Abstract
Low levels of serum calcium, elevated levels of serum phosphorus and absent or abnormally low levels of serum parathyroid hormone characterize hypoparathyroidism, a rare endocrine deficiency illness. Hypoparathyroidism is caused by injury to the parathyroid gland as a result of surgery or autoimmune disease. In addition, hypoparathyroidism may develop due to genetic causes or infiltrative diseases. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is characterized by multi-organ involvement, including the dysfunction of endocrine glands. Previous studies have demonstrated that SARS-CoV-2 infection induces endocrine tissue damage via various mechanisms, including direct cell damage from viral entry to the glands by binding to the angiotensin converting enzyme 2 receptors and replication, vasculitis, arterial and venous thrombosis, hypoxic cell damage, immune response and the cytokine storm. The effects of the new coronavirus, coronavirus disease 2019 (COVID-19) on the parathyroid glands have received limited attention. Hypoparathyroidism has been observed in a small number of individuals as a result of SARS-CoV-2 infection. The present study describes the case of a patient with primary hypoparathyroidism induced by COVID-19. Clinicians should also keep in mind that, despite the fact that SARS-CoV-2 has no known tropism for the parathyroid glands, it can result in primary hypoparathyroidism and decompensation of old primary hypoparathyroidism.
Keywords: hypoparathyroidism, COVID-19, endocrine glands, parathyroid glands, hypocalcemia
Introduction
Hypoparathyroidism is an infrequent endocrine deficiency disorder characterized by low levels of serum calcium, increased levels of serum phosphorus and absent or inappropriately low levels of serum parathyroid hormone (PTH) (1). Hypoparathyroidism usually occurs as a result of post-surgical or autoimmune damage to the parathyroid gland (2). The most common cause is neck surgery, which accounts for 75% of all cases. The incidence of hypoparathyroidism following neck surgery is ~8%; however, in 75% of these cases, the condition is temporary and is resolved within 6 months. As a result, the incidence of persistent hypoparathyroidism following neck surgery is ~2% (3).
The most common cause of non-surgical hypoparathyroidism is autoimmune hypoparathyroidism. This disorder can present on its own or as a part of autoimmune polyglandular syndrome type I (4). The destruction of the parathyroid glands can develop secondary to infiltration in the context of granulomatous diseases, such as sarcoidosis, amyloidosis and Riedel thyroiditis (5). Genetic causes account for <10% of all cases of hypoparathyroidism, with DiGeorge syndrome the most common genetic cause (6). The destruction of the parathyroid glands leading to hypoparathyroidism is rarely observed due to infiltrating metastatic cancer (7).
Hypoparathyroidism can also be caused by external or internal radiation, although this is rarely mentioned due to the fact that the parathyroid glands are extremely resistant to radiation (8). Moreover, hypoparathyroidism may develop due to the accumulation or deposition of minerals, such as copper (Wilson's disease) and iron (hemochromatosis) in the parathyroid gland (4). Furthermore, numerous mitochondrial diseases have been found to be associated with hypoparathyroidism (4). In addition, hypoparathyoidism may develop with no obvious etiology. In such cases, patients are most likely suffering from an autoimmune disorder (8).
The novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is responsible for corona virus disease 2019 (COVID-19), has rapidly spread across the globe, posing a severe threat to human health due to a pandemic (9). The distribution of the angiotensin converting enzyme 2 (ACE2) protein in human tissues, identified by previous studies, has allowed researchers to recognize probable infection pathways and speculate on the pathogenetic consequences of SARS-CoV-2 infection. According to these studies, ACE2 expression is highest in the lungs and small intestine enterocytes, and lowest in the testes, thyroid, adipose tissue, ovaries and endothelium. ACE2 has also been found in the adrenals, prostate, pituitary and hypothalamus (10,11). In addition, the increased expression of ACE2 receptors has been found in acidophilic cells of the parathyroid glands (12).
Hypocalcemia has been identified as a prevalent symptom of COVID-19, and it appears to be a biochemical trait that distinguishes COVID-19 from other acute respiratory distress syndromes (13). Furthermore, it appears to be a predictor of the development of severe COVID-19 infection. These findings, however, appear to be predominantly linked to vitamin D insufficiency (14).
Research into the effects of the novel coronavirus on parathyroid glands is limited. Hypoparathyroidism due to SARS-CoV-2 infection has been reported in a small number of cases (15,16). The present study describes a rare case of primary hypoparathyroidism induced by COVID-19.
