Abstract
Fear-associated conditions such as posttraumatic stress disorder (PTSD) and panic disorder (PD) are highly prevalent. There is considerable interest in understanding contributory risk and vulnerability factors. Accumulating evidence suggests that chronically elevated inflammatory load may be a potential risk factor for these disorders. In this regard, an association of asthma, a chronic inflammatory condition, with PTSD and PD has been reported. Symptoms of PD and PTSD are more prevalent in severe asthmatics, compared to those with mild or moderate asthma suggesting that factors that influence the severity of asthma, may also influence susceptibility to the development of fear-related disorders. There has been relatively little progress in identifying contributory factors and underlying mechanisms, particularly, the translation of severe asthma-associated lung inflammation to central neuroimmune alterations and behavioral manifestations remains unclear. The current study investigated the expression of behaviors relevant to PD and PTSD (CO2 inhalation and fear conditioning/extinction) in A/J mice using a model of severe allergic asthma associated with a mixed T helper 2 (Th2) and Th17 immune response. We also investigated the accumulation of Th2- and Th17-cytokine expressing cells in lung and brain tissue, microglial alterations, as well as neuronal activation marker, delta FosB (ΔFosB)) in fear and panic regulatory brain areas. HDM-exposed mice elicited higher freezing during fear extinction. CO2-associated spontaneous and conditioned freezing, as well as, anxiety or depression-relevant exploratory and coping behaviors were not altered by HDM treatment. A significant increase in brain Th17-associated inflammatory mediators was observed prior to behavioral testing, accompanied by microglial alterations in specialized blood brain barrier-compromised circumventricular area, subfornical organ. Post extinction measurements revealed increased ΔFosB staining within the medial prefrontal cortex and basolateral amygdala in HDM-treated mice. Collectively, our data show modulation of brain immune mechanisms and fear circuits by peripheral airway inflammation, and is relevant to understanding the risk and comorbidity of asthma with fear-associated disorders such as PTSD.
Keywords: asthma, fear, PTSD, Th17, IL-17A, neuroimmune
1. Introduction
Anxiety and fear-associated conditions such as posttraumatic stress disorder (PTSD) and panic disorder (PD) afflict 4%−7% of the US population (Gros et al., 2011; Kessler et al., 1995, 2005). These disorders are associated with functional impairment leading to disability and significant societal economic burden. Therefore, there is considerable interest in understanding contributory risk and vulnerability factors. Accumulating clinical evidence supports an association between inflammatory and psychiatric conditions (Michopoulos et al., 2017) suggesting that chronically elevated inflammatory load may be a potential risk factor for development of fear-associated disorders. In this regard, individuals with asthma, a chronic inflammatory condition, show higher incidence of fear-associated disorders (Del Giacco et al., 2016). A frequent co-occurrence of PTSD and asthma has been reported (Kean et al., 2006; Spitzer et al., 2011) and the association between asthma and PTSD is stronger than that observed for other physical illnesses such as cardiovascular or digestive disease (O’Toole and Catts, 2008). Interestingly, a recent genome-wide association study (GWAS) reported a high genetic correlation between PTSD and asthma (Nievergelt et al., 2019). An increased asthma-PTSD co-occurrence was observed in US veterans of the Vietnam War after controlling for factors such as smoking, body mass index, and depression (Goodwin et al., 2007). Similarly, a strong, bi-directional panic-asthma association, with a dose-reponse relationship between the two conditions, was reported in a community sample of young adults (Hasler et al., 2005).
Despite considerable evidence supporting asthma comorbidity with fear-related disorders, there has been relatively little progress in identifying contributory factors and underlying mechanisms. Unlike many other panic and PTSD-comorbid conditions, asthma often develops in children, promoting long-term sensitization and risk (Stowman et al., 2015). Indeed, asthma currently afflicts about 11% of children between the ages of 15 and 19 in the US (CDC, 2018). Importantly, symptoms of PD and PTSD are more prevalent in severe asthmatics, compared to those with mild or moderate asthma (Vanderbilt et al., 2008), suggesting that factors that influence the severity of asthma may also influence susceptibility to fear-related disorders.
CD4+ T helper (Th) cells are central mediators of the adaptive immune responses that underlie the development of many chronic inflammatory conditions. In response to innate immune system-derived cues, Th cells can differentiate into several lineages (e.g. Th1, Th2, Th17) which produce unique panels of cytokines when stimulated through antigen specific T cell receptors (Zhu et al., 2010). Asthma is typically driven by a dysregulated T helper cell, Th2-dominated immune response (associated with excessive production of cytokines like IL-4, IL-5, IL-9 and IL-13) to normally innocuous environmental allergens (Lambrecht et al., 2019). Although asthma symptoms are typically well controlled in individuals with mild to moderate asthma, severe forms of asthma are characterized by significant symptomology (airflow limitation, poor quality of life, frequent exacerbations) despite high levels of immunosuppressive drugs (Chung et al 2014). Importantly, compared to mild/moderate disease, severe asthma is more frequently associated with psychiatric comorbidities. Given that severe asthma is often associated with a mixed Th2/Th17 response (Chesné et al., 2014; Gurczynski and Moore, 2018), and the recent observations that IL-17A signatures can be found in patients with PTSD (Zhou et al., 2014), it is conceivable that the unique inflammatory profiles associated with more severe allergic asthma (i.e. production of IL-17A) may contribute to the development of fear-associated disorders.
Translation of asthma-associated lung inflammation to central neuroimmune alterations and behavioral manifestations relevant to PD and PTSD remains unclear. In previous studies, chronic exposure to methacholine-induced labored breathing or house dust mite (HDM)-induced airway inflammation during development modulated anxiety and depression-associated behaviors, respectively (Caulfield et al., 2017, 2018). BALB/cJ animals used in these studies represent a Th2-dominated model of allergic asthma that recapitulates the mild/moderate asthma phenotype in humans. Recently, we reported a unique model of severe allergic airway inflammation using house dust mite (HDM) treatment in A/J mice (Lewkowich et al., 2008; Lajoie et al., 2010; Kim et al., 2019). HDM-exposed A/J mice develop severe airway dysfunction characterized by a mixed Th2/Th17 immune profile comparable to that observed in humans with more severe forms of disease (Al-Ramli et al., 2009; Irvin et al., 2014; Hasegawa et al., 2017), and display evidence of treatment refractory responses (Serra et al 2018), as reported in human severe asthmatics. Although there have been relatively few behavioral studies in A/J mice, this model represents a useful paradigm in which to study the effects of severe allergic asthma on the development of fear and panic-associated behaviors.
With all these considerations, the current study investigated delayed expression of behaviors relevant to panic (CO2 inhalation) and PTSD (fear conditioning and extinction) in A/J mice using a paradigm of severe allergic asthma associated with a mixed Th2/Th17 immune response. Furthermore, to gain insights on how asthma-related inflammation may translate into behavior, we assessed accumulation of Th2- and Th17-cytokine expressing cells in brain, regional microglial alterations including specialized blood brain barrier compromised circumventricular areas as well as, neuronal activation (ΔFosB) in fear and panic regulatory brain areas. We hypothesized an association of HDM treatment with enhanced fear and panic-associated behaviors, increased Th17-associated inflammatory mediators in the brain and altered forebrain neuronal activation in HDM treated animals.
2. Materials and Methods
2.1. Animals
Adult, male A/J mice (n= 58) were obtained from Jackson Laboratories (Bar Harbor, Maine, USA) at 7 weeks of age. Mice were group-housed in a climate-controlled vivarium (temperature averages 23 ± 4 °C, humidity averages 30 ± 6%, light-dark cycle 6am–8pm). All behavioral tests were performed between 8am–2pm. Study protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Cincinnati Children’s Hospital Medical Center and University of Cincinnati, in a vivarium accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All experiments were performed between 10–14 weeks of age. Cohort 1 underwent testing for exploratory and defensive behaviors relevant to anxiety, panic, depression and fear one week following HDM/PBS treatment (Fig 1). For assessing HDM-induced alterations in inflammatory mediators in brain and lungs, a behavior naïve cohort (Cohort 2, Fig 1) was exposed to HDM/PBS and tissue was collected 72h following the last administration when peak lung inflammation has been reported (Lewkowich et al., 2008). Home cage activity was assessed in a separate cohort of HDM/PBS-exposed mice.
