Abstract
An increase in oxacillin activity was observed against methicillin-resistant coagulase-negative staphylococci (MRCNS) and methicillin-resistant Staphylococcus aureus (MRSA) in the presence of a sub-MIC of vancomycin. Vancomycin and oxacillin were synergistic against 14 of 21 strains of MRCNS and MRSA. A pattern of enhanced killing was also supported by time-kill studies. These results suggest that combinations of sub-MICs of vancomycin and oxacillin may have therapeutic benefits against methicillin-resistant staphylococci.
Vancomycin is the drug of choice for most methicillin-resistant staphylococcus infections, and therefore, the recent emergence of decreased vancomycin susceptibility in methicillin-resistant staphylococci presents a significant clinical problem (7, 15; F. A. Waldvodel, Editorial, N. Engl. J. Med. 7:556–557, 1999). Reduced susceptibility to vancomycin in Staphylococcus spp. appears to occur on exposure to vancomycin and under selective pressure, rather than by gene transfer as in enterococci. In vitro experiments have demonstrated that selective pressure can produce vancomycin resistance but have also revealed that increases in vancomycin resistance can induce concurrent decreases in resistance to β-lactams in both methicillin-resistant coagulase-negative staphylococci (MRCNS) and methicillin-resistant Staphylococcus aureus (MRSA) (5, 12; B. E. Domaracki, A. Evans, K. E. Preston, H. Fraimow, and R. A. Venezia, Abstr. 96th Gen. Meet. Am. Soc. Microbiol. 1996, abstr. A-32, p. 138, 1996; B. E. Domaracki, A. Evans, K. E. Preston, and R. A. Venezia, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-133, p. 107, 1998). This study shows that clinical isolates of vancomycin-susceptible MRCNS and MRSA become increasingly susceptible to oxacillin when grown in the presence of a sub-MIC of vancomycin. In addition, checkerboard assays and time-kill curves demonstrate a synergistic interaction of combinations of sub-MICs of oxacillin and vancomycin against clinical isolates of both MRCNS and MRSA.
The staphylococcal isolates used were from clinical specimens submitted for routine culture to the Clinical Microbiology Laboratory at Albany Medical Center, Albany, N.Y. The isolates were identified using the Vitek GPI identification system (bioMerieux Vitek). Antimicrobial susceptibility testing was performed by broth dilution MIC tests according to the National Committee for Clinical Laboratory Standards (8). E-tests (AB Biodisk, Dalvagen, Sweden) were carried out according to the manufacturer's instructions. Oxacillin E-tests were therefore performed on medium with 2% NaCl to enhance detection of resistance. All assays were performed in duplicate.
To study the combined antibiotic activity of oxacillin and vancomycin, a modification of the E-test was performed. Briefly, oxacillin E-test strips were applied to inoculated (0.5 McFarland) Mueller-Hinton agar plates (Difco Laboratories, Detroit, Mich.) and vancomycin (Eli Lilly, Indianapolis, Ind.)-supplemented plates of concentrations ranging from 0.25 to 2.0 μg/ml (5). All plates contained 2% NaCl. The MIC of oxacillin alone was divided by the MIC of oxacillin in the presence of a sub-MIC of vancomycin to determine the degree of reduction. The lowest concentration of vancomycin that achieved the highest reduction in the oxacillin MIC was recorded.
The checkerboard titration method was used to test for vancomycin and oxacillin synergy against 15 strains of MRCNS and 6 strains of MRSA (1, 5; Domaracki et al., Abstr. 96th Gen. Meet. Am. Soc. Microbiol. 1996; Domaracki et al., 38th ICAAC). Microtiter plates containing 96 wells contained concentrations of vancomycin ranging from 0.125 to 8.0 μg/ml in combination with concentrations of oxacillin ranging from 1.0 to 1,024 μg/ml. The concentration of antibiotics needed to inhibit growth was recorded. Fractional inhibitory concentrations (FICs) were calculated as the MIC of the antibiotic in combination divided by the MIC of the antibiotic alone. The vancomycin and oxacillin FICs were then summed to derive the FIC index, which indicated synergy when index values were ≤0.5 (1). Antagonism was defined by FIC index values of >4.0.
