Abstract
A fundamental task in medicine is the understanding of the causes of diseases. Preeclampsia and eclampsia, an enigmatic and elusive disorder, have been labeled the “disease of theories.” Preeclampsia is one of the “great obstetrical syndromes” in which multiple and sometimes overlapping pathologic processes activate a common pathway composed of endothelial cell activation, intravascular inflammation, and syncytiotrophoblast stress. This article addresses the potential etiologies, or causal explanations, for preeclampsia. The role of uteroplacental ischemia is well established, based upon a solid body of clinical and experimental evidence. A causal role for microorganisms has gained recognition through the realization that periodontal disease and maternal gut dysbiosis are linked to atherosclerosis, thus possibly to a subset of patients with preeclampsia. The recent reports indicating that SARS-CoV-2 infection appears to be causally linked to preeclampsia are reviewed as well as the mechanisms whereby this viral infection can cause this syndrome. Particular etiological factors, such as the breakdown of maternal-fetal immune tolerance, thought to account for the excess of preeclampsia in primipaternity and egg donation—may operate, in part, through uteroplacental ischemia, while another, such as placental aging, may operate largely through syncytiotrophoblast stress. This article also examines the nature of the association between gestational diabetes mellitus and maternal obesity in preeclampsia. The various roles of autoimmunity, fetal diseases, and endocrine disorders are also discussed. A greater understanding of the etiologic factors of preeclampsia is essential to improve treatment and prevention.
Keywords: Angiotensin receptor II, atherosclerosis, autoantibodies, Ballantyne syndrome, body mass index, COVID-19, Cushing’s syndrome, endothelial cell dysfunction, genetic incompatibility, gestational diabetes mellitus, hydatidiform mole, hydrops fetalis, hyperaldosteronism, hyperparathyroidism, hypertension, infection, inflammation, insulin resistance, intestinal dysbiosis, maternal anti-fetal rejection, metabolic syndrome, mirror syndrome, molar pregnancy, obesity, placental aging, placental ischemia, placental lesions of maternal vascular malperfusion, primipaternity, proteinuria, SARS-CoV-2, sleep-disordered breathing, sleep disorders, snoring, tolerance
Condensation:
This article describes the causal risk factors, or etiology, of preeclampsia.
Introduction
A fundamental task in medicine is the understanding of the causes of diseases. Preeclampsia and eclampsia, the enigmatic, elusive disorders of pregnancy, have been labeled the “diseases of theories”. Preeclampsia and eclampsia are among the “great obstetrical syndromes” in which multiple and sometimes overlapping pathologic processes activate a common pathway that leads to the clinical recognition of these disorders. Just as the syndrome of preterm labor is recognized by the clinical manifestations of activation of the common pathway of parturition (ie increased uterine contractility, cervical remodeling, and membrane and decidual activation), so are preeclampsia and eclampsia. Their common pathway consists of endothelial cell activation, intravascular inflammation, and syncytiotrophoblast stress, and the diagnosis has traditionally rested in the detection of hypertension and proteinuria, although professional organizations had recently recommended that the diagnosis can be made in the absence of proteinuria when patients have evidence of multi-systemic involvement.
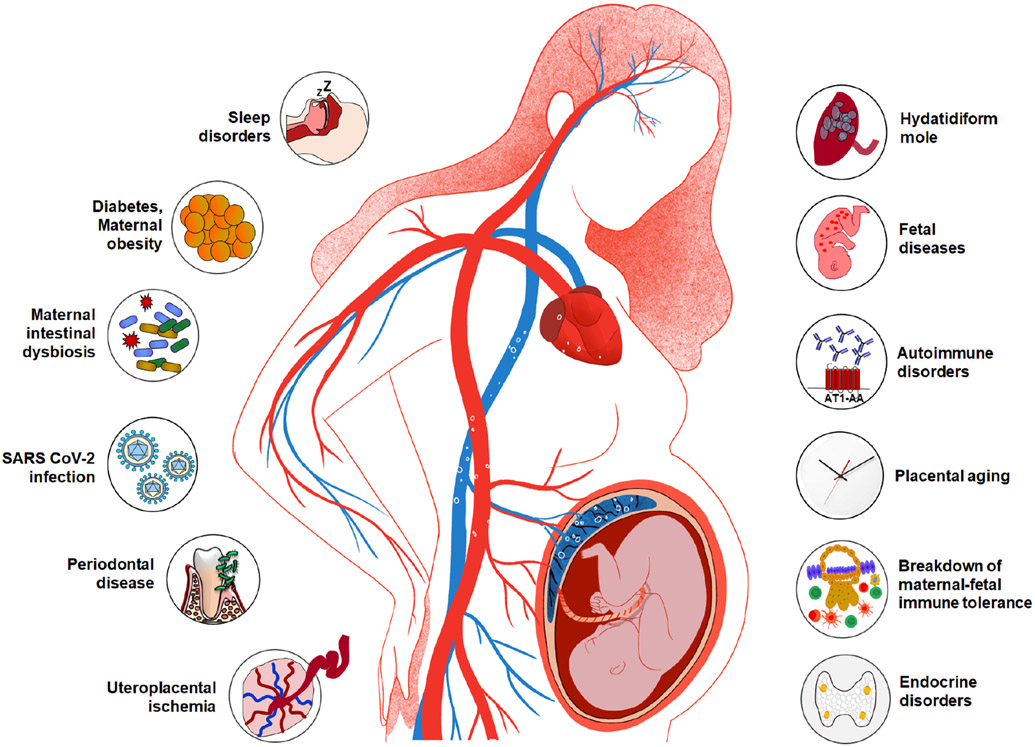
A rich body of literature describes the risk factors for preeclampsia and eclampsia (ie the conditions that increase the likelihood of these syndromes but are not necessarily causal). However, the elucidation of etiologic, or causal, factors is necessary to successfully treat and prevent disease. This article reviews the evidence linking these etiologic factors with preeclampsia and eclampsia, which is summarized in Figure 1.
Figure 1.
Multiple etiologies implicated in preeclampsia. Uteroplacental ischemia, maternal infection and inflammation (eg periodontal disease, urinary tract infection, COVID-19), maternal intestinal dysbiosis, maternal obesity, sleep disorders, hydatidiform mole, fetal diseases (eg hydrops fetalis, viral infection, Trisomy 13, and unique complications of multiple gestation), autoimmune disorders, placental aging, breakdown of maternal-fetal immune tolerance, and endocrine disorders (eg hyperparathyroidism, Cushing’s syndrome, hyperaldosteronism).
Uteroplacental ischemia
The principal mechanism of disease implicated in the etiology of preeclampsia and eclampsia is uteroplacental ischemia. In 1914, James Young proposed that interference of the uterine blood supply to the placenta would lead to placental infarctions that, in turn, would release toxins into the maternal circulation, thus causing eclampsia.1 This theory was based on the demonstration that placental infarctions were observed in patients with eclampsia and on animal studies that showed subcutaneous injections of extracts of the autolyzed human placenta into guinea pigs elicited convulsions, hepatic focal necrosis, and renal lesions, similar to those observed in women who died of eclampsia.1 Dixon and Taylor reported that intravenous injections of extracts of fresh human placenta induced an increase in blood pressure in cats, rabbits, and dogs, resembling the effects of adrenaline.2 Therefore, the search for the causes of preeclampsia and eclampsia and for the identity of the circulating “toxins” began more than 100 years ago.
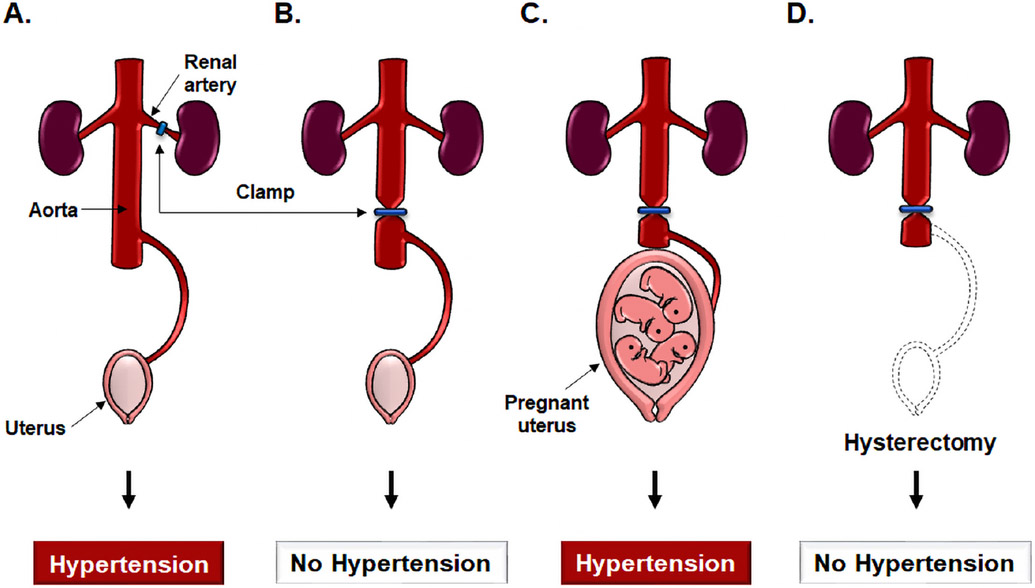
Additional evidence supporting a role for uteroplacental ischemia came from studies by Ogden, Hildebrand, and Page who reported that clamping of the abdominal aorta below the renal arteries (to avoid renovascular hypertension) in dogs reduced uteroplacental perfusion; furthermore, this response was followed by maternal hypertension that resolved after the clamp was released.3 Because this hypertensive response was not observed in non-pregnant animals, the investigators concluded that the signals responsible for hypertension must have originated within the gravid uterus.3 Another observation buttressed this interpretation: after removal of the pregnant uterus, the clamping of the aorta did not elicit hypertension (Figure 2).3, 4
Figure 2.
A diagrammatic depiction of experiments demonstrating that uterine ischemia in pregnant animals, but not in non-pregnant animals, can cause hypertension. A. In the Goldblatt model of renovascular hypertension, clamping the renal artery leads to development of hypertension through renal ischemia and the release of renin in non-pregnant animals. B. By contrast, clamping the aorta below the renal arteries does not induce hypertension in non-pregnant animals. C. Clamping of the aorta in pregnant animals below the renal arteries leads to hypertension. D. The hypertension disappears after a hysterectomy has been performed; this suggests that the source of the signals leading to maternal systemic hypertension are derived from the gravid uterus. Modified from Chaiworapongsa T. et al. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014 Aug; 10 (8):466-80.
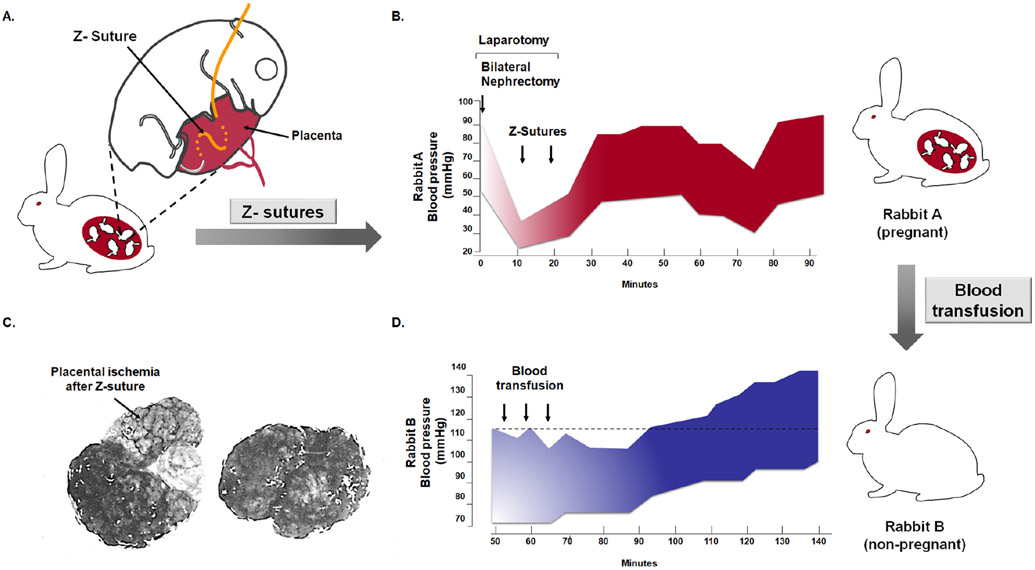
Subsequent studies strengthened this concept, demonstrating that placental ischemia, generated by placing silver clips in the uterine arteries of pregnant dogs, led to hypertension and proteinuria.5 Two lines of evidence supported the view that placental ischemia rather than uterine ischemia was key. First, patients with an abdominal pregnancy were known to develop preeclampsia given that the implantation site was outside of the uterus.6 Second, the placement of a Z suture through the placenta to generate ischemia resulted in the development of hypertension and proteinuria (Figure 3A-3C).7 Moreover, the existence of a circulating “toxin,” supported by the observation that the administration of blood from a rabbit with experimental placental ischemia caused by the Z suture, resulted in hypertension in non-pregnant animals (Figure 3D).7
Figure 3.
Experimental demonstration that placental ischemia causes maternal hypertension and that a soluble factor in the blood of an animal with placental ischemia can induce hypertension in a non-pregnant animal. A. The Z suture is placed in the uterus to generate placental ischemia. B. Placental ischemia causes hypertension. Rabbit A had undergone a bilateral nephrectomy; therefore, the kidney is not a cause of the hypertension. After Z sutures were placed through several placentas, hypertension developed. C. Gross evidence that the suture has caused a placental infarction. The pale portion of the placenta with the arrow represents a large infarction. A control placenta of the same animal is illustrated on the right. D. Transfusions of blood from Rabbit A (a pregnant rabbit with placental ischemia) to a non-pregnant rabbit (Rabbit B) caused hypertension; this suggests that a circulating factor generated after placental ischemia is present in the maternal blood and that it can induce hypertension in a non-pregnant rabbit. Modified from Berger M. and Cavanagh D. Toxemia of pregnancy: The hypertensive effect of acute experimental placental ischemia. Am J Obstet Gynecol. 1963 Oct 1;87:293-305.
The first in vivo evidence indicating that women with preeclampsia had decreased maternal-placental blood flow was reported by McClure Browne and Veall,8 who described the injection of radioactive sodium into the choriodecidual space of women with a normal pregnancy and in those affected by preeclampsia. The investigators noted that the blood flow at term was 600 mL/minute, but it was substantially lower in patients with preeclampsia. These observations have been confirmed with different radioactive tracers in subsequent studies.9-13 For example, Lunell et al 13 reported that uteroplacental blood flow was reduced by 50% in patients with preeclampsia and that the reduction was greater in those with severe preeclampsia than in those with mild disease.
The role of placental ischemia in the pathogenesis of preeclampsia is now well established. Indeed, the most frequently implemented animal model of the syndrome is chronic reduction of uteroplacental perfusion in pregnant rats, generated by the placement of a constriction clip around the aorta below the renal arteries and before the origin of the uterine arteries at 14 days of gestation.14 In addition, this effect led to a reduction in placental blood flow by approximately 40%, an increase in arterial blood pressure by 20-25 mm Hg by day 19 of pregnancy, increased vascular resistance, decreased cardiac output and glomerular filtration rate, and the frequent appearance of proteinuria.14 This model recapitulated two of the many findings of preeclampsia: (1) an increase in circulating concentrations of soluble vascular endothelial growth factor receptor-1 (sVEGFR-1; also known as soluble fms-like tyrosine kinase 1 [sFlt-1]) and endoglin, and (2) a rise in the concentrations of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6). A similar non-human primate model of preeclampsia, developed in pregnant baboons by selective ligation of one uterine artery, led to the development of hypertension, proteinuria, and increased production of sFlt-1.15 Importantly, the administration of short interfering RNAs, which silence three of the sFlt-1 mRNA isoforms, suppressed sFlt-1 overexpression and reduced hypertension and proteinuria.16 These studies suggest that the soluble factor, or “toxin,” responsible for hypertension is, at least in part, sFlt-1. The reader is referred to the review by Bakrania, George, and Granger17 in this Supplement for more details about the model and the pathophysiologic events caused by uteroplacental ischemia.
