Abstract
Background
To illustrate the molecular mechanism of mycoheterotrophic interactions between orchids and fungi, we assembled chromosome-level reference genome of Gastrodia menghaiensis (Orchidaceae) and analyzed the genomes of two species of Gastrodia.
Results
Our analyses indicated that the genomes of Gastrodia are globally diminished in comparison to autotrophic orchids, even compared to Cuscuta (a plant parasite). Genes involved in arbuscular mycorrhizae colonization were found in genomes of Gastrodia, and many of the genes involved biological interaction between Gatrodia and symbiotic microbionts are more numerous than in photosynthetic orchids. The highly expressed genes for fatty acid and ammonium root transporters suggest that fungi receive material from orchids, although most raw materials flow from the fungi. Many nuclear genes (e.g. biosynthesis of aromatic amino acid L-tryptophan) supporting plastid functions are expanded compared to photosynthetic orchids, an indication of the importance of plastids even in totally mycoheterotrophic species.
Conclusion
Gastrodia menghaiensis has the smallest proteome thus far among angiosperms. Many of the genes involved biological interaction between Gatrodia and symbiotic microbionts are more numerous than in photosynthetic orchids.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-022-03573-1.
Keywords: Gastrodia, Genome evolution, Mycoheterotrophy, Mycorrhizal roots
Background
Orchid family is among the largest plant families with approximately 27 000 species in 750 genera [1]. The germination of dust-like seeds depends on mycorrhizal fungi for nutrients, including organic carbon (C), phosphorus (P) and nitrogen (N) [2, 3]. With plants becoming autotrophy by photosynthesis, heterotrophic orchid seedlings switch to autotrophic adults. Many plants have maintained the ability to live on fungal carbon and gradually lost the capacity to photosynthesize, and these groups range from partially photosynthetic green species to obligate mycoheterotrophs that completely lack chlorophyll and are fully dependent on their fungal associates [4–9].
It is estimated that there are approximately 47 independent origins of full mycoheterotrophy in land plants [10]. Three major fungal lineages, i.e., Ascomycota, Basidiomycota and Glomeromycota, have been involved in the mycoheterotrophic interactions, out of which the Glomeromycota supports the greatest number of fully mycoheterotrophic species [11–13]. The evolutionary dynamics and genetic composition of plant–fungus interactions are largely unknown [14–18].
Gastrodia (Orchidaceae; Epidedroideae) comprises ~ 100 species distributed in the Old World subtropical and tropics [10, 19–22] and is the largest genus of orchid obligate mycoheterotrophs. Like most orchids, Gastrodia species depend on fungi for seed germination and initially their source of organic carbon, but in Gastrodia and relatives (tribe Gastrodieae) this dependence continues throughout their life cycle [4, 23–25]. Compared with photosynthetic orchids, the species of Gastrodia exhibit massive changes in their body plans and consist of solely leafless swollen stems (tubers) [9, 14, 24]. Most species of Gastrodia, such as G. menghaiensis (Supplementary Figure S1a-b), form well-developed mycorrhizal roots, whereas other species, such as G. elata (Supplementary Figure S1c), are rootless with their fungal associate directly connected to their tubers [14, 26, 27]. To date, G. elata has the smallest known angiosperm genome, containing approximately 18,969 protein-coding genes [9, 28] (but see [29]) with some genes families associated with its mycoheterotrophic lifestyle, such strigolactone signaling and digestion of hyphae, expanded.
These features make Gastrodia an important model to study plant–fungus interactions and obligate mycoheterotrophy. Here, we present a high-quality chromosome-level assembly of the G. menghaiensis genome and demonstrate that the G. menghaiensis genome has experienced massive alterations of the number and kinds of genes. We have found that many of the genes involved biological interaction between Gatrodia and symbiotic microbionts are more numerous than in photosynthetic orchids.
Results and discussions
Assembly, annotation of genome of Gastrodia menghaiensis
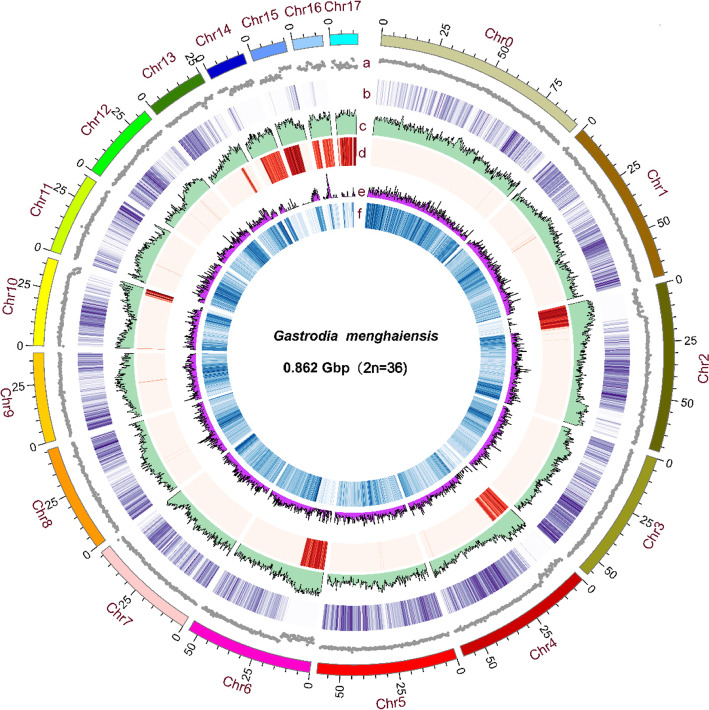
The k-mer-based genome size estimate of G. menghaiensis is 0.987 Gb with a low level of heterozygosity (0.1%) and high repeats (65.08%) (Supplementary Table S1, Supplementary Figure S2). Whole-genome shotgun sequencing was performed with the PacBio Sequel platform (~ 102.70 × coverage), Illumina Hiseq X-ten (read length of 150 bp, ~ 122.50 × coverage) and 10X Genomics (~ 131.90 × coverage) (Supplementary Table S2). Finally, the assembly consisted of 1,595 scaffolds, with a scaffold N50 of 6.82 Mb (total length = 862.84 Mb) and contig N50 of 2.37 Mb (total length = 859.12 Mb) (Supplementary Table S3). Overall, our results showed that 97.66% of the raw sequence reads could be mapped to the assembly, suggesting that our assembly was nearly complete (Supplementary Table S4). This was further assessed using EST (Expressed Sequence Tag), CEGMA (conserved core eukaryotic gene mapping approach), BUSCO (benchmarking universal single-copy orthologs analysis) [30] and transcriptome data. Approximately 99.8% ETS sequences are covered by our assembly; 232 of 248 (93.55%) conserved core eukaryotic genes from CEGMA were captured in our assembly, and 212 (85.48%) of these were complete (Supplementary Table S5 and S6). BUSCO revealed that 1046 of 1440 (72.7%) highly conserved genes were captured in our assembly (Supplementary Tables S7 and S8). We further revised the G. menghaiensis genome assembly using high-throughput chromosome conformation capture (Hi-C) data. The full genome comprises 1506 scaffolds with a scaffold N50 of 54.12 Mb, and 785.36 Mb of the assembly were distributed across 18 chromosome-level pseudomolecules (Fig. 1, Supplementary Tables S3, S9 and S10).
Fig. 1.
Genome characteristics of G. menghaiensis. Track a-f: a chromosome, b GC density (gray), c gene density (purple), d transposon element density (green), e transposon element density (red), f LTR-Copia density (pink), g LTR-Gypsy density (blue). All were drawn in a window size of 300 kb, chromosomes units = 1,000,000 bp
We annotated 539.84 Mb of repetitive elements occupying 62.57% of the G. menghaiensis genome (Supplementary Table S3). The majority of the repeats are long terminal repeats (LTRs), about 49.49% of the genome (supplementary tables S11 and S12). Based on a combination of homology search, de novo prediction, and RNA sequence-aided prediction, 17,948 protein-coding genes (PCGs) were annotated with an average length of 13,657 bp (Supplementary Figure S3, Supplementary Table S13). Additionally, 16,402 (91.39%) PCGs were supported by at least one of the transcriptome datasets from tubers, flowers, flower buds and fruits, indicating a high level of annotation accuracy (Supplementary Table S14). The statistics results show that each gene contains 5.15 exons with an average length of 221.78 bp (Supplementary Table S14). Approximately 84.4% of PCGs were functionally annotated by similarity searches against homologs sequences and protein domains (Supplementary Table S15). In addition, we identified noncoding RNA (ncRNA) genes in G. menghaiensis, including 157 rRNA, 292 tRNA, 191 miRNA, and 2725 snRNA genes (Supplementary Table S16).
