Abstract
Microscopic colitis (MC) is a chronic inflammatory disease of the large intestine and as a relatively late recognized condition, its relationship with other disorders of the gastrointestinal tract is gradually being understood and investigated. As a multifactorial disease, MC interacts with inflammatory bowel disease, celiac disease, and irritable bowel syndrome through genetic overlap, immunological factors, and gut microflora. The risk of colorectal cancer was significantly lower in MC, gastrointestinal infections increased the risk of developing MC, and there was an inverse association between Helicobacter pylori infection and MC. A variety of associations are found between MC and other gastrointestinal disorders, where aspects such as genetic effects, resemblance of immunological profiles, and intestinal microecology are potential mechanisms behind the relationships. Clinicians should be aware of these connections to achieve a better understanding and management of MC.
Keywords: microscopic colitis, inflammatory bowel disease, celiac disease, irritable bowel syndrome, management
Introduction
Microscopic colitis (MC) is a chronic inflammatory disease of the large intestine that primarily affects the elderly and has a female predominance [1]. Persistent watery diarrhea is indicated as the main manifestation and the disease can be recurrent in course [2, 3]. MC is characterized by a nearly normal endoscopic appearance with microscopic abnormalities identified on histology—a feature that distinguishes it from classical inflammatory bowel disease (IBD) [4]. Histologically, it comprises two main types: one with an increase in lymphocytes in the sub-epithelial layer of the colon (≥20 lymphocytes/100 colonic epithelial cells) without a thickened collagen lamina termed lymphocytic colitis (LC), and the other type, collagenous colitis (CC), which is presented as lymphocytosis with a collagen band of >10 μm.
Since MC was first described in 1982, the incidence and prevalence have been increasing overall, with recent epidemiological studies showing that it has expanded many times compared with the situation in around 2000 [5–8]. MC constitutes ∼10%–15% of the population suffering from chronic diarrhea and has emerged as the leading cause of diarrhea in the elderly [9]. Aberrant response of the immune system to intestinal antigens is the key pathogenic mechanism of MC and a host of immune-related diseases are associated with it, such as type 1 diabetes, autoimmune thyroid disease, and other autoimmune disorders [10]. In the context of the digestive system, MC is also commonly associated with immune disorders such as celiac disease and IBD. Furthermore, MC is predisposed to developing in individuals with susceptibility genes that are shared with those implicated in IBD [4]. Several intestinal infections are followed by an increased risk of acquiring MC, putting this condition in contact with a couple of specific infections [11–13]. The overlap between irritable bowel syndrome (IBS) and MC in terms of symptoms and diagnostic methods also renders the two conditions confusing [14]. This makes for a cross-linked relationship between MC and these gastrointestinal disorders. Behind these disease relationships lies the clinical aspect of management, and the interaction between MC and them makes it more complex and comprehensive in terms of management.
Here, we aim to clarify the interfaces between MC and other digestive disorders, illustrate the mechanisms involved, and explore the impact on disease management. With the understanding of these aspects, we hope to offer a better insight into MC as a disease group and provide clinical perspectives on the precise location of MC in gastroenterology.
Method
The electronic databases PubMed and Embase were retrieved manually to obtain relevant literature. The reference lists in the majority of the included literature were also checked internally to search for matches. Only publications in the English language were included. There was no restriction on the year of publication for the documents. The databases were queried using a combination of MeSH terms and entry terms, including “microscopic colitis,” “lymphocytic colitis,” “collagenous colitis,” “colitis,” “inflammatory bowel disease,” “irritable bowel syndrome,” “celiac disease,” “cancer,” “colon,” and “treatment.” All included publications were critically reviewed. Endnote X9 software was used for literature management.
Possible proposed pathogenic mechanisms for MC
An overview of the pathogenesis of MC
A recent systematic review provided a detailed summary of the possible pathogenic mechanisms involved in MC [10]. Although the etiology of MC remains unclear, an understanding of its plausible pathogenesis may allow a rational explanation of the link between MC and other digestive tract diseases. Therefore, a thorough overview of the proposed possible etiopathogenic mechanisms of MC is fundamental and crucial to facilitate understanding its interaction with related diseases. The pathogenesis of MC is summarized in Table 1.
Table 1.
Proposed possible pathogenic mechanisms of MC
| Proposed pathogenesis of MC | Altered components | Specific changes | Influencing factor | Mechanism description | Comments | References |
|---|---|---|---|---|---|---|
| Environmental factors | Smoking and alcohol consumption | Smoking: alteration of the gut microbiome, and inflammation induction; alcohol consumption: integrity of the intestinal epithelial barrier; endotoxin-producing bacteria | NA | Smoking: epithelial barrier dysfunction and intestinal inflammation; alcohol consumption: increasing the trans-epithelial and paracellular passage of luminal antigens, dysbiosis, and intestinal bacterial overgrowth | Smoking: a pooled OR of 2.99 for current smokers and 1.63 for former smokers compared with never smokers; alcohol consumption: aHRs of MC were 1.20 for consumers of 0.1–4.9 g/day of alcohol, 1.90 for consumers of 5–14.9 g/day, and 2.31 for consumers of ≥15 g/day | [15, 16] |
| Medication | PPIs, NSAIDs, statins, and SSRIs | Intraluminal environment and bacterial flora; bowel integrity and colonic permeability | NA | PPIs: acid suppression-related colonic dysbiosis and affected immune reaction; NSAIDs: intestinal damage and increased bile salt cytotoxicity; statins: unknown; SSRIs: aggravation of colitis symptoms by interference with the gastrointestinal motility and secretion | Poor understanding of the pathogenic mechanisms | [17–20] |
| Infectious agents | Clostridioides difficile, norovirus, Escherichia species, Campylobacter concisus, and H. pylori | Enteric flora; intestinal microenvironment | NA | Further dysbiosis of the enteric flora; intestinal epithelial sodium channel dysfunction and claudin-8-dependent gut barrier dysfunction; alteration of the intestinal microenvironment activates the immune pathway | H. pylori may reduce the risk of MC and the “hygiene hypothesis” may be the relevant mechanism. | [11, 12, 22–26] |
| Autoimmune disorders | HLA haplotypes; autoantibodies; hypersensitivity response | Concomitant autoimmune conditions and shared HLA genotype; serum antinuclear antibodies, IgM, antigliadin IgA, anti-endomysial, ASCA; drug or food allergy | Some drugs | Underlying autoimmune diseases affecting the gut or cross-reactivity of antigens due to increased intestinal permeability | No direct evidence | [10] |
| Genetic factors | Genetic predisposition | HLA region and the extended haplotype 8.1 | NA | HLA-DQ2 as a shared genetic predisposition to celiac disease; haplotype 8.1 in association with collagenous colitis | HLA regions play a role in MC | [10] |
aHR, adjusted hazard ratio; ASCA, anti-Saccharomyces cerevisiae antibody; HLA, human leukocyte antigen; H. pylori, Helicobacter pylori; MC, microscopic colitis; NA, not available; NSAIDs, non-steroidal anti-inflammatory drugs; OR, odds ratio; PPIs, proton-pump inhibitors; SSRIs, selective serotonin reuptake inhibitors.
Risk factors associated with MC
Also, environmental factors such as smoking [15] and alcohol consumption [16], certain medications such as non-steroidal anti-inflammatory drugs (NSAIDs) [17], statins [18], selective serotonin reuptake inhibitors (SSRIs) [19], and proton-pump inhibitors (PPIs) [17, 20] have been shown to be associated with an increased risk of MC. These risk factors may therefore act as a contributing component in the pathogenesis of MC (Table 1).
The possible role of gastrointestinal infections
Intestinal microflora dysbiosis in MC is one of the possible pathogenic mechanisms and therefore infections of the gastrointestinal tract, especially certain specific bacterial infections, could potentially be associated with the risk of MC. A previous systematic review found that gastrointestinal infections were involved in the risk of IBD, with several specific infections being associated with an increased risk of IBD and Helicobacter pylori infection reducing the risk of IBD [21]. There is supposedly also a risk profile for MC as a possible attenuated form of IBD associated with gastrointestinal infections.
In a nationwide case–control study, gastrointestinal infections were significantly associated with an increased risk of MC, with an adjusted odds ratio (OR) of 2.63 (95% confidence interval [CI], 2.42–2.85) [11]. Several specific infections, Clostridium difficile, norovirus, and Escherichia species, increased the odds of developing MC, whereas no association was found for Salmonella species. The increased risk due to gastrointestinal infections was higher in CC than in LC. Another cohort study demonstrated a significantly elevated risk of MC following Campylobacter concisus infection [12]. Several case series have also reported new-onset MC following recurrent C. difficile infection [22–24]. An inverse association was found between H. pylori infection and MC, similar to that in IBD [25, 26]. Differences in the prevalence of H. pylori in distinct regions may provide an explanation for the differing ethnic distribution of patients with MC [25].
Infection of the gastrointestinal tract leads to further dysbiosis of the enteric flora in patients with MC, initiating associated immune pathways and thus increasing the risk of MC. Specific infections such as C. difficile infection and C. concisus infection have a higher risk of developing MC, which seems to indicate that these bacteria have a more sustained pro-inflammatory effect [11, 12]. Campylobacterconcisus has also been found to be associated with intestinal epithelial sodium channel dysfunction and claudin-8-dependent gut barrier dysfunction—a dysregulation that leads to a translocation of the intestinal flora, making it even further dysregulated [27]. Alternatively, gastrointestinal infections may have activated immune pathways in MC by altering the intestinal microenvironment, since CC, a subtype in which more immune mechanisms are involved [28], is more strongly implicated in infections of the gastrointestinal tract.