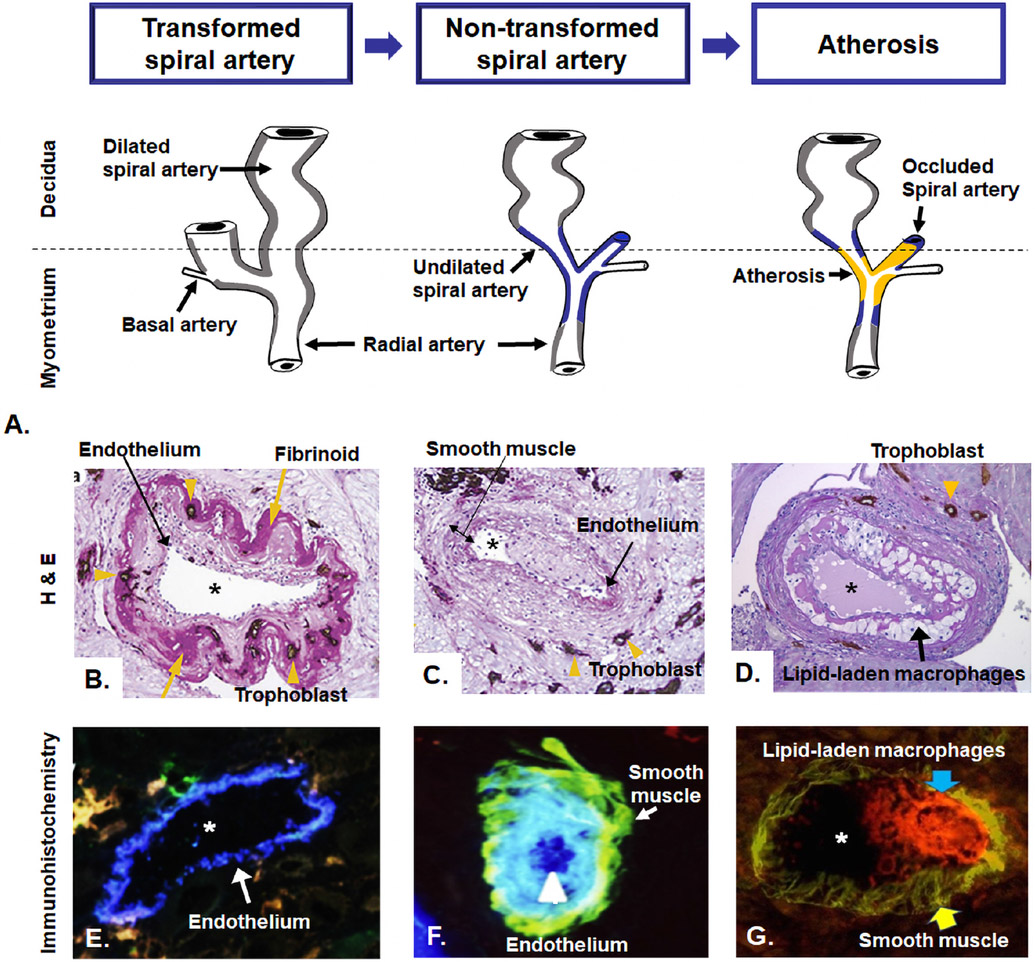
What is the cause of placental ischemia in women with preeclampsia? The traditional explanation has been that a defect in placentation leads to ischemia18, 19 but more recently, a dysfunctional maternal cardiovascular system has been implicated.20 The developmental abnormalities include failure of physiologic transformation of the spiral arteries which are characterized by a narrow diameter and retention of the muscle in the media of the vessel wall.18, 19 The persistence of the muscular coat is thought to make the vessels susceptible to the effect of vasoconstrictive agents. In addition, arteries affected by failure of physiologic transformation are more likely to develop atherosis (Figure 4), which also narrows the vessel lumen and further compromises placental perfusion.21-26 Atherosis is a lesion specific to the spiral arteries, which is equivalent to the atherosclerotic lesions observed in the coronary arteries. Figure 5 illustrates the typical lesions of atherosis in the spiral arteries and shows lipid-laden macrophages with the deposition of fat droplets detected with oil red-O stain. A systematic review and meta-analysis showed that placental lesions consistent with maternal vascular malperfusion (eg placental infarction, failure of physiologic transformation of the spiral arteries, acute atherosis) is four-fold to seven-fold higher in patients with preeclampsia than in those with a normal pregnancy.27 A comprehensive review of the failure of physiological transformation and spiral artery atherosis by Staff et al 28 is available as part of this Supplement. Atherosis is not specific to preeclampsia, and this type of lesion has been reported in other pregnancy-related conditions, such as spontaneous abortion,29 preterm labor,30 preterm prelabor rupture of the membranes,31 fetal growth restriction,32, 33 and fetal death.25, 29 We have proposed that placental ischemia is a major mechanism of disease in various obstetrical syndromes and that the timing, severity, and duration of the insult may explain the clinical occurrence of different obstetrical syndromes.34
Figure 4.
A. Diagram of maternal blood supply to the placenta. The spiral arteries undergo physiologic changes in normal pregnancy (grey). In preeclampsia, the myometrial segment of the spiral artery fails to undergo physiological transformation (blue), which is thought to explain the uteroplacental ischemia observed in preeclampsia. Moreover, non-transformed spiral arteries are prone to atherosis (yellow), characterized by the presence of lipid-laden macrophages within the lumen. Placental basal plate spiral arteries with hematoxylin-eosin stain (B, C, and D). B. Transformed spiral arteries are characterized by the presence of intramural trophoblasts (arrowheads) and fibrinoid degeneration (arrows) of the arterial wall. C. Non-transformed spiral arteries lack intramural trophoblasts and fibrinoid degeneration, and they retain smooth muscle. Arrowheads indicate the presence of trophoblasts in myometrium, but not in the wall of the spiral artery. D. Acute atherosis in decidual spiral artery. Many lipid-laden macrophages (arrows) are seen in the spiral artery with the lack of invasion of the trophoblast (arrowhead) into myometrial segment of the spiral artery. Images (B, C, and D) stained with cytokeratin 7 (brown) and periodic acid–Schiff (pink), original magnification ×200. Immunohistochemistry of placental basal plate spiral arteries (E, F, and G). E. Presence of endothelium (arrow, blue) in vessels with normal trophoblastic invasion, original magnification × 640. F. spiral artery with presence of endothelium (blue, arrowhead) and smooth muscle cells (green, arrow), original magnification ×640. G. Atherosis lesions show the presence of numerous macrophages CD36-reactive (red reactivity, blue arrow) and smooth muscle cells in the vessel wall (green reactivity, yellow arrow), original magnification x400. *lumen of spiral artery. Modified from McMaster-Fay RA. Uteroplacental vascular syndromes: theories, hypotheses and controversies. Clin Obstet Gynecol Reprod Med 2018;4:1–5. Espinoza J et al. Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med. 2006;34(6):447-58. Labarrere CA et al. Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. Am J Obstet Gynecol. 2017 Mar;216(3):287.e1-287.e16.
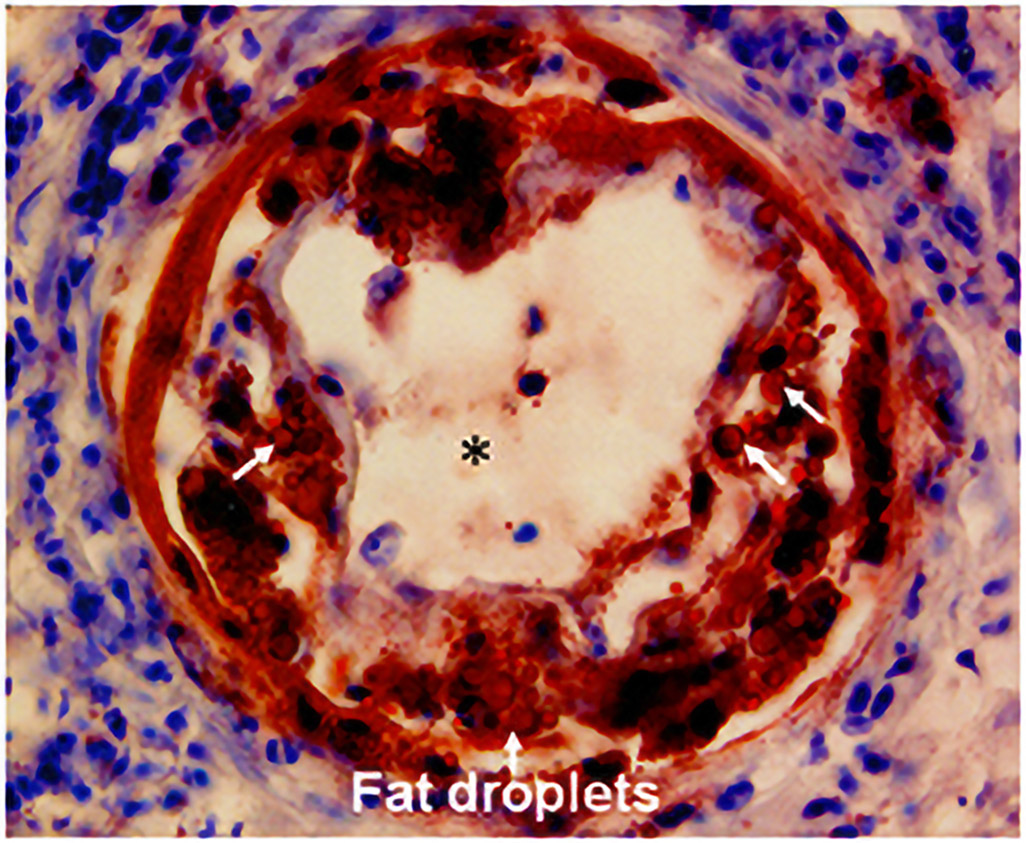
Figure 5.
Acute atherosis on oil-red O staining. Fat droplets (arrows) in the non-transformed spiral artery are stained red. *Lumen of spiral artery. Cover, Am J Obstet Gynecol. November 2014 Yeon Mee Kim, Roberto Romero.
The following evidence supports a causal link between placental ischemia and preeclampsia: (1) experimentally induced ischemia in several animal models leads to hypertension and proteinuria;3, 5, 7 (2) uterine blood flow is lower in patients with preeclampsia than in normal pregnant women;8, 11, 13 (3) placental histopathologic lesions indicative of ischemia (often referred to as maternal vascular malperfusion) are frequent, consistent findings in preeclampsia and eclampsia; 27 (4) the failure of physiologic transformation of the spiral arteries and atherosis are typical features of preeclampsia;28, 35 (5) the pulsatility index of the uterine artery (a parameter to assess resistance to flow) is higher in patients with preeclampsia than in normal pregnant women; this finding can be observed during the midtrimester of pregnancy, weeks or even months before the development of the disease;36, 37 (6) the maternal plasma placental growth factor (PlGF)/sFlt-1 ratio, a non-invasive marker of lesions of maternal vascular malperfusion, is elevated at the time of the onset of disease and prior to the development of preeclampsia;38-44 and (7) a blockade of sFlt-1 mRNA reduces hypertension and proteinuria.16 The frequency of placental lesions of maternal vascular malperfusion, of abnormalities in the uterine artery Doppler, and of alterations of biomarkers (eg PlGF/sFlt-1) is higher in preterm preeclampsia than in term preeclampsia, suggesting that ischemia plays a different role in early-onset versus late-onset preeclampsia.45-48
The case for ischemia as an etiologic factor could be even more persuasive if the treatment of ischemia could prevent the occurrence of preeclampsia. This evidence is difficult to generate in pregnant humans. Nonetheless, it can be argued that the efficacy of aspirin in reducing the rate of preterm preeclampsia49 is achieved through the prevention of arterial thrombosis in the spiral arteries and intervillous space, as this is the proposed mechanism for aspirin in the prevention of myocardial infarction in atherosclerosis.50 This interpretation would also explain the lack of efficacy of aspirin in preventing preeclampsia at term, given that ischemia seems to play a lesser role.
Maternal infection
Maternal infection has been implicated in the etiology for preeclampsia and eclampsia since the beginning of the 20th century. Albert51 proposed that the “toxins” responsible for eclampsia were the product of putrefactive changes in the uterine cavity caused by the action of bacteria (“a latent microbic endometritis”). Indeed, a microorganism ‘Bacillus eclampsiae’ was proposed to be the cause.52, 53 This view progressively fell out of favor because preeclampsia and eclampsia do not present the typical features, eg a fever, of an infectious disease. Nonetheless, the idea that microorganisms may be involved in the genesis of preeclampsia and eclampsia recurs in the literature every few years, and it has reemerged recently based on the relationship between periodontal disease, urinary tract infection, SARS-CoV-2 infection, and maternal gut dysbiosis.
Periodontal disease
The best evidence to support a relationship between microorganisms and preeclampsia derives from studies of periodontal disease, which increases the risk of developing this pregnancy-related syndrome (OR 1.76; 95% confidence interval (CI) 1.43-2.18).54 The term periodontal disease refers to an inflammatory condition caused by immune dysfunction initiated by bacteria within the oral cavity.55 The spectrum of disease ranges from gingivitis (inflammation of the soft tissues only) to the destruction of the connective tissue attachment and alveolar bone, which can eventually lead to tooth loss.55 Bacteria in the periodontal space can be released during dental procedures or in the course of severe disease, leading to a systemic inflammatory response that can cause damage and seed sites in the cardiovascular system.56 Indeed, strong evidence indicates that periodontal disease is a risk factor for atherosclerotic cardiovascular diseases, including atherosclerosis, coronary artery disease, stroke, and atrial fibrillation.57, 58 In brief, such evidence denotes that 1) microorganisms found in the periodontal space can cause bacteremia;59 2) bacteria from the oral cavity have been found in atheromatous plaques;60 and 3) periodontal infections can induce vascular lesions in the aorta and coronary arteries.57, 61 In an animal model of hyperlipidemia, ie apolipoprotein E null mice, an oral infection with Porphyromonas gingivalis led to plaque in the aorta (Figure 6).61 Similar findings have been reported in an integrin β6 null mice model with polymicrobial infection and periodontal pathogens.62 The etiologic role of periodontal disease in preeclampsia is predicated on the same mechanisms linking periodontal disease and atherosclerosis.
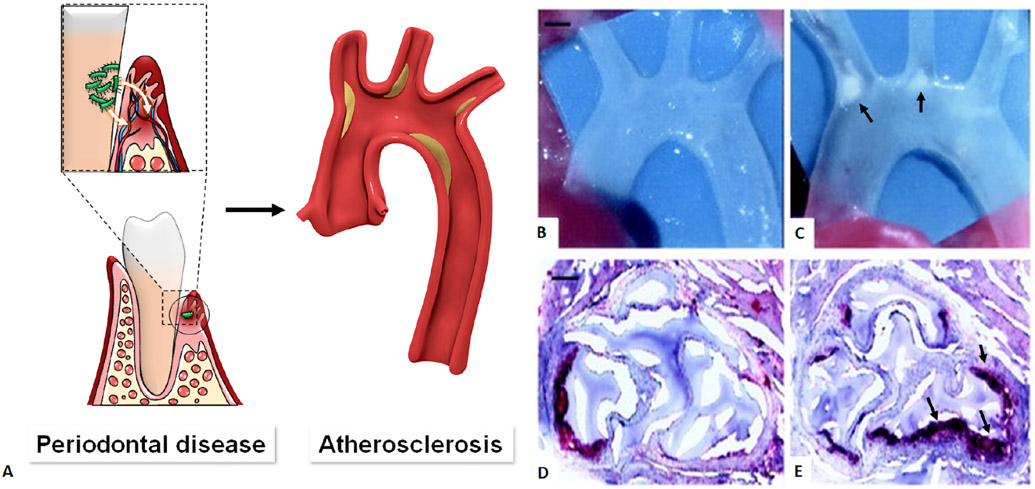
Figure 6.
Periodontal diseases and association with atherosclerotic disease. A. Bacteria found in the periodontal space can enter the bloodstream (bacteremia) and eventually the heart, resulting in atherosclerotic plaque in the heart blood vessels. Compared with uninfected control (B), periodontal infection with Porphyromonas gingivalis causes atherosclerotic aortic arch plaques (C, arrow) in apoE-null mice. Scale bar =1 mm. Oil red O staining of cryosections at the aortic sinus shows few small fatty streaks (control, D), whereas atherosclerotic lesions were greater in number and size (arrow) in infected animals (E). Scale bar=50 μm. Modified from Hajishengallis G et al. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021 Jul;21(7):426-440 and Lalla E. et al, Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2003 Aug 1;23(8):1405-11.
Offenbacher, Beck, Boggess, and Murtha63 have provided clinical, epidemiologic, and experimental evidence that periodontal disease is causally linked to preeclampsia. For example, women with periodontal disease who have an elevated C-reactive protein (CRP) concentration are at increased risk for the development of preeclampsia compared to those without periodontal disease (adjusted relative risk (aRR) 5.8; 95% CI 1.2–26.9).63 An elevated CRP would indicate that periodontal disease has led to a systemic inflammatory process, thus it provides a link between periodontal disease and preeclampsia.
Urinary tract infection
The relationship between microbial colonization of the maternal urinary tract and preeclampsia has also been reported. A systematic review noted that urinary tract infections are associated with preeclampsia (OR 1.57; 95% CI 1.45-1.70).54 However, the case definitions have been broad and have included pyelonephritis, lower urinary tract infections, and asymptomatic bacteriuria as a group.64, 65 When subgroup analysis is performed, evidence for the association with preeclampsia either weakens or disappears. We have doubts that asymptomatic bacteriuria, which is not associated with a systemic inflammatory response,66 could cause preeclampsia. Isolated cases have documented instances in which preeclampsia is associated with malaria,67-69 cytomegalovirus (CMV),70, 71 human immunodeficiency virus,72, 73 but the evidence is insufficient to support causality.
Experimental observations supporting a causal relationship between infection and systemic inflammation and preeclampsia indicate that intravenous low-dose endotoxin administration in pregnant rats on day 14 of gestation results in the development of high blood pressure, proteinuria, low platelet count, and glomerular fibrinogen deposits.74 Bacterial endotoxin is a method utilized to induce a systemic inflammatory response and the activation of thrombin through the release of tissue factor. These mechanisms are implicated in the pathogenesis of preeclampsia.