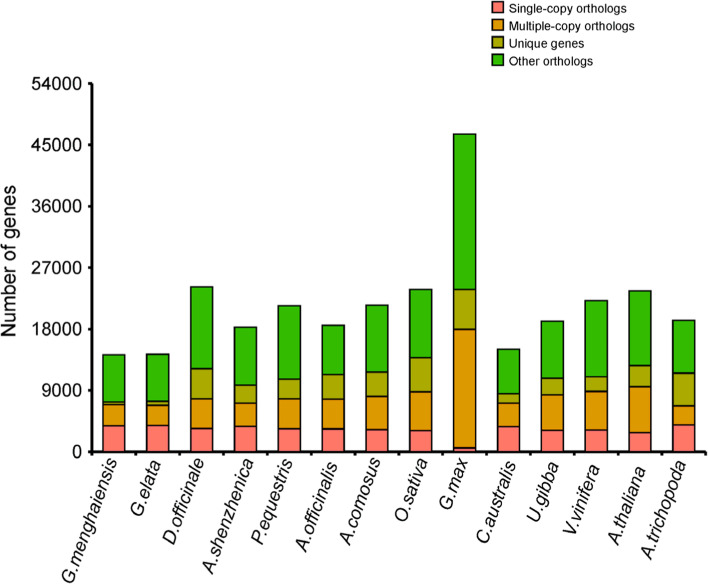
Extensive loss of genes and gene families in Gastrodia menghaiensis genome
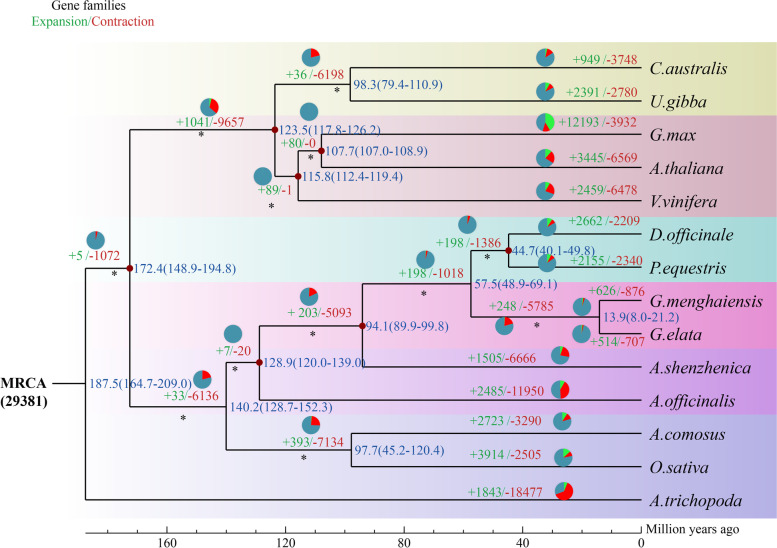
The divergence of Gastrodia from D. officinale/P. equestris was estimated at ~ 57.5 million years, and that of Gastrodia menghaiensis from G. elata at ~ 13.9 million years ago (Fig. 2). A total of 14,233 G. menghaiensis genes (79%) were clustered into four groups, including single-copy, multiple-copy, unique and other orthologs, containing 3,827, 3,100, 379, and 6,927 genes, respectively (Fig. 3). Among the 14 angiosperm species used in the phylogenetic analysis, G. menghaiensis had the smallest number of gene families and on average fewer genes in these families (Fig. 2, and Supplementary Table S13). Of 8,139 gene families shared by these five orchid species, 5,785 had decreased in Gastrodia, whereas 248 gene families had expanded (Fig. 2). KEGG (Kyoto Encyclopedia of Genes and Genomes) [31, 32] enrichment (FDR < 0.05) of expanded gene families of G. menghaiensis include tyrosine metabolism, steroid hormone biosynthesis, prolactin signal pathway, and endocytosis (Supplementary Figure S4); and contracted gene families include vitamin digestion and absorption, prolactin signal pathway, plant-pathogen interactions etc. (Supplementary Figure S5).
Fig. 2.
Phylogenetic position and gene families of G. menghaiensis. a, Inferred phylogenetic tree with 254 single-copy genes of 14 plant species. Gene family expansions are indicated in green, and gene family contractions are indicated in red. Expansions of Gene families are indicated in green, contractions of gene families are indicated in purple. Estimated divergence times (in millions of years) are indicated by light blue boxes, the red star represents the divergence time between Gastrodia. MRCA, most recent common ancestor
Fig. 3.
Bar graph of the number of protein-coding genes in each of 14 species. Single-copy orthologs, common orthologs with one copy in specific species; multi-copy orthologs, common orthologs with multiple copy numbers in specific species; unique gene, genes belonging to only one specific species; other orthologs, genes from families shared in 2–13 species
Compared to green orchids with 854–1,182 unique gene families, G. menghaiensis has 286 unique gene families (Fig. 2, Supplementary Figure S6). GO enrichment (FDR < 0.05) showed that the unique genes were mainly enriched in regulation of cyclin-dependent protein serine/threonine kinase activity, potassium channel activity, potassium ion transmembrane transport, nutrient reservoir activity (Supplementary Figure S7; Supplementary Tables S17 and S18). From most recent common ancestor of Gastrodia (MRCAG), 876 gene families contracted and 626 gene families expanded in G. menghaiensis, and 707 gene families had contracted and 516 gene families expanded in G. elata (Fig. 2). Compared to G. elata, genes related to regulation of autophagy and nitrogen compound transport increased in G. menghaiensis (Supplementary Tables S18, S19 and S20). Compared to P. equestris [33] (29,334 PCGs), D. officinale (29,099 PCGs), G. elata [9, 29] (18,950–21, 115PCGs), and A. shenzhenica [34] (21,676 PCGs), G. menghaiensis has a relatively small proteome (17,948 PCGs), making it the smallest proteome thus far among angiosperms (Supplementary Table S13).
Among the eight species KEGG [31] annotation 132 map results, G. menghaiensis had significant contraction in 69 KEGG maps (the other three orchids, C. australis, O. sativa, A. thaliana and the two Gastrodia species), such as anthocyanin biosynthesis, limonene and pinene degradation, photosynthesis and pyrimidine metabolism, etc. (Supplementary Tables S21 and S22). Notably, compared with A. shenzhenica, P. equestris, D. officinale, the G. menghaiensis genome lost approximately 1073, 2590 and 2794 genes, respectively (Supplementary Table S23).
The rooting pattern of G. menghaiensis is characterized by well-developed branched lateral roots extending along the soil surface in the tropical forests in which it grows. We found there are 410 genes involved in root development in G. menghaiensis, which is similar to 411 of A. shenzhenica, 417 of D. officinale, and 429 genes for P. equestris (Supplementary Table S24). Many genes involved in adventitious root development are more numerous in Gastrodia menghaiensis, such as RPT2b [35], MKK6 [36], PLGG1 [37] (Supplementary Table S24). Some genes involved in root development, such as UTR7 (lateral root emergence) [38], RSL2 (required for root-hair growth) [39], and SIEL (involved in root patterning) [40], were found in G. menghaiensis.
The petals and sepals of Gastrodia are united into a floral tube, which is different from most orchids [20]. We found that genes involved in boundaries of organs, such as AS2, TP3, LOB1, LOF2, LBD1, are fewer or absent in Gastrodia compared to the orchids with free sepals and petals (Supplementary Table S25). The petal lobes are small in size with almost dorsoventral symmetry in Gastrodia, which is different from most orchids. We found that genes involved dorsoventral asymmetry of petals and sepals, such as DICH [41], CYC [42, 43], RAD [44], are fewer or absent in Gastrodia (Supplementary Table S25).
The loss and relative expansion of nuclear genome copies of genes that function in plastids
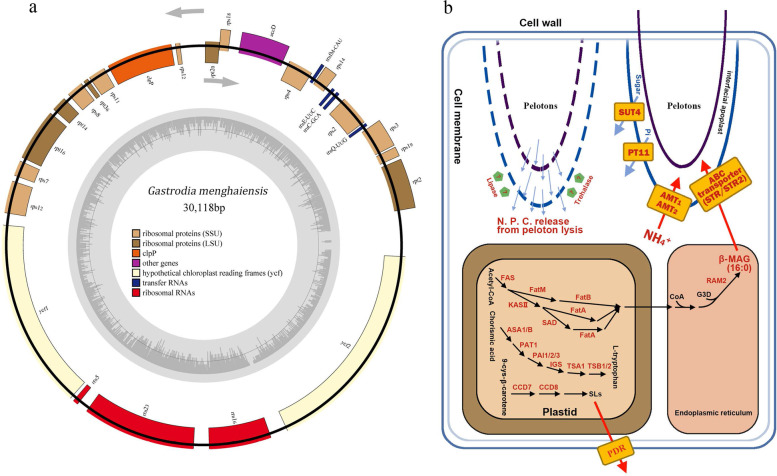
All species of Gastrodia are leafless [20], so we specifically searched the G. menghaiensis genome for genes that mediate leaf development and found that a number of these are absent in the G. menghaiensis genome (Supplementary Table S24, Supplementary Figure S8). To better understand the putative functions of missing genes, we examined nuclear genes of the photosynthesis apparatus, especially chlorophyll, photosystem I, photosystem II, cytochrome b6f, cytochrome C6m, ATP synthase, and rubisco. Our results showed that that chlorophyll a oxygenase required for the chlorophyll b synthesis, together with chlorophyll degradation genes, were absent (Supplementary Tables S26 and S27)). Of the 31 nuclear genes for photosynthetic apparatus proteins (NEP), none was present in the G. menghaiensis genome. The plastid genome of G. menghaiensis (30,118 bp) was dramatically reduced in size (Fig. 4a) compared to the plastid genomes of photosynthetic orchids (see [45])( (Supplementary Table S28, Supplementary Figures S9). Most plastid genes involved in photosynthesis were lost in a manner similar to its counterpart in nuclear genome.
Fig. 4.