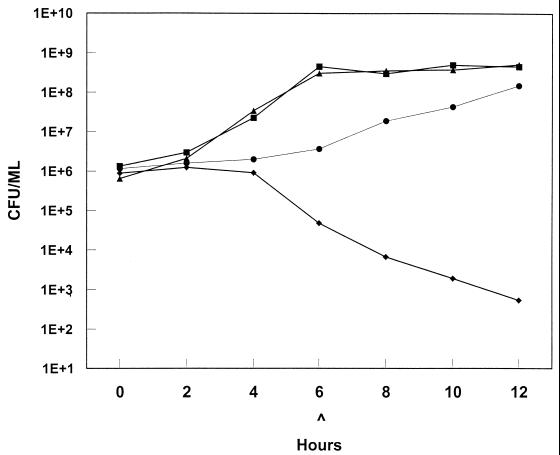
Time-kill curves were utilized to study the effect of combinations of sub-MICs of oxacillin and vancomycin on the growth of MRCNS and MRSA throughout a 12-h incubation (1, 5; Domaracki et al., Abstr. 96th Gen. Meet. Am. Soc. Microbiol. 1996). Four staphylococcal species were tested: S. haemolyticus, S. epidermidis, S. hominis, and S. aureus. The isolates were grown in cation-adjusted Mueller-Hinton broth plus 2% NaCl with a sub-MIC of either vancomycin or oxacillin (vancomycin, 1 μg/ml; oxacillin, 8 or 64 μg/ml) or with a combination of sub-MICs of the two antibiotics (vancomycin, 1 μg/ml, and oxacillin, 8 μg/ml; or vancomycin, 1 μg/ml, and oxacillin, 64 μg/ml). The antibiotic sub-MICs used for the S. aureus isolate (vancomycin, 0.5 μg/ml; oxacillin, 8 μg/ml; and vancomycin, 0.5 μg/ml, plus oxacillin, 8 μg/ml) were readministered at 6 h to simulate clinical conditions (Fig. 1). Antibiotic concentrations were selected to be one-fourth to one-half the MIC of that antibiotic. Viability counts were performed at 0, 3, 6, and 12 h. Inocula were diluted in sterile saline and plated in small volumes (100 and 10 μl) to prevent antibiotic carryover.
FIG. 1.
Time-kill curve for approximately 106 MRSA bacteria (oxacillin MIC, 24 μg/ml; vancomycin MIC, 1 μg/ml), in the presence of no antibiotics (▴), 0.5 μg of vancomycin (■) per ml, 8 μg of oxacillin (●) per ml, and 0.5 μg of vancomycin plus 8 μg of oxacillin (⧫) per ml. All agents were readministered at 6 h (∧). E, exponential log10.
As measured by E-test, subinhibitory levels of vancomycin (one-fourth to one-half the MIC) added to the agar medium caused measurable increases in oxacillin susceptibility regardless of the species tested (Table 1). The eight strains of MRCNS tested showed 5- to 670-fold increases in oxacillin susceptibility in the presence of vancomycin. Of the five MRSA strains tested, the oxacillin MIC did not change in the presence of vancomycin for one isolate, and for the remaining four isolates, the susceptibility to oxacillin increased 2-fold to 96-fold.
TABLE 1.
