Abstract
Introduction
The approach to evaluating nephrotoxins in studies of drug-associated acute kidney injury varies. Some studies use a list of under ten drugs for evaluation whereas others include over 100 drugs. Drugs are typically assigned a binary classification, nephrotoxic or not nephrotoxic. This oversimplifies the nephrotoxic potential of the drugs under investigation.
Objective
This study aimed to assign a nephrotoxin potential for 167 drugs used in the adult critical care setting.
Methods
A three-round, international, interdisciplinary, web-based modified-Delphi study was used to evaluate nephrotoxins used in adult critically ill patients. Twenty-four international experienced clinicians were identified through the Acute Disease Quality Initiative group and professional affiliations. Included individuals represented the fields of intensive care, nephrology, and pharmacy. One hundred and fifty-nine medications were identified from the literature, with eight additional medications added after the first round, for a total of 167 medications. The primary outcome was consensus achieved for nephrotoxicity ratings. Scores were evaluated each round to determine if a consensus was met.
Results
Our nephrotoxin potential index rating indicated that 20 drugs were nephrotoxicity probable or probable/definite per consensus. Nephrotoxic potential was assessed based on the standard use of medications in intensive care and the following consensus scores: 0 = no nephrotoxic potential, 1 = possible nephrotoxic potential, 2 = probable nephrotoxic potential, 3 = definite nephrotoxic potential.
Conclusions
The nephrotoxin potential index rating allows for prioritization of targeted drugs with greater nephrotoxic potential for institutional nephrotoxin stewardship programs. Furthermore, the nephrotoxin potential index rating provides homogeneity for research and guidance on detailed assessments by severity for each drug.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-022-01173-4.
Key Points
| Consensus ratings of the nephrotoxicity of 167 medications used in critically ill patients were generated. |
| Twenty medications were identified as having probable to definite nephrotoxic potential. |
| Further validation of nephrotoxic potential ratings of medications is important for standardization in research and drug therapy evaluation. |
Introduction
Acute kidney injury (AKI) occurs in approximately 22% of adults who are hospitalized. Almost 57% of patients in intensive care units experience AKI within a week of admission, with 13% requiring renal replacement therapy [1] . The consequences associated with this syndrome are significant, with all-cause mortality rates of 24% within 6 months of AKI [2]. Acute kidney injury creates a significant economic burden on healthcare systems with incremental costs of US$9100 per episode in surgical patients and up to US$81,000 in non-surviving dialysis patients [3].
There are numerous causes of AKI that include sepsis, trauma, cardiac surgery, major non-cardiac surgery, and nephrotoxic agents [4]. In fact, drugs are the third to fifth leading cause of AKI (D-AKI), contributing to 14–26% of events in hospitalized patients [5–7]. Identifying D-AKI causes depends on premarketing studies that are often limited in sample size and not focused on kidney injury as a primary outcome. Subsequently, clinicians rely on large post-marketing studies to identify D-AKI causes, but it can take years to accrue sufficient data to be considered definitive evidence [8].
Healthcare professionals depend on primary, secondary, and tertiary information sources to enumerate potentially nephrotoxic drugs for practice and research. This is a tedious process and can be complex with the substantial discrepancy in nephrotoxins provided by various sources [9, 10]. Further, rating the identified nephrotoxins based on their potential or likelihood of causing AKI is elusive given the lack of evidentiary support for such a comparison. Potentially nephrotoxic agents are “known to have potential adverse effects on kidney function either through direct toxicity or by impairment of kidney perfusion, with the recognition that their toxicity may depend on the clinical context” [2]. The appraised nephrotoxic potential (NxP) for drugs is study specific and includes a modified Delphi method and a structured approach with an agreement between various drug compendia [8, 11–14]. Perceived lists of nephrotoxins in pediatric populations determined by experienced clinicians’ consensus recommendations has demonstrated to be useful in guiding nephrotoxin surveillance leading to decreasing the days of AKI, AKI severity, and AKI incidence [13, 15]. In the absence of large epidemiologic association studies, consensus agreement by clinicians about nephrotoxic drugs has utility in clinical practice and the standardization of research studies.
A standardized list of nephrotoxins and associated ratings for potential kidney injury is needed to facilitate and prioritize nephrotoxin stewardship in clinical practice and provide homogeneity for comparative studies [10]. In addition, these ratings should be specific to patient populations because the frequency of use and nephrotoxin risk potential vary between critically ill and non-critically ill patients or adults compared with pediatrics. To date, a consensus-generation study assessing nephrotoxins is limited to pediatric patients [11, 13]. A previous study noted significant differences in the nephrotoxins used in pediatric and adult critically ill populations [16]. Therefore, our objective was to conduct a modified Delphi study to obtain consensus from a group of interdisciplinary, international, experienced critical care clinicians to determine the NxP index ratings for 167 drugs in adult critically ill patients.