SARS-CoV-2 Infection
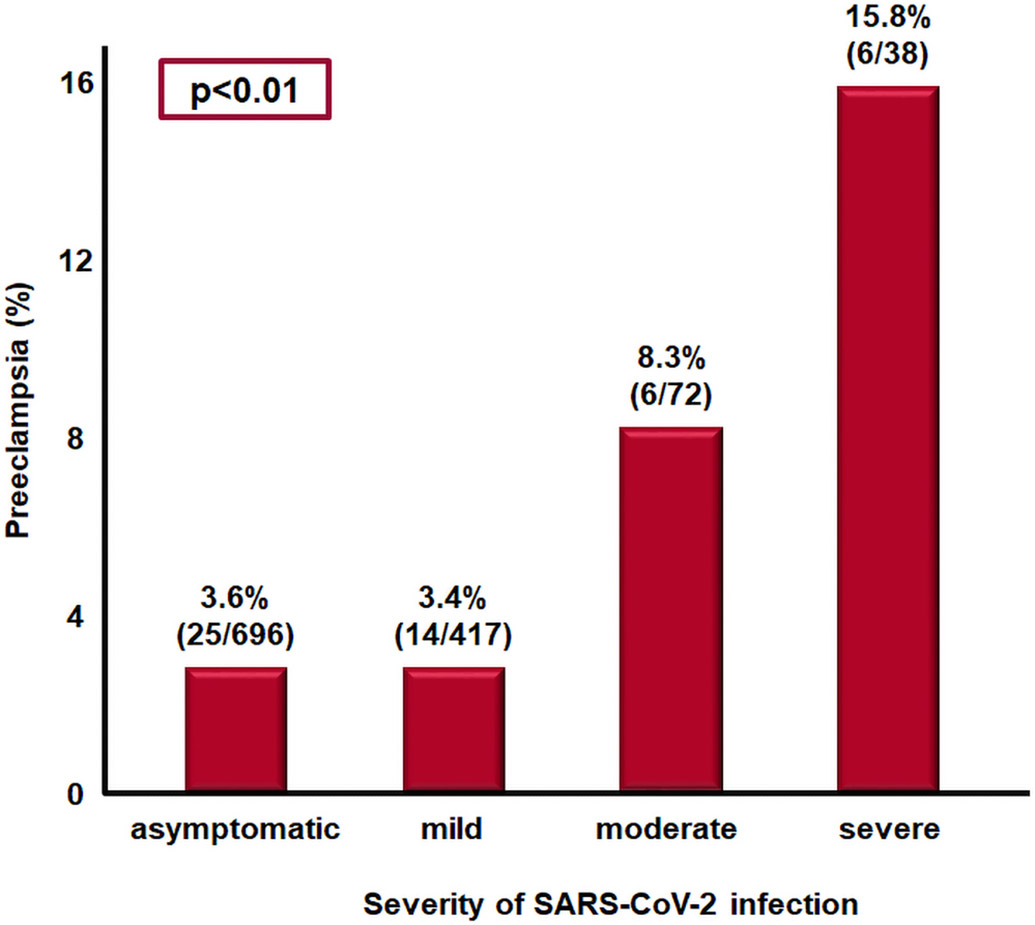
Early during the COVID-19 pandemic, it was recognized that a subset of non-pregnant patients developed hypertension,75-77 proteinuria,78-81 thrombocytopenia,82, 83 and elevated liver enzymes,84, 85 which resemble preeclampsia and hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome. A recent meta-analysis demonstrated that SARS-CoV-2 infection during pregnancy is associated with a significant increase in the odds of developing preeclampsia (OR 1.58; 95% CI 1.39-1.8), preeclampsia with severe features (OR 1.76; 95% CI 1.18-2.63), eclampsia (OR 1.97; 95% CI 1.01-3.84), and HELLP syndrome (OR 2.01; 95% CI 1.48-2.97).86 In addition, there is a dose-response relationship between SARS-CoV-2 infection and the subsequent development of preeclampsia (Figure 7).87 Patients with severe COVID-19 had a 5-fold greater risk of preeclampsia than those with asymptomatic COVID-19.87 The median interval between maternal SARS-CoV-2 infection and the subsequent development of preeclampsia is 3.8 weeks (interquartile range, 0.29-11.5).88
Figure 7.
Association between SARS-CoV-2 infection severity and subsequent development of preeclampsia. The most severe COVID-19, the greater the risk of preeclampsia.
Modified from Lai J et al. SARS-COV-2 and the subsequent development of preeclampsia and preterm birth: evidence of a dose response relationship supporting causality. Am J Obstet Gynecol. 2021 Aug 26:S0002-9378(21)00947-9.
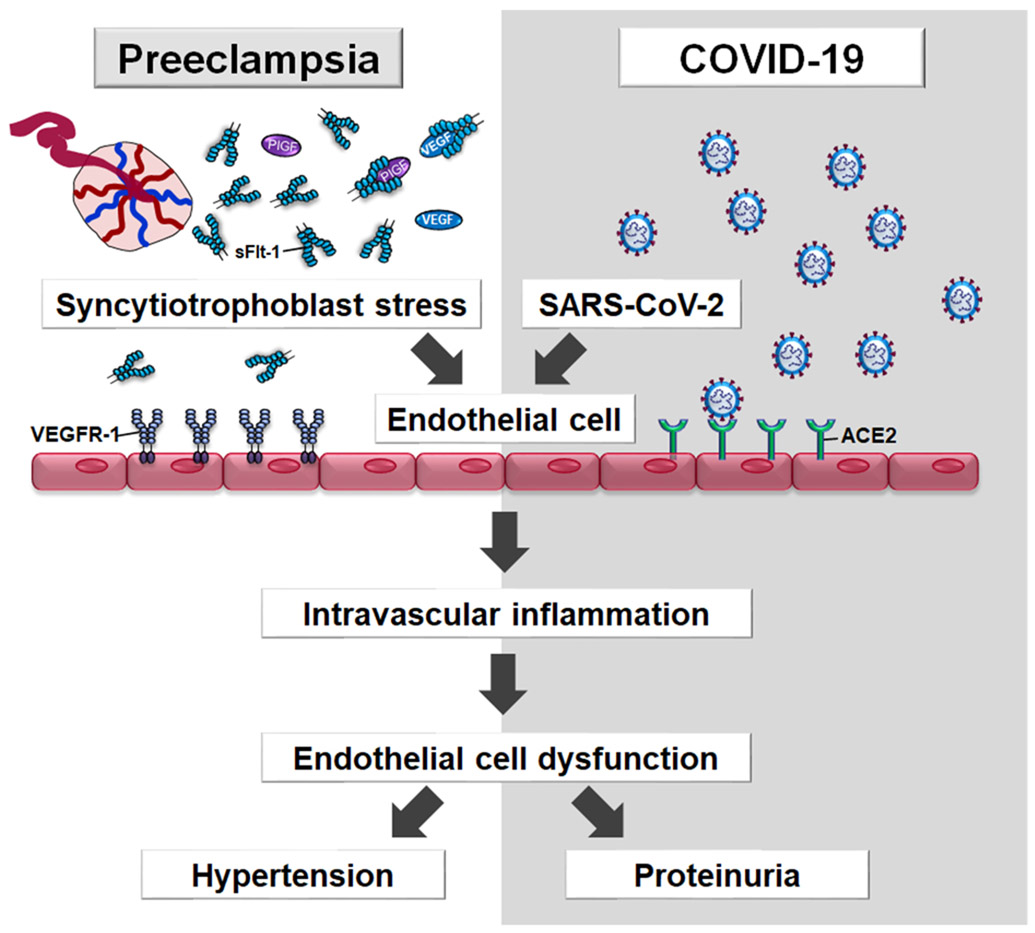
One mechanism whereby SARS-CoV-2 infection can be causally linked to preeclampsia has been proposed to involve endothelial dysfunction. Indeed, SARS-CoV-2 can infect endothelial cells that normally express angiotensin-converting enzyme 2 (ACE2), one of the cell entry receptors for the virus, leading to endothelitis.89, 90 Endothelial infection can induce the activation of thrombin, intravascular inflammation (ie a cytokine storm), and damage of the microvasculature in target organs; this susceptibility ultimately leads to the multi-systemic nature of the syndrome, which includes not only renal involvement but also central nervous system dysfunction and seizures.91-93 Therefore, an infectious process, which targets the endothelium, could lead to a syndrome similar to preeclampsia and eclampsia (Figure 8).
Figure 8.
Placental syncytiotrophoblast stress induces excessive sFlt-1 into the maternal circulation. sFlt-1 binds to free PlGF or VEGF (proangiogenic factors) with high affinity, thus preventing their interaction with their cell-surface receptors (i.e. VEGR-1) on the endothelial cells, leading to endothelial dysfunction. SARS-CoV-2 also targets the endothelium which normally express angiotensin-converting-enzyme 2 (ACE-2), one of the cell entry receptors for the virus, leading to endothelitis, which induces intravascular inflammation (i.e. cytokine storm) and endothelial dysfunction.
ACE2; angiotensin-converting enzyme 2, COVID-19; Coronavirus disease 2019, SARS-CoV-2; severe acute respiratory syndrome coronavirus 2, sFlt-1; Soluble Fms-Like Tyrosine Kinase-1, VEGFR-1; vascular endothelial growth factor receptor-1
Of interest, pregnant women with COVID-19 infection who developed preeclampsia-like symptoms: their recovery from SARS-CoV-2 infection, followed by the resolution of hypertension, occurred without the delivery of the fetus and placenta.94 It remains an open question whether preeclampsia, after SARS-CoV-2 infection, may or may not require placental involvement. Serum and plasma concentrations of sFlt-1, a marker of endothelial dysfunction, were elevated in non-pregnant subjects with COVID-19.95 This finding is consistent with another study in pregnant women with severe COVID-19 who presented an elevated maternal plasma concentration of sFlt-1 and a high sFlt-1/PlGF ratio.96 Genetic susceptibility may explain why some women with COVID-19 infection develop preeclampsia but others do not.97, 98
Maternal intestinal dysbiosis
The human gut microbiota plays an important role in host nutrition, harvesting of energy, and immune responses to potential pathogens.99-103 Normal pregnancy represents a state in which a major reorganization of energy distribution (harvesting, storage, and expenditure) is related to the need to support the growth and development of the fetus and placenta. Remodeling of the gut microbiota during normal human pregnancy, reported for the third trimester of pregnancy, indicated an overall increase in Proteobacteria and Actinobacteria and a reduction in microbial richness.104 When the intestinal content from normal pregnant women in the third trimester was administered to germ-free mice, it increased adiposity and insulin resistance,105 which have been attributed to the proinflammatory effects of the gut microbiota.104
Gut dysbiosis, or an imbalance among the human gut’s microbial communities, is now implicated in the development of atherosclerosis,106, 107 hypertension,108, 109 proteinuria,110 cardiometabolic syndrome,111, 112 and recently, preeclampsia.113-117 A causal link between gut dysbiosis and cardiovascular disease has been derived from an observation that the transplant of fecal material from hypertensive, non-pregnant, human subjects to germ-free mice led to hypertension.108 Similarly, fecal transplants from mice prone to atherosclerosis can transmit the condition to susceptible mice (eg apolipoprotein E null mice).118 This effect has been attributed, at least in part, to trimethylamine-N-oxide (TMAO), a bacteria-derived metabolite from choline and carnitine that is present in gut dysbiosis and has been shown to accelerate the development of atherosclerosis.107, 118
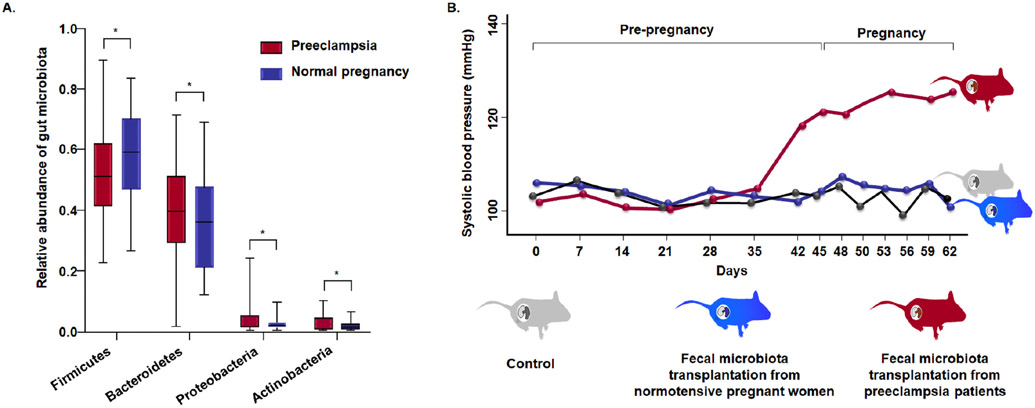
Changes in the human gut microbiota have been reported in preeclampsia,114, 115, 117, 119 which persist 6 weeks postpartum.120 The changes include a reduction in microbial burden with Firmicutes, Clostridia, Clostridiales, and Ruminococcus and an increase in Bacteroidetes, Proteobacteria, Actinobacteria, Bacteroidia, Gammaproteobacteria, and Enterobacteriaceae (Figure 9A).119 Moreover, the study provides evidence to support causality: fecal transplants from pregnant women with preeclampsia into mice led to the development of the syndrome.115 The experimental paradigm required the administration of fecal material from pregnant women with and without preeclampsia to non-pregnant mice that had received antibiotics for 5 days (to change gut flora); the mice were housed in pathogen-free facilities.115 Six weeks after the first fecal inoculation, mice were mated.115 Pregnant mice that had received the fecal microbiota transplantation from mothers with preeclampsia developed high systolic blood pressure and proteinuria, and they delivered less live pups than mice that receive fecal microbiota transplantation from women with normotensive pregnancy (Figure 9B).115 Collectively, this evidence suggests a role for gut dysbiosis in preeclampsia. However, further investigation into the precise mechanisms that may explain this phenomenon as well as the susceptibility of some pregnant women compared to others is necessary.
Figure 9.
(A) Gut microbiota in patients with preeclampsia (red) and those with normal pregnancy (blue). The significantly different genera (Firmicutes, Bacteroidetes, Proteobacterias, and Actinobacteria) at the phylum level in the two groups. The boxes represent the interquartile range (IQR) between first and third quartiles and the line inside represents the median. *p < 0.05. (B) Maternal intestinal dysbiosis and preeclampsia. Mice that received fecal microbiota from patients with preeclampsia (red) had higher systolic blood pressure than mice that received fecal microbiota from normotensive pregnant women (blue) or those that received the phosphate-buffered saline (control, grey). Modified from Wang J et al. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients with Preeclampsia. Front Cell Infect Microbiol. 2019 Dec 3;9:409 and Chen X. et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut. 2020 Mar;69(3):513-522.
Gestational diabetes mellitus, maternal obesity, and the metabolic syndrome
Gestational diabetes mellitus
Gestational diabetes mellitus is an independent risk factor for preeclampsia, after adjusting for confounders.121, 122 In a retrospective study of 647,392 pregnancies, women with gestational diabetes had an increased risk of preeclampsia (adjusted odds ratio (aOR) 1.29; 95% CI 1.19-1.41).121 Preexisting diabetes mellitus had also been linked to the development of preeclampsia: a systematic review reported that diabetes mellitus prior to pregnancy was associated with an increased risk of preeclampsia (aRR 3.56; 95% CI 2.54-4.99).123 Diabetes mellitus is considered to be strongly associated with late-onset rather than early-onset preeclampsia (early onset: aOR 1.87, 95% CI 1.6-2.18; late onset: aOR 2.46, 95% CI 2.32-2.61).124 The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Cooperative Research Group reported a significant positive association between the degree of maternal hyperglycemia and preeclampsia: the odds ratio for each 1-SD increase in glucose concentrations (fasting, one hour, and two hours after a 75mg glucose tolerance test) ranged from 1.21 to 1.28.125
A causal role was strengthened by the observation that the treatment of gestational diabetes mellitus with diet,126 insulin,127, 128 and metformin129-132 reduces the risk of preeclampsia. Two randomized clinical trials have shown that the treatment of gestational diabetes mellitus with insulin reduces the risk of preeclampsia (adjusted treatment effect: 0.70; 95% CI 0.51-0.95).127, 128 Metformin is associated with a reduced risk of preeclampsia (RR 0.68; 95% CI 0.48–0.95)131 and prolongation of gestation in women with preterm preeclampsia (median, 18 days).133 Prenatal exercise has also been reported to decrease the rate of gestational diabetes mellitus by 38% and preeclampsia by 41% based on the results of a systematic review and meta-analysis.134
Collectively, the evidence suggests a causal relationship between gestational diabetes mellitus and preeclampsia given the consistency of association, its magnitude, the temporal relationship, and the efficacy of interventions, such as insulin and metformin, in reducing the rate of preeclampsia.