Case report
A 53-year-old male patient, who was a non-smoker, presented to the Emergency Department of Laiko General Hospital (Athens, Greece) complaining of fever and cough over the past 10 days. He had a medical history of a surgically resected colon cancer 10 years prior, with no relapse in follow-up. He was receiving no medication.
A clinical examination revealed a febrile patient with crackles on auscultation at the bases of both lungs. The clinical evaluation of the other organs/systems did not reveal any notable findings. Blood pressure was 130/80 mmHg, heart rate was 96 beats per minute, oxygen saturation was 92% in room air and body temperature was 37.6˚C. An electrocardiography did not reveal any abnormalities upon admission.
The analysis of arterial blood gas revealed partial pressure of oxygen (pO2) levels of 54 mmHg, pressure of carbon dioxide (pCO2) levels of 41 mmHg, pH 7.47 and HCO3- at 29.8 mmol/l in room air. An X-ray of the chest revealed patchy diffuse opacities in both lower lung lobes (Fig. 1).
Figure 1.
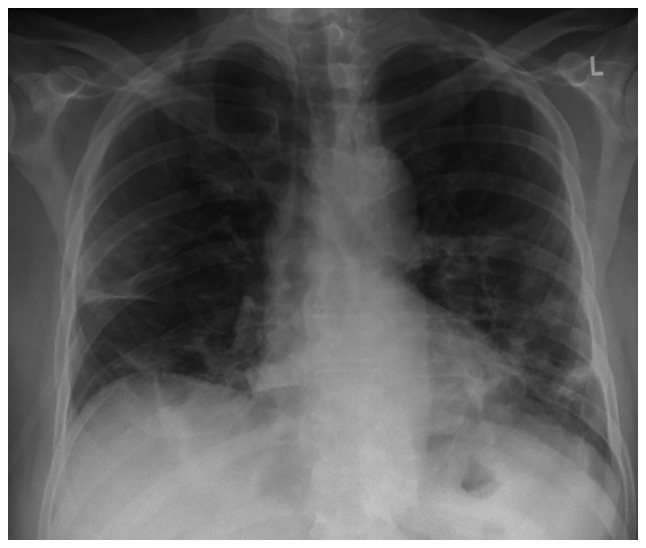
Chest X-ray illustrating patchy diffuse opacities in both lower lung lobes.
Laboratory tests included a complete blood cell count, biochemistry serum parameters and blood clotting testing. The notable findings were a white blood cell count of 2.99x103/µl (normal count, 4-11x103/µl), lymphocyte count of 0.58x103/µl (normal count, 1.2-3.4x103/µl), platelet count of 104x103/µl (normal count, 140-440x103/µl), creatinine levels of 0.61 mg/dl (normal range, 0.6-1 mg/dl), serum calcium levels of 6.9 mg/dl (normal range, 8.6-10.2 mg/dl), serum phosphorus levels of 4.7 mg/dl (normal range, 2.5-4.5 mg/dl), serum albumin levels of 37.4 g/l (normal range, 35-50 g/l), C-reactive protein levels of 53.29 mg/l (normal levels, <6 mg/l) and ferritin levels of 759 ng/ml (normal range, 15-150 ng/ml).
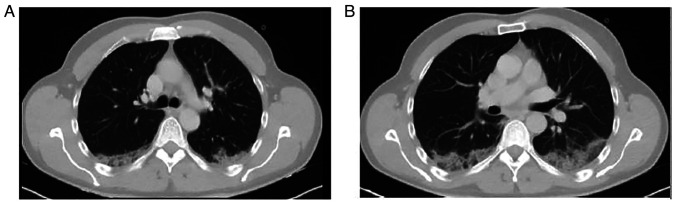
A nasopharyngeal swab was obtained and the patient tested positive for SARS-CoV-2 infection using reverse transcription-polymerase chain reaction (RT-PCR). The patient had not been vaccinated against SARS-CoV-2. He was transferred to the COVID-19 unit and received oxygen therapy with a Venturi mask delivering 50% oxygen. He also received intravenous dexamethasone, remdesivir and subcutaneous enoxaparin. A computed tomography (CT) scan of the patient's chest revealed nodular ground glass opacities in the posterior segments of the upper and lower lobes (Fig. 2).
Figure 2.
Computed tomography scan of the chest illustrating nodular ground glass opacities in the posterior segments of the (A) upper lobes and (B) lower lobes.
