Abstract
CD155 serves an important role in tumor progression by promoting cell proliferation and migration. CD155 is also involved in the immune evasion of tumor cells, which may cause the development and progression of tumors. Accordingly, CD155 has emerged as a novel target in cancer immunotherapy; however, its expression in lung cancer remains unclear. To assess CD155 expression and its prognostic significance, 96 patients with completely resected pathologic stage I adenocarcinoma of the lung were retrospectively reviewed. Immunohistochemical staining was performed to evaluate CD155 expression on tumor cells. Expression levels of programmed death-ligand 1 (PD-L1), another molecule participating in immune evasion, were also evaluated immunohistochemically. CD155 expression was positive in 37 patients (38.5%). CD155-positivity was associated with aggressive tumor behavior, such as pleural invasion and vascular invasion. In addition, CD155-positivity was a significant factor to predict a poor prognosis (5-year overall survival (OS) rate, 63.3% for CD155-positive patients vs. 93.1% for CD155-negative patients; P<0.001). Patients harboring tumors with positive CD155 and PD-L1 expression showed the poorest prognosis (5-year OS rate, 44.4% for both-positive patients vs. 85.4% for the other patients; P<0.001). The positive expression status of both CD155 and PD-L1 was a significant and independent unfavorable prognostic factor (hazard ratio, 3.86; 95% confidence interval, 1.51-9.89; P=0.004; in a multivariate analysis). In conclusion, CD155-positivity was associated with aggressive tumor behavior, and was a factor to predict a poor prognosis. Its prognostic impact was enhanced when combined with PD-L1 expression status. These results should be validated in a large-scale study.
Keywords: CD155, adenocarcinoma, lung, PD-L1
Introduction
Non-small cell lung cancer (NSCLC) accounts for 80–90% of lung cancer which is the leading cause of cancer-related deaths worldwide (1). Platinum-based chemotherapy had only provided a modest survival benefit, but recent advances in systemic treatment have improved the prognosis of patients with advanced NSCLC. Immunotherapy with or without platinum-based chemotherapy is recommended for patients with NSCLC harboring no oncogenic gene alteration (2).
Cancer immunity is co-regulated through a balance in stimulatory and inhibitory signals (‘immune checkpoints’). Programmed cell death 1 (PD-1) is the most important immune-inhibitory molecule expressed on activated cytotoxic T-lymphocytes (CTLs). When PD-1 binds to its ligand such as programmed death-ligand 1 (PD-L1), the ability of CTLs to kill cancer cells is inhibited. Cancer cells expressing PD-L1 may evade immune attack by CTLs, which leads to tumor progression. Blockade of the PD-1/PD-L1 axis may restore cancer immunity to kill cancer cells, and plays a pivotal role in modern systemic treatment for a variety of malignant tumors including NSCLC (3,4). However, only ~30% of all NSCLC patients may respond to immunotherapy using an anti-PD-1/PD-L1 antibody (2,5).
The T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory domain (TIGIT) is also an immune-inhibitory molecule on CTLs. Cancer cells may evade cancer immunity by expressing their ligands, such as CD155 [also known as poliovirus receptor (PVR)] (6–8). Accordingly, blockade of the TIGIT/CD155 axis has emerged as a novel therapeutic strategy for a variety of malignant tumors (6,7,9). A recent randomized phase 2 study (CITYSCAPE trial) assessing the efficacy of an anti-TIGIT antibody (tiragolumab) in addition to an anti-PD-L1 antibody (atezolizumab) for advanced NSCLC showed promising results. The study revealed a higher overall response rate (ORR, 37 vs. 20%) and longer progression-free survival (PFS, 5.6 vs. 3.9 months) in the group receiving combination therapy compared to the control group receiving placebo, respectively (9). Despite the potential clinical significance of CD155 expression on cancer cells as a biomarker for the prediction of prognosis and response to anti-TIGIT/CD155 antibody, the clinical significance of CD155 expression in NSCLC remains unclear. We previously reported the prognostic significance of PD-L1 expression in completely resected pathologic (p-) stage I lung adenocarcinoma that was the most common histological subtype of NSCLC (10). Here, we examined CD155 expression in the same patient population, and assessed its clinical significance in correlation with PD-L1 expression.
