease are present.
Several limitations of this study should be addressed. First, this was a retrospective single-center study. The retrospective nature is prone to biases from missing data and the power to detect differences in risk factors for the development of CIP-R was limited by the relatively low number of cases. Second, the mechanisms of CIP were still unknown, with previous study suggesting that early-onset and late-onset CIP may be two different disease phenotypes (13). Unprovoked and provoked CIP-R may also have different mechanisms; however, they were analyzed together in this study. Lastly, there were six different types of PD-1 inhibitors and two different types of PD-L1 inhibitors in this study, and different drugs have distinct toxicity profiles, which may contribute to heterogeneity when analyzed together.
Conclusions
In conclusion, the results of this study showed that the recurrence of CIP in a real-world lung cancer patients’ cohort receiving ICIs-therapies is not an uncommon event. Recurrence of CIP was more frequently reported in males, squamous histology or patients who received chest radiotherapy. Squamous NSCLC patients and a duration of prednisolone equivalent dose ≥15 mg/day more than 4 weeks have been associated with an decreased risk of CIP recurrence. Based on these findings a duration of prednisolone equivalent dose ≥15 mg/day of at least 4 weeks during the tapering process of corticosteroids may be warranted. Further studies including larger cohorts of patients are needed to verify this preliminary observation.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This study was supported by the Major Research plan of the National Health Commission (No. GWJJ2021100304).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Chinese PLA General Hospital (No. S2021-562-01). Individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-168/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-168/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-168/coif). FF received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from BMS, Roche and BeiGene. FP declared consulting/advisory board fee from Astrazeneca, Janssen, Amgen, Termofisher Scientific, Beigene, and Sanofi. The other authors have no conflicts of interest to declare.
References
- 1.Mazieres J, Kowalski D, Luft A, et al. Health-Related Quality of Life With Carboplatin-Paclitaxel or nab-Paclitaxel With or Without Pembrolizumab in Patients With Metastatic Squamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:271-80. 10.1200/JCO.19.01348 [DOI] [PubMed] [Google Scholar]
- 2.Paz-Ares L, Vicente D, Tafreshi A, et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J Thorac Oncol 2020;15:1657-69. 10.1016/j.jtho.2020.06.015 [DOI] [PubMed] [Google Scholar]
- 3.Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:1505-17. 10.1200/JCO.19.03136 [DOI] [PubMed] [Google Scholar]
- 4.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 5.Gray JE, Villegas A, Daniel D, et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J Thorac Oncol 2020;15:288-93. 10.1016/j.jtho.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AR, Manser R. The knowns & unknowns of pulmonary toxicity following immune checkpoint inhibitor therapies: a narrative review. Transl Lung Cancer Res 2021;10:2752-65. 10.21037/tlcr-20-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su Q, Zhu EC, Wu JB, et al. Risk of Pneumonitis and Pneumonia Associated With Immune Checkpoint Inhibitors for Solid Tumors: A Systematic Review and Meta-Analysis. Front Immunol 2019;10:108. 10.3389/fimmu.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Guo X, Zhou J, et al. Clinical diagnosis and treatment of immune checkpoint inhibitor-associated pneumonitis. Thorac Cancer 2020;11:191-7. 10.1111/1759-7714.13240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suresh K, Voong KR, Shankar B, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thorac Oncol 2018;13:1930-9. 10.1016/j.jtho.2018.08.2035 [DOI] [PubMed] [Google Scholar]