Due to detected hypocalcemia and hyperphosphatemia, a further laboratory investigation was performed, revealing PTH levels of 11.7 pg/ml (normal range, 12-65 pg/ml) and 25 hydroxyvitamin D levels of 38.4 ng/ml (levels of sufficiency, >30 ng/ml). The patient had no vitamin D deficiency and no chronic renal insufficiency. Therefore, the presence of hypocalcemia, hyperphosphatemia and inappropriately low serum levels of PTH confirmed the diagnosis of primary hypoparathyroidism.
The patient had no symptoms related to this condition. In addition, he had a normal level of serum calcium at 8.9 mg/dl (normal range, 8.6-10.2 mg/dl) at ~6 months prior when evaluated as a part of his general health checkup. This fact, along with his age, ruled out a genetic cause of hypoparathyroidism. Moreover, he had no history of surgery, trauma, infiltrative disease or regional radiation. PTH-related peptide levels were determined to be within the normal range, thus ruling out paraneoplastic syndrome as a cause of low PTH levels. Autoantibody screening for autoimmune diseases did not reveal any abnormal findings.
The patient underwent a CT scan of the neck without revealing any abnormal findings, which ruled out the infiltration of the parathyroid glands by metastases. He also underwent an abdominal CT scan with no abnormalities. Thus, primary hypoparathyroidism was considered to have been caused by SARS-CoV-2 infection.
The patient received oral calcium supplementation. After 5 days of hospitalization, his clinical condition and serum calcium levels improved. At 1 month following discharge, he presented with normal levels of serum calcium and serum phosphorus.
Discussion
Researchers have reported a small number of cases of primary hypoparathyroidism induced by SARS-CoV-2 infection and the decompensation of old primary hypoparathyroidism during the course of COVID-19 infection. In 2020, Elkattawy et al (15) reported the first case of primary hypoparathyroidism induced by SARS-CoV2 infection in a 46-year-old male patient with no marked past medical history, who was hospitalized with hypoxic respiratory failure and had a prolonged hospital stay. In that case, hyperphosphatemia and low PTH levels were detected incidentally (15). Dianatfar et al (16) described a 44-year old, previously healthy, female patient who was hospitalized due to COVID-19, who then entered a depressive state, and had one tonic-clonic seizure at ~1 week after being discharged from the hospital. Laboratory tests revealed a low serum calcium level and an increased serum phosphorus level. In addition, the serum PTH level was significantly low, establishing the diagnosis of primary hypoparathyroidism (16). The case described herein is the third, to the best of our knowledge, to report primary hypoparathyroidism induced by SARS-CoV-2 infection. The patient in the present study, similar to the other cases (15,16) developed hypocalcemia and hyperphosphatemia, with low levels of PTH in the context of COVID-19 pneumonia. Malignancy and paraneoplastic syndrome and autoimmune diseases were ruled out. The patient had a positive response to therapy with oral calcium supplementation. SARS-CoV-2 infection induced hypoparathyroidism, which was not persistent.
COVID-19 disease has also been reported to induce the decompensation of previously well-tolerated primary hypoparathyroidism. Bossoni et al (17) reported a case of a 72-year-old female patient with a past history of thyroidectomy who experienced mild COVID-19 infection and presented with acute perioral paresthesia and dysarthria. Laboratory investigations revealed a low serum calcium level, an increased serum phosphorus level and a low serum PTH level, suggesting that SARS-CoV-2 infection precipitated severe hypocalcemia in the context of a subclinical post-surgical hypoparathyroidism (17).
Pla et al (18) described a case of a 76-year-old male patient with a known history of hypocalcemia who presented with perioral paresthesia during COVID-19 infection, upper extremity paresthesia, anorexia, and with hypocalcemia, hyperphosphatemia and inappropriately normal levels of PTH, establishing the decompensation of a well-tolerated primary hypoparathyroidism due to SARS-CoV-2 infection. Moreover, Bonnet et al (19) reported decompensated primary hypoparathyroidism in an 82-year-old male patient with COVID-19. The cases of primary hypoparathyroidism and the decompensation of old primary hypoparathyroidism due to SARS-CoV-2 infection in the literature are summarized in Table I.
Table I.
