Abstract
Acquired resistance to tyrosine kinase inhibitors (TKIs) limits the duration of antitumor effects and impairs the survival of patients with oncogene-driven non-small cell lung cancer (NSCLC). At present, little is known about the immunomodulatory ability of TKIs during the entire treatment period, including the drug-sensitive and drug-resistant periods. The present review aimed to comprehensively explore the dynamic changes in the tumor microenvironment (TME) during TKI treatment in NSCLC. Previous clinical and preclinical studies from medical and health databases related to NSCLC are reviewed. During the response period, cytotoxic immune cells accumulate in the TME and contribute to the formation of an inflammatory microenvironment. During the resistance period, the number of immunosuppressive cells increases, as does the expression of immune checkpoint proteins, which are critical mechanisms for tumor progression. The combination of targeted therapy and immunotherapy has been explored in multiple studies, and preliminary data showed controversial results. Extensive studies are needed to confirm the criteria of the selected patient subgroups and the toxicity profiles of EGFR TKIs and immune checkpoint inhibitors (ICIs). At present, the reagents targeting other immune cells, cytokines and related pathways remain underexplored compared with the revolutionary effect of ICIs in lung cancer. In the future, the precisely selected regimens for combination treatment should be further investigated in carefully designed xenograft models and clinical trials.
Keywords: non-small cell lung cancer, tyrosine kinase inhibitors, acquired resistance, tumor microenvironment, combination treatment
1. Introduction
Lung cancer remains the leading cause of cancer-related death worldwide. During the last decade, the treatment paradigm for non-small cell lung cancer (NSCLC) has been significantly changed by targeted tyrosine kinase inhibitors (TKIs) based on molecular features (1). However, the limited response prevents patients from receiving the benefits of the targeted therapy. For example, in the case of EGFR, most patients gain resistance to gefitinib and erlotinib within a median period of 14 months (2). Overcoming the dilemma of recurrent resistance to TKIs is challenging. PD-1/PD-L1 inhibitors are gaining attention for the treatment of NSCLC. Published clinical data have shown that immune checkpoint inhibitors (ICIs) exhibit little antitumor efficacy in NSCLC with oncogene mutations (3,4). Accumulating evidence has demonstrated that the response rate of ICI is closely associated with the immune microenvironment (5). Changes in the tumor immune microenvironment during TKI treatment remain unclear. Therefore, a better understanding of the tumor microenvironment (TME) during targeted treatment is required to provide clues for functional treatment strategies (6,7).
The present review summarizes the last 10 years of research into the immune alterations occurring before and after resistance to targeted therapy in NSCLC. Furthermore, the present review summarizes how different components of the TME contribute to TKI resistance as well as to the disease relapse and discusses combination strategies to achieve long-lasting responses in patients with NSCLC.
2. Short-term effects on the TME
EGFR inhibitors
Numerous immune cells coexist in the TME, including T and B lymphocytes, macrophages, polymorphonuclear cells, mast cells, natural killer cells, dendritic cells and myeloid-derived suppressor cells (MDSCs). These cell clusters can infiltrate into the TME and alter it after targeted therapy (6).
During the response period to TKIs, CD8+ T cells and dendritic cells expand, while immunosuppressive cells such as Foxp3+ regulatory T cells (Tregs) and M2-like polarization of macrophages are inhibited. T-cell infiltration in the TME is elevated by TKIs (8). Increased lymphocyte infiltration generates a local inflammatory TME. For example, skin rash is a common side effect of EGFR-TKIs. EGFR-TKIs have been reported to alter the levels of circulating cytokines and lymphocytes (9). A significant increase in peripheral natural killer cells and interferon-γ (IFN-γ) levels is observed after 4 weeks of gefitinib treatment. In addition, erlotinib and gefitinib improve the susceptibility of cancer cells to natural killer cells (10). EGFR inhibitors induce the expression of major histocombatibility complex (MHC) class I and II molecules and promote T cell-mediated tumor death (11). Circulating interleukin 6 (IL-6) levels are significantly decreased, which might predict better progression-free survival (7). EGFR TKIs decrease pro-inflammatory cytokines such as CC chemokine ligand (CCL)2, CCL5, C-X-C motif chemokine ligand (CXCL)8, CXCL10, and IFN-γ-induced protein 10, and increase the level of IFN-γ (9,12).