Plastid genome of G. menghaiensis and proposed model of biological interaction between G. menghaiensis and symbiotic fungi. a The plastid genomes of G. menghaiensis. SSU, small subunit; LSU, large subunit. b Model of biological interaction between G. menghaiensis and symbiotic microbials. ASA1/B, anthranilate synthase; PAT1, phosphoribosyl tranferase; PAI1/2/3, PRA isomerase; IGS, InGP synthase; TSA1, Trytophan synthase; TSB1/2, Trytophan synthase; SLs, Strigolactone; FAS, fatty acid synthase; KASII, ketoacyl-ACP synthase II; SAD, stearoyl-ACP desaturase; FatA, acyl-ACP thioesterase A; FatB, acyl-ACP thioesterase B; FatC, acyl-ACP thioesterase C; FatM, acyl-ACP thioesterase M; ABC transporter, ATP binding cassette transporter; CoA, coenzyme A; MAG, monoacylglycerol; SUT4, sugar transporter 4; PT11/PT4, Phosphorus transporter 11; AMT1, AMT4, ammonium transporters 1 and 4; CCD 7, CCD 8, carotenoid cleavage dioxygenases; PDR, ATP binding cassette transporter. The schematic diagrams of strigolactone, monoacylglycerol and L-trytophan pathway were edited according to KEGG and reported references [9, 46, 47]
We found that there are approximately 696 nuclear-encoded plastid genes (NPGs) in the G. menghaiensis genome (Supplementary Table S26). Genes related to plastid biosynthesis of aromatic and branched amino acids and fatty acids are intact (Supplementary Tables S26 and S27). Compared to the other orchids, 28 and 38 NPGs had expanded in the genomes of G. menghaiensis and G. elata, respectively. These genes are enriched for GO terms G. menghaiensis in related to following metabolic processes/molecular functions (Supplementary Tables S26 and S27): (1) biosynthesis of aromatic amino acid L-tryptophan (CM1, PAT1); (2) amino acid transmembrane transport (CAT6); (3) starch biosynthetic process (SS4); (4) defense response to oomycetes (APK2, CLT2); (5) transmembrane transporter (SAMC1, NDT1); (6) monoterpenoid biosynthetic process (TPS10); (7) plastid ribosomal large subunit (RPL10, RPL11, RPL28); (8) lipase that hydrolyzes phosphatidylcholine, glycolipids as well as triacylglycerols (DALL1); (9) pentatricopeptide repeat (PPR) proteins for RNA editing (PCMP-E95 or AEF1); (10) plastidal glycolate/glycerate translocator 1 (PLGG1). For G. elata, NPGs enriched for GO terms include (Supplementary Tables S26 and S27): (1) branched chain amino acid (ALS); (2) biosynthesis of aromatic amino acid L-tryptophan (ASB, PAT1); (3) fatty acid biosynthetic process (accD); (4) lipase that hydrolyzes phosphatidylcholine, glycolipids as well as triacylglycerols (DALL1); (5) transmembrane transporter (SAMC1, DIT1); (6) plastid ribosomal subunits (RPL12, RPL14, RPL2-A, RPL9, RPS11, RPS12, RPS3); (7) strigolactone biosynthetic process (CCD7); (8) starch biosynthetic process (SBE3); (9) starch degradation (R1); (10) plastid division protein (CDP1).
Biological interaction between G. menghaiensis and symbiotic microbionts
Mycorrhizal symbioses have been important mutualistic associations between plants and soil fungi for 460 million years, and this link is likely an ancestral feature of all terrestrial plants [48, 49]. Plants depend on soil fungi for uptake of minerals and water, and fungi obtain essential nutrients (carbohydrates and amino acids) from their partners [5, 49–51]. It is hypothesized that mycorrhizal symbioses have triggered the contemporaneous radiations of fungi and plants [48, 49]. Some green orchids obtain organic carbon from both photosynthesis and their mycorrhizal fungi [8, 18]. With total loss of photosynthesis function, leafless G. menghaiensis fully depends on its mycorrhizal partners for organic carbon throughout its life cycle. Mycoheterotrophic plants are an ideal model system to illustrated plant–fungus interactions.
In general for photosynthetic orchids, fungi provide amino acids to the orchids in exchange for minerals and water [52]. It has been hypothesized that interactions between some orchids and their symbiotic microbionts are similar to those between other plants and arbuscular mycorrhizae (AM) [18, 53]. Genes related to colonization of AM were found in five orchids specie genomes, including the development of AM symbiosis (EXO70, SNARE family) [54, 55], fatty acid biosynthesis in plastid and endoplasmic reticulum (FatM, KASI, FAS, RAM1, RAM2) [53, 56, 57], fatty acid transporter (STR/STR2) [57], ammonium transporters (AMT1, AMT2) [58, 59], phosphate transporter (PT11-PT4) [60] (Fig. 4b; Supplementary Table S29), and sugar transporter (SUT4) [14] (Supplementary Table S30). Transcriptome data indicated that most genes were expressed or highly expressed in roots of G. menghaiensis (Supplementary Figures S10 and S11). Suetsugu et al. (2017) indicated that fully mycoheterotrophic albino individuals of Epipactis helleborine (Orchidaceae) had upregulated expression of genes related to AM [25]. The loss of NTR (nitrate transporters) and expansion of the number of genes for urease in Gastrodia indicated that its uptake of nitrogen is mainly in form of ammonium (Supplementary Table S30). SUT4 has been revealed to mediate the transport of sugar from mycorrhizal fungi to G. elata [14].
The interaction with mycorrhizae is crucial for survival of G. menghaiensis. LysM receptor-like kinases (LysM-RLK) mediate this process with AM fungi in plants [61]. Four LysM-RLK were found in the G. menghaiensis genome (Supplementary Table S31). Transcriptome data indicated that two of them were highly expressed in roots (Supplementary Figure S12). It is well known that strigolactones, a class of plant hormones, stimulate AM fungal pre-symbiotic growth [62, 63]. Specifically, strigolactone can stimulate hyphal branching and development of symbiotic fungus A. mellea in G. elata [9]. Key genes for biosynthesis and secretion of strigolactone (CCDS, PDKs) were expanded in G. menghaiensis genome (Supplementary Table S32).
There are lots of debates about the way in which carbon transferred from fungi to orchids [64–66]. Trehalose is an abundant fungal soluble carbohydrate [67]. Smith (1967) suggested that trehalose moved from fungi hyphae to orchid cells as carbon nutrients [65]. Ponert et al. (2021) indicated that orchid protocorms possess an efficient and trehalase-dependent pathway for utilizing exogenous trehalose [64]. Expansion of genes encoding trehalase in genomes of Gastrodia indicated that G. menghaiensis might have developed the ability to use trehalose as its organic carbon source. The pelotons are highly dynamic, and degradation of pelotons also releases large amounts of organic carbon and nitrogen to orchids [68]. Glucans and chitin are two main components of fungal cell walls [69, 70]. There are 36 beta-glucosidase genes (Supplementary Table S29) and four glycoside hydrolase family 18 (GH18) chitinases (Supplementary Table S30). These genes may be involved in the degradation of the cell wall of fungi to provide nutrients for G. menghaiensis, although extensive degradation of fungal tissues in orchids is not typical.
Hyphae of orchid mycorrhizal fungi usually form pelotons in root cells of orchids. The rooting pattern of G. menghaiensis is characterized by well-developed branched lateral roots extending along the soil surface in the tropical forests in which it grows (Figure S1a). We found there are 410 genes involved in root development in G. menghaiensis, which is similar to the 411 of A. shenzhenica, 417 of D. officinale, and 429 genes for P. equestris (Supplementary Table S24). Many genes involved in adventitious root development are more numerous in Gastrodia menghaiensis, such as RPT2b [35], MKK6 [36], PLGG1 [37] (Supplementary Table S24). Some genes involved in root development, such as UTR7 (lateral root emergence) [38], RSL2 (required for root-hair growth) [39], and SIEL (involved in root patterning) [40], were found in G. menghaiensis but absent in the other orchids including G. elata. In particular, two genes related to lateral root development, ASL18a and NF-Y [71, 72], are present in the G. menghaiensis genome but absent from the other orchid genomes. ASL18a and NF-Y together regulate nodule organogenesis in legumes [71, 72]. Although G. elata has lost roots, there are 424 genes involved in root development in G. elata. However, many genes essential for root development, such as PSP, VPS26C, PI-4KBETA2 and PI-4KBETA1, were lost in G. elata (Supplementary Table S24).
Although G. menghaiensis depends on mycorrhizal fungi for life, it still requires protection against attack by pathogens and thus retains defense-related genes. The G. menghaiensis genome contains 28 terpene synthase genes, which defend against pathogens [73, 74], but there are 15, 29 and 43 TPSs in the other orchids (Supplementary Table S33). The G. menghaiensis genome contains 65 R genes R (resistance), which are important components of plant defense system, which is similar to the number in A. shenzhenica but fewer than the other two autotrophic orchid species. The G. menghaiensis genome contains 145 P450s, whereas there are 123 P450s in the genome A. shenzhenica (Supplementary Table S33). Compared to the other orchids, 143 genes involved in plant resistance to pathogens, such as DIR15, SBT3.3, TL1 [39], are increased in the G. menghaiensis genome (Supplementary Table S34).
Materials and methods
Genome sequencing
The Gastrodia menghaiensis used for sequencing was collected from Mengsong, Menghai County, Yunnan Province, China. We had permission from local Forest Department to collect plants for this study. Healthy flowering plants were collected and washed three times with ultrapure water. Then, the plants were immediately frozen in liquid nitrogen and stored at -80 °C prior to DNA extraction. Total DNA was extracted from inflorescences of G. menghaiensis [26, 75] (removing corms and roots) with the DNAsecure Plant Kit (TIANGEN) and cut into random fragments.
We constructed the DNA sequencing libraries and paired-end library with insert size of 350 bp following the standard Illumina library preparation protocols and the manufacturer’s instructions (Illumina, San Diego, CA), respectively. Short-read libraries were sequenced on Illumina HiSeq 2500. We filtered out adapter sequences and the low-quality and duplicated reads, a total of 122.51 Gb of data remained for the assembly.
For Pacbio libraries, at least 10 μg of sheared DNA was required. The SMRT bell template preparation involved DNA concentration, damage repair, end repair, ligation of hairpin adapters, and template purification. SMRT Bell libraries with an insert size of 40 kb were constructed and then sequenced on the PacBio Sequel platform (Pacific Biosciences, USA) using the P6 polymerase/C4 chemistry combination, based on the manufacturer’s procedure (Pacific Biosciences, CA, USA). A total of 100.48 Gb of (102.7-fold coverage of whole genome) data were retained (Supplementary Table S2).
For 10X Genomics libraries, approximately 1 ng of input DNA with 50 kb length was used for the GEM reaction procedure during PCR, and 16-bp barcodes were introduced into droplets. The plant cells (removed the corms and roots) were lysed and HindIII endonuclease was used for digesting the fixed chromatin. The 5’ overhangs of the DNA were recovered with biotin-labeled nucleotides and the resulting blunt ends were ligated to each other using DNA ligase. Proteins were removed with protease to release the DNA molecules from the crosslinks. Then, the droplets were fractured following the purification of the intermediate DNA library. The libraries were finally sequenced on the Illumina Hiseq 2500. Finally, a total of 129.04 Gb (131.9-fold coverage of the genome) data were retained (Supplementary Table S2).