Cases of primary hypoparathyroidism and the decompensation of primary hypoparathyroidism due to COVID-19 found in the literature.
| Author/(Refs), year | Age, years/sex | Symptoms/signs related to hypoparathyroidism | Type of dysfunction | Course of COVID-19 infection | Outcome |
|---|---|---|---|---|---|
| Elkattawy et al (15), 2020 | 46/M | None, incidental finding | Primary hypoparathyroidism | Critical | Recovery |
| Dianatfar et al (16), 2021 | 44/F | Tonic-clonic seizure; depressed mood | Primary hypoparathyroidism | Severe | Recovery |
| Bossoni et al (17), 2020 | 72/F | Acute-onset dysarthria; perioral paresthesia | Decompensation of primary hypoparathroidism | Mild | Recovery |
| Pla et al (18), 2021 | 76/M | Perioral paresthesia; upper extremity paresthesia; anorexia; positive Trousseau sign | Decompensation of primary hypoparathroidism | Severe | Recovery |
| Bonnet et al (19), 2021 | 82/M | Prolongation of the QTc interval at 470 msec on electrocardiography | Decompensation of primary hypoparathroidism | Severe | Recovery |
| The present study | 53/M | None, incidental finding | Primary hypoparathyroidism | Severe | Recovery |
M, male; F, female.
The frequency of parathyroid dysfunction among patients with COVID-19, as well as the exact underlying mechanisms, duration and reversibility of this phenomenon, remain unclear. Due to the paucity of data linking SARS-CoV-2 to parathyroid glands, studies on the previous generation of coronavirus (SARS-Co-V), which caused the SARS pandemic in 2003, may elucidate this topic. SARS-Co-V RNA and antigenic materials have been detected in parathyroid gland acidophilic cells in tissue samples obtained from patients with SARS who did not survive (20). In addition, the increased expression of ACE2 receptors has been detected in acidophilic cells of parathyroid glands (9). Therefore, SARS-CoV-2 may potentially directly affect the parathyroid glands by binding to the ACE2 receptors on acidophilic cells (21).
Previous pathological research on SARS-CoV-1- or SARS-CoV-2-infected patients has revealed a range of endocrine tissue damage, including direct cell damage from viral entry and replication, vasculitis, arterial and venous thrombosis, hypoxic cell damage, immune response and the cytokine storm (22). Thrombosis is more common in patients with COVID-19, particularly in small vessels and extrapulmonary organs, than in patients with SARS (23). This SARS-CoV-2-specific pathogenetic action may cause damage to highly vascularized organs, such as the endocrine glands, and in particular those with a dense vascular network (24).
Of note, a previous study demonstrated that chronic respiratory alkalosis increased resistance of renal PTH receptors to PTH, resulting in hypocalcemia and hyperphosphatemia with no increase in PTH levels, indicating that chronic respiratory alkalosis might lead to relative hypoparathyroidism (25). These findings suggest an additional potential mechanism of indirect affection of parathyroid gland function during COVID-19 disease, which is characterized by an increased effort for respiration and carbon dioxide washout, leading to respiratory alkalosis (25). Further studies are required to investigate the causative association between SARS-CoV-2 infection and the effects on parathyroid glands.
In conclusion, SARS-CoV-2 infection can lead to multi-organ involvement, including the dysfunction of the endocrine glands. The present study highlighted the effect of the novel coronavirus on parathyroid glands. Clinicians should also consider that although SARS-CoV-2 does not present a known tropism for the parathyroid glands, it can cause the decompensation of old primary hypoparathyroidism that was well-tolerated prior to infection.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
PA, AB and PP conceptualized the study. VEG, EK and CD advised on patient treatment, wrote and prepared the draft of the manuscript and made a substantial contribution to data analysis. AG and MSV analyzed patient data. NT and DAS analyzed patient data and provided critical revisions. PS and DP obtained the medical images and made substantial contributions to data interpretation. VEG and AB confirm the authenticity of all the raw data. All authors contributed to manuscript revision and have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have not competing interests.