In mouse models with sensitive EGFR mutations, erlotinib increases the infiltration of T lymphocytes and natural killer cells other than B cells. Dendritic cells and macrophages with increased MHC class II expression may enhance antigen-presenting activity. The levels of immune checkpoints in the TME were reduced (13). Several EGFR downstream signaling pathways [IL-6/Janus kinase (JAK)/STAT3, NF-κB, and phospho-ERK1/2/phospho-c-Jun] regulate PD-L1 expression in tumor cells (3,7). An NSCLC specimen study illustrated that PD-L1 expression was downregulated after 4 weeks of gefitinib treatment (14). EGFR TKI treatment led to a decrease in PD-1, cytotoxic T lymphocyte-associated antigen 4 and T cell immunoglobulin and mucin domain-containing protein 3 expression in T cells in mouse models (8). EGFR TKIs and anti-PD-1 antibody combination treatment produced no synergistic antitumor effect in an in vitro co-culture system (3). Reduced expression of immune checkpoints within the TME may explain these clinical observations (15).
ALK inhibitors
ALK inhibitors have been reported to increase T-lymphocyte infiltration. Recently, whole-exome and RNA sequencing indicated that immune-related genes were altered in response to ALK TKI treatment. Antigen presentation genes, IFN-γ signaling genes, inhibitory checkpoint genes, and stimulatory checkpoint genes increased apparently after response to ALK TKIs (Table I) (16).
Table I.
Alterations in tumor microenvironment before and after tyrosine kinase inhibitor resistance.
| Function category | Immune component | iEGFR sensitive | iEGFR resistance | iALK resistance |
|---|---|---|---|---|
| Immune cells | CD8 + T cells | Increase (8,16,25) | Stable (16) | Increase (16), |
| Decrease (31) | Decrease (39) | |||
| NK cells | Increase (7,8,14,16) | Stable (16) | Increase (16) | |
| B cells | Increase (16) | NA | NA | |
| MDSCs | NA | Increase (8) | NA | |
| Dendritic cells | Increase (8) | NA | NA | |
| M2-macrophage | Decrease (8,25) | Increase (25) | Increase (39) | |
| Regulatory T cells | Decrease (8) | NA | Increase (39) | |
| Antigen presentation genes | CALR | NA | Decrease (16) | Decrease (16) |
| CANX | NA | Decrease (16) | Decrease (16) | |
| PDIA3 | NA | Decrease (16) | Decrease (16) | |
| MHC-I | Increase (11) | Decrease (23) | NA | |
| MHC-II | Increase (11) | NA | NA | |
| Immune checkpoints | STAT5B | NA | Increase (16) | Increase (16) |
| PD-1 | NA | Increase (16) | Increase (16) | |
| BTLA | NA | Increase (16) | Increase (16) | |
| CD27 | NA | Increase (16) | Increase (16) | |
| PD-L1 | Decrease (3,36) | Increase (29,31,36) | Increase (39) | |
| Inflammatory cytokines | IL-4 | NA | NA | Increase (39) |
| IL-6 | Decrease (7,14) | NA | NA | |
| IL-10 | Increase (8) | NA | NA | |
| CCL-2 | Increase (8) | NA | NA | |
| CCL-5 | Increase (9,12) | NA | NA | |
| CXCL8 | Increase (9) | NA | NA | |
| CXCL10 | Increase (9) | NA | NA | |
| IFN-γ | Increase (7,39) | Increase (16) | Increase (16) | |
| Mutational load | Decrease (6) | Increase (6,31) | Decrease (16) |
iEGFR, EGFR inhibitors; iALK, ALK inhibitors; MDSCs, myeloid-derived suppressor cells; CCL, CC chemokine ligand; CXCL, C-X-C motif chemokine ligand; MHC, major histocompatibility complex; PD-L1, programmed death-ligand 1; PD-1, programmed cell death protein-1; BTLA, B- and T-lymphocyte attenuator; CALR, calreticulin; CANX, calnexin; PDIA3, protein disulfide isomerase A3; NA, not applicable; NK cells, natural killer cells; IL, interleukin; IFN, interferon.
In conclusion, short-term exposure to TKIs not only eliminates tumor cells, but also modulates immune-mediated cytotoxicity in the TME during the initial response period. One study provided a rationale for the potential combination of erlotinib and immunotherapies for the treatment of lung carcinomas in first-line treatment (17). However, other pre-clinical studies confirmed that synergistic effects of EGFR-TKIs combined with anti-PD-1 antibody were not present in an in vitro co-culture system (3). According to the latest edition of the National Comprehensive Cancer Network guidelines for NSCLC (18), PD-1/PD-L1 inhibitor may not be recommended for EGFR+/ALK+ NSCLC due to the negative results generated in a retrospective study (19). Therefore, TKI monotherapy is preferred for oncogene-driven NSCLC in the first-line treatment.