Maternal obesity
Obesity, defined by a body mass index (BMI) of 30.0 kg/m2 or more, is strongly associated with preeclampsia.135-137 A meta-analysis of 29 prospective cohort studies (1,980,761 participants and 67,075 cases of preeclampsia) showed that maternal obesity was significantly associated with the development of preeclampsia (aOR 2.93; 95% CI 2.58-3.33), and the risk was even higher in severe obesity (BMI ≥ 35 kg/m2; aOR 4.14; 95% CI 3.61-4.75).138 A similar finding was reported overweight women (BMI: 26.1-29.0 kg/m2) who had an increased risk of preeclampsia in comparison to women with a normal BMI (RR 1.57; 95% CI 1.49-1.64).139
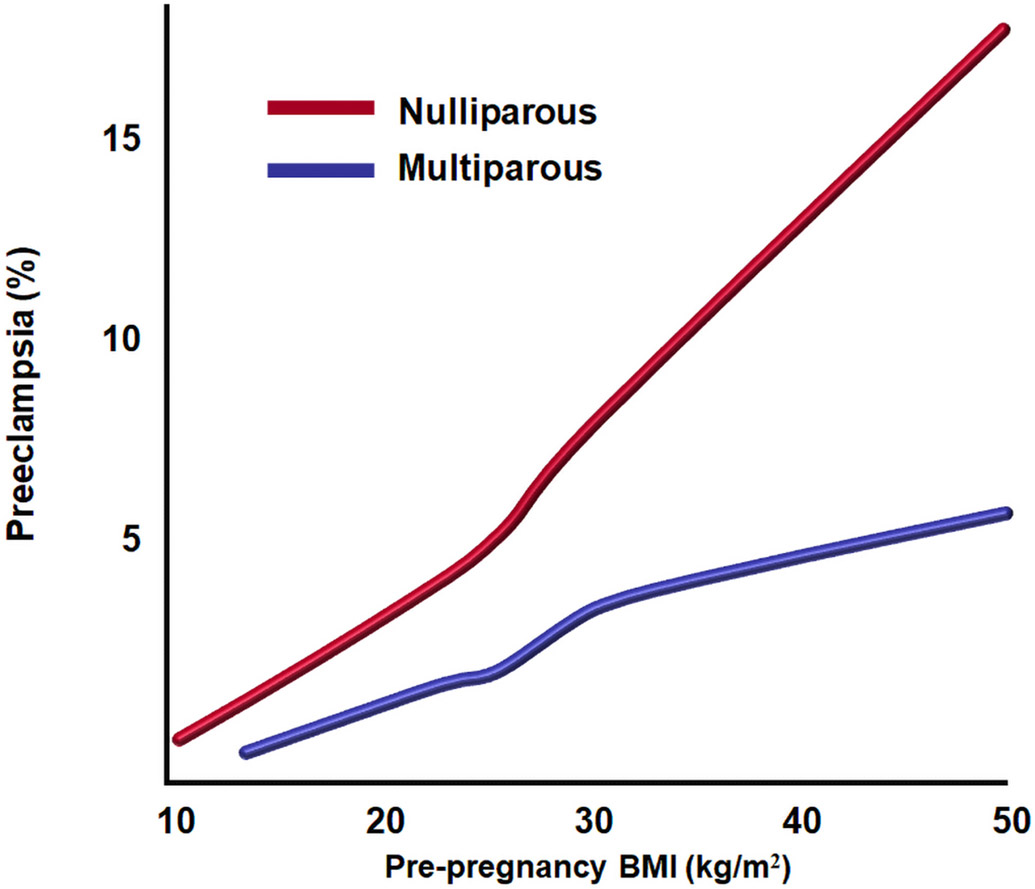
Moreover, a dose-dependent relationship was found between pre-pregnancy BMI and the risk of preeclampsia in both nulliparous and parous women in a large epidemiological study (41,000 nulliparous and 29,000 multiparous) (Figures 10).140 Data from the USA Collaborative Perinatal Project indicated similar results for Caucasian and for African-American women (severe preeclampsia: African American, OR 3.2, 95% CI 2.5-5.0; Caucasian, OR 3.4, 95% CI 2.1-5.6).141 Most studies considered that obesity predisposes mainly to late-onset preeclampsia.123, 141 However, a recent population-based study, which used US vital statistics data and included 15.8 million women (48,007 cases of early-onset disease and 777,715 cases of late-onset disease), demonstrated that maternal obesity is associated with an increased risk of both early-onset and late-onset disease (eg BMI 40 kg/m2 or greater: early-onset disease, aRR 2.18, 95% CI 2.12–2.24; late-onset disease: aRR 3.93, 95% CI 3.91–3.96).142 In addition, preconceptional maternal weight loss, either with lifestyle modification or by bariatric surgery, was shown to be effective in reducing the risk of preeclampsia (OR 0.67; 95% CI 0.51-0.88).143
Figure 10.
Probability of preeclampsia according to the pre-pregnancy body mass index in both nulliparous (top line, red) and multiparous (bottom line, blue) women. Modified from Catov JM et al. Risk of early or severe pre-eclampsia related to pre-existing conditions. Int J Epidemiol. 2007 Apr;36(2):412-9.
Metabolic syndrome
The term “metabolic syndrome” refers to a cluster of metabolic abnormalities that includes central obesity, insulin resistance, atherogenic dyslipidemia, and hypertension.144, 145 This syndrome is strongly associated with systemic inflammation, oxidative stress, and endothelial dysfunction, all of which are features of preeclampsia.146-149 A retrospective cohort study of 212,463 women showed that the presence of pre-pregnancy metabolic syndrome was associated with an increased risk of preeclampsia (aOR 1.48; 95% CI 1.26-1.74).150 Preeclampsia is also a risk factor for the subsequent development of metabolic syndrome after delivery.151-154 Moreover, bariatric surgery performed before pregnancy as a treatment for metabolic syndrome was associated with a lower rate of preeclampsia or eclampsia (aOR 0.2; 95% CI 0.09-0.44).155
The mechanisms whereby insulin resistance predisposes to the development of preeclampsia are related to intravascular inflammation and endothelial cell dysfunction,156, 157 which is the common pathway of the syndrome. Nonetheless, insulin resistance is neither required nor sufficient for the development of preeclampsia. The reason why some, but not other, patients with insulin resistance develop preeclampsia is unknown.
Sleep disorders
Sleep-disordered breathing, a term encompassing obstructive sleep apnea, snoring, periodic episodes of hypoxia, central apnea, and sleep hypopnea, during pregnancy is a risk factor of preeclampsia. A systematic review and meta-analysis of 120 studies, which included a total of 58,123,250 pregnant women, indicated that maternal sleep disturbances during pregnancy were associated with an increased risk of preeclampsia (OR 2.80; 95% CI 2.38-3.30);158 these disturbances included subjective sleep-disordered breathing (OR 3.52; 95% CI 2.58-4.79), obstructive sleep apnea (OR 2.36; 95% CI 2.00-2.79), and the restless legs syndrome (OR 1.83; 95% CI 1.04-3.21).158
Snoring is defined as the vibration of respiratory structures resulting from turbulent flow when the upper airway narrows during sleep.159 It is more common in pregnant women than in non-pregnant women (14%-23% vs 4%)160, 161 and increases the risk of hypertension,162 and new-onset snoring during pregnancy is associated with preeclampsia (OR 1.59; 95% CI 1.06-2.37).163 Evidence supporting a causal relationship between snoring and preeclampsia indicated that treatment with nasal continuous positive airway pressure (CPAP) improved blood pressure in women with preeclampsia.164, 165 Edwards et al164 reported that autosetting nasal CPAP administered through sleep resulted in a marked reduction in blood pressure (before treatment: mean systolic 146 mm Hg and mean diastolic 92 mm Hg; after treatment: mean systolic 128 mm Hg and mean diastolic 73 mm Hg). Recently, Bourjeily et al166 reported that pregnant women with obstructive sleep apnea presented a higher maternal plasma sFlt-1/PlGF concentration ratio than those in a control group and that maternal sFlt-1 concentrations were decreased after CPAP treatment.167
In normal pregnancy, blood pressure has a circadian rhythm and is at its highest during the daytime. A reversed diurnal blood-pressure rhythm (ie the nocturnal blood pressure was higher than the diurnal blood pressure) has been reported in preeclampsia.168, 169 Sleep architecture, using polysomnography, has shown that patients with preeclampsia had altered sleeping patterns,170 specifically spending more time in slow-wave sleep, in comparison to those with a normal pregnancy (43 ± 3 vs 21 ± 2; p < 0.001).170 One possible explanation for this finding is that cytokines, such as IL-1 and TNF-α, can increase the amount of slow-wave sleep,170 as they are elevated in the maternal circulation of those with preeclampsia.171-173 Collectively, the proposed mechanisms linking sleep disorders and preeclampsia involve intravascular inflammation and endothelial cell dysfunction.
Molar pregnancy
A hydatidiform mole, a gestational trophoblastic disease characterized by abnormal proliferation of trophoblast and hydropic changes of the chorionic villi, is associated with preeclampsia174 and sometimes presents before 20 weeks of gestation.175-178 The frequency of preeclampsia in patients with a hydatidiform mole ranges from 27% to 40% and is probably higher in patients who are left untreated until the second trimester.175-178
The mechanism whereby a complete hydatidiform mole causes preeclampsia involves the excess production of sFlt-1. Maternal serum sFlt-1 concentrations were 2-fold to 3-fold higher in patients with a hydatidiform mole than in those with a gestational age-matched control;179 an increased expression of sFlt-1 within the molar tissue has also been reported.180 Moreover, placentas with a hydatidiform mole had an increased expression of LIGHT (TNFSF14, or TNF superfamily member 14), which was co-localized with sFlt-1 in the molar tissue.181 Chorionic villi in the molar tissue are edematous or hydropic, which are often avascular or display markedly reduced vessel density.175 These villus capillary changes may lead to excessive production of sFlt-1 that enters maternal circulation and, consequently, to the development of the preeclampsia syndrome.
Fetal diseases
Specific fetal diseases associated with the development of preeclampsia include 1) Ballantyne or mirror syndrome;225, 226 2) Trisomy 13 or triploidy;227, 228 and 3) a unique complication of multiple gestations (eg twin-to-twin transfusion syndrome or selective fetal growth restriction).229 Ballantyne or mirror syndrome reflects the simultaneously edematous state of the mother, fetus, and placenta (also called triple edema).230, 231 This syndrome has been observed in fetal hydrops caused by rhesus isoimmunization,230 CMV,232 or parvovirus 19 infections,233 Ebstein’s anomaly,234 aneurysms of the vein of Galen,235 fetal supraventricular tachycardia,236, 237 and placental chorioangioma.238 In mirror syndrome, the mother may develop proteinuria, hypertension, and even severe preeclampsia.225, 226, 234, 239 The frequency of hypertension in patients with mirror syndrome is about 60%.225, 226, 234, 239 The reversal of preeclampsia and Ballantyne syndrome can occur after intrauterine transfusion in parvovirus-induced hydrops without delivery.240 All patients with mirror syndrome presented an increased maternal plasma concentration of sFlt-1 (above the 95th percentile for gestational age).241 In addition, it has been shown that the maternal sFlt-1 concentration returns to normal after intrauterine transfusion.242, 243
Another example of preeclampsia with fetal disease is Trisomy 13 or triploidy.227, 228, 244 A case control study of 25 cases with Trisomy 13 showed that the incidence of preeclampsia was significantly higher than detected in the normal karyotype control (44% vs 8%, p=0.001).245 Women with a Trisomy 13 pregnancy have a higher serum concentration of sFlt-1/PlGF ratio,246 and the placentas had an increased staining for sFlt-1 when compared to euploid as well as Trisomy 21.247 Interestingly, sFlt-1 is located on chromosome 13, suggesting the possibility that an extra copy of chromosome 13 may lead to increased production of sFlt-1.244, 247
Multiple gestations with twin-to-twin transfusion syndrome or selective fetal growth restriction can also predispose to the development of preeclampsia.229 Resolution of hypertension and proteinuria generally occur after the selective termination of pregnancy248-251 or after the fetal death of one twin.252 An improvement in angiogenic and anti-antiangiogenic profiles has also been demonstrated after selective termination or fetal demise of one twin.253, 254 Collectively, these observations indicate that preeclampsia can resolve without delivery and suggest that fetal compromise may induce the syndrome in some cases.
Autoimmune mechanisms: a role for antibodies against angiotensin II type I receptor
Preeclampsia is not traditionally considered an autoimmune disorder. However, a role for autoimmune mechanisms of disease has been investigated for several decades, given that patients with a systemic autoimmune disease, such as systemic lupus erythematosus (SLE) or antiphospholipid syndrome (APS), were at an increased risk for preeclampsia (SLE: RR 1.91, 95% CI 1.44-2.53; APS: RR 1.81, 95% CI 1.33-2.45).255-258 Similarly, several studies have shown that some specific autoantibodies, such as anti-β2 glycoprotein-I (ab2GPI), anti-cardiolipin antibodies (aCL), or lupus anti-coagulant (LA), are associated with preeclampsia.256, 259 However, the most compelling case for a role of an autoimmune mechanism is exemplified by antibodies against the angiotensin II receptor.
Nearly two decades ago, Wallukat et al260 reported that women with preeclampsia had antibodies that bind to the angiotensin II type I receptor (AT1-AA). Subsequently, substantial evidence has accumulated that supports a role for AT1-AA in the pathogenesis of preeclampsia. Such evidence comprises the following findings: 1) 80% of women with preeclampsia have increased concentrations of AT1-AA in maternal serum at the time of diagnosis;261 2) the concentration of AT1-AA in maternal serum correlates with the severity of hypertension and proteinuria;262 and 3) the administration of AT1-AA (either produced endogenously in response to transgenic expression of human renin and angiotensinogen, or administered by an injection of purified AT1-AA from women with preeclampsia) to pregnant rats led to hypertension, proteinuria, glomerular capillary endotheliosis,263 and the increased production of sFlt-1 as well as soluble endoglin,264 which can be attenuated by the administration of an AT1-receptor blocker (Losartan). However, one longitudinal study, conducted to determine whether the concentration of AT1-AA is increased before the onset of preeclampsia, did not produce such a result.265
What causes the production of AT1-AA? Placental ischemia after a reduction in uterine perfusion pressure has been shown to increase the concentration of serum AT1-AA.266 Also, the inhibition of AT1-AA by the administration of an epitope-binding peptide (‘n7AAc’) can reduce maternal blood pressure and plasma concentrations of endothelin-1, sFlt-1, and isoprostanes, a marker of oxidative stress.267 Moreover, the administration of the inflammatory mediators TNF-α,266, 268 IL-6,268, 269 and IL-17270 to pregnant rats can induce preeclampsia and enhance AT1-AA activity, which is abrogated by an AT1-AA blockade.267
Placental Aging
Cells and organisms have a finite lifespan. Human cells in culture can multiply (mitosis) a limited number of times (Hayflick limit) before they stop dividing and, subsequently, undergo programmed cell death, or apoptosis.271 The placenta is also thought to have a pre-specified lifetime, and as age advances, the functional capacity of placental cells declines. Placental aging has been described for several decades,272, 273 and premature aging has been implicated as a mechanism of disease for obstetric outcomes, eg preeclampsia,274, 275 fetal growth restriction,274, 276 fetal death,277 and preterm labor.278
Cytotrophoblasts are replicating cells, but the syncytiotrophoblast is generated by fusion and, therefore, is in a state of senescence (ie defined as the inability to multiply). Evidence supporting the concept that senescence is present in the normal syncytiotrophoblast includes the following findings: 1) high lysosomal activity indicated by staining with beta-galactosidase; 2) increased expression of cyclin-dependent kinase, p53, p21, p16; and 3) the presence of syncytial knots, proposed to be equivalent to senescence-associated heterochromatic foci, which are clusters of chromatin in the nucleus of senescent cells.279-281 Cindrova-Davies et al 280 demonstrated that markers of senescence increase in normal placentas as a function of advancing gestational age.
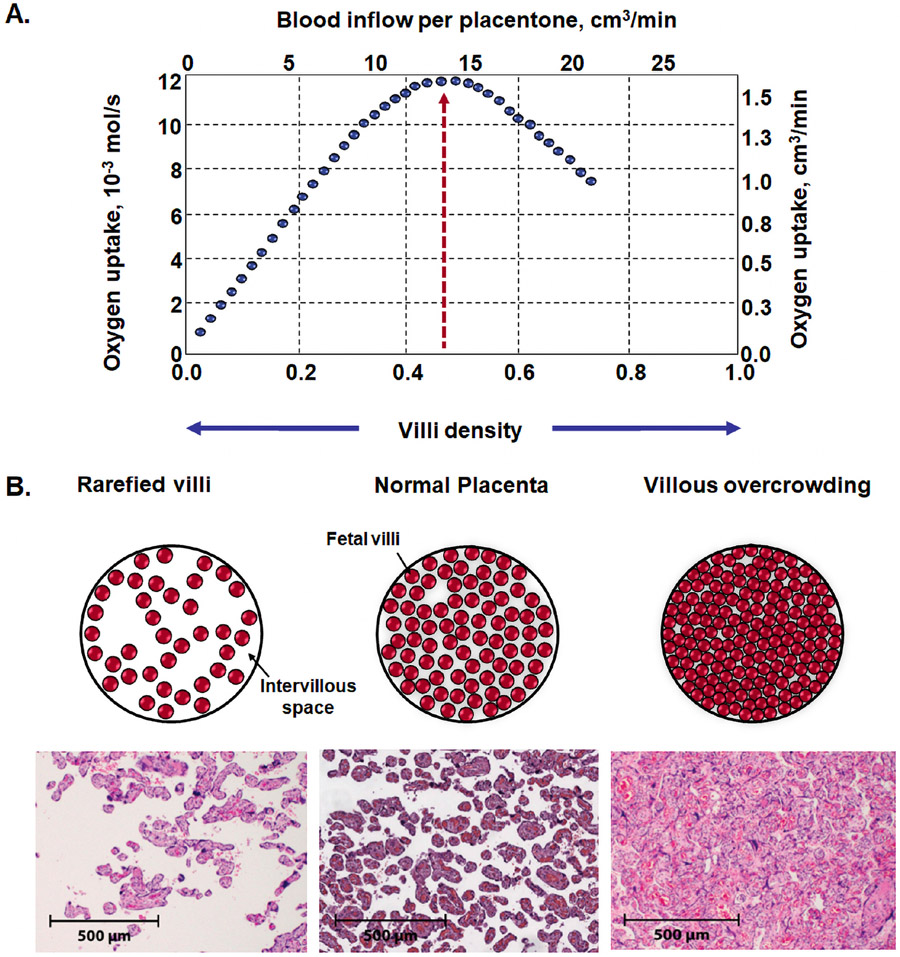
Placentas of patients with preeclampsia exhibit greater expression of p53, p21, and p16, shorter telomeres,274 and reduced telomerase activity.282, 283 Redman et al 279 proposed that with advancing gestational age some patients develop a “twilight placenta,” a condition in which the organ is affected by senescence. When placental growth reaches its limits at term, terminal villi become overcrowded with diminished intervillous pore size, leading to respiratory failure of the placenta (Figure 12) and contributing to intervillous hypoxia and syncytiotrophoblast stress.284-286 A twilight placenta has been invoked as a potential cause for late pregnancy problems such as late-onset preeclampsia,46, 287 unexplained term stillbirth, or fetal death in prolonged pregnancy.277, 283, 288 At present, there is not an easy or practical way to determine placental age; however, recent studies have identified an epigenetic clock for placental dating,289 which may be used to examine the role of placental aging in pregnancy complications in the future.
