Abstract
Myasthenic crisis (MC) is a life-threatening condition for patients with myasthenia gravis (MG). Seronegative patients represent around 10–15% of MG, but data on outcome of seronegative MCs are lacking. We performed a subgroup analysis of patients who presented with MC with either acetylcholine-receptor-antibody-positive MG (AChR-MG) or seronegative MG between 2006 and 2015 in a retrospective German multicenter study. We identified 15 seronegative MG patients with 17 MCs and 142 AChR-MG with 159 MCs. Seronegative MCs were younger (54.3 ± 14.5 vs 66.5 ± 16.3 years; p = 0.0037), had a higher rate of thymus hyperplasia (29.4% vs 3.1%; p = 0.0009), and were more likely to be female (58.8% vs 37.7%; p = 0.12) compared to AChR-MCs. Time between diagnosis of MG and MC was significantly longer in seronegative patients (8.2 ± 7.6 vs 3.1 ± 4.4 years; p < 0.0001). We found no differences in duration of mechanical ventilation (16.2 ± 15.8 vs 16.5 ± 15.9 days; p = 0.94) and length of stay at intensive care unit (17.6 ± 15.2 vs 17.8 ± 15.4 days; p = 0.96), or in-hospital mortality (11.8% vs. 10.1%; p = 0.69). We conclude that MC in seronegative MG affects younger patients after a longer period of disease, but that crisis treatment efficacy and outcome do not differ compared to AChR-MCs.
Keywords: Myasthenia gravis, Myasthenic crisis, Antibody status, Seronegative, Outcome
Introduction
Myasthenia gravis (MG) is an autoimmune disease with antibodies (Abs) targeting the postsynaptic neuromuscular junction. Ultimately, muscle fatigability and weakness are caused by disrupted neuromuscular signaling. Nearly 90% of all MG patients have positive test results for AChR, Muscle-specific kinase (MuSK), or low-density lipoprotein receptor-related protein (LRP4) autoantibodies, with the majority tested positive for AChR-Abs [1]. However, in around 10–15% of MG patients no specific autoantibodies can be found. This group of seronegative patients is also thought to include patients with very low antibody titers, low-affinity antibodies and yet to be defined autoantigens [1].
Myasthenic crisis (MC) is the most severe form of MG and is potentially life threatening. MC is mostly provoked by infections, but also fever, aspiration, inadequate treatment, various medications, or following surgery [2]. In the first two years after diagnosis, around 15–20% of MG patients suffer from a MC [2, 3]. Characteristic symptoms are extensive weakness, dysphagia, and dyspnea which can result in respiratory insufficiency. The clinical management of MC is well defined and has led to a significant decline in mortality from around 40% in the early 1960s to 5% to 12% in recent studies [2–8].
However, to date little is known about the management of MC in seronegative MG. Here, we therefore investigated seronegative patients with MC and compared their crises to AChR-MCs regarding clinical features, therapeutic management, and outcome.
Methods
Study design and patient selection
We performed a subgroup analysis of seronegative MC needing mechanical ventilation (MV) compared to AChR-MC treated at eight German Departments of Neurology with specialized Neuro-Intensive Care Units (NICU) or neurologically associated interdisciplinary ICU [2]. For identification, records of all patients discharged with the diagnosis of MG according to the International Classification of Diseases (ICD10: G70.0–70.3) who were treated and ventilated on an ICU between 2006 and 2015 were reviewed. MC was defined as an exacerbation of myasthenic symptoms with bulbar and/or general weakness requiring MV. Seronegative MG was defined as absence of AChR and MuSK autoantibodies. Per protocol, antibody status was confirmed by routine laboratory testing using certified assays. Most AChR-Abs and MuSK-Abs were tested by radio-receptor assay, but the method is not known in all cases due to the retrospective character. Diagnosis of MG had to be established clinically according to national guidelines and confirmed by specific tests (antibody testing or repetitive stimulation or improvement after cholinergic medication) [9]. New episodes of MC were counted separately if patients were discharged in their prehospital status and if new triggers for the next crisis could be determined. For this analysis, we only included AChR-MCs treated at the same centers as the seronegative MCs to reduce treatment and data acquisition bias.