3. Long-term effects on the TME
After the long-term use of TKIs, the positive therapeutic response is reversed, especially when resistance emerges. Under the selective pressure of targeted therapy, certain tumor cells evade the host immunity through a variety of intrinsic mechanisms. Multiple signaling pathways and related molecules comprise the complexity of the TME. In summary, a wide range of immunosuppressive mechanisms may evolve the acquired resistance to TKIs (20,21).
EGFR inhibitors
A decrease in cytotoxic T cell populations was observed in EGFR inhibitor-resistant lung adenocarcinoma when compared with that in the initial biopsy from an identical patient (22). Some resistant tumors are deficient for MHC class I as a result of decreased mRNA levels and related genes (23). Thus, targeted treatment may influence different aspects of tumor antigen presentation and T-cell effector function. Mitogen-activated protein kinase is the most common compensatory signaling pathway responsible for the development of acquired resistance (24). In the examination of tumor biopsies, cytotoxic T cells are found to decrease after TKI resistance and T-cell infiltration. Tregs are the main component of the TME. Conversely, the number of macrophages, especially those expressing indoleamine 2,3-dioxygenase 1 (IDO1), increase with disease progression (25).
MDSCs are immature myeloid cells that mainly inhibit T-cell activation; they help create a favorable environment for tumor survival. In an in vivo study an increased percentage of MDSCs was detected during long-term use of EGFR-TKI in mouse models (8,26). Recruitment and expansion of MDSCs were detected in the serum of patients with NSCLC who achieved EGFR TKI resistance, compared with the baseline (27). In the TME, MDSCs impair host antitumor immunity and the effect of immunotherapy (28).
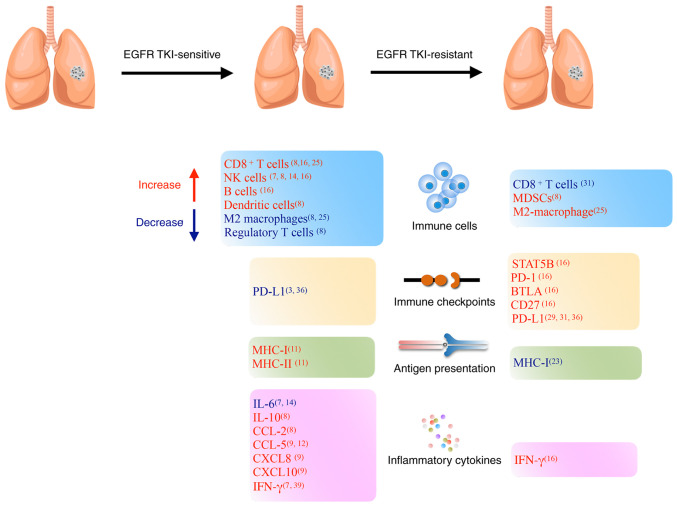
PD-L1 expression levels in tumor cells were found to notably increase after gefitinib treatment in a subset of patients (29). PD-L1 is a well-known immune checkpoint gene, and knockout of PD-L1 helps to restore the function of human cytotoxic T lymphocytes (30,31). The histological transformation of the tumor and metastasis sites affects the PD-L1 expression levels after resistance (32). In one study, paired analysis indicated that tumors with TPS ≥50% increased from 14 to 28% after acquired resistance, especially obvious in the EGFR T790M-negative tumor (31). High expression levels of PD-L1 have been reported to be a negative prognostic marker (33) and although lung cancer cells develop resistance to EGFR TKIs, EGFR phosphorylation is still suppressed in the resistant cells. However, the downstream ERK signaling is reactivated upon drug resistance, leading to PD-L1 restoration (34,35). Several studies have revealed that PD-L1 is a downstream target of the EGFR pathway, which is interceded via the IL-6/JAK/STAT3, NF-κB, and phospho-ERK1/2/phospho-c-Jun pathways (3,36–38). Single-cell RNA sequencing of biopsy samples showed that immunosuppressive genes, such as IDO1, kynureninase and quinolinate phosphoribosyltransferase levels were elevated in TKI-resistant tumors compared with those in naïve tumors (25). Dynamic changes are shown in Figure 1.