Apart from epidemiological evidence, the protective effect of H. pylori infection on MC remains largely unknown at present. However, as a similar inverse association has been found in IBD, it is possible to speculate that the mechanisms involved may be consistent. In mouse models of experimental colitis, H. pylori exposure exhibits a blocking or mitigating effect on colitis [29]. In this context, the NLRP3 inflammasome and interferon-18 (IL-18) are involved in the protective mechanism of H. pylori. Meanwhile, helper T-cell (Th), Th17/Th1-related cytokines were found to be downregulated while cytokines secreted by Th2 were upregulated [30–32]. This suppression of pro-inflammatory cytokines is likely to be implicated in the protective mechanism [33]. This evidence suggests that H. pylori may reduce the inflammation of IBD through immunomodulatory effects. Interestingly, not all strains of H. pylori exhibit this effect. The specific component of H. pylori, CagA, may be an integral component of the protective mechanism. In patients with IBD who were seronegative for CagA, no significant protective effect was demonstrated [34].
Another plausible cause of this inverse relationship may be the “hygiene hypothesis.” This hypothesis was originally proposed by Strachan [35], who found that early sibling infections were associated with a decrease in future autoimmune diseases or allergies. Based on this hypothesis, some of the infectious agents that grow with us may be able to prevent the development of a range of immune-related diseases [36]. The clearance of H. pylori has been shown to lead to a disturbance of the intestinal flora of the colon [37]. A study showing the therapeutic effect of Schistosoma mansoni and Ancylostoma caninum soluble proteins on experimental colitis sidesteps this hypothesis [38]. Thus, the inverse association observed between H. pylori infection and MC may be attributed to that H. pylori acts as a surrogate marker of a commensal flora that reduces the occurrence of MC. However, no studies on MC are currently available and further validation of this hypothesis is needed in the future (Table 1).
Similarities and differences in the genetic susceptibility and immunology of LC and CC
Although CC and LC are covered under the umbrella term MC and are similar in many aspects such as clinical presentation and prognosis, there are still some essential differences and hence the two diseases should be perceived as separate entities. They are distinguished by histological findings in pathological biopsies, and otherwise have marked distinctions at the level of immunology and susceptibility genes [10, 28, 39–43]. These molecular and cellular aspects may have contributed to their dissimilar relationship with other gastrointestinal diseases. Understanding these distinctions is consequently mandatory to appreciate the differences that may arise between CC and LC in this interaction. The similarities and differences in immunology and genetic susceptibility between them are synthesized in Table 2.
Table 2.
Similarities and differences between CC and LC in terms of immunological profile and genetic susceptibility
| Subtypes of MC | Immunological profile |
Genetic predisposition | References | |||||
|---|---|---|---|---|---|---|---|---|
| Chemokine and receptor | Cytokine | Prostaglandin | Growth factor | T-lymphocyte | Others | |||
| CC and LC | ↑: CXCL8, CXCL9, CXCL10, CXCL11, CCL2, CCL3, CCL20, CX3CL1, CX3CL2, CXCR1, CXCR2 | ↑: TNF-α mRNA, IFN-γ mRNA, IL-17-A mRNA, IL-10, IL-21, IL-23 | ↑: COX-2 | – | ↑: Th1/Tc1, Th17/Tc17, Ki67+ T, CD45RO+ T, FoxP3+ Treg | ↓: TRECs | – | [10, 39–42] |
| CC | ↑: CXCR3, CX3CR1, CCR3, CCR5, CCR7, CCR8, CCR10 | ↑: IL-6 | – | ↑: VEGF, CTGF, TGF-β1, bFGF | – | ↑: TIMP1, CfB, miR-31, T-bet | HLA haplotype 8.1 | [10, 28, 39, 40, 42, 43] |
| ↓: CXCL5, CXCL7, CXCL8, CXCL9, CXCL12, CXCL13, XCL1, CCL7, CCL8, CCL16 | ||||||||
| LC | ↑: CXCL11, CXCL8, CCL3, CCL5 | ↑: IL-15 mRNA | – | – | ↓: CD4+T, CD4+CD8+T, CD4-CD8-T; | ↑: T-bet/GATA-3. | – | [10, 42] |
| ↑: CD8+T, CD4+γδ +T | ↓: TcRβ V-J: eveness, diversity | |||||||
↑: increased indicator level; ↓: decreased indicator level; –, not mentioned; CC, collagenous colitis; LC, lymphocytic colitis; MC, microscopic colitis; CXCL, C-X-C motif chemokine ligand; CCL, chemokine c-c motif ligand; CX3CL, C-X-3-C motif chemokine ligand; CXCR, C-X-C motif chemokine receptor; TNF, tumor necrosis factor; IFN, interferon; IL, interleukin; COX, cyclooxygenases; Th, helper T-cell; CD, Cluster of Differentiation; FoxP3, forkhead box protein P3; Treg, regulatory T-cell; TREC, T-cell receptor excision circle; CX3CR, C-X-3-C motif chemokine receptor; CCR, C-C chemokine receptor; XCL, X-C motif chemokine ligand; VEGF, vascular endothelial growth factor; CTGF, connective tissue growth factor; TGF, transforming growth factor; bFGF, basic fibroblast growth factor; TIMP, tissue inhibitor of metalloproteinase; CfB, complement factor B; miR, miRNA; HLA, human leukocyte antigen; T-bet, T-box transcription factor; GATA, a class of transcriptional regulators that normally recognize the consensus sequence WGATAR (W = T or A; R = G or A); TcRβ V-J, T-cell antigen receptor beta chain variable-J.
The interrelationship between MC and IBD
New onset of IBD after MC diagnosis
Status summary of the MC-to-IBD transition
When a diagnosis of either LC or CC is established, a small proportion of patients may subsequently develop IBD, irrespective of whether it presents as Crohn's disease (CD) or ulcerative colitis (UC). However, the overwhelming majority of these reports are case reports or case series, with three of them reporting the transformations of CC to UC [44–46], four reporting the transformations of CC to CD [47–50], and one reporting the transformation of LC to UC [49]. Apart from these scattered cases, only a single recent cohort study [4] and a recent case–control study [51] have examined this conversion relationship. The cohort study was conducted as a nationwide prospective study in which researchers included 13,957 patients with MC and found a remarkable association of MC with IBD, with an adjusted hazard ratio of 12.6 (95% CI, 8.8–18.1) for CD, 17.3 (95% CI, 13.7–21.8) for UC, and 16.8 (95% CI, 13.9–20.3) for IBD, and when comparing patients with MC with their non-affected siblings, they were still at significant risk of developing IBD. Another nationwide case–control study that included 15,597 patients with MC explored the relationship between 16 autoimmune diseases and MC, and found a significant association with MC and UC as well as CD, along with a higher risk of UC. Another study examining the link between MC and IBD based on clinical and pathological features revealed that the interval required for progression from CC or LC to IBD was relatively short, with an average of 14 months [52]. In this direction of disease evolution, the mean age of new-onset IBD patients is 66.5 years, which is inconsistent with the usual age of onset of IBD. These findings may suggest that MC may be the initial manifestation in a proportion of older patients with IBD, as an attenuated form of IBD undergoing transition and transformation.
Possible mechanisms involved in the MC-to-IBD conversion
Genetic overlap
In the genome-wide association study (GWAS) carried out in UK Biobank [53], the researchers used single nucleotide polymorphisms (SNPs) to calculate genetic risk scores (GRS) for IBD and its two phenotypes, CD and UC, to investigate the possible genetically related overlap between MC and IBD, and to compare their mean GRS to MC and controls without any related disease, respectively, comparisons. The results presented using the OR and 95% CI found genetic overlap between MC and both CD (P = 0.035) and IBD (P = 0.019) but failed to find a statistically significant higher genetic risk in UC (P = 0.261). A further genetic association study yielded 15 pleiotropic signals and these results identified a common genetic link between CC and IBD and celiac disease [54]. Similarly, another systematic gene discovery study also documented the genetic overlap between CC and IBD and its two subtypes, and suggested that there may be shared genetic risk loci between CC and IBD, rather than resemblance in the human leukocyte antigen (HLA) region [55]. The above genetic correlation studies provide a scientific basis for possible mechanisms of interaction between MC and IBD at the level of genetic susceptibility (Table 3).
Table 3.
Association of MC (both CC and LC) with genetic aspects of IBD (both CD and UC) and celiac disease
| Genetically linked diseases | Author, year | Country | Study design | Study cohort | Detection methods | Genetic linkage to MC (both CC and LC) | P-value |
|---|---|---|---|---|---|---|---|
| MC and IBD | Green et al. [53], 2019 | UK | GWAS | 483 MC cases and 450,616 controls | Genetic risk scores calculated using ORs for previously published SNPs | Mean (95% CI) score of 0.9286 (0.9237–0.9335) for patients with MC; 0.9230 (0.9229–0.9231) for controls | 0.019 |
| MC and CD | Mean (95% CI) score of 0.9688 (0.9632–0.9739) for patients with MC; 0.9634 (0.9632–0.9636) for controls | 0.035 | |||||
| CC, CD, and UC | Stahl et al. [54], 2021 | US | Genetic association study | 84,922 SNPs for 804 CC cases, 11,700 celiac disease cases, 17,342 CD cases, 13,436 UC cases, and 27,101 controls | Using ASSET and CPBayes to identify loci with common genetic effects | rs6702421 chr1:195651049 near CRB1, DENND1B; rs1981525 chr5:131699561 near SLC22A4/5, and rs10114531 chr9:4988855 near INSL4/6, TAK2 | NA |
| CC, celiac disease, and CD | rs10806425 chr6:90868580 near BACH2, rs9482850 chr6:128307943 near PTPRK, and rs1250566 chr10:80702538 near ZMIZ1 | ||||||
| CC, celiac disease, and UC | rs4142969 chr6:138003826 and rs6927172 chr6:138000928 | ||||||
| CC and celiac disease | rs4525910 chr3:161108490 near IL12A, rs653178 chr12:110492139 near ATXN2, and rs516246 chr19:53897984 near RASIP1 | ||||||
| CC, celiac disease, CD, and UC | rs12131796, chr1:199137377 near KIF21B; rs12656877, chr5:141418152 near NDFIP1, rs56086356 chr11:127881686 near ETS1, and rs243317 chr16:11254549 near PRM1/2/3, SOCS1, and TNP2 | ||||||
| CC and IBD | Westerlind et al. [55], 2017 | Germany, Sweden | Genetic association study | 314 patients with CC and 4,299 controls from three separate North European cohorts | Retrieval of summary statistics from the GWAS/Immunochip meta-analysis from the IIBDGC and comparison with the CC Immunochip summary statistics from the current study to perform the validated SECA | Significant associations of IBD/CD/UC risk loci in CC: rs2930047, rs3851228, rs6920220, rs9297145, rs6651252, rs10761659, rs1250546, and rs1893217 | <0.001 |
| CC and CD | <0.001 | ||||||
| CC and UC | <0.01 | ||||||
| CC and celiac disease | Fernández-Bañares et al. [86], 2005 | Spain | Genetic association study | 25 patients with LC, 34 with CC, and 70 healthy controls | HLA-DQ2 and HLA-DQ8 were investigated in patients with MC using PCR-SSP | 24.3% healthy controls were DQ2-positive, 32.3% with CC were DQ2-positive | 0.38 |
| LC and celiac disease | 24.3% healthy controls were DQ2-positive, 48% patients with LC were DQ2-positive | 0.027 | |||||
| MC and celiac disease | Fine et al. [85], 2000 | US | Genetic association study | 53 patients with MC and 429 normal controls | Serological cytotoxicity methods and PCR based DNA sequence-specific primer methods to detect HLA typing | HLA-DQ2 or DQ1,3 is more common in patients with MC compared with controls | <0.02 |
MC, microscopic colitis; CC, collagenous colitis; LC, lymphocytic colitis; IBD, inflammatory bowel disease; CD, Crohn's disease; UC, ulcerative colitis; GWAS, genome-wide association study; OR, odds ratio; SNP, single nucleotide polymorphism; CI, confidence interval; ASSET, Association analysis of SubSETs; CPBayes, Cross-Phenotype Bayesian meta-analysis approach; NA, not available; IIBDGC, the International Inflammatory Bowel Disease Genetics Consortium; SECA, SNP effect concordance analysis; HLA, human leukocyte antigen; PCR, polymerase chain reaction; SSP, sequence-specific primer.