Methods
Delphi Design and Clinician Panel
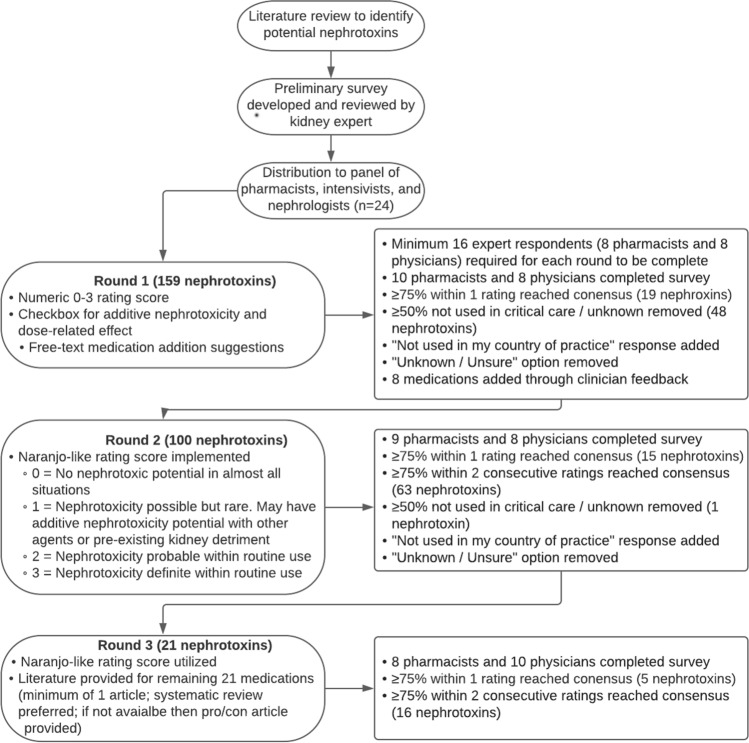
This study was reviewed by the University of Pittsburgh International Review Board (STUDY19120256) and designated as exempt for minimal risk prior to study commencement. A list of medications previously reported to have nephrotoxic effects was created through a literature review [17–19]. Clinicians actively working in an intensive care environment were identified through the Acute Disease Quality Initiative group and professional affiliations, including an international collective of intensivists, nephrologists, and pharmacists [20]. Participants represented Belgium (n = 1); Canada (n = 2); Chile (n = 2); Finland (n = 1); France (n = 1); Germany (n = 2); Ireland (n = 1); Jordan (n = 1); Singapore (n = 2); Sweden (n = 1); UK (n = 3); and USA (n = 7). A modified Delphi method was used to generate a consensus NxP index rating [21, 22]. A Delphi method is a structured technique that uses a series of rounds to gather group consensus [22]. Clinicians were invited to participate in an anonymous web-based survey via e-mail [23]. All surveys were in English. Twenty-four clinicians (12 physicians and 12 pharmacists) were recruited based on prior recommendations for panel size and heterogeneity [22]. A round of evaluation was considered complete when a total of 16 clinicians finished the surveys, with a minimum of eight pharmacists and eight physicians in each round. All experts’ input was equally weighted using anonymous responses to limit stature, seniority, or authority from influencing responses [24]. Figure 1 displays the details of the three rounds for the modified Delphi method.
Fig. 1.
Delphi method flowchart. The Delphi process was between March 2020 and April 2021. A total of three rounds were completed. Unless otherwise listed, all parameters from the previous round were carried forward for the following round
Round 1 (March–June 2020)
The survey tasked clinicians to independently provide a numeric rating from 0 (none) to 3 (very high) for NxP based on clinical experience for each of the 159 medications. As the original list of 159 medications was not specific to critical care, a response indicating that medication was not used in critical care was included, and a response for “unknown” to allow clinicians to skip medications they were not familiar with. Finally, a free-text comment field was provided to allow clinicians to provide additional context to their responses, as well as identifying any medications that they believed to have NxP that were not included in the original 159 medication list. Consensus was achieved when ≥ 75% of clinicians (minimum 16) agreed on the chosen rating between 0 and 3. Consensus for removal/unknown was achieved when ≥ 50% of respondents were in agreement. Medications reaching consensus were not included in subsequent rounds.