Figure 1.
Alterations in the tumor microenvironment during EGFR-TKI treatment. TKI, tyrosine kinase inhibitor; MDSCs, myeloid-derived suppressor cells; CCL, CC chemokine ligand; CXCL, C-X-C motif chemokine ligand; MHC, major histocompatibility complex; NK cells, natural killer cells; PD-L1, programmed death-ligand 1; PD-1, programmed cell death protein-1; BTLA, B- and T-lymphocyte attenuator; IFN, interferon; IL, interleukin.
ALK inhibitors
Limited data have been reported on the impact of ALK inhibitors on the TME after resistance in ALK-positive NSCLC. A preclinical study explored the changes in the tumor immune microenvironment in mouse models. The results indicated that ALK-positive NSCLC was as an immune ‘desert’ before the initiation of targeted therapy (39). After resistance to ceritinib the immunogenic features of TME changed, including increased PD-L1 expression levels, increased number of functionally impaired CD8+ T cells, antigen-presenting cells, Tregs and MDSCs, and increased IFN-γ secretion. RNA sequencing analysis revealed increased transcription of IFN-γ-related genes, Treg-related genes and immune suppressive macrophage-related genes. However, whole-exome sequencing revealed no obvious difference in tumor mutation burden and T cell receptor clonality between untreated and resistant ALK-positive tumors (39). Thus, due to the lack of immunogenicity and impaired antitumor immunity, ICIs are expected to be less effective in ALK-TKI-resistant tumors.
In conclusion, along with the emergence of acquired resistance, the TME begins to gain immunosuppressive features, such as the presence of inhibitory ligands, restoration of the MDSCs and Tregs, impaired functions of tumor-infiltrating lymphocytes, decreased antigen presentation, increased level of IFN-γ and increased immune checkpoint molecules (Table I) (8). Subsequently, immune evasion and T-cell exhaustion lead to disease progression (40). The potential mechanisms that are involved in these changes include: i) The reactivation of EGFR downstream signaling pathways; ii) the secretion of tumor-related exosomes by EGFR-mutated cells; and iii) the induction of MHC expression. The change in the TME indicates the possibility of initiating combination strategies in resistant patients.
4. Combination treatment strategies
At present, ICIs have achieved great success in the treatment area of lung cancer and are considered ideal for combination treatment. According to preclinical data, combination of EGFR-TKIs with ICIs showed promising antitumor effect in a mouse model (41–43). Two retrospective studies suggested that nivolumab had a favorable response rate in NSCLC, especially in EGFR T790M-negative disease (44).
In first-line treatment, a phase Ib study investigated the safety and efficacy of durvalumab in combination with gefitinib. However, this study was halted due to liver dysfunction. The objective response rate was 77.8% (45). The KEYNOTE-021 study evaluated the safety of the combination of erlotinib or gefitinib and pembrolizumab. Compared with erlotinib, pembrolizumab plus gefitinib increased the incidence of toxicity and resulted in treatment discontinuation. The overall objective response rate was 41.7% (46).
In second-line treatment, the response rate of combination therapy between nivolumab and erlotinib was 15% and the response lasted as long as 38.2 months. A total of 20% of the participants experienced grade three toxicities (47). Long-term analysis of a phase Ib study of erlotinib plus atezolizumab showed tolerable adverse effects of combination therapy, with a median overall survival time of 32.7 months (48). In the TATTON trial, the objective response rate was 42% in the osimertinib and durvalumab group. The most common adverse events were arm rash, vomiting and diarrhea (49). Another phase III trial, CAURAL, reported that 64% (9/14) of participants responded to osimertinib and durvalumab. The main toxicity presented as a rash (50). Unfortunately, the TATTON and CAURAL trials were terminated by AstraZeneca owing to the increased incidence of interstitial lung disease (51). Related data were collected from the FAERS database, where 70 patients with NSCLC were treated with EGFR-TKI plus nivolumab, and the incidence of interstitial pneumonitis was 25.7% (52).
Due to the lack of immunogenicity, preclinical and clinical data have indicated that PD-1/PD-L1 inhibitors combined with ALK inhibitors are not superior to ALK inhibitors alone, and the incidence of drug-related hepatotoxicity has increased (39,53). However, carefully selected drugs for combination therapy, such as avelumab plus lorlatinib, could produce antitumor activity with an acceptable safety profile (54).