Figure 12.
A. Optimal villi density for maximal oxygen uptake in the human placenta. Placentas with low villi density (rarefied villi) have low oxygen uptake, as fetal villi are rare. By contrast, in placentas with high villous density (villous overcrowding), there is no space for intervillous space for oxygen exchange. The optimal villi density for the oxygen uptake was 0.47 ± 0.06, calculated from histomorphometrical data for villi and intervillous volumes of placentas. B. Cross-section of the placenta: 1) rarefied villi in preeclampsia; 2) normal placenta; and 3) villous overcrowding in diabetes mellitus. H&E stained and scale represents 50 μm. Modified from Serov AS et al. Optimal villi density for maximal oxygen uptake in the human placenta. J Theor Biol. 2015 Jan 7;364:383-96.
Breakdown of maternal-fetal immune tolerance
Viviparity involves the temporal coexistence of two individuals (mother and fetus) with different genetic makeup. The placenta and fetus together are viewed as the most successful semi-allograft (with maternal and paternal contributions), and the mechanisms responsible for the immune tolerance have been the subject of investigation for decades.182-187 Two typical features of the adaptive immune system are memory and specificity.188-190 A role for the immune system has been proposed because preeclampsia is more common in primigravidas than in parous women and in multigravidas with different fathers (primipaternity) than in subsequent pregnancies with the same father.191, 192 The predilection for primigravidas has been attributed to a memory-like phenomenon that protects mothers against paternal antigens in subsequent pregnancies.193 The higher frequency of preeclampsia in multigravidas whose pregnancy is from a different father supports the concept that the immune phenomenon is specific to paternally derived antigens.194, 195 Moreover, this syndrome is more common in pregnancies that occur from an egg donation in which the placenta and fetus represent a full allograft rather than a semi-allograft.196
The placenta and fetus express both paternal and maternal antigens; they are a semiallograft.197-199 The syncytiotrophoblast is in direct contact with maternal blood and the decidua, thus the maternal immune system is exposed to paternal antigens expressed by the fetus.182 Immune tolerance is a requirement for a successful pregnancy,185, 200, 201 and a breakdown of tolerance leads to maternal anti-fetal rejection, placental damage, and pregnancy complications that may include preeclampsia. Placental lesions, which represent manifestations of maternal anti-fetal rejection, are villitis of unknown etiology,202-205 massive perivillous fibrin deposition,206, 207 chronic chorioamnionitis,208-210 and chronic deciduitis of the placental basal plate.209 The frequency of chronic villitis is higher in preeclampsia than in normal pregnancy (25% vs 17%).203, 211 Massive perivillous fibrin deposition was present in 20% of patients with preeclampsia.212
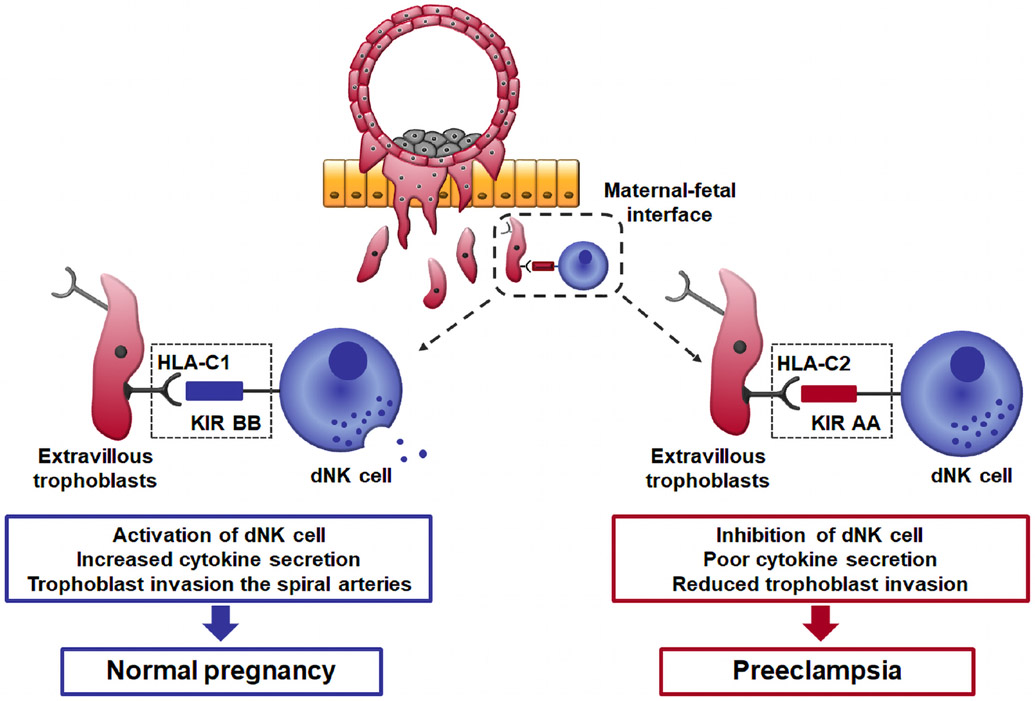
Maternal-fetal genetic incompatibility has been implicated in preeclampsia. Placentation is regulated by interactions between members of the killer cell immunoglobulin-like receptors (KIRs) expressed by decidual natural killer (dNK) cells and trophoblast human leukocyte antigen-C (HLA-C) molecules (Figure 11).213-216 Extravillous trophoblasts are specialized fetal cells that invade the decidua basalis, where they come into direct contact with maternal immune cells, eg dNK cells, macrophages, T cells, B cells, and dendritic cells.217 Extravillous trophoblasts do not express major T-cell ligands such as HLA-A and HLA-B molecules. Instead, these fetal cells express HLA-C, HLA-E, and HLA-G.218 The latter two are largely monomorphic, while HLA-C is polymorphic, and therefore will vary according to the genetic makeup of the father.219 On the other hand, the dNK cells express the receptors for HLA-C proteins; these receptors are called KIRs.220 Both maternal KIR and fetal HLA-C genes are highly polymorphic, and the interaction between HLA-C and KIR has a unique role in placentation through the facilitation of trophoblast invasion of the decidua and the physiologic transformation of the spiral artery. The molecular machinery implicated in this process involves chemokines (CXCL-10 and CXCL8, or IL-8) that can attract trophoblasts expressing their receptors (CXCR3 and CXCR1), metalloproteinases, and growth factors, including the angiogenic factors (PlGF, VEGF, etc.).221, 222 Moffett et al 213-215 has shown that normal pregnancy is more common when a mother has the KIR BB genotype and the fetus has HLA-C1 genes, whereas preeclampsia is more frequent when a mother has the KIR AA genotype with additional fetal HLA-C2 genes (HLA-C2 vs HLA-C1 in KIR AA mothers: 45% vs 20%; OR 2.38; 95% CI 1.45-3.90). By contrast, Staff et al 223 reported that fetal HLA-C2 combined with maternal KIR-BB was associated with placental lesions of acute atherosis. Patients with preeclampsia and acute atherosis presented this specific genetic combination in 60% of cases.223 The mechanisms whereby a breakdown of maternal-fetal immune tolerance leads to preeclampsia appear to involve defective placentation, an example of the convergence of an immune disorder with uteroplacental ischemia. Such can be the case for other etiologic factors in preeclampsia. For further details of immunological mechanisms of preeclampsia, the reader is referred to the review article by Robillard et al 224 in this Supplement.
Figure 11.
Interactions between maternal KIR and fetal HLA-C at the site of placentation. If the mother has a KIR BB genotype, which binds to trophoblast HLA-C1 molecules, this activates dNK cells, producing increased levels of cytokines such as GM-CSF that can enhance placentation. In contrast, when the mother has a KIR AA genotype and fetal HLA-C2 alleles, this inhibits dNK cells, leading to defective placentation.
KIR; killer cell immunoglobulin like receptors, HLA; human leukocyte antigen, dNK; decidual natural killer cells, GM-CSF; granulocyte-macrophage colony-stimulating factor. Modified from https://www.ivi-rmainnovation.com/maternal-killer-immunoglobulin-receptors-predictive-live-birth-rate/
Endocrine disorders
An association among several endocrine disorders and preeclampsia has been reported,290-297 yet the evidence for causality is the weakest among those reviewed in this article. Two explanations are plausible: the low frequency of endocrine disorders in pregnancy and the paucity of experimental evidence from animal studies of endocrine disorders. These investigations have not focused on the effect of such disorders in the development of preeclampsia. Hence, this section will review the evidence of an association and the proposed mechanism for activation of the common pathway.
Hyperparathyroidism
Normal pregnancy is characterized by changes in maternal calcium hemostasis, which are thought to occur to meet fetal demands. By term, the fetus has 30g of calcium, 20g of phosphorus, and 0.8g of magnesium, and 80% of calcium is deposited during the third trimester.298 The evidence that links altered calcium hemostasis to preeclampsia is as follows: 1) hypocalcemia is a risk factor for preeclampsia (OR 7.63; 95% CI 1.64-35.37);290 2) low vitamin D concentration in early pregnancy is associated with a 5-fold increased risk for preeclampsia;299 3) hyperparathyroidism is commonly found in pregnant women with low calcium and vitamin D;300, 301 4) patients diagnosed with parathyroid adenoma are at an increased risk for preeclampsia (OR 6.89; 95% CI 2.30-20.58);291 5) the absence of a vitamin D prophylaxis in the mother’s childhood is associated with a subsequent higher risk of preeclampsia;302 and 6) the administration of vitamin D combined with calcium or a multivitamin supplement has been shown to reduce blood pressure and the frequency of preeclampsia in a randomized trial.303, 304 The proposed mechanisms whereby primary hyperparathyroidism leads to preeclampsia are thought to involve endothelial cell damage, increased insulin resistance, left ventricular hypertrophy, and dyslipidemia.305, 306
Cushing’s syndrome, aldosteronism, pheochromocytoma, and paraganglioma
Cushing’s syndrome is associated with an increased risk of preeclampsia (aOR 2.20; 95% CI 1.18–4.41).292, 293 In non-pregnant subjects, the chronic glucocorticoid excess of Cushing’s syndrome is commonly associated with an increased cardiometabolic risk through an increase in pro-inflammatory adipokine production, alteration of coagulation and thrombosis factor, platelet function, and endothelial activation.307-309
Primary aldosteronism is the most common form of endocrine hypertension, and it can be caused by aldosterone-producing adenoma or bilateral adrenal hyperplasia.310, 311 Increased aldosterone secretion suppresses renin activity, leading to hypertension, hypokalemia, and hypernatremia.312, 313 Twenty-five percent of women with this disorder develop preeclampsia.294, 295
Pheochromocytoma and paraganglioma are rare catecholamine-producing tumors, with a reported frequency of 1/54,000 pregnancies.314 An excess of catecholamine released by these tumors can elicit signs or symptoms similar to preeclampsia (eg hypertension, headache, and proteinuria), making the diagnosis of pheochromocytoma during pregnancy difficult.314, 315 A recent meta-analysis that included 204 pregnant patients with pheochromocytoma paraganglioma showed that 20% of patients are initially misdiagnosed as preeclampsia.316 Maternal and fetal mortality are as high as 28% and 27%, respectively, when there is a delay in diagnosis and treatment.316, 317 Only a few studies have reported that patients with pheochromocytoma developed superimposed preeclampsia;296, 297 therefore, the existence of such a relationship is not clear yet.316
Conclusion
Multiple and sometimes overlapping insults can induce an adaptive homeostatic vascular response in pregnancy, which can be recognized clinically by the presence of hypertension and proteinuria (or other signs of multi-systemic involvement). When this response becomes maladaptive, it can cause target organ damage and becomes potentially life-threatening to the mother and fetus. This article has reviewed the etiological factors responsible for preeclampsia and eclampsia.
At present, the classification of the syndrome is largely based on the gestational age at the time of diagnosis (early-onset vs late-onset preeclampsia). Early-onset preeclampsia is associated with defective placentation, while late-onset preeclampsia appears to be related to the mismatch between maternal perfusion and feto-placental demands, together with a maternal predisposition to cardiovascular disease. However, it is necessary to identify the fundamental causes of the vascular dysfunction responsible for preeclampsia and to develop comprehensive predictive models and preventive interventions. This review highlights the multiple etiologies of the syndrome of preeclampsia. We propose that multiple etiologic factors converge to cause endothelial cell dysfunction, intravascular inflammation, and syncytiotrophoblast stress. The recognition that a viral infection as a result of SARS-CoV-2 infection can induce preeclampsia brings challenging questions of whether preeclampsia is a pregnancy-specific disorder caused by the placenta and cured only by delivery. Further research is required to assess the relative contribution of each cause and the effect of interventions to prevent this syndrome. A greater understanding of the differences in the etiology of each subtype of preeclampsia and the pathophysiology of other great obstetrical syndromes are required in order to improve the understanding of this elusive disease.
Funding:
This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health (NG-L).