Data acquisition
Data on baseline demographics, clinical information, medication and comorbidities were obtained through review of medical records and institutional databases. Characteristics reviewed included antibody-status, evidence of thymoma and Myasthenia Gravis Foundation of America (MGFA) Score prior to MC. Assessed treatment regimens were intravenous immunoglobulins (IVIG), plasma exchanging therapy (PE), immunoadsorption (IA), use of intravenous pyridostigmine, and continuous potassium infusion. Analyzed data regarding the MC included time at intensive care unit (ICU-LOS), days in hospital, duration of MV, in-hospital mortality and referral/discharge. In addition, we performed a survey about LRP4- and Agrin-antibody-positive MGs in our study group in June 2021.
Statistics
GraphPad Prism 5® (GraphPad Software, La Jolla, USA) was used for statistical analysis. Data were presented as mean with standard deviation or range (as indicated) or total number with percentage. Group comparison was tested with either Student’s t test or Fisher’s exact test (with odds ratios (OR)). The significance level was set to α = 0.05 both sided.
Results
Characteristics of study group
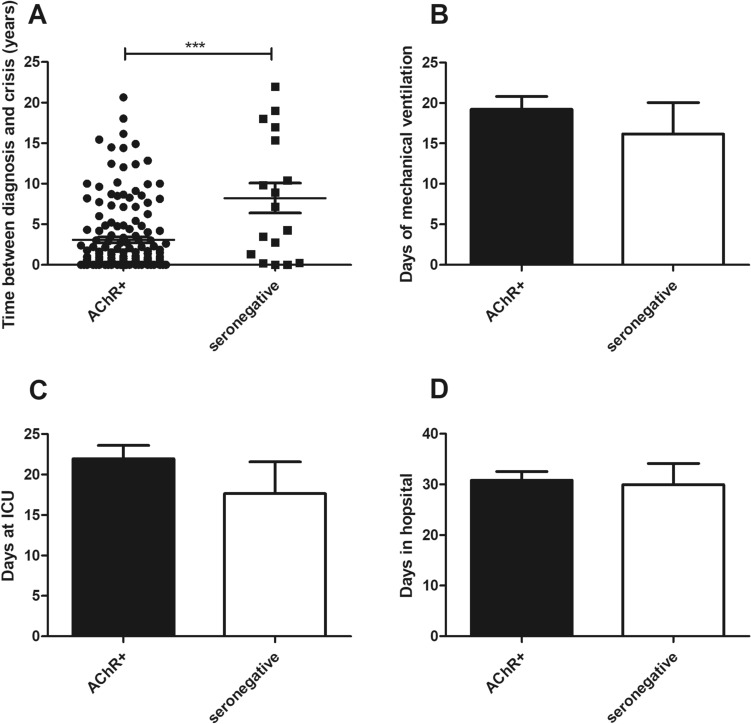
The cohort consisted of 15 patients with 17 seronegative MCs and 142 AChR antibody-positive patients with 159 MCs requiring MV. Patients from both groups were treated at the same centers (Table 1). Seronegative patients were responsible for 6.8% of the crises in our whole cohort (n = 250 crises). Patients with seronegative MC were significantly younger (54.3 ± 14.5 vs 66.5 ± 16.3; p = 0.0037) and more likely to be female (58.8 vs 37.7%; p = 0.12) than AChR-MCs. AChR-MCs were significantly more often late-onset MGs (85.5% vs 41.2%; p = 0.0001; OR = 0.12), whereas seronegative MCs belonged mainly to the early-onset group (Table 1) and had significantly more frequently a thymus hyperplasia (29.4% vs 3.1%; p = 0.0009; OR 12.83). Thymus hyperplasias were resected in all patients prior to crisis, except in one patient in the AChR-group. Importantly, the time between diagnosis of MG and onset of MC was significantly longer in seronegative patients (8.2 ± 7.6 vs 3.1 ± 4.4 years; p < 0.0001) (Fig. 1A). Due to the higher age, patients with AChR-MCs had more comorbidities, yet without reaching statistical significance (Table 1). We also did not find statistically significant differences in the status before crisis, MGFA classification before crisis, dosage of pyridostigmine treatment before crisis (252.4 ± 243.3 vs 251.1 ± 206.6 mg/d; p = 0.99) or number of myasthenic worsening/crises before present MC (Table 1). The number of days between first symptoms of MC and hospitalization were similar (9.9 ± 13.1 vs 9.6 ± 14.9; p = 0.95).
Table 1.