Recent studies have shown that the response rate to PD-1/PD-L1 inhibitors is closely associated with PD-L1 expression levels, tumor-infiltrating lymphocytes, gut microbiota, and the levels of IFN-γ, TGF-β, VEGF-A, IL-6, IL-10 and other biomarkers (8,55). In TKI-resistant patients with favorable immune features, there should be more confidence in attempting combined therapy with ICIs and TKIs.
Researchers seek to discover novel combination treatments. An in vitro study found that inhibition of EGFR signaling and IL-8 signaling triggered tumor apoptosis in resistant lung cancer (17). Blockade of TGF-β signaling combined with EGFR TKIs decreased the motility of resistant tumor cells in the cell culture system (56). Erlotinib plus bevacizumab produced a synergy effect in the EGFR-TKI-resistant xenograft model (57). These studies provide different treatment strategies to overcome the acquired resistance to TKIs in NSCLC.
5. Conclusion and future perspectives
It is known that the host immunity undergoes dynamic changes following TKI treatment. During the response period, cytotoxic immune cells accumulate in the TME and contribute to the formation of an inflammatory TME. Once the acquired resistance develops, immunosuppressive cells as well as immune checkpoints begin to increase and function in the TME, which is a critical mechanism for tumor progression. Although the response rate of ICIs shows negative results in advanced oncogene-driven NSCLC at frontline treatment, the combination of targeted therapy and immunotherapy remains a feasible strategy to achieve synergistic effects in selected resistant patients. Combination treatment has been explored in multiple studies, and preliminary data have shown both positive and negative results. Thus, extensive exploration is still needed to confirm the selection criteria of patient subgroups and the toxicity profiles of EGFR-TKIs plus ICIs. At present, reagents targeting MDSCs, Tregs, IL-8, TGF-β and related pathways remain underexplored as compared with the revolutionary effect of ICIs in lung cancer. In the future, the precisely selected regimens for combination therapy should be further investigated in carefully designed xenograft models and clinical trials.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- NSCLC
non-small cell lung cancer
- TKIs
tyrosine kinase inhibitors
- MDSCs
myeloid-derived suppressor cells
- TME
tumor microenvironment
- Tregs
regulatory T cells
- ICIs
immune checkpoint inhibitors
- IDO1
indoleamine 2,3-dioxygenase 1
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
LJ performed the literature search and drafted the manuscript. JYL conceived the review and revised the manuscript. Both authors have read and approved the manuscript. Data sharing is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cappuzzo F. Springer International Publishing; Cham: 2015. Therapy options for advanced NSCLC. In: Guide to Targeted Therapies: Treatment Resistance in Lung Cancer; pp. 5–25. [Google Scholar]
- 2.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 3.Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, Zhang Y, He X, Zhou T, Qin T, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: Implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10:910–923. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 4.Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, Coluzzi P, Ledezma B, Mendenhall M, Hunt J, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol. 2018;13:1138–1145. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y, Matsui S, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. 2020;21:1346–1358. doi: 10.1038/s41590-020-0769-3. [DOI] [PubMed] [Google Scholar]
- 6.Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, Zhou Q, Tu HY, Xu CR, Yan LX, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6:e1356145. doi: 10.1080/2162402X.2017.1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soo RA, Lim SM, Syn NL, Teng R, Soong R, Mok TSK, Cho BC. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: Current controversies and future directions. Lung Cancer. 2018;115:12–20. doi: 10.1016/j.lungcan.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Jia Y, Li X, Jiang T, Zhao S, Zhao C, Zhang L, Liu X, Shi J, Qiao M, Luo J, et al. EGFR-targeted therapy alters the tumor microenvironment in EGFR-driven lung tumors: Implications for combination therapies. Int J Cancer. 2019;145:1432–1444. doi: 10.1002/ijc.32191. [DOI] [PubMed] [Google Scholar]