Genome assembly
We estimated the genome size of G. menghaiensis by analyzing the K-mer frequency. Based on 122.59 Gb pair-end reads (350 bp) and the k-mer analysis, we found that the distribution of the17-mer depends on the characteristics of the genome and follows a Poisson’s distribution. The G. menghaiensis genome size was estimated about 988.74 Mb (Supplementary Table S1, Supplementary Figure S2).
De novo assembly of the long reads from the PacBio SMRT Sequencer was performed using FALCON (https://github.com/PacificBiosciences/FALCON/) [76]. To obtain enough corrected reads, the longest coverage of subreads was first selected as seed reads to correct sequence errors. Then, error-corrected reads were aligned to each other and assembled into genomic contigs using FALCON with the following parameters: length_cutoff_pr = 10,000, max_diff = 95, and max_cov = 105. Then, genomic contigs were polished using Quiver [77], which yielded an assembly with a contig N50 size of 2.37 Mb. The total length of this assembly version was 859.11 Mb. Then, we used BWA-MEM [78] to align the 10X Genomics data to the assembly using default settings. Scaffolding was performed by FragScaff [79] with the barcoded sequencing reads. Last, Pilon [80] was used to perform error correction based on the Illumina sequences, generating a genome with a scaffold N50 size of 6.82 Mb. The total length of this assembly version was 862.84 Mb. Subsequently, the Hi-C sequencing data were aligned to the assembled scaffolds by BWA-mem [78] and the scaffolds were clustered onto chromosomes with LACHESIS [81] (http://shendurelab.github.io/LACHESIS/), the final genome was 862.86 Mb and the contig and scaffold N50 were 2.02 Mb and 54.12 Mb, respectively (Supplementary Table S3).
RNA sequencing and assembly
Five tissues of G. menghaiensis, including flower buds, blooming flowers, stems, young fruits, roots, were collected from Menghai County, Yunnan Province, China. All collected samples were washed with ultrapure water then immediately kept in RNALater and stored at -80 °C prior to RNA extraction. For each tissue, three biological replicate samples were analyzed. The total RNA was extracted from all samples using genomic DNA contamination and removed using RNase-Free DNase I (TIANGEN). The integrity of RNA was evaluated on a 1.0% agarose gel stained with ethidium bromide (EB), and its quality and quantity were assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies). The cDNA library was constructed using the NEBNext Ultra RNA Library Prep Kit for Illumina, following the manufacturer’s recommendations. Library preparations were sequenced on an Illumina Hiseq 2500 platform, generating 150 bp paired-end reads.
Clean data were obtained by removing reads containing adapter, reads containing ploy-N and low-quality reads from raw data. We mapped clean reads and high-quality reads to the draft reference genomes by TopHat2 [82] with following the parameters: –max-intron-length 500,000, –read-gap-length 10, –read-edit-dist 15, –max-insertion-length 5 and –max-deletion-length 5. RPKM value for each protein-coding gene was calculated by HTSeq [83] using default parameters. DESeq2 [84] were used for nomorlizing gene expression (BaseMean) in each sample, and identified differentially expressed genes (DEGs) for each compared group by using P-adj (adjusted p value) < 0.05 as the threshold. GO enrichment analysis of DEGs was implemented by the GOseq R package [85], in which the gene length bias was corrected. GO terms with corrected P-values less than 0.05 were considered significantly enriched by DEGs. We used KOBASsoftware [31] to test the statistical enrichment of DEGs in KEGG pathways. Pathways with q-values < 0.05 were considered significantly enriched.
Genome annotation
To predict protein-coding genes in the G. menghaiensis genome, we used homology-based prediction, de novo prediction and transcriptome prediction. Homolog proteins from six plant genomes, Gastrodia elata [9], Dendrobium officinale [86], Apostasia shenzhenica [34], Phalaenopsis equestris [33], Oryza sativa [87], Ananas comosus [88], were downloaded from Ensemble Plants (http://plants.ensembl.org/index.html, ensembl.plant.v32).
Protein sequences from these genomes the aligned to the G. menghaiense genome assembly using TblastN [89] with an E-value cutoff of 1e−5. The BLAST hits were conjoined by Solarsoftware [90], and low-quality records were removed. GeneWise [91] was used to predict the exact gene structure of the corresponding regions for each BLAST hit (Homo-set). For transcriptome-based prediction methods, RNA based prediction methods, RNA-seq data seq data were mapped to the assembly using Tophat (version 2.0.13) [82] and Cufflinks [92] (version 2.1.1), and then the transcripts were assembled into gene models (Cufflinks-set). In addition, RNA-seq data were assembled by Trinity [93] (r20140413p1), creating pseudo-ESTs and ESTs. These pseudo-ESTs were also mapped to the assembly, and gene models were predicted by PASA [94]. This gene set was denoted PASA-T-set (PASA Trinity set) and was used to train ab initio gene prediction programs. Five ab initio gene prediction programs, Augustus (version 2.5.5) [95], Genscan (version 1.0) [96], GlimmerHMM (version 3.0.1) [97], Geneid (version 1.4) (23) [98] and SNAP [99] (version 2006–07-28) were used to predict coding regions in the repeat-masked genome. Gene model evidence from the Homo–set, Cufflinks–set, PASAT–set and ab initio programs set and ab initio programs was combined by EvidenceModeler (EVM) [100] into a non-redundant set of gene structures. Finally, a total of 17,948 genes were predicted genes were predicted from the G. menghaiensis genome (Supplementary Table S13).
The functional annotation of the protein-coding genes was achieved using BLASTP (version 2.2.28) (with an E-cutoff of 1e−5) against two integrated protein sequence databases: SwissProt (https://web.expasy.org/docs/swis--protprot/guideline.html) and NR (version 20,190,709). Protein domains were annotated by searching against the InterPro (version 32.0) [101] and Pfam (version 3.0) databases using InterProScan (version 4.8) and HMMER [102] (version 3.1b1), respectively. The Gene Ontology (GO) terms for each gene were obtained from the corresponding InterPro or Pfam entry. The pathways in which the genes might be involved were assigned by BLAST against the KEGG databases (release 20,190,601) with an E-value cutoff 1e−5. We used the same method to re-annotate six reference genomes (Dendrobium officinale, Apostasia shenzhenica, Phalaenopsis equestris, Asparagus officinalis, Oryza sativa, Arabidopsis thaliana). A total of 15,152 genes were predicted to be functional, accounting for 84.42% of all genes in the G. menghaiensis genome (Supplementary Table S15). Annotation features such as the distributions of mRNA length, exon length, exon number, intron length and CDS length are shown in (Supplementary Figure S2, Supplementary Table S14). Gene structures were predicted with a combination of homology-based prediction, de novo prediction and transcriptome-based prediction. We then generated functional assignments of the G. menghaiensis genes with BLAST in public protein databases, including SwissProt (https://web.expasy.org/docs/swis--protprot/guideline.html), NR (version 20,190,709), Protein domains were annotated by searching against the InterPro (version 32.0), Pfam (version 3.0) and KEGG (release 20,190,601)(https://www.kegg.jp/).
A total of 62.57% repeat sequences in the genome were annotated. Among them, TEs were identified by combining de novo-based and homology-based approaches using RepeatModeler (version 1.0.4) (http://www.repeatmasker.org/RepeatModeler/ RepeatModeler/), LTR_FINDER (version 1.07) (http://tlife.fudan.edu.cn/ltr_finder/ finder/), RepeatScout (version 1.0.5) (http://www.repeat masker.org/) and Piler (version 1.0) (http://www.drive5.com/piler/), RepeatMasker (version 3.3.0) (http://www.repeatmasker.org/org/) and RepeatProteinMask (http://www.repeatmasker.org/org/). Tandem repeats were detected using Tandem Repeats Finder (TRF) (Supplementary Table S12).
Noncoding RNA was predicted using de novo and homology search methods. The tRNA genes were identified by tRNAscanSE software [103] (version 1.3.1). The rRNA fragments were predicted by aligning to the rRNA sequences using BlastN with an E-value value cutoff 1e−10. The miRNA and snRNA genes were predicted by INFERNAL softwares (version 1.1) [104] against the Rfam database (release 11.0) [105]. Finally, we predicted the transfer RNA genes, miRNA genes, small nuclear RNA genes, and ribosomal RNA genes in the G. menghaiensis genome (Supplementary Table S16).
Quality evaluation for genome assembly
We evaluated draft assembly by mapping the high-quality reads from short insert-size PE libraries to the scaffolds using BWA-mem [106]. The distribution of the sequencing depth at each position was calculated using SAMtools to assess the completeness of the genome assembly. Approximately 97.66% of the reads could be mapped to the assembly, which covered 99.55% of the assembled sequence (Supplementary Table S4).
To assess the quality of the genome assembly, we assembled the transcriptome data of G. menghaiensis using Trinity [93], and generated 100,217 unigenes. These unigenes were then mapped to the scaffolds using BLAT [107]). More than 99.63% of these unigenes could be identified in the assembly, indicating that the assembly has good coverage of the gene regions (Supplementary Table S5). The CEGMA (Core Eukaryotic Genes Mapping Approach) pipeline was used to assess the completeness of the genome assembly or annotations. Analysis of the genome assembly for core eukaryotic genes revealed homologs for 93.55% of conserved genes in the assembly (Supplementary Table S6 and S8). We also used BUSCO (Benchmarking Universal Single-Copy Orthologs) to quantitatively assess of genome assembly, gene set, and transcriptome completeness based on evolutionarily informed expectations of gene content from near-universal single-copy orthologs selected from embryophyta_odb9. We found 67.9% conserved genes in the G. menghaiensis genome (Supplementary Table S8).