Figure 1:

Schematic representation of experimental layout. Cohort 1 mice were exposed to three intratracheal administrations of PBS or HDM separated by one week. After a week of recovery mice underwent behavioral testing on the elevated zero maze (EZM), carbon dioxide (CO2) inhalation paradigm, the forced swim test (FST) and contextual fear conditioning paradigm. Tissue was collected 24 h post behavior. For assessing HDM-induced alterations in inflammatory mediators in brain and lungs, a behavior naïve cohort (Cohort 2) was exposed to intratracheal HDM/PBS and tissue was collected at 72 h following the last administration.
2.2. HDM administration
HDM was administered to mice intratracheally. Briefly, following acclimation to the colony, mice were anesthetized with isoflurane and intratracheally administered 40μl of 200μg HDM (Greer Labs, Lenoir, NC) thrice, spaced one week apart. Animals in Cohort 1 (Fig 1) subsequently underwent behavioral testing (see below), whereas animals in Cohort 2 were euthanized 72 hours after the final allergen exposure to simultaneously assess inflammatory cell infiltration into the lung and the brain (Fig 1). Control mice were intratracheally administered the same volume of phosphate buffered saline (PBS).
2.3. Home Cage Activity
Home cage activity in PBS and HDM exposed mice was measured for 2 days post treatment using previously published procedures (Vollmer et al., 2013). A SmartFrame stainless steel cage rack frame (Lafayette Instrument Company, Lafayette, IN, USA) with infrared photo beam interruption sensors (7X and 15Y) was used. Data was then analyzed using the hmm100 motormonitor software.
2.4. Behavioral Tests
Animals in Cohort 1 underwent behavioral tests which were temporally laid out from the least to most invasive. The behavioral testing layout was based on previous studies that showed prior testing had a significant impact on exploration tests, such as mazes, while fear-associated behaviors were not significantly impacted by previous behavioral experiences (McIlwain et al., 2001; Võikar et al., 2004). This layout was also adopted for consistency with rodent models simulating delayed development of PTSD-associated behaviors where testing is conducted in the same animal.
2.4.1. Elevated Zero Maze (EZM)
Animals were tested on the EZM following previous studies (Schubert et al., 2018). The maze (Stoelting Co., Wood Dale, IL) was a circular track divided in four quadrants of equal lengths with two opposing open quadrants with 1 cm high clear acrylic curbs to prevent falls and two opposing closed quadrants with black acrylic walls 20 cm in height. Dim halogen lamp lighting (24 lux on the open quadrant) was used during testing. Mice were placed in a closed quadrant and allowed to explore for 5min, during which they were videotaped with an overhead video camera. The track and walls were cleaned with 10% ethanol between trials. Time spent in the open and closed quadrants, latency to enter the open quadrant and distance travelled were scored offline using TopScan software (CleverSys. Inc., Reston, VA).
2.4.2. CO2 context conditioning paradigm
CO2 inhalation is a widely-studied interoceptive stimulus, that produces intense fear and physiological responses that can evoke panic attacks in individuals with panic disorder (Papp et al 1993; Rassovsky and Kyshner 2003). CO2 evoked fear (freezing) in rodents has been used by us and others as a translational model of panic (Leibold et al 2016, Vollmer et al 2015b, Ziemann et al 2009). A CO2 inhalation context conditioning paradigm (see Fig 3D) was used as previously described by our group (Vollmer et al., 2016; McMurray et al., 2019; Winter et al., 2019). This test provides a measure of spontaneous CO2-evoked fear, as well as conditioned fear specific to the exposure context relevant to fear and avoidance of panic-associated contexts observed in panic disorder (Lissek et al 2010). The setup was a dual vertical Plexiglas chamber (25.5cm × 29cm × 28cm per chamber). CO2 (5%; custom industrial mix in breathing air, Wright Brothers Inc., Cincinnati, OH) was infused in the upper chamber while the mice were placed in the lower compartment to avoid direct blowing of the gas which is highly aversive to rodents. A flow meter with a steady infusion rate of 10 L/min was used for all animals. Ambient concentration of CO2 within the lower chamber was verified (5.0 ± 0.5%) by the CARBOCAP® GM70 carbon dioxide meter (GMP221 probe with accuracy specification +/− 0.5%) (Vaisala, Helsinki, Finland). Briefly, mice were habituated to the CO2 chamber for 7min on Day 1, the day prior to CO2 exposure. On Day 2, mice were placed back in the chamber and exposed to 5% CO2 for 10min. This concentration of CO2 is translationally relevant to studies reported in humans (Sanderson and Wetzler, 1990). The following day, Day 3, animals were re-exposed to CO2 context for 5min in the absence of CO2 for assessment of context-conditioned behaviors. Mice were video recorded during habituation, CO2 inhalation and context exposure. Freezing, the complete lack of movement except for respiration, was analyzed using FreezeScan software (CleverSys Inc., Reston, VA).
Figure 3:
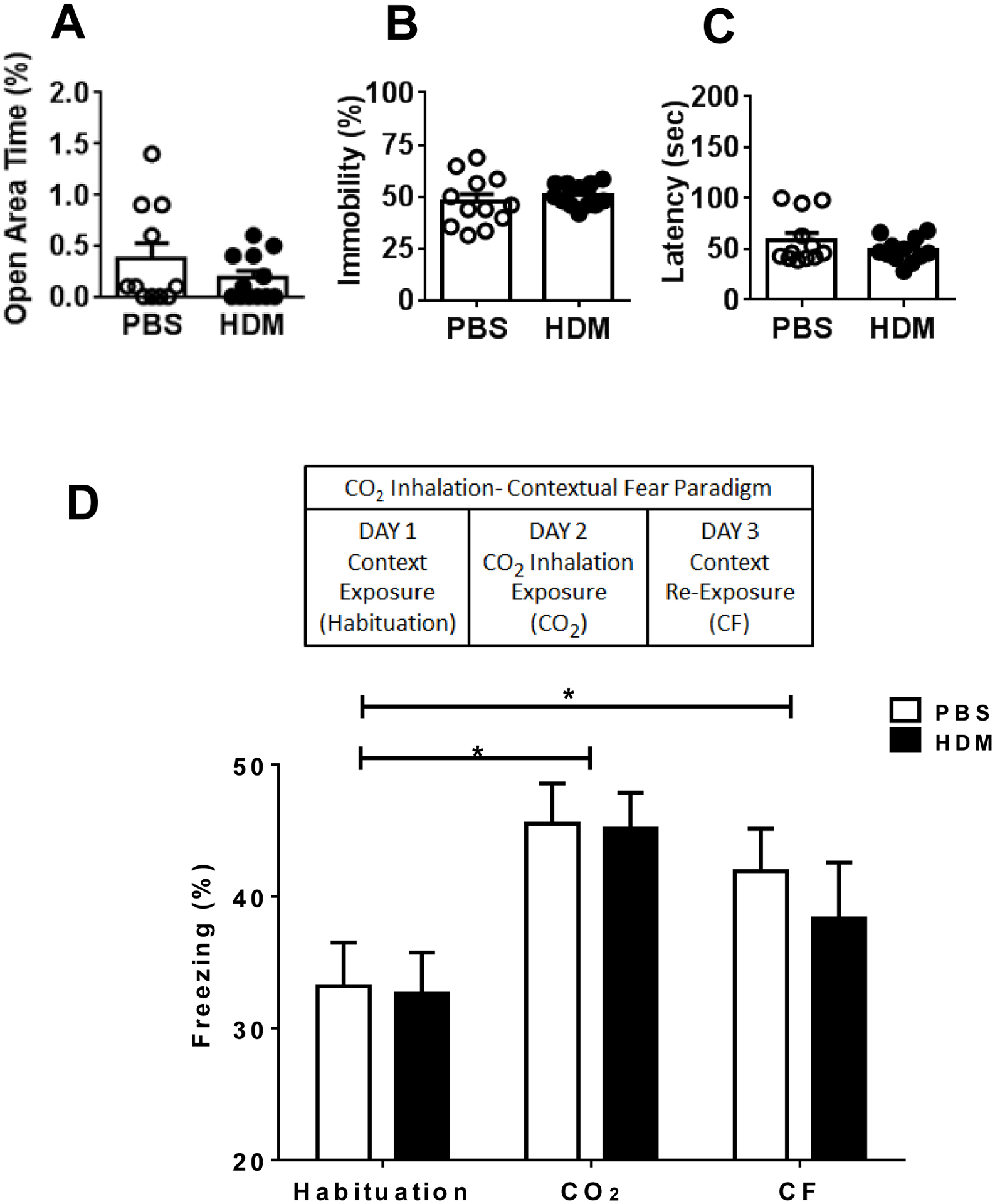
HDM exposure does not impact exploratory and coping behaviors in the elevated zero maze (EZM), forced swim test (FST) and CO2 inhalation-evoked spontaneous and conditioned behaviors. PBS and HDM mice showed similar time spent in the open area of the EZM (A). In the FST, immobility (B) and latency to immobility (C) showed no differences between PBS and HDM groups. In the CO2 inhalation-contextual fear paradigm (see D schematic), PBS and HDM cohorts showed similar novelty evoked freezing during habituation, CO2 evoked spontaneous freezing, as well as, contextual freezing (CF) upon re-exposure to CO2 context (panel D). Data are mean ± SEM; n= 12/group.