Patients and methods
Patients
We retrospectively evaluated consecutive patients with p-stage I lung adenocarcinoma who underwent complete resection without preoperative treatment at our hospital [Second Department of Surgery (Chest Surgery), University of Occupational and Environmental Health, Japan] from January 2003 through December 2006. All patients underwent lung resection through minimal thoracotomy. Lobectomy was performed in the 89 (92.7%) patients who were fit for lobectomy. Sub-lobar resection was performed in the remaining 7 patients who did not tolerate lobectomy (segmentectomy in 5 patients and wedge resection in 2 patients).
Patients who did not agree to give informed consent for participating in the study were excluded. In addition, patients who did not provide sufficient tumor samples for immunohistochemistry (IHC) were deemed ineligible. A total of 96 patients were finally included in this study. P-stage was determined according to the TNM classification (Union for International Cancer Control TNM staging system, 7th edition). Patients with p-stage IB disease who were eligible for adjuvant chemotherapy were encouraged to participate in clinical trials (11,12), and 10 patients received adjuvant chemotherapy (carboplatin-based chemotherapy in 8 patients and tegafur plus uracil in 2 patients). Other patient characteristics are shown in Table I.
Table I.
Patient characteristics according to tumoral CD155 status.
| Tumoral CD155 expression | ||||
|---|---|---|---|---|
|
|
||||
| Variables | Total | Positive | Negative | P-value |
| All patients, n (%) | 96 | 37 (38.5) | 59 (61.5) | |
| Age, years | ||||
| Median | 72 | 73 | 70 | 0.232 |
| Range | 40-88 | 45-86 | 40-88 | |
| Sex, n (%) | ||||
| Male | 54 | 25 (46.3) | 29 (53.7) | 0.093 |
| Female | 42 | 12 (28.6) | 30 (71.4) | |
| Smoking, n (%) | ||||
| Former or current | 55 | 27 (49.1) | 28 (50.9) | 0.019 |
| Never | 41 | 10 (24.4) | 31 (75.6) | |
| Cell differentiation, n (%) | ||||
| Well | 51 | 13 (25.5) | 38 (74.5) | 0.005 |
| Moderately or poorly | 33 | 19 (57.6) | 14 (42.4) | |
| Tumor size, n (%) | ||||
| >2 cm | 54 | 29 (53.7) | 25 (46.3) | <0.001 |
| ≤2 cm | 42 | 8 (19.0) | 34 (81.0) | |
| Lympho-vascular invasion, n (%) | ||||
| Yes | 23 | 12 (52.2) | 11 (47.8) | 0.302 |
| No | 47 | 17 (36.2) | 30 (63.8) | |
| Vascular invasion, n (%) | ||||
| Yes | 15 | 11 (73.3) | 4 (26.7) | 0.007 |
| None | 58 | 19 (32.8) | 39 (67.2) | |
| Pleural invasion, n (%) | ||||
| Yes | 14 | 9 (64.3) | 5 (35.7) | 0.040 |
| None | 82 | 28 (34.1) | 54 (65.9) | |
| Pathologic stage, n (%) | ||||
| IA | 69 | 16 (23.2) | 53 (76.8) | <0.001 |
| IB | 27 | 21 (77.8) | 6 (22.2) | |
| Tumoral PD-L1 expression, n (%) | ||||
| Positive | 14 | 9 (64.2) | 5 (35.7) | 0.041 |
| Negative | 82 | 28 (34.1) | 54 (65.9) | |
PD-L1, programmed death-ligand 1.
Immunohistochemistry (IHC)
For evaluation of CD155 expression, serial sections were cut from each formalin-fixed and paraffin-embedded primary tumor specimen and served for IHC using the Histofine Simple Stain, MAX-PO (Nichirei Biosciences, Inc.) according to the manufacturer's protocol. Sections were incubated with an anti-CD155 antibody (clone B6; Santa Cruz Biotechnology, Inc.) diluted at 1:100 for 1 h at room temperature.
Each slide was examined independently by two investigators (R.O. and M.M.) who were blinded for any clinical data. In case of disagreement between the two investigators, a consensus was reached through the simultaneous examination by both investigators using a double-headed microscope. Each cancer cell was judged as positively stained for CD155 if the membrane or cytoplasm was stained at any intensity. Each patient was classified into ‘CD155-negative (CD155−)’ group or ‘CD155-positive (CD155+)’ group according to the percentage of CD155-positive cancer cells [tumor proportion score (TPS) for CD155], and the optimal cut-off value was determined using a receiver operating characteristic (ROC) curve analysis.