References
- 1.Bilezikian JP, Khan AA, Potts JT Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: Summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94:335–339. doi: 10.1210/jc.2008-1763. Third International Workshop on the Management of Asymptomatic Primary Hyperthyroidism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilezikian JP, Khan A, Potts JT Jr, Brandi ML, Clarke BL, Shoback D, Jüppner H, D'Amour P, Fox J, Rejnmark L, et al. Hypoparathyroidism in the adult: Epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26:2317–2337. doi: 10.1002/jbmr.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke BL, Brown EM, Collins MT, Jüppner H, Lakatos P, Levine MA, Mannstadt MM, Bilezikian JP, Romanischen AF, Thakker RV. Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab. 2016;101:2284–2299. doi: 10.1210/jc.2015-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siraj N, Hakami Y, Khan A. Medical hypoparathyroidism. Endocrinol Metab Clin North Am. 2018;47:797–808. doi: 10.1016/j.ecl.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Brinkane A, Peschard S, Leroy-Terquem E, Bergheul S, Raheriarisoa H, Hubert N, Crickx L, Levy R. Association rare d'une hypoparathyroïdie et d'une sarcoïdose médiastino-pulmonaire: Rare association of hypoparathyroidism and mediastinal-pulmonary sarcoidosis. Ann Med Interne (Paris) 2001;152:63–64. (In French) [PubMed] [Google Scholar]
- 6.Goddard CJ, Mbewu A, Evanson JM. Symptomatic hypocalcaemia associated with metastatic invasion of the parathyroid glands. Br J Hosp Med. 1990;43(72) [PubMed] [Google Scholar]
- 7.Bilezikian JP. Hypoparathyroidism. J Clin Endocrinol Metab. 2020;105:1722–1736. doi: 10.1210/clinem/dgaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med. 2008;359:391–403. doi: 10.1056/NEJMcp0803050. [DOI] [PubMed] [Google Scholar]
- 9.Georgakopoulou VE, Avramopoulos P, Papalexis P, Bitsani A, Damaskos C, Garmpi A, Gkoufa A, Garmpis N, Mantzouranis K, Chlapoutakis S, et al. Exacerbation of bronchiectasis by Pseudomonas putida complicating COVID-19 disease: A case report. Exp Ther Med. 2021;22(1452) doi: 10.3892/etm.2021.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;525:135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazartigues E, Qadir MMF, Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2's reliance on ACE2. Endocrinology. 2020;161(bqaa108) doi: 10.1210/endocr/bqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He L, Ding Y, Zhang Q, Che X, He Y, Shen H, Wang H, Li Z, Zhao L, Geng J, et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Filippo L, Formenti AM, Rovere-Querini P, Carlucci M, Conte C, Ciceri F, Zangrillo A, Giustina A. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine. 2020;68:475–478. doi: 10.1007/s12020-020-02383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Han P, Wu J, Gong J, Tian D. Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J Infect Public Health. 2020;13:1224–1228. doi: 10.1016/j.jiph.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkattawy S, Alyacoub R, Ayad S, Pandya M, Eckman A. A novel case of hypoparathyroidism secondary to SARS-CoV-2 infection. Cureus. 2020;12(e10097) doi: 10.7759/cureus.10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dianatfar M, Sanjari M, Dalfardi B. Hypoparathyroidism after COVID-19 Pneumonia. Shiraz E-Med J. 2021;22(e115832) [Google Scholar]
- 17.Bossoni S, Chiesa L, Giustina A. Severe hypocalcemia in a thyroidectomized woman with Covid-19 infection. Endocrine. 2020;68:253–254. doi: 10.1007/s12020-020-02326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pla B, Silva M, Arranz A, Marazuela M. Hipocalcemia severa y resistente al tratamiento en paciente con neumonía bilateral COVID-19. Severe and treatment-resistant hypocalcemia in patient with bilateral COVID-19 pneumonia. Endocrinol Diabetes Nutr. 2021;68:518–519. doi: 10.1016/j.endinu.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnet JB, Berchoux E, Sultan A. Decompensated primary hypoparathyroidism in a patient with COVID-19. Ann Endocrinol (Paris) 2021;82:123–124. doi: 10.1016/j.ando.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abobaker A, Alzwi A. The effect of COVID-19 on parathyroid glands. J Infect Public Health. 2021;14:724–725. doi: 10.1016/j.jiph.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Y, Korteweg C, McNutt MA, Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133:4–12. doi: 10.1016/j.virusres.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 24.Piticchio T, Le Moli R, Tumino D, Frasca F. Relationship between betacoronaviruses and the endocrine system: A new key to understand the COVID-19 pandemic-A comprehensive review. J Endocrinol Invest. 2021;44:1553–1570. doi: 10.1007/s40618-020-01486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krapf R, Jaeger P, Hulter HN. Chronic respiratory alkalosis induces renal PTH-resistance, hyperphosphatemia and hypocalcemia in humans. Kidney Int. 1992;42:727–734. doi: 10.1038/ki.1992.340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.