2.4.3. Forced Swim Test (FST)
Mice were tested in the FST following previous studies (Vollmer et al., 2015a) with modifications. Mice were immersed for 6min in a glass cylinder (14 cm diameter, 19 cm high) filled with 14 cm water (maintained at 24 ± 0.5 °C). Behavior was recorded and scored for percent immobility (no active movements or floating in the water without struggling) by a rater blind to treatment. The total immobility counts for minutes 2–6 were summed for individual animals and were averaged within each treatment group.
2.4.4. Contextual Fear Conditioning
We selected a contextual fear conditioning paradigm (see Fig 4 layout) for our study based on previous evidence reporting poor performance of A/J mice in cued tone-shock fear conditioning (Bolivar et al., 2001). Fear acquisition, conditioned fear and extinction was investigated as reported previously by us (Vollmer et al., 2013; Schubert et al., 2018) with modifications. Operant chambers housed in sound attenuated isolation cabinets were used (San Diego Instruments). The floors of the chambers consisted of stainless-steel grid bars that delivered scrambled electric shocks. The grid, floor trays and chamber walls were wiped with 10% ethanol and allowed to dry completely. Each animal was acclimated to the chamber for 5min, then received 3 shocks of 0.5mA intensity, 1s duration administered 1min apart. The animals were placed in the chamber the next 6 days and recorded for 5min without shocks to measure conditioned fear and extinction. Freezing, defined as complete lack of movement except respiration (Fanselow, 1980), was measured on Day 1 (acquisition), Day 2 (conditioned fear), and Day 3–8 (extinction), using the Freeze Scan software (Clever Sys Inc.).
Figure 4:

Freezing response in PBS and HDM mice in a contextual fear conditioning paradigm (see layout) to assess fear acquisition (A), conditioned fear (B) and extinction (C). PBS and HDM groups showed similar pre- shock (baseline, B) and post-shock freezing during fear acquisition. At 24h post-acquisition, no significant difference in freezing was observed during testing for conditioned fear (B). Context re-exposure for extinction revealed a significant effect of treatment with the HDM cohort showing higher overall freezing compared to the PBS cohort across extinction (C). Comparison of Extinction Day 1 to Day 6 freezing (inset) revealed significantly higher freezing in the HDM group. (* p < 0.05 overall extinction freezing versus PBS mice(C) and Day 1 to Day 6 freezing versus PBS (inset). Data are shown as mean ± SEM (n= 12 mice/group).
2.5: Tissue preparation and Flow Cytometry
Tissue for flow cytometric analyses was collected at 72h after the final PBS/HDM administration (Fig 1, Cohort 2). This particular time point after the final HDM exposure was selected, as this is when the inflammatory response in the lung is as its peak (Lewkowich et al 2008).
Lung tissue: Lungs were removed, minced and placed in 6 ml of RPMI 1640 containing Liberase CI (0.5 mg/ml)(Roche Diagnostics) and DNase I (0.5 mg/ml)(Sigma) at 37°C for 45min. The remaining tissue was forced through a 70-micron cell strainer, and red blood cells were lysed with ACK lysis buffer (Thermo Fisher Scientific). Cells were washed with RPMI containing 10% FBS, viable cells were counted via trypan blue exclusion.
Brain tissue: Mononuclear cells were isolated from whole brain at 72h following the last administration of PBS/HDM as described by previous studies (Frank et al., 2006; Pino and Cardona, 2011) with modifications. Briefly, mice were anesthetized and perfused transcardially with ice-cold 1X HBSS without Ca++ or Mg++. Brains were homogenized in RPMI media. Resulting homogenates were centrifuged at 600 × g for 6min. Supernatants were removed and cell pellets were resuspended in 70% isotonic Percoll (GE Healthcare) at room temperature. The cell suspension was layered over a discontinuous Percoll density gradient using 70%– 30% percoll and centrifuged for 30 min at 500 ×g Cells were washed and then resuspended in sterile RPMI till further processing.
Flow cytometry: Single cell suspensions of brain and lung tissue were first incubated with FcBlock (mAb clone 2.4G2) to prevent non-specific Ab binding for 15min. Following this, cells were incubated with fluorochrome labeled antibodies to surface markers, and incubated with a fixable Live-Dead Dye to exclude dead cells (Thermo Fisher Scientific). Cells were then fixed and permeabilized using Foxp3 staining kit (Thermo Fisher Scientific) for 1 hour. Intracellular Fc receptors were blocked using FcBlock suspended in Permeabilization Buffer (Thermo Fisher Scientific), followed by staining with cytokine-specific or transcription factor-specific mAbs suspended in Permeabilization Buffer. Data were acquired with an LSR-Fortessa flow cytometer equipped with lasers tuned to 355 nm, 405 nm, 488 nm, 561 nm, and 640 nm, and digital DiVa Software. Spectral overlap was compensated, and data was analyzed using FlowJo software (Treestar Inc., Ashland, OR). All staining reagents used were purchased from Thermo Fisher Scientific, unless otherwise indicated. Clones used were as follows: BV605-conjugated anti-mouse CD90.2 (clone 53-2.1 (BioLegend), APC-Cy7-conjugated anti-mouse TCRβ (clone H57–597), AlexaFluor700-conjugated anti-mouse CD3ε (clone 17A2), Brilliant Violet 711-conjugated anti-mouse CD4 (clone RM4–5 (BioLegend)); PE-eFluor-610-conjugated anti-mouse CD44 (clone IM7), Fixable Live/Dead e506 (Thermo Fisher Scientific), PE-conjugated anti-mouse Rorγt (clone AFKJS-9), eFluor 660 conjugated anti-human/mouse GATA3 (clone TWAJ).
2.6: Immunohistochemistry
A subset of mice in Cohort 2 were perfused transcardially with 4% paraformaldehyde 72h following final PBS/HDM administration for analysis of microglial morphology. Cohort 1 mice were perfused the day after behavioral testing for assessment of delta FosB, a marker for prolonged neuronal activation (see Fig 1 for experimental layout). Brains were removed and sectioned at 30μm on a sliding microtome and the resulting sections were stored in cryoprotectant (0.1 M phosphate buffer, 30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylene glycol) at −20°C until processed for immunohistochemistry. Pre- and post-behavior tissues were stained for anti-ionized calcium binding adapter molecule (IBA-1) (1:1000, Synaptic Systems Inc., cat. 234–003, Germany). Tissue obtained post-behavior was also stained for delta FosB (ΔFosB) (H-75) (1:5000, Santa Cruz Biotechnology, sc-7203, Santa Cruz, CA) to assess persistent neuronal activation. Slices were transferred to 50 mM PBS (pH 7.4; 40 mM potassium phosphate dibasic, 10 mM potassium phosphate monobasic, and 0.9% sodium chloride) and rinsed five times for 5min at RT. Sections were transferred to 0.3% H2O2 in PBS and incubated for 10min at RT. Slices were rinsed five times for 5min in PBS and transferred to blocking solution [50 mM PBS, 0.5% bovine serum albumin (BSA), and 0.4% Triton X-100] for 1h at RT. Slices were incubated overnight at 4°C in primary antibody diluted in blocking solution. The following day, sections stained for IBA-1 were rinsed (five times for 5min) in PBS and incubated in secondary antibody (Cy-3 anti-rabbit; Jackson Immunoresearch) diluted (1:500) in 50 mM PBS plus 0.5% BSA for 1h at RT on a shaker in the dark. Tissue was rinsed five times for 5min in PB, mounted onto UltraStick micro slides (Gold Seal Products, cat. 3039, Portsmouth, NH) and coverslipped with Gelvatol (Sigma, Milwaukee, WI). Sections stained for ΔFosB were incubated in biotinylated anti-rabbit secondary antibody (Vector Laboratories, Inc., Burlingame, CA; 1:400 in blocking buffer for 1h). Sections were washed again five times for 5min in PBS, and incubated in avidin-biotin complex using ABC Vectastain kit, diluted 1:800 for 1h. Following washes, sections were incubated in diaminobenzadine (DAB, Pierce, Rockford, IL) for 10min. Sections were washed again in PB and mounted onto microscope slides followed by dehydration in xylene solutions. Finally, slides were cover-slipped using DPX (Sigma, 44581).