Involvement of immunological profiles
Within the immunological landscape of MC (both CC and LC), two main types of T-cells are involved. One type is the regulatory T-lymphocytes (Treg), which control the immune responsiveness of the body (also called suppressor T-lymphocytes). In the intestinal mucosa of CC and LC, both CD4+CD25+FOXP3+ Treg and non-Treg and CD4+CD25-FOXP3+ T-cells are shown to be upregulated and the latter has also been shown to be associated with both regulatory and immunosuppressive effects [39]. Treg-secreted IL-10, known for its function in triggering immunosuppression, is also increased in expression in patients with CC and LC [39]. The second group of T-cells, Th1 and Th17 lymphocytes, are also involved in the immune response as helper T-cells. Th1 secretes mainly interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), whereas Th-17 secretes primarily IL-21, IL-22, and IL-17-A. Both cytokines have a role in promoting inflammation and their increment reflects the clinical activity and severity of inflammation [40]. In both CC and LC, mRNA levels of cytokines increased by flow cytometry, while no elevation of Th1 or Th17 at the cellular level or protein level was found [39].
A 14-year study including 2,324 patients with MC identified 20 cases of conversion to IBD [56]. Comparing the immunological profiles of 13 “IBD transformers” with 22 MC regressions, researchers noticed that IFN-γ, TNF-α, and the specific transcription factor T-bet for Th1 were increased in the transformers. Notably, these immune-related factors were all associated with Th1. This intriguing result indicates that in some subgroups of patients with MC, there is a spread of their pre-existing inflammation. One possible explanation is that in this subgroup, the anti-inflammatory effect of IL-10 fails, thus allowing Th1 to deregulate and thereby proliferate, secreting more pro-inflammatory factors, and leading to increased inflammation with consequent disease transformation [57].
Disturbed intestinal microflora
Substantial alterations in the composition and function of the gut microbiota have been found in both MC and IBD, and this microbiota disruption affects the respective pathophysiological courses of both diseases and is involved in the pathogenesis underlying each [58–60].
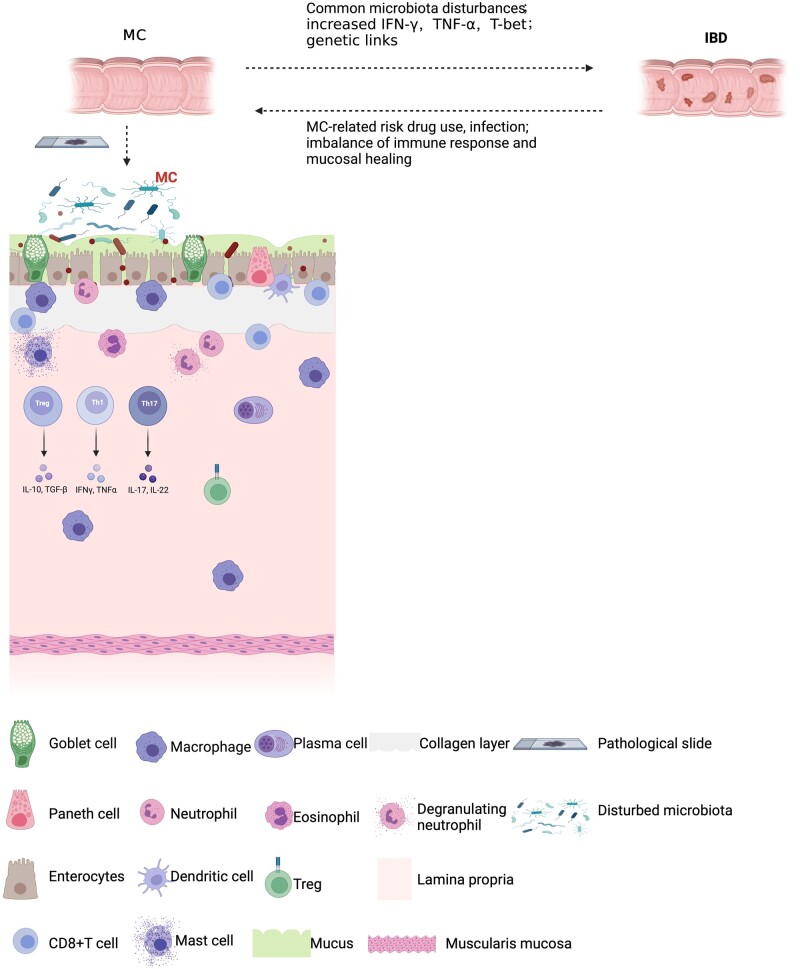
In fecal samples from patients with active MC, there was a significant decrease in biodiversity, known as alpha diversity [61]. This decrease in species richness of microorganisms was also found in patients with IBD [62] and was more prominent in patients with CD [63]. Another study comparing the microbiota of stool samples from patients with CC and IBD using Taxa-specific analysis revealed a decrease in the abundance of 10 operational taxonomic units regarding the Ruminococcaceae family in patients with active CC or on continuous corticosteroid treatment [64]. Nine of these species were also observed in patients with CD and four had a consistent decrease in abundance in patients with UC (Figure 1).
Figure 1.
A graphical summary of the conversion of MC and IBD to each other. MC and IBD share some intestinal dysbiosis, increased pro-inflammatory factors such as IFN-γ and TNF-α in the conversion of MC to IBD, and common genetic effects between the two. The transformation of IBD to MC may then be triggered by certain risk factors such as MC-related drug use, infection, and mechanistically may be due to an imbalance of the immune response and mucosal repair. MC, microscopic colitis; IBD, inflammatory bowel disease; IFN, interferon; TNF, tumor necrosis factor; Treg, regulatory T-cell; T-bet, T-box transcription factor; IL, interleukin; TGF, transforming growth factor.
New-onset MC after IBD diagnosis
Status summary of IBD-to-MC transition
A fair number of case reports or case series have reported that a small proportion of patients with IBD, including CD and UC, converted to CC or LC after treatment [49, 50, 65–70]. In fact, patients with IBD often experience symptoms such as diarrhea during the remission period after drug maintenance therapy, which may be confused with a relapse of IBD [65]. A retrospective observational study reported a possible shift to MC in 2.6% of patients with IBD [50]. Transformation is typically temporary and can be clinically dissipated by treating MC. In contrast to the disease transition from MC to IBD, it usually takes >10 years from the diagnosis of IBD to the detection of new-onset MC, and the average age of MC patients is usually younger [52]. This trend seems to suggest that new-onset MC patients are a continuation of IBD in the quiescent phase.
Risk factors and possible mechanisms
Proposed risk factors for the emergence of emerging MC may be medication use and infections [65]. In some case reports, a proportion of patients have used drugs that can be linked to the development of MC, such as NSAIDs, PPIs, and statins, and one case report even described a case of new-onset MC following high-dose chemotherapy drugs and autologous stem cell transplantation [71]. However, not all patients have a history of taking the relevant risk medication and a case series did not find an association between drug use and new-onset MC [67]. A second potential cause relates to infection, particularly C. difficile infection. Some patients have been reported to present with MC after refractory C. difficile infection [65], one of whom was treated with fecal microbial transplantation after C. difficile infection and eventually identified the presence of MC [69].
Little is known about the mechanisms underlying the onset of MC in patients with IBD in remission. The coexistence of some autoimmune diseases such as autoimmune thyroid disease and diabetes mellitus in some patients prior to the onset of MC seems to point to the presence of an inappropriate immune response in some affected individuals [67, 72]. During the remission period of IBD, the intestinal mucosa is in a healing state and certain risk factors such as drug use and infections may lead to an aberrant response of the intestinal mucosa to these factors, and this imbalance between the immune response and mucosal repair could be responsible for the development of MC (Figure 1).
Management aspects in interaction with IBD
Clinicians should investigate the possibility of IBD when a patient with confirmed MC has not improved over a relatively long duration after standard treatment or has demonstrated more frequent inflammatory activity. This condition may involve very extensive lesions in some patients and therefore sufficient regard should be given to this unusual presentation [45, 67]. Furthermore, incidental detection of frequent episodes of watery diarrhea in patients with IBD in remission after decades with possible risk medications or co-infection requires vigilance for new onset of MC. Meanwhile, the suspicion of a new development should be confirmed by endoscopic observation and multiple biopsies.