PD-L1 expression was also evaluated with IHC as described in a previous study (10). Briefly, an anti-PD-L1 antibody (clone E1L3N; Cell Signaling Technology, Inc.) was used as a primary antibody, and each patient was also classified into ‘PD-L1-positive (PD-L1+)’ group or ‘PD-L1-negative (PD-L1−)’ group with the cut-off value of 5% as the percentage of cancer cells with membrane-staining for PD-L1 (TPS for PD-L1).
Statistical analysis
The proportions of the categorical data were compared using the chi-square test. Continuous data were compared using a non-parametric test (Mann-Whitney U test). To determine an optimal cut-off value of TPS for CD155, an ROC curve was generated by plotting the false-positive rate of a model against its true positive rate for prediction of tumor recurrence and the area under the curve (AUC) was calculated.
The Kaplan-Meier method was used to estimate the probability of OS and recurrence-free survival (RFS), and survival differences were analyzed using the log-rank test.
To identify independent prognostic factors, univariate and multivariate analyses were performed using a Cox proportional hazards regression model. Sex, smoking status and pathologic stage (IA or IB), which have been already founded to be a significant prognostic factors, were included in the multivariate analyses (13).
All statistical analyses were performed using EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a modified version of R (The R Foundation for Statistical Computing).
For each patient, a routine follow-up was performed at the outpatient clinic as follows: chest roentgenography every 3 months, as well as chest computed tomography, brain magnetic resonance imaging, and bone scan every 6 months for the first 3 years after surgery; all examinations were performed annually thereafter. Additional examinations were performed when any symptoms or signs of recurrence were detected. A telephone follow-up would be made if the patient did not come to our clinic for a routine follow-up.
Results
CD155 expression in lung adenocarcinoma
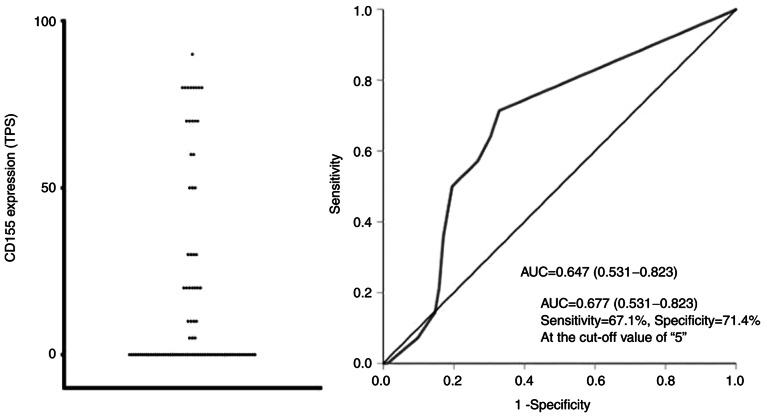
The distribution of TPS for CD155 is indicated in Figs. 1 and 2. The area under the ROC curve (AUC-ROC) for prediction of recurrence was 0.677 with a 95% confidence interval (CI) of 0.531-0.823, suggesting that CD155 was a significant prognostic marker. The ROC curve also indicated that the TPS value of 5% was the optimal cut-off value with sensitivity of 71.4% and specificity of 67.1% (Fig. 2). Based on these results, each patient was classified according to the TPS value into the CD155+ group (TPS, ≥5%) or the CD155− group (TPS, <5%). Thirty-seven patients (38.5%) were classified into the CD155+ group. When CD155+ patients were further classified by using the cut-off TPS value of 50%, the number of CD155-low (TPS, 5–49%) patients and CD155-high (TPS, ≥50%) patients were 18 and 19, respectively.
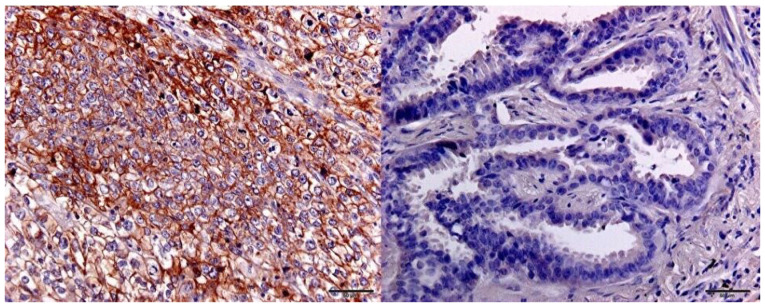
Figure 1.