2.7. Imaging, Quantification and Analysis
Immunolabeled sections were imaged using the AxioImager ZI microscope (Zeiss) equipped with apotome (z-stack) imaging capability (Axiocam MRm camera and AxioVision Release 4.6 software; Zeiss). Processing for IBA-1 and ΔFosB imaging and quantification was performed following previously published procedures from our lab (Vollmer et al., 2016; McMurray et al., 2019). Microglial analysis was conducted in sensory circumventricular areas, subfornical organ (SFO), organum vasculosum laminae terminalis (OVLT), and area postrema (AP), that lack a traditional blood brain barrier representing brain-body interface (Johnson and Gross 1993), and are well-recognized hubs for neuroimmune signaling (Quan and Banks 2007), as well as,areas regulating fear and emotional regulation; including, the amygdala, medial prefrontal cortex and hippocampal dentate gyrus (Izquierdo et al 2016). Briefly, for IBA-1 labeled tissue, Z-stacks were acquired using the 20x air objective lens at 568nm. Images were analyzed using Image J software (NIH open access). For IBA-1 positive microglial cells, soma perimeter was analyzed using the ImageJ software tool “Freehand line” and recorded using the “Analyze and Measure” option. Images were collected for each region and 6 microglia per image were measured. For post behavior ΔFosB analysis, areas were selected based on their role in stress, fear and emotional regulation, including, the amygdala, medial prefrontal cortex, hippocampal dentate gyrus, bed nucleus of stria terminalis and paraventricular nucleus of the hypothalamus (Ulrich-Lai and Herman 2009; Lebow and Chen 2016; Izquierdo et al 2016). Regions were delineated using characteristics of each nucleus taken from the atlas of Franklin and Paxinos (Franklin and Paxinos, 2008). At least, four images per region of interest per mouse were collected. To quantify the number of immunoreactive nuclei the Image-J “cell counter” tool was used by an investigator blind to experimental group. Regions were quantified at a similar distance from bregma within all animals. Cell counts for each section were averaged for each animal and individual means averaged to derive group means.
2.8. Data Analysis and Statistics
Data are represented as mean ± SEM and were analyzed by two-factorial ANOVA, or student t-test. Normality was tested formally for all behavior data and met assumptions of the statistical tests being used. Welch’s correction was used when variances were unequal. Behavior in the home cage activity, fear conditioning and CO2 inhalation paradigms was analyzed by two-way repeated measures ANOVA using treatment and time as variables. Sidak’s post hoc analysis was applied where main effects were significant. Measures on the EZM and FST were assessed by two-tailed student’s t-test. Quantified microglia morphological measures, ΔFosB and flow cytometry data were analyzed by two-tailed student’s t-test using HDM and PBS control groups as variables. Statistical significance was taken as p < 0.05. Prism 8.0 software was used for statistical analysis (GraphPad Software, Inc., La Jolla, CA).
3. Results
HDM treatment induces short-term deficits in motor activity
To determine whether HDM treatment has persisting effects on motor activity, we measured 2 day home cage activity in HDM and PBS cohorts. This is relevant as effects of HDM on motor activity have not been assessed before and persistent alterations in motor activity may confound behavioral testing. HDM-treated mice showed a significant reduction in motor activity as compared with PBS-treated mice that persisted for one day (Fig 2). A 2-way RM ANOVA analysis revealed a significant effect of treatment [F (1, 48) = 7.206; p= 0.009] and time [F (5, 48) = 4.854; p=0.001] but no treatment × time interaction [F (5, 48) = 0.5748; p=0.720]. These effects of HDM were normalized on Day 2 (Fig 2), as no significant treatment [F (1, 48) = 1.57; p= 0.25], or treatment × time interaction [F (5, 48) = 0.2778; p=0.9] was observed. A significant effect of time [F (5, 48) = 13.9; p<0.0001] was observed in both groups.
Figure 2:

Locomotor activity in the home cage measured over 2 days in mice following exposure to three intratracheal administrations of HDM or PBS. (* p<0.05 versus PBS). Data are mean ± SEM; n= 5/group.
HDM treatment does not impact CO2-associated freezing or defensive behaviors relevant to anxiety and depression
A week after their last airway exposures, PBS and HDM-treated mice in Cohort 1 (Fig 1) were tested on the elevated zero maze (EZM) and the forced swim test (FST) (Figure 3 A–C) to test exploration and coping associated with anxiety and depression-like behaviors. Consistent with previous observations (Molenhuis et al., 2014; Moloney et al., 2015), A/J mice exhibited low exploration and high immobility indicative of high trait anxiety and depression-relevant phenotype of this strain. Percent time spent in the open arm of the EZM was not statistically different between the treatment groups (t test, t13=1.16; p>0.05 using Welch’s correction) (Fig 3A). In the FST test, immobility (Fig 3B), or latency to first immobility bout (Fig 3C) were not significantly different between HDM-treated and PBS control mice [immobility (t test, t15=0.76; p>0.05 using Welch’s correction), latency (t test, t15=1.3; p>0.05 using Welch’s correction).
We next assessed panic-relevant behaviors using our model of CO2 inhalation contextual fear paradigm (Fig 3D schematic) (Vollmer et al., 2016; McMurray et al., 2019; Winter et al., 2019). In both PBS and HDM-exposed A/J mice, high freezing (immobility) was observed on day 1 during habituation to context (CO2 chamber) (Fig 3D). Exposure to CO2 inhalation the next day evoked increased freezing but no differences between PBS and HDM-treated mice were observed. Re-exposure to context on day 3 in the absence of CO2 produced conditioned freezing in both groups. Repeated measure 2-way ANOVA revealed a significant effect of day [F (2, 44) = 9.983; p=0.0003], but no treatment [F (1, 22) = 0.1984. p=0.66] or treatment × time interaction [F (2, 44) = 0.2080; p= 0.81]
HDM-exposed A/J mice show increased freezing during extinction
PBS and HDM-exposed A/J mice were tested on a contextual fear paradigm. High immobility (~20%) during the baseline pre-shock context habituation was observed in both PBS and HDM groups (Fig 4A), indicative of low novelty-induced exploration in this strain. Exposure to foot shocks resulted in increased freezing in both cohorts with no group differences suggestive of similar fear acquisition in both groups. A 2-way ANOVA revealed a significant effect of time [F (1, 44) = 6.304; p = 0.016], but no treatment [F (1, 44); p= 0.98] or treatment × time interaction [F (1, 44) = 0.1662; p=0.68]. Exposure to context 24h post acquisition elicited similar context conditioned freezing in PBS and HDM groups (Fig 4B) (t test t22 =0.9909 p=0.16). Measurement of extinction freezing during re-exposure to context (Fig 4C) revealed significantly higher freezing in the HDM group. Two-way RM ANOVA revealed a significant effect of treatment [F(1,132)= 6.266, p= 0.014], but no significant effect of day [F (5, 132) = 0.6575; p= 0.65] or day × treatment interaction [F(5, 132) = 0.4082, p=0.84]. Absence of a day effect indicates poor extinction learning in this strain that appears to be worsened in HDM-treated mice. Comparison of extinction day 1 to extinction day 6 freezing revealed reduced freezing in the PBS group, an effect that was significantly blunted in HDM-exposed mice (Fig 4C, inset, [F (1, 44) = 4.248, p= 0.04]. Overall, our data revealed a high fear response in A/J mice that appears to be exacerbated in HDM-exposed mice specifically during extinction.