Oxacillin MICs and FICs in the presence of vancomycin determined by E-test and checkerboard titration
| Species | MIC (μg/ml) by E-test
|
Value by checkerboard titrationa
|
|||
|---|---|---|---|---|---|
| Vancomycin alone | Oxacillin alone | Oxacillin with vancomycinb | FIC index | Synergy | |
| S. epidermidis | 2 | 24 | 0.5 (1) | NDc | ND |
| S. epidermidis | 3 | >256 | 0.38 (1) | 0.56 | No |
| S. epidermidis | 3 | >256 | 0.75 (1) | 0.13 | Yes |
| S. epidermidis | 3 | 64 | 0.38 (1) | 0.63 | No |
| S. epidermidis | 3 | >256 | 3 (1) | 0.26 | Yes |
| S. epidermidis | 3 | >256 | 48 (2) | ND | ND |
| S. epidermidis | 2 | 3 | 0.19 (1.5) | 0.63 | No |
| S. epidermidis | 2 | 24 | 1 (0.75) | 0.38 | Yes |
| S. epidermidis | 1.5 | >256 | 0.5 (0.75) | 0.27 | Yes |
| S. epidermidis | 2 | 32 | 0.75 (0.75) | 0.31 | Yes |
| S. epidermidis | 1.5 | 64 | 0.5 (0.75) | 0.27 | Yes |
| S. epidermidis | 2 | 3 | 0.5 (1) | 0.75 | No |
| S. epidermidis | 3 | 3 | 0.5 (1.5) | 0.28 | Yes |
| S. epidermidis | 2 | 32 | 0.5 (0.75) | 0.28 | Yes |
| S. haemolyticus | 2 | >256 | 0.38 (1) | 0.28 | Yes |
| S. hominis | 3 | >256 | 0.5 (1) | 0.26 | Yes |
| S. hominis | ND | ND | ND (ND) | 0.33 | Yes |
| S. aureus | 1 | 24 | 0.25 (0.5) | 0.5 | Yes |
| S. aureus | 1.5 | 64 | 6 (0.5) | 0.27 | Yes |
| S. aureus | 1 | >256 | 128 (0.5) | 0.53 | No |
| S. aureus | 0.5 | >256 | >256 (0.25) | 1 | No |
| S. aureus | 1 | 48 | 6 (0.5) | 0.31 | Yes |
| S. aureus | 1.5 | >256 | 64 (0.5) | 0.53 | No |
FIC index indicates synergy when value is ≤0.5 or antagonism when value is >4.
Values in parentheses are concentrations (micrograms per milliliter) of vancomycin added to agar.
ND, not done.
Synergy was observed using the checkerboard method for 1 of 1 isolate of S. haemolyticus, 2 of 2 isolates of S. hominis, 8 of 12 isolates of S. epidermidis, and 3 of 6 isolates of MRSA (Table 1). The FIC indices of the 12 MRCNS strains for which synergy (FIC of ≤0.5) was observed were ≤0.38 (range, 0.13 to 0.38). The remaining four MRCNS strains had FIC indices ranging from 0.56 to 0.75. The FIC indices for the six MRSA isolates were 0.27, 0.31, 0.5, 0.53, and 1.0. No antagonism was detected. These results were similar to those from a study showing synergy or an additive effect of cefpirome or cefoperazone with vancomycin by the checkerboard method for MRSA isolates (11).
Time-kill curves showed an enhancement of killing of all four staphylococcal species in the presence of combinations of sub-MICs of vancomycin and oxacillin. Synergy, defined as ≥2 logs of killing compared to the starting inoculum, was observed for the S. aureus isolate. After 12 h of growth in the presence of vancomycin (1.0 μg/ml) plus oxacillin (8.0 μg/ml) and without readministration of antibiotics at 6 h, the assays showed 1.16 logs of killing for the S. epidermidis isolate, 1.53 logs of killing for the S. haemolyticus isolate, and 1.62 logs of killing for S. hominis isolates. In the presence of vancomycin (0.5 μg/ml) plus oxacillin (8.0 μg/ml) and with readministration at 6 h, the S. aureus isolate showed 3.22 logs of killing at 12 h. No vancomycin resistance was detected at 12 h.
By a variety of in vitro tests, we found that vancomycin and oxacillin were synergistic against many clinical isolates of MRCNS and MRSA. The synergistic action of these antibiotics was achieved with sub-MIC combinations of one-fourth to one-half of the MICs of vancomycin and oxacillin. However, not all isolates for which synergy was detected by the checkerboard method showed synergy (≥2 logs of killing) in time-kill studies. In the time-kill studies, synergy was detected only when the antibiotics were readministered after 6 h of growth to maintain antibiotic concentrations and to simulate clinical conditions. In vitro studies have shown that vancomycin is removed from the growth medium by some staphylococci and is sequestered in a biologically active form within the cell wall structure (12, 14). This suggests that the 1.2 to 1.6 logs of killing observed at 12 h for MRCNS strains would have likely been extended to ≥2 logs with appropriate readministration.