Footnotes
Disclosure: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young J The etiology of eclampsia and albuminuria and their relation to accidental hemorrhage. Transactions Edinburgh Obstetrical Society 1914;39:153–202. [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon WE, Taylor FE. An Epidiascopic Demonstration on "The Physiological Action of the Placenta.". Proc R Soc Med 1908;1:11–3. [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden E, Hildebrand G, Page EW. Rise of blood pressure during ischemia of the gravid uterus. Proc Soc Exp Biol Med 1940;43:49–51. [Google Scholar]
- 4.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nature reviews Nephrology 2014;10:466–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar D Chronic placental ischemia in relation to toxemias of pregnancy: a preliminary report. Am J Obstet Gynecol 1962;84:1323–29. [Google Scholar]
- 6.Piering WF, Garancis JG, Becker CG, Beres JA, Lemann J Jr. Preeclampsia related to a functioning extrauterine placenta: report of a case and 25-year follow-up. Am J Kidney Dis 1993;21:310–3. [DOI] [PubMed] [Google Scholar]
- 7.Berger M, Cavanagh D. Toxemia of pregnancy. The hypertensive effect of acute experimental placental ischemia. Am J Obstet Gynecol 1963;87:293–305. [PubMed] [Google Scholar]
- 8.Browne JC, Veall N. The maternal placental blood flow in normotensive and hypertensive women. J Obstet Gynaecol Br Emp 1953;60:141–7. [DOI] [PubMed] [Google Scholar]
- 9.Janisch H, Leodolter S. Results of placental circulation measurements in hazard pregnancies. Z Geburtshilfe Perinatol 1973;177:74–80. [PubMed] [Google Scholar]
- 10.Olkkonen HS, Suonio S, Haring P. Determination of placental blood flow by external monitoring of 113In. Nuklearmedizin 1976;15:168–72. [PubMed] [Google Scholar]
- 11.Lippert TH, Cloeren SE, Fridrich R. Assessment of uteroplacental hemodynamics in complicated pregnancy. Int J Gynaecol Obstet 1978;16:274–80. [DOI] [PubMed] [Google Scholar]
- 12.Käär K, Jouppila P, Kuikka J, Luotola H, Toivanen J, Rekonen A. Intervillous blood flow in normal and complicated late pregnancy measured by means of an intravenous 133Xe method. Acta Obstet Gynecol Scand 1980;59:7–10. [DOI] [PubMed] [Google Scholar]
- 13.Lunell NO, Nylund LE, Lewander R, Sarby B. Uteroplacental blood flow in pre-eclampsia measurements with indium-113m and a computer-linked gamma camera. Clin Exp Hypertens B 1982;1:105–17. [DOI] [PubMed] [Google Scholar]
- 14.Alexander BT, Kassab SE, Miller MT, et al. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 2001;37:1191–5. [DOI] [PubMed] [Google Scholar]
- 15.Makris A, Thornton C, Thompson J, et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int 2007;71:977–84. [DOI] [PubMed] [Google Scholar]
- 16.Turanov AA, Lo A, Hassler MR, et al. RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat Biotechnol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakrania BA, George EM, Granger JP. Animal models of preeclampsia: investigating pathophysiology and therapeutic targets. Am J Obstet Gynecol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brosens I [Spiraled arterioles of the decidua basalis in the hypertensive complications of pregnancy. Anatomoclinical study]. Bull Soc R Belge Gynecol Obstet 1963;33:61–72. [PubMed] [Google Scholar]
- 19.Brosens I A STUDY OF THE SPIRAL ARTERIES OF THE DECIDUA BASALIS IN NORMOTENSIVE AND HYPERTENSIVE PREGNANCIES. J Obstet Gynaecol Br Commonw 1964;71:222–30. [DOI] [PubMed] [Google Scholar]
- 20.Melchiorre K, Giorgione V, Thilaganathan B. The placenta and preeclampsia: villain or victim? Am J Obstet Gynecol 2021. [DOI] [PubMed] [Google Scholar]
- 21.Hertig A Vascular pathology in hypertensive albuminuric toxemias of pregnancy. Clinics 1945;4:602. [Google Scholar]
- 22.De Wolf F, Robertson WB, Brosens I. The ultrastructure of acute atherosis in hypertensive pregnancy. Am J Obstet Gynecol 1975;123:164–74. [DOI] [PubMed] [Google Scholar]
- 23.Staff AC, Johnsen GM, Dechend R, Redman CW. Preeclampsia and uteroplacental acute atherosis: immune and inflammatory factors. J Reprod Immunol 2014;101-102:120–6. [DOI] [PubMed] [Google Scholar]
- 24.Kim YM, Chaemsaithong P, Romero R, et al. Placental lesions associated with acute atherosis. J Matern Fetal Neonatal Med 2015;28:1554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labarrere CA, DiCarlo HL, Bammerlin E, et al. Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. Am J Obstet Gynecol 2017;216:287 e1–87 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMaster-Fay R Uteroplacental vascular syndromes: Theories, hypotheses and controversies. Clinical Obstetrics, Gynecology and Reproductive Medicine 2018;4. [Google Scholar]
- 27.Falco ML, Sivanathan J, Laoreti A, Thilaganathan B, Khalil A. Placental histopathology associated with pre-eclampsia: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017;50:295–301. [DOI] [PubMed] [Google Scholar]
- 28.Staff AC, Fjeldstad HE, Fosheim IK, et al. Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia. Am J Obstet Gynecol 2021. [DOI] [PubMed] [Google Scholar]
- 29.Kim YM, Chaemsaithong P, Romero R, et al. The frequency of acute atherosis in normal pregnancy and preterm labor, preeclampsia, small-for-gestational age, fetal death and midtrimester spontaneous abortion. J Matern Fetal Neonatal Med 2015:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YM, Bujold E, Chaiworapongsa T, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2003;189:1063–9. [DOI] [PubMed] [Google Scholar]
- 31.Kim YM, Chaiworapongsa T, Gomez R, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol 2002;187:1137–42. [DOI] [PubMed] [Google Scholar]
- 32.Labarrere C, Alonso J, Manni J, Domenichini E, Althabe O. Immunohistochemical findings in acute atherosis associated with intrauterine growth retardation. Am J Reprod Immunol Microbiol 1985;7:149–55. [DOI] [PubMed] [Google Scholar]
- 33.Khong TY. Acute atherosis in pregnancies complicated by hypertension, small-for-gestational-age infants, and diabetes mellitus. Arch Pathol Lab Med 1991;115:722–5. [PubMed] [Google Scholar]
- 34.Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract Res Clin Obstet Gynaecol 2011;25:313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espinoza J, Romero R, Mee Kim Y, et al. Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med 2006;34:447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallo DM, Poon LC, Akolekar R, Syngelaki A, Nicolaides KH. Prediction of preeclampsia by uterine artery Doppler at 20-24 weeks' gestation. Fetal Diagn Ther 2013;34:241–7. [DOI] [PubMed] [Google Scholar]
- 37.Khalil A, Garcia-Mandujano R, Maiz N, Elkhouli M, Nicolaides KH. Longitudinal changes in uterine artery Doppler and blood pressure and risk of pre-eclampsia. Ultrasound Obstet Gynecol 2014;43:541–7. [DOI] [PubMed] [Google Scholar]
- 38.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006;355:992–1005. [DOI] [PubMed] [Google Scholar]
- 39.Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med 2008;21:9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med 2008;21:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusanovic JP, Romero R, Chaiworapongsa T, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med 2009;22:1021–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med 2011;24:1187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaisbuch E, Whitty JE, Hassan SS, et al. Circulating angiogenic and antiangiogenic factors in women with eclampsia. Am J Obstet Gynecol 2011;204:152.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaiworapongsa T, Romero R, Korzeniewski SJ, et al. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med 2014;27:132–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espinoza J, Romero R, Nien JK, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol 2007;196:326.e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogge G, Chaiworapongsa T, Romero R, et al. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med 2011;39:641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rana S, Schnettler WT, Powe C, et al. Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens Pregnancy 2013;32:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orabona R, Donzelli CM, Falchetti M, Santoro A, Valcamonico A, Frusca T. Placental histological patterns and uterine artery Doppler velocimetry in pregnancies complicated by early or late pre-eclampsia. Ultrasound Obstet Gynecol 2016;47:580–5. [DOI] [PubMed] [Google Scholar]
- 49.Rolnik DL, Wright D, Poon LC, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med 2017;377:613–22. [DOI] [PubMed] [Google Scholar]
- 50.Relman AS. Aspirin for the primary prevention of myocardial infarction. N Engl J Med 1988;318:245–6. [DOI] [PubMed] [Google Scholar]
- 51.Albert. Archiv für Gynaekologie 1901;Ixiii:488 [Google Scholar]
- 52.Loudon I Some historical aspects of toxaemia of pregnancy. A review. BJOG 1991;98:853–58. [DOI] [PubMed] [Google Scholar]
- 53.DeLee J Theories of eclampsia. Am J Obstet 1905;51:325–30. [Google Scholar]
- 54.Conde-Agudelo A, Villar J, Lindheimer M. Maternal infection and risk of preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol 2008;198:7–22. [DOI] [PubMed] [Google Scholar]
- 55.Williams RC. Periodontal disease. N Engl J Med 1990;322:373–82. [DOI] [PubMed] [Google Scholar]
- 56.Sanz M, Del Castillo AM, Jepsen S, et al. Periodontitis and Cardiovascular Diseases. Consensus Report. Global heart 2020;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrera D, Molina A, Buhlin K, Klinge B. Periodontal diseases and association with atherosclerotic disease. Periodontol 2000 2020;83:66–89. [DOI] [PubMed] [Google Scholar]
- 58.Sen S, Redd K, Trivedi T, et al. Periodontal Disease, Atrial Fibrillation and Stroke. Am Heart J 2021;235:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol 2021;21:426–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Velsko IM, Chukkapalli SS, Rivera MF, et al. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One 2014;9:e97811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lalla E, Lamster IB, Hofmann MA, et al. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol 2003;23:1405–11. [DOI] [PubMed] [Google Scholar]
- 62.Velsko IM, Chukkapalli SS, Rivera-Kweh MF, et al. Periodontal pathogens invade gingiva and aortic adventitia and elicit inflammasome activation in αvβ6 integrin-deficient mice. Infect Immun 2015;83:4582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruma M, Boggess K, Moss K, et al. Maternal periodontal disease, systemic inflammation, and risk for preeclampsia. Am J Obstet Gynecol 2008;198:389.e1–5. [DOI] [PubMed] [Google Scholar]
- 64.Easter SR, Cantonwine DE, Zera CA, Lim KH, Parry SI, McElrath TF. Urinary tract infection during pregnancy, angiogenic factor profiles, and risk of preeclampsia. Am J Obstet Gynecol 2016;214:387.e1–7. [DOI] [PubMed] [Google Scholar]
- 65.Yan L, Jin Y, Hang H, Yan B. The association between urinary tract infection during pregnancy and preeclampsia: A meta-analysis. Medicine (Baltimore) 2018;97:e12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheterized patients. Arch Intern Med 2000;160:678–82. [DOI] [PubMed] [Google Scholar]
- 67.Sartelet H, Rogier C, Milko-Sartelet I, Angel G, Michel G. Malaria associated pre-eclampsia in Senegal. Lancet 1996;347:1121. [DOI] [PubMed] [Google Scholar]
- 68.Ndao CT, Dumont A, Fievet N, Doucoure S, Gaye A, Lehesran JY. Placental malarial infection as a risk factor for hypertensive disorders during pregnancy in Africa: a case-control study in an urban area of Senegal, West Africa. Am J Epidemiol 2009;170:847–53. [DOI] [PubMed] [Google Scholar]
- 69.Mruma HA, McQuillan R, Norrie J. The association of malaria infection and gestational hypertension in Africa: Systematic review and meta-analysis. Journal of global health 2020;10:020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carreiras M, Montagnani S, Layrisse Z. Preeclampsia: a multifactorial disease resulting from the interaction of the feto-maternal HLA genotype and HCMV infection. Am J Reprod Immunol 2002;48:176–83. [DOI] [PubMed] [Google Scholar]
- 71.von Dadelszen P, Magee LA, Krajden M, et al. Levels of antibodies against cytomegalovirus and Chlamydophila pneumoniae are increased in early onset pre-eclampsia. BJOG 2003;110:725–30. [PubMed] [Google Scholar]
- 72.Mattar R, Amed AM, Lindsey PC, Sass N, Daher S. Preeclampsia and HIV infection. Eur J Obstet Gynecol Reprod Biol 2004;117:240–1. [DOI] [PubMed] [Google Scholar]
- 73.Nourollahpour Shiadeh M, Riahi SM, Khani S, et al. Human Immunodeficiency Virus and risk of pre-eclampsia and eclampsia in pregnant women: A meta-analysis on cohort studies. Pregnancy Hypertens 2019;17:269–75. [DOI] [PubMed] [Google Scholar]
- 74.Faas MM, Schuiling GA, Baller JF, Visscher CA, Bakker WW. A new animal model for human preeclampsia: ultra-low-dose endotoxin infusion in pregnant rats. Am J Obstet Gynecol 1994;171:158–64. [DOI] [PubMed] [Google Scholar]
- 75.Somani SS, Richter F, Fuster V, et al. Characterization of Patients Who Return to Hospital Following Discharge from Hospitalization for COVID-19. J Gen Intern Med 2020;35:2838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiong S, Liu L, Lin F, et al. Clinical characteristics of 116 hospitalized patients with COVID-19 in Wuhan, China: a single-centered, retrospective, observational study. BMC Infect Dis 2020;20:787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet 2021;397:220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nature reviews Nephrology 2020;16:747–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zahid U, Ramachandran P, Spitalewitz S, et al. Acute Kidney Injury in COVID-19 Patients: An Inner City Hospital Experience and Policy Implications. Am J Nephrol 2020;51:786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez-Rojas MA, Vega-Vega O, Bobadilla NA. Is the kidney a target of SARS-CoV-2? Am J Physiol Renal Physiol 2020;318:F1454–f62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020;97:829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rahi MS, Jindal V, Reyes SP, Gunasekaran K, Gupta R, Jaiyesimi I. Hematologic disorders associated with COVID-19: a review. Ann Hematol 2021;100:309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castro RA, Frishman WH. Thrombotic Complications of COVID-19 Infection: A Review. Cardiol Rev 2021;29:43–47. [DOI] [PubMed] [Google Scholar]
- 84.Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. Journal of clinical and translational hepatology 2020;8:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saviano A, Wrensch F, Ghany MG, Baumert TF. Liver Disease and Coronavirus Disease 2019: From Pathogenesis to Clinical Care. Hepatology 2021;74:1088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Conde-Agudelo A, Romero R. SARS-COV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai J, Romero R, Tarca AL, et al. SARS-COV-2 and the subsequent development of preeclampsia and preterm birth: evidence of a dose response relationship supporting causality. Am J Obstet Gynecol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosenbloom JI, Raghuraman N, Carter EB, Kelly JC. Coronavirus disease 2019 infection and hypertensive disorders of pregnancy. Am J Obstet Gynecol 2021;224:623–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. The Lancet 2020;395:1417–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020;383:120–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020;98:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharifian-Dorche M, Huot P, Osherov M, et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci 2020;417:117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thakur V, Ratho RK, Kumar P, et al. Multi-Organ Involvement in COVID-19: Beyond Pulmonary Manifestations. Journal of clinical medicine 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mendoza M, Garcia-Ruiz I, Maiz N, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG 2020;127:1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giardini V, Carrer A, Casati M, Contro E, Vergani P, Gambacorti-Passerini C. Increased sFLT-1/PlGF ratio in COVID-19: A novel link to angiotensin II-mediated endothelial dysfunction. Am J Hematol 2020;95:E188–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Espino-y-Sosa S, Martinez-Portilla RJ, Torres-Torres J, et al. Novel Ratio Soluble Fms-like Tyrosine Kinase-1/Angiotensin-II (sFlt-1/ANG-II) in Pregnant Women Is Associated with Critical Illness in COVID-19. Viruses 2021;13:1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee JW, Lee IH, Sato T, Kong SW, Iimura T. Genetic variation analyses indicate conserved SARS-CoV-2-host interaction and varied genetic adaptation in immune-response factors in modern human evolution. Dev Growth Differ 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choudhary S, Sreenivasulu K, Mitra P, Misra S, Sharma P. Role of Genetic Variants and Gene Expression in the Susceptibility and Severity of COVID-19. Ann Lab Med 2021;41:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004;101:15718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444:1022–3. [DOI] [PubMed] [Google Scholar]
- 101.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- 102.Jia W, Li H, Zhao L, Nicholson JK. Gut microbiota: a potential new territory for drug targeting. Nature reviews Drug discovery 2008;7:123–9. [DOI] [PubMed] [Google Scholar]
- 103.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 2012;336:1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koren O, Goodrich JK, Cullender TC, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012;150:470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kimura I, Miyamoto J, Ohue-Kitano R, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 2020;367. [DOI] [PubMed] [Google Scholar]
- 106.Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol 2017;14:79–87. [DOI] [PubMed] [Google Scholar]
- 107.Sanchez-Rodriguez E, Egea-Zorrilla A, Plaza-Díaz J, et al. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim S, Goel R, Kumar A, et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond) 2018;132:701–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kanbay M, Onal EM, Afsar B, et al. The crosstalk of gut microbiota and chronic kidney disease: role of inflammation, proteinuria, hypertension, and diabetes mellitus. Int Urol Nephrol 2018;50:1453–66. [DOI] [PubMed] [Google Scholar]