Intratracheal HDM exposure increases Th17/ IL17A associated inflammatory mediators in brain and induces microglial alterations in selective, BBB-compromised circumventricular organs
To determine whether HDM exposure increased severe asthma-relevant Th17/IL17A immune mediators, we measured the frequency of immune cells expressing cytokines associated with Th2 (IL-13), or Th17 (IL-17A) differentiation in the lungs at 72 hours post HDM in mice from Cohort 2 (Fig 1). Importantly, this is a time point that correlates with peak inflammatory responses in the lung (Lewkowich et al., 2008). Based on our flow cytometric panel we were able to identify cytokine producing γδ T cells (CD90.2+, CD3+, TCRβneg), CD4+ T cells (CD90.2+, CD3+, TCRβ+, CD4+), CD8+ T cells (CD90.2+, CD3+, TCRβ+, CD4neg), and innate lymphoid cells (ILCs; CD90.2+, CD3neg, TCRβneg) (Supplemental Figure 1). Consistent with an allergic phenotype, a significant increase in the frequency of cells expressing IL-13, was observed in the lungs of HDM-exposed A/J mice (Fig 5A) (t test, t13 =2.97; p=0.01). HDM exposure also increased the frequency of IL-17A-expressing cells in the lungs (Fig 5B) (t test, t13 =3.81; p=0.002). Similar results were observed when we examined the frequency of cells expressing Th2- and Th17-associated transcription factors GATA3 and Rorγt (data not shown).
Figure 5:

Pulmonary allergen exposure selectively increases the frequency of IL-17A-producing cells in the brain. Flow cytometric data from lung (panels A, B) and brain (panels C-F) tissue is shown. 72 hours after the last intratracheal HDM/PBS administration, the frequency of pulmonary IL-13 (A) and IL-17A-secreting cells (B) was increased in HDM-exposed mice. In the same animals, brain mononuclear cells were isolated, and the frequency of IL-13-producing cells (C) was not altered, however, the frequency of IL-17A-producing cells (D) was increased in the brains of HDM-exposed mice. As an alternative approach, all IL-13- (E) and IL-17A- (F) producing cells present in brain mononuclear cell preparations were identified by flow cytometry, and the relative proportions of CD4+ T cells, CD8+ T cells, ILCs and γδ T cells within each cytokine-producing population was compared in PBS and HDM-exposed animals. HDM treatment had no effect on the proportion of cell types. (*p< 0.05 versus PBS). Data are represented as mean ± SEM; (n = 8/group).
To determine whether changes in pulmonary cytokine-producing cells were mirrored in the brain, we also examined the frequency of Th2- or Th17-associated cytokines in the brain in mice from Cohort 2 (Fig 1). In contrast to the increased frequency of IL-13-expressing cells in the lung following exposure to HDM at that time, no increase in IL-13-expressing cells was observed in the brain after HDM exposure (Fig 5C) (t test, t12 =0.71; p=0.49). However, a significant increase in the frequency of IL-17A-secreting cells was observed in the brain after HDM exposure (Figure 5D) (t test, t12 = 3.92; p=0.002). More than 95% of all IL-13+ or IL-17A+ in the brain were ILCs; CD4+ T cells, CD8+ T cells and γδ T cells combined represented <5% of all IL-13+ or IL-17A+ cells, and this ratio was not impacted by HDM exposure (Fig 5E, F). This suggests that HDM is not altering the type of cell making IL-17A in the brain, but increasing the overall number of IL-17A-secreting cells. Thus, while pulmonary allergen exposure induces increased numbers of Th2 and Th17 cells in the lungs, an increase in only IL-17A-producing cells is observed in the brain.
To assess whether these global changes in asthma-relevant cytokine mediators in the brain were accompanied by alterations in microglia, we also measured regional microglial morphology in diverse areas within and outside of the blood brain barrier (BBB) at 72 h post HDM treatment in mice from Cohort 2 (Fig 1). These included forebrain and hind brain BBB-compromised sensory circumventricular areas (CVOs): subfornical organ (SFO), organum vasculosum laminae terminalis (OVLT) and area postrema (AP) (Fig 6 D–F) as well as areas regulating fear and anxiety: amygdala (basolateral nucleus, BLA), medial prefrontal cortex (mPFC) and hippocampus (dentate gyrus, DG), (Supplemental Figure 2). A significant increase in microglial soma perimeter was observed within the SFO of HDM-treated mice compared to the PBS group (Fig 6A–C) (t test, t10=4.56; p= 0.001) Interestingly, no differences were observed within other CVOs, OVLT (t test, t10=0.47;p= 0.65) and the AP (t test, t10= 1.28;p= 0.24). No differences in microglial morphology between PBS and HDM groups was observed in the mPFC (t test, t10=0.28; p= 0.78), BLA (t test, t10=0.77;p= 0.46) and DG (t test, t10=0.25;p= 0.80).
Figure 6:

Morphological analysis of microglia in HDM and PBS mice within forebrain and hindbrain sensory circumventricular organs showing microglial morphological alterations within the subfornical organ (SFO) at 72 h post administration. (A) Illustration of a coronal section showing location of the SFO within the lateral and dorsal third ventricles (black). (B) Representative images showing IBA-1 (ionized calcium binding adaptor molecule 1) stained microglia within the SFO of PBS and HDM-exposed mice. Bar graphs show quantified microglial soma perimeter in the SFO (D), organum vasculosum laminae terminalis (OVLT) (E) and area postrema (AP) (F). Significant increase in soma perimeter was observed in the SFO (D). (*p< 0.05 versus PBS). Data are represented as mean +/− SEM. (n = 6/group).
Post behavior increases in neuronal (FosB) activation within the prefrontal cortex and basolateral amygdala of HDM mice
Given the fear phenotype of HDM-treated mice during extinction, we investigated ΔFosB immunoreactivity, a measure of persistent neuronal activity in fear and stress regulatory areas (Fig 7). HDM-treated mice had significantly higher FosB cell counts in the medial prefrontal cortex (t22=2.21; p= 0.03) and the BLA (t test, t22= 2.15; p= 0.04) as compared with PBS-treated mice. No significant differences were observed in other fear and stress regulatory areas such as the dentate gyrus (t22=0.81; p= 0.42), central nucleus of the amygdala CeA (t22=0.26; p= 0.79) bed nucleus of stria terminalis (BNST) (t22=1.08; p= 0.29) or the hypothalamic PVN (t test, t22=0.39; p= 0.70).
Figure 7:

HDM alters neuronal activation in selective forebrain fear regulatory regions. Significant increase in delta (Δ) FosB immunopositive (Δ FosB+) cells was observed inHDM versus PBS treated mice following behavior within: the medial prefrontal cortex (mPFC) (A) and basolateral amygdala (BLA) (B). No significant differences were observed in the central nucleus of amygdala (CeA) (C), bed nucleus of stria terminalis (BNST) (D), hippocampal dentate gyrus (DG) or the paraventricular nucleus of hypothalamus (PVN) (F). Bar graphs show mean ± SEM of cell counts from N = 12 mice/group, averaged from at least 3–4 images per side, per animal. *p < .05 versus PBS.
4. Discussion
The present study shows that repeated exposure to the aeroallergen, HDM, exacerbates fear during extinction in a mouse strain that exhibits severe allergen-induced airway hyperreactivity due to the development of a mixed Th2/Th17 immune response. Our data also revealed increased prevalence of IL-17A/Rorγt-expressing cells in the brain and morphologically altered microglia in a select circumventricular organ, SFO at a time point when peak HDM-induced lung inflammation has been reported. Furthermore, alterations in persistent neuronal activation were also observed within fear regulatory regions post behavior. Collectively, these data suggest that airway inflammation can modulate fear memory and alter neuroimmune function in a region-specific manner.
To our knowledge, our studies are the first to investigate brain neuroimmune alterations and behavioral effects in a model of severe airway hypersensitivity using repeated HDM treatment in A/J mice. Severe asthma, which is clinically defined as the presence of persistent asthma symptoms, despite high dose immunosuppressive drugs (Chung et al 2014), is reported in about 6–10% of all asthma patients (Backman et al 2018), and has been associated with increased risk for psychiatric disorders, particularly fear-associated conditions such as PD and PTSD. Among several physical illnesses, asthma showed that highest association with PTSD (odds ratio, OR of 5.38 after controlling for trauma and other psychiatric illnesses) (O’Toole and Catts, 2008). PTSD symptoms have been found to be significantly correlated with the severity of asthma (Vanderbilt et al., 2008) and individuals with the highest severity of PTSD symptoms had increased likelihood of having asthma even after controlling for depression, demographics and smoking (Goodwin et al., 2007). Panic disorder (PD) has also been identified as one of the anxiety disorders that is most strongly associated with asthma (Goodwin et al., 2010).