- 9.Mascia F, Mariani V, Girolomoni G, Pastore S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303–312. doi: 10.1016/S0002-9440(10)63654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H, Kim SH, Kim MJ, Kim SJ, Park SJ, Chung JS, Bae JH, Kang CD. EGFR inhibitors enhanced the susceptibility to NK cell-mediated lysis of lung cancer cells. J Immunother. 2011;34:372–381. doi: 10.1097/CJI.0b013e31821b724a. [DOI] [PubMed] [Google Scholar]
- 11.Im JS, Herrmann AC, Bernatchez C, Haymaker C, Molldrem JJ, Hong WK, Perez-Soler R. Immune-modulation by epidermal growth factor receptor inhibitors: Implication on Anti-tumor immunity in lung cancer. PLoS One. 2016;11:e0160004. doi: 10.1371/journal.pone.0160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul T, Schumann C, Rüdiger S, Boeck S, Heinemann V, Kächele V, Steffens M, Scholl C, Hichert V, Seufferlein T, Stingl JC. Cytokine regulation by epidermal growth factor receptor inhibitors and epidermal growth factor receptor inhibitor associated skin toxicity in cancer patients. Eur J Cancer. 2014;50:1855–1863. doi: 10.1016/j.ejca.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Venugopalan A, Lee MJ, Niu G, Medina-Echeverz J, Tomita Y, Lizak MJ, Cultraro CM, Simpson RM, Chen X, Trepel JB, Guha U. EGFR-targeted therapy results in dramatic early lung tumor regression accompanied by imaging response and immune infiltration in EGFR mutant transgenic mouse models. Oncotarget. 2016;7:54137–54156. doi: 10.18632/oncotarget.11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng J, Fang W, Liu X, Xing S, Zhan J, Ma Y, Huang Y, Zhou N, Zhao H, Zhang L. Impact of gefitinib in early stage treatment on circulating cytokines and lymphocytes for patients with advanced non-small cell lung cancer. Onco Targets Ther. 2017;10:1101–1110. doi: 10.2147/OTT.S112158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin Cancer Res. 2016;22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Y, Wang Y, Zeng D, Zhi S, Shu T, Huang N, Zheng S, Wu J, Liu Y, Huang G, et al. Comprehensive analyses reveal TKI-induced remodeling of the tumor immune microenvironment in EGFR/ALK-positive non-small-cell lung cancer. OncoImmunology. 2021;10:1951019. doi: 10.1080/2162402X.2021.1951019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominguez C, Tsang KY, Palena C. Short-term EGFR blockade enhances immune-mediated cytotoxicity of EGFR mutant lung cancer cells: Rationale for combination therapies. Cell Death Dis. 2016;7:e2380. doi: 10.1038/cddis.2016.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network (NCCN), corp-author NCCN; Plymouth Meeting, PA: 2021. The NCCN Clinical Practice Guidelines in Oncology, Non-small Cell Lung Cancer (version 1.2021) [Google Scholar]
- 19.Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, Thai AA, Mascaux C, Couraud S, Veillon R, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann Oncol. 2019;30:1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 21.Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawana S, Saito R, Miki Y, Kimura Y, Abe J, Sato I, Endo M, Sugawara S, Sasano H. Suppression of tumor immune microenvironment via microRNA-1 after epidermal growth factor receptor-tyrosine kinase inhibitor resistance acquirement in lung adenocarcinoma. Cancer Med. 2021;10:718–727. doi: 10.1002/cam4.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrido G, Rabasa A, Garrido C, López A, Chao L, García-Lora AM, Garrido F, Fernández LE, Sánchez B. Preclinical modeling of EGFR-specific antibody resistance: Oncogenic and immune-associated escape mechanisms. Oncogene. 2014;33:3129–3139. doi: 10.1038/onc.2013.288. [DOI] [PubMed] [Google Scholar]
- 24.Chapman PB. Mechanisms of resistance to RAF inhibition in melanomas harboring a BRAF mutation. Am Soc Clin Oncol Educ Book. doi: 10.14694/EdBook_AM.2013.33.e80. doi: 10.1200/EdBook_AM.2013.33.e80. [DOI] [PubMed] [Google Scholar]