- 10.Cathcart-Rake EJ, Sangaralingham LR, Henk HJ, et al. A Population-based Study of Immunotherapy-related Toxicities in Lung Cancer. Clin Lung Cancer 2020;21:421-427.e2. 10.1016/j.cllc.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tone M, Izumo T, Awano N, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer 2019;10:2006-12. 10.1111/1759-7714.13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Zhao Y, Zhang X, et al. Clinical characteristics and management of immune checkpoint inhibitor-related pneumonitis: A single-institution retrospective study. Cancer Med 2021;10:188-98. 10.1002/cam4.3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui P, Huang D, Wu Z, et al. Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer. Ther Adv Med Oncol 2020;12:1758835920922033. 10.1177/1758835920922033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishino M, Chambers ES, Chong CR, et al. Anti-PD-1 Inhibitor-Related Pneumonitis in Non-Small Cell Lung Cancer. Cancer Immunol Res 2016;4:289-93. 10.1158/2326-6066.CIR-15-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jong C, Peters BJM, Schramel FMNH. Recurrent Episodes of Nivolumab-Induced Pneumonitis after Nivolumab Discontinuation and the Time Course of Carcinoembryonic Antigen Levels: A Case of a 58-Year-Old Woman with Non-Small Cell Lung Cancer. Chemotherapy 2018;63:272-7. 10.1159/000494841 [DOI] [PubMed] [Google Scholar]
- 16.Asher N, Marom EM, Ben-Betzalel G, et al. Recurrent Pneumonitis in Patients with Melanoma Treated with Immune Checkpoint Inhibitors. Oncologist 2019;24:640-7. 10.1634/theoncologist.2018-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolladille C, Ederhy S, Sassier M, et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol 2020;6:865-71. 10.1001/jamaoncol.2020.0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan PX, Huang W, Liu PP, et al. Dynamic changes in the radiologic manifestation of a recurrent checkpoint inhibitor related pneumonitis in a non-small cell lung cancer patient: A case report. World J Clin Cases 2021;9:9108-13. 10.12998/wjcc.v9.i30.9108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buttgereit F. Views on glucocorticoid therapy in rheumatology: the age of convergence. Nat Rev Rheumatol 2020;16:239-46. 10.1038/s41584-020-0370-z [DOI] [PubMed] [Google Scholar]
- 20.Johkoh T, Lee KS, Nishino M, et al. Chest CT Diagnosis and Clinical Management of Drug-related Pneumonitis in Patients Receiving Molecular Targeting Agents and Immune Checkpoint Inhibitors: A Position Paper from the Fleischner Society. Radiology 2021;298:550-66. 10.1148/radiol.2021203427 [DOI] [PubMed] [Google Scholar]
- 21.Nishino M, Ramaiya NH, Awad MM, et al. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin Cancer Res 2016;22:6051-60. 10.1158/1078-0432.CCR-16-1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atchley WT, Alvarez C, Saxena-Beem S, et al. Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer: Real-World Incidence, Risk Factors, and Management Practices Across Six Health Care Centers in North Carolina. Chest 2021;160:731-42. 10.1016/j.chest.2021.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miura Y, Mouri A, Kaira K, et al. Chemoradiotherapy followed by durvalumab in patients with unresectable advanced non-small cell lung cancer: Management of adverse events. Thorac Cancer 2020;11:1280-7. 10.1111/1759-7714.13394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avrillon V, Bota Ouchlif S, Merle P, et al. First real life data on durvalumab after definitive concomitant chemoradiotherapy (cCRT) in unresectable stage (St) III non-small cell lung cancer (NSCLC) in France: Analysis of 591 patients (pts) enrolled in the French cohort (c) temporary authorization of use (ATU). Ann Oncol 2019;30:v591-v601. 10.1093/annonc/mdz259.013 [DOI] [Google Scholar]
- 27.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pozzessere C, Bouchaab H, Jumeau R, et al. Relationship between pneumonitis induced by immune checkpoint inhibitors and the underlying parenchymal status: a retrospective study. ERJ Open Res 2020;6:e00165-2019. 10.1183/23120541.00165-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin 2020;70:86-104. 10.3322/caac.21596 [DOI] [PubMed] [Google Scholar]
- 30.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson JA, Schneider BJ, Brahmer J, et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J Natl Compr Canc Netw 2020;18:230-41. 10.6004/jnccn.2020.0012 [DOI] [PubMed] [Google Scholar]
- 32.Kanai O, Kim YH, Demura Y, et al. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer 2018;9:847-55. 10.1111/1759-7714.12759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma K, Lu Y, Jiang S, et al. The Relative Risk and Incidence of Immune Checkpoint Inhibitors Related Pneumonitis in Patients With Advanced Cancer: A Meta-Analysis. Front Pharmacol 2018;9:1430. 10.3389/fphar.2018.01430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: A retrospective analysis. Lung Cancer 2018;125:212-7. 10.1016/j.lungcan.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 35.Ikeda S, Kato T, Kenmotsu H, et al. A Phase 2 Study of Atezolizumab for Pretreated NSCLC With Idiopathic Interstitial Pneumonitis. J Thorac Oncol 2020;15:1935-42. 10.1016/j.jtho.2020.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as