In this study, we investigated fear-associated behaviors to CO2 inhalation (relevant to panic) and fear conditioning-extinction (relevant to PTSD) as well as approach-avoidance and coping behaviors in the EZM and FST. Consistent with our study, a previous study by Caulfield et al, reported no effect of repeated challenges with early life intranasal HDM treatment (to induce airway inflammation) on anxiety-associated behavior on the elevated maze (Caulfield et al., 2018) although in that study, a reduced latency to immobility was observed in the FST suggestive of reduced coping behaviors. Differences in FST behavior between studies may be due to a less chronic allergen exposure model (3 exposures over 3 weeks herein, versus 22 exposures over 7 weeks (Caulfield et al., 2018) or strain differences. A/J mice exhibit poor exploration and avoidance of open arms in the EZM, and high immobility in the FST suggesting that this strain may exhibit high baseline “anxiety-like” and “depression-relevant” phenotypes consistent with previous observations (Molenhuis et al., 2014; Moloney et al., 2015). A/J mice also showed high immobility and freezing during the habituation phases of the CO2 inhalation and fear conditioning tests consistent with enhanced suppression of novelty-induced locomotor behavior in this strain also reported in previous studies (Molenhuis et al., 2014; Moloney et al., 2015).
In the fear conditioning paradigm, A/J mice showed normal fear acquisition but slow decrements in extinction freezing across days that could be suggestive of persisting generalized fear or compromised extinction learning as these mice have previously been reported to have poor cognitive abilities (Molenhuis et al., 2014). Interestingly, despite high generalized freezing in A/J mice, HDM treatment exacerbated freezing selectively during the extinction phase but not during fear acquisition and conditioned fear. Although the exact explanation for this selectivity is not evident, it may be an outcome of altered function of brain circuits, primarily cortical areas, that regulate extinction. This inference is supported by the post-behavior ΔFosB data showing selective increases within the medial prefrontal cortex and the basolateral amygdala. We did not determine cellular phenotypes of labelled cells, therefore no inferences can be made whether these represent excitatory or inhibitory neurons. Prefrontal top-down control of amygdala activity is important during extinction learning and retrieval (Quirk and Mueller, 2008; Motzkin et al., 2015); differences in neuronal activation between HDM and PBS groups within these areas further support altered behavior during extinction. Surprisingly, we did not observe significant effects of HDM on CO2 inhalation-associated spontaneous and contextual freezing. Higher behavioral sensitivity to CO2, an interoceptive trigger is observed in individuals with PD (Vollmer et al., 2015b; Leibold et al., 2016) and evidence suggests high comorbidity of respiratory disorders such as asthma with PD (Goodwin et al., 2003; Hasler et al., 2005). On exposure to 5% CO2 inhalation, A/J mice exhibited high freezing that may have masked any potential behavioral effects of HDM. Collectively, our behavioral observations suggest significant effect of HDM on extinction freezing consistent with increased persistent neuronal activation in mPFC and amygdala.
We determined that HDM-treatment induced behavioral changes were preceded by altered patterns of recruitment of cytokine producing immune cells in the brain and periphery. As previously reported, allergen exposure in A/J animals is associated with increased frequency of both IL-13-producing, and IL-17A-producing immune cells to the lung (Lewkowich et al., 2008; Lajoie et al., 2010) and development of treatment refractory responses (Serra et al 2018). However, pulmonary allergen exposure was also associated with an increased frequency of IL-17A-producing cells into the brain 72 hours after pulmonary allergen exposure. Although this time point precedes the observed HDM-induced behavioral changes, it represents the peak of allergen-induced lung inflammation, and suggests a potential contribution of IL-17A-producing cells in the induction of HDM-induced behavioral changes, however, a direct association needs to be determined. This possibility is consistent with recent reports demonstrating a role for IL-17A-producing T cells in the brain in models of anxiety/depression like behavior (Beurel and Lowell, 2018). The mechanisms surrounding increased numbers of these cells to the brain remains unclear. However, passage of pathogenic IL-17A-producing T cells through peripheral organs like the lung has been reported to “license” them with the appropriate adhesion molecules needed for entry into the brain in a mouse model of experimental autoimmune encephalomyelitis (EAE) (Odoardi et al., 2012; Tan et al., 2017). It is thus conceivable that HDM-induced recruitment of IL-17A-producing cells to the lung serves a similar function, and that IL-17A-producing cells in the brain are derived from IL-17A-producing cells in the lung. In this context, it is interesting to note that the majority of IL-17A-producing cells in the brain were ILC in both PBS- and HDM-exposed animals. However, in a model of EAE, recruitment of IL-17A-producing ILCs to the meninges was required for subsequent entry of pathogenic IL-17A-secreting T cells into the brain (Kwong et al., 2017). Thus, it will be interesting to examine recruitment of IL-17A-secreting cells at later times post HDM exposure to determine if increased recruitment of IL-17A-producing T cells can be observed. Nonetheless, our observations of the accumulation of IL-17A-secreting cells in the brain, prior to observed behavioral changes, support the possibility that local production of IL-17A within the brain may regulate these effects.
Previous work reported T cells within blood brain barrier compromised nodes, such as the circumventricular organs (CVOs), at baseline non-inflammatory conditions (Song et al., 2016) suggesting that these cells can have ready access to brain compartments via these sites. The sensory CVOs are highly vascularized structures located around the ventricles, with a compromised BBB facilitating communication between blood, brain parenchyma, and CSF. Our data supports a potential role of sensory circumventricular organ, SFO in transducing peripheral HDM-induced immune signals to the brain, as an increase in morphologically activated microglia was observed in HDM-treated mice. Interestingly, other CVOs such as the area postrema and OVLT did not elicit significant alterations, at least under our experimental conditions and timeframe. Although an exact explanation for the sensitivity of the SFO to intra-tracheal HDM-associated immune mediators is not evident, differential immune responsivity of CVOs to peripheral challenges may arise due to differences in cellular expression of receptors that respond to circulating signals. Previous studies have shown that lesions of the SFO (but not OVLT and AP) attenuate intravenous LPS-induced fever response (Takahashi et al., 1997) suggesting differences in sensitivity between these sites. It would be important to investigate HDM/Th17/IL17-associated neuroimmune mechanisms within the SFO, as well as, projection areas of the SFO that may regulate fear. Interestingly, the SFO has direct projections to the infralimbic cortex (Swanson and Lind, 1986), an area regulating fear extinction. The SFO may offer a potential site for sensing systemic disturbances including immune challenges related to asthma and their translation into emotional behaviors. However, more investigation is warranted to understand specific neuroimmune mechanisms contributing to HDM effects on fear behaviors.
Limitations and future directions:
Our study provides important information on the association between airway inflammation, brain inflammatory mediators and fear behavior. However, future work is required to identify causal relationships and underlying mechanisms. This initial study was conducted under baseline, non-stressed conditions and it is possible that behaviors not impacted under the baseline conditions of this study will show sensitization in HDM-treated cohorts following stress. Given the relevance of stress and trauma in both asthma and posttraumatic physiology, it would be important to investigate the contribution of stress, its interaction with airway inflammation and impact on behavior. As there is a bi-directional relationship between allergic inflammation and psychiatric illness, it would also be interesting to investigate how posttraumatic stress manipulations impact allergic inflammatory responses. The current investigation focused on behavioral outcomes, however it would be important to investigate the long term effects of HDM on physiological endpoints such as cardiovascular responses and respiratory outcomes that are relevant to PTSD and PD. Although our study provided important behavioral information on the A/J mouse model of severe asthma, exploration of other strains should be undertaken. In this regard, our group has previously demonstrated that BALBc mice treated with a complement component 5 (C5) depleting monoclonal antibody (mAb), develop more severe HDM-induced asthma symptoms compared to isotype control mAb-treated animals due to the specific expansion of Th17 cells (Lajoie et al., 2010). It would be interesting to determine if HDM-exposure alters behaviors observed in anti-C5 mAb-treated, but not isotype mAb-treated BALB/c mice.