Immunohistochemical detection of CD155 expression. Positive (left) and negative (right) staining in lung adenocarcinoma.
Figure 2.
Distribution of CD155 expression (left), and receiver operating characteristic curve for prediction of recurrence (right). TPS, tumor proportion score; AUC, area under curve.
CD155-positivity was significantly associated with advanced stage (p-stage IB) and pleural/vascular invasion. CD155+ patients were less frequent in never smoker and in patients with well-differentiated tumor. CD155-positivity was significantly correlated with PD-L1 positivity (Table I).
CD155-status and prognosis
The median follow-up time after surgery was 1,835 days. Twenty patients (9 patients in the CD155+ group and 11 patients in the CD155− group) were lost to follow-up within 5 years after surgery. The 5-year RFS rates of CD155+ patients and CD155− patients were 52.5 and 89.6%, respectively. There was a significant difference in the RFS according to the CD155-status (P<0.001, Fig. 3). Similarly, the 5-year OS rates were 63.3 and 93.1%, respectively, with a significant difference in the OS according to the CD155-status (P<0.001, Fig. 4). In multivariate analyses, the prognostic impact of the CD155 status was not significant with the hazard ratio (HR) of 2.33 (95% CI, 0.97-5.57; P=0.056) for RFS and with the HR of 2.18 (95% CI, 0.84-5.67; P=0.107) for OS. Exploratory analyses showed no significant difference in the prognosis between CD155-low patients and CD155-high patients (5-year RFS rate, 42.9% vs. 62.2%; P=0.096; 5-year OS rate, 48.6 vs. 77.7%; P=0.083).
Figure 3.
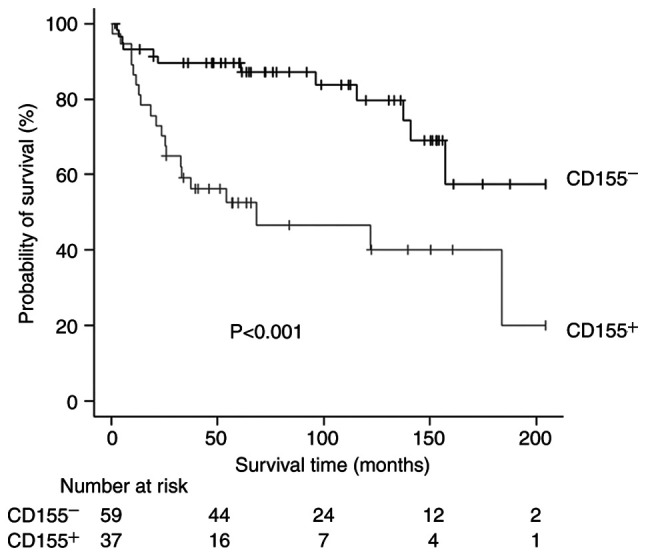
Recurrence-free survival curves according to CD155 expression. CD155−, CD155-negative; CD155+, CD155-positive.
Figure 4.
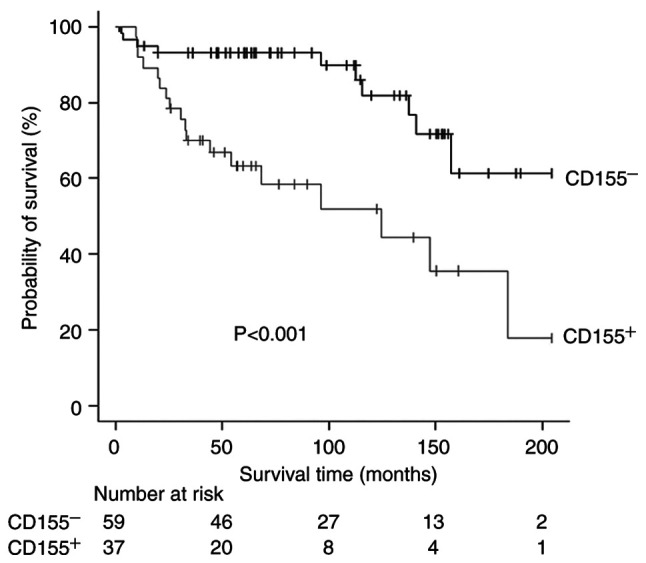
Overall survival (OS) curves according to CD155 expression. CD155−, CD155-negative; CD155+, CD155-positive.