Comparison of episodes of myasthenic crisis with AChR-Abs and seronegative patients
| Myasthenic crises | AChR-positive (n = 159) | Seronegative (n = 17) | P value | Odds ratio |
|---|---|---|---|---|
| Age | 66.5 ± 16.3 (14–89) | 54.3 ± 14.5 (25–81) | 0.0037 | |
| Male/female | 99 (62.3%)/60 (37.7%) | 7 (41.2%)/10 (58.8%) | 0.12 | 0.42 |
| Pulmonary disease | 38 (23.9%) | 2 (11.8%) | 0.37 | 0.42 |
| Heart disease | 61 (38.4%) | 5 (29.4%) | 0.60 | 0.67 |
| Diabetes mellitus | 48 (30.2%) | 2 (11.8%) | 0.16 | 0.31 |
| Tumour (other than thymoma) | 24 (15.1%) | 0 (0%) | 0.13 | 0.16 |
| Dialysis | 1 (0.6%) | 0 (0%) | 1.00 | 3.02 |
| Smoker | 16 (10.1%) | 2 (11.8%) | 0.69 | 1.19 |
| Alcohol addicted | 5 (3.1%) | 2 (11.8%) | 0.14 | 4.11 |
| ≥ 3 diseases (kidney, heart, lung, diabetes, tumour) | 21 (13.2%) | 0 (0%) | 0.23 | 0.18 |
| Myasthenia gravis | ||||
| Early onset | 22 (13.8%; 1 unknown) | 10 (58.8%) | < 0.0001 | 8.90 |
| Late onset | 136 (85.5%) | 7 (41.2%) | 0.0001 | 0.12 |
| Paraneoplastic MG (Thymoma) | 53 (33.3%) | 3 (17.6%) | 0.27 | 0.43 |
| Thymus hyperplasia | 5 (3.1%) | 5 (29.4%) | 0.0009 | 12.83 |
| Number of myasthenic worsenings/crises before present myasthenic crisis | 0.7 ± 1.3 | 0.9 ± 1.3 | 0.49 | |
| Time between first diagnosis and crisis (years) | 3.1 ± 4.4 (0–18.2) | 8.2 ± 7.6 (0–22) | < 0.0001 | |
| MGFA-classification before crisis | ||||
| First manifestation of MG | 38 (23.9%) | 3 (17.6%) | 0.77 | 0.68 |
| Class I | 8 (5.0%) | 1 (5.9%) | 1.00 | 1.18 |
| Class II | 44 (27.7%) | 4 (23.5%) | 1.00 | 0.80 |
| Class III | 40 (25.2%) | 4 (23.5%) | 1.00 | 0.92 |
| Class IV | 16 (10.1%) | 2 (11.8%) | 0.69 | 1.19 |
| Unknown | 13 (8.2%) | 2 (11.8%) | ||
| Status before crisis | ||||
| Independent at home | 73 (45.9%) | 7 (41.2%) | 0.80 | 0.82 |
| At home dependent on help | 23 (14.5%) | 3 (17.6%) | 0.72 | 1.27 |
| In a care facility or hospital | 49 (30.8%) | 7 (41.2%) | 0.42 | 1.57 |
| Unknown | 14 (8.8%) | 0 (0%) | ||
| Cause of crisis | ||||
| Infection | 80 (50.3%) | 8 (47.1%) | n.s | |
| First episode | 36 (22.6%) | 3 (17.6%) | ||
| Poor treatment compliance | 14 (8.8%) | 0 (0%) | ||
| Intake of contraindicated medication | 2 (1.2%) | 0 (0%) | ||
| Idiopathic/unknown | 32 (20.1%) | 6 (35.3%) | ||
| Therapy | ||||
| IVIG | 93 (58.5%) | 4 (23.5%) | 0.009 | 0.22 |
| Plasma exchange/immunoadsorption in total | 75 (47.2%) | 10 (58.8%) | 0.45 | 1.60 |
| PE or IA as first line therapy | 60 (37.7%) | 10 (58.8%) | 0.12 | 2.34 |
| IVIG + plasma exchange or immunoadsorption | 30 (18.9%) | 1 (5.9%) | 0.31 | 0.27 |
| Continuous pyridostigmine infusion | 61 (38.4%) | 5 (29.4%) | 0.60 | 0.67 |
| Complications | ||||
| CPR | 19 (11.9%) | 2 (11.8%) | 1.00 | 0.98 |
| Pneumonia | 86 (54.1%) | 9 (52.9%) | 1.00 | 0.95 |
| Sepsis | 32 (20.1%) | 3 (17.6%) | 1.00 | 0.85 |
| Outcome | ||||
| Days of mechanical ventilation at ICU | 19.2 ± 19.5 (1–119) | 16.2 ± 15.8 (1–55) | 0.54 | |
| Days at ICU | 22.0 ± 20.5 (1–135) | 17.6 ± 15.2 (3–56) | 0.42 | |
| Days in hospital | 30.8 ± 21.4 (3–144) | 29.9 ± 16.5 (3–71) | 0.87 | |
| In-hospital mortality | 16 (10.1%) | 2 (11.8%) | 0.69 | 1.19 |