In conclusion, we report an association of aeroallergen HDM exposure with increased Th17-IL17A-associated inflammatory mediators in the brain, enhanced fear-associated behavior and altered neuronal activation in fear regulatory areas. Modulation of brain immune mechanisms and fear circuits by peripheral airway inflammation is relevant to understanding the risk and comorbidity of asthma with fear-associated disorders such as PTSD.
Supplementary Material
Supplemental Figure 1: Gating Strategy to quantify cytokine producing cells in the lung. Lungs were removed 72 hours after final HDM/PBS exposure, processed, and stained as described in Materials and Methods. (A) Viability dye versus SSC gating was used to identify cells live cells, (B) FSC versus SSC gating was used to enrich for lymphocytic cells, (C) and CD90.2+ cells were gated to further identify lymphocytes. (D Cells were then gated based on TCRβ versus CD3 expression which facilitated identification of Innate Lymphoid Cells (CD90.2+, CD3neg, TCRβneg), γδ T cells (CD90.2+, CD3ε+, TCRβneg) and conventional αβ T cells (CD90.2+, CD3+, TCRβ+). (E) Conventional αβ T cells were subsequently assessed for CD4 expression to identify CD4+ T cells (CD90.2+, CD3+, TCRβ+, CD4+), and CD8+ T cells (CD90.2+, CD3+, TCRβ+, CD4neg). In both PBS-treated (G) and HDM-treated (F) animals, activated, cytokine producing γδ T cells, CD4+ T cells, and CD8+ T cells were then identified by gating on CD44+, IL-13+, or CD44+, IL-17A+ cells. As CD44 expression has not been demonstrated to be a reliable activation marker on ILCs, both CD44+ and CD44neg ILCs were considered activated cytokine producing cells. All gates were set based on FMO-stained controls. Representative plot from one of 6 animals used for flow cytometric staining shown.
Supplemental Figure 2: Morphological analysis of microglia in HDM and PBS mice within forebrain limbic fear regulatory regions at 72 h post administration Bar graphs show quantified microglial soma perimeter within the medial prefrontal cortex (mPFC) (A), the basolateral amygdala (BLA) and the hippocampal dentate gyrus (DG). Data are represented as mean +/− SEM. (n=6 mice/ group)
Acknowledgements
The authors would like to acknowledge support from NIH grant MH117483-01 (RS and IL). Financial support was also provided by the UC pilot translation research project (PTRP) grant (RS) and CCHMC Mind Brain and Behavior Research Innovation Program (IL). We thank Peter König (University of Lübeck) for providing additional assistance.
References
- Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. (2012). Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief; 94:1–8. [PubMed] [Google Scholar]
- Al-Ramli W, Préfontaine D, Chouiali F, Martin JG, Olivenstein R, Lemière C, Hamid Q (2009) T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol 123:1185–1187 [DOI] [PubMed] [Google Scholar]
- Backman H, Jansson SA, Stridsman C, Muellerova H, Wurst K, Hedman L, Lindberg A, Rönmark E. (2018) Chronic airway obstruction in a population-based adult asthma cohort: Prevalence, incidence and prognostic factors. Respir Med. 138:115–122 [DOI] [PubMed] [Google Scholar]
- Beurel E, Lowell JA (2018) Th17 cells in depression. Brain Behav Immun 69: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Pooler O, Flaherty L (2001) Inbred strain variation in contextual and cued fear conditioning behavior. Mamm Genome 12:651–656 [DOI] [PubMed] [Google Scholar]
- Caulfield JI, Caruso MJ, Bourne RA, Chirichella NR, Klein LC, Craig T, Bonneau RH, August A, Cavigelli SA (2018) Asthma Induction During Development and Adult Lung Function, Behavior and Brain Gene Expression. Front Behav Neurosci 12:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield JI, Caruso MJ, Michael KC, Bourne RA, Chirichella NR, Klein LC, Craig T, Bonneau RH, August A, Cavigelli SA (2017) Peri-adolescent asthma symptoms cause adult anxiety-related behavior and neurobiological processes in mice. Behav Brain Res 326:244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Center for Disease Control and Prevention) Most recent National Asthma Data. Source: 2018 National Health Interview Survey (NHIS) Data. .(https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm).
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jajour NN, Mauad T, Sorkness RL, Teague WG. (2014) International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 43(2):343–73. [DOI] [PubMed] [Google Scholar]
- Chesné J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A (2014) IL-17 in Severe Asthma. Where Do We Stand? Am J Respir Crit Care Med 190:1094–1101 [DOI] [PubMed] [Google Scholar]
- Del Giacco SR, Cappai A, Gambula L, Cabras S, Perra S, Manconi PE, Carpiniello B, Pinna F (2016) The asthma-anxiety connection. Respir Med 120:44–53 [DOI] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF (2006) Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: Immunophenotypic and functional characteristics. J Neurosci Methods 151:121–130 [DOI] [PubMed] [Google Scholar]
- Franklin K and Paxinos G (2008) The Mouse Brain in Stereotaxic Coordinates, Compact-3rd Edition Academic Press, San Diego, CA [Google Scholar]
- Goodwin RD, Fischer ME, Goldberg J (2007) A twin study of post-traumatic stress disorder symptoms and asthma. Am J Respir Crit Care Med 176:983–987 [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Jacobi F, Thefeld W (2003) Mental disorders and asthma in the community. Arch Gen Psychiatry 60:1125–1130. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Pagura J, Cox B, Sareen J (2010) Asthma and mental disorders in Canada: impact on functional impairment and mental health service use. J Psychosom Res 68:165–173 [DOI] [PubMed] [Google Scholar]
- Gros DF, Frueh BC, Magruder KM Prevalence and features of panic disorder and comparison to posttraumatic stress disorder in VA primary care. Gen Hosp Psychiatry 33:482–488 [DOI] [PubMed] [Google Scholar]
- Gurczynski SJ, Moore BB (2018) IL-17 in the lung: the good, the bad, and the ugly. Am J Physiol Cell Mol Physiol 314:L6–L16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Uga H, Mori A, Kurata H (2017) Increased serum IL-17A and Th2 cytokine levels in patients with severe uncontrolled asthma. Eur Cytokine Netw 28:8–18 [DOI] [PubMed] [Google Scholar]
- Hasler G, Gergen PJ, Kleinbaum DG, Ajdacic V, Gamma A, Eich D, Rössler W, Angst J (2005) Asthma and panic in young adults: a 20-year prospective community study. Am J Respir Crit Care Med 171:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. (2003) Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 24(3):151–80 [DOI] [PubMed] [Google Scholar]
- Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, Martin RJ, Alam R (2014) Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol 134:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Furini CR, Myskiw JC.(2016) Fear Memory. Physiol. Rev 96(2):695–750 [DOI] [PubMed] [Google Scholar]
- Johnson AK, Gross PM (1993) Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 7(8):678–86. [DOI] [PubMed] [Google Scholar]
- Kean EM, Kelsay K, Wamboldt F, Wamboldt MZ (2006) Posttraumatic stress in adolescents with asthma and their parents. J Am Acad Child Adolesc Psychiatry 45:78–86 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995) Posttraumatic stress disorder in the National Comorbidity Survey. ArchGenPsychiatry 52:1048–1060. [DOI] [PubMed] [Google Scholar]
- Kim D, McAlees JW, Bischoff LJ, Kaur D, Houshel LK, Gray J, Hargis J, Davis X, Dudas PL, Deshmukh H, Lewkowich IP (2019) Combined administration of anti-IL-13 and anti-IL-17A at individually sub-therapeutic doses limits asthma-like symptoms in a mouse model of Th2/Th17 high asthma. Clin Exp Allergy 49:317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong B, Rua R, Gao Y, Flickinger J, Wang Y, Kruhlak MJ, Zhu J, Vivier E, McGavern DB, Lazarevic V (2017) T-bet-dependent NKp46+ innate lymphoid cells regulate the onset of TH17-induced neuroinflammation. Nat Immunol 18:1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M (2010) Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol 11:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H, Fahy JV (2019) The Cytokines of Asthma. Immunity 50:975–991 [DOI] [PubMed] [Google Scholar]
- Leibold NK, van den Hove DLA, Viechtbauer W, Buchanan GF, Goossens L, Lange I, Knuts I, Lesch KP, Steinbusch HWM, Schruers KRJ (2016) CO2 exposure as translational cross-species experimental model for panic. Transl Psychiatry 6:e885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, Grillon C.(2010) Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry. 167:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow MA, Chen A (2016) Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 21(4):450–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M (2008) Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS One 3:e3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R (2001) The use of behavioral test batteries: effects of training history. Physiol Behav 73:705–717 [DOI] [PubMed] [Google Scholar]