Relationship between MC and other lymphocytic disorders of the gastrointestinal tract
Summary of the association of MC with other lymphocytic disorders of the gastrointestinal tract
About 14% of patients with MC are affected by other concurrent gastrointestinal lymphocytic disorders, including celiac disease, duodenal intraepithelial lymphocytosis (DIL), lymphocytic gastritis (LyG), and lymphocytic esophagitis (LyE) [73]. Coeliac disease is the most common concomitant autoimmune disorder in patients with MC [74] and there is an equally bidirectional relationship that can be established between the two entities. The latest case–control study revealed a >10-fold risk of celiac disease in patients with MC compared with the general population (OR = 10.15; 95% CI, 8.20–12.6) [51]. The conclusion is similar to those of other population-based and epidemiological studies [73, 75–78]. A meta-analysis summarized the prevalence of each of these two diseases in refractory cases [79]. In patients with refractory coeliac disease, the prevalence of MC was 4.5% and similarly in patients with refractory MC, the prevalence of coeliac disease was 6.7%. These results indicate an overlap of the two diseases. In patients with coeliac disease complicating MC, the individuals are usually more elderly and exhibiting significantly more duodenal mucosal atrophy [75] whereby in patients with MC presenting with concomitant coeliac disease, the patients are younger than in those without co-morbidity [73]. LyG is a histopathological pattern characterized by lymphocytosis within gastric epithelium [80]. Whereas it is not a specific disease group, it is usually seen in coincidence with other lymphocytic disorders and is closely associated with MC [73, 81]. A cross-sectional study including 3,038 patients with LyG reported that 19% of patients can develop MC as a co-morbidity [82]. DIL is the most common lymphocytic disorder of the gastrointestinal tract. Although only a small proportion of patients with DIL may co-morbidly develop MC, pathological evidence has suggested that it may manifest in ≤8% of those with MC [73].
Possible shared etiological linkages
Shared genetic effects
Celiac disease is associated with genotypes such as HLA-DQ2 and DQ-8 (mainly HLA-DQ2) [83, 84]. In one study, the authors found an increase in both HLA-DQ2 and DQ-8 in celiac disease and MC compared with normal controls [85]. Another study employing polymerase chain reaction amplification using sequence-specific primers (PCR-SSP) found an increase in HLA-DQ2 in LC compared with controls, but not in CC [86]. Further confirmation of the overlap between the two diseases at the genetic level was provided by a recent study that identified 15 polygenic pleiotropic associations (using SNPs) for CC, UC, CD, and celiac disease [54]. These have demonstrated that celiac disease and MC do present a joint genetic risk. The shared immunogenic molecules allow a possible link between the two etiologically (Table 3).
Resembling mucosal immunological profiles
Celiac disease is a T-cell-mediated immune disorder mainly induced by gluten [87]. As a multifactorial disease, immune-related factors are central to the pathogenesis. In terms of immunopathogenesis, exposure to dietary antigens is presented to T-cells by the antigen-presenting cells (APCs) of the body, which induce intraepithelial cytotoxic CD8+ γδ, αβ T-cells to migrate to the targeted locations resulting in inflammation and injury [88, 89]. Another essential component is the involvement of helper T-cells in the lamina propria, mainly Th1, Th17, and regulatory T-cells, which differentiate and respectively produce cytokines to mediate inflammation via engagement with the APC [90–92]. Previously referred to as an increase in the immunological profile of the Th1-associated cytokine IFN-γ in patients with MC, similar alterations were demonstrated in celiac disease [93]. Also, E-cadherin was found to be significantly reduced around inflammation in celiac disease [94], which was paralleled by MC [93]. This resemblance in mucosal cytokines may explain the possible common pathophysiological mechanisms that both share in lymphocytic disorders.
Impact on clinical management decisions
In some patients with MC coupled with coeliac disease, a gluten-free diet may be able to mitigate disease progression and decrease medication for MC [95]. Coeliac disease and MC share numerous clinical manifestations, with diarrhea being the predominant symptom. The diagnosis of coeliac disease in most patients with MC is likely to be due to a lack of response to medication or the presence of persistent diarrhea and the suspicion of other conditions, and vice versa in patients with coeliac disease [75]. Therefore, special attention needs to be drawn to possible complications in these populations. A bidirectional approach to endoscopy is also required in refractory cases when necessary to establish the diagnosis to help avoid a missed presentation. Notably, in patients with MC, intraepithelial lymphocytosis of the duodenum is occasionally observed, which may be analogous to the presentation of celiac disease [96]. Similarly in patients with celiac disease, intraepithelial lymphomatosis of the colon may also be expected in the absence of LC [97]. These highlight the relevance of random biopsy and meticulous monitoring.
Overlap of MC and IBS
Irritable bowel syndrome-like symptoms in patients with MC
IBS is the most common form of functional gastrointestinal disorder (FBD) with abdominal pain and altered bowel habits as the main clinical manifestations [98, 99]. It is characterized by the absence of any abnormalities on clinical examination and the current diagnostic criteria are the Rome IV criteria [100]. IBS can be divided into diarrhea-predominant IBS (IBS-D), constipation-predominant IBS (IBS-C), and IBS-mixed (IBS-M) on the basis of the number of days on which the predominant bowel habit is present in the abnormal stool pattern [101]. IBS-D presents clinically with prolonged recurrent abdominal pain and diarrhea, and similarly MC has diarrhea as the main symptom, and some patients also have abdominal pain.
There is a significant overlap regarding symptoms between the two. As the diagnosis of a patient with IBS requires the exclusion of the presence of organic gastrointestinal disease, the condition seen in MC should be referred to as IBS-like symptoms. IBS-like symptoms are not uncommon in patients with MC. Many studies have reported the prevalence of symptoms that meet the diagnostic criteria for IBS in patients with MC [102–106] whereas the prevalence varies between studies; two systematic reviews and meta-analyses have concluded that IBS-compatible symptoms are found in approximately one-third of patients with MC [14, 107]. Besides the typical abdominal pain and diarrhea, these symptoms include psychological abnormalities such as anxiety and depression [103, 108]. Compared with those without IBS-like symptoms, the quality of life of this group is more compromised and the gastrointestinal symptoms are more frequent and severe in individuals with MC [108–111]. These patients are also younger and more likely to be female [104]. Smoking may contribute to the increased risk of developing IBS-like symptoms [112].
Detection of MC in patients with IBS
A proportion of patients with confirmed IBS are found to have pathological MC on screening colonoscopy and biopsy [113–118]. The prevalence of MC in FBD is at ∼7%, while in IBS-D, MC can be detected at 9.8% [14]. IBS-D is the type of IBS in which MC is most frequently detected, while IBS-C is rarely found [119, 120]. MC were typically detected predominantly in older women [119]. This possible overlap has also appeared to be controversial, with one meta-analysis showing that the OR of MC in patients with IBS did not reach statistical significance when compared with other patients with diarrhea [107]. However, as there are so few relevant available studies, this relationship may require more consideration. An evidence-based research has shown that the characteristics of individuals including being >50 years old, nocturnal stools, weight loss, duration of diarrhea for <12 months, and medication use are associated with increased risk of MC, and that comorbid immune disorders are also a risk factor for MC [121]. The prevalence of organic gastrointestinal disease is higher when alert symptoms are present in IBS than in its absence, but organic gastrointestinal disease may still be found in one in six patients in the population without alert features [122]. Strikingly, one-third of patients with IBS may have delayed treatment due to misdiagnosis [105] and it is estimated that 25% of patients with MC fail to receive timely biopsies in the IBS population [120].
Management implications regarding the diagnostic overlap of MC and IBS
Management of IBS-like symptoms in MC patients is a critical issue given that one-third of MC patients develop IBS-like symptoms. Anxiety and depression as psychological disorders impair the quality of life of patients with MC and therefore the administration of psychological medications such as 5-hydroxytryptamine reuptake inhibitors may improve the prognosis of MC patients and reduce the burden of the disease. This overlap in symptoms can have a noticeable impact on the treatment of MC [103].
The detection of possible MC in patients with IBS, especially in patients with IBS-D, is paramount as it may prevent incorrect medication use and the progression of MC leading to a reduced quality of life for the patient [123]. Concerning the necessity for colonoscopy and tissue biopsy in patients with IBS because of a suspected diagnosis of MC is an issue worth noting and weighing up. On the one hand, excessive invasive testing would increase potentially unnecessary costs and lead to a psychological burden on the patient and reinforcement of the associated symptoms [14]. Hence, the diagnosis of IBS is still largely based on clinical data and simple diagnostic techniques, and colonoscopy is only performed if suspicious symptoms of organic diseases are present. On the other hand, possibly under-diagnosed MC can delay treatment because a definitive diagnosis is not carried out. As the prevalence of MC is not higher in patients with IBS than that in other diarrheal populations, it seems justified to assume that routine colonoscopy is not necessary [107]. Colonoscopy and biopsy to rule out and diagnose possible MC are indicated in patients with IBS when factors that may suggest an increased risk of MC are identified. Persistent diarrhea in older women, for example, warrants prompt colonoscopy as well as biopsy to confirm the presence of MC. The clinical background and demographic characteristics of the patient may also reduce the incidence of misdiagnosis in patients with IBS [124]. Several biomarkers including NGAL/LCN2 [125] and fecal calprotectin [126] may also play a role in the differential diagnosis of IBS and MC. However, the discriminatory value of these markers is questionable and fecal calprotectin was not associated with IBS-like symptoms in a cross-sectional study conducted by Pagoldh et al. [102]. Finally, both IBS and MC patients can be triggered by infection. Post-infectious IBS can occur in >10% of patients following infectious enterocolitis [127] and gastrointestinal infections are also a possible causative factor for MC. Therefore, MC detected in patients with IBS or IBS-like symptoms presented in patients with MC can be triggered by infection and this possibility needs to be clarified in clinical practice.