Age, “Days of mechanical ventilation at ICU”, “Days at ICU”, “Days in hospital” and “Time between first diagnosis and crisis (years)”are depicted as mean ± Standard Deviation and range, other parameters are total number with percentage in brackets. MGFA Myasthenia Gravis Foundation of America, MG Myasthenia Gravis, IVIG Intravenous Immunoglobulin, PE Plasma exchange, IA Immunoadsorption, CPR Cardio Pulmonal Resuscitation, n.s. not significant. t test was used for statistical analysis of age-differences and for comparison of “Days of mechanical ventilation at ICU”, “Days at ICU” and “Days in hospital”. For other parameters Fisher`s exact test with odds ratio was used. Significant result (p ≤ 0.05) are shown in bold letters
Fig. 1.
A Time between first diagnosis of MG to first MC in years. Every dot or square symbolizes one patient. Long line shows mean, short lines show SD (t test). ***p < 0.001 B Days of mechanical ventilation, C Days at ICU, D Days in hospital in 159 MCs with AChR-Abs and 17 MCs with MuSK-Abs. B–D Bars show mean ± SD (t test). No significant results were found
To further characterize the patients with seronegative MCs, we surveyed all participating centers on retesting for LRP4 and Agrin antibodies in these seronegative patients. 6 of 15 seronegative MGs were tested for LRP4-antibodies and 5 of 15 for Agrin-antibodies. However, all tests remained negative. At the centers of our study group 28 patients with LRP4 and 0 patients with Agrin-antibodies are treated, but none developed an MC needing ICU-treatment within the period of observation.
Treatment and outcome
Seronegative MCs were significantly less frequently treated with IVIGs (23.5% vs 58.5%; p = 0.009; OR = 0.22) (Table 1), and although they received PE or IA more frequently than AChR-MCs, no statistically significant difference could be found (58.8% vs 47.2%; p = 0.45; OR = 1.60). Likewise, although AChR-MCs were more frequently treated with the combination of PE or IA and IVIG, this was not statistically significant (18.9% vs 5.9%; p = 0.31; OR = 0.27). Furthermore, days of MV at ICU (16.2 ± 15.8 vs 19.2 ± 19.5; p = 0.54), ICU-LOS (17.6 ± 15.2 vs 22.0 ± 20.5; p = 0.42) and hospital-LOS (29.9 ± 16.5 vs 30.8 ± 21.4; p = 0.87) were not statistically significantly different (Table 1 and Fig. 1B-D). The in-hospital mortality was similar between both groups (11.8% vs 10.1%; p = 0.69; OR = 1.19). Importantly, seronegative patients were less frequently discharged while still needing MV (5.9% vs 20.8%; p = 0.20; OR = 0.24) compared to AChR-MG patients, but without a statistically significant difference. Consequently, after matching 17 seronegative to 51 AChR-positive MCs regarding age (54.3 ± 14.5 vs 53.9 ± 16.3; p = 0.93) and sex (58.8% vs 53.0% female; p = 0.78) we found no differences in days of MV (16.2 ± 15.8 vs 16.5 ± 15.9; p = 0.94) and ICU-LOS (17.6 ± 15.2 vs 17.8 ± 15.4; p = 0.96). Treatment and outcome details for all 17 seronegative MCs are shown in Table 2. We found no difference between the treatment options IVIG and PE/IA or the additional use of intravenous pyridostigmine regarding the endpoint duration of MV in seronegative MCs.
Table 2.