- 25.Maynard A, McCoach CE, Rotow JK, Harris L, Haderk F, Kerr DL, Yu EA, Schenk EL, Tan W, Zee A, et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell. 2020;182:1232–1251.e22. doi: 10.1016/j.cell.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Tang J, Liu X, Ma Q, Deng Q, Li K, Zhang B, Wang Y. A38 Gemcitabine improves suppressive immune microenvironment induced by long-term treatment with EGFR-TKIs: Implications for combination chemotherapy and immunotherapy. J Thoracic Oncol. 2020;15((Suppl)):S25. doi: 10.1016/j.jtho.2019.12.068. [DOI] [Google Scholar]
- 27.Tang J, Liu X, Gong Y, Zhu J, Huang M, Ding Z, Yu M, Tie Y, Li Q, Wang Y. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) impact on immune microenvironment in non-small cell lung cancer (NSCLC) J Clin Oncol. 2018;36:e21154. doi: 10.1200/JCO.2018.36.15_suppl.e21154. [DOI] [Google Scholar]
- 28.Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-derived suppressor cells: Critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res. 2015;128:95–139. doi: 10.1016/bs.acr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han JJ, Kim DW, Koh J, Keam B, Kim TM, Jeon YK, Lee SH, Chung DH, Heo DS. Change in PD-L1 expression after acquiring resistance to gefitinib in EGFR-mutant non-small-cell lung cancer. Clin Lung Cancer. 2016;17:263–270.e2. doi: 10.1016/j.cllc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Peng S, Wang R, Zhang X, Ma Y, Zhong L, Li K, Nishiyama A, Arai S, Yano S, Wang W. EGFR-TKI resistance promotes immune escape in lung cancer via increased PD-L1 expression. Mol Cancer. 2019;18:165. doi: 10.1186/s12943-019-1073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isomoto K, Haratani K, Hayashi H, Shimizu S, Tomida S, Niwa T, Yokoyama T, Fukuda Y, Chiba Y, Kato R, et al. Impact of EGFR-TKI treatment on the tumor immune microenvironment in EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res. 2020;26:2037–2046. doi: 10.1158/1078-0432.CCR-19-2027. [DOI] [PubMed] [Google Scholar]
- 32.Suda K, Murakami I, Yu H, Kim J, Ellison K, Rivard CJ, Mitsudomi T, Hirsch FR. Heterogeneity in immune marker expression after acquisition of resistance to EGFR kinase inhibitors: Analysis of a case with small cell lung cancer transformation. J Thorac Oncol. 2017;12:1015–1020. doi: 10.1016/j.jtho.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Lu ZY, Fang YC, Chen XF, Liu GT. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: A meta-analysis. Eur J Surg Oncol. 2015;41:450–456. doi: 10.1016/j.ejso.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Ercan D, Xu C, Yanagita M, Monast CS, Pratilas CA, Montero J, Butaney M, Shimamura T, Sholl L, Ivanova EV, et al. Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discov. 2012;2:934–947. doi: 10.1158/2159-8290.CD-12-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang L, Guo F, Liu X, Li X, Qin Q, Shu P, Li Y, Wang Y. Continuous targeted kinase inhibitors treatment induces upregulation of PD-L1 in resistant NSCLC. Sci Rep. 2019;9:3705. doi: 10.1038/s41598-018-38068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang N, Zeng Y, Du W, Zhu J, Shen D, Liu Z, Huang JA. The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. Int J Oncol. 2016;49:1360–1368. doi: 10.3892/ijo.2016.3632. [DOI] [PubMed] [Google Scholar]
- 38.Lin K, Cheng J, Yang T, Li Y, Zhu B. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-κB. Biochem Biophys Res Commun. 2015;463:95–101. doi: 10.1016/j.bbrc.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 39.Pyo KH, Lim SM, Park CW, Jo HN, Kim JH, Yun MR, Kim D, Xin CF, Lee W, Gheorghiu B, et al. Comprehensive analyses of immunodynamics and immunoreactivity in response to treatment in ALK-positive non-small-cell lung cancer. J Immunother Cancer. 2020;8:e000970. doi: 10.1136/jitc-2020-000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philip M, Schietinger A. Beyond genomics: Multidimensional analysis of cancer therapy resistance. Trends Immunol. 2015;36:665–667. doi: 10.1016/j.it.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugiyama E, Togashi Y, Takeuchi Y, Shinya S, Tada Y, Kataoka K, Tane K, Sato E, Ishii G, Goto K, et al. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR-mutated non-small cell lung cancer. Sci Immunol. 2020;5:eaav3937. doi: 10.1126/sciimmunol.aav3937. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Han C, Dong C, Shen A, Hsu E, Ren Z, Lu C, Liu L, Zhang A, Timmerman C, et al. Hypofractionated EGFR tyrosine kinase inhibitor limits tumor relapse through triggering innate and adaptive immunity. Sci Immunol. 2019;4:eaav6473. doi: 10.1126/sciimmunol.aav6473. [DOI] [PubMed] [Google Scholar]