- 111.Leshem A, Horesh N, Elinav E. Fecal Microbial Transplantation and Its Potential Application in Cardiometabolic Syndrome. Front Immunol 2019;10:1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kazemian N, Mahmoudi M, Halperin F, Wu JC, Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome 2020;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dunlop AL, Mulle JG, Ferranti EP, Edwards S, Dunn AB, Corwin EJ. Maternal Microbiome and Pregnancy Outcomes That Impact Infant Health: A Review. Adv Neonatal Care 2015;15:377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hampton T Do Gut Bacteria Play a Role in Preeclampsia? JAMA 2020;323:2120–21. [DOI] [PubMed] [Google Scholar]
- 115.Chen X, Li P, Liu M, et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 2020;69:513–22. [DOI] [PubMed] [Google Scholar]
- 116.Beckers KF, Sones JL. Maternal microbiome and the hypertensive disorder of pregnancy, preeclampsia. Am J Physiol Heart Circ Physiol 2020;318:H1–h10. [DOI] [PubMed] [Google Scholar]
- 117.Altemani F, Barrett HL, Gomez-Arango L, et al. Pregnant women who develop preeclampsia have lower abundance of the butyrate-producer Coprococcus in their gut microbiota. Pregnancy Hypertens 2021;23:211–19. [DOI] [PubMed] [Google Scholar]
- 118.Gregory JC, Buffa JA, Org E, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem 2015;290:5647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang J, Gu X, Yang J, Wei Y, Zhao Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients With Preeclampsia. Frontiers in cellular and infection microbiology 2019;9:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lv LJ, Li SH, Li SC, et al. Early-Onset Preeclampsia Is Associated With Gut Microbial Alterations in Antepartum and Postpartum Women. Frontiers in cellular and infection microbiology 2019;9:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schneider S, Freerksen N, Röhrig S, Hoeft B, Maul H. Gestational diabetes and preeclampsia--similar risk factor profiles? Early Hum Dev 2012;88:179–84. [DOI] [PubMed] [Google Scholar]
- 122.Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep 2015;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ 2005;330:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early-versus late-onset disease. Am J Obstet Gynecol 2013;209:544.e1–44.e12. [DOI] [PubMed] [Google Scholar]
- 125.Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 126.Kinshella MW, Omar S, Scherbinsky K, et al. Maternal Dietary Patterns and Pregnancy Hypertension in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. Adv Nutr 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477–86. [DOI] [PubMed] [Google Scholar]
- 128.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Romero R, Erez O, Huttemann M, et al. Metformin, the aspirin of the 21st century: its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am J Obstet Gynecol 2017;217:282–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kalafat E, Sukur YE, Abdi A, Thilaganathan B, Khalil A. Metformin for prevention of hypertensive disorders of pregnancy in women with gestational diabetes or obesity: systematic review and meta-analysis of randomized trials. Ultrasound Obstet Gynecol 2018;52:706–14. [DOI] [PubMed] [Google Scholar]
- 131.Alqudah A, McKinley MC, McNally R, et al. Risk of pre-eclampsia in women taking metformin: a systematic review and meta-analysis. Diabet Med 2018;35:160–72. [DOI] [PubMed] [Google Scholar]
- 132.Tarry-Adkins JL, Ozanne SE, Aiken CE. Impact of metformin treatment during pregnancy on maternal outcomes: a systematic review/meta-analysis. Sci Rep 2021;11:9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cluver CA, Hiscock R, Decloedt EH, et al. Use of metformin to prolong gestation in preterm pre-eclampsia: randomised, double blind, placebo controlled trial. BMJ 2021;374:n2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davenport MH, Ruchat SM, Poitras VJ, et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br J Sports Med 2018;52:1367–75. [DOI] [PubMed] [Google Scholar]
- 135.Sebire NJ, Jolly M, Harris JP, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 2001;25:1175–82. [DOI] [PubMed] [Google Scholar]
- 136.Roberts JM, Bodnar LM, Patrick TE, Powers RW. The Role of Obesity in Preeclampsia. Pregnancy Hypertens 2011;1:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chaemsaithong P, Leung TY, Sahota D, et al. Body mass index at 11-13 weeks' gestation and pregnancy complications in a Southern Chinese population: a retrospective cohort study. J Matern Fetal Neonatal Med 2019;32:2056–68. [DOI] [PubMed] [Google Scholar]
- 138.Wang Z, Wang P, Liu H, et al. Maternal adiposity as an independent risk factor for pre-eclampsia: a meta-analysis of prospective cohort studies. Obes Rev 2013;14:508–21. [DOI] [PubMed] [Google Scholar]
- 139.Conde-Agudelo A, Belizan JM. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG 2000;107:75–83. [DOI] [PubMed] [Google Scholar]
- 140.Catov JM, Ness RB, Kip KE, Olsen J. Risk of early or severe pre-eclampsia related to pre-existing conditions. Int J Epidemiol 2007;36:412–9. [DOI] [PubMed] [Google Scholar]
- 141.Bodnar LM, Catov JM, Klebanoff MA, Ness RB, Roberts JM. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology 2007;18:234–9. [DOI] [PubMed] [Google Scholar]
- 142.Bicocca MJ, Mendez-Figueroa H, Chauhan SP, Sibai BM. Maternal Obesity and the Risk of Early-Onset and Late-Onset Hypertensive Disorders of Pregnancy. Obstet Gynecol 2020;136:118–27. [DOI] [PubMed] [Google Scholar]
- 143.Schenkelaars N, Rousian M, Hoek J, Schoenmakers S, Willemsen S, Steegers-Theunissen R. Preconceptional maternal weight loss and hypertensive disorders in pregnancy: a systematic review and meta-analysis. Eur J Clin Nutr 2021. [DOI] [PubMed] [Google Scholar]
- 144.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–7. [DOI] [PubMed] [Google Scholar]
- 145.Kaur J A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014;2014:943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 146.Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res 2009;335:165–89. [DOI] [PubMed] [Google Scholar]
- 147.Funk SD, Yurdagul A Jr., Orr AW. Hyperglycemia and endothelial dysfunction in atherosclerosis: lessons from type 1 diabetes. Int J Vasc Med 2012;2012:569654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stekkinger E, Scholten R, van der Vlugt MJ, van Dijk AP, Janssen MC, Spaanderman ME. Metabolic syndrome and the risk for recurrent pre-eclampsia: a retrospective cohort study. BJOG 2013;120:979–86. [DOI] [PubMed] [Google Scholar]
- 149.Spradley FT. Metabolic abnormalities and obesity's impact on the risk for developing preeclampsia. Am J Physiol Regul Integr Comp Physiol 2017;312:R5–r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cho GJ, Park JH, Shin SA, Oh MJ, Seo HS. Metabolic syndrome in the non-pregnant state is associated with the development of preeclampsia. Int J Cardiol 2016;203:982–6. [DOI] [PubMed] [Google Scholar]
- 151.Hubel CA. Dyslipidemia, iron, and oxidative stress in preeclampsia: assessment of maternal and feto-placental interactions. Semin Reprod Endocrinol 1998;16:75–92. [DOI] [PubMed] [Google Scholar]
- 152.Pouta A, Hartikainen AL, Sovio U, et al. Manifestations of metabolic syndrome after hypertensive pregnancy. Hypertension 2004;43:825–31. [DOI] [PubMed] [Google Scholar]
- 153.Forest JC, Girouard J, Massé J, et al. Early occurrence of metabolic syndrome after hypertension in pregnancy. Obstet Gynecol 2005;105:1373–80. [DOI] [PubMed] [Google Scholar]
- 154.Smith GN, Pudwell J, Walker M, Wen SW. Risk estimation of metabolic syndrome at one and three years after a pregnancy complicated by preeclampsia. J Obstet Gynaecol Can 2012;34:836–41. [DOI] [PubMed] [Google Scholar]
- 155.Bennett WL, Gilson MM, Jamshidi R, et al. Impact of bariatric surgery on hypertensive disorders in pregnancy: retrospective analysis of insurance claims data. BMJ 2010;340:c1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jonk AM, Houben AJ, de Jongh RT, Serné EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda) 2007;22:252–60. [DOI] [PubMed] [Google Scholar]
- 157.Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord 2013;14:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: A systematic review and meta-analysis. Sleep Med Rev 2021;58:101436. [DOI] [PubMed] [Google Scholar]
- 159.Dalmasso F, Prota R. Snoring: analysis, measurement, clinical implications and applications. Eur Respir J 1996;9:146–59. [DOI] [PubMed] [Google Scholar]
- 160.Loube DI, Poceta JS, Morales MC, Peacock MD, Mitler MM. Self-reported snoring in pregnancy. Association with fetal outcome. Chest 1996;109:885–9. [DOI] [PubMed] [Google Scholar]
- 161.Franklin KA, Holmgren PA, Jönsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest 2000;117:137–41. [DOI] [PubMed] [Google Scholar]
- 162.Hu FB, Willett WC, Colditz GA, et al. Prospective study of snoring and risk of hypertension in women. Am J Epidemiol 1999;150:806–16. [DOI] [PubMed] [Google Scholar]
- 163.O'Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol 2012;207:487.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med 2000;162:252–7. [DOI] [PubMed] [Google Scholar]
- 165.Whitehead C, Tong S, Wilson D, Howard M, Walker SP. Treatment of early-onset preeclampsia with continuous positive airway pressure. Obstet Gynecol 2015;125:1106–9. [DOI] [PubMed] [Google Scholar]
- 166.Bourjeily G, Curran P, Butterfield K, Maredia H, Carpenter M, Lambert-Messerlian G. Placenta-secreted circulating markers in pregnant women with obstructive sleep apnea. J Perinat Med 2015;43:81–7. [DOI] [PubMed] [Google Scholar]
- 167.Daly A, Robertson A, Bobek G, Middleton S, Sullivan C, Hennessy A. Sleep disordered breathing controlled by CPAP and sFlt-1 in a pregnant patient with chronic hypertension: Case report and literature review. Obstetric medicine 2018;11:32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Redman CW, Beilin LJ, Bonnar J. Reversed diurnal blood pressure rhythm in hypertensive pregnancies. Clin Sci Mol Med Suppl 1976;3:687s–89s. [DOI] [PubMed] [Google Scholar]
- 169.Beilin LJ, Deacon J, Michael CA, et al. Diurnal rhythms of blood pressure, plasma renin activity, angiotensin II and catecholamines in normotensive and hypertensive pregnancies. Clin Exp Hypertens B 1983;2:271–93. [DOI] [PubMed] [Google Scholar]
- 170.Edwards N, Blyton CM, Kesby GJ, Wilcox I, Sullivan CE. Pre-eclampsia is associated with marked alterations in sleep architecture. Sleep 2000;23:619–25. [PubMed] [Google Scholar]
- 171.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 1998;179:80–6. [DOI] [PubMed] [Google Scholar]
- 172.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999;180:499–506. [DOI] [PubMed] [Google Scholar]
- 173.Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol 2001;185:792–7. [DOI] [PubMed] [Google Scholar]
- 174.Vassilakos P, Riotton G, Kajii T. Hydatidiform mole: two entities. A morphologic and cytogenetic study with some clinical consideration. Am J Obstet Gynecol 1977;127:167–70. [DOI] [PubMed] [Google Scholar]
- 175.Kohorn EI. Molar pregnancy: presentation and diagnosis. Clin Obstet Gynecol 1984;27:181–91. [DOI] [PubMed] [Google Scholar]
- 176.Soto-Wright V, Bernstein M, Goldstein DP, Berkowitz RS. The changing clinical presentation of complete molar pregnancy. Obstet Gynecol 1995;86:775–9. [DOI] [PubMed] [Google Scholar]
- 177.Jauniaux E Partial moles: from postnatal to prenatal diagnosis. Placenta 1999;20:379–88. [DOI] [PubMed] [Google Scholar]
- 178.Zilberman Sharon N, Maymon R, Melcer Y, Jauniaux E. Obstetric outcomes of twin pregnancies presenting with a complete hydatidiform mole and coexistent normal fetus: a systematic review and meta-analysis. BJOG 2020;127:1450–57. [DOI] [PubMed] [Google Scholar]
- 179.Koga K, Osuga Y, Tajima T, et al. Elevated serum soluble fms-like tyrosine kinase 1 (sFlt1) level in women with hydatidiform mole. Fertil Steril 2010;94:305–8. [DOI] [PubMed] [Google Scholar]
- 180.Kanter D, Lindheimer MD, Wang E, et al. Angiogenic dysfunction in molar pregnancy. Am J Obstet Gynecol 2010;202:184.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Iriyama T, Wang G, Yoshikawa M, et al. Increased LIGHT leading to sFlt-1 elevation underlies the pathogenic link between hydatidiform mole and preeclampsia. Sci Rep 2019;9:10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol 2006;6:584–94. [DOI] [PubMed] [Google Scholar]
- 183.Leslie M. Immunology. Fetal immune system hushes attacks on maternal cells. Science 2008;322:1450–1. [DOI] [PubMed] [Google Scholar]
- 184.Burlingham WJ. A lesson in tolerance--maternal instruction to fetal cells. N Engl J Med 2009;360:1355–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 2012;490:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Betz AG. Immunology: Tolerating pregnancy. Nature 2012;490:47–8. [DOI] [PubMed] [Google Scholar]
- 187.Williams Z Inducing tolerance to pregnancy. N Engl J Med 2012;367:1159–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol 2005;5:807–17. [DOI] [PubMed] [Google Scholar]
- 189.Alegre ML, Florquin S, Goldman M. Cellular mechanisms underlying acute graft rejection: time for reassessment. Curr Opin Immunol 2007;19:563–8. [DOI] [PubMed] [Google Scholar]
- 190.Kim IK, Bedi DS, Denecke C, Ge X, Tullius SG. Impact of innate and adaptive immunity on rejection and tolerance. Transplantation 2008;86:889–94. [DOI] [PubMed] [Google Scholar]
- 191.Robillard PY, Dekker G, Chaouat G, Hulsey TC, Saftlas A. Epidemiological studies on primipaternity and immunology in preeclampsia--a statement after twelve years of workshops. J Reprod Immunol 2011;89:104–17. [DOI] [PubMed] [Google Scholar]
- 192.Robillard PY, Dekker G, Chaouat G, Elliot MG, Scioscia M. High incidence of early onset preeclampsia is probably the rule and not the exception worldwide. 20th anniversary of the reunion workshop. A summary. J Reprod Immunol 2019;133:30–36. [DOI] [PubMed] [Google Scholar]
- 193.Gamliel M, Goldman-Wohl D, Isaacson B, et al. Trained Memory of Human Uterine NK Cells Enhances Their Function in Subsequent Pregnancies. Immunity 2018;48:951–62.e5. [DOI] [PubMed] [Google Scholar]
- 194.Trupin LS, Simon LP, Eskenazi B. Change in paternity: a risk factor for preeclampsia in multiparas. Epidemiology 1996;7:240–4. [DOI] [PubMed] [Google Scholar]
- 195.Li DK, Wi S. Changing paternity and the risk of preeclampsia/eclampsia in the subsequent pregnancy. Am J Epidemiol 2000;151:57–62. [DOI] [PubMed] [Google Scholar]
- 196.Perni SC, Predanik M, Cho JE, Baergen RN. Placental pathology and pregnancy outcomes in donor and non-donor oocyte in vitro fertilization pregnancies. J Perinat Med 2005;33:27–32. [DOI] [PubMed] [Google Scholar]
- 197.Koch CA, Platt JL. Natural mechanisms for evading graft rejection: the fetus as an allograft. Springer Semin Immunopathol 2003;25:95–117. [DOI] [PubMed] [Google Scholar]
- 198.Chaouat G, Petitbarat M, Dubanchet S, Rahmati M, Ledée N. Tolerance to the foetal allograft? Am J Reprod Immunol 2010;63:624–36. [DOI] [PubMed] [Google Scholar]
- 199.Erlebacher A Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol 2013;13:23–33. [DOI] [PubMed] [Google Scholar]
- 200.Erlebacher A Why isn't the fetus rejected? Curr Opin Immunol 2001;13:590–3. [DOI] [PubMed] [Google Scholar]
- 201.Trowsdale J, Betz AG. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol 2006;7:241–6. [DOI] [PubMed] [Google Scholar]
- 202.Knox WF, Fox H. Villitis of unknown aetiology: its incidence and significance in placentae from a British population. Placenta 1984;5:395–402. [DOI] [PubMed] [Google Scholar]
- 203.Labarrere C, Althabe O. Chronic villitis of unknown etiology and maternal arterial lesions in preeclamptic pregnancies. European Journal of Obstetrics and Gynecology and Reproductive Biology 1985;20:1–11. [DOI] [PubMed] [Google Scholar]
- 204.Kim MJ, Romero R, Kim CJ, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol 2009;182:3919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Benzon S, Zekić Tomaš S, Benzon Z, Vulić M, Kuzmić Prusac I. Involvement of T lymphocytes in the placentae with villitis of unknown etiology from pregnancies complicated with preeclampsia. J Matern Fetal Neonatal Med 2016;29:1055–60. [DOI] [PubMed] [Google Scholar]
- 206.Whitten AE, Romero R, Korzeniewski SJ, et al. Evidence of an imbalance of angiogenic/antiangiogenic factors in massive perivillous fibrin deposition (maternal floor infarction): a placental lesion associated with recurrent miscarriage and fetal death. Am J Obstet Gynecol 2013;208:310 e1–10 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Romero R, Whitten A, Korzeniewski SJ, et al. Maternal floor infarction/massive perivillous fibrin deposition: a manifestation of maternal antifetal rejection? Am J Reprod Immunol 2013;70:285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lee J, Romero R, Dong Z, et al. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology 2011;59:928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 2015;213:S53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Maymon E, Romero R, Bhatti G, et al. Chronic inflammatory lesions of the placenta are associated with an up-regulation of amniotic fluid CXCR3: A marker of allograft rejection. J Perinat Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Stanek J Histological Features of Shallow Placental Implantation Unify Early-Onset and Late-Onset Preeclampsia. Pediatr Dev Pathol 2019;22:112–22. [DOI] [PubMed] [Google Scholar]
- 212.Devisme L, Chauvière C, Franquet-Ansart H, et al. Perinatal outcome of placental massive perivillous fibrin deposition: a case-control study. Prenat Diagn 2017;37:323–28. [DOI] [PubMed] [Google Scholar]
- 213.Hiby SE, Walker JJ, O'Shaughnessy KM, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med 2004;200:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hiby SE, Apps R, Chazara O, et al. Maternal KIR in combination with paternal HLA-C2 regulate human birth weight. J Immunol 2014;192:5069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nakimuli A, Chazara O, Hiby SE, et al. A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc Natl Acad Sci U S A 2015;112:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Colucci F The role of KIR and HLA interactions in pregnancy complications. Immunogenetics 2017;69:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Faas MM, de Vos P. Uterine NK cells and macrophages in pregnancy. Placenta 2017;56:44–52. [DOI] [PubMed] [Google Scholar]