Association of MC and colorectal neoplasia
Possible reduced risk of colorectal cancer and precancerous conditions
The relationship between MC as a chronic inflammatory disease of the intestine and colorectal cancer (CRC) and precancerous lesions has been investigated (Table 4). Other intestinal chronic inflammatory diseases such as IBD [128–130] and celiac disease [131, 132] have been shown to increase the risk of CRC, although the risk of CRC in coeliac disease appears to be disputed [133, 134]. Paradoxically, the prevalence of CRC and its precancerous lesions may not only not increase in patients with MC [106, 135–137] but has been demonstrated in a notable number of studies to reduce the risk of CRC [138–144], which is a noteworthy protective effect. Cancer-related deaths were also lower in patients with MC than in matched controls [145].
Table 4.
Studies on the association of MC (including CC and LC) with colorectal neoplastic lesions
| Type of MC | Author, year | Country | Study design | Patient cohort | Age, mean ± SD, years | Colorectal neoplastic lesion type | Effect size (95% CI) | Adjustment factors |
|---|---|---|---|---|---|---|---|---|
| MC | Borsotti et al. [138], 2021 | Italy | Prospective cohort study | 43 (28 CC; 15 LC) | 60 ± 16 | Colorectal neoplasia | OR: 0.39 (0.22–0.67) | Age, gender |
| MC | Weimers et al. [139], 2021 | Denmark | Nationwide cohort study | 14,302 (8,437 CC; 5,865 LC) | 65 ± 14 | CRC | RR: 0.47 (0.38–0.59) | Charlson co-morbidity index |
| MC | Bergman et al. [140], 2020 | Sweden | Population-based cohort study | 11,758 (3,734 CC; 8,024 LC) | 59 ± 17 | CRC | HR: 0.52 (0.40–0.66) | Age, sex, county of residence, calendar year, education level, diabetes, coeliac disease |
| MC | Levy et al. [135], 2019 | US | Retrospective cohort study | 221 (112 CC; 109 LC) | 65.7 ± 15.4 | Colorectal neoplasia | OR: 1.07 (0.69–1.66) for tubular adenoma; 1.26 (0.17–9.42) for villous adenomaa | Age, gender, smoking, BMI |
| MC | Sonnenberg et al. [144], 2015 | US | Case–control study | 11,176b | 64.2 ± 13.8 | Colon polyps | OR: 0.46 (0.43–0.49) for hyperplastic polyps; 0.24 (0.19–0.30) for serrated adenomas; 0.35 (0.33–0.38) for tubular adenomas | Age, sex |
| MC | Tontini et al. [143], 2014 | Italy | Prospective cohort study | 43 (30 CC; 13 LC) | 67 ± 15 | Colorectal neoplasia | OR: 0.22 (0.05–0.97) | NA |
| MC | Yen et al. [142], 2012 | US | Case–control study | 647 (281 CC; 386 LC) | 68.0 ± 14.7 | CRC and adenoma | OR: 0.34 (0.16–0.73) for CRC; 0.52 (0.39–0.69) for colorectal adenoma | Family history of CRC, tobacco use, alcohol use, BMI |
| MC | Kao et al. [106], 2009 | US | Retrospective analysis | 547 (171 CC; 376 LC) | 61.7c | CRC | NAd | NA |
| CC | Chan et al. [137], 1999 | US | NAe | 117 | 61 ± 13 | CRC | NAd | Age, race, sex, calendar time |
| CC | Larsson et al. [141], 2019 | UK; Sweden | Two-stage observational study | 738; 1141 | 68 (58–77); 67 (57–76)f | Colon cancer | SIR: 0.23 | Year of onset, sex, age group |
| CC | Bonderup et al. [136], 1999 | Denmark | Retrospective follow-upg | 24 | NA | CRC | NAd | NA |
MC, microscopic colitis; CC, collagenous colitis; LC, lymphocytic colitis; SD, standard deviation; CI, confidence interval; OR, odds ratio; CRC, colorectal cancer; SD, standard deviation; RR, relative risk; HR, hazard ratio; BMI, body mass index; NA, not available; SIR, standardized incidence ratio.
Compared with the general US population, MC was not associated with an increased risk of CRC in either men (SIR 1.59, 95% CI 0.27–5.24) or women (SIR 1.97, 95% CI 0.86–3.89).
No numbers reported for CC and LC respectively.
No SD reported.
All these studies did not report cases of CRC.
A study reporting on the incidence of cancer in CC.
The interquartile range (IQR) is reported instead of SD.
A retrospective follow-up of patients with CC diagnosed during 1979–1990.
Possible factors and mechanisms explaining the protective effect
First, as the diagnosis of MC requires colonoscopy, the increased activity in the utilization of colonoscopy may result in an improved detection rate of early lesions in CRC, which is a protective effect from colonoscopy [146]. Second, medications such as NSAIDs and statins that may be prescribed in patients with MC have a preventive effect on the development of CRC [147, 148]. In addition, body mass index (BMI) is relatively low in patients with MC and high BMI is positively associated with the development of CRC, thus lowering the risk of CRC [139]. Furthermore, the increased intestinal intraepithelial lymphocytes of the MC enhance immune surveillance and thus reduce the prevalence of CRC. This form of immune surveillance is primarily engaged by T-lymphocytes in the epithelium; when carcinogenic antigens are present in the gut, it first activates the NKG2d receptor on natural killer cells, which in turn can activate γδ T-cells to kill cells in the presence of DNA damage and stress [142]. Such T-cells are essential for the clearance of mutant and abnormal cells [138]. The absence of γδ T-cells leaves the mice model vulnerable to epithelial tumors, suggesting a protective role for the T-cells in immune surveillance [149]. Finally, a comparable process of carcinogenesis may not have been present in MC as in IBD. NF-κB, a transcription factor for inducible nitric oxide synthase (iNOS), may mediate the mucosal damage caused by colonic inflammation. Over-activation is found in both MC and IBD, whereas in MC it is confined to the intestinal epithelium and in UC it spreads to macrophages in the lamina propria [150]. This indicates that chronic inflammation in MC is not as severe as in IBD and may not lead to epithelial damage and dysfunction [138]. Chronic inflammation can be responsible for cancer development through DNA damage but the severity of inflammation in MC, which is not as significant as in IBD, probably does not activate the relevant carcinogenic pathways [135, 151].
Conclusions
As a relatively recently understood immune-mediated chronic inflammatory disease of the large intestine, MC is associated with several gastrointestinal disorders. MC may interact with classical IBD as an attenuated form of IBD and the two may be convertible. Genetic, immunological, and gut microbiological factors may be involved in this process. Both MC and celiac disease, as lymphocytic disorders, can occur in conjunction with each other. Clinical misdiagnosis is possible between MC and IBS due to the similarity of symptoms and diagnostic overlap. MC is likely to be detected in patients with IBS and should be ruled out by colonoscopy and biopsy when suspicious symptoms point to MC, and IBS-like symptoms in patients with MC should be addressed to improve their quality of life. Patients with MC have a reduced risk of CRC and colonic adenoma, and screening for CRC in individuals with MC is therefore not required. Gastrointestinal infections and increased risk of MC are associated. Clostridiumdifficile and C. concisus infections significantly increase the risk of MC, whereas H. pylori demonstrates an inverse relationship.
Authors’ Contributions
Y.L. proposed the idea for the article, carried out the literature search, and wrote the manuscript, as well as prepared the illustrations and tables. M.C. revised the manuscript as the corresponding author and provided comments. All authors read and approved the final version of the manuscript.
Funding
None.
Acknowledgements
None.
Conflict of Interest
None declared.