Round 2 (September–October 2020)
A summary of each medication’s previous rating was provided to participants for rounds 2 and 3. Eight additional drugs (e.g., loop diuretics) were identified by respondents in round 1 and added in round 2. In response to feedback from the participants regarding a lack of clear delineation between the numeric rating levels, additional context was provided in round 2 using the Naranjo Adverse Drug Reaction Causality Scale (0 for no NxP in almost all situations; 1 for NxP possible but rare, may have additive NxP with other agents or pre-existing kidney detriment; 2 for NxP probable within routine use; 3 for NxP definite within routine use) [25]. To allow for differences in regional drug use, clinicians could also respond that the medication was not used or not available in their country (e.g., rofecoxib in the USA). These responses were not counted in the consensus calculations. At the end of round 1, it was evident that many medications were unlikely to reach the original definition of ≥ 75% consensus for a single rating of 0–3, regardless of the number of rounds completed. To address this, in rounds two and three, the two most commonly reported consecutive ratings were combined. If they achieved ≥ 75% consensus, the final rating was defined as falling between the two categories. For example, out of 17 total responses, a medication receiving ten ratings of “1” and five ratings of “2” would generate a final consensus rating of 1.5. This combined approach was still consistent with the goal of clinician consensus for an index rating of nephrotoxin potential.
Round 3 (February–April 2021)
The scoring system implemented in round 2 was utilized for the third and final round. In addition to receiving an invitation to complete the survey, clinicians were also provided with a link to a repository containing articles describing each remaining medication’s effect on kidney function. A minimum of one article and a maximum of two articles were provided for each drug. Systematic reviews were preferred, and if a review was unavailable, then an article providing a pro/con evaluation of nephrotoxicity was provided. Based on the scoring from rounds 1 and 2, it was known that the remaining 21 medications would have final ratings above “0,” and the goal in providing these articles was to aid clinicians in gauging the relative nephrotoxicity of each remaining medication with additional information.
Analysis
In total, 167 drugs were evaluated (159 original, eight added based on participant feedback in round 1). Aggregate scores by participants were assessed in each round to determine if consensus was met. When consensus was reached, then drugs were not included in subsequent rounds.
Results
In round 1, 67 medications reached consensus; 19 were those with ≥75% responses for one single rating, and 48 medications with ≥ 50% indicating “unknown” or that the medication was not used in critical care. There were 92 drugs from the original list of 159 and eight drugs added per recommendations of the participants for a total of 100 drugs to be evaluated in round 2. In round 2, 79 medications reached consensus; 63 reached consensus using an updated combined rating definition of ≥ 75% consensus within two consecutive rating scores, 15 reached consensus using the original definition of ≥ 75% consensus within a single category, and one medication indicated as not being used in critical care. Following round 3, the remaining 21 medications all reached consensus, with five achieving ≥ 75% consensus within a single rating score and 16 achieving ≥ 75% consensus within two consecutive rating scores.
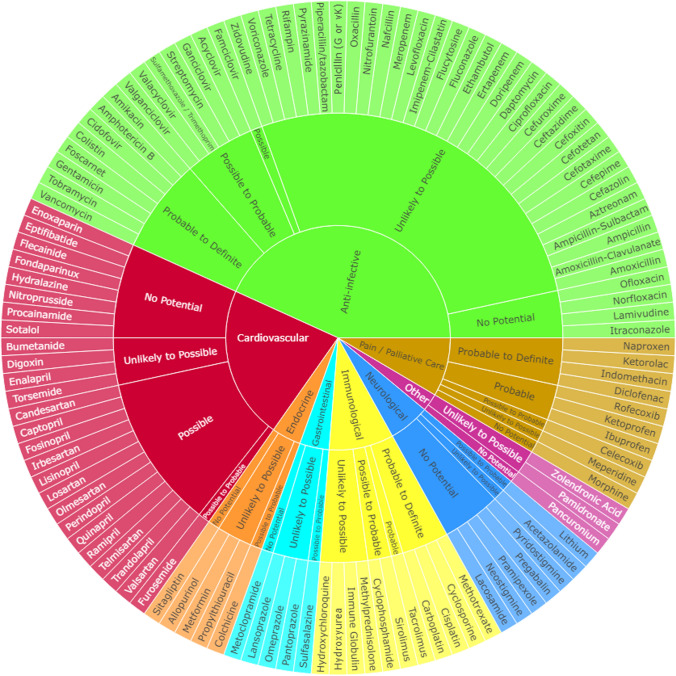
The NxP index rating indicates (1) 21 drugs that were considered to have no NxP, (2) 51 drugs considered nephrotoxicity unlikely to be possible, (3) 12 drugs with nephrotoxicity possible, (4) 12 drugs deemed nephrotoxicity possible to probable, (5) four drugs were nephrotoxicity probable, and (6) 16 drugs rating highest with nephrotoxicity probable to definite and zero ranked exclusively as definite per consensus. The NxP of 20 drugs was rated as nephrotoxicity probable or probable to definite with a final consensus rating of 2.0 or higher (Table 1). Physicians and pharmacists reported different consensus scores for 41 medications (Table 2). When the consensus score between clinician groups differed, physicians reported the higher nephrotoxicity ratings for 37 of the 41 medications. Figure 2 illustrates the NxP index ratings by drug class.
Table 1.