Indications that vancomycin resistance develops under selective pressure with exposure to vancomycin have led to concerns that treatment with vancomycin will lead to the emergence of increased vancomycin resistance in staphylococci. Indeed, evidence indicates that vancomycin resistance can be selected in the laboratory (2, 5, 6, 9, 10, 13; Domaracki et al., Abstr. 96th Gen. Meet. Am. Soc. Microbiol. 1996; Domaracki et al., 38th ICAAC), and the recent clinical cases of staphylococci with reduced vancomycin susceptibilities in patients being treated with vancomycin also support this concern (3, 4, 7, 14). However, this study indicates that sub-MICs of vancomycin and oxacillin are effective in killing methicillin-resistant staphylococci and yet do not select for vancomycin resistance. Using a combination of a sub-MIC of vancomycin with a sub-MIC of oxacillin may prevent an increase in vancomycin-resistant staphylococci in vivo, but this remains to be tested.
Further investigations should be conducted with animal models to see if a vancomycin-oxacillin combination is synergistic in vivo. Additional testing should also be performed to determine the prevalence of a synergistic effect in different species of staphylococci and in the clinical staphylococcal population. Use of an oxacillin E-test strip on vancomycin-containing agar would provide a quick and easy test with which to screen isolates for potential synergy. The therapeutic implications of vancomycin and oxacillin synergy should be explored further.
REFERENCES
- 1.Bajaksouzian S, Visalli M A, Jacobs M R, Appelbaum P C. Activities of levofloxacin, ofloxacin, and ciprofloxacin, alone and in combination with amikacin, against acinetobacters as determined by checkerboard and time-kill studies. Antimicrob Agents Chemother. 1997;41:1073–1076. doi: 10.1128/aac.41.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biavasco F, Giovanetti E, Montanari M P, Lupidi R, Varaldo P E. In vitro selection of resistance to vancomycin in bloodstream isolates of Staphylococcus haemolyticus and Staphylococcus epidermidis. Eur J Clin Microbiol Infect Dis. 1991;27:71–79. doi: 10.1007/BF01984921. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morbid Mortal Weekly Rep. 1997;46:765–766. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1998. Morbid Mortal Weekly Rep. 1997;46:813–815. [PubMed] [Google Scholar]
- 5.Domaracki B E, Evans A, Preston K E, Fraimow H, Venezia R A. Increased oxacillin activity associated with glycopeptides in coagulase-negative staphylococci. Eur J Clin Microbiol Infect Dis. 1998;17:143–150. doi: 10.1007/BF01691109. [DOI] [PubMed] [Google Scholar]
- 6.Herwaldt L, Boyken L, Pfaller M. In vitro selection of resistance to vancomycin in bloodstream isolates of Staphylococcus haemolyticus and Staphylococcus epidermidis. Eur J Clin Microbiol Infect Dis. 1991;10:1007–1012. doi: 10.1007/BF01984921. [DOI] [PubMed] [Google Scholar]
- 7.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–146. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1996. [Google Scholar]
- 9.Schwalbe R S, Stapelton J T, Gilligan P H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987;316:927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- 10.Schwalbe R S, Ritz W J, Verma P R, Barranco E A, Gilligan P H. Selection for vancomycin resistance in clinical isolates of Staphylococcus haemolyticus. J Infect Dis. 1990;161:45–51. doi: 10.1093/infdis/161.1.45. [DOI] [PubMed] [Google Scholar]
- 11.Seibert G, Isert D, Klesel N, Limbert M, Markus A, Schrinner E. The in-vitro antibacterial activity of a combination of cefpirome or cefoperazone with vancomycin against enterococci and Staphylococcus aureus. J Antimicrob Chemother. 1992;29(Suppl. A):25–30. doi: 10.1093/jac/29.suppl_a.25. [DOI] [PubMed] [Google Scholar]
- 12.Sieradzki K, Tomasz A. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J Bacteriol. 1997;179:2557–2566. doi: 10.1128/jb.179.8.2557-2566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sieradzki K, Villari P, Tomasz A. Decreased susceptibilities to teicoplanin and vancomycin among coagulase-negative methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother. 1998;42:100–107. doi: 10.1128/aac.42.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sieradzki K, Roberts P R B, Haber S W, Tomasz A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med. 1999;340:517–523. doi: 10.1056/NEJM199902183400704. [DOI] [PubMed] [Google Scholar]
- 15.Smith T L, Pearson M L, Wilcox K R, Cruz C, Lancaster M V, Robinson-Dunn B, Tenover F C, Zervos M J, Band J D, White E, Jarvis W R for the Glycopeptide-Intermediate Staphylococcus aureus Working Group. Emergence of vancomycin resistance in Staphylococcus aureus. N Engl J Med. 1999;340:493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]