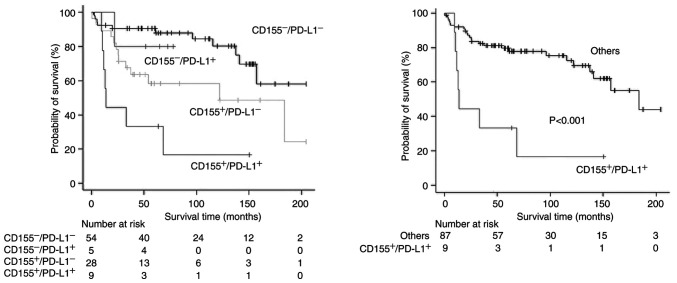
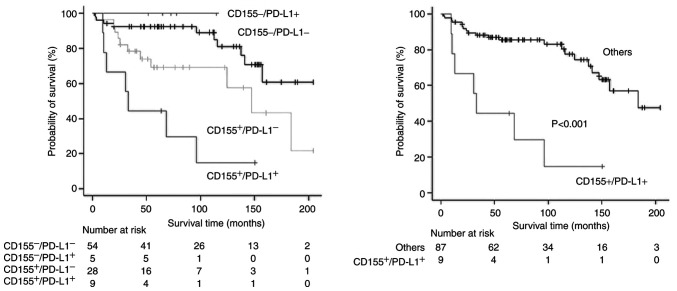
PD-L1-expression status was also significantly associated with a poor prognosis in univariate analyses (Tables II and III), but the prognostic impact failed to be significant in multivariate analyses (HR=2.12 [95% CI, 0.89-5.01] and P=0.087 for RFS; HR=2.24 [95% CI, 0.90-5.57] and P=0.080 for OS). As PD-L1-positivity was a potential prognostic factor (10), survival analyses according to a combination of CD155 status and PD-L1 status were conducted. Patients with both-positive (CD155+/PD-L1+) tumor showed a significantly poor prognosis (Figs. 5 and 6). The status of CD155+/PD-L1+ was a significant factor to predict poor prognosis in both univariate and multivariate analyses (Tables II and III).
Table II.
Univariate and multivariate Cox model of prognostic factors for overall survival (OS).
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
|
|
|||
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age (continuous) | 1.02 (0.98-1.06) | 0.291 | ||
| Sex (male vs. female) | 3.14 (1.27-7.76) | 0.013 | 3.12 (1.24-7.81) | 0.014 |
| Smoking (former/current vs. never) | 2.04 (0.89-4.64) | 0.089 | ||
| Cell differentiation (well vs. others) | 1.56 (0.69-3.49) | 0.279 | ||
| Tumor size (>2 cm vs. ≤2 cm) | 3.70 (1.49-9.16) | 0.005 | ||
| Lympho-vascular invasion (no vs. yes) | 0.44 (0.26-1.79) | 0.449 | ||
| Vascular invasion (no vs. yes) | 0.85 (0.28-2.54) | 0.782 | ||
| Pleural invasion (no vs. yes) | 0.88 (0.26-2.95) | 0.845 | ||
| Pathologic stage (IB vs. IA) | 3.09 (1.46-6.51) | 0.003 | 2.28 (1.03-5.03) | 0.041 |
| CD155 expression (positive vs. negative) | 3.74 (1.71-8.15) | <0.001 | ||
| PD-L1 (positive vs. negative) | 3.04 (1.25-7.34) | 0.014 | ||
| CD155 expression and PD-L1 expression (CD155+/PD-L1+ vs. others) | 5.26 (2.19-12.61) | <0.001 | 3.86 (1.51-9.89) | 0.004 |
| Mode of lung resection (sub-lobar resection vs. lobectomy) | 0.58 (0.13-2.54) | 0.477 | ||
| Adjuvant chemotherapy (not performed vs. performed) | 1.28 (0.38-4.27) | 0.682 | ||
95% CI, 95% confidence interval; CD155+, CD155 expression-positive; HR, hazard ratio; PD-L1, programmed death-ligand 1; PD-L1+, PD-L1 expression-positive.