Gene family construction
Protein sequences from G. menghaiensis and the thirteen other sequenced plant genomes with representatives from Gastrodia elata [9], Dendrobium officinale [86], Apostasia shenzhenica [34], Phalaenopsis equestris [33], Asparagus officinalis [108], Oryza sativa [87], Ananas comosus [88], Cuscuta australis [109], Utricularia gibba [110], Vitis vinifera [111], Glycine max [112], Arabidopsis thaliana [113] and Amborella trichopoda [114] were used for gene family clustering. These 14 species include the angiosperm sister to all others, five core dicots, and seven monocots. Four out of the five orchids with sequenced genome were studied. Arabidopsis thaliana [113], Glycine max[112], Oryza sativa, are model plants; Asparagus officinalis and the orchids belong to same monocot order, Asparagales. All 14 species protein datasets were clustered into paralogous and orthologous using the program OrthoMCL (http://orthomcl.org/orthomcl/) with the inflation parameter 1.5.
Phylogenetic tree and divergence estimation
We aligned all 254 single-copy gene protein sequences by MUSCLE (http://www.drive5.com/muscle/) and combined alignment results to build a super alignment matrix. Then, the phylogenetic tree of 14 species was constructed using RAxML (version 8.0.19) (http://sco.h-its.org/exelixis/web/software/raxml/index.html) with the maximum likelihood method and a bootstrap of 100. A. trichopoda was used as outgroup. The MCMC tree program (http://abacus.gene.ucl.ac.uk/software/paml.html) implemented in phylogenetic analysis by maximum likelihood (PAML) was applied to infer the divergence time based on the phylogenetic tree constructed. The calibration times of the divergence between Dendrobium officinale and Phalaenopsis equestris, Apostasia shenzhenica and other orchid species, Oryza sativa and Ananas comosus, Glysine max and other monocots were obtained from the TimeTree database (http://www.time.org/) and previous results [19, 115, 116].
Expansion and contraction of gene families
We determined expansion and contraction of the gene families by comparing the cluster size differences between the ancestor and each species using the CAFÉ [117] (version 4.0). Functional categories that were enriched for significant gene family expansions mainly included terpene synthase activity, magnesium ion binding, serine-type endopeptidase activity and so on (Supplementary Table S22).
Analysis of R genes, terpene synthase and P450s
To discover R genes in G. menghaiensis genome, we screened for the presence of NB-ARC domain (PF00931) with HMMER(version 3.1b1), resulting in a total of 65 R genes in G. menghaiensis. The NB-ARC domain was also identified for the 6 Ref-Species to discover the R genes in these reference species (Supplementary Table S23). We identified 28 terpene synthase (TPS) by requiring the presence of both the N-terminal domain PF01397 and C-terminal domain PF03936 [118, 119] in the G. menghaiensis genome. The same method was also applied to search for TPSs of the 6 Ref-Species (Supplementary Table S24). P450 genes were identified using PFAM with PF00067 using HMMER (version 3.1b1) (Supplementary Table S23).
The assembly and analysis of plastid genome of G. menghaiensis
The cleaned reads approximately 5 Gb from Illumina HiSeq 2500 were used to assemble the plastid genome (plastome) of G. menghaiensis following methods in Li et al. [120]. The finished plastome scaffolds were reoriented according to the C. triplicata reference plastome. Linear plastome maps were drawn using OGDRAW.
Completed plastomes were annotated using PGA [121].
Identification of genes involved in leaf and root development, fusion of sepals and petals and floral symmetry in G. menghaiensis
To discover leaf development genes in G. menghaiensis, the complete published list of Arabidopsis leaf development genes (327 genes) [122, 123] were used as the queries to blast to 6 Ref-Species to identify the candidate genes. The BLAST hits were conjoined by Solar software, then we compared the consistency of domain of the query and ref-genes use HMMER (version 3.1b1)(Supplementary Table S35). To discover root development genes in G. menghaiensis, the complete published list of Arabidopsis root development genes [113, 124–126] (540 genes) were used as the queries to blast to 6 Ref-Species to identify the candidate genes. To discover the genes involved the fusion of petals and petals and floral symmetry in G. menghaiensis, we used the genes mentioned in as queries to blast to 6 Ref-Species to identify the candidate genes. The BLAST hits were conjoined by Solar software, the blast result was filtered and compared the consistency of domain of the query and ref-genes using HMMER(version 3.1b1) (Supplementary Table S36).
Cytological studies on Gastrodia menghaiensis
Chromosomes of Gastrodia spp. are often diffuse and indistinct, and the size is small [127]. Karyotype of Gastrodia menghaiensis was studied in 2019 and 2020 following methods of Jin et al. [128]. Briefly, fresh root tips about 0.2 cm in length were cut in field, pretreated in 0.002 M 8-hydroxyquinoline at 20 °C about 3 to 4 h. Experiments were repeated five times, we observed ten or more slides for each time. The chromosome numbers of G. menghaiensis are 2n = 36 (Supplementary Fig. 13).
Supplementary Information
Acknowledgements
The authors thank Prof. Yan Luo, Dr. Zhanghai Li, Dr. Deyi Wang, Dr. Xiao Ma for their help on data analyses.
Abbreviations
- BUSCO
Benchmarking universal single-copy orthologs analysis
- CEGMA
Conserved core eukaryotic gene mapping approach
- EST
Expressed Sequence Tag
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LTRs
Long terminal repeats
- PCGs
Protein-coding genes
Authors’ contributions
XJ conceived and supervised the study. YJ, XH, XG, JF, KL, WJ performed the experiments. YJ, XH, XG, JF and YW analyzed the data. XJ, YJ, XH and JF wrote the manuscript. YJ, XJ, MC, XH, XG, JF, YJ, SG, YY and WJ revised the manuscript. All authors read and approved last manuscript.
Funding
This study was supported by from the National Natural Science Foundation of China (31870195 to XJ).
Availability of data and materials
The genome assembly and annotation data of Gastrodia menghaiensis have been deposited to the Figshare database (https://figshare.com/s/f759784e78e86ba71c7c).
The raw sequencing data used for de novo whole-genome assembly and the RNA-seq data for the annotation of G. menghaiensis have been deposited to the NCBI Sequence Read Archive (SRA) under GenBank Bioproject PRJNA695369. The biosample for raw sequencing data is SAMN17216907 and for RNA-seq data SAMN17216905. When the manuscript is accepted, all genome assembly and annotation data will be publicly available.
Declarations
Ethics approval and consent to participate
Plant materials used in this study were collected in field with necessary permissions from local authorities of Forest Department. Voucher specimens (Jin X.H. 18195, identified by XJ & JL) have been deposited in publicly available herbaria (PE Institute of Botany, Chinese Academy of Sciences). All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chase MW, Cameron KM, Freudenstein JV, Pridgeon AM, Salazar G, Van den Berg C, Schuiteman A. An updated classification of Orchidaceae. Bot J Linn Soc. 2015;177(2):151–174. [Google Scholar]
- 2.Yeung EC. A perspective on orchid seed and protocorm development. Botanical Studies. 2017;58:33. doi: 10.1186/s40529-017-0188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen HN, Dixon KW, Jersakova J, Tesitelova T. Germination and seedling establishment in orchids: a complex of requirements. Ann Bot. 2015;116(3):391–402. doi: 10.1093/aob/mcv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett CF, Davis JI. The plastid genome of the mycoheterotrophic corallorhiza striata (Orchidaceae) is in the relatively early stages of degradation. Am J Bot. 2012;99(9):1513–1523. doi: 10.3732/ajb.1200256. [DOI] [PubMed] [Google Scholar]
- 5.Gebauer G, Meyer M. N-15 and C-13 natural abundance of autotrophic and mycoheterotrophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytol. 2003;160(1):209–223. doi: 10.1046/j.1469-8137.2003.00872.x. [DOI] [PubMed] [Google Scholar]
- 6.Graham SW, Lam VK, Merckx VS. Plastomes on the edge: the evolutionary breakdown of mycoheterotroph plastid genomes. New Phytol. 2017;214(1):48–55. doi: 10.1111/nph.14398. [DOI] [PubMed] [Google Scholar]
- 7.Merckx V, Freudenstein JV. Evolution of mycoheterotrophy in plants: a phylogenetic perspective. New Phytol. 2010;185(3):605–609. doi: 10.1111/j.1469-8137.2009.03155.x. [DOI] [PubMed] [Google Scholar]
- 8.Gebauer G, Preiss K, Gebauer AC. Partial mycoheterotrophy is more widespread among orchids than previously assumed. New Phytol. 2016;211(1):11–15. doi: 10.1111/nph.13865. [DOI] [PubMed] [Google Scholar]
- 9.Yuan Y, Jin X, Liu J, Zhao X, Zhou J, Wang X, Wang D, Lai C, Xu W, Huang J et al. The Gastrodia elata genome provides insights into plant adaptation to heterotrophy. Nat Commun. 2018;9:1615. [DOI] [PMC free article] [PubMed]
- 10.Merckx VS, Mennes CB, Peay KG, Geml J. Evolution and diversification. In: Mycoheterotrophy. Springer New York Heidelberg Dordrecht London; 2013:215–44.
- 11.Bidartondo MI, Redecker D, Hijri I, Wiemken A, Bruns TD, Dominguez L, Sersic A, Leake JR, Read DJ. Epiparasitic plants specialized on arbuscular mycorrhizal fungi. Nature. 2002;419(6905):389–392. doi: 10.1038/nature01054. [DOI] [PubMed] [Google Scholar]
- 12.Merckx VS, Freudenstein JV, Kissling J, Christenhusz MJ, Stotler RE, Crandall-Stotler B, Wickett N, Rudall PJ, Maas-Van De Kamer H, Maas PJ. Taxonomy and classification. In: Mycoheterotrophy. Springer New York Heidelberg Dordrecht London; 2013:19–101.