References
- 1. Burke KE, D'Amato M, Ng SC. et al. Microscopic colitis. Nat Rev Dis Primers 2021;7:39. [DOI] [PubMed] [Google Scholar]
- 2. Tome J, Kamboj AK, Pardi DS.. Microscopic colitis: a concise review for clinicians. Mayo Clin Proc 2021;96:1302–8. [DOI] [PubMed] [Google Scholar]
- 3. Verhaegh BPM, Münch A, Guagnozzi D. et al. Course of disease in patients with microscopic colitis: a European prospective incident cohort study. J Crohns Colitis 2021;15:1174–83. [DOI] [PubMed] [Google Scholar]
- 4. Khalili H, Burke KE, Roelstraete B. et al. Microscopic colitis and risk of inflammatory bowel disease in a nationwide cohort study. Gastroenterology 2020;158:1574–83.e2. [DOI] [PubMed] [Google Scholar]
- 5. Maye H, Safroneeva E, Godat S. et al. Increasing incidence of microscopic colitis in a population-based cohort study in Switzerland. Clin Gastroenterol Hepatol 2021;19:2205–6. [DOI] [PubMed] [Google Scholar]
- 6. Tome J, Sehgal K, Kamboj AK. et al. The epidemiology of microscopic colitis in Olmsted County, Minnesota: population-based study from 2011 to 2019. Clin Gastroenterol Hepatol 2021;S1542-3565(21)00691-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weimers P, Ankersen DV, Lophaven S. et al. Incidence and prevalence of microscopic colitis between 2001 and 2016: a Danish nationwide cohort study. J Crohns Colitis 2020;14:1717–23. [DOI] [PubMed] [Google Scholar]
- 8. Bergman D, Clements MS, Khalili H. et al. A nationwide cohort study of the incidence of microscopic colitis in Sweden. Aliment Pharmacol Ther 2019;49:1395–400. [DOI] [PubMed] [Google Scholar]
- 9. Pardi DS, Kelly CP.. Microscopic colitis. Gastroenterology 2011;140:1155–65. [DOI] [PubMed] [Google Scholar]
- 10. Zabana Y, Tontini G, Hultgren-Hörnquist E. et al. Pathogenesis of microscopic colitis: a systematic review. J Crohns Colitis 2021;16:143–61. [DOI] [PubMed] [Google Scholar]
- 11. Khalili H, Axelrad JE, Roelstraete B. et al. Gastrointestinal infection and risk of microscopic colitis: a nationwide case-control study in Sweden. Gastroenterology 2021;160:1599–607.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nielsen HL, Dalager-Pedersen M, Nielsen H.. High risk of microscopic colitis after Campylobacter concisus infection: population-based cohort study. Gut 2020;69:1952–8. [DOI] [PubMed] [Google Scholar]
- 13. Rizzo AG, Orlando A, Gallo E. et al. Is Epstein-Barr virus infection associated with the pathogenesis of microscopic colitis? J Clin Virol 2017;97:1–3. [DOI] [PubMed] [Google Scholar]
- 14. Guagnozzi D, Arias Á, Lucendo AJ.. Systematic review with meta-analysis: diagnostic overlap of microscopic colitis and functional bowel disorders. Aliment Pharmacol Ther 2016;43:851–62. [DOI] [PubMed] [Google Scholar]
- 15. Jaruvongvanich V, Poonsombudlert K, Ungprasert P.. Smoking and risk of microscopic colitis: a systematic review and meta-analysis. Inflamm Bowel Dis 2019;25:672–8. [DOI] [PubMed] [Google Scholar]
- 16. Niccum B, Casey K, Burke K. et al. Alcohol consumption is associated with an increased risk of microscopic colitis: results from 2 prospective US cohort studies. Inflamm Bowel Dis 2021;26:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verhaegh BP, de Vries F, Masclee AA. et al. High risk of drug-induced microscopic colitis with concomitant use of NSAIDs and proton pump inhibitors. Aliment Pharmacol Ther 2016;43:1004–13. [DOI] [PubMed] [Google Scholar]
- 18. Weimers P, Vedel Ankersen D, Lophaven SN. et al. Microscopic colitis in Denmark: regional variations in risk factors and frequency of endoscopic procedures. J Crohns Colitis 2021;16:49–56. [DOI] [PubMed] [Google Scholar]
- 19. Bonderup OK, Fenger-Grøn M, Wigh T. et al. Drug exposure and risk of microscopic colitis: a nationwide Danish case-control study with 5751 cases. Inflamm Bowel Dis 2014;20:1702–7. [DOI] [PubMed] [Google Scholar]
- 20. Mori S, Kadochi Y, Luo Y. et al. Proton pump inhibitor induced collagen expression in colonocytes is associated with collagenous colitis. World J Gastroenterol 2017;23:1586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Axelrad JE, Cadwell KH, Colombel JF. et al. Systematic review: gastrointestinal infection and incident inflammatory bowel disease. Aliment Pharmacol Ther 2020;51:1222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Erim T, Alazmi WM, O'Loughlin CJ. et al. Collagenous colitis associated with Clostridium difficile: a cause effect? Dig Dis Sci 2003;48:1374–5. [DOI] [PubMed] [Google Scholar]
- 23. Byrne MF, McVey G, Royston D. et al. Association of Clostridium difficile infection with collagenous colitis. J Clin Gastroenterol 2003;36:285. [DOI] [PubMed] [Google Scholar]
- 24. Khan MA, Brunt EM, Longo WE. et al. Persistent Clostridium difficile colitis: a possible etiology for the development of collagenous colitis. Dig Dis Sci 2000;45:998–1001. [DOI] [PubMed] [Google Scholar]
- 25. Sonnenberg A, Turner KO, Genta RM.. Interaction of ethnicity and H. pylori infection in the occurrence of microscopic colitis. Dig Dis Sci 2017;62:1009–15. [DOI] [PubMed] [Google Scholar]
- 26. Sonnenberg A, Genta RM.. Inverse association between helicobacter pylori gastritis and microscopic colitis. Inflamm Bowel Dis 2016;22:182–6. [DOI] [PubMed] [Google Scholar]
- 27. Nattramilarasu PK, Bücker R, Lobo de Sá FD. et al. Campylobacter concisus impairs sodium absorption in colonic epithelium via ENaC dysfunction and claudin-8 disruption. Int J Mol Sci 2020;21:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Westerlind H, Bonfiglio F, Mellander MR. et al. HLA associations distinguish collagenous from lymphocytic colitis. Am J Gastroenterol 2016;111:1211–3. [DOI] [PubMed] [Google Scholar]
- 29. Engler DB, Leonardi I, Hartung ML. et al. Helicobacter pylori-specific protection against inflammatory bowel disease requires the NLRP3 inflammasome and IL-18. Inflamm Bowel Dis 2015;21:854–61. [DOI] [PubMed] [Google Scholar]
- 30. Wu YZ, Tan G, Wu F. et al. H. pylori attenuates TNBS-induced colitis via increasing mucosal Th2 cells in mice. Oncotarget 2017;8:73810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang H, Dai Y, Liu Y. et al. Helicobacter pylori colonization protects against chronic experimental colitis by regulating Th17/Treg balance. Inflamm Bowel Dis 2018;24:1481–92. [DOI] [PubMed] [Google Scholar]
- 32. Higgins PD, Johnson LA, Luther J. et al. Prior Helicobacter pylori infection ameliorates Salmonella typhimurium-induced colitis: mucosal crosstalk between stomach and distal intestine. Inflamm Bowel Dis 2011;17:1398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luther J, Owyang SY, Takeuchi T. et al. Helicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitis. Gut 2011;60:1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tepler A, Narula N, Peek RM Jr.. et al. Systematic review with meta-analysis: association between Helicobacter pylori CagA seropositivity and odds of inflammatory bowel disease. Aliment Pharmacol Ther 2019;50:121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strachan DP. Hay fever, hygiene, and household size. BMJ 1989;299:1259–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Okada H, Kuhn C, Feillet H. et al. The “hygiene hypothesis” for autoimmune and allergic diseases: an update. Clin Exp Immunol 2010;160:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen CC, Liou JM, Lee YC. et al. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes 2021;13:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruyssers NE, De Winter BY, De Man JG. et al. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel Dis 2009;15:491–500. [DOI] [PubMed] [Google Scholar]
- 39. Carrasco A, Esteve M, Salas A. et al. Immunological differences between lymphocytic and collagenous colitis. J Crohns Colitis 2016;10:1055–66. [DOI] [PubMed] [Google Scholar]
- 40. Kumawat AK, Strid H, Tysk C. et al. Microscopic colitis patients demonstrate a mixed Th17/Tc17 and Th1/Tc1 mucosal cytokine profile. Mol Immunol 2013;55:355–64. [DOI] [PubMed] [Google Scholar]
- 41. Kumawat AK, Strid H, Elgbratt K. et al. Microscopic colitis patients have increased proportions of Ki67(+) proliferating and CD45RO(+) active/memory CD8(+) and CD4(+)8(+) mucosal T cells. J Crohns Colitis 2013;7:694–705. [DOI] [PubMed] [Google Scholar]
- 42. Carrasco A, Esteve M, Pedrosa E. et al. Lymphocytic and collagenous colitis: two clinically similar entities but with a distinct immunological pattern. Journal of Crohn's and Colitis 2014;8:S82–3. [Google Scholar]
- 43. Zhang C, Zhao Z, Osman H. et al. Differential expression of miR-31 between inflammatory bowel disease and microscopic colitis. Microrna 2014;3:155–9. [DOI] [PubMed] [Google Scholar]
- 44. Aqel B, Bishop M, Krishna M. et al. Collagenous colitis evolving into ulcerative colitis: a case report and review of the literature. Dig Dis Sci 2003;48:2323–7. [DOI] [PubMed] [Google Scholar]
- 45. Freeman HJ, Berean KW, Nimmo M.. Evolution of collagenous colitis into severe and extensive ulcerative colitis. Can J Gastroenterol 2007;21:315–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pokorny CS, Kneale KL, Henderson CJ.. Progression of collagenous colitis to ulcerative colitis. J Clin Gastroenterol 2001;32:435–8. [DOI] [PubMed] [Google Scholar]
- 47. Chandratre S, Bramble MG, Cooke WM. et al. Simultaneous occurrence of collagenous colitis and Crohn's disease. Digestion 1987;36:55–60. [DOI] [PubMed] [Google Scholar]
- 48. O'Beirne JP, Ireland A.. Progression of collagenous colitis to Crohn's disease. Eur J Gastroenterol Hepatol 2005;17:573–5. [DOI] [PubMed] [Google Scholar]
- 49. Rönnblom A, Holmström T, Tanghöj H. et al. Celiac disease, collagenous sprue and microscopic colitis in IBD: observations from a population-based cohort of IBD (ICURE). Scand J Gastroenterol 2015;50:1234–40. [DOI] [PubMed] [Google Scholar]
- 50. Wickbom A, Bohr J, Nyhlin N. et al. ; Swedish Organisation for the Study of Inflammatory Bowel Disease (SOIBD). Microscopic colitis in patients with ulcerative colitis or Crohn's disease: a retrospective observational study and review of the literature. Scand J Gastroenterol 2018;53:410–6. [DOI] [PubMed] [Google Scholar]
- 51. Wildt S, Munck LK, Winther-Jensen M. et al. Autoimmune diseases in microscopic colitis: a Danish nationwide case-control study. Aliment Pharmacol Ther 2021;54:1454–62. [DOI] [PubMed] [Google Scholar]