Table III.
Univariate and multivariate Cox model of prognostic factors for recurrence-free survival (RFS).
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
|
|
|||
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age (continuous) | 1.01 (0.97-1.04) | 0.651 | ||
| Sex (male vs. female) | 2.76 (1.23-6.16) | 0.013 | 2.46 (1.00-6.05) | 0.048 |
| Smoking (former/current vs. never) | 2.26 (1.04-4.89) | 0.038 | 1.14 (0.47-2.78) | 0.758 |
| Cell differentiation (well vs. others) | 1.72 (0.80-3.68) | 0.157 | ||
| Tumor size (>2 cm vs. ≤2 cm) | 2.42 (1.11-5.25) | 0.025 | ||
| Lympho-vascular invasion (no vs. yes) | 1.06 (0.44-2.51) | 0.889 | ||
| Vascular invasion (no vs. yes) | 0.70 (0.24-2.07) | 0.527 | ||
| Pleural invasion (no vs. yes) | 0.97 (0.33-2.78) | 0.956 | ||
| Pathologic stage (IB vs. IA) | 2.69 (1.34-5.39) | 0.005 | 1.89 (0.88-4.05) | 0.099 |
| CD155 expression (positive vs. negative) | 3.44 (1.67-7.09) | <0.001 | ||
| PD-L1 (positive vs. negative) | 2.92 (1.28-6.64) | 0.011 | ||
| CD155 expression and PD-L1 expression (CD155+/PD-L1+ vs. others) | 4.41 (1.8-10.30) | <0.001 | 3.20 (1.24-8.22) | 0.016 |
| Mode of lung resection (sub-lobar resection vs. lobectomy) | 0.78 (0.18-3.32) | 0.741 | ||
| Adjuvant chemotherapy (not performed vs. performed) | 1.36 (0.41-4.47) | 0.612 | ||
95% CI, 95% confidence interval; CD155+, CD155 expression-positive; HR, hazard ratio; PD-L1, programmed death-ligand 1; PD-L1+, PD-L1 expression-positive.
Figure 5.
Recurrence-free survival curves according to CD155 expression and PD-L1 expression. (Left) Comparison among all groups and (right) comparison between the CD155+/PD-L1+ group and others (CD155+/PD-L1−, CD155-/PD-L1+ and CD155-/PD-L1−). CD155−, CD155 expression-negative; CD155+, CD155 expression-positive; PD-L1, programmed death-ligand 1; PD-L1−, PD-L1 expression-negative; PD-L1+, PD-L1 expression-positive.
Figure 6.
Overall survival curves according to CD155 expression and PD-L1 expression. (Left) Comparison among all groups and (right) comparison between the CD155+/PD-L1+ group and others (CD155+/PD-L1−, CD155-/PD-L1+ and CD155-/PD-L1−). CD155−, CD155 expression-negative; CD155+, CD155 expression-positive; PD-L1, programmed death-ligand 1; PD-L1−, PD-L1 expression-negative; PD-L1+, PD-L1 expression-positive.
Discussion
The present study revealed the detailed CD155 expression in lung adenocarcinoma. As the TIGIT/CD155 axis has emerged as a novel therapeutic target in a variety of malignant tumors, several studies on the CD155 expression in NSCLC including lung adenocarcinoma have been reported (14–21). However, no study has previously reported detailed distribution of tumoral CD155 expression. Accordingly, we quantitatively evaluated tumoral CD155, and determined the optimal cut-off value (TPS, 5%) using ROC-curve analysis.
Next, we showed that CD155-positivity was significantly associated with aggressive cancer behavior such as pleural/vascular invasion and was a significant factor to predict a poor prognosis. Previous clinical studies in NSCLC also showed that CD155-positivity was correlated with a poor prognosis (14,16–21). However, characteristics of patients included in previous studies were too heterogenous to draw a definitive conclusion. For example, stage I–IV patients were included in 3 studies (14,18,19). Accordingly, the present study is the first clinical study to reveal the prognostic impact of CD155 status in homogenous patients with early-stage lung adenocarcinoma. CD155 is a member of the immunoglobulin superfamily, and plays important biological roles in cell proliferation and migration as well as modulation of immune responses (6–8,22). CD155 expression is not detected in most normal tissues, but is upregulated in a variety of malignant tumors. Several experimental studies have shown that CD155 overexpression cause tumor progression through promoting migration and invasion of cancer cells and through inducing immune escape (22), which may reasonably explain the poor prognosis associated with CD155-positivity.