- 13.Leake JR. THE BIOLOGY OF MYCO-HETEROTROPHIC (SAPROPHYTIC) PLANTS. New Phytol. 1994;127(2):171–216. doi: 10.1111/j.1469-8137.1994.tb04272.x. [DOI] [PubMed] [Google Scholar]
- 14.Ho L-H, Lee Y-I, Hsieh S-Y, Lin IS, Wu Y-C, Ko H-Y, Klemens PA, Neuhaus HE, Chen Y-M, Huang T-P, et al. GeSUT4 mediates sucrose import at the symbiotic interface for carbon allocation of heterotrophic Gastrodia elata (Orchidaceae) Plant, Cell Environ. 2021;44(1):20–33. doi: 10.1111/pce.13833. [DOI] [PubMed] [Google Scholar]
- 15.Barrett CF, Sinn BT, Kennedy AH. Unprecedented Parallel Photosynthetic Losses in a Heterotrophic Orchid Genus. Mol Biol Evol. 2019;36(9):1884–1901. doi: 10.1093/molbev/msz111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebauer G, Clemens S. Stealing sugar from the honey fungus. Plant, Cell Environ. 2021;44(1):17–19. doi: 10.1111/pce.13909. [DOI] [PubMed] [Google Scholar]
- 17.Delannoy E, Fujii S. des Francs-Small CC, Brundrett M, Small I: Rampant gene loss in the underground orchid Rhizanthella gardneri hghlights hvolutionary constraints on plastid genomes. Mol Biol Evol. 2011;28(7):2077–2086. doi: 10.1093/molbev/msr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suetsugu K, Yamato M, Miura C, Yamaguchi K, Takahashi K, Ida Y, Shigenobu S, Kaminaka H. Comparison of green and albino individuals of the partially mycoheterotrophic orchid Epipactis helleborine on molecular identities of mycorrhizal fungi, nutritional modes and gene expression in mycorrhizal roots. Mol Ecol. 2017;26(6):1652–1669. doi: 10.1111/mec.14021. [DOI] [PubMed] [Google Scholar]
- 19.Li Y-X, Li Z-H, Schuiteman A, Chase MW, Li J-W, Huang W-C, Hidayat A, Wu S-S, Jin X-H. Phylogenomics of Orchidaceae based on plastid and mitochondrial genomes. Mol Phylogenet Evol. 2019;139:106540. [DOI] [PubMed]
- 20.Pridgeon AM, Cribb PJ, Chase MW. F.N. R: Genera Orchidacearum Vol. 4. Epidendroideae (Part 1) Oxford: Oxford University Press; 2005. [Google Scholar]
- 21.Suetsugu K. Gastrodia longiflora (Orchidaceae: Epidendroideae: Gastrodieae), a new mycoheterotrophic species from Ishigaki Island, Ryukyu Islands. Japan Phytotaxa. 2021;502(1):107–110. [Google Scholar]
- 22.Suetsugu K. Gastrodia amamiana (Orchidaceae; Epidendroideae; Gastrodieae), a new completely cleistogamous species from Japan. Phytotaxa. 2019;413(3):225–230. [Google Scholar]
- 23.Cha JY, Igarashi T. Armillaria species associated with Gastrodia elata in Japan. Eur J For Pathol. 1995;25(6–7):319–326. [Google Scholar]
- 24.Park E-J, Lee WY. In vitro symbiotic germination of myco-heterotrophic Gastrodia elata by Mycena species. Plant Biotechnology Reports. 2013;7(2):185–191. [Google Scholar]
- 25.Suetsugu K, Matsubayashi J, Tayasu I. Some mycoheterotrophic orchids depend on carbon from dead wood: novel evidence from a radiocarbon approach. New Phytol. 2020;227(5):1519–1529. doi: 10.1111/nph.16409. [DOI] [PubMed] [Google Scholar]
- 26.Chen X-Q, Liu Z-J, Zhu G-H, Lang K-Y, Ji Z-H, Luo Y-B, Jin X-H, Cribb PJ, Wood JJ, Gale SW, et al. Flora of China. Beijing: Science Press; 2009. [Google Scholar]
- 27.Liu H, Luo Y, Liu H. Studies of Mycorrhizal Fungi of Chinese Orchids and Their Role in Orchid Conservation in China-A Review. Bot Rev. 2010;76(2):241–262. [Google Scholar]
- 28.Bae E-K, An C, Kang M-J, Lee S-A, Lee SJ, Kim K-T, Park E-J. Chromosome-level genome assembly of the fully mycoheterotrophic orchid Gastrodia elata. G3 (Bethesda, Md) 2022;12(3): jkab433. [DOI] [PMC free article] [PubMed]
- 29.Xu Y, Lei Y, Su Z, Zhao M, Zhang J, Shen G, Wang L, Li J, Qi J, Wu J. A chromosome-scale Gastrodia elata genome and large-scale comparative genomic analysis indicate convergent evolution by gene loss in mycoheterotrophic and parasitic plants. Plant J. 2021;108(6):1609–1623. doi: 10.1111/tpj.15528. [DOI] [PubMed] [Google Scholar]
- 30.Simao FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 31.Mao XZ, Cai T, Olyarchuk JG, Wei LP. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005;21(19):3787–3793. doi: 10.1093/bioinformatics/bti430. [DOI] [PubMed] [Google Scholar]
- 32.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32:D277–D280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai J, Liu X, Vanneste K, Proost S, Tsai W-C, Liu K-W, Chen L-J, He Y, Xu Q, Bian C, et al. The genome sequence of the orchid Phalaenopsis equestris. Nat Genet. 2015;47(1):65-+. doi: 10.1038/ng.3149. [DOI] [PubMed] [Google Scholar]
- 34.Zhang G-Q, Liu K-W, Li Z, Lohaus R, Hsiao Y-Y, Niu S-C, Wang J-Y, Lin Y-C, Xu Q, Chen L-J, et al. The Apostasia genome and the evolution of orchids. Nature. 2017;549(7672):379-+. doi: 10.1038/nature23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueda M, Matsui K, Ishiguro S, Kato T, Tabata S, Kobayashi M, Seki M, Shinozaki K, Okada K. Arabidopsis RPT2a Encoding the 26S Proteasome Subunit is Required for Various Aspects of Root Meristem Maintenance, and Regulates Gametogenesis Redundantly with its Homolog, RPT2b. Plant Cell Physiol. 2011;52(9):1628–1640. doi: 10.1093/pcp/pcr093. [DOI] [PubMed] [Google Scholar]
- 36.Zeng Q, Sritubtim S, Ellis BE. AtMKK6 and AtMPK13 are required for lateral root formation in Arabidopsis. Plant Signal Behav. 2011;6(10):1436–1439. doi: 10.4161/psb.6.10.17089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong H, Bai L, Chang J. Song C-p: Chloroplast protein PLGG1 is involved in abscisic acid-regulated lateral root development and stomatal movement in Arabidopsis. Biochem Biophys Res Commun. 2018;495(1):280–285. doi: 10.1016/j.bbrc.2017.10.113. [DOI] [PubMed] [Google Scholar]
- 38.Handford M, Rodriguez-Furlan C, Marchant L, Segura M, Gomez D, Alvarez-Buylla E, Xiong G-Y, Pauly M, Orellana A. Arabidopsis thaliana AtUTr7 Encodes a Golgi-Localized UDP-Glucose/UDP-Galactose Transporter that Affects Lateral Root Emergence. Mol Plant. 2012;5(6):1263–1280. doi: 10.1093/mp/sss074. [DOI] [PubMed] [Google Scholar]
- 39.Yi K, Menand B, Bell E, Dolan L. A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet. 2010;42(3):264–U108. doi: 10.1038/ng.529. [DOI] [PubMed] [Google Scholar]
- 40.Koizumi K, Wu S, MacRae-Crerar A, Gallagher KL. An Essential Protein that Interacts with Endosomes and Promotes Movement of the SHORT-ROOT Transcription Factor. Curr Biol. 2011;21(18):1559–1564. doi: 10.1016/j.cub.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Luo D, Carpenter R, Copsey L, Vincent C, Clark J, Coen E. Control of organ asymmetry in flowers of Antirrhinum. Cell. 1999;99(4):367–376. doi: 10.1016/s0092-8674(00)81523-8. [DOI] [PubMed] [Google Scholar]
- 42.Menges M, Samland AK, Planchais S, Murray JAH. The D-type cyclin CYCD3;1 is limiting for the G1-to-S-phase transition in Arabidopsis. Plant Cell. 2006;18(4):893–906. doi: 10.1105/tpc.105.039636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valoroso MC, Sobral R, Saccone G, Salvemini M, Ribeiro Costa MM, Aceto S. Evolutionary Conservation of the Orchid MYB Transcription Factors DIV, RAD, and DRIF. Frontiers in Plant Science 2019, 10:10.3389/fpls.2019.01359. [DOI] [PMC free article] [PubMed]
- 44.Baxter CEL, Costa MMR, Coen ES. Diversification and co-option of RAD-like genes in the evolution of floral asymmetry. Plant J. 2007;52(1):105–113. doi: 10.1111/j.1365-313X.2007.03222.x. [DOI] [PubMed] [Google Scholar]
- 45.Feng Y-L, Wicke S, Li J-W, Han Y, Lin C-S, Li D-Z, Zhou T-T, Huang W-C, Huang L-Q, Jin X-H. Lineage-Specific Reductions of Plastid Genomes in an Orchid Tribe with Partially and Fully Mycoheterotrophic Species. Genome Biol Evol. 2016;8(7):2164–2175. doi: 10.1093/gbe/evw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bravo A, Brands M, Wewer V, Doermann P, Harrison MJ. Arbuscular mycorrhiza-specific enzymes FatM and RAM2 fine-tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytol. 2017;214(4):1631–1645. doi: 10.1111/nph.14533. [DOI] [PubMed] [Google Scholar]
- 47.Radwanski ER, Last RL. TRYPTOPHAN BIOSYNTHESIS AND METABOLISM - BIOCHEMICAL AND MOLECULAR-GENETICS. Plant Cell. 1995;7(7):921–934. doi: 10.1105/tpc.7.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lutzoni F, Nowak MD, Alfaro ME, Reeb V, Miadlikowska J, Krug M, Arnold AE, Lewis LA, Swofford DL, Hibbett D, et al. Contemporaneous radiations of fungi and plants linked to symbiosis. Nat Commun. 2018;9:5451. doi: 10.1038/s41467-018-07849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heckman DS, Geiser DM, Eidell BR, Stauffer RL, Kardos NL, Hedges SB. Molecular evidence for the early colonization of land by fungi and plants. Science. 2001;293(5532):1129–1133. doi: 10.1126/science.1061457. [DOI] [PubMed] [Google Scholar]