- 52. Yuan L, Wu TT, Chandan V. et al. Clinicopathological significance of lymphocytic colitis/collagenous colitis in inflammatory bowel disease. Hum Pathol 2020;96:87–95. [DOI] [PubMed] [Google Scholar]
- 53. Green HD, Beaumont RN, Thomas A. et al. Genome-wide association study of microscopic colitis in the UK biobank confirms immune-related pathogenesis. J Crohns Colitis 2019;13:1578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stahl E, Roda G, Dobbyn A. et al. Collagenous colitis is associated with HLA signature and shares genetic risks with other immune-mediated diseases. Gastroenterology 2020;159:549–61.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Westerlind H, Mellander MR, Bresso F. et al. Dense genotyping of immune-related loci identifies HLA variants associated with increased risk of collagenous colitis. Gut 2017;66:421–8. [DOI] [PubMed] [Google Scholar]
- 56. Li J, Yan Y, Meng Z. et al. Microscopic colitis evolved into inflammatory bowel diseases is characterized by increased Th1/Tc1 cells in colonic mucosal lamina propria. Dig Dis Sci 2017;62:2755–67. [DOI] [PubMed] [Google Scholar]
- 57. Carrasco A, Fernández-Bañares F.. Th1 pathway: the missing link between inflammatory bowel disease and microscopic colitis? Dig Dis Sci 2017;62:2609–11. [DOI] [PubMed] [Google Scholar]
- 58. Pavel FM, Vesa CM, Gheorghe G. et al. Highlighting the relevance of gut microbiota manipulation in inflammatory bowel disease. Diagnostics (Basel) 2021;11:1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hertz S, Durack J, Kirk KF. et al. Microscopic colitis patients possess a perturbed and inflammatory gut microbiota. Dig Dis Sci 2021. [DOI] [PubMed] [Google Scholar]
- 60. Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008;134:577–94. [DOI] [PubMed] [Google Scholar]
- 61. Morgan DM, Cao Y, Miller K. et al. Microscopic colitis is characterized by intestinal dysbiosis. Clin Gastroenterol Hepatol 2020;18:984–6. [DOI] [PubMed] [Google Scholar]
- 62. Kostic AD, Xavier RJ, Gevers D.. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology 2014;146:1489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Manichanh C, Rigottier-Gois L, Bonnaud E. et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 2006;55:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Carstens A, Dicksved J, Nelson R. et al. The gut microbiota in collagenous colitis shares characteristics with inflammatory bowel disease-associated dysbiosis. Clin Transl Gastroenterol 2019;10:e00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Megna B, Saha S, Wald A. et al. Symptomatic microscopic colitis atop quiescent inflammatory bowel disease: a case series. ACG Case Rep J 2017;4:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jegadeesan R, Liu X, Pagadala MR. et al. Microscopic colitis: is it a spectrum of inflammatory bowel disease? World J Gastroenterol 2013;19:4252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Saad RE, Shobar RM, Jakate S. et al. Development of collagenous colitis in inflammatory bowel disease: two case reports and a review of the literature. Gastroenterol Rep (Oxf) 2019;7:218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Haque M, Florin T.. Progression of ulcerative colitis to collagenous colitis: chance, evolution or association? Inflamm Bowel Dis 2007;13:1321. [DOI] [PubMed] [Google Scholar]
- 69. Tariq R, Smyrk T, Pardi DS. et al. New-onset microscopic colitis in an ulcerative colitis patient after fecal microbiota transplantation. Am J Gastroenterol 2016;111:751–2. [DOI] [PubMed] [Google Scholar]
- 70. Silva M, Nunes AC, Andrade P. et al. Collagenous colitis and Crohn's disease: guilty or innocent bystander? Dig Liver Dis 2016;48:1261–2. [DOI] [PubMed] [Google Scholar]
- 71. Janczewska I, Mejhert M, Hast R. et al. Primary AL-amyloidosis, ulcerative colitis and collagenous colitis in a 57-year-old woman: a case study. Scand J Gastroenterol 2004;39:1306–9. [DOI] [PubMed] [Google Scholar]
- 72. Fernández-Bañares F, de Sousa MR, Salas A. et al. Epidemiological risk factors in microscopic colitis: a prospective case-control study. Inflamm Bowel Dis 2013;19:411–7. [DOI] [PubMed] [Google Scholar]
- 73. Sonnenberg A, Turner KO, Genta RM.. Associations of microscopic colitis with other lymphocytic disorders of the gastrointestinal tract. Clin Gastroenterol Hepatol 2018;16:1762–7. [DOI] [PubMed] [Google Scholar]
- 74. Vigren L, Tysk C, Ström M. et al. Celiac disease and other autoimmune diseases in patients with collagenous colitis. Scand J Gastroenterol 2013;48:944–50. [DOI] [PubMed] [Google Scholar]
- 75. Green PH, Yang J, Cheng J. et al. An association between microscopic colitis and celiac disease. Clin Gastroenterol Hepatol 2009;7:1210–6. [DOI] [PubMed] [Google Scholar]
- 76. Stewart M, Andrews CN, Urbanski S. et al. The association of coeliac disease and microscopic colitis: a large population-based study. Aliment Pharmacol Ther 2011;33:1340–9. [DOI] [PubMed] [Google Scholar]
- 77. Thörn M, Sjöberg D, Ekbom A. et al. Microscopic colitis in Uppsala health region, a population-based prospective study 2005-2009. Scand J Gastroenterol 2013;48:825–30. [DOI] [PubMed] [Google Scholar]
- 78. Kane JS, Rotimi O, Ford AC.. Macroscopic findings, incidence and characteristics of microscopic colitis in a large cohort of patients from the United Kingdom. Scand J Gastroenterol 2017;52:1– 94. [DOI] [PubMed] [Google Scholar]
- 79. Aziz M, Haghbin H, Khan RS. et al. Celiac disease is associated with microscopic colitis in refractory cases in adults: a systematic review and meta-analysis of observational studies. Dig Dis Sci 2021. [DOI] [PubMed] [Google Scholar]
- 80. Yip RHL, Lee LH, Lee LH. et al. Topography, morphology, and etiology of lymphocytic gastritis: a single institution experience. Virchows Arch 2020;476:551–9. [DOI] [PubMed] [Google Scholar]
- 81. Carmack SW, Lash RH, Gulizia JM. et al. Lymphocytic disorders of the gastrointestinal tract: a review for the practicing pathologist. Adv Anat Pathol 2009;16:290–306. [DOI] [PubMed] [Google Scholar]
- 82. Genta RM, Singhal A, Turner KO. et al. Lymphocytic gastritis and its relationships with other gastrointestinal disorders. Aliment Pharmacol Ther 2021;54:1170–8. [DOI] [PubMed] [Google Scholar]
- 83. Ting YT, Dahal-Koirala S, Kim HSK. et al. A molecular basis for the T cell response in HLA-DQ2.2 mediated celiac disease. Proc Natl Acad Sci USA 2020;117:3063–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brown NK, Guandalini S, Semrad C. et al. A clinician's guide to celiac disease HLA genetics. Am J Gastroenterol 2019;114:1587–92. [DOI] [PubMed] [Google Scholar]
- 85. Fine KD, Do K, Schulte K. et al. High prevalence of celiac sprue-like HLA-DQ genes and enteropathy in patients with the microscopic colitis syndrome. Am J Gastroenterol 2000;95:1974–82. [DOI] [PubMed] [Google Scholar]
- 86. Fernández-Bañares F, Esteve M, Farré C. et al. Predisposing HLA-DQ2 and HLA-DQ8 haplotypes of coeliac disease and associated enteropathy in microscopic colitis. Eur J Gastroenterol Hepatol 2005;17:1333–8. [DOI] [PubMed] [Google Scholar]
- 87. van der Graaf A, Zorro MM, Claringbould A. et al. ; BIOS Consortium. Systematic prioritization of candidate genes in disease loci identifies TRAFD1 as a master regulator of IFNγ signaling in celiac disease. Front Genet 2020;11:562434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lammers KM, Lu R, Brownley J. et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 2008;135:194–204.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Escudero-Hernández C, Martín Á, de Pedro Andrés R. et al. Circulating dendritic cells from celiac disease patients display a gut-homing profile and are differentially modulated by different gliadin-derived peptides. Mol Nutr Food Res 2020;64:e1900989. [DOI] [PubMed] [Google Scholar]
- 90. Zygmunt B, Veldhoen M.. T helper cell differentiation more than just cytokines. Adv Immunol 2011;109:159–96. [DOI] [PubMed] [Google Scholar]
- 91. D'Avino P, Serena G, Kenyon V. et al. An updated overview on celiac disease: from immuno-pathogenesis and immuno-genetics to therapeutic implications. Expert Rev Clin Immunol 2021;17:269–84. [DOI] [PubMed] [Google Scholar]
- 92. Serena G, Yan S, Camhi S. et al. Proinflammatory cytokine interferon-γ and microbiome-derived metabolites dictate epigenetic switch between forkhead box protein 3 isoforms in coeliac disease. Clin Exp Immunol 2017;187:490–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tagkalidis PP, Gibson PR, Bhathal PS.. Microscopic colitis demonstrates a T helper cell type 1 mucosal cytokine profile. J Clin Pathol 2007;60:382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Barshack I, Goldberg I, Chowers Y. et al. Immunohistochemical analysis of candidate gene product expression in the duodenal epithelium of children with coeliac sprue. J Clin Pathol 2001;54:684–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu PH, Lebwohl B, Burke KE. et al. Dietary gluten intake and risk of microscopic colitis among US women without celiac disease: a prospective cohort study. Am J Gastroenterol 2019;114:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Brown I, Mino-Kenudson M, Deshpande V. et al. Intraepithelial lymphocytosis in architecturally preserved proximal small intestinal mucosa: an increasing diagnostic problem with a wide differential diagnosis. Arch Pathol Lab Med 2006;130:1020–5. [DOI] [PubMed] [Google Scholar]
- 97. Fine KD, Lee EL, Meyer RL.. Colonic histopathology in untreated celiac sprue or refractory sprue: is it lymphocytic colitis or colonic lymphocytosis? Hum Pathol 1998;29:1433–40. [DOI] [PubMed] [Google Scholar]
- 98. Ford AC, Sperber AD, Corsetti M. et al. Irritable bowel syndrome. Lancet 2020;396:1675–88. [DOI] [PubMed] [Google Scholar]
- 99. Enck P, Aziz Q, Barbara G. et al. Irritable bowel syndrome. Nat Rev Dis Primers 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mearin F, Lacy BE, Chang L. et al. Bowel disorders. Gastroenterology 2016;S0016-5085(16)00222-5. [DOI] [PubMed] [Google Scholar]
- 101. Palsson OS, Whitehead W, Törnblom H. et al. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020;158:1262–73.e3. [DOI] [PubMed] [Google Scholar]