Finally, we found that the status of CD155+/PD-L1+ was a significant factor to predict the poorest prognosis, and that the status of CD155+/PD-L1+ was a significant factor to predict a poor prognosis. Lee and coworkers also reported that CD155+/PD-L1+ patients showed the poorest prognosis in lung squamous cell carcinoma (19). Cancer cells may survive by expressing PD-L1 to evade immune attack, which may be associated with aggressive cancer behavior. Accordingly, when CD155-status and PD-L1-status were combined, CD155+/PD-L1+ tumor may represent a highly aggressive behavior associated with the poorest prognosis.
There are several limitations in the present study. First, this study is a retrospective, single-center study on a small number of patients. Second, a large proportion of patients were lost to follow-up. Finally, the present study provided no data on CD155 expression in p-stage II–III diseases, although two previous studies showed that CD155 expression was significantly higher in more advanced stages (14,18). We are now planning to conduct a large-scale study to assess CD155 expression in other histological types of NSCLC such as squamous cell carcinoma in addition to that in p-stage II–III diseases.
In conclusion, CD155 expression was positive in 37 patients (38.5%) of all the 96 patients with completely resected p-stage I adenocarcinoma of the lung. CD155-positivity was associated with aggressive tumor behavior, and was a significant predictor of a poor prognosis. Its prognostic impact was enhanced when it was combined with the expression status of PD-L1, where CD155+/PD-L1+ patients showed the poorest prognosis. A large-scale study should be conducted to draw a convinced conclusion.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- NSCLC
non-small cell lung cancer
- PD-1
programmed cell death 1
- PD-L1
programmed death-ligand 1
- CTL
cytotoxic T-lymphocyte
- TIGIT
T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory domain
- PVR
poliovirus receptor
- IHC
immunohistochemistry
- TPS
tumor proportion score
- ROC
receiver operating characteristic
- AUC-ROC
area under receiver operating characteristic curve
- OS
overall survival
- RFS
recurrence-free survival
Funding Statement
This work was supported in part by the Japan Society for the Promotion of Science (Grants-in-Aid for Scientific Research; grant nos. 18K08806, 19K09293, 19K16786 and 20K97688), and Research Grant for Promotion of Occupational Health by the University of Occupational and Environmental Health (grant no. UOEH-R3).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
KK, KY and FT designed the study. RO, MK and AT performed immunohistochemical staining. RO, KY, MT and MM evaluated the results of IHS. HM, AT, SS, MT and KK collected the clinical data. RO, MM, MT and KK confirm the authenticity of all the raw data. HM, AT and SS performed statistical analyses. KY, AT and FT wrote the manuscript. SS and MT helped to write the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board of the University of Occupational and Environmental Health (approval no. H26-15; Kitakyushu, Japan). All participants provided written informed consent to participate in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29((Suppl 4)):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 3.Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21:687–692. doi: 10.1158/1078-0432.CCR-14-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews LP, Yano H, Vignali DAA. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: Breakthroughs or backups. Nat Immunol. 2019;20:1425–1434. doi: 10.1038/s41590-019-0512-0. [DOI] [PubMed] [Google Scholar]
- 7.Yeo J, Ko M, Lee DH, Park Y, Jin HS. TIGIT/CD226 axis regulates anti-tumor immunity. Pharmaceuticals (Basel) 2021;14:200. doi: 10.3390/ph14030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Attili I, Tarantino P, Passaro A, Stati V, Curigliano G, de Marinis F. Strategies to overcome resistance to immune checkpoint blockade in lung cancer. Lung Cancer. 2021;154:151–160. doi: 10.1016/j.lungcan.2021.02.035. [DOI] [PubMed] [Google Scholar]