Treatment and outcome details of all 17 seronegative MCs
| Patient no. | Age | IVIG | PE/IA | Pyridostigmine intravenous | Days of mechanical ventilation | Death |
|---|---|---|---|---|---|---|
| 1 | 80 | 5 × 30 g | No | No | 38 | No |
| 2 | 66 | No | 5 cycles of PE | 10,8 mg/24 h | 39 | No |
| 3 | 25 | No | 5 cycles of PE | 9,6 mg/24 h | 20 | No |
| 4 | 45 | 3 × 30 g | No | No | 3 | No |
| 5 | 48 | No | No | 7,2 mg/24 h | 12 | No |
| 6 | 46 | 5 × 20 g | No | 2,4 mg/24 h | 13 | No |
| 7 | 58 | No | No | No | 30 | Septic shock |
| 8 | 55 | No | 5 cycles of PE | 12 mg/24 h | 5 | No |
| 9 | 81 | 5 × 30 g | 5 cycles of IA | No | 26 | No |
| 10 | 68 | No | No | No | 1 | Septic shock |
| 11 | 60 | No | 5 cycles of IA | No | 55 | No |
| 12 | 45 | No | 7 cycles of PE | No | 10 | No |
| 13 | 47 | No | 5 cycles of PE | No | 2 | No |
| 14 | 66 | No | unknown cycles of PE | No | 3 | No |
| 15 | 52 | No | 6 cycles of PE | No | 8 | No |
| 16 | 42 | No | 3 cycles of PE | No | 4 | No |
| 17 | 39 | No | 5 cycles of PE | No | 16 | No |
IVIG intravenous immunoglobulin, PE plasma exchange, IA immunoadsorption
Discussion
Here, we investigated clinical features of seronegative MC compared to AChR-MC requiring mechanical ventilation based on our multicenter cohort of MC [2]. In contrast to MuSK-MCs, which are as old as AChR-MCs [5], we found that in seronegative MC, patients were younger but that the time between diagnosis of MG and onset of MC was longer compared to AChR-MC. Interestingly, 8 of 17 seronegative MCs had thymic abnormalities. Although seronegative MCs were less frequently treated with IVIg, there was no difference in other MC treatments. Furthermore, we did not find any difference in baseline characteristics, in the rate of complications or outcome between the patient groups, which is in contrast to more severely affected MuSK MC patients [5].
Seronegative patients represent 10–15% of all MGs. However, there are only very limited data on the clinical management and outcome of MC. In our cohort of MCs, 17 of 250 (6.8%) events were from seronegative patients [2], which may indicate that MC is less prevalent in seronegative patients. Yet, our study was not designed to unambiguously address this question. While MC occurs in most MG patients within the first 2 years after diagnosis [3], seronegative patients in our cohort developed MC significantly later compared to AChR-MG thus suggesting a less severe disease onset in these patients.
Interestingly, LRP4-positive MGs treated at our centers until now never experienced a MC and all retested seronegative patients in our cohort (40%) were negative for LRP4. Moreover, we did not find any publication about a LRP4-MC suggesting that this subgroup is even less severely affected than seronegative MG.
Seronegative MG patients are a heterogeneous group of patients and although we had stringent inclusion criteria, we cannot rule out that some seronegative patients have low-affinity antibodies or would be positive for complement deposition at the neuromuscular junction [10, 11]. Especially the high portion of thymus hyperplasia in our cohort might argue for low-affinity antibodies since thymic pathologies in MG are known to produce AChR-antibodies [12], but 75% of double negative patients (AChR and MuSK) showed lymph node-type infiltrates in thymus similar to AChR-MG [13].
Limitations of this study arise from its retrospective nature and the relatively small sample size. Nevertheless, our study is by far the largest analysis of seronegative MCs and therefore provides important evidence on the treatment of this understudied patient population. Nevertheless, large prospective multicenter studies are needed to further elucidate the character of seronegative myasthenic crisis and whether specific treatment is warranted compared to AChR-MC or MuSK-MC. Another limitation is that antibody tests were done in different labs and therefore false negative or false positive results due to unspecificity of the test technique or positive/negative results near the threshold range cannot be ruled out in every case, like in previous studies in myasthenia gravis.
We conclude that patients with seronegative MC are younger, with a longer course of disease until first crisis needing MV than AChR-MC but that there is no difference in outcome between these patient groups.