Nephrotoxicity consensus ratings for 167 medications used in adults in critical care
| No nephrotoxic potential in almost all situations (rating = 0) | |||||
| Enoxaparin | Fondaparinux | Lacosamide | Morphine | Ofloxacin | Procainamide |
| Eptifibatide | Hydralazine | Lamivudine | Neostigmine | Pancuronium | Pyridostigmine |
| Flecainide | Itraconazole | Metoclopramide | Nitroprusside | Pramipexole | Sitagliptin |
| Norfloxacin | Pregabalin | Sotalol | |||
| No nephrotoxic potential to nephrotoxicity possible (rating = 0.5) | |||||
| Acetazolamide | Cefepime | Digoxin | Imipenem-cilastatin | Nitrofurantoin | Rifampin |
| Allopurinol | Cefotaxime | Doripenem | Immune globulin | Omeprazole | Sulfasalazine |
| Amoxicillin | Cefotetan | Enalapril | Lansoprazole | Oxacillin | Tetracycline |
| Amoxicillin-clavulanate | Cefoxitin | Ertapenem | Levofloxacin | Pamidronate | Torsemide |
| Ampicillin | Ceftazidime | Fluconazole | Meperidine | Pantoprazole | Ampicillin-sulbactam |
| Aztreonam | Cefuroxime | Flucytosine | Meropenem | Penicillin | Voriconazole |
| Bumetanide | Ciprofloxacin | Hydroxychloroquine | Metformin | Piperacillin/tazobactam | Zidovudine |
| Cefazolin | Daptomycin | Hydroxyurea | Methylprednisolone | Propylthiouracil | Zoledronic acid |
| Nafcillin | Pyrazinamide | Ethambutol | |||
| Nephrotoxic potential possible but rare (rating = 1.0) | |||||
| Candesartan | Famciclovir | Irbesartan | Losartan | Perindopril | Telmisartan |
| Captopril | Fosinopril | Lisinopril | Olmesartan | Ramipril | Trandolapril |
| Valsartan | |||||
| Nephrotoxic potential possible to probable (rating = 1.5) | |||||
| Acyclovir | Colchicine | Furosemide | Lithium | Streptomycin | Valacyclovir |
| Celecoxib | Cyclophosphamide | Ganciclovir | Sirolimus | Sulfamethoxazole/trimethoprim | Valganciclovir |
| Nephrotoxic potential probable within routine use (rating = 2.0) | |||||
| Ibuprofen | Ketoprofen | Rofecoxib | Tacrolimus | ||
| Nephrotoxic potential probable to definite (rating = 2.5) | |||||
| Amikacin | Carboplatin | Cisplatin | Diclofenac sodium | Ketorolac | Tobramycin |
| Amphotericin B | Cidofovir | Colistin | Gentamicin | Methotrexate | Vancomycin |
| Cyclosporine | Indomethacin | Naproxen | Foscarnet | ||
| Nephrotoxic potential unknown/not used in critical care | |||||
| Abacavir | Capreomycin | Diflunisal | Flubiprofen | Mesalamine | Quinidine |
| Acarbose | Chloroquine | Disopyramide | Gallamine | Metocurine | Stavudine |
| Acetohexamide | Chlorpropamide | Dofetilide | Glyburide | Mitomycin | Sulindac |
| Adefovir | Clofibrate | Doxacurium | Idarubicin | Mivacurium | Temozolomide |
| Amantadine | Cycloserine | Etodolac | Ifosfamide | Moexipril | Tenofovir |
| Benazepril | Cytarabine | Etoposide | Indinavir | Nabumetone | Tocainide |
| Bevacizumab | Dapsone | Exenatide | Meloxicam | Pentostatin | Tolmetin |
| Bretylium | Didanosine | Fenoprofen | Melphalan | Piroxicam | Topotecan |
| Trimetrexate | |||||
Table 2.