- 10.Hirai A, Yoneda K, Shimajiri S, Kuroda K, Hanagiri T, Fujino Y, Tanaka F. Prognostic impact of programmed death-ligand 1 expression in correlation with human leukocyte antigen class I expression status in stage I adenocarcinoma of the lung. J Thorac Cardiovasc Surg. 2018;155:382–392.e1. doi: 10.1016/j.jtcvs.2017.05.106. [DOI] [PubMed] [Google Scholar]
- 11.Sugaya M, Uramoto H, Uchiyama A, Nagashima A, Nakanishi R, Sakata H, Nakanishi K, Hanagiri T, Yasumoto K. Phase II trial of adjuvant chemotherapy with bi-weekly carboplatin plus paclitaxel in patients with completely resected non-small cell lung cancer. Anticancer Res. 2010;30:3039–3044. [PubMed] [Google Scholar]
- 12.Uramoto H, Nakanishi R, Nagashima A, Uchiyama A, Inoue M, Osaki T, Yoshimatsu T, Sakata H, Nakanishi K, Yasumoto K. A randomized phase II trial of adjuvant chemotherapy with bi-weekly carboplatin plus paclitaxel versus carboplatin plus gemcitabine in patients with completely resected non-small cell lung cancer. Anticancer Res. 2010;30:4695–4699. [PubMed] [Google Scholar]
- 13.Talbot D, Massamba VK. A descriptive review of variable selection methods in four epidemiologic journals: There is still room for improvement. Eur J Epidemiol. 2019;34:725–730. doi: 10.1007/s10654-019-00529-y. [DOI] [PubMed] [Google Scholar]
- 14.Nakai R, Maniwa Y, Tanaka Y, Nishio W, Yoshimura M, Okita Y, Ohbayashi C, Satoh N, Ogita H, Takai Y, Hayashi Y. Overexpression of Necl-5 correlates with unfavorable prognosis in patients with lung adenocarcinoma. Cancer Sci. 2010;101:1326–1330. doi: 10.1111/j.1349-7006.2010.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Luo J, Chen Y, Cui J, Lei Y, Cui Y, Jiang N, Jiang W, Chen L, Chen Y, et al. Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma) Int Immunopharmacol. 2020;80:106198. doi: 10.1016/j.intimp.2020.106198. [DOI] [PubMed] [Google Scholar]
- 16.Lee BR, Chae S, Moon J, Kim MJ, Lee H, Ko HW, Cho BC, Shim HS, Hwang D, Kim HR, Ha SJ. Combination of PD-L1 and PVR determines sensitivity to PD-1 blockade. JCI Insight. 2020;5:e12863. doi: 10.1172/jci.insight.128633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang WC, Kuo KT, Wang CH, Yeh CT, Wang Y. Cisplatin resistant lung cancer cells promoted M2 polarization of tumor-associated macrophages via the Src/CD155/MIF functional pathway. J Exp Clin Cancer Res. 2019;38:180. doi: 10.1186/s13046-019-1166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You H, Zhang YZ, Lai HL, Li D, Liu YQ, Li RZ, Khan I, Hsiao WW, Duan FG, Fan XX, et al. Prognostic significance of tumor poliovirus receptor and CTLA4 expression in patients with surgically resected non-small-cell lung cancer. J Cancer Res Clin Oncol. 2020;146:1441–1450. doi: 10.1007/s00432-020-03189-8. [DOI] [PubMed] [Google Scholar]
- 19.Lee JB, Hong MH, Park SY, Chae S, Hwang D, Ha SJ, Shim HS, Kim HR. Overexpression of PVR and PD-L1 and its association with prognosis in surgically resected squamous cell lung carcinoma. Sci Rep. 2021;11:8551. doi: 10.1038/s41598-021-87624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Yang Z, Du G, Cao L, Tan B. CD155-prognostic and immunotherapeutic implications based on multiple analyses of databases across 33 human cancers. Technol Cancer Res Treat. 2021;20:1533033820980088. doi: 10.1177/1533033820980088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller S, Mayer S, Möller P, Barth TFE, Marienfeld R. Spatial distribution of immune checkpoint proteins in histological subtypes of lung adenocarcinoma. Neoplasia. 2021;23:584–593. doi: 10.1016/j.neo.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao J, Zheng Q, Xin N, Wang W, Zhao C. CD155, an onco-immunologic molecule in human tumors. Cancer Sci. 2017;108:1934–1938. doi: 10.1111/cas.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.