Acknowledgements
Co-investigators: Kornelius Fuchs, (Department of Neurology, University Medical Center Regensburg, Regensburg, Germany): Data acquisition, supervision of personnel. Berthold Schalke (Department of Neurology, University Medical Center Regensburg, Regensburg, Germany): Data acquisition, supervision of personnel. Amelie Vidal (Department of Neurology, University Medical Center Regensburg, Regensburg, Germany): Data acquisition. Izabela Brachaczek, Jana Maidhof (Charité-Universitätsmedizin Berlin, Berlin, Department of Neurology, NeuroCure Clinical Research Center): Data acquisition. Arno Wenke (Charité-Universitätsmedizin Berlin, Berlin, Department of Neurology, NeuroCure Clinical Research Center): Data acquisition, supervision of personnel. Manuel Hagen (Department of Neurology, University Hospital Erlangen, Erlangen, Germany): Data acquisition. Jan Rahmig (Department of Neurology, University Hospital, Technische Universität Dresden, Dresden, Germany): Data acquisition. Eik Schimmel (Department of Neurology, University Hospital, Technische Universität Dresden, Dresden, Germany. Now: Department of Neurology, Staedtisches Klinikum Dresden, Dresden, Germany): Data acquisition. Wolf Niesen (Department of Neurology, University of Freiburg, Freiburg, Germany): Data acquisition, supervision of personnel. Christine Fahrendorf (Department of Neurology, St. Josef Hospital, Ruhr University Bochum, Bochum, Germany): Data acquisition, supervision of personnel.
Author contributions
All authors have made a substantial contribution to the design, data collection and analysis of the research and the drafting of the manuscript and reviewed and accepted the contents of the manuscript prior to its submission. PM, HRS, AM, and BN: conception and design of the study, acquisition and analysis of data and drafting or revising a significant portion of the manuscript or figures. CD and KA: conception and design of the study and drafting or revising a significant portion of the manuscript or figures. SK, STG, HBH, HF, JZ, AA, IK, CS-G, CR, JD, AS, and AT: acquisition and analysis of data. SS, JB, and HS: acquisition and analysis of data and drafting or revising a significant portion of the manuscript or figures. HR, BB, D-HL, and RL: drafting or revising a significant portion of the manuscript or figures.
Funding
Open Access funding enabled and organized by Projekt DEAL. PM is Einstein Junior Fellow of the Einstein Foundation Berlin and has been supported by the Charité-BIH Clinician Scientist Program and the Bundesministerium für Bildung und Forschung (Grant nos. 16GW0191 and NUM-COVID 19—Organo-Strat 01KX2021). This research did not receive further specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
Anonymized data will be made available upon reasonable request.
Declarations
Conflicts of interest
Philipp Mergenthaler is on the Advisory Board of HealthNextGen Inc. and has equity interest in the company, within the scope of the present work, but not directly related; Henning R. Stetefeld reports no disclosures; Christian Dohmen reports no disclosures; Siegfried Kohler reports no disclosures; Silvia Schönenberger reports no disclosures; Julian Bösel reports personal fees from Medtronic, personal fees from Zoll, personal fees from Boehringer Ingelheim, personal fees from Sedana Medical, personal fees from PCORI, outside the submitted work. Stefan T. Gerner reports no disclosures; Hagen B. Huttner reports personal fees from Daiichi Sanyko, grants and personal fees from Novartis, grants and personal fees from UCB Pharma, grants and personal fees from Portola Pharmaceuticals, personal fees from Bayer AG, personal fees from Boehringer Ingelheim, personal fees from Prediction BioSciences, outside the submitted work; Hauke Schneider reports no disclosures; Heinz Reichmann reports no disclosures; Hannah Fuhrer reports no disclosures; Benjamin Berger received travel grants and/or training expenses from Bayer Vital GmbH, Ipsen Pharma GmbH, Novartis, Biogen GmbH, Merck Serono GmbH, and Genzyme, as well as lecture fees from Ipsen Pharma GmbH, Alexion Pharma Germany GmbH, Merck Serono GmbH, Sanofi Genzyme, and Roche Pharma AG outside the submitted work; Jan Zinke reports no disclosures; Anke Alberty reports no disclosures; Ingo Kleiter reports personal fees from Biogen, personal fees from Novartis, personal fees from Merck, personal fees from Sanofi Genzyme, personal fees from Roche, personal fees from Mylan, personal fees from Alexion, personal fees from Celgene, grants and personal fees from Chugai, personal fees from IQVIA, outside the submitted work; Christiane Schneider-Gold has received consulting and/or speaker's honoraria from Alexion Pharmaceuticals, Amicus Therapeutics, Bayer Schering, CSL Behring, Grünenthal, Hormosan, Lupin Pharmaceuticals, and TEVA outside the submitted work; Christian Roth reports no disclosures; Juliane Dunkel reports no disclosures; Andreas Steinbrecher reports no disclosures; Andrea Thieme reports no disclosures; De-Hyung Lee reports no disclosures; Ralf Linker reports no disclosures; Klemens Angstwurm reports no disclosures; Andreas Meisel reports personal fees from Hormosan, personal fees from Grifols, grants and personal fees from Alexion, personal fees from Argnx, personal fees from Novartis, personal fees from UCB, grants from Octapharma, outside the submitted work. Bernhard Neumann reports no disclosures. The authors declare no financial or other conflicts of interest related to the manuscript. Statistical analysis was conducted by Dr. Bernhard Neumann, University Medical Center Regensburg/Donau-Isar-Klinikum Deggendorf.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Local ethic committees and institutional review boards of the participating centers approved the study based on the central vote of the ethics committee at University of Regensburg (No. 15–101-0259). Due to the retrospective character of the study, patient`s consent was not necessary according to the decisions of the ethic committees and institutional review boards.