Differences in physician and pharmacist nephrotoxicity ratings
| Medication | Physician rating | Pharmacist rating | Medication | Physician rating | Pharmacist rating |
|---|---|---|---|---|---|
| Amikacin | 3 | 2 | Hydroxyurea | 1 | 0 |
| Amoxicillin | 1 | 0 | Imipenem/cilistatin | 1 | 0 |
| Ampcillin/sulbactam | 1 | 0 | Lacosamide | 1 | 0 |
| Amoxicillin-clavulanate | 1 | 0 | Lansoprazole | 1 | 0 |
| Cefazolin | 1 | 0 | Meropenem | 1 | 0 |
| Cefotaxime | 1 | 0 | Methylprednisolone | 0 | 1 |
| Cefotetan | 1 | 0 | Naproxen | 3 | 2 |
| Cefoxitin | 1 | 0 | Nitrofurantoin | 1 | 0 |
| Ceftazidime | 1 | 0 | Pantoprazole | 1 | 0 |
| Cefuroxime | 1 | 0 | Penicillin (G or vK) | 1 | 0 |
| Celecoxib | 3 | 1 | Propylthiouracil | 1 | 0 |
| Cisplatin | 3 | 2 | Pyrazinamide | 1 | 0 |
| Colistin | 3 | 2 | Rifampin | 1 | 0 |
| Daptomycin | 2 | 1 | Streptomycin | 1 | 3 |
| Diclofenac | 3 | 2 | Sulfamethoxazole/trimethoprim | 1 | 2 |
| Doripenem | 1 | 0 | Tobramycin | 3 | 2 |
| Ertapenem | 1 | 0 | Valganciclovir | 2 | 1 |
| Ethambutol | 1 | 0 | Vancomycin | 3 | 2 |
| Flucytosine | 0 | 1 | Voriconazole | 2 | 0 |
| Foscarnet | 3 | 2 | Zidovudine | 1 | 0 |
| Hydroxychloroquine | 1 | 0 |
Notes: Nephrotoxicity ratings provided for medications where physician and pharmacist ratings differed. Medications not listed received the same consensus ratings by both physicians and pharmacists. Ratings provided are the most commonly reported responses in each clinician group during the round the medication reached consensus. Excludes medications where the consensus rating was “Unknown or Not used in Critical Care” and medications where both clinician groups reported the same ratings. In the instance of a tie, the higher of the two ratings is provided. Rating definitions are as follows: 0 = no nephrotoxic potential in almost all situations, 1 = nephrotoxic potential possible but rare, 2 = nephrotoxic potential probable within routine use, 3 = nephrotoxic potential definite within routine use
Fig. 2.
Consensus nephrotoxicity ratings by medication therapeutic class. Numeric ratings corresponding to each nephrotoxicity category are as follows: 0 = “No Potential”; 0.5 = “Unlikely to Possible”; 1.0 = “Possible”; 1.5 = “Possible to Probable”; 2.0 = “Probable”; 2.5 = “Probable to Definite”; 3.0 = “Definite” (no medication received a 3 rating). An interactive version of this figure is available as Electronic Supplementary Material
Discussion
A NxP index rating was determined by an interdisciplinary international experienced group of critical care clinicians to be applied to adult critically ill patients. This ranked list aimed to assist in the selection of drugs for nephrotoxin stewardship in clinical practice with an ability to prioritize surveillance based on drugs with greater NxP, especially in settings of resource constraints [10]. This NxP rating can also assist with developing knowledge for rule-based clinical decision support systems to ensure that alerts are specific to patients at high risk for developing D-AKI. Increasing the specificity of alerts could reduce false positives and alert fatigue by improving alert accuracy [26]. The NxP rating provided with this modified Delphi method can guide surveillance in clinical practice.
It is stated that up to 87% of critically ill patients receive an average of nine nephrotoxins throughout their intensive care unit stay [14, 27]. Furthermore, among the 100 commonly prescribed drugs in critically ill patients, about 20% were considered to be nephrotoxic [16]. These assessments were based on comprehensive lists of potentially nephrotoxic drugs that may have little or hypothetical potential kidney injury, including acetaminophen and fentanyl in previous studies. Interestingly, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers were considered nephrotoxic despite the suggested benefits of these agents in preventing kidney disease progression, thus allowing for a permissive AKI [14, 28]. Even the comprehensive lists of potentially nephrotoxic drugs contained variation between studies, with 41 and 47 included for evaluation in two different studies. There is value in understanding the NxP index rating so that the exposure to nephrotoxins in critically ill patients can be further assessed by rating the potential for injury instead of providing equal weighting.
The nephrotoxic burden is “cumulative or aggregate exposure to nephrotoxins, with consideration to nephrotoxin potential for each drug, evaluated at a given time or within a reasonable time frame depending on the elimination half-life of the drug in the body” [10]. This has also been described as nephrotoxic intensity, although it does not consider cumulative exposure [29]. Previous studies have indicated a 40–53% greater likelihood of developing AKI for each nephrotoxin a patient received [30, 31]. This assessment of burden is potentially due to the cumulative NxP of each drug added. For example, two drugs considered to have only possible nephrotoxicity potential would be expected to have less of a burden on the kidney than two drugs deemed probable/definite nephrotoxicity potential. A recent study designed to assess nephrotoxin burden reported that a higher drug burden score was significantly associated with subsequent AKI occurrence or worsening of AKI for patients with a lower severity of disease [32]. However, this study did not account for the NxP of each drug considered in the burden assessment. Clearly, this requires further investigation, but the first step is provided in this study with an NxP index rating that can be used in future studies.