- McMurray KMJ, Strawn JR, Sah R (2019) Fluoxetine Modulates Spontaneous and Conditioned Behaviors to Carbon Dioxide (CO2) Inhalation and Alters Forebrain–Midbrain Neuronal Activation. Neuroscience 396:108–118 [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T (2017) Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 42:254–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenhuis RT, de Visser L, Bruining H, Kas MJ (2014) Enhancing the value of psychiatric mouse models; differential expression of developmental behavioral and cognitive profiles in four inbred strains of mice. Eur Neuropsychopharmacol 24:945–954 [DOI] [PubMed] [Google Scholar]
- Moloney RD, Dinan TG, Cryan JF (2015) Strain-dependent variations in visceral sensitivity: relationship to stress, anxiety and spinal glutamate transporter expression. Genes Brain Behav 14:319–329 [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M (2015) Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry 77:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt CM et al. (2019) International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole BI, Catts SV. (2008) Trauma, PTSD, and physical health: An epidemiological study of Australian Vietnam veterans. J Psychosom Res 64:33–40 [DOI] [PubMed] [Google Scholar]
- Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schläger C, Lodygin D, Heckelsmiller K, Nietfeld W, Ellwart J, Klinkert WEF, Lottaz C, Nosov M, Brinkmann V, Spang R, Lehrach H, Vingron M, Wekerle H, Flügel-Koch C, Flügel A (2012) T cells become licensed in the lung to enter the central nervous system. Nature 488:675–679 [DOI] [PubMed] [Google Scholar]
- Papp LA, Klein DF, Gorman JM (1993) Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. Am J Psychiatry 150:1149–57. [DOI] [PubMed] [Google Scholar]
- Pino PA, Cardona AE (2011) Isolation of Brain and Spinal Cord Mononuclear Cells Using Percoll Gradients. J Vis Exp 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Banks WA. (2007) Brain-immune communication pathways. Brain Behav Immun. 21(6):727–35 [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33:56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassovsky Y, Kushner MG (2003) Carbon dioxide in the study of panic disorder: issues of definition, methodology, and outcome. J Anxiety Disord. 17(1):1–32. [DOI] [PubMed] [Google Scholar]
- Sanderson WC, Wetzler S (1990) Five percent carbon dioxide challenge: Valid analogue and marker of panic disorder? Biol Psychiatry 27:689–701 [DOI] [PubMed] [Google Scholar]
- Schubert I, Ahlbrand R, Winter A, Vollmer L, Lewkowich I, Sah R (2018) Enhanced fear and altered neuronal activation in forebrain limbic regions of CX3CR1-deficient mice. Brain Behav Immun 68:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra MF, Cotias AC, Pão CRR, Daleprane JB, Jurgilas PB, Couto GC, Anjos-Valotta EA, Cordeiro RSB, Carvalho VF, Silva PMR, Martins MA (2018) Repeated Allergen Exposure in A/J Mice Causes Steroid-Insensitive Asthma via a Defect in Glucocorticoid Receptor Bioavailability. J Immunol 201:851–860. [DOI] [PubMed] [Google Scholar]
- Song C, Nicholson JD, Clark SM, Li X, Keegan AD, Tonelli LH (2016) Expansion of brain T cells in homeostatic conditions in lymphopenic Rag2−/− mice. Brain Behav Immun 57:161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer C, Koch B, Grabe HJ, Ewert R, Barnow S, Felix SB, Ittermann T, Obst A, Volzke H, Glaser S, Schaper C (2011) Association of airflow limitation with trauma exposure and post-traumatic stress disorder. Eur Respir J 37:1068–1075 [DOI] [PubMed] [Google Scholar]
- Stowman S, Kearney CA, Daphtary K (2015) Mediators of Initial Acute and Later Posttraumatic Stress in Youth in a PICU*. Pediatr Crit Care Med 16:e113–e118 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Lind RW (1986) Neural projections subserving the initiation of a specific motivated behavior in the rat: new projections from the subfornical organ. Brain Res 379:399–403 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Smith P, Ferguson A, Pittman QJ (1997) Circumventricular organs and fever. Am J Physiol - Regul Integr Comp Physiol 273. [DOI] [PubMed] [Google Scholar]
- Tan C, Wandu WS, Lee RS, Hinshaw SH, Klinman DM, Wawrousek E, Gery I (2017) Shedding New Light on the Process of “Licensing” for Pathogenicity by Th Lymphocytes. J Immunol 198:681–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderbilt D, Young R, MacDonald HZ, Grant-Knight W, Saxe G, Zuckerman B (2008) Asthma severity and PTSD symptoms among inner city children: a pilot study. J Trauma Dissociation 9:191–207 [DOI] [PubMed] [Google Scholar]
- Võikar V, Vasar E, Rauvala H (2004) Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes Brain Behav 3:27–38 [DOI] [PubMed] [Google Scholar]
- Vollmer L, Ghosal S, Rush JA, Sallee FR, Herman JP, Weinert M, Sah R (2013) Attenuated stress-evoked anxiety, increased sucrose preference and delayed spatial learning in glucocorticoid-induced receptor-deficient mice. Genes, Brain Behav 12:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer LL, Schmeltzer SN, Ahlbrand R, Sah R (2015a) A potential role for the acid-sensing T cell death associated gene-8 (TDAG8) receptor in depression-like behavior. Physiol Behav 150:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer LL, Strawn JR, Sah R (2015b) Acid-base dysregulation and chemosensory mechanisms in panic disorder: A translational update. Transl Psychiatry 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer LL, Ghosal S, McGuire JL, Ahlbrand RL, Li K-Y, Santin JM, Ratliff-Rang CA, Patrone LGA, Rush J, Lewkowich IP, Herman JP, Putnam RW, Sah R (2016) Microglial Acid Sensing Regulates Carbon Dioxide-Evoked Fear. Biol Psychiatry 80:541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A, Ahlbrand R, Sah R (2019) Recruitment of central angiotensin II type 1 receptor associated neurocircuits in carbon dioxide associated fear. Prog Neuro-Psychopharmacology Biol Psychiatry 92:378–386 [DOI] [PubMed] [Google Scholar]
- Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J, Nagarkatti M (2014) Dysregulation in microRNA Expression Is Associated with Alterations in Immune Functions in Combat Veterans with Post-Traumatic Stress Disorder Gonzalez P, ed. PLoS One 9:e94075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE (2010) Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28:445–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA 3rd, Welsh MJ, Wemmie JA (2009) The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 139:1012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Gating Strategy to quantify cytokine producing cells in the lung. Lungs were removed 72 hours after final HDM/PBS exposure, processed, and stained as described in Materials and Methods. (A) Viability dye versus SSC gating was used to identify cells live cells, (B) FSC versus SSC gating was used to enrich for lymphocytic cells, (C) and CD90.2+ cells were gated to further identify lymphocytes. (D Cells were then gated based on TCRβ versus CD3 expression which facilitated identification of Innate Lymphoid Cells (CD90.2+, CD3neg, TCRβneg), γδ T cells (CD90.2+, CD3ε+, TCRβneg) and conventional αβ T cells (CD90.2+, CD3+, TCRβ+). (E) Conventional αβ T cells were subsequently assessed for CD4 expression to identify CD4+ T cells (CD90.2+, CD3+, TCRβ+, CD4+), and CD8+ T cells (CD90.2+, CD3+, TCRβ+, CD4neg). In both PBS-treated (G) and HDM-treated (F) animals, activated, cytokine producing γδ T cells, CD4+ T cells, and CD8+ T cells were then identified by gating on CD44+, IL-13+, or CD44+, IL-17A+ cells. As CD44 expression has not been demonstrated to be a reliable activation marker on ILCs, both CD44+ and CD44neg ILCs were considered activated cytokine producing cells. All gates were set based on FMO-stained controls. Representative plot from one of 6 animals used for flow cytometric staining shown.
Supplemental Figure 2: Morphological analysis of microglia in HDM and PBS mice within forebrain limbic fear regulatory regions at 72 h post administration Bar graphs show quantified microglial soma perimeter within the medial prefrontal cortex (mPFC) (A), the basolateral amygdala (BLA) and the hippocampal dentate gyrus (DG). Data are represented as mean +/− SEM. (n=6 mice/ group)