Footnotes
Bernhard Neumann: on behalf of the Initiative of German NeuroIntensive Trial Engagement (IGNITE) and with support from the German Neurocritical Care Society (DGNI)
Contributor Information
Philipp Mergenthaler, Email: philipp.mergenthaler@charite.de.
Henning R. Stetefeld, Email: henning.stetefeld@uk-koeln.de
Christian Dohmen, Email: christian.dohmen@lvr.de.
Siegfried Kohler, Email: siegfried.kohler@charite.de.
Silvia Schönenberger, Email: Silvia.Schoenenberger@med.uni-heidelberg.de.
Julian Bösel, Email: julian.boesel@klinikum-kassel.de.
Stefan T. Gerner, Email: stefan.gerner@neuro.med.uni-giessen.de
Hagen B. Huttner, Email: direktion@neuro.med.uni-giessen.de
Hauke Schneider, Email: hauke.schneider@uk-augsburg.de.
Heinz Reichmann, Email: heinz.reichmann@uniklinikum-dresden.de.
Hannah Fuhrer, Email: hannah.fuhrer@uniklinik-freiburg.de.
Benjamin Berger, Email: benjamin.berger@uniklinik-freiburg.de.
Jan Zinke, Email: jan.zinke@med.uni-jena.de.
Anke Alberty, Email: anke.alberty@mariahilf.de.
Ingo Kleiter, Email: Ingo.Kleiter@ruhr-uni-bochum.de.
Christiane Schneider-Gold, Email: Christiane.Schneider-Gold@ruhr-uni-bochum.de.
Christian Roth, Email: roth@drk-nh.de.
Juliane Dunkel, Email: dunkel@drk-nh.de.
Andreas Steinbrecher, Email: andreas.steinbrecher@helios-gesundheit.de.
Andrea Thieme, Email: andrea.thieme@helios-gesundheit.de.
De-Hyung Lee, Email: dehyung.lee@medbo.de.
Ralf A. Linker, Email: ralf.linker@medbo.de
Klemens Angstwurm, Email: klemens.angstwurm@medbo.de.
Andreas Meisel, Email: andreas.meisel@charite.de.
Bernhard Neumann, Email: bernhard.neumann@donau-isar-klinikum.de.