Another utility of the NxP index rating is to standardize lists of nephrotoxins for evaluation in research. Two studies ranked NxP based on drug information resources. They indicated that drugs cited in more references (three out of four) suggested known toxicity, with fewer references indicating a less established association [8, 12]. Another study used a modified Delphi approach in pediatric hospitalized patients and ranked potential as high and moderate risk, although this was not the primary purpose of the study [11]. Details of the pediatric nephrotoxic consensus study indicated that authors and clinical pharmacists were included, but it did not state the criteria for reaching consensus for the drugs evaluated. Our study provides a clear process for the NxP index rating and is specific for adult critically ill patients. Notably, the list of assessed nephrotoxins in our modified Delphi study and the pediatric consensus study had several similarities with 16 of the 20 drugs on our list of probable or probable/definite linking to the drugs ranked as high risk in the other study (Table 3).
Table 3.
Comparison of probable or probable/definite nephrotoxic potential in the current critical care adult modified Delphi study to high-risk nephrotoxic potential in the pediatric consensus study [11]
| Nephrotoxic potential | Nephrotoxins |
|---|---|
| Agreement in rating between studies (n = 16) | Amikacin, amphotericin B, cidofovir, colistin, cyclophosphamide, cyclosporine, fludarabine, gentamicin, ibuprofen, indomethacin, ketorolac, methotrexate, naproxen, tacrolimus, tobramycin, vancomycin |
| Discrepancy in rating between studies (n = 5) |
Acyclovir (possible/probable vs high) Foscarnet (probable/definite vs moderate) Immune globulin (unlikely/possible vs high) Sirolimus (possible/probable vs high) Streptomycin (possible/probable vs high) |
More inconsistencies in the nephrotoxins were identified in the literature and intended for evaluation with nine drugs not overlapping between lists. The references used to generate the NxP lists for evaluation, including individual drugs or all drugs in a drug class, and the decision to include drugs that indirectly affect kidney function through intraglomerular hemodynamics created divergence [33–35]. Additional discrepancies could be because 49 drugs from the original list of 159 in our study were removed because of a lack of use in critically ill patients. An assessment of NxP varies depending on context and requires evaluation in various patient populations such as pediatrics and non-critically ill patients. Additionally, we noted differences in the ratings provided by physicians and critical care pharmacists, with the physicians providing consistently higher NxP scores, with the full differences seen in Table 2. These differences demonstrate that physicians may be more cautious when evaluating the nephrotoxicity of a particular regimen, and supports the need for standardization in research to avoid inconsistencies in perceived NxP of medications across clinical backgrounds.
An interesting element we observed in NxP rating was disagreement for drugs within the same drug class. For example, there are different NxP ratings provided for tenofovir and remdesivir [36, 37]. Both are nucleotide analogs that act as reverse-transcriptase inhibitors with concern for their metabolites causing mitochondrial injury in renal tubular epithelial cells, as documented for tenofovir after longer treatment, but not yet for remdesivir. The explanation for the difference between AKI rates with these two antivirals could be variations in molecules, but it may also be because of exposure with shorter treatment durations for remdesivir. Another biologically plausible explanation for remdesivir being associated with AKI and liver necrosis is excipient accumulation in patients with an estimated glomerular filtration rate of less than 30 mL/min. Favipiravir, another nucleotide analog, is not associated with AKI, but it is administered orally and not intravenously. Therefore, it does not have the excipient found in injectable remdesivir. Another example of medications within the same class receiving different ratings is furosemide and torsemide, which were ranked as possible to probable and unlikely to possible, respectively. However, this perceived variation for NxP for drugs furosemide and torsemide, as observed in the modified Delphi method could be influenced by torsemide being limited to an oral formulation, which may reduce critical care clinicians’ familiarity with this medication in their severely ill patient population. Deviations in NxP between drugs within a drug class will require further investigation. Additionally, the next steps for the NxP index rating in this study are to evaluate the clinicians’ perceptions provided in the Delphi using large real-world data in future epidemiologic studies.
Limitations
This study is the first step in providing an NxP index rating for drugs intended for use in a critically ill patient population. We attempted to provide a comprehensive list of drugs for evaluation but it is still possible that some potential nephrotoxins were missed, could vary depending on the patient population, or were excluded because of a perceived lack of use in the intensive care unit by the study participants. The references used to generate our list of potential nephrotoxins did not include some classes of medications such as vasopressors, contrast dyes, or fluids, which have been previously examined and can highly influence the risk for AKI [33–35]. The use of a modified Delphi method allowed for a flexible process to generate consensus using a standardized definition; however, the definition of NxP used was refined based on participant feedback, and the interpretation of this changing definition may influence our results. Next, our study asked clinicians to identify the perceived nephrotoxicity of medications within the general course of their use, but the nephrotoxicity of medications can be affected by patient and environmental-specific factors, which may limit the generalizability of our results. The group of experienced clinicians was selected internationally, which had advantages and disadvantages. The advantage was a more global and generalizable context for the NxP rating. Still, some drugs were not familiar to clinicians depending on the availability in specific countries, requiring us to adjust the denominator for consensus when use was limited and preventing the generation of consensus on other medications if the denominator dropped below 50% the panel size. Perception and experience are the keys for a consensus assessment, and newer drugs included for evaluation may lack familiarity and allow for lower ratings. As more data and new drugs become available, consensus ratings will need to be updated. This is a consensus method approach to developing an NxP index rating limited to clinician perception that needs to be validated in epidemiologic association studies.