- 50.Bonfante P, Genre A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat Commun. 2010;1:48. 10.1038/ncomms1046. [DOI] [PubMed]
- 51.Bravo AB, Brands M, Wewer V, Doermann PH, Maria J. Arbuscular mycorrhiza-specific enzymes FatM and RAM2 fine-tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytologist. 2017;214(4):1631–1645. doi: 10.1111/nph.14533. [DOI] [PubMed] [Google Scholar]
- 52.Fochi V, Falla N, Girlanda M, Perotto S, Balestrini R. Cell-specific expression of plant nutrient transporter genes in orchid mycorrhizae. Plant Sci. 2017;263:39–45. doi: 10.1016/j.plantsci.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 53.Garcia K, Delaux P-M, Cope KR, Ane J-M. Molecular signals required for the establishment and maintenance of ectomycorrhizal symbioses. New Phytol. 2015;208(1):79–87. doi: 10.1111/nph.13423. [DOI] [PubMed] [Google Scholar]
- 54.MacLean AM, Bravo A, Harrison MJ. Plant Signaling and Metabolic Pathways Enabling Arbuscular Mycorrhizal Symbiosis. Plant Cell. 2017;29(10):2319–2335. doi: 10.1105/tpc.17.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huisman R, Hontelez J, Mysore KS, Wen J, Bisseling T, Limpens E. A symbiosis-dedicated SYNTAXIN OF PLANTS 13II isoform controls the formation of a stable host-microbe interface in symbiosis. New Phytol. 2016;211(4):1338–1351. doi: 10.1111/nph.13973. [DOI] [PubMed] [Google Scholar]
- 56.Lanfranco L, Fiorilli V, Gutjahr C. Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytol. 2018;220(4):1031–1046. doi: 10.1111/nph.15230. [DOI] [PubMed] [Google Scholar]
- 57.Jiang Y, Wang W, Xie Q, Liu N, Liu L, Wang D, Zhang X, Yang C, Chen X, Tang D, et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science. 2017;356(6343):1172–1175. doi: 10.1126/science.aam9970. [DOI] [PubMed] [Google Scholar]
- 58.Fochi V, Chitarra W, Kohler A, Voyron S, Singan VR, Lindquist EA, Barry KW, Girlanda M, Grigoriev IV, Martin F, et al. Fungal and plant gene expression in the Tulasnella calospora-Serapias vomeracea symbiosis provides clues about nitrogen pathways in orchid mycorrhizas. New Phytol. 2017;213(1):365–379. doi: 10.1111/nph.14279. [DOI] [PubMed] [Google Scholar]
- 59.Dearnaley JDW, Cameron DD. Nitrogen transport in the orchid mycorrhizal symbiosis - further evidence for a mutualistic association. New Phytol. 2017;213(1):10–12. doi: 10.1111/nph.14357. [DOI] [PubMed] [Google Scholar]
- 60.Yang S-Y, Gronlund M, Jakobsen I, Grotemeyer MS, Rentsch D, Miyao A, Hirochika H, Kumar CS, Sundaresan V, Salamin N, et al. Nonredundant Regulation of Rice Arbuscular Mycorrhizal Symbiosis by Two Members of the PHOSPHATE TRANSPORTER1 Gene Family. Plant Cell. 2012;24(10):4236–4251. doi: 10.1105/tpc.112.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He J, Zhang C, Dai H, Liu H, Zhang X, Yang J, Chen X, Zhu Y, Wang D, Qi X, et al. A LysM Receptor Heteromer Mediates Perception of Arbuscular Mycorrhizal Symbiotic Signal in Rice. Mol Plant. 2019;12(12):1561–1576. doi: 10.1016/j.molp.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 62.Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435(7043):824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 63.Besserer A, Becard G, Jauneau A, Roux C, Sejalon-Delmas N. GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol. 2008;148(1):402–413. doi: 10.1104/pp.108.121400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ponert J, Soch J, Vosolsobe S, Cihakova K, Lipavska H. Integrative study supports the role of trehalose in carbon transfer from fungi to mycotrophic orchid. Front Plant Sci. 2021;12:793876. 10.3389/fpls.2021.793876. [DOI] [PMC free article] [PubMed]
- 65.Smith SE. Carbohydrate translocation in orchid mycorrhizas. New Phytol. 1967;66(3):371–380. [Google Scholar]
- 66.Selosse M-A, Boullard B, Richardson D. Noel Bernard (1874–1911): orchids to symbiosis in a dozen years, one century ago. Symbiosis. 2011;54(2):61–68. [Google Scholar]
- 67.Jorge JA, Polizeli M, Thevelein JM, Terenzi HF. Trehalases and trehalose hydrolysis in fungi. FEMS Microbiol Lett. 1997;154(2):165–171. doi: 10.1111/j.1574-6968.1997.tb12639.x. [DOI] [PubMed] [Google Scholar]
- 68.Kuga Y, Sakamoto N, Yurimoto H. Stable isotope cellular imaging reveals that both live and degenerating fungal pelotons transfer carbon and nitrogen to orchid protocorms. New Phytol. 2014;202(2):594–605. doi: 10.1111/nph.12700. [DOI] [PubMed] [Google Scholar]
- 69.Chen W, Jiang X, Yang Q. Glycoside hydrolase family 18 chitinases: The known and the unknown. Biotechnol Adv. 2020;43:107553. 10.1016/j.biotechadv.2020.107553. [DOI] [PubMed]
- 70.Ruiz-Herrera J, Ortiz-Castellanos L. Cell wall glucans of fungi. A review. Cell Surf. 2019;5:100022–100022. doi: 10.1016/j.tcsw.2019.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soyano T, Shimoda Y, Kawaguchi M, Hayashi M. A shared gene drives lateral root development and root nodule symbiosis pathways in Lotus. Science. 2019;366(6468):1021-+. doi: 10.1126/science.aax2153. [DOI] [PubMed] [Google Scholar]
- 72.Bu F, Rutten L, Roswanjaya YP, Kulikova O, Rodriguez-Franco M, Ott T, Bisseling T, van Zeijl A, Geurts R. Mutant analysis in the nonlegume Parasponia andersonii identifies NIN and NF-YA1 transcription factors as a core genetic network in nitrogen-fixing nodule symbioses. New Phytol. 2020;226(2):541–54. [DOI] [PMC free article] [PubMed]
- 73.Block AK, Vaughan MM, Schmelz EA, Christensen SA. Biosynthesis and function of terpenoid defense compounds in maize (Zea mays) Planta. 2019;249(1):21–30. doi: 10.1007/s00425-018-2999-2. [DOI] [PubMed] [Google Scholar]
- 74.Ye J, Zhang L, Zhang X, Wu X, Fang R. Plant defense networks against insect-borne pathogens. Trends Plant Sci. 2020;26(3):272–87. [DOI] [PubMed]
- 75.Chen S-C. Flora Republicae Popularis Sinicae 18. Beijing: Science Press; 1999. [Google Scholar]
- 76.Chin C-S, Peluso P, Sedlazeck FJ, Nattestad M, Concepcion GT, Clum A, Dunn C, O'Malley R, Figueroa-Balderas R, Morales-Cruz A, et al. Phased diploid genome assembly with single-molecule real-time sequencing. Nat Methods. 2016;13(12):1050-+. doi: 10.1038/nmeth.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chin C-S, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10(6):563-+. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 78.Li H. Toward better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics. 2014;30(20):2843–2851. doi: 10.1093/bioinformatics/btu356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adey A, Kitzman JO, Burton JN, Daza R, Kumar A, Christiansen L, Ronaghi M, Amini S, Gunderson KL, Steemers FJ, et al. In vitro, long-range sequence information for de novo genome assembly via transposase contiguity. Genome Res. 2014;24(12):2041–2049. doi: 10.1101/gr.178319.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PloS one. 2014; 9(11):0112963. 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed]
- 81.Burton JN, Adey A, Patwardhan RP, Qiu R, Kitzman JO, Shendure J. Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions. Nat Biotechnol. 2013;31(12):1119-+. doi: 10.1038/nbt.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anders S, Pyl PT, Huber W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R10. 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed]
- 85.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11(2):R14. 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed]
- 86.Yan L, Wang X, Liu H, Tian Y, Lian J, Yang R, Hao S, Wang X, Yang S, Li Q, et al. The Genome of Dendrobium officinale Illuminates the Biology of the Important Traditional Chinese Orchid Herb. Mol Plant. 2015;8(6):922–934. doi: 10.1016/j.molp.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 87.Ouyang S, Zhu W, Hamilton J, Lin H, Campbell M, Childs K, Thibaud-Nissen F, Malek RL, Lee Y, Zheng L, et al. The TIGR Rice Genome Annotation Resource: Improvements and new features. Nucleic Acids Res. 2007;35:D883–D887. doi: 10.1093/nar/gkl976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen L-Y, VanBuren R, Paris M, Zhou H, Zhang X, Wai CM, Yan H, Chen S, Alonge M, Ramakrishnan S, et al. The bracteatus pineapple genome and domestication of clonally propagated crops. Nat Genet. 2019;51(10):1549-+. doi: 10.1038/s41588-019-0506-8. [DOI] [PubMed] [Google Scholar]