- 43.Creelan BC, Yeh TC, Kim SW, Nogami N, Kim DW, Chow LQM, Kanda S, Taylor R, Tang W, Tang M, et al. A Phase 1 study of gefitinib combined with durvalumab in EGFR TKI-naive patients with EGFR mutation-positive locally advanced/metastatic non-small-cell lung cancer. Br J Cancer. 2021;124:383–390. doi: 10.1038/s41416-020-01099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, Hayashi K, Tomida S, Chiba Y, Yonesaka K, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol. 2017;28:1532–1539. doi: 10.1093/annonc/mdx183. [DOI] [PubMed] [Google Scholar]
- 45.Gibbons DL, Chow LQ, Kim DW, Kim SW, Yeh T, Song X, Jiang H, Taylor R, Karakunnel J, Creelan B. 57O Efficacy, safety and tolerability of MEDI4736 [durvalumab (D)], a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): A phase I expansion in TKI-naïve patients (pts) with EGFR mutant NSCLC. J Thoracic Oncol. 2016;11((Suppl)):S79. doi: 10.1016/S1556-0864(16)30171-X. [DOI] [Google Scholar]
- 46.Yang JC, Gadgeel SM, Sequist LV, Wu CL, Papadimitrakopoulou VA, Su WC, Fiore J, Saraf S, Raftopoulos H, Patnaik A. Pembrolizumab in combination with erlotinib or gefitinib as first-line therapy for advanced NSCLC with sensitizing EGFR mutation. J Thorac Oncol. 2019;14:553–559. doi: 10.1016/j.jtho.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 47.Gettinger S, Hellmann MD, Chow LQM, Borghaei H, Antonia S, Brahmer JR, Goldman JW, Gerber DE, Juergens RA, Shepherd FA, et al. Nivolumab plus erlotinib in patients with EGFR-mutant advanced NSCLC. J Thorac Oncol. 2018;13:1363–1372. doi: 10.1016/j.jtho.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Rudin C, Cervantes A, Dowlati A, Besse B, Ma B, Costa D, Schmid P, Heist R, Villaflor V, Sarkar I, et al. MA15.02 long-term safety and clinical activity results from a phase Ib study of erlotinib plus atezolizumab in advanced NSCLC. J Thoracic Oncol. 2018;13((Suppl)):S407. doi: 10.1016/j.jtho.2018.08.440. [DOI] [Google Scholar]
- 49.Oxnard GR, Yang JC, Yu H, Kim SW, Saka H, Horn L, Goto K, Ohe Y, Mann H, Thress KS, et al. TATTON: A multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol. 2020;31:507–516. doi: 10.1016/j.annonc.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 50.Yang JC, Shepherd FA, Kim DW, Lee GW, Lee JS, Chang GC, Lee SS, Wei YF, Lee YG, Laus G, et al. Osimertinib plus durvalumab versus osimertinib monotherapy in EGFR T790M-positive NSCLC following previous EGFR TKI therapy: CAURAL brief report. J Thorac Oncol. 2019;14:933–939. doi: 10.1016/j.jtho.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Gourd K. AstraZeneca halts two lung cancer drug trials. Lancet Respir Med. 2015;3:926. doi: 10.1016/S2213-2600(15)00464-6. [DOI] [PubMed] [Google Scholar]
- 52.Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4:1112–1115. doi: 10.1001/jamaoncol.2017.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel SP, Pakkala S, Pennell NA, Reckamp KL, Lanzalone S, Polli A, Tarazi J, Robert-Vizcarrondo F. Phase Ib study of crizotinib plus pembrolizumab in patients with previously untreated advanced non-small cell lung cancer with ALK translocation. Oncologist. 2020;25:562–e1012. doi: 10.1634/theoncologist.2020-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw AT, Felip E, Bauer TM, Besse B, Navarro A, Postel-Vinay S, Gainor JF, Johnson M, Dietrich J, James LP, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, Wu K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17:129. doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serizawa M, Takahashi T, Yamamoto N, Koh Y. Combined treatment with erlotinib and a transforming growth factor-β type I receptor inhibitor effectively suppresses the enhanced motility of erlotinib-resistant non-small-cell lung cancer cells. J Thorac Oncol. 2013;8:259–269. doi: 10.1097/JTO.0b013e318279e942. [DOI] [PubMed] [Google Scholar]
- 57.Masuda C, Yanagisawa M, Yorozu K, Kurasawa M, Furugaki K, Ishikura N, Iwai T, Sugimoto M, Yamamoto K. Bevacizumab counteracts VEGF-dependent resistance to erlotinib in an EGFR-mutated NSCLC xenograft model. Int J Oncol. 2017;51:425–434. doi: 10.3892/ijo.2017.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.