Conclusions
Twenty drugs were considered to have probable to probably/definite nephrotoxicity by an international group of clinicians with experience in adult intensive care units. This NxP index rating allows for prioritization of nephrotoxin stewardship targeted at drugs with greater NxP. Furthermore, the NxP index rating provides homogeneity for research evaluations and opportunities for more detailed assessments by severity ratings.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was reviewed by the University of Pittsburgh International Review Board (STUDY19120256) and designated as exempt for minimal risk prior to study commencement.
Consent to participate
Recruitment materials explained that completion of the survey constituted consent to have the respondent’s anonymous results included in the study, but that the respondent could opt out at any time.
Consent for publication
Recruitment materials explained that completion of the survey constituted consent to have the respondent’s anonymous results included in the study, but that the respondent could opt out at any time.
Availability of data and material
Available upon request to the corresponding author.
Code availability
Not applicable.
Author contributions
MG: concept generation, materials creation, ethics approval, data analysis, manuscript drafting and revision. EB: concept generation, material review, manuscript review. DS: concept generation, material review, manuscript review. JK: subject matter expert, manuscript review. KS: material review, manuscript review. KK: subject matter expert, manuscript review. AR: subject matter expert, manuscript review. SKG: concept generation, material review and distribution, manuscript drafting and revision. All authors read and approved the final version.
References
- 1.Griffin BR, Liu KD, Teixeira JP. Critical care nephrology: core curriculum 2020. Am J Kidney Dis. 2020;75(3):435–452. doi: 10.1053/j.ajkd.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasta JF, Kane-Gill S. Review of the literature on the costs associated with acute kidney injury. J Pharm Pract. 2019;32(3):292–302. doi: 10.1177/0897190019852556. [DOI] [PubMed] [Google Scholar]
- 4.Kane-Gill SL, Goldstein SL. Drug-induced acute kidney injury: a focus on risk assessment for prevention. Crit Care Clin. 2015;31(4):675–684. doi: 10.1016/j.ccc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66(4):1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 7.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 8.Welch HK, Kellum JA, Kane-Gill SL. Drug-associated acute kidney injury identified in the United States Food and Drug Administration Adverse Event Reporting System Database. Pharmacotherapy. 2018;38(8):785–793. doi: 10.1002/phar.2152. [DOI] [PubMed] [Google Scholar]
- 9.Bicalho MD, Soares DB, Botoni FA, Reis AM, Martins MA. Drug-induced nephrotoxicity and dose adjustment recommendations: agreement among four drug information sources. Int J Environ Res Public Health. 2015;12(9):11227–11240. doi: 10.3390/ijerph120911227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kane-Gill SL. Nephrotoxin stewardship. Crit Care Clin. 2021;37(2):303–320. doi: 10.1016/j.ccc.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, McGregor TL, Jones DP, Bridges BC, Fleming GM, Shirey-Rice J, et al. Electronic health record-based predictive models for acute kidney injury screening in pediatric inpatients. Pediatr Res. 2017;82(3):465–473. doi: 10.1038/pr.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sileanu FE, Murugan R, Lucko N, Clermont G, Kane-Gill SL, Handler SM, et al. AKI in low-risk versus high-risk patients in intensive care. Clin J Am Soc Nephrol. 2015;10(2):187–196. doi: 10.2215/CJN.03200314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goswami E, Ogden RK, Bennett WE, Goldstein SL, Hackbarth R, Somers MJG, et al. Evidence-based development of a nephrotoxic medication list to screen for acute kidney injury risk in hospitalized children. Am J Health Syst Pharm. 2019;76(22):1869–1874. doi: 10.1093/ajhp/zxz203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soares DB, Mambrini JVM, Botelho GR, Girundi FF, Botoni FA, Martins MAP. Drug therapy and other factors associated with the development of acute kidney injury in critically ill patients: a cross-sectional study. PeerJ. 2018;6:e5405. doi: 10.7717/peerj.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein SL, Dahale D, Kirkendall ES, Mottes T, Kaplan H, Muething S, et al. A prospective multi-center quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int. 2020;97(3):580–588. doi: 10.1016/j.kint.