- 218.Papúchová H, Meissner TB, Li Q, Strominger JL, Tilburgs T. The Dual Role of HLA-C in Tolerance and Immunity at the Maternal-Fetal Interface. Front Immunol 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol 2013;13:133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 2009;127:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006;12:1065–74. [DOI] [PubMed] [Google Scholar]
- 222.Karimi K, Blois SM, Arck PC. The upside of natural killers. Nat Med 2008;14:1184–5. [DOI] [PubMed] [Google Scholar]
- 223.Johnsen GM, Storvold GL, Drabbels JJM, et al. The combination of maternal KIR-B and fetal HLA-C2 is associated with decidua basalis acute atherosis in pregnancies with preeclampsia. J Reprod Immunol 2018;129:23–29. [DOI] [PubMed] [Google Scholar]
- 224.Robillard P-Y, Dekker G, Scioscia M, Saito S. Progress in the understanding of the pathophysiology of immunological maladaptation related to early onset preeclampsia and metabolic syndrome related to late onset preeclampsia. Am J Obstet Gynecol 2021;Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 225.John AH, Duncan AS. The maternal syndrome associated with hydrops fetalis. J Obstet Gynaecol Br Commonw 1964;71:61–5. [DOI] [PubMed] [Google Scholar]
- 226.Gedikbasi A, Oztarhan K, Gunenc Z, et al. Preeclampsia due to fetal non-immune hydrops: mirror syndrome and review of literature. Hypertens Pregnancy 2011;30:322–30. [DOI] [PubMed] [Google Scholar]
- 227.Broekhuizen FF, Elejalde R, Hamilton PR. Early-onset preeclampsia, triploidy and fetal hydrops. J Reprod Med 1983;28:223–6. [PubMed] [Google Scholar]
- 228.Dotters-Katz SK, Hardisty E, Campbell E, Vora N. Trisomy 13-confined placental mosaicism: is there an increased risk of gestational hypertensive disorders? Prenat Diagn 2017;37:938–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Giorgione V, Bhide A, Bhate R, Reed K, Khalil A. Are Twin Pregnancies Complicated by Weight Discordance or Fetal Growth Restriction at Higher Risk of Preeclampsia? Journal of clinical medicine 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.O'Driscoll DT. A fluid retention syndrome associated with severe iso-immunization to the rhesus factor. J Obstet Gynaecol Br Emp 1956;63:372–4. [DOI] [PubMed] [Google Scholar]
- 231.Kaiser IH. Ballantyne and triple edema. Am J Obstet Gynecol 1971;110:115–20. [DOI] [PubMed] [Google Scholar]
- 232.Rana S, Venkatesha S, DePaepe M, Chien EK, Paglia M, Karumanchi SA. Cytomegalovirus-induced mirror syndrome associated with elevated levels of circulating antiangiogenic factors. Obstet Gynecol 2007;109:549–52. [DOI] [PubMed] [Google Scholar]
- 233.Brochot C, Collinet P, Provost N, Subtil D. Mirror syndrome due to parvovirus B19 hydrops complicated by severe maternal pulmonary effusion. Prenat Diagn 2006;26:179–80. [DOI] [PubMed] [Google Scholar]
- 234.Braun T, Brauer M, Fuchs I, et al. Mirror syndrome: a systematic review of fetal associated conditions, maternal presentation and perinatal outcome. Fetal Diagn Ther 2010;27:191–203. [DOI] [PubMed] [Google Scholar]
- 235.Ordorica SA, Marks F, Frieden FJ, Hoskins IA, Young BK. Aneurysm of the vein of Galen: a new cause for Ballantyne syndrome. Am J Obstet Gynecol 1990;162:1166–7. [DOI] [PubMed] [Google Scholar]
- 236.Sherer DM, Sadovksy E, Menashe M, Mordel N, Rein AJ. Fetal ventricular tachycardia associated with nonimmunologic hydrops fetalis. A case report. J Reprod Med 1990;35:292–4. [PubMed] [Google Scholar]
- 237.Midgley DY, Harding K. The Mirror Syndrome. European Journal of Obstetrics & Gynecology and Reproductive Biology 2000;88:201–02. [DOI] [PubMed] [Google Scholar]
- 238.Dorman SL, Cardwell MS. Ballantyne syndrome caused by a large placental chorioangioma. Am J Obstet Gynecol 1995;173:1632–3. [DOI] [PubMed] [Google Scholar]
- 239.Allarakia S, Khayat HA, Karami MM, et al. Characteristics and management of mirror syndrome: a systematic review (1956-2016). J Perinat Med 2017;45:1013–21. [DOI] [PubMed] [Google Scholar]
- 240.Duthie SJ, Walkinshaw SA. Parvovirus associated fetal hydrops: reversal of pregnancy induced proteinuric hypertension by in utero fetal transfusion. Br J Obstet Gynaecol 1995;102:1011–3. [DOI] [PubMed] [Google Scholar]
- 241.Espinoza J, Romero R, Nien JK, et al. A role of the anti-angiogenic factor sVEGFR-1 in the 'mirror syndrome' (Ballantyne's syndrome). J Matern Fetal Neonatal Med 2006;19:607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Stepan H, Faber R. Elevated sFlt1 level and preeclampsia with parvovirus-induced hydrops. N Engl J Med 2006;354:1857–58. [DOI] [PubMed] [Google Scholar]
- 243.Goa S, Mimura K, Kakigano A, et al. Normalisation of angiogenic imbalance after intrauterine transfusion for mirror syndrome caused by parvovirus B19. Fetal Diagn Ther 2013;34:176–9. [DOI] [PubMed] [Google Scholar]
- 244.Kakigano A, Mimura K, Kanagawa T, et al. Imbalance of angiogenic factors and avascular edematous cystic villi in a trisomy 13 pregnancy: a case report. Placenta 2013;34:628–30. [DOI] [PubMed] [Google Scholar]
- 245.Tuohy JF, James DK. Pre-eclampsia and trisomy 13. Br J Obstet Gynaecol 1992;99:891–4. [DOI] [PubMed] [Google Scholar]
- 246.Bdolah Y, Palomaki GE, Yaron Y, et al. Circulating angiogenic proteins in trisomy 13. Am J Obstet Gynecol 2006;194:239–45. [DOI] [PubMed] [Google Scholar]
- 247.Silasi M, Rana S, Powe C, et al. Placental expression of angiogenic factors in Trisomy 13 . Am J Obstet Gynecol 2011;204:546.e1–4. [DOI] [PubMed] [Google Scholar]
- 248.Heyborne KD, Chism DM. Reversal of Ballantyne syndrome by selective second-trimester fetal termination. A case report. J Reprod Med 2000;45:360–2. [PubMed] [Google Scholar]
- 249.Audibert F, Salomon LJ, Castaigne-Meary V, Alves K, Frydman R. Selective termination of a twin pregnancy as a treatment of severe pre-eclampsia. BJOG 2003;110:68–9. [PubMed] [Google Scholar]
- 250.Heyborne KD, Porreco RP. Selective fetocide reverses preeclampsia in discordant twins. Am J Obstet Gynecol 2004;191:477–80. [DOI] [PubMed] [Google Scholar]
- 251.Okby R, Mazor M, Erez O, Beer-Weizel R, Hershkovitz R. Reversal of mirror syndrome after selective feticide of a hydropic fetus in a dichorionic diamniotic twin pregnancy. J Ultrasound Med 2015;34:351–3. [DOI] [PubMed] [Google Scholar]
- 252.Sarhanis P, Pugh DH. Resolution of pre-eclampsia following intrauterine death of one twin. Br J Obstet Gynaecol 1992;99:159–60. [DOI] [PubMed] [Google Scholar]
- 253.Hladunewich MA, Steinberg G, Karumanchi SA, et al. Angiogenic factor abnormalities and fetal demise in a twin pregnancy. Nature reviews Nephrology 2009;5:658–62. [DOI] [PubMed] [Google Scholar]
- 254.Fox CE, Lash GE, Pretlove SJ, Chan BC, Holder R, Kilby MD. Maternal plasma and amniotic fluid angiogenic factors and their receptors in monochorionic twin pregnancies complicated by twin-to-twin transfusion syndrome. Ultrasound Obstet Gynecol 2010;35:695–701. [DOI] [PubMed] [Google Scholar]
- 255.Bundhun PK, Soogund MZ, Huang F. Impact of systemic lupus erythematosus on maternal and fetal outcomes following pregnancy: A meta-analysis of studies published between years 2001-2016. J Autoimmun 2017;79:17–27. [DOI] [PubMed] [Google Scholar]
- 256.Saccone G, Berghella V, Maruotti GM, et al. Antiphospholipid antibody profile based obstetric outcomes of primary antiphospholipid syndrome: the PREGNANTS study. Am J Obstet Gynecol 2017;216:525.e1–25.e12. [DOI] [PubMed] [Google Scholar]
- 257.Liu L, Sun D. Pregnancy outcomes in patients with primary antiphospholipid syndrome: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e15733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dong Y, Yuan F, Dai Z, Wang Z, Zhu Y, Wang B. Preeclampsia in systemic lupus erythematosus pregnancy: a systematic review and meta-analysis. Clin Rheumatol 2020;39:319–25. [DOI] [PubMed] [Google Scholar]
- 259.Lee RM, Brown MA, Branch DW, Ward K, Silver RM. Anticardiolipin and anti-beta2-glycoprotein-I antibodies in preeclampsia. Obstet Gynecol 2003;102:294–300. [DOI] [PubMed] [Google Scholar]
- 260.Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 1999;103:945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yang X, Wang F, Chang H, et al. Autoantibody against AT1 receptor from preeclamptic patients induces vasoconstriction through angiotensin receptor activation. J Hypertens 2008;26:1629–35. [DOI] [PubMed] [Google Scholar]
- 262.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension 2010;55:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhou CC, Zhang Y, Irani RA, et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 2008;14:855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Parrish MR, Murphy SR, Rutland S, et al. The effect of immune factors, tumor necrosis factor-alpha, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens 2010;23:911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Aggarwal S, Sunderland N, Thornton C, Xu B, Hennessy A, Makris A. A longitudinal analysis of angiotensin II type 1 receptor antibody and angiogenic markers in pregnancy. Am J Obstet Gynecol 2017;216:170.e1–70.e8. [DOI] [PubMed] [Google Scholar]
- 266.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension 2008;52:1168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cunningham MW Jr., Castillo J, Ibrahim T, et al. AT1-AA (Angiotensin II Type 1 Receptor Agonistic Autoantibody) Blockade Prevents Preeclamptic Symptoms in Placental Ischemic Rats. Hypertension 2018;71:886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol 2012;303:H1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lamarca B, Speed J, Ray LF, et al. Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation. International journal of interferon, cytokine and mediator research 2011;2011:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Dhillion P, Wallace K, Herse F, et al. IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am J Physiol Regul Integr Comp Physiol 2012;303:R353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 1961;25:585–621. [DOI] [PubMed] [Google Scholar]
- 272.Martin BJ, Spicer SS. Ultrastructural features of cellular maturation and aging in human trophoblast. J Ultrastruct Res 1973;43:133–49. [DOI] [PubMed] [Google Scholar]
- 273.Burstein R, Frankel S, Soule SD, Blumenthal HT. Aging of the placenta: autoimmune theory of senescence. Am J Obstet Gynecol 1973;116:271–6. [DOI] [PubMed] [Google Scholar]
- 274.Biron-Shental T, Sukenik-Halevy R, Sharon Y, et al. Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol 2010;202:381.e1–7. [DOI] [PubMed] [Google Scholar]
- 275.Mayne BT, Leemaqz SY, Smith AK, Breen J, Roberts CT, Bianco-Miotto T. Accelerated placental aging in early onset preeclampsia pregnancies identified by DNA methylation. Epigenomics 2017;9:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sultana Z, Maiti K, Dedman L, Smith R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am J Obstet Gynecol 2018;218:S762–s73. [DOI] [PubMed] [Google Scholar]
- 277.Maiti K, Sultana Z, Aitken RJ, et al. Evidence that fetal death is associated with placental aging. Am J Obstet Gynecol 2017;217:441 e1–41 e14. [DOI] [PubMed] [Google Scholar]
- 278.Gomez-Lopez N, Romero R, Plazyo O, et al. Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. Am J Obstet Gynecol 2017;217:592.e1–92.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Redman CWG, Staff AC, Roberts JM. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol 2021. [DOI] [PubMed] [Google Scholar]
- 280.Cindrova-Davies T, Fogarty NME, Jones CJP, Kingdom J, Burton GJ. Evidence of oxidative stress-induced senescence in mature, post-mature and pathological human placentas. Placenta 2018;68:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Higuchi S, Miyamoto T, Kobara H, et al. Trophoblast type-specific expression of senescence markers in the human placenta. Placenta 2019;85:56–62. [DOI] [PubMed] [Google Scholar]
- 282.Londero AP, Orsaria M, Marzinotto S, et al. Placental aging and oxidation damage in a tissue micro-array model: an immunohistochemistry study. Histochem Cell Biol 2016;146:191–204. [DOI] [PubMed] [Google Scholar]
- 283.Cox LS, Redman C. The role of cellular senescence in ageing of the placenta. Placenta 2017;52:139–45. [DOI] [PubMed] [Google Scholar]
- 284.Mayhew TM, Barker BL. Villous trophoblast: morphometric perspectives on growth, differentiation, turnover and deposition of fibrin-type fibrinoid during gestation. Placenta 2001;22:628–38. [DOI] [PubMed] [Google Scholar]
- 285.Redman CW, Sargent IL, Staff AC. IFPA Senior Award Lecture: making sense of pre-eclampsia - two placental causes of preeclampsia? Placenta 2014;35 Suppl:S20–5. [DOI] [PubMed] [Google Scholar]
- 286.Serov AS, Salafia CM, Brownbill P, Grebenkov DS, Filoche M. Optimal villi density for maximal oxygen uptake in the human placenta. J Theor Biol 2015;364:383–96. [DOI] [PubMed] [Google Scholar]
- 287.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta 2012;33:816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Smith R, Maiti K, Aitken RJ. Unexplained antepartum stillbirth: a consequence of placental aging? Placenta 2013;34:310–3. [DOI] [PubMed] [Google Scholar]
- 289.Tekola-Ayele F, Zeng X, Ouidir M, et al. DNA methylation loci in placenta associated with birthweight and expression of genes relevant for early development and adult diseases. Clin Epigenetics 2020;12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Okoror CEM, Enabudoso EJ, Okoror OT, Okonkwo CA. Serum calcium-magnesium ratio in women with pre-eclampsia at a tertiary hospital in Nigeria. Int J Gynaecol Obstet 2020;149:354–58. [DOI] [PubMed] [Google Scholar]
- 291.Hultin H, Hellman P, Lundgren E, et al. Association of parathyroid adenoma and pregnancy with preeclampsia. J Clin Endocrinol Metab 2009;94:3394–9. [DOI] [PubMed] [Google Scholar]
- 292.Caimari F, Valassi E, Garbayo P, et al. Cushing's syndrome and pregnancy outcomes: a systematic review of published cases. Endocrine 2017;55:555–63. [DOI] [PubMed] [Google Scholar]
- 293.Baghlaf HA, Badeghiesh AM, Suarthana E, Dahan MH. The effect of Cushing's syndrome on pregnancy complication rates: analysis of more than 9 million deliveries. J Matern Fetal Neonatal Med 2021:1–7. [DOI] [PubMed] [Google Scholar]
- 294.Landau E, Amar L. Primary aldosteronism and pregnancy. Ann Endocrinol (Paris) 2016;77:148–60. [DOI] [PubMed] [Google Scholar]
- 295.Quartermaine G, Lambert K, Rees K, et al. Hormone-secreting adrenal tumours cause severe hypertension and high rates of poor pregnancy outcome; a UK Obstetric Surveillance System study with case control comparisons. BJOG 2018;125:719–27. [DOI] [PubMed] [Google Scholar]
- 296.Oliva R, Angelos P, Kaplan E, Bakris G. Pheochromocytoma in pregnancy: a case series and review. Hypertension 2010;55:600–6. [DOI] [PubMed] [Google Scholar]
- 297.Dusitkasem S, Herndon BH, Paluzzi D, Kuhn J, Small RH, Coffman JC. From Bad to Worse: Paraganglioma Diagnosis during Induction of Labor for Coexisting Preeclampsia. Case reports in anesthesiology 2017;2017:5495808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Kovacs CS. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol Rev 2016;96:449–547. [DOI] [PubMed] [Google Scholar]
- 299.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 2007;92:3517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Scholl TO, Chen X, Stein TP. Vitamin D, secondary hyperparathyroidism, and preeclampsia. Am J Clin Nutr 2013;98:787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wheeler BJ, Taylor BJ, de Lange M, et al. A Longitudinal Study of 25-Hydroxy Vitamin D and Parathyroid Hormone Status throughout Pregnancy and Exclusive Lactation in New Zealand Mothers and Their Infants at 45° S. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hyppönen E, Hartikainen AL, Sovio U, Järvelin MR, Pouta A. Does vitamin D supplementation in infancy reduce the risk of pre-eclampsia? Eur J Clin Nutr 2007;61:1136–9. [DOI] [PubMed] [Google Scholar]
- 303.Marya RK, Rathee S, Manrow M. Effect of calcium and vitamin D supplementation on toxaemia of pregnancy. Gynecol Obstet Invest 1987;24:38–42. [DOI] [PubMed] [Google Scholar]
- 304.Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. The Cochrane database of systematic reviews 2019;7:Cd008873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Nilsson IL, Aberg J, Rastad J, Lind L. Left ventricular systolic and diastolic function and exercise testing in primary hyperparathyroidism-effects of parathyroidectomy. Surgery 2000;128:895–902. [DOI] [PubMed] [Google Scholar]
- 306.Hagström E, Lundgren E, Rastad J, Hellman P. Metabolic abnormalities in patients with normocalcemic hyperparathyroidism detected at a population-based screening. Eur J Endocrinol 2006;155:33–9. [DOI] [PubMed] [Google Scholar]
- 307.Trementino L, Arnaldi G, Appolloni G, et al. Coagulopathy in Cushing's syndrome. Neuroendocrinology 2010;92 Suppl 1:55–9. [DOI] [PubMed] [Google Scholar]
- 308.Isidori AM, Minnetti M, Sbardella E, Graziadio C, Grossman AB. Mechanisms in endocrinology: The spectrum of haemostatic abnormalities in glucocorticoid excess and defect. Eur J Endocrinol 2015;173:R101–13. [DOI] [PubMed] [Google Scholar]
- 309.Pivonello R, De Martino MC, Iacuaniello D, et al. Metabolic Alterations and Cardiovascular Outcomes of Cortisol Excess. Front Horm Res 2016;46:54–65. [DOI] [PubMed] [Google Scholar]
- 310.Monticone S, Burrello J, Tizzani D, et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol 2017;69:1811–20. [DOI] [PubMed] [Google Scholar]
- 311.Käyser SC, Deinum J, de Grauw WJ, et al. Prevalence of primary aldosteronism in primary care: a cross-sectional study. Br J Gen Pract 2018;68:e114–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int 2004;66:1–9. [DOI] [PubMed] [Google Scholar]
- 313.Liang W, Chen C, Shi J, et al. Disparate effects of eplerenone, amlodipine and telmisartan on podocyte injury in aldosterone-infused rats. Nephrol Dial Transplant 2011;26:789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lenders JWM, Langton K, Langenhuijsen JF, Eisenhofer G. Pheochromocytoma and Pregnancy. Endocrinol Metab Clin North Am 2019;48:605–17. [DOI] [PubMed] [Google Scholar]
- 315.Mohamed Ismail NA, Abd Rahman R, Abd Wahab N, Muhammad R, Nor Azmi K. Pheochromocytoma and pregnancy: a difficult and dangerous ordeal. Malays J Med Sci 2012;19:65–8. [PMC free article] [PubMed] [Google Scholar]
- 316.Langton K, Tufton N, Akker S, et al. Pregnancy and phaeochromocytoma/paraganglioma: clinical clues affecting diagnosis and outcome - a systematic review. BJOG 2021;128:1264–72. [DOI] [PubMed] [Google Scholar]
- 317.Kamari Y, Sharabi Y, Leiba A, Peleg E, Apter S, Grossman E. Peripartum hypertension from pheochromocytoma: a rare and challenging entity. Am J Hypertens 2005;18:1306–12. [DOI] [PubMed] [Google Scholar]