- 102. Pagoldh J, Lundgren D, Suhr OB. et al. Irritable bowel syndrome-like symptoms in treated microscopic colitis patients compared with controls: a cross-sectional study. Gastroenterol Rep (Oxf) 2020;8:374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kane JS, Irvine AJ, Derwa Y. et al. High prevalence of irritable bowel syndrome-type symptoms in microscopic colitis: implications for treatment. Therap Adv Gastroenterol 2018;11:175628481878360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Abboud R, Pardi DS, Tremaine WJ. et al. Symptomatic overlap between microscopic colitis and irritable bowel syndrome: a prospective study. Inflamm Bowel Dis 2013;19:550–3. [DOI] [PubMed] [Google Scholar]
- 105. Limsui D, Pardi DS, Camilleri M. et al. Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm Bowel Dis 2007;13:175–81. [DOI] [PubMed] [Google Scholar]
- 106. Kao KT, Pedraza BA, McClune AC. et al. Microscopic colitis: a large retrospective analysis from a health maintenance organization experience. Wjg 2009;15:3122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kamp EJ, Kane JS, Ford AC.. Irritable bowel syndrome and microscopic colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:659–68.e1. quiz e54–5. [DOI] [PubMed] [Google Scholar]
- 108. Kane JS, Irvine AJ, Derwa Y. et al. Fatigue and its associated factors in microscopic colitis. Therap Adv Gastroenterol 2018;11:175628481879959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Parker CH, Naliboff BD, Shih W. et al. The role of resilience in irritable bowel syndrome, other chronic gastrointestinal conditions, and the general population. Clin Gastroenterol Hepatol 2020;19:2541–50.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Roth B, Bengtsson M, Ohlsson B.. Diarrhoea is not the only symptom that needs to be treated in patients with microscopic colitis. Eur J Intern Med 2013;24:573–8. [DOI] [PubMed] [Google Scholar]
- 111. Roth B, Ohlsson B.. Gastrointestinal symptoms and psychological well-being in patients with microscopic colitis. Scand J Gastroenterol 2013;48:27–34. [DOI] [PubMed] [Google Scholar]
- 112. Roth B, Gustafsson RJ, Jeppsson B. et al. Smoking- and alcohol habits in relation to the clinical picture of women with microscopic colitis compared to controls. BMC Womens Health 2014;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Staller K, Olén O, Söderling J. et al. Diagnostic yield of endoscopy in irritable bowel syndrome: a nationwide prevalence study 1987-2016. Eur J Intern Med 2021;94:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ebeid B, Eid RA, Attia D. et al. Prevalence of microscopic colitis in diarrhea-predominant irittable bowel syndrome patients: cohort study from Upper Egypt. J Clin Gastroenterol 2021;56:e232–e8. [DOI] [PubMed] [Google Scholar]
- 115. Ohlsson B, Gustafsson R, Swahn F. et al. Endoscopic full-thickness biopsy, a novel method in the work up of complicated abdominal symptoms. Therap Adv Gastroenterol 2018;11:1756283x17730747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pan CH, Chang CC, Su CT. et al. Trends in irritable bowel syndrome incidence among Taiwanese adults during 2003-2013: a population-based study of sex and age differences. PLoS One 2016;11:e0166922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hilpüsch F, Johnsen PH, Goll R. et al. Microscopic colitis: a missed diagnosis among patients with moderate to severe irritable bowel syndrome. Scand J Gastroenterol 2017;52:173–7. [DOI] [PubMed] [Google Scholar]
- 118. Chey WD, Nojkov B, Rubenstein JH. et al. The yield of colonoscopy in patients with non-constipated irritable bowel syndrome: results from a prospective, controlled US trial. Am J Gastroenterol 2010;105:859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ozdil K, Sahin A, Calhan T. et al. The frequency of microscopic and focal active colitis in patients with irritable bowel syndrome. BMC Gastroenterol 2011;11:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Asghar Z, Thoufeeq M, Kurien M. et al. Diagnostic yield of colonoscopy in patients with symptoms compatible with Rome IV functional bowel disorders. Clin Gastroenterol Hepatol 2020;20:334–41.e3. [DOI] [PubMed] [Google Scholar]
- 121. Macaigne G, Lahmek P, Locher C. et al. Microscopic colitis or functional bowel disease with diarrhea: a French prospective multicenter study. Am J Gastroenterol 2014;109:1461–70. [DOI] [PubMed] [Google Scholar]
- 122. Patel P, Bercik P, Morgan DG. et al. Prevalence of organic disease at colonoscopy in patients with symptoms compatible with irritable bowel syndrome: cross-sectional survey. Scand J Gastroenterol 2015;50:816–23. [DOI] [PubMed] [Google Scholar]
- 123. Aziz I, Simrén M.. The overlap between irritable bowel syndrome and organic gastrointestinal diseases. Lancet Gastroenterol Hepatol 2021;6:139–48. [DOI] [PubMed] [Google Scholar]
- 124. Quigley EMM. The patient with irritable bowel syndrome-type symptoms: when to investigate and how? Curr Opin Gastroenterol 2021;37:39–43. [DOI] [PubMed] [Google Scholar]
- 125. Bakke I, Walaas GA, Bruland T. et al. Mucosal and faecal neutrophil gelatinase-associated lipocalin as potential biomarkers for collagenous colitis. J Gastroenterol 2021;56:914–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Caviglia GP, Pantaleoni S, Touscoz GA. et al. Fecal calprotectin is an effective diagnostic tool that differentiates inflammatory from functional intestinal disorders. Scand J Gastroenterol 2014;49:1419–24. [DOI] [PubMed] [Google Scholar]
- 127. Klem F, Wadhwa A, Prokop LJ. et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology 2017;152:1042–54.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Beyaert R, Beaugerie L, Van Assche G. et al. Cancer risk in immune-mediated inflammatory diseases (IMID). Mol Cancer 2013;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lutgens MW, van Oijen MG, van der Heijden GJ. et al. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis 2013;19:789–99. [DOI] [PubMed] [Google Scholar]
- 130. Axelrad JE, Lichtiger S, Yajnik V.. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 2016;22:4794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Han Y, Chen W, Li P. et al. Association between coeliac disease and risk of any malignancy and gastrointestinal malignancy: a meta-analysis. Medicine (Baltimore) 2015;94:e1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Askling J, Linet M, Gridley G. et al. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology 2002;123:1428–35. [DOI] [PubMed] [Google Scholar]
- 133. Pereyra L, Gonzalez R, Mohaidle A. et al. Risk of colorectal neoplasia in patients with celiac disease: a multicenter study. J Crohns Colitis 2013;7:e672–7. [DOI] [PubMed] [Google Scholar]
- 134. Elfström P, Granath F, Ye W. et al. Low risk of gastrointestinal cancer among patients with celiac disease, inflammation, or latent celiac disease. Clin Gastroenterol Hepatol 2012;10:30–6. [DOI] [PubMed] [Google Scholar]
- 135. Levy A, Borren NZ, Maxner B. et al. Cancer risk in microscopic colitis: a retrospective cohort study. BMC Gastroenterol 2019;19:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bonderup OK, Folkersen BH, Gjersøe P. et al. Collagenous colitis: a long-term follow-up study. Eur J Gastroenterol Hepatol 1999;11:493–5. [PubMed] [Google Scholar]
- 137. Chan JL, Tersmette AC, Offerhaus GJ. et al. Cancer risk in collagenous colitis. Inflamm Bowel Dis 1999;5:40–3. [DOI] [PubMed] [Google Scholar]
- 138. Borsotti E, Barberio B, D'Incà R. et al. Low prevalence of colorectal neoplasia in microscopic colitis: a large prospective multi-center study. Dig Liver Dis 2021;53:846–51. [DOI] [PubMed] [Google Scholar]
- 139. Weimers P, Vedel Ankersen D, Lophaven S. et al. Disease activity patterns, mortality, and colorectal cancer risk in microscopic colitis: a Danish nationwide cohort study, 2001 to 2016. J Crohns Colitis 2021;15:594–602. [DOI] [PubMed] [Google Scholar]
- 140. Bergman D, Khalili H, Roelstraete B. et al. Microscopic colitis and risk of cancer—a population-based cohort study. J Crohns Colitis 2020;15:212–21. [DOI] [PubMed] [Google Scholar]
- 141. Larsson JK, Dabos KJ, Höglund P. et al. Cancer risk in collagenous colitis. J Clin Med 2019;8:1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yen EF, Pokhrel B, Bianchi LK. et al. Decreased colorectal cancer and adenoma risk in patients with microscopic colitis. Dig Dis Sci 2012;57:161–9. [DOI] [PubMed] [Google Scholar]
- 143. Tontini GE, Pastorelli L, Spina L. et al. Microscopic colitis and colorectal neoplastic lesion rate in chronic nonbloody diarrhea: a prospective, multicenter study. Inflamm Bowel Dis 2014;20:882–91. [DOI] [PubMed] [Google Scholar]
- 144. Sonnenberg A, Genta RM.. Low prevalence of colon polyps in chronic inflammatory conditions of the colon. Am J Gastroenterol 2015;110:1056–61. [DOI] [PubMed] [Google Scholar]
- 145. Khalili H, Bergman D, Roelstraete B. et al. Mortality of patients with microscopic colitis in Sweden. Clin Gastroenterol Hepatol 2020;18:2491–9.e3. [DOI] [PubMed] [Google Scholar]
- 146. Shergill AK, Conners EE, McQuaid KR. et al. Protective association of colonoscopy against proximal and distal colon cancer and patterns in interval cancer. Gastrointest Endosc 2015;82:529–37.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rostom A, Dubé C, Lewin G. et al. ; U.S. Preventive Services Task Force. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med 2007;146:376–89. [DOI] [PubMed] [Google Scholar]
- 148. Lytras T, Nikolopoulos G, Bonovas S.. Statins and the risk of colorectal cancer: an updated systematic review and meta-analysis of 40 studies. World J Gastroenterol 2014;20:1858–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Girardi M, Oppenheim DE, Steele CR. et al. Regulation of cutaneous malignancy by gammadelta T cells. Science 2001;294:605–9. [DOI] [PubMed] [Google Scholar]
- 150. Andresen L, Jørgensen VL, Perner A. et al. Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut 2005;54:503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rutter M, Saunders B, Wilkinson K. et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004;126:451–9. [DOI] [PubMed] [Google Scholar]