- 89.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. BASIC LOCAL ALIGNMENT SEARCH TOOL. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 90.Yu X-J, Zheng H-K, Wang J, Wang W, Su B. Detecting lineage-specific adaptive evolution of brain-expressed genes in human using rhesus macaque as outgroup. Genomics. 2006;88(6):745–751. doi: 10.1016/j.ygeno.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 91.Bateman A, Birney E, Durbin R, Eddy SR, Howe KL, Sonnhammer ELL. The Pfam protein families database. Nucleic Acids Res. 2000;28(1):263–266. doi: 10.1093/nar/28.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28(5):511–U174. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campbell MA, Haas BJ, Hamilton JP, Mount SM, Buell CR. Comprehensive analysis of alternative splicing in rice and comparative analyses with Arabidopsis. Bmc Genomics. 2006;7:327. 10.1186/1471-2164-7-327. [DOI] [PMC free article] [PubMed]
- 95.Stanke M, Steinkamp R, Waack S, Morgenstern B. AUGUSTUS: a web server for gene finding in eukaryotes. Nucleic Acids Res. 2004;32:W309–W312. doi: 10.1093/nar/gkh379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aggarwal G, Ramaswamy R. Ab initio gene identification: prokaryote genome annotation with GeneScan and GLIMMER. J Biosci. 2002;27(1):7–14. doi: 10.1007/BF02703679. [DOI] [PubMed] [Google Scholar]
- 97.Majoros WH, Pertea M, Salzberg SL. TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene-finders. Bioinformatics. 2004;20(16):2878–2879. doi: 10.1093/bioinformatics/bth315. [DOI] [PubMed] [Google Scholar]
- 98.Alioto T, Blanco E, Parra G, Guigo R. Using geneid to Identify Genes. Curr Protoc Bioinformatics. 2018;64(1):e56. doi: 10.1002/cpbi.56. [DOI] [PubMed] [Google Scholar]
- 99.Bromberg Y, Rost B. SNAP: predict effect of non-synonymous polymorphisms on function. Nucleic Acids Res. 2007;35(11):3823–3835. doi: 10.1093/nar/gkm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haas BJ, Salzberg SL, Zhu W, Pertea M, Allen JE, Orvis J, White O, Buell CR, Wortman JR. Automated eukaryotic gene structure annotation using EVidenceModeler and the program to assemble spliced alignments. Genome Biol. 2008;9(1):R7. 10.1186/gb-2008-9-1-r7. [DOI] [PMC free article] [PubMed]
- 101.Hunter S, Apweiler R, Attwood TK, Bairoch A, Bateman A, Binns D, Bork P, Das U, Daugherty L, Duquenne L, et al. InterPro: the integrative protein signature database. Nucleic Acids Res. 2009;37:D211–D215. doi: 10.1093/nar/gkn785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25(5):955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nawrocki EP, Kolbe DL, Eddy SR. Infernal 1.0: inference of RNA alignments. Bioinformatics. 2009;25(10):1335–1337. doi: 10.1093/bioinformatics/btp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Griffiths-Jones S, Moxon S, Marshall M, Khanna A, Eddy SR, Bateman A. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res. 2005;33(Database issue):D121–124. doi: 10.1093/nar/gki081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kathiresan N, Temanni MR, Al-Ali R, Ieee. Performance Improvement of BWA MEM Algorithm using Data-parallel with Concurrent Parallelization; 2014.
- 107.Kent WJ. BLAT - The BLAST-like alignment tool. Genome Res. 2002;12(4):656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harkess A, Zhou J, Xu C, Bowers JE, Van der Hulst R, Ayyampalayam S, Mercati F, Riccardi P, McKain MR, Kakrana A, et al. The Asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat Commun. 2017;8:1279. doi: 10.1038/s41467-017-01064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun G, Xu Y, Liu H, Sun T, Zhang J, Hettenhausen C, Shen G, Qi J, Qin Y, Li J, et al. Large-scale gene losses underlie the genome evolution of parasitic plant Cuscuta australis. Nat Commun. 2018;9:2683. 10.1038/s41467-018-04721-8. [DOI] [PMC free article] [PubMed]
- 110.Carretero-Paulet L, Librado P, Chang T-H, Ibarra-Laclette E, Herrera-Estrella L, Rozas J, Albert VA. High Gene Family Turnover Rates and Gene Space Adaptation in the Compact Genome of the Carnivorous Plant Utricularia gibba. Mol Biol Evol. 2015;32(5):1284–1295. doi: 10.1093/molbev/msv020. [DOI] [PubMed] [Google Scholar]
- 111.Jaillon O, Aury J-M, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C, et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007;449(7161):463–U465. doi: 10.1038/nature06148. [DOI] [PubMed] [Google Scholar]
- 112.Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, et al. Genome sequence of the palaeopolyploid soybean (vol 463, pg 178, 2010) Nature. 2010;465(7294):120–120. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- 113.Lamesch P, Berardini TZ, Li D, Swarbreck D, Wilks C, Sasidharan R, Muller R, Dreher K, Alexander DL, Garcia-Hernandez M, et al. The Arabidopsis Information Resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 2012;40(D1):D1202–D1210. doi: 10.1093/nar/gkr1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Albert VA, Barbazuk WB, dePamphilis CW, Der JP, Leebens-Mack J, Ma H, Palmer JD, Rounsley S, Sankoff D, Schuster SC, et al. The Amborella Genome and the Evolution of Flowering Plants. Science. 2013;342(6165):1467-+. doi: 10.1126/science.1241089. [DOI] [PubMed] [Google Scholar]
- 115.Xiang X-G, Mi X-C, Zhou H-L, Li J-W, Chung S-W, Li D-Z, Huang W-C, Jin W-T, Li Z-Y, Huang L-Q, et al. Biogeographical diversification of mainland Asian Dendrobium (Orchidaceae) and its implications for the historical dynamics of evergreen broad-leaved forests. J Biogeogr. 2016;43(7):1310–1323. [Google Scholar]
- 116.Givnish TJ, Spalink D, Ames M, Lyon SP, Hunter SJ, Zuluaga A, Iles WJD, Clements MA, Arroyo MTK, Leebens-Mack J, et al. Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proceedings of the Royal Society B-Biological Sciences. 1814;2015(282):171–180. doi: 10.1098/rspb.2015.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Han MV, Thomas GWC, Lugo-Martinez J, Hahn MW. Estimating Gene Gain and Loss Rates in the Presence of Error in Genome Assembly and Annotation Using CAFE 3. Mol Biol Evol. 2013;30(8):1987–1997. doi: 10.1093/molbev/mst100. [DOI] [PubMed] [Google Scholar]
- 118.Bohlmann J, Meyer-Gauen G, Croteau R. Plant terpenoid synthases: Molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA. 1998;95(8):4126–4133. doi: 10.1073/pnas.95.8.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu W, Lu H, Li Y, Yao H, Luo H. The new advance of terpene synthase research in the plant. Plant Physiology Journal. 2017;53(7):1139–1149. [Google Scholar]
- 120.Li Z-H, Ma X, Wang D-Y, Li Y-X, Wang C-W, Jin X-H. Evolution of plastid genomes of Holcoglossum (Orchidaceae) with recent radiation. BMC Evol Biol. 2019;19:63. 10.1186/s12862-019-1384-5. [DOI] [PMC free article] [PubMed]
- 121.Qu X-J, Moore MJ, Li D-Z, Yi T-S. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods. 2019;15:50.10.1186/s13007-019-0435-7. [DOI] [PMC free article] [PubMed]
- 122.Szakonyi D. LEAFDATA: a literature-curated database for Arabidopsis leaf development. Plant Methods. 2016;12:15. 10.1186/s13007-016-0115-9. [DOI] [PMC free article] [PubMed]
- 123.Capella M, Ribone PA, Arce AL, Chan RL. Arabidopsis thaliana HomeoBox 1 (AtHB1), a Homedomain-Leucine Zipper I (HD-Zip I) transcription factor, is regulated by phytochrome-interacting factor 1 to promote hypocotyl elongation. New Phytol. 2015;207(3):669–682. doi: 10.1111/nph.13401. [DOI] [PubMed] [Google Scholar]
- 124.Wachsman G, Sparks EE, Benfey PN. Genes and networks regulating root anatomy and architecture. New Phytol. 2015;208(1):26–38. doi: 10.1111/nph.13469. [DOI] [PubMed] [Google Scholar]
- 125.van Gelderen K, Kang C, Paalman R, Keuskamp D, Hayes S, Pierik R. Far-red light detection in the shoot regulates lateral root development through the HY5 transcription factor. Plant Cell. 2018;30(1):101–116. doi: 10.1105/tpc.17.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tian H, De Smet I, Ding Z. Shaping a root system: regulating lateral versus primary root growth. Trends Plant Sci. 2014;19(7):426–431. doi: 10.1016/j.tplants.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 127.Dawson MI, Molloy BPJ, Beuzenberg EJ. Contributions to a chromosome atlas of the New Zealand flora-39 orchidaceae. N Z J Bot. 2007;45(4):611–684. [Google Scholar]
- 128.Jin X-H, Zhang T, Gu Z-J, Li D-Z. Cytological studies on the genus Holcoglossum (Orchidaceae) Bot J Linn Soc. 2007;154(2):283–288. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome assembly and annotation data of Gastrodia menghaiensis have been deposited to the Figshare database (https://figshare.com/s/f759784e78e86ba71c7c).
The raw sequencing data used for de novo whole-genome assembly and the RNA-seq data for the annotation of G. menghaiensis have been deposited to the NCBI Sequence Read Archive (SRA) under GenBank Bioproject PRJNA695369. The biosample for raw sequencing data is SAMN17216907 and for RNA-seq data SAMN17216905. When the manuscript is accepted, all genome assembly and annotation data will be publicly available.