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Taber SS, Mueller BA. Drug-associated renal dysfunction. Crit Care Clin. 2006;22(2):357–374. doi: 10.1016/j.ccc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Cox ZL, McCoy AB, Matheny ME, Bhave G, Peterson NB, Siew ED, et al. Adverse drug events during AKI and its recovery. Clin J Am Soc Nephrol. 2013;8(7):1070–1078. doi: 10.2215/CJN.11921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta RL, Awdishu L, Davenport A, Murray PT, Macedo E, Cerda J, et al. Phenotype standardization for drug-induced kidney disease. Kidney Int. 2015;88(2):226–234. doi: 10.1038/ki.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivosecchi RM, Kellum JA, Dasta JF, Armahizer MJ, Bolesta S, Buckley MS, et al. Drug class combination-associated acute kidney injury. Ann Pharmacother. 2016;50(11):953–972. doi: 10.1177/1060028016657839. [DOI] [PubMed] [Google Scholar]
- 20.Ostermann M, Chawla LS, Forni LG, Kane-Gill SL, Kellum JA, Koyner J, et al. Drug management in acute kidney disease: report of the Acute Disease Quality Initiative XVI meeting. Br J Clin Pharmacol. 2018;84(2):396–403. doi: 10.1111/bcp.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003;41(4):376–382. doi: 10.1046/j.1365-2648.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- 22.Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS ONE. 2011;6(6):e20476. doi: 10.1371/journal.pone.0020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qualtrics. 2020. Available from: https://www.qualtrics.com. Accessed 16 Mar 2022.
- 24.Helmer-Hirschberg O. Analysis of the future: the Delphi method. Rand Paper. Santa Monica, CA: RAND Corporation; 1967.
- 25.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 26.Kane-Gill SL, O'Connor MF, Rothschild JM, Selby NM, McLean B, Bonafide CP, et al. Technologic distractions (part 1): summary of approaches to manage alert quantity with intent to reduce alert fatigue and suggestions for alert fatigue metrics. Crit Care Med. 2017;45(9):1481–1488. doi: 10.1097/CCM.0000000000002580. [DOI] [PubMed] [Google Scholar]
- 27.Slater MB, Gruneir A, Rochon PA, Howard AW, Koren G, Parshuram CS. Identifying high-risk medications associated with acute kidney injury in critically ill patients: a pharmacoepidemiologic evaluation. Paediatr Drugs. 2017;19(1):59–67. doi: 10.1007/s40272-016-0205-1. [DOI] [PubMed] [Google Scholar]
- 28.Parikh CR, Coca SG. "Permissive AKI" with treatment of heart failure. Kidney Int. 2019;96(5):1066–1068. doi: 10.1016/j.kint.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Moffett BS, Goldstein SL. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol. 2011;6(4):856–863. doi: 10.2215/CJN.08110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu AJ, Tamma PD. Impact of an antibiotic stewardship program on the incidence of vancomycin-associated acute kidney injury in hospitalized children. J Pediatr Pharmacol Ther. 2019;24(5):416–420. doi: 10.5863/1551-6776-24.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cartin-Ceba R, Kashiouris M, Plataki M, Kor DJ, Gajic O, Casey ET. Risk factors for development of acute kidney injury in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Res Pract. 2012;2012:691013. doi: 10.1155/2012/691013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehrmann S, Helms J, Joret A, Martin-Lefevre L, Quenot JP, Herbrecht JE, et al. Nephrotoxic drug burden among 1001 critically ill patients: impact on acute kidney injury. Ann Intensive Care. 2019;9(1):106. doi: 10.1186/s13613-019-0580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bangash MN, Kong M-L, Pearse RM. Use of inotropes and vasopressor agents in critically ill patients. Br J Pharmacol. 2012;165(7):2015–2033. doi: 10.1111/j.1476-5381.2011.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Futier E, Garot M, Godet T, Biais M, Verzilli D, Ouattara A, et al. Effect of hydroxyethyl starch vs saline for volume replacement therapy on death or postoperative complications among high-risk patients undergoing major abdominal surgery: the FLASH randomized clinical trial. JAMA. 2020;323(3):225–236. doi: 10.1001/jama.2019.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammed NM, Mahfouz A, Achkar K, Rafie IM, Hajar R. Contrast-induced nephropathy. Heart Views. 2013;14(3):106–116. doi: 10.4103/1995-705X.125926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adamsick ML, Gandhi RG, Bidell MR, Elshaboury RH, Bhattacharyya RP, Kim AY, et al. Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol. 2020;31(7):1384–1386. doi: 10.1681/ASN.2020050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettit NN, Pisano J, Nguyen CT, Lew AK, Hazra A, Sherer R, et al. Remdesivir use in the setting of severe renal impairment: a theoretical concern or real risk? Clin Infect Dis. 2021;73(11):e3990–e3995. doi: 10.1093/cid/ciaa1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.