References
- 1.Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S. Myasthenia gravis—autoantibody characteristics and their implications for therapy. Nat Rev Neurol. 2016;12(5):259–268. doi: 10.1038/nrneurol.2016.44. [DOI] [PubMed] [Google Scholar]
- 2.Neumann B, Angstwurm K, Mergenthaler P, Kohler S, Schönenberger S, Bösel J, Neumann U, Vidal A, Huttner HB, Gerner ST, Thieme A, Steinbrecher A, Dunkel J, Roth C, Schneider H, Schimmel E, Fuhrer H, Fahrendorf C, Alberty A, Zinke J, Meisel A, Dohmen C, Stetefeld HR, German Myasthenic Crisis Study Group Myasthenic crisis demanding mechanical ventilation: a multicenter analysis of 250 cases. Neurology. 2020;94(3):e299–e313. doi: 10.1212/WNL.0000000000008688. [DOI] [PubMed] [Google Scholar]
- 3.Thomas CE, Mayer SA, Gungor Y, Swarup R, Webster EA, Chang I, Brannagan TH, Fink ME, Rowland LP. Myasthenic crisis: clinical features, mortality, complications, and risk factors for prolonged intubation. Neurology. 1997;48(5):1253–1260. doi: 10.1212/wnl.48.5.1253. [DOI] [PubMed] [Google Scholar]
- 4.Angstwurm K, Vidal A, Stetefeld H, Dohmen C, Mergenthaler P, Kohler S, Schönenberger S, Bösel J, Neumann U, Lee DH, Gerner ST, Huttner HB, Thieme A, Dunkel J, Roth C, Schneider H, Schimmel E, Reichmann H, Fuhrer H, Berger B, Kleiter I, Schneider-Gold C, Alberty A, Zinke J, Schalke B, Steinbrecher A, Meisel A, Neumann B. Early tracheostomy is associated with shorter ventilation time and duration of ICU stay in patients with myasthenic crisis-a multicenter analysis. J Intensive Care Med. 2020;25:885066620967646. doi: 10.1177/0885066620967646. [DOI] [PubMed] [Google Scholar]
- 5.König N, Stetefeld HR, Dohmen C, Mergenthaler P, Kohler S, Schönenberger S, Bösel J, Lee DH, Gerner ST, Huttner HB, Schneider H, Reichmann H, Fuhrer H, Berger B, Zinke J, Alberty A, Kleiter I, Schneider-Gold C, Roth C, Dunkel J, Steinbrecher A, Thieme A, Schlachetzki F, Linker RA, Angstwurm K, Meisel A, Neumann B. MuSK-antibodies are associated with worse outcome in myasthenic crisis requiring mechanical ventilation. J Neurol. 2021;268(12):4824–4833. doi: 10.1007/s00415-021-10603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alshekhlee A, Miles JD, Katirji B, Preston DC, Kaminski HJ. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology. 2009;72(18):1548–1554. doi: 10.1212/WNL.0b013e3181a41211. [DOI] [PubMed] [Google Scholar]
- 7.Spillane J, Hirsch NP, Kullmann DM, Taylor C, Howard RS. Myasthenia gravis–treatment of acute severe exacerbations in the intensive care unit results in a favourable long-term prognosis. Eur J Neurol. 2014;21(1):171–173. doi: 10.1111/ene.12115. [DOI] [PubMed] [Google Scholar]
- 8.Ramos-Fransi A, Rojas-García R, Segovia S, Márquez-Infante C, Pardo J, Coll-Cantí J, Jericó I, Illa I. Myasthenia gravis: descriptive analysis of life-threatening events in a recent nationwide registry. Eur J Neurol. 2015;22(7):1056–1061. doi: 10.1111/ene.12703. [DOI] [PubMed] [Google Scholar]
- 9.Wiendl H (2015). Diagnostik und Therapie der Myasthenia gravis und des Lambert-Eaton-Syndroms. Leitlinien für Diagnostik und Therapie in der Neurologie
- 10.Leite MI, Jacob S, Viegas S, Cossins J, Clover L, Morgan BP, Beeson D, Willcox N, Vincent A. IgG1 antibodies to acetylcholine receptors in ‘seronegative’ myasthenia gravis. Brain. 2008;131(Pt 7):1940–1952. doi: 10.1093/brain/awn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann S, Harms L, Schuelke M, Rückert JC, Goebel HH, Stenzel W, Meisel A. Complement deposition at the neuromuscular junction in seronegative myasthenia gravis. Acta Neuropathol. 2020;139(6):1119–1122. doi: 10.1007/s00401-020-02147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent A, Scadding GK, Thomas HC, Newsom-Davis J. In-vitro synthesis of anti-acetylcholine-receptor antibody by thymic lymphocytes in myasthenia gravis. Lancet. 1978;1(8059):305–307. doi: 10.1016/s0140-6736(78)90073-9. [DOI] [PubMed] [Google Scholar]
- 13.Leite MI, Ströbel P, Jones M, Micklem K, Moritz R, Gold R, Niks EH, Berrih-Aknin S, Scaravilli F, Canelhas A, Marx A, Newsom-Davis J, Willcox N, Vincent A. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol. 2005;57(3):444–448. doi: 10.1002/ana.20386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be